Abstract
A successful experience of lymphatic filariasis control in the Republic of Korea is briefly reviewed. Filariasis in the Republic of Korea was exclusively caused by infection with Brugia malayi. Over the past several decades from the 1950s to 2006, many investigators exerted their efforts to detection, treatment, and follow-up of filariasis patients in endemic areas, and to control filariasis. Mass, combined with selective, treatments with diethylcarbamazine to microfilaria positive persons had been made them free from microfilaremia and contributed to significant decrease of the microfilarial density in previously endemic areas. Significant decrease of microfilaria positive cases in an area influenced eventually to the endemicity of filariasis in the relevant locality. Together with remarkable economic growth followed by improvement of environmental and personal hygiene and living standards, the factors stated above have contributed to blocking the transmission cycle of B. malayi and led to disappearance of this mosquito-borne ancient disease in the Republic of Korea.
Keywords: Brugia malayi, lymphatic filariasis, control, diethylcarbamazine
INTRODUCTION
Historically, the Republic of Korea had been an endemic area for lymphatic filariasis, caused by Brugia malayi, since more than 1,000 years ago. It is not well described and is still curious when lymphatic filariasis became prevalent in the Republic of Korea. According to scientific literature [1], it seems likely that, at least, filariasis was not endemic on this peninsula prior to the Goryeo Dynasty (935-1392 AD, Korea). During the Goryeo Dynasty, the import and export transportations and communications between the Goryeo and the Song (960-1279 AD, China) or Yuan Dynasty (1280-1368 AD, China) and south-east and middle Asian countries increased substantially via sea routes from central coastal areas of China (e.g., Shanghai) through southwestern islands (the Heuksan Islands) to Yeongam-gun, Jeollanam-do, the Republic of Korea. In addition, some castaways arrived from southern China as well as other Southeast Asian countries such as Sri Lanka, Indonesia, the Philippines, and Malaysia where lymphatic filariasis might be endemic. Based on these descriptions, lymphatic filariasis is thought to be an imported disease in the Republic of Korea.
HISTORICAL REVIEW OF LYMPHATIC FILARIASIS IN THE REPUBLIC OF KOREA
Status of lymphatic filariasis during the 1910s-1950s
The microfilariae found in the cattle were firstly described in Korea [2,3]. They examined the blood specimens of Korean cattle and identified microfilariae of Setaria species. Nakagawa [4] also observed some kinds of microfilariae in the blood samples of Korean sparrows, but he did not identify the exact species.
Japanese scientists [5,6] reported the presence of elephantiasis in Korea for the first time. However, the first Korean case of lymphatic filariasis was found at autopsied materials of inguinal lymph nodes of a man, who was born in Buyeo-gun, Chungcheongnam-do [7]. The patient visited Kyoto University Hospital with chief complaints of swollen legs and enlarged inguinal lymph nodes, which had developed several years earlier. Yun [7] had incidentally recognized swelling of the left inguinal lymph node in this patient during the past 8 years. This swelling became gradually enlarged up to the hen's egg size. Lower extremities were also enlarged with fever attacks. The symptoms subsided within approximately 1 month, while several minor attacks of these symptoms recurred 5-6 times in a year, particularly in autumn. Approximately 8 months before he died, swelling of both legs with varicose veins and engorgement of inguinal lymph nodes at both sides were noticed. His blood smear revealed no microfilariae. On histopathological examinations, lymphangiectasis, thickening of the lymphatic walls, disappearance of adipose tissues, and atrophic changes of the lymph follicles in hilar, mesenteric, and retroperitoneal lymph nodes were observed.
Oh [8] also observed microfilariae in the peripheral blood of 24 Koreans and described nocturnal periodicity of the microfilariae. An epidemiological survey of 527 elephantiasis cases in Chungcheongnam-do demonstrated that 12 patients were microfilaria positive in their peripheral blood. A series of epidemiological surveys were performed in Chungcheongnam-do, Jeollabuk-do, and Jeju-do, and it was revealed that hydrocele or chyluria was hardly associated with these patients. It was later found that Korean elephantiasis mainly affected lower extremities and occasionally arms but never involved the external genitalia [9].
Moon [10] conducted an epidemiological survey on endemic elephantiasis in Nonsan and Buyeo in Chungcheongnam-do and Namwon-myeon in Jeju-do. He analyzed the clinical symptoms observed from a total of 204 cases, and particularly he observed that there was no case with hydrocele or chyluria.
During the period, the patients had been thought to be infected with Wuchereria bancrofti and secondarily with Streptococcus spp. [7]. However, clinical manifestations of these patients were substantially different from those observed in bancroftian filariasis. Neither chyluria nor scrotal involvement was observed even in cases with advanced stages. Senoo [11] identified the microfilariae in these patients to be those of B. malayi, but not W. bancrofti.
Nelson et al. [12] also reported the occurrence of filarial infections (under the name Wuchereria malayi) in 8.5% of 570 Korean prisoners of the World War II. Senoo and Lincicome [13] reported the distribution of brugian filariasis in Korea by examining 5,001 patients representing 25 villages from Korea. They found that 604 (12.1%) of the patients were microfilariae positive in their peripheral blood, all of the causative agent was identified as B. malayi. Their epidemiological surveys concluded that the highest incidence occurred in Jeju-do, the next in the southwestern areas, and the lowest in the southeastern areas of the Korean peninsula. The mosquito vectors, however, were not identified by this time.
Epidemiological surveys and control strategies during the 1950s-1970s
An epidemiological survey for endemic filariasis conducted in the 1950s demonstrated 9.2% microfilaria positive rate (19/206 cases) [14] in Chungcheongnam-do. A subsequent survey reported positive rates of 11.4% (26/229 schoolchildren) and 22.2% (79/356 inhabitants) in Jeju-do [15,16]. Night blood specimens collected from 2,139 inhabitants who resided in 15 villages in Jeju-do showed 8.6% microfilaria positive rate [17]. It was later found that the distribution of filariasis was throughout the South Korea except Gyeonggi-do and Gyeongsangnam-do. A total of 601 cases out of 30,534 persons examined (2.0%) were found to be infected with B. malayi. There were 3 major endemic foci of brugian filariasis in Korea, including the northeastern part (inland) of Gyeongsangbuk-do, the western coastal areas of Jeollanam-do and Jeju-do. Jeju-do was found to be a highly endemic area, whereas the other 2 localities were found to be moderate to low endemic areas, and several investigators thereafter plotted out endemic areas in Namwon-myeon and Pyosun-myeon which were the most highly endemic areas in Namjeju-gun, Jeju-do [17]. In these areas, mass chemotherapy with diethylcarbamazine was extensively conducted during 1968 and 1973. In the inland of the northeastern areas of Gyeongsangbuk-do, the prevalence of lymphatic filariasis was reported to be in the range from 3.1 to 13.4% [18-22]. In the endemic areas of Yeongju-gun, Gyeongsangbuk-do (one of these inland areas), a mass and selective treatment with diethylcarbamazine was carried out on B. malayi microfilaria [23].
Chemotherapy of lymphatic filariasis was conducted in Jeju-do during the 1960s and the early 1970s. The conventional dosage of diethylcarbamazine was applied in the initial stage of the control program [24]. However, many of the treated cases had severe side reactions with febrile attacks for the first several days of the drug administration and this had seriously hampered further implementation of the filariasis control programs. This problem had been overcome after introduction of low dosages daily or with a gradual increase of daily dosages after several days of initial administration, totaling 36 mg/kg in a full course during the early 1970s. In Yeongju area of Gyeongsangbuk-do, microfilaria positive cases were medicated with diethylcarbamazine 1 mg/kg daily for 36 days [23].
According to Ree [25], there were 9 genera of mosquitoes such as Anopheles, Culex, Aedes, Armigeres, Mansonia, Heizmannia, Tripteroides, Culiseta, and Tozorhynchites found in Korea. Among them, Aedes togoi was recognized as the main vector mosquito of B. malayi in Jeju-do, Korea [16,26]. In addition, Anopheles sinensis was also found to be the vector mosquito responsible for transmission of B. malayi in inland areas [21,27].
Control activities after the 1980s
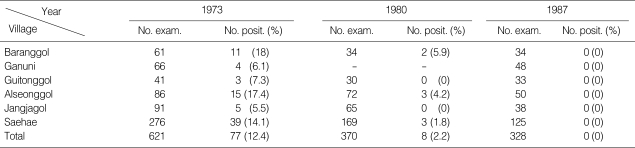
Jeju-do had long been known as the highest endemic area of lymphatic filariasis in Korea with the highest microfilaremia of 19.5% in Taeheung-ri, Namjeju-gun until the 1970s. The prevalence of filariasis decreased to a significantly low level of 0.5% following mass and selective treatments conducted since 1968 [28]. A survey done in 1988 in 4 villages of Jeju-do that were formerly well known as endemic areas revealed that microfilaremia among the inhabitants was 0.3% out of a total of 357 persons [29]. On the other hand, in the eastern inland area, Gyeongsangbuk-do, the moderate endemicity of lymphatic filariasis during the early 1970s was considered to be almost eliminated in the 1980s with selective treatments. The average microfilaria positive rate of this area in the late 1960s and early 1970s were 3.1% and 8.1%, respectively. Long term evaluation surveys during 1973 and 1987 at 7-year intervals in 7 sample villages have revealed that the microfilaria rates decreased from 12.4% in 1973 to 2.2% in 1980 and 0% in 1987 [28,30,33,36,54] (Table 1).
Table 1.
Transition of microfilaria rates in inhabitants of Yeongju-si, Gyeongsangbuk-do in 1973, 1980, and 1987
The transmission of filariasis in the formerly known endemic areas in Korea had been thought to be almost ceased around the middle of the 1980s [28,30,31]. However, in a series of extensive investigations conducted in the 1980s, groups of islands including Daeheuksan-do of Heuksan-myeon, located in the south-western part of the Korean Peninsula were belatedly found to be moderately endemic with B. malayi filariasis [32-37]. Surveys in these areas demonstrated relatively high microfilaria rates of an average 11.2% out of a total of 2,159 persons examined by 120 µl of nightblood in 29 villages of 15 small islands from 1985 to 1988 [32-34]. All of the islands surveyed were found to be endemic and the microfilaria rate on each island ranged from 2.2% to 22.4%. The positive cases were found in all age groups, being increased gradually in older age groups. Microfilarial density of the positive cases was relatively low. The average microfilaria count for 198 positive cases was 33.4 per 120 µl of night blood. In Wando-gun, Jindo-gun, and Yeosu-si of Jeollanam-do in southern areas, the microfilaria rate was 2.5% with a moderate density in 1990 to 1992 [36]. The microfilaria positives found on the islands in Sinan-gun were treated with low dosage of diethylcarbamazine schedule from 1986 to 1992 and these treatment decreased microfilaria rates from 12.3% to 1.4% in 2000.
The important factors that contributed to block the transmission of lymphatic filariasis in inland Korea might include selective chemotherapy with diethylcarbamazine, as well as improvement of the quality of life including housing [30,33]. The situation was similar in remote islands. In addition, changes of the outdoor environment, improvement of personal sanitation, and decrease in the number of residents in remote areas on these islands may have contributed to blocking of the transmission of filariasis in Korea.
Evaluation of the elimination program during the 1980s-1990s
An epidemiological survey performed in 1988 on 4 villages of Jeju-do that were formerly well-known endemic areas has observed that microfilaremia among the inhabitants was 0.3% out of a total of 357 persons [29]. On the other hand, Gyeongsangbuk-do, the moderately endemic area of B. malayi filariasis during the early 1970s was found to be completely controlled in the 1980s.
After the mid-1980s, groups of islands including Daeheuksan-do of Heuksan-myeon, located off the southwestern part of the Korean peninsula, were newly found to be moderately endemic with lymphatic filariasis [32]. The infected people in Sinan-gun were treated with diethylcarbamazine during 1986 to 1992 with the low dosage schedule, and finally these treatments resulted in a positive rate lower than 2%. A small-scale survey was conducted in this area in 2000. A total of 378 people, 151 male and 227 female, living in 8 villages (6 on Daeheuksan-do, 1 on Daejang-do, and 1 on Yeongsan-do) were subjected to a night blood survey, and physical examination for elephantiasis on their extremities. There were 6 (1.6%) microfilaria positive cases, all females aged 57-72 years, and from only 2 villages of Daeheuksan-do. There were 4 patients with lower leg elephantiasis, but they showed no microfilaremia [38].
Chemotherapeutic control
Administration of first doses of diethylcarbamazine to lymphatic filariasis patients often caused fever and other serious untoward effects. In addition, there were carriers whose microfilaria count rapidly decreased by successive administration of diethylcarbamazine in a short time but gradually increased again. Clinical and physical observations of filariasis cases were carried out in Yeongju area as well [22,23]. To decrease the severe side reactions induced by the conventional regimen of diethylcarbamazine (72 mg/kg) maintaining its efficacy, efficacy of diethylcarbamazine citrate against brugian filariasis was evaluated with 3 modified low dosage schedules prior to mass treatment in this area [23,39]. Mass chemotherapy with diethylcarbamazine was carried out with low dosage schedules (36 mg/kg) of 1 mg/kg daily for 36 days, which showed the most acceptable result.
When Jeju-do was found to be a highly epidemic area for lymphatic filariasis, many researchers conducted epidemiological surveys and administered diethylcarbamazine to microfilaria positive people [24,40,41]. Paik [42] prescribed diethylcarbamazine at the dose of 5 mg/kg/day for 5 days (total 30 mg/kg) to 34 of 52 microfilaria positive cases as a short-term concentration method. The total microfilarial count in blood prior to administration was 3,558 and the average count was 104.6. Two weeks after the treatment, the total microfilarial count decreased to 2 with an average count of 0.07. Although the microfilarial count increased slightly to 16 with an average count of 0.5 at 4 months after the medication, administration of diethylcarbamazine was found to have relatively excellent microfilaricidal effects. The negative conversion rate of microfilaremia was determined to be 93% at 2 weeks after the drug administration, and 74.2% at 4 months post-treatment. More conspicuous finding other than the negative conversion rate of microfilaremia was the decrease of microfilarial density in the administered group. The successful treatment of patients based on individual microfilaria negative conversion was important; however, a sharp decrease of microfilarial density would also have great impact on the removal and decrease of local transmission of filariasis in endemic areas. Many investigators insisted that the decrease of microfilarial density in a certain group is more conspicuous and more important than the negative conversion rate of microfilaremia. Conclusively, it is believed that the decrease of microfilarial density in the administered group has profound impact on removal of filariasis in certain local endemic areas.
One remarkable finding was that the microfilaria negative conversion rate and decrease rate reached a plateau when 1 mg/kg of diethylcarbamazine was administered for 36 days (total 36 mg/kg) for B. malayi positive cases in Sinan-gun, Jindo-gun, and Wando-gun in Jeollanam-do between 1986 and 1992 [36]. The average microfilarial density of 213 positive persons in Sinan-gun was initially 31.7. Among these 213 people, 142 were subjected to re-examination 3 months after the medication. Of 142 people, 110 (77.5%) showed negative conversion and the remaining microfilaria positives showed a decrease of microfilarial density from 29.6 to 17.9. However, some people did not respond to the drug, thus resulting in a failure of the drug treatment. This result might be ascribed to the fact that different physiological reactions of individuals to the drug. Chai et al. [38] administered a mixture of albendazole (400 mg) and ivermectin (150 µg/kg) to 6 microfilaria positive persons found in Sinan-gun, Jeollanam-do as a single dose therapy. They were all found to have negative conversion.
CURRENT STATUS IN PREVIOUSLY ENDEMIC AREAS
Jeju-do
In Jeju-do, Senoo and Lincicome [43] reported the high infection rate of 26.6% in Wimi-ri, Namwon-myeon, of which the rate varied from 0.8 to 47% in the 1960s to 1970s [9,15-17,26,44].
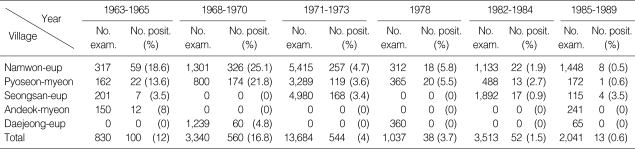
In Namjeju-gun, the microfilaria positive rate was 12-16.8% during the 1960s [15,16,19,45,46]. The highest endemic area was found to be Namwon-eup. During the 1970s, the infection rate ranged between 3.4 and 7.8% [26,47]. However, the infection rate dropped to approximately 1% during the 1980s, when a total of 5,554 residents (ranged between 0.6 and 1.5%) were examined [16,19,24,27,29,31-35,41,44-46] (Table 2). Most recently, Cheun et al. [48] collected blood specimens from a total of 1,801 dwellers in 9 different villages in these areas and examined the presence of microfilariae in their blood, but found no positive case in 2005.
Table 2.
Decrease of microfilaria rates in Namjeju-gun, Jeju-do from 1963 to 1989
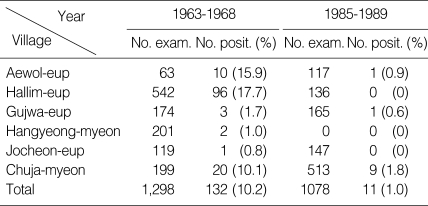
In Bukjeju-gun, the microfilaria positive rate in the 1960s was reported to be 10.2% (0.8-17.7%) among 1,298 investigated people [15,16,44-46]. However, the infection rate decreased to below 1.0% in the 1980s [16,17,19,29,31-35,42,44,46] (Table 3). In 2005, peripheral blood smears of a total of 1,543 residents in 11 different local villages in these areas showed no positive cases [48].
Table 3.
Decrease of microfilaria rates in Bukjeju-gun, Jeju-do in 1963 to 1989
Jeollanam-do
Sinan-gun areas
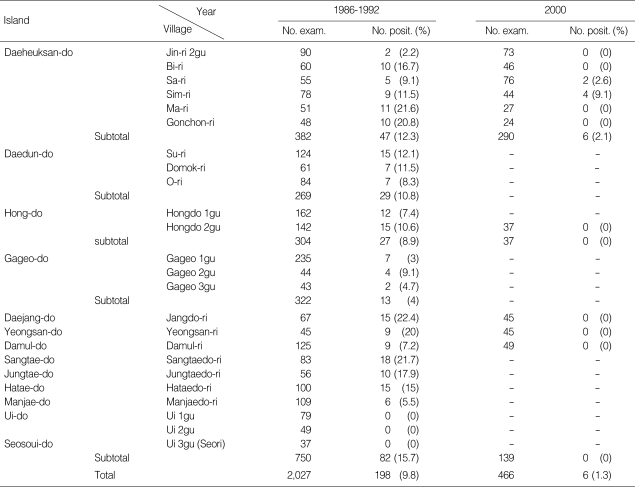
The microfilaria rates on the islands of Sinan-gun from 1986 to 2000 are shown in Table 4. Until the early 1980s, the positive rates on respective islands of Sinan-gun were found to be 12.3% (47/382 dwellers) in 6 villages of Daeheuksan-do, 8.9% (27/304 residents) in Hong-do, 10.8% (29/269 dwellers) in Daedun-do, 22.4% (15/67 residents) in Daejang-do, 20.0% (9/45 dwellers) in Yeongsan-do, 7.2% (9/125 dwellers) in Damul-do, 4.0% (13/322 dwellers) in Gageo-do, 21.7% (18/83 dwellers) in Sangtae-do, 17.9% (10/56 residents) in Joongtae-do, 15.0% (15/100 dwellers) in Hatae-do, and 5.5% (6/109 dwellers) in Manjae-do [28,32-36,38,49] (Table 4). Epidemiological surveys done during 1986 to 1992 also revealed that 198 cases out of 2,027 dwellers (9.8%) were found to be microfilaria positive in their blood. When a follow-up survey was done in 2000, all of these positive cases except 2 (0.2%, 2/1,251 dwellers) converted to negative (Table 4).
Table 4.
Decrease of microfilaria rates on the islands in Sinan-gun, Jeollanam-do from 1986 to 2000
Jindo-gun areas
In 1992, the positive rate of microfilariae in these regions were shown to be 3.7% in Donggeocha-do (2/54 people), 7.1% in Cheongdeung-do (2/28 dwellers), and 1.1% (1/94 residents) in Gwanmae-do. These areas were found to be relatively low endemic areas when compared to other remote islands among Jeollanam-do [36].
An epidemiological survey done 10 years later in 2002 in these areas revealed no microfilaria positive cases [48]. The study subjects were composed of 248 people in Donggeocha-do, Cheongdeung-do, and Gwanmae-do, and 631 residents (246 males and 358 females) in 18 villages of 15 islands in Jindo-gun.
Wando-gun areas
Wando-gun is located in the southwestern part of Jeollanam-do. During the mid-1980s, Wando-gun area was belatedly found to be an endemic area (0 to 1.6% positive rate) [36]. However, Wando-gun showed lower infection rates compared to other endemic areas in Jeollanam-do including Sinan-gun (9.8-21.6%), Jindo-gun (1.7-7.1%), and Yeosu-si (0.9-2.4%) during 1985 and 1992 [32-36]. When a total of 500 inhabitants (245 male and 255 female) were examined in 4 villages in Bogil-do, Wando-gun, 3 microfilaria positive cases were detected in 1992 [36]. The positive rate was 0.6%. They were all aged male cases. However, another survey carried out in the same areas in 2003 revealed that, among 465 examinees, no positive case was detected [48]. This result suggests that transmission of lymphatic filariasis has been terminated in these areas.
Yeosu-si, Tongyeong-si, and Yeonggwang-gun areas
An epidemiological survey of 723 blood specimens, which were collected from 312 males and 411 females, from 10 villages and 5 islands in Yeosu-si during the mid-1990s revealed no positive cases with microfilariae [48]. This number accounted for 58.6% of whole dwellers in this area. A total of 230 samples collected from 97 males and 133 females from 3 village inhabitants of Geomun-do revealed 0.9% of positive rate of microfilariae (1.0% for male and 0.3% for female) [36]. However, no positive case was detected in 2004 [48], when 423 people (178 men and 245 women) from 4 villages were subjected to examinations.
A total of 594 people consisting of 270 males and 324 females, from 16 villages on 8 different islands of Tongyeong-si were examined their blood for microfilariae in 2004 [48]. There was found no positive case. The number examined accounted for 78.4% of whole inhabitants of the areas covered.
Also an investigation on 76% of whole inhabitants, which included 266 people (127 males and 139 females) from 5 villages on 4 islands which belong to Yeonggwang-gun, revealed no positive case of microfilaremia in 2005 [48].
Evaluation of the elimination program in 2006 by filarial antibody tests
The efficacy of the elimination program for elementary school children and residents who had resided in the endemic areas (Jeju-do, Jeollanam-do, or Gyeongsangbuk-do) of brugian filariasis was evaluated. In order to properly evaluate the control program, establishment of comparative criteria, technical indicators, and reliable methods for evaluation are pre-requisites. Effective control and prevention of brugian filariasis should be accompanied with trials on a large scale administration of anthelmintics with extensive geographical coverage over a long period of time. Blockage of the transmission of the lymphatic filariasis should cover up the whole endemic areas.
In order to detect antibodies aginst B. malayi in young age groups, i.e., primary school children of 3rd to 6th grade (10-13 years old), the BRUGIArapid™ dipstick kit (Malaysian Bio-Diagnositcs Research, Malaysia) was used as recommended by World Heath Organization Advisory Committee, WHO in 2005. In short, the dipstick is prepared with a goat anti-mouse antibody control line and B. malayi recombinant-antigen test line. Antifilarial antibodies in patient's sera react with this antigen, followed by binding of this complex with monoclonal anti-human IgG4 conjugated to colloidal gold. The overall results of the evaluation for brugian filariasis showed 97% sensitivity, 99% specificity, 97% positive predictive value, and 99% negative predictive value [50]. Further evaluations of the test were reported [51-53].
National Institute of Health, Republic of Korea (KNIH) selected 11 elementary schools in Jeju-do, 14 in Jeollanam-do, 2 in Gyeongsangbuk-do, and 4 Gyeongsangnam-do where brugian filariasis had been endemic [48]. No case was found microfilaria positive out of 1,329 children (720 males and 609 females) in Jeju-do, 1,369 (739 males and 630 females) in Gyeongsangbuk-do, 60 (30 males and 30 females) in Gyeongsangnam-do, and 291 (161 males and 130 females) in Jeollanam-do.
In addition, a total of 1,526 residents were randomly selected from formerly endemic areas of Jeollanam-do, Gyeongsangbuk-do, and Jeju-do [48]. Out of 446 persons (162 males and 284 females) from 5 different areas in Jeju-do, of 865 persons (335 males and 535 females) from 25 remote island areas in Jeollanam-do, and of 215 persons of 5 areas in Gyeongsangbuk-do, none were found positive for microfilariae. These data strongly indicate that transmission of filariasis has been blocked in Korea.
ASSESSMENT OF THE SUCCESS OF THE ELIMINATION PROGRAM
Brief contents of the elimination
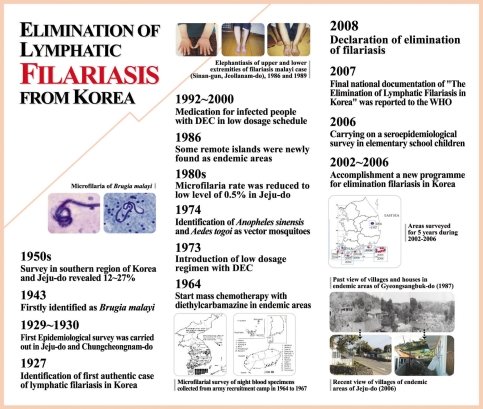
Lymphatic filariasis was first recorded in Korea in 1927 [7] and was had been found in Chungcheongnam-do and Jeju-do [8]. During the 1930s, the microfilaria positive cases had been described in the southern region of Korea and Jeju-do [13,43]. To review the changing patterns of microfilaria positive rates in Bukjeju-gun, Jeju-do, where lymphatic filariasis was highly epidemic during the 1960s, Seo et al. [17,19] reported the positive rate of 10.2% from 1,298 inhabitants. However, this positive rate conspicuously decreased to below 1.0% during the 1980s [31,35]. In 2005, an epidemiological survey of the filariasis done by the KNIH could not find any microfilaria positive persons from 1,543 subjects by microscopic examinations [48]. For Namjeju-gun, the positive rate was reported to have ranged from 12 to 16.8% according to the investigators during the 1960s [15-17,19,44]. During the 1970s, however, it considerably decreased from 3.7 to 4.0% [40]. It decreased to 1.5% in the first half and further down to 0.6% in the second half during the 1980s [31,32]. In 2005, no microfilaria positive people were found among 1,801 people examined, which clearly indicated that the microfilaremia cases completely disappeared from Jeju-do which once was an endemic area [48].
In Yeongju-gun, Gyeongsangbuk-do, an inland area of Korea, Hwang et al. [18] examined 378 people and detected 29 microfilaria positive cases (7.7%) for the first time. In 1970s, through an epidemiological investigation conducted in 1970 to 1973, the prevalence rate of microfilaremia was shown to be 8.1% on the average out of a total of 2,178 persons examined from 32 villages with the range of microfilaria rate of 2.6-18.0% [20,27,54]. For an evaluation of the transmission of B. malayi in this area, transition of the prevalence of microfilaremia was determined for 14 years at 7-year interval from 1973. The prevalence rate was 12.4% out of 621 people examined from 6 villages in 1973. The prevalence rate in 1980 was 2.2% out of 370 persons examined, and in 1987, none were found positive out of 328 persons examined [28,30,33]. These results indicate that the transmission of B. malayi filariasis was probably terminated around this time in this area.
On the other hand, southwestern remote islands including Heuksan-do (islands) of Sinan-gun, Jeollanam-do were belatedly found to be endemic foci in Korea during the mid-1980s [32]. Large scale epidemiological surveys were extensively carried out in these areas by several investigators [34,35,49]. From 1986 to 1990, a total of 213 cases of microfilaria positive persons were detected out of 2,533 subjects (8.4%) in Sinan-gun. However, microfilarial density of the positive cases was relatively low. The average count of microfilariae for 198 positives was 33.4/120 µl night blood. In this region, 3 (0.1%) positive cases were found even among young age group under 10, which strongly suggested that there was propagation although the transmission was minimal. An epidemiological survey done in 2000 found 6 positive cases (1.6%) among 380 persons examined [38]. In addition, 2 positive cases among 1,393 samples tested (0.2%) were found in 2002 from an examination by KNIH. However, all these people were older than 60, and the microfilarial density was 1.5/120 µl.
In Jindo-gun, 5 people was shown to be microfilaria positive in their peripheral blood smears when a total of 296 inhabitants were examined in 1992 (1.7%), but no more positive case was detected among 631 examined in an epidemiological survey in 2003 [48]. In addition, KNIH found a total of 3 cases among 500 inhabitants in 1991 (0.6%) in Wando-gun. However, no positive case was found among 2,488 people reexamined in 2003 by KNIH [48]. Also no microfilaria positive cases was found from these areas among 3,049 elementary school children of 10-13 years old and 1,526 inhabitants in 2006 [48].
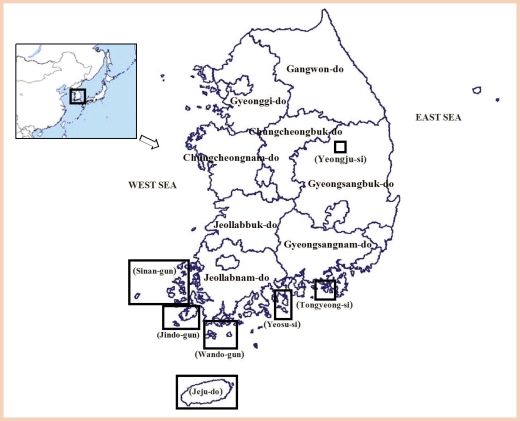
These data indicate that transmission of B. malayi has been ceased in Gyeongsangbuk-do, Jeollanam-do, and Jeju-do areas where B. malayi infection was prevalent in the Korean Peninsula (Fig. 1).
Fig. 1.
Endemic and surveyed areas of lymphatic filariasis in Korea, 2002-2006.
Impact of environmental changes and new town and village construction
It is well known in Korea that the vector of B. malayi is Ae. togoi in the coast and islands, and that of inland is An. sinensis. After the discovery of infective larvae of B. malayi in Ae. togoi mosquitoes in Jeju-do in 1960 [16], many researchers examined the presence of larvae within the mosquito and confirmed that the mosquito involved in the transmission of B. malayi in Jeju-do was Ae. togoi [1,55,56]. Seo et al. [17] reported that there are many holes in rocks in the coast of Jeju-do, and they might provide the optimum inhabitable conditions for the larvae of Ae. togoi. Lee [57] found that Ae. togoi was the most prevalent species in Jeju-do. Its prevalence reached as high as 70-90% according to the villages. In the natural status, the infection rate of Ae. togoi with the filarial larvae is proportional to the infection rate of residents. Therefore, the natural infection of mosquitoes is directly associated with human infections [30], which implies that transmission of lymphatic filariasis could be successfully controlled through mass chemotherapy of humans.
The improved life standard of residents is believed to have greatly decreased the opportunities for residents to be exposed to the blood sucking of vector mosquitoes. In the past, there were many agricultural and fishing villages that did not have electricity and many of the residents slept outside during the hot summer and easily exposed to mosquito biting. However, this is no longer the case with the supply of electricity, and the use of mosquito net and pesticides such as mosquito repellents and aerosols have created a situation that is unfavorable to the spread of mosquito-borne diseases.
Furthermore, all the residences in farm villages and islands now have protection nets on windows to block biting of insects including mosquitoes, and most houses in islands have installed chassis at the end of the eaves to shut the always strong wind, so the inside of the house is not directly exposed to the outside, further preventing the intrusion of mosquitoes.
Effects of changes in human behaviors avoiding mosquito bites
The improvement of living standards, residences, and villages by modernization and economic development that began from the 1970's, the gradual urbanization of farm villages and industrialization, wide usage of agricultural pesticides, and chemical extermination of insects to prevent mosquito-borne diseases, have significantly decreased the habitats and population density of mosquitoes. Moreover, the heightened health consciousness of individuals accompanied by economic affluence partly contributed to the extermination of some endemic diseases. The virtual active period of mosquitoes is 6-7 months in the Republic of Korea, which is shorter than the tropical and subtropical zones. These various environmental causes are also believed to minimize the exposure to mosquitoes and contributed to the prevention of propagation of the disease in Korea.
Most importantly, the microfilaria positive patients were continuously searched for and should be treated, and this resulted in free of microfilaremia in these persons. Although they were not converted to microfilaremia negatives, the microfilarial density was greatly decreased, which was the principal factors responsible for the extermination of filariasis. In particular, Jeju-do and other islands have turned into tourist destinations, and the improved residential environment and development of the community decreased the habitats of mosquitoes. Another cause was the increased interest of people in self-protection against mosquitoes, which decreased the opportunities for people to be exposed to mosquitoes. In these areas, the positive people decreased greatly since the 1980s and most of them are now adults in their sixth decades or older. The population of farm villages and islands is continuously decreasing. The young people migrate from islands to inland and cities for education and employment, which resulted in almost no young natives in these areas. Some of the positive people have naturally decreased with emigration or death. Such complex causes as movement to other regions, death, and natural cure have contributed together to the extermination of filariasis in previously endemic areas in Korea (Fig. 2).
Fig. 2.
Korea: one century of progress in lymphatic filariasis. There are some notable milestones-from first authentic case of lymphatic filariasis in 1927 to elimination of lymphatic filariasis in 2008.
ACKNOWLEDGEMENTS
We thank the following colleagues very much for their devoted support and efforts: the Province and City Bureau of Health Center, the Research Institute of Health and Environment and Universities. These works were supported by a grant from the National Institute of Health (NIH-091-4800-4845-300), Ministry of Health and Welfare, Republic of Korea.
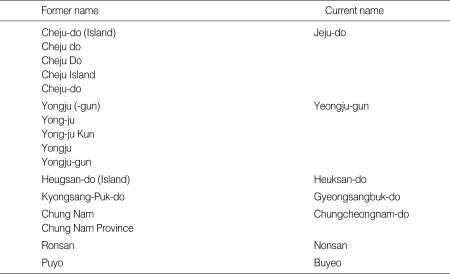
Appendix 1
Former and current names of surveyed areas
※Former names are shown in "References".
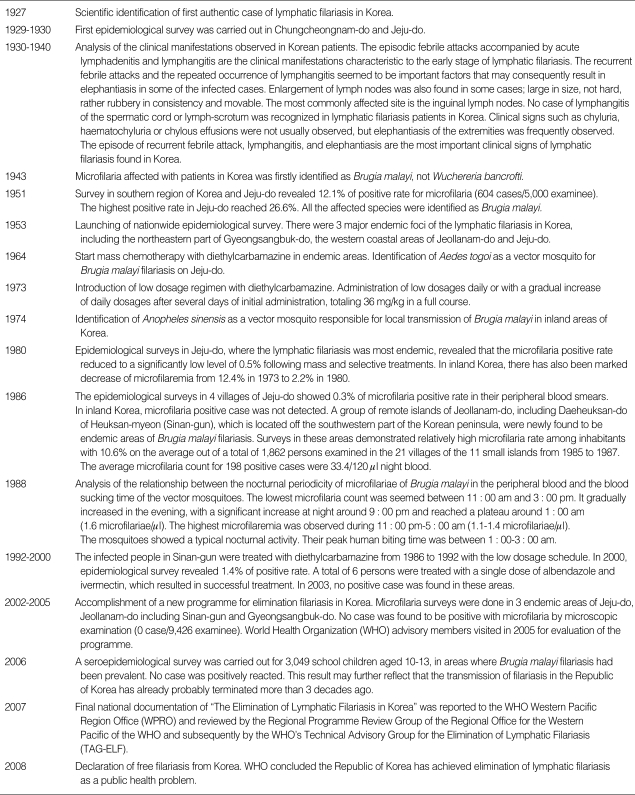
Appendix 2
Important events of filariasis in Korea
References
- 1.Seo BS. Malayan filariasis in Korea. Korean J Parasitol. 1978;16(suppl):1–108. doi: 10.3347/kjp.1978.16.suppl.5. [DOI] [PubMed] [Google Scholar]
- 2.Yamada K, Mizugi G. Gyu-Eki Ken-Kyu Ji-Ko. 1912. Filariasis in Korean cattle; pp. 141–145. [Google Scholar]
- 3.Kawamura R. The 2nd Annual Report of Animal Dis Serum Lab. 1915. A survey on the microfilaria in the blood of Korean cattle; pp. 142–167. [Google Scholar]
- 4.Nakagawa Y. On the microfilaria in the blood of sparrow. Chosen Iggakai Zasshi. 1914;13:53. [Google Scholar]
- 5.Fujimori K. A disease like elephantiasis in Korea. Koseikan Iji Kenkukaishi. 1924;20:63–77. [Google Scholar]
- 6.Murakami T. Ueber den therapeutischen wert der kondoleonschen operation zur heilung elephantiastischer oedeme, nebst einer Krankheit. "Pitzin" in Korea. Geka Hokan. 1925;2:1–11. [Google Scholar]
- 7.Yun IS. Elephantiasis due to filaria in Korea. Chosen Iggakai Zasshi. 1927;76:326–334. [Google Scholar]
- 8.Oh HY. Filariasis in Korea. Chinese Med J. 1929;43:16–21. [Google Scholar]
- 9.Moon IJ. Studies on the endemic elephantiasis in Korea. Part III. Study on the pathogenicity. Chosen Iggakai Zasshi. 1940;30:1136–1159. [Google Scholar]
- 10.Moon IJ. Studies on the endemic elephantiasis in Korea. Part 1. Survey in Nonsan and Puyo areas in Chung-Nam. Chosen Iggakai Zasshi. 1939a;29:553–575. [Google Scholar]
- 11.Senoo T. Detection of microfilaria malayi brug in Korea. Nippon Kiseichu Gakkai Kiji. 1943;15:36. [Google Scholar]
- 12.Nelson EC, Webb JC, Bayliss M, Starkey GS. Studies of filariasis development of Wuchereria bancrofti in Culex quinquefasciatus of Oahu. Am J Trop Med. 1946;26:707–713. [PubMed] [Google Scholar]
- 13.Senoo T, Lincicome DR. Malayan filariasis: incidence and distribution in southern Korea. US Armed Forces Med J. 1951;2:1483–1489. [PubMed] [Google Scholar]
- 14.Paik YH, Ah HS, Huh RS, Yang YJ. Filariasis investigation on filariasis in Ronsan (Chung Nam Province) Korean Med J. 1957;2:1175–1179. [Google Scholar]
- 15.Lee KT. Malayan filariasis. The lst report on incidences and distribution among children in Cheju-Do. Report NIH Korea. 1961;4:107–111. [Google Scholar]
- 16.Lee KT, Kim SH, Kong TH, Song JS. Malayan filariasis. 2nd report: epidemiological investigations on filariasis due to Brugia malayi in the residents of southern Cheju-Do island. J Korean Med Assoc. 1964;7:657–664. [Google Scholar]
- 17.Seo BS, Rim HJ, Seong SH, Park YH, Kim BC, Lim TB. The epidemiological studies on the filariasis in Korea I. Filariasis in Cheju-do (Quelpart Island) Korean J Parasitol. 1965;3:139–145. doi: 10.3347/kjp.1965.3.3.139. [DOI] [PubMed] [Google Scholar]
- 18.Hwang CH, Kahn CM, Lee CS, Song JS, Hong HK. A report on elephantiasis and microfilariasis found in Yong-ju Kun, Kyongsang-Puk-do, in 1963. Korea Central J Med. 1965;9:491–496. [Google Scholar]
- 19.Seo BS, Rim HJ, Lim YC, Kang IK, Park YO. Epidemiological studies on the filariasis in Korea II: distribution and prevalence of malayan filariasis in southern Korea. Korean J Parasitol. 1968;6:132–141. doi: 10.3347/kjp.1968.6.3.132. [DOI] [PubMed] [Google Scholar]
- 20.Kim DC, Lee OY, Kim TW, Han EJ, Lee KW, Choi SH. Epidemiological studies of human filariasis of inland Korea: endemicity and transmission of human filariasis in Yongju area. Report NIH Korea. 1971;8:147–165. [Google Scholar]
- 21.Kim DC, Lee OY, Lee KW. Epidemiology of malayan filariasis in Inland Korea. II. Vector finding and transmission of Brugia malayi in Yongju area. Yonsei Rep Trop Med. 1977;8:23–32. [Google Scholar]
- 22.Soh CT, Kim DC. Clinical and physical observation of malayan filariasis cases in Yongju-gun, Korea. Yonsei Rept Trop Med. 1974;5:104–116. [Google Scholar]
- 23.Soh CT, Kim DC. Efficacy of diethylcarbamazine citrate against filariasis malayi in modified low dosage schedule. Yonsei Rep Trop Med. 1977;8:51–56. [Google Scholar]
- 24.Seo BS, Lee JW. Effectiveness of diethylcarbamazine in the mass treatment of malayan filariasis with low dosage schedule. Korean J Parasitol. 1973;11:61–69. doi: 10.3347/kjp.1973.11.2.61. [DOI] [PubMed] [Google Scholar]
- 25.Ree HI. Medical Entomology. Seoul, Korea: Komoon Sa; 1978. pp. 1–294. [Google Scholar]
- 26.Kim JS, Lee WY, Chun SL. Ecology of filariasis on Cheju Island. Korean J Parasitol. 1973;11:33–53. doi: 10.3347/kjp.1973.11.1.33. [DOI] [PubMed] [Google Scholar]
- 27.Kim DC. Epidemiological studies of filariasis in inland Korea. 4. Vector determination of filariasis malayi in Yongju Area; Abstracts of the 16th Annual Meeting of The Korean Society for Parasitology; 1974. [Google Scholar]
- 28.Kim DC. Lymphatic filariasis in the Republic of Korea. Yonsei Rep Trop Med. 1994;25:1–12. [Google Scholar]
- 29.Paik YH, Cho YJ, Koo DS, Ree HI, Shim JC. Studies on the current epidemiological situation of brugian filariasis in endemic areas of Korea. Korean J Parasitol. 1988;26:255–262. doi: 10.3347/kjp.1988.26.4.255. [DOI] [PubMed] [Google Scholar]
- 30.Kim DC, Lee OY, Jeong EB, Jeong MG. Natural transition of endemicity of malayan filariasis in Inland Korea: pattern of change in microfilaria rate among inhabitants of Yongpung (formerly Yongju) area during the period of the last seven years. Korean J Parasitol. 1980;18:171–178. doi: 10.3347/kjp.1980.18.2.171. [DOI] [PubMed] [Google Scholar]
- 31.Lee OY, Lee JS, Kim TS, Kim DC, Son SC, Kim JB, Song CH. Epidemiological studies on filariasis malayi on Chejudo. Report NIH Korea. 1985;22:241–253. [Google Scholar]
- 32.Lee OY, Lee JS, Son SC, Yong TS, Kim DC, Kim JB, Lee SS. Epidemiological studies on filariasis malayi on Cheju Do and the southern islands. Report NIH Korea. 1986;23:407–422. [Google Scholar]
- 33.Lee OY, Lee JS, Son SC, Yong TS, Lee IS, Kim SS, Kim DC. Epidemiological studies on filariasis malayi on the southern islands and inland Korea. Report NIH Korea. 1987;23:519–538. [Google Scholar]
- 34.Lee OY, Lee JS, Yong TS, Kim TS, Lee IS, Kim SS, Seo BJ, Kim DC. Epidemiological studies of filariasis malayi on the southern islands, Korea. Report NIH Korea. 1988;25:411–425. [Google Scholar]
- 35.Lee OY, Lee JS, Kim TS, In TS, Lee IS, Seo BJ, Kim DJ, Kim DC. Epidemiological studies of filariasis malayi on the southern islands of Korea (II) Report NIH Korea. 1989;26:247–265. [Google Scholar]
- 36.Lee JS, Kim TS, Lee WJ, In TS, Kim H, Lee OY, Kim DC. Epidemiology of filariasis malayi on the southern islands and inland Korea (III) Report NIH Korea. 1992;29:114–122. [Google Scholar]
- 37.Lee JS, Hong HK. Effects of nutrient and salinity in egg and larval development of Aedes togoi. Korean J Parasitol. 1995;33:9–18. doi: 10.3347/kjp.1995.33.1.9. [DOI] [PubMed] [Google Scholar]
- 38.Chai JY, Lee SH, Choi SY, Lee JS, Yong TS, Park KJ, Yang KA, Lee KH, Park MJ, Park HR, Kim MJ, Rim HJ. A survey of Brugia malayi infection on the Heuksan island, Korea. Korean J Parasitol. 2003;41:69–73. doi: 10.3347/kjp.2003.41.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim DC, Soh CT. Efficacy of diethylcarbamazine of filariasis malayi in modified schedule; Abstracts of the 16th Annual Meeting of The Korean Society for Parasitology; 1974. [Google Scholar]
- 40.Kim JS, Moon OR, Lee WY, Chun SL. Efficacy of mass treatment for control of human filariasis. Korean J Parasitol. 1973;11:54–60. doi: 10.3347/kjp.1973.11.1.54. [DOI] [PubMed] [Google Scholar]
- 41.Seo BS, Whang KI. Evaluation of mass treatment of malayan filariasis by diethylcarbamazine in Cheju Island. Korean J Parasitol. 1974;12:21–32. doi: 10.3347/kjp.1974.12.1.21. [DOI] [PubMed] [Google Scholar]
- 42.Paik YH. Effect of diethylcarbamazine against Brugia malayi infection on Cheju Island evaluated in 1965. Korean J Parasitol. 1986;24:201–204. doi: 10.3347/kjp.1986.24.2.201. [DOI] [PubMed] [Google Scholar]
- 43.Senoo T, Lincicome DR. The presence of malayan filariasis in Korea. Trans R Soc Trop Med Hyg. 1951;45:269–273. doi: 10.1016/s0035-9203(51)90997-2. [DOI] [PubMed] [Google Scholar]
- 44.Moon OR. An epidemiological study and clinical evaluation of mass chemotherapy with supatonin for filariasis in southern area of Cheju Do. Korean J Public Health. 1968;5:113–121. [Google Scholar]
- 45.Soh CT, Lee KT, Im SW, Lee JH. Clinical manifestation of Brugia malayi infection in Korea. Korean J Parasitol. 1966;4:1–6. doi: 10.3347/kjp.1966.4.2.1. [DOI] [PubMed] [Google Scholar]
- 46.Kim BC, Hahn SS, Seo BS, Rim HJ, Ko YH, Lim DB. Mass treatment of malayan filariasis with diethylcarbamazine citrate in Cheju Do. Korean J Intern Med. 1968;11:799–805. [Google Scholar]
- 47.Seo BS. The periodicity of microfilariae of Brugia malayi in Cheju Island, Korea. Korean J Parasitol. 1974;12:95–100. doi: 10.3347/kjp.1974.12.2.95. [DOI] [PubMed] [Google Scholar]
- 48.Cheun HI, Lee JS, Cho SH, Kong Y, Kim TS. Elimination of lymphatic filariasis in the Republic of Korea: an epidemiological survey of formerly endemic areas, 2002-2006. Trop Med Int Health. 2009;14:1–5. doi: 10.1111/j.1365-3156.2009.02240.x. [DOI] [PubMed] [Google Scholar]
- 49.Yong TS, Lee OY, Lee JS, Kim TS, Kim DC. Clinical observation of malayan filariasis cases on the Heugsan Islands, Korea. Report NIH Korea. 1988;25:427–441. [Google Scholar]
- 50.Rahmah N, Lim BH, Khairul Anuar A, Shenoy RK, Kumaraswami V, Lokman Hakim S, Chotechuang P, Kanjanopas K, Ramachandran CP. A recombinant antigen-based IgG4 ELISA for the specific and sensitive detection of Brugia malayi infection. Trans R Soc Trop Med Hyg. 2001;95:280–284. doi: 10.1016/s0035-9203(01)90234-2. [DOI] [PubMed] [Google Scholar]
- 51.Rahmah N, Shenoy RK, Nutman TB, Weiss N, Gilmour K, Maizels RM, Yazdanbakhsh M, Sartono E. Multicentre laboratory evaluation of Brugia rapid dipstick test for detection of brugian filariasis. Trop Med Int Health. 2003;8:895–900. doi: 10.1046/j.1365-3156.2003.01102.x. [DOI] [PubMed] [Google Scholar]
- 52.Lammie PJ, Weil G, Noordin R, Kaliraj P, Steel C, Goodman D, Lakshmikanthan VB, Ottesen E. Recombinant antigen-based antibody assays for the diagnosis and surveillance of lymphatic filariasis-a multicenter trial. Filaria J. 2004;3:9–13. doi: 10.1186/1475-2883-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischer P, Bonow I, Supali T, Rückert P, Rahmah N. Detection of filaria-specific IgG4 antibodies and filarial DNA for the screening of blood spots for Brugia timori. Ann Trop Med Parasitol. 2005;99:53–60. doi: 10.1179/136485905X13339. [DOI] [PubMed] [Google Scholar]
- 54.Kim DC, Lee OY, Lee KW. Epidemiology of malayan filariasis of inland Korea. 1. Endemicity of filariasis malayi in Yongju area. Yonsei Rep Trop Med. 1977;8:9–22. [Google Scholar]
- 55.Chun SR. A preliminary survey of mosquitoes of Cheju do related to filariasis, on the species, biology and infection status. Korean J Public Health. 1968;5:113–121. [Google Scholar]
- 56.Wada Y, Katamine D, Oh MY. Studies on malayan filariasis in Cheju Island, Korea. 2. Vector mosquitoes of malayan filariasis. Jpn J Trop Med Hyg. 1973;1:197–210. [Google Scholar]
- 57.Lee WY. A study on Aedes togoi as vector of filariasis in Cheju Island. Korean J Parasitol. 1969;7:153–159. doi: 10.3347/kjp.1969.7.3.153. [DOI] [PubMed] [Google Scholar]