Abstract
Male life history and resource allocation is not frequently studied in aging and life span research. Here we verify that males of long-lived fruit-feeding butterfly species have reduced longevity on restricted diets (Beck 2007 Oecologia), in contrast to the common finding of longevity extension in dietary restriction experiments in Drosophila and some other organisms. Males of some of the most long-lived species of fruit-feeding butterflies were collected from Kibale Forest, Uganda, and kept on diets of either sugar or mashed banana. Seven out of eight species had non-significantly longer life spans on mashed banana diets. Data analysis using a time-varying Cox-model with species as covariate showed that males had reduced survival on the sugar diet during the first 35 days of captive life, but the effect was absent or reversed at more advanced ages. These results challenge the generality of dietary restriction as a way to extend life span in animals. We argue that such studies on males are promising tools for better understanding life history evolution and aging because males display a wider variety of tactics for obtaining reproductive success than females.
Keywords: mating tactics, reproduction, time-varying Cox-model, wing-wear
Introduction
The majority of studies on effects of dietary restriction on longevity show life span extension under dietary restriction (Partridge et al., 2005), including model organisms ranging from yeasts (e.g., Saccharomyces cerevisaie Lin et al., 2002) to invertebrates (e. g., the nematode Caenorhabditis elegans Walker et al., 2005) and mammals (e.g., the rhesus monkey, Macaca mulatta Lane et al., 2004). Common belief that similar mechanisms can be inherent to humans stimulates intense scrutiny of the longevity benefits in model organisms (see recent review in Kennedy et al., 2007). However, recent studies that better represent the integrative nature of diet and resource allocation (using dietary restriction gradients, analyses of body composition, or measurement or control of food intake) show that these manipulations merely scratch the surface of the biological changes that are induced by diet (Carey et al., 2008a; Lee et al., 2008; Maklakov et al., 2008; Skorupa et al., 2008). Important model organisms in this field, such as lab rats, Drosophila melanogaster and Tephritid flies, have an evolutionary history on more or less complete diets.
The evolutionary history of Lepidoptera is probably dominated by adult diets consisting mainly of nectar sugars (Barth, 1991). Therefore, sugar solutions are regarded as the control to test for the effects of added amino acids or other nutrients on reproduction in this order. Usually, these additions do not affect the life span of female butterflies (Fischer and Fiedler, 2001; Hill and Pierce, 1989; Mevi-Schütz and Erhardt, 2003, 2005; Romeis and Wackers, 2002). A remarkable exception is found in the pollen feeding Heliconius butterflies where females deprived of pollen (which supplies them with amino acids for egg-production, O'Brien et al., 2003) live much shorter (Dunlap-Pianka, 1979; Dunlap-Pianka et al., 1977; Gilbert, 1972). Thus, while Heliconius diet is more complete like in Drosophila, dietary restriction via changes in diet composition had the opposite effect from that seen in Drosophila.
Recent data suggest that the life histories of fruit-feeding butterflies may tend towards reliance on sources of amino acids in the adult diet (Molleman et al., 2009), and Beck and Fiedler (2009) summarized circumstantial evidence for amino acids in the adult diet playing a crucial role in extended longevity in butterflies. Within this feeding guild, small effects of amino acids on longevity were found in B. anynana where females being additionally fed amino acids had lower survival rates compared to females fed on sugar only or banana (Bauerfeind and Fischer, 2005; Molleman et al., 2008b). In the larger Charaxes fulvescens, females fed banana lived shorter than those feeding on a sugar solution, while addition of amino acids to the sugar solution did not significantly affect longevity (Molleman et al., 2008a). Diet significantly affected mortality in captivity in a time-dependent manner in female Euphaedra butterflies, where butterflies fed a sugar-only diet initially survived better than those fed banana (see Table 1 and discussion for more data and details; Molleman et al., 2009).
Table 1.
Summary statistics for male butterflies collected from Kibale Forest, Uganda with; sample sizes (number of censored observations in brackets), median life span in captivity in days and maximum life span. Data for con-specific females are given in italics for comparison but statistical details for females are treated elsewhere. The longest median and maximum life span for each species and sex is in bold. As banana fed males had higher survival rates during the first 35 days, median lifespan tended to be longer for the banana cohorts, but as older (>45 days) males showed the opposite effect, sugar fed males often had the longest captive life span for a species.
| N (censored) | Median Life Span | Maximum Life Span | ||||
|---|---|---|---|---|---|---|
| Species | sugar | banana | sugar | banana | sugar | Banana |
| E. medon ♂ | 17 (4) | 20 (2) | 26 | 44 | 123 | 86 |
| ♀ | 18 (4) | 21 | 31.5 | 39 | 59 | 80 |
| E. alacris ♂ | 23 (2) | 20 | 22 | 39 | 64 | 112 |
| ♀ | 19 (3) | 23 (1) | 29 | 32 | 50 | 83 |
| Charaxes comb. ♂ | 22 | 15 | 26 | 40 | 108 | 62 |
| ♀ | 12 (2) | 14 | 10 | 30 | 76 | 52 |
| C. numenes ♂ | 6 | 5 | 32.5 | 33 | 83 | 40 |
| ♀ | 6 (1) | 5 | 21.5 | 33 | 76 | 43 |
| C. bipunctatus♂ | 3 | 1 | 27 | 58 | 108 | 58 |
| ♀ | 2 (1) | 3 | 3 | 39 | 3 | 52 |
| C. tiridates♂ | 2 | 1 | 47 | 43 | 52 | 43 |
| ♀ | 0 | 2 | 10.5 | 15 | ||
| C. protoclea ♂ | 5 | 4 | 25 | 43.5 | 51 | 62 |
| ♀ | 2 | 2 | 22.5 | 26.5 | 40 | 30 |
| C. Cynthia ♂ | 4 | 2 | 22.5 | 25.5 | 63 | 45 |
| ♀ | 1 | 0 | 45 | 45 | ||
| C. pollux ♂ | 2 | 2 | 13 | 43 | 22 | 50 |
| ♀ | 1 | 2 | 4 | 36 | 4 | 42 |
Studies on life span effects of caloric restriction in Lepidoptera are scarce. In the butterfly Speyeria mormonia, adult survival was lower in both males and females subjected to larval semi-starvation (Boggs and Freeman, 2005), while adult caloric restriction had no effect on male or female life span but reduced female fecundity (Boggs and Ross, 1993) and male rates of spermatophore formation (Boggs unpub.). Individuals of Jalmenus evagoras attained the greatest longevity on a medium (25% wt/wt) sugar diet (Hill and Pierce, 1989). In the fruit-feeding butterfly Bicyclus anynana, life span is reduced under caloric restriction (Ferkau and Fischer, 2006; Molleman et al., 2008b).
Because information on life history of females is putatively most important for demography and population biology, and insect female reproduction is more readily measured in the laboratory (number of eggs), male life histories and resource allocation strategies are less frequently studied. Males, on the other hand, have attracted much attention from ethologists because of the wide range of mating tactics they can adopt (i.e. Alcock, 1994; Arlet et al., 2008; Waltz and Wolf, 1984). Therefore, the study of male life histories offers largely ignored opportunities to learn more about the evolution of life histories and the interrelatedness of behavior and life history.
Beck (2007) compared survival in captivity on a sugar solution versus an amino acid enriched sugar solution (butterflies also had access to salt) on Bornean wild-caught rainforest butterflies and caught mainly males. He found that males of some long lived butterfly species (including the fruit-feeding Faunis gracilis Morphinae) lived significantly longer when they had access to amino acids. His sample sizes per species and diet treatment varied between 5 and 19, while life spans ranged from 2 days to 4 weeks, and data were analyzed using two-tailed t-tests on log-transformed life span data.
For the one fruit-feeding butterfly species included (which responded significantly to amino acids), there were only 5 individuals in each diet treatment (Beck, 2007). Therefore, we further test the hypothesis that long-lived fruit-feeding butterfly males live longer when they have access to a diet that contains amino acids, using larger sample sizes and more appropriate statistical tools.
Experimental methods
We used the two Euphaedra species with the longest life span records (Molleman et al., 2007) and several large Charaxes species (Table 1: Molleman et al., 2005a). Male butterflies were collected from Kibale National Park in April-May 2007 using baited traps, regardless of wing-wear, and kept singly in cages at Makerere University Biological Field Station. The locally manufactured cages were cylindrical (30 cm height, 21 cm width), made of fine mesh and 2 metal rings and fitted with a zipper. Butterflies and cages were numbered and hung in a room with four mesh covered windows (allowing ventilation and indirect sunlight) and a high (3-4 meters) wooden ceiling under a tin roof.
Wing length was measured using a ruler, and percent scale loss and wing surface loss were estimated and any missing appendages were recorded. Individuals were randomly assigned to either a mashed banana or a 10% (wt:vol) sugar solution diet. The mashed banana was prepared by slicing and mashing about 30 bananas in a clean bucket. Bananas contain about 1 gram of protein and 14 grams of sugar per 100 grams (NutritionData, 2008), and their sugar composition is very variable with about 50% sucrose, 25% glucose and 25% fructose (Molleman et al., 2008b). The sugar solution was made of locally obtained cane sugar and boiled rainwater. Banana was presented on a plastic dish and replaced twice a week and sugar solution was presented in a small vial mounted on a piece of cardboard, topped off daily and refreshed once every other week. Butterflies were generally observed to find the sugar solution quickly and to feed from the vial, but this was not verified for all individuals. Water was presented as wet tissue paper on a plastic dish inside the cages. The tissue paper was moistened by spraying water daily and was replaced weekly.
Differences with the method of Beck (2007) include: no restriction on butterfly wear at capture, use of mashed banana instead of a sugar solution with amino acids, and no salt was provided. By including more worn and thus presumably older individuals, information on aging in the wild could potentially be gained (Carey et al., 2008b; Molleman et al., 2008a; Molleman et al., 2009). Kibale National Park features extraordinary high butterfly population densities (Molleman et al., 2006) that enabled us to obtain our sample sizes in a matter of days for the Euphaedra included.
Results
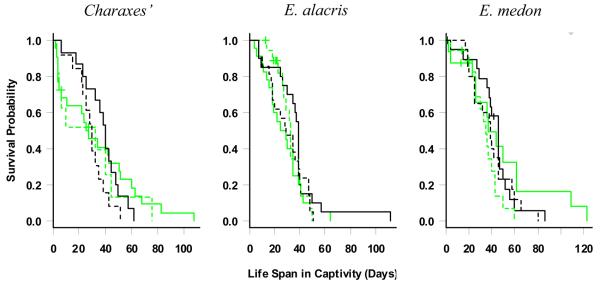
Eight of 117 male butterflies died accidently or escaped from cages and were treated as censored observations. The median life span in captivity of the banana cohorts was insignificantly longer than that of the sugar cohort in all but one species (C. tiridates) which had very small sample sizes (7 out of 8 species; Table 1). In E. medon, E. alacris and the Charaxes species combined, banana diet seemed beneficial in the early period of capture but later seemed to reduce survival compared to the sugar diet (Figure 1). To formally test this hypothesis, we pooled all data and constructed a time-varying Cox model to fit the lifespan in captivity (Table 2). Banana diet was compared with sugar using three parameters for three different periods: in the first 35 days in captivity, from the 36th day to the 45th day, and afterwards. Species (with Charaxes pooled) were adjusted as a covariate in the model.
Figure 1.
Kaplan-Meier Estimation of Survival Probability of a mix of large Charaxes species and two Euphaedra species. Life span of males are presented as solid lines, female butterflies are represented by dashed lines. The grey/green is for sugar and black is for banana diets. Note that the survival probability here is not adjusted for other covariates such as scale loss.
Table 2.
Parameter estimation in the time-varying Cox model with E. medon at zero scale loss as the baseline. Results are significant when the confidence interval does not contain 1. Note that time effects vary among species and are most pronounced in the pooled Charaxes species. When Charaxes are excluded, the <35days period still shows a significant diet effect, but the >45 days is no longer significant.
| Variable | Parameter Estimation |
Standard Error |
p-value | Hazard Ratio |
95% CI for Hazard Ratio |
|---|---|---|---|---|---|
| Diet <35 day | −0.883 | 0.317 | 0.005 | 0.414 | [0.222, 0.769] |
| Diet36~45 day | 0.206 | 0.379 | 0.586 | 1.299 | [0.585, 2.581] |
| Diet >45 day | 0.769 | 0.378 | 0.042 | 2.157 | [1.028, 4.525] |
| Charaxes | 0.384 | 0.250 | 0.124 | 1.468 | [0.900, 2.395] |
| E. alacris | 0.568 | 0.248 | 0.022 | 1.765 | [1.085, 2.869] |
| Scale loss | 0.035 | 0.026 | 0.188 | 1.035 | [0.983, 1.090] |
The loss of wing scales, as a surrogate of age at capture (Ehrlich and Gilbert, 1973; Kemp, 2001), was also included as a predictor of life span in captivity. Both ‘species’ and ‘loss of wing scales’ satisfied the proportional hazards model assumptions. During the first 35 days in captivity, the butterflies fed sugar had a higher risk of death than those fed banana. More precisely, within this period the instant mortality of a butterfly fed with banana is only 41.4% (exponent of −0.883 reported in table 2) of that of a butterfly fed with sugar (with p-value=0.005 and 95% confidence interval 22.3%, 77.0%), if they belonged to the same species and had the same level of scale loss at capture. During the second period in captivity, the two diet cohorts were not significantly different. After day 45, the butterflies fed banana had lower survival probabilities than those fed sugar (Table 2). A similar experiment on female butterflies found the opposite effect for three species of Euphaedra: compared to female butterflies fed sugar, the females fed banana had a lower survival rate at first and survived better after a month in captivity (see Figure 1 for 2 Euphaedra species; Molleman et al., 2009). In contrast to Euphaedra, the diet effect was similar for Charaxes males and females (Figure 1). Different species also had significantly different male life spans in captivity regardless of diet: E. medon lived significantly longer than E. alacris, but was not significantly different from the Charaxes species pooled (table 2). Within the current sample, we did not find that male butterflies with more scale loss lived significantly shorter (table 2), which was the case for female butterflies of the same species (Molleman et al., 2009). Although the estimated effect size of scale loss was similar (about 1.04) for both sexes, the standard error for that estimate was much higher for males.
Discussion
Our results corroborate the suggestion of Beck (2007) that nutrients in fruit can phenotypicaly extend life span of male butterflies, although a negative effect on survival was noted later in life. Interestingly, such patterns have not been found in females of Euphaedra butterfly species (Molleman et al., 2009), or females and males of the small fruit-feeding butterfly Bicyclus anynana (Molleman et al., 2008b). Females of Charaxes fulvescens had higher survival rates on sugar based diets than on a (moistened) slice of banana (Molleman et al., 2008a). There are several important caveats for interpreting these results; (1) although we often observed butterflies feeding from the vials with sugar solution we did not verify whether each individual fed from the vial from the first day it entered captivity, while in the banana cohort this issue is not expected to play any role because these butterflies are attracted to the smell of bananas (Molleman et al., 2005c), (2) sticky mashed banana could affect mortality in other ways than through its nutritional value. Because butterflies can be trapped with their wings or other appendages in the sticky mashed banana, sugar fed males may have better survived better later on in captive life; (3) while females were mated and had the opportunity to lay eggs, males in our and Beck's (2007) experiment had no opportunity to mate. In some insect species mating has a male life span cost (i. e. Ferkau and Fischer, 2006; Martin and Hosken, 2004). However, this may not be an important issue here because it is likely that many male butterflies never obtain matings in nature (Beck, 2007; Boggs, 1979), moreover, mating does not confer a life span cost in monarch butterflies (Oberhauser, 1989). The differences between the sexes in Euphaedra could be explained by a survival cost of reproduction in females fed banana, not incurred by males.
Despite these caveats, we have the following evidence that male fruit-feeding butterflies survive better when they feed on a more complete diet; 1) we found large effects in particular for the mixed Charaxes group; 2) the results of Beck (2007) in which the first caveat (males ability to find the vial) was avoided; and 3) the opposite diet effects in females of Euphaedra species so that we would also need to assume a sex-differential in the ability to find vials with sugar solution in this genus.
Beck (2007) pointed out the implications of his findings for butterfly ecology. More insight into mechanisms may be gained if male-specific hypotheses were developed on resource allocation and life history evolution, and if we had more insight into the cause of death of old captive butterflies. A possible explanation for our finding is a decrease in muscle power in males on poor diets, because this (and possibly decrease in coordination), seems to precede death in insects in captivity. Examples of this include; medflies start to spend time laying on their backs (supine behavior) several days before they die (Papadopoulos et al., 2002); and Drosophila with artificially impaired legs appeared to die because the missing of legs decreased their ability to escape from sticky food (Carey et al., 2007).
Muscle mass and power output of insects can increase with adult nutrient intake, or decrease with age or severity of microbial infections, as is well documented in odonates (i. e. Anholt et al., 1991; Marden, 1998; Matsubara et al., 2005; Schilder, 2007). The maintenance (or increase) of muscle power may be aided by nutrients in the adult diet, and in particular amino acids that are important building blocks of muscle proteins. This was found in the damselfly Calopteryx maculata, in which males fly away from their stream after emergence to forage without harassment by other males and several days later return to the stream after significantly increasing their muscle mass, to then defend territories and obtain matings (Kirkton and Schultz, 2001).
When muscle power plays a role in survival, extension of life span through dietary enrichment could be explained by allocation of nutrients in the adult diet to muscles, or these butterflies having no need to resorb muscles to allocate amino acids to other goals (Karlsson, 1994; Stjernholm and Karlsson, 2000). In contrast, females may allocate these nutrients preferentially to eggs, and as a result life span of females is not extended on a more complete diet. Interestingly, for Bicyclus anynana, no such male-female differential response to diet was observed (Molleman et al., 2008b). In this genus, males use pheromones to court females (Nieberding et al., 2007) and produce large spermatophores (but see Ferkau and Fischer, 2006; Molleman et al., 2005a) that not every male is able to produce on consecutive days (Lewis and Wedell, 2007; Molleman et al., 2004). On the other hand, sexual flight dimorphism is slight or absent in this group (Molleman et al., 2005a). The males of B. anynana may thus invest more in pheromone and spermatophore production (even though they did not mate in that experiment) than in muscles, resulting in a trade off between life span and reproduction (Boivin et al., 2005; Lewis and Wedell, 2007) similar to females (Molleman et al., 2008b).
In summary, we hypothesize that (a) male reproductive success in these butterflies is largely dependent on muscle power for male-male competition and courtship, so (b) because investment in muscles increases competitive abilities in male-male interactions and in courtship displays as well as ability to evade predators or free themselves from sticky substances, investment in reproduction and longevity is synergistic in these males. Similar principles may apply to other traits that enhance both competitive ability and defense against predators, such as horns or antlers in ungulates and canine teeth in primates and pigs. Because males also tend to display risky behavior (e. g. by spending a larger proportion of their time flying in the case of butterflies, or defending their social groups in mammals), the investments in these features may not be associated directly with higher survival in the wild (i. e. Arlet and Isbell, 2009; Moore, 1987).
Amino acids in the adult diet could extend life span by supporting other systems such as immune response (Boggs, 2009; Stoehr, 2007) as well. However, given the differences found between males and females in their survival response, we searched for processes that are more prevalent in or unique to males.
Over all, our data suggest that fruit-feeding provides butterflies with the opportunity to ingest larger quantities of amino acids as adults, and this may have enabled males to evolve mating strategies that require higher amino acid intakes (i. e. intensive patrolling and territorial defense or amino acid enriched spermatophores) over a longer life span. Longer reproductive life spans can be expected to be adaptive for males in these tropical butterfly species because receptive females are available any time (Gotthard et al., 2000) since they have overlapping generations, and females mate multiply and probably in intervals (Molleman et al., 2005a).
Further experiments that (1) use sugar solution based diets (as in Beck 2007), (2) include verification of feeding of each individual, (3) include longitudinal (time series of individuals) mass (and if possible muscle mass) and activity measures, and (4) explore dietary-restriction gradients (Carey et al., 2008a; Lee et al., 2008; Maklakov et al., 2008; Skorupa et al., 2008), are needed to provide more definite answers on how nutrients affect male survival patterns in captivity. In addition, the results for Charaxes need further verification because the pooling of data from different species was statistically convenient, but possibly not biologically appropriate. The crucial question of how representative such captive studies are for life in the wild might then be approached by measuring the nutritional and muscular state of butterflies in the wild using mark-recapture or captive cohort protocols. This may be particularly important for these tropical butterflies because the keeping conditions in cages may be poor, or they need much space to exert their agile life-style and some species had to be excluded from my captive studies for this reason (Kelson, 2008; Molleman et al., 2005b; Molleman et al., 2007). In addition, Beck and Fiedler (2009) found a large discrepancy between the life span data from captive and field-studies and assumed that poor keeping conditions in caged tropical studies may be responsible for this finding.
That wing-damage was a not a significant predictor of remaining lifespan in captivity may indicate that rating butterfly age by wear is not so reliable in these species (see for further discussion Molleman et al., 2008a; Molleman et al., 2009).
Our results indicate that nutrient allocation strategies of males and females of the same species can differ more from each other, than from individuals of the same sex of distantly related species. Even though male insects are not considered important in demography and do not provide the advantage of convenient longitudinal measurement of an important aspect of reproductive success (egg production), they merit further investigation. In particular, studies on males can aid in gaining a better understanding of life history evolution because they display a wider variety of tactics for obtaining reproductive success. Our results further challenge the generality of dietary restriction as a way to extend life span in animals.
Acknowledgements
We thank Boniface Balyeganira, Harriet Kesiime, Christopher Aliganyira, Francis Katuramu Kaywanii, John Koojo, and Mary Alum, for their invaluable assistance in the field and in the laboratory and Francis Akoch Edigu for data entry. Thanks also to Shannon McCauley for putting us on the track of the dragonfly literature and Jan Beck and an anonymous reviewer for helpful comments on an earlier version of this manuscript. This study was conducted with kind permission of the Uganda Wildlife Authority (U.W.A.) and the Ugandan National Council for Science and Technology (U.N.C.S.T.). The funding was provided by the National Institute on Aging (PO1 AG022500-01 and PO1 AG608761-10 to JRC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcock J. Alternative mate-locating tactics in Chlosyne californica (Lepidoptera, Nymphalidae) Ethology. 1994;16:103–118. [Google Scholar]
- Anholt BR, Marden JH, Jenkins DM. Pattterns of mass gain and sexual dimorphism in adult dragonflies (Insecta, Ododnata) Canadian Journal of Zoology-Revue Canadienne De Zoologie. 1991;69:1156–1163. [Google Scholar]
- Arlet ME, Molleman F, Chapman CA. Mating tactics in male grey-cheeked mangabeys (Lophocebus albigena) Ethology. 2008 [Google Scholar]
- Arlet ME, Isbell LA. Variation in behavioral and hormonal responses of adult male gray-cheeked mangabeys (Lophocebus albigena) to crowned eagles (Stephanoaetus coronatus) in Kibale National Park, Uganda. Behavioral Ecology and Sociobiology. 2009;63:491–499. [Google Scholar]
- Barth FG. Insects and flowers: The biology of a partnership. Princeton University Press; Princeton: 1991. [Google Scholar]
- Bauerfeind SS, Fischer K. Effects of adult-derived carbohydrates, amino acids and micronutrients on female reproduction in a fruit-feeding butterfly. Journal of Insect Physiology. 2005;51:545–554. doi: 10.1016/j.jinsphys.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Beck J. The importance of amino acids in the adult diet of male tropical rainforest butterflies. Oecologia. 2007;151:741–747. doi: 10.1007/s00442-006-0613-y. [DOI] [PubMed] [Google Scholar]
- Beck J, Fiedler K. Adult life spans of butterflies (Lepidoptera: Papilionoidea plus Hesperioidea): broadscale contingencies with adult and larval traits in multi-species comparisons. Biological Journal of the Linnean Society. 2009;96:166–184. [Google Scholar]
- Boggs CL. Resource allocation and reproductive strategies in several heliconiine butterfly species. University of Texas; Austin: 1979. PhD thesis. [Google Scholar]
- Boggs CL, Ross CL. The effect of adult food limitation on life-history traits in Speyeria mormonia (Lepidoptera, Nymphalidae) Ecology. 1993;74:433–441. [Google Scholar]
- Boggs CL, Freeman KD. Larval food limitation in butterflies: effects on adult resource allocation and fitness. Oecologia. 2005;144:353–361. doi: 10.1007/s00442-005-0076-6. [DOI] [PubMed] [Google Scholar]
- Boggs CL. Understanding insect life histories and senescence through a resource allocation lens. Functional Ecology. 2009;23:27–37. [Google Scholar]
- Boivin G, Jacob S, Damiens D. Spermatogeny as a life-history index in parasitoid wasps. Oecologia. 2005;143:198–202. doi: 10.1007/s00442-004-1800-3. [DOI] [PubMed] [Google Scholar]
- Carey JR, Pinter-Wollman N, Wyman M, Müller HG, Molleman F, Zhang N. A search for principles of disability using experimental impairment of Drosophila melanogaster. Experimental Gerontology. 2007;42:166–172. doi: 10.1016/j.exger.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Harshman LG, Liedo P, Muller HG, Wang JL, Zhang Z. Longevity-fertility trade-offs in the tephritid fruit fly, Anastrepha ludens, across dietary-restriction gradients. Aging Cell. 2008a;7:470–477. doi: 10.1111/j.1474-9726.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Papadopoulos NT, Müller HG, Katsoyannos BI, Kouloussis NA, Wang JL, Wachter K, Yu W, Liedo P. Age structure changes and extraordinary lifespan in wild medfly populations. Aging Cell. 2008b;7:426–437. doi: 10.1111/j.1474-9726.2008.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap-Pianka HL, Boggs CL, Gilbert LE. Ovarian dynamics in Heliconiine butterflies: programmed senescence versus eternal youth. Science. 1977;197:487–490. doi: 10.1126/science.197.4302.487. [DOI] [PubMed] [Google Scholar]
- Dunlap-Pianka HL. Ovarian dynamics in Heliconius butterflies: correlations among daily oviposition rates, egg weights, and quantitative aspects of oogenesis. Journal of Insect Physiology. 1979;25:741–749. [Google Scholar]
- Ehrlich PR, Gilbert LE. Population structure and dynamics of the tropical butterfly Helicoinius ethilla. Biotropica. 1973;5:69–82. [Google Scholar]
- Ferkau C, Fischer K. Costs of reproduction in male Bicyclus anynana and Pieris napi butterflies: Effects of mating history and food limitation. Ethology. 2006;112:1117–1127. [Google Scholar]
- Fischer K, Fiedler K. Effects of adult feeding and temperature regime on fecundity and longevity in the butterfly Lycaena hippothoe (Lycaenidae) Journal of the Lepidopterists' Society. 2001;53:91–95. [Google Scholar]
- Gilbert LE. Pollen feeding and reproductive biology of Heliconius butterflies. Proceedings of the National Academy of Science, USA. 1972;69:1403–1407. doi: 10.1073/pnas.69.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotthard K, Nylin S, Wiklund C. Mating opportunity and the evolution of sex-specific mortality rates in a butterfly. Oecologia. 2000;8:36–43. doi: 10.1007/PL00008833. [DOI] [PubMed] [Google Scholar]
- Hill CJ, Pierce NE. The effect of adult diet on the biology of butterflies 1: the common imperial blue, Jalmenus evagoras. Oecologia. 1989;81:249–257. doi: 10.1007/BF00379812. [DOI] [PubMed] [Google Scholar]
- Karlsson B. Feeding-habits and change of body-composition with age in 3 Nymphalid butterfly species. Oikos. 1994;69:224–230. [Google Scholar]
- Kelson R. Searching for Methuselah: butterfly longevity revisited; Proceedings of the invertebartes in education and conservation conference; 2008. pp. 51–57. [Google Scholar]
- Kemp DJ. Age-related site fidelity in the territorial butterfly Hypolimnas bolina (L.) (Lepidoptera : Nymphalidae) Australian Journal of Entomology. 2001;4:65–68. [Google Scholar]
- Kennedy BK, Steffen KK, Kaeberlein M. Ruminations on dietary restriction and aging. Cell Mol Life Sci. 2007;64:1323–8. doi: 10.1007/s00018-007-6470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkton SD, Schultz TD. Age-specific behavior and habitat selection of adult male damselflies, Calopteryx maculata (Odonata : Calopterygidae) Journal of Insect Behavior. 2001;14:545–556. [Google Scholar]
- Lane MA, Mattison JA, Roth GS, Brant LJ, Ingram DK. Effects of long-term diet restriction on aging and longevity in primates remain uncertain. J Gerontol A Biol Sci Med Sci. 2004;59:405–7. doi: 10.1093/gerona/59.5.b405. [DOI] [PubMed] [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JWO, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis Z, Wedell N. Effect of adult feeding on male mating behaviour in the butterfly, Bicyclus anynana (Lepidoptera : Nymphalidae) Journal of Insect Behavior. 2007;20:201–213. [Google Scholar]
- Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–8. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- Maklakov AA, Simpson SJ, Zajitschek F, Hall MD, Dessmann J, Clissold F, Raubenheimer D, Bonduriansky R, Brooks RC. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Current Biology. 2008;18:1062–1066. doi: 10.1016/j.cub.2008.06.059. [DOI] [PubMed] [Google Scholar]
- Marden JH. From molecules to mating success: Integrative biology of muscle maturation in a dragonfly. American Zoologist. 1998;38:528–544. [Google Scholar]
- Martin OY, Hosken DJ. Copulation reduces male but not female longevity in Saltella sphondylli (Diptera : Sepsidae) Journal of Evolutionary Biology. 2004:357–362. doi: 10.1046/j.1420-9101.2003.00668.x. [DOI] [PubMed] [Google Scholar]
- Matsubara K, Tojo S, Suzuki N. Age-related changes in flight muscle mass, lipid reserves and flight capacity during adult maturation in males of the territorial damselfly Calopteryx atrata (Odonata : Calopterygidae) Zoological Science. 2005;22:587–592. doi: 10.2108/zsj.22.587. [DOI] [PubMed] [Google Scholar]
- Mevi-Schütz J, Erhardt A. Effects of nectar amino acids on fecundity of the wall brown butterfly (Lasiommata megera L.) Basic and Applied Ecology. 2003;4:413–421. [Google Scholar]
- Mevi-Schütz J, Erhardt A. Amino acids in nectar enhance butterfly fecundity: a long-awaited link. American Naturalist. 2005;165:411–419. doi: 10.1086/429150. [DOI] [PubMed] [Google Scholar]
- Molleman F, Zwaan BJ, Brakefield PM. The effect of male sodium diet and mating history on female reproduction in the puddling squinting bush brown Bicyclus anynana (Lepidoptera) Behavioral Ecology and Sociobiology. 2004;56:404–411. [Google Scholar]
- Molleman F, Grunsven RHA, Liefting M, Zwaan BJ, Brakefield PM. Is male puddling behaviour of tropical butterflies targeted at sodium for nuptial gifts or activity? Biological Journal of the Linnean Society. 2005a;86:345–361. [Google Scholar]
- Molleman F, Krenn HW, Van Alphen ME, Brakefleld PM, Devries PJ, Zwaan BJ. Food intake of fruit-feeding butterflies: evidence for adaptive variation in proboscis morphology. Biological Journal of the Linnean Society. 2005b;86:333–343. [Google Scholar]
- Molleman F, van Alphen ME, Brakefield PM, Zwaan BJ. Preferences and food quality of fruit-feeding butterflies in Kibale Forest, Uganda. Biotropica. 2005c;37:657–663. [Google Scholar]
- Molleman F, Kop A, Brakefield PM, De Vries PJ, Zwaan BJ. Vertical and temporal patterns of biodiversity of fruit-feeding butterflies in a tropical forest in Uganda. Biodiversity and Conservation. 2006;15:107–121. [Google Scholar]
- Molleman F, Zwaan BJ, Brakefield PM, Carey JR. Extraordinary long life spans in fruit-feeding butterflies can provide window on evolution of life span and aging. Experimental Gerontology. 2007;42:472–482. doi: 10.1016/j.exger.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molleman F, Ding J, Wang J-L, Brakefield PM, Carey JR, Zwaan BJ. Adult diet affects life span and reproduction of the fruit-feeding butterfly Charaxes fulvescens. Entomologia Experimentalis et applicata. 2008a;129:54–65. doi: 10.1111/j.1570-7458.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molleman F, Ding J, Wang J-L, Brakefield PM, Carey JR, Zwaan BJ. Amino acid sources in the adult diet do not affect life span and fecundity in the fruit-feeding butterfly Bicyclus anynana. Ecological Entomology. 2008b;33:429–438. doi: 10.1111/j.1365-2311.2008.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molleman F, Ding J, Wang J-L, Carey JR. Nutrients in fruit increase fertility in wild-caught females of long-lived Euphaedra species (Lepidoptera, Nymphalidae) The Journal of Insect Physiology. 2009;55:375–383. doi: 10.1016/j.jinsphys.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SD. Male-biased mortality in the butterfly Euphydryas editha: a novel cost of mate acquisition. American Naturalist. 1987;130:306–309. [Google Scholar]
- Nieberding C, Schneider V, De Vos H, Lassance JM, Lofstedt C, Brakefield PM. Role of male sexual pheromones in sexual selection in the African butterfly Bicyclus anynana. Journal of Insect Science 7. 2007 [Google Scholar]
- 2008 http://www.nutritiondata.com/facts-C00001-01c20Tm.html [Google Scholar]
- O'Brien DM, Boggs CL, Fogel ML. Pollen feeding in the butterfly Heliconius charitonia: isotopic evidence for essential amino acid transfer from pollen to eggs. Proceedings of the Royal Society of London Series B-Biological Sciences. 2003;270:2631–2636. doi: 10.1098/rspb.2003.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser KS. Effects of spermatophores on male and female monarch butterfly reproductive success. Behavioral Ecology and Sociobiology. 1989;25:237–246. [Google Scholar]
- Papadopoulos NT, Carey JR, Katsoyannos BI, Kouloussis NA, Muller HG, Liu XL. Supine behaviour predicts the time to death in male Mediterranean fruitflies (Ceratitis capitata) Proceedings of the Royal Society of London Series B-Biological Sciences. 2002;269:1633–1637. doi: 10.1098/rspb.2002.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Piper MDW, Mair W. Dietary restriction in Drosophila. Mechanisms of Ageing and Development. 2005;126:938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Romeis J, Wackers FL. Nutritional suitability of individual carbohydrates and amino acids for adult Pieris brassicae. Physiological Entomology. 2002;9:148–156. [Google Scholar]
- Schilder RJ. Parasites, proteomics and performance: effects of gregarine gut parasites on dragonfly flight muscle composition and function. Journal of Experimental Biology. 2007;210:4298–4306. doi: 10.1242/jeb.011114. [DOI] [PubMed] [Google Scholar]
- Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7:478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjernholm F, Karlsson B. Nuptial gifts and the use of body resources for reproduction in the green-veined white butterfly Pieris napi. Proceedings of the Royal Society of London Series B-Biological Sciences. 2000;267:807–811. doi: 10.1098/rspb.2000.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoehr AM. Inter- and intra-sexual variation in immune defence in the cabbage white butterfly, Pieris rapae L. (Lepidoptera : Pieridae) Ecological Entomology. 2007;32:188–193. [Google Scholar]
- Walker G, Houthoofd K, Vanfleteren JR, Gems D. Dietary restriction in C. elegans: from rate-of-living effects to nutrient sensing pathways. Mech Ageing Dev. 2005;126:929–37. doi: 10.1016/j.mad.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Waltz EC, Wolf LL. By Jove! Why do alternative mating tactics assume so many different forms? American Zoologist. 1984;24:333–343. [Google Scholar]