Abstract
A survey to determine the geographical distribution and relative abundance of potential vectors of scrub typhus was conducted from October to November 2006 at 13 localities throughout the Republic of Korea. Apodemus agrarius accounted for 97.6% (80/82) of all rodents, while only 2 Myodes regulus (2/82) were collected. A total of 10,860 chiggers were collected from A. agrarius belonging to 4 genera and 8 species, while only Walchia fragilis (40) was collected from Myodes regulus. Leptotrombidium pallidum (8,137; 74.9%), a vector of scrub typhus, was the predominant species collected from A. agrarius followed by Leptotrombidium scutellare (2,057, 18.9%), Leptotrombidium palpale (279; 2.7%), Leptotrombidium orientale (232; 2.1%), and Leptotrombidium zetum (79; 0.7%), Neotrombicula tamiyai (58; 0.5%), Euschoengastica koreaensis (16; 0.1%), and Cheladonta ikaoensis (2; < 0.1%). L. pallidum was the predominant chigger collected at collection sites in Gangwon (100%), Gyeonggi (87.2%), Chungnam (100%), Chungbuk (100%), Jeonbuk (73.9%), Jeonnam (77.0%), and Gyeongbuk (66.1%) provinces, whereas L. scutellare was the predominant chigger collected in Gyeongnam province (77.9%) and Jeju Island (62.3%). Data suggest a correlation between chigger population abundance and human cases of scrub typhus in Korea.
Keywords: Leptotrombidium, chigger mites, geographical distribution, scrub typhus
INTRODUCTION
Chigger mites are important veterinary and medical pests and vectors of Orientia tsutsugamushi, the causative agent of scrub typhus. O. tsutsugamushi is an obligate intracellular gram-negative bacterium that is responsible for large numbers of febrile illness in Korea, Japan, China, Thailand, and other East Asian countries [1,2]. Since 2005, the annual number of cases of scrub typhus has increased to more than 4,000 in the Republic of Korea (ROK) raising serious public health and military concerns. While scrub typhus cases are reported throughout the year in Korea, most occur from October through December [3-5].
In Korea, a total of 39 species of chigger mites have been reported; 36, 2, and 1 species are parasitic on mammals, birds, or both, respectively [6]. Leptotrombidium pallidum and Leptotrombidium scutellare are the primary vectors [7-11]. L. pallidum has the most widespread distribution, while L. scutellare is usually confined to the southern part of the Korean peninsula and islands [12,13]. Ree et al. [14] identified O. tsutsugamushi from 3 other less commonly collected species, Leptotrombidium palpale, Leptotrombidium orientale, and Leptotrombidium zetum, in addition to the 2 previously confirmed vectors in Korea. The relative population density of chigger vectors corresponds with annual increases in reported cases from October through December.
The objective of this study was to determine the relative abundance of chiggers and their hosts and geographic distribution to provide a more accurate assessment of human health risks throughout the Korean peninsula during the season of greatest disease incidence.
MATERIALS AND METHODS
Collection sites
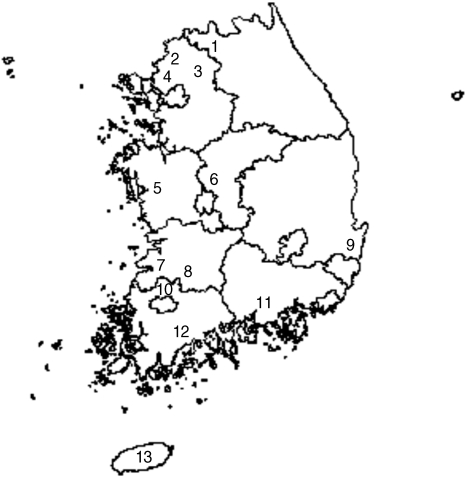
A total of 13 study sites in 9 provinces (Gangwon, Gyeonggi, Chungnam, Chungbuk, Jeonbuk, Jeonnam, Gyeongbuk, Gyeongnam, and Jeju) were surveyed throughout the Korean Peninsula where scrub typhus is endemic (Fig. 1). Collections were conducted from October through November 2006 when peak vector mite populations and numbers of scrub typhus cases were previously reported.
Fig. 1.
Map of field rodents collection at 13 study sites during October-November, 2006. 1. Cheorwon-gun (Gangwon province, N38° 00'47.6'', E127° 15'14.2''). 2. Yeoncheon-gun (Gyeonggi province, N38° 02'55.34'', E127° 06'18.35''). 3. Pochenon-gun (Gyeonggi province, N38° 00'47.6'', E127° 15'14.2''). 4. Paju-si (Gyeonggi province, N 37° 53'14.6'', E126° 55'56.1''). 5. Cheongyang-gun (Chungnam province, N36° 27'10.7'', E126° 43'50.0''). 6. Cheongwon-gun (Chungbuk province, N 36° 36'35.8'', E127° 20'36.0''). 7. Buan-gun (Jeonbuk province, N35° 36'37.1'', E126° 29'33.0''). 8. Namwon-si (Jeonbuk province, N35° 24'08.6'', E127° 26'05.2''). 9. Gyeongju-si (Gyeongbuk province, N35° 47'09.41'', E129° 20' 15.5''). 10. Jangseong-gun (Jeonnam province, N35° 13'24.2'', E126° 41'27.4''). 11. Jinju-si (Gyeongnam province, N35° 14'43.6'', E128° 16'43.8''). 12. Suncheon-si (Jeonnam province, N35°06' 11.5'', E127°30'12.8''). 13. Aaeweol (Jeju province, N33° 19'39.8'', E126°21'20.1'').
Rodent and chigger collections
Sylvatic rodents were collected by Sherman® live traps (7.6 × 8.9 × 22.9 cm; H.B. Sherman, Tallahassee, Florida, USA) baited with peanut butter placed between 2 saltine crackers. The traps were set out in the late afternoon and collected early the following morning. Rodents were transported to the laboratory, where they were euthanized, in accordance with an animal use protocol (Yonsei University), and identified to species.
The bodies of the euthanized rodents were hung individually over a 1,000 ml beaker filled to a depth of 1 cm with tap water for harvesting the larval mites. The mites which detached from the host and fell into the water were removed with a fine brush and placed in 75% ethanol until mounted on slides with polyvinyl alcohol. Larval mites were identified under a microscope at 400 × using morphological keys prepared by Ree [6]. A final electronic data record for each small mammal and the number of each mite species with locality and collection period was prepared.
RESULTS
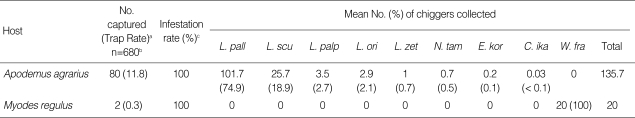
A total of 82 rodents were collected at the 13 study sites. The striped field mouse, Apodemus agrarius (80; 97.6%) was the predominant species collected, while only 2 Korean red backed voles, Myodes regulus (2; 2.4%), were collected. All rodents of both species were infested with larval mites. The chigger index (mean number of chiggers per infested rodent) for A. agrarius was 135.7, being predominantly infested by L. pallidum (101.7) and L. scutellare (25.7) (Table 1).
Table 1.
Rodent trap rates, chigger-infestation rates, and the mean number of chiggers collected per infested rodent captured from October to November, 2006
aNumber of rodents collected/number of trap nights; (% of all rodents captured); bNumber of traps; cPercent of each species infested with chigger mites.
L. pall, Leptotrombidium pallidum; L. scu, L. scutellare; L. palp, L. palpale; L. ori, L. orientale; L. zet, L. zetum; N. tam, Neotrombicula tamiyai; E. kor, Euschoengastica koreaensis; C. ika, Cheladonta ikaoensis; W. fra, Walchia fragilis.
A total of 10,860 chiggers belonging to 4 genera and 8 species were collected from A. agrarius. The most predominant species collected from A. agrarius was L. pallidum (74.9%), followed by L. scutellare (18.9%), L. palpale (2.7%), L. orientale (2.1%), L. zetum (0.7%), Neotrombicula tamiyai (0.5%), Euschoengastica koreaensis (0.1%), and Cheladonta ikaoensis (< 0.1%). Walchia fragilis (40) was collected only from M. regulus and none were collected from A. agrarius (Table 2).
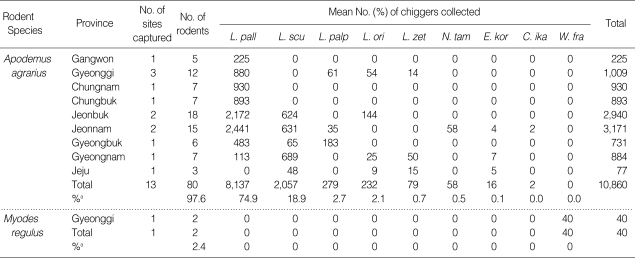
Table 2.
Provincial geographical distribution and the number of chiggers collected from field rodents captured from October to November, 2006
aPercent of each chigger species captured by rodent species.
L. pall, Leptotrombidium pallidum; L. scu, L. scutellare; L. palp, L. palpale; L. ori, L. orientale; L. zet, L. zetum; N. tam, Neotrombicula tamiyai; E. kor, Euschoengastica koreaensis; C. ika, Cheladonta ikaoensis; W. fra, Walchia fragilis.
Rodent infestation rates for L. pallidum were highest at Gangwon (100%), Gyeonggi (87.2%), Chungnam (100%), Chungbuk (100%), Jeonbuk (73.9%), Jeonnam (77.0%), and Gyeongbuk (66.1%) provinces and was collected from all provinces except Jeju Island (province). Rodent infestations for L. scutellare were the highest at Gyeongnam province (77.9%) and Jeju Island (62.3%), while none of the rodents were infested from Gangwon, Gyeonggi, Chungnam, and Chungbuk provinces. Rodent infestation rates for L. palpale were the highest at Gyeongbuk province (25.0%), while few rodents (0-6.1%) were infested at the other provinces. Rodent infestation rates for L. orientale and L. zetum were the highest at Jeju Island (11.7% and 19.5%, respectively), while few were infested from those captured at other provinces (Table 3).
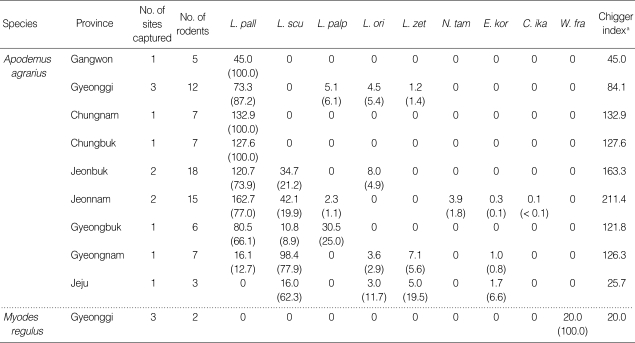
Table 3.
Geographical distribution, the mean number of chigger-infested Apodemus agrarius and Myodes regulus, and the percentage of each chigger species collected by province and rodent species from October to November, 2006
aChigger index = Mean number of chiggers collected per rodent.
L. pall, Leptotrombidium pallidum; L. scu, L. scutellare; L. palp, L. palpale; L. ori, L. orientale; L. zet, L. zetum; N. tam, Neotrombicula tamiyai; E. kor, Euschoengastica koreaensis; C. ika, Cheladonta ikaoensis; W. fra, Walchia fragilis.
The highest chigger index for L. pallidum was reported at Jeonnam province (162.7), while none were collected at Jeju Island. Geographical distribution of L. scutellare varied by locality (chigger indices ranging from 0-98.4) and was only collected in the southern provinces (Jeonbuk, Jeonnam, Gyeongbuk, Gyeongnam, and Jeju). L. palpale demonstrated limited geographical distributions and relatively low chigger indices ranging from 0-30.5. L. orientale and L. zetum also demonstrated limited geographical distributions and low chigger indices, ranging from 0-8.0 and 0-7.1, respectively (Table 3).
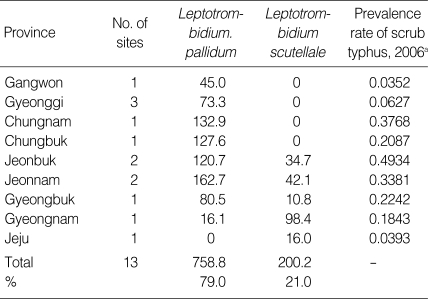
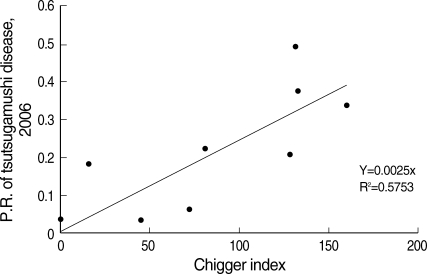
The high prevalence rate of scrub typhus was correlated with higher population densities based on chigger indices (r = 0.7674) of the 2 primary vectors, L. pallidum and L. scutellare (Table 4; Fig. 2). Chigger indices of the 2 primary vectors, L. pallidum and L. scutellare, while highly variable, were highest for Jeonnam (204.8), Chungnam (132.9), and Jeonbuk (155.4) provinces where scrub typhus prevalence rates ranged from 0.3381-0.4934/1,000, during 2006 (Table 4).
Table 4.
Chigger indices for Leptotrombidium pallidum and Leptotrombidium scutellare collected from Apodemus agrarius and prevalence rate of scrub typhus for each province, 2006
aPrevalence rate (%) = number of cases per total population of area × 1,000 (Data from Korea Centers for Disease Control and Prevention, 2006).
Fig. 2.
Correlation between chigger indices of Leptotrombidium pallidum and scrub typhus prevalence rates (Number of cases/total population of selected province × 1,000) of scrub in ROK, 2006.
DISCUSSION
As vectors of scrub typhus, chiggers pose a serious health threat among civilian and military populations throughout the Korean peninsula. In Korea, scrub typhus is regarded as one of the most prevalent zoonoses, with most cases reported from October to December [3-5]. A prerequisite to a comprehensive scrub typhus epidemiology program is knowledge of chigger bionomics, including their species distribution, population density, and larval habitats.
In the present descriptive study, L. pallidum was the predominant chigger collected in Gangwon, Gyeonggi, Chungnam, Chungbuk, Jeonbuk, Jeonnam, and Gyeongbuk provinces, except Jeju Island, whereas L. scutellare was the predominant chigger collected in Gyeongnam province and Jeju Island, while none of the rodents were infested from Gangwon, Gyeonggi, Chungnam, and Chungbuk provinces. L. palpale, L. orientale, and L. zetum demonstrated limited geographical distributions and relatively low chigger indices during the season of greatest disease incidence (Table 3). L. pallidum is found throughout the Korean peninsula, while L.scutellare is found only in the southern part of the Korean peninsula where its distribution is limited to annual temperatures ≥ 10℃ [14]. L. orientale is found highest during the autumn-spring seasons, while L. palpale and L. zetum are found highest in the late fall-winter and winter-spring seasons, respectively, and may variously contribute to low levels of human transmission of scrub typhus during periods of greatest abundance [3,13,14].
The high incidence of scrub typhus in humans during October through December is due to high populations of L. pallidum and L. scutellare that peak from September through November, and which sharply decline and remain low throughout December through August. Population densities of L. palpale, L. orientale, and L. zetum are relatively low and unevenly distributed and their impact on human diseases is uncertain. Additionally, the geographical distribution of chiggers and host indices is strongly correlated by species, with various chigger species predominating spatially during the same season and is supported by other studies [12-18].
As shown in Table 4, L. pallidum and L. scutellare, while highly variable, were highest for Jeonbuk, Chungnam, and Jeonnam provinces where scrub typhus is endemic. These findings suggest that transmission of O. tsutsugamushi is maintained by both vector species. The prevalence rate was the highest in Jeonbuk followed by Chungnam, Jeonnam, Gyeongbuk, and Chungbuk provinces (Table 4). The prevalence of scrub typhus cases in Jeonbuk, Chungnam, and Jeonnam provinces were higher than in any other provinces and were correlated with higher mean chigger indices of L. pallidum and L. scutellare. These data support seasonal and geographical risks of transmission of scrub typhus to human populations, especially when harvesting of crops and recreational activities increase exposure during the fall period when vector populations are high.
In the present study, strong evidence was found that seasonal and geographical distributions of chigger populations L. pallidum and L. scutellare were correlated with the prevalence of human cases. An increased density of chigger mite populations over their distribution increases the probability of human bites by mites and provides evidence for the high seasonal prevalence rates. Further geographical, ecological, and environmental studies that identify chigger mite hosts, relative chigger indices (population densities), and infection rates will provide a better understanding of disease risks throughout the Korean peninsula.
ACKNOWLEDGEMENTS
This work was supported by the Armed Forces Health Surveillance Center, Global Emerging Infections Surveillance and Response System, Silver Spring, Maryland, USA.
Disclaimer: The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Department of the Army or Department of Defense.
References
- 1.Traub R, Wisseman CL., Jr The ecology of chigger-borne rickettsiosis (scrub typhus) J Med Entomol. 1974;11:237–303. doi: 10.1093/jmedent/11.3.237. [DOI] [PubMed] [Google Scholar]
- 2.Sasa M. Biology of chiggers. Annu Rev Entomol. 1961;6:221–244. [Google Scholar]
- 3.Chang WH. Current status of tsutsugamushi disease in Korea. J Korean Med Sci. 1995;10:227–238. doi: 10.3346/jkms.1995.10.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim DM, Won KJ, Park CY, Yu KD, Kim HS, Yang TY, Lee JH, Kim HK, Song HJ, Lee SH, Shin H. Distribution of eschars on the body of scrub typhus patients: a prospective study. Am J Trop Med Hyg. 2004;76:806–809. [PubMed] [Google Scholar]
- 5.Bang HA, Lee MJ, Lee WC. Comparative research on epidemiological aspects of tsutsugamushi disease (scrub typhus) between Korea and Japan. Jpn J Infect Dis. 2008;61:148–150. [PubMed] [Google Scholar]
- 6.Ree HI. Fauna and key to the chigger mites of Korea (Acarina: Trombiculidae and Leeuwenhoekiidae) Korean J Syst Zool. 1990;6:57–70. [Google Scholar]
- 7.Jackson EB, Danaska JX, Smadel JE, Fuller HS, Coale MC, Bozeman FM. Occurrence of Rickettsia tustsugamushi in Korea rodents and chiggers. Am J Hyg. 1957;66:309–320. doi: 10.1093/oxfordjournals.aje.a119904. [DOI] [PubMed] [Google Scholar]
- 8.Ree HI, Lee HS, Lee IY, Yoshida Y. Epidemiological studies on host animals of tsutsugamushi disease in Korea. Korean J Parasitol. 1991;29:181–188. doi: 10.3347/kjp.1991.29.2.181. [DOI] [PubMed] [Google Scholar]
- 9.Ree HI, Lee HS, Lee IY. Study on the population density of chigger mites, the vector of Tsutsugamushi disease in Korea. Korean J Zool. 1991;34:257–264. [Google Scholar]
- 10.Ree HI, Kim TE, Lee IY, Jeon SH, Hwang UK, Chang WH. Determination and geographical distribution of Orientia tsutsugamushi serotypes in Korea by nested polymersae chain reaction. Am J Trop Med Hyg. 2001;65:528–534. doi: 10.4269/ajtmh.2001.65.528. [DOI] [PubMed] [Google Scholar]
- 11.Ree HI, Lee IY, Cho MK. Determination of the vector species of tsutsugamushi disease in Korea. Korean J Parasitol. 1991;29:87–92. doi: 10.3347/kjp.1991.29.1.87. [DOI] [PubMed] [Google Scholar]
- 12.Ree HI, Lee IY, Cho MK. Study on vector mites of tsutsugamushi disease in Cheju Island. Korean J Parasitol. 1992;30:341–348. doi: 10.3347/kjp.1992.30.4.341. [DOI] [PubMed] [Google Scholar]
- 13.Lee IY, Ree HI, Hong HK. Seasonal prevalence and geographical distribution of trombiculid mites (Acarina: Trombiculidae) in Korea. Korean Zool. 1993;36:408–415. [Google Scholar]
- 14.Ree HI, Lee IY, Jeon SH, Yoshida Y. Geographical distribution of vectors and sero-strains of tsutsugamushi disease at mid-south inland of Korea. Korean J Parasitol. 1997;35:171–179. doi: 10.3347/kjp.1997.35.3.171. [DOI] [PubMed] [Google Scholar]
- 15.O'Guinn ML, Klein TA, Lee JS, Richards AL, Kim HC, Ha SJ, Shim SH, Baek LJ, Song KJ, Chong ST, Turell MJ, Burkett DA, Schuster A, Lee IY, Yi SH, Sames WJ, Song JW. Serological surveillance of scrub typhus, murine typhus, and leptospirosis in small mammals captured at firing points 10 and 60, Gyeonggi Province, Republic of Korea, 2001-2005. Vector-borne Zoon Dis. 2009;9 doi: 10.1089/vbz.2008.0123. (In press) [DOI] [PubMed] [Google Scholar]
- 16.Ree HI, Lee MC, Lee IY. Study on the population density of chigger mites, the vector of tsutsugamushi disease in Korea. Korean J Zool. 1991;34:257–264. [Google Scholar]
- 17.Ree HI, Cho MK, Lee IY, Jeon SH. Comparative epidemiological studies on vector/reservoir animals of tsutsugamushi disease between high and low endemic areas in Korea. Korean J Parasitol. 1995;33:27–36. doi: 10.3347/kjp.1995.33.1.27. [DOI] [PubMed] [Google Scholar]
- 18.Song HJ, Kim KH, Kim SC, Hong SS, Ree HI. Population density of chigger mites, the vector of tsutsugamushi disease in Chollanam-do, Korea. Korean J Parasitol. 1996;34:27–33. doi: 10.3347/kjp.1996.34.1.27. [DOI] [PubMed] [Google Scholar]