Abstract
Timely cell cycle regulation is conducted by sequential activation of a family of serine-threonine kinases called cycle dependent kinases (CDKs). Tight CDK regulation involves cyclin dependent kinase inhibitors (CKIs) which ensure the correct timing of CDK activation in different phases of the cell cycle. One CKI of importance is p27KIP1. The regulation and cellular localization of p27KIP1 can result in biologically contradicting roles when found in the nucleus or cytoplasm of both normal and tumor cells. The p27KIP1 protein is mainly regulated by proteasomal degradation and its downregulation is often correlated with poor prognosis in several types of human cancers. The protein can also be functionally inactivated by cytoplasmic localization or by phosphorylation. The p27KIP1 protein is an unconventional tumor suppressor because mutation of its gene is extremely rare in tumors, implying the normal function of the protein is deranged during tumor development. While the tumor suppressor function is mediated by p27KIP1's inhibitory interactions with the cyclin/CDK complexes, its oncogenic function is cyclin/CDK independent, and in many cases correlates with cytoplasmic localization. Here we review the basic features and novel aspects of the p27KIP1 protein, which displays genetically separable tumor suppressing and oncogenic functions.
Keywords: cell cycle, cyclin-dependent kinase inhibitor p27, cyclin-dependent kinases, tumor suppressor proteins
Introduction
Extracellular environments initiate cell cycle division or arrest by activating or deactivating cycle dependent kinase (CDK) complexes. Since timely regulation of CDK complexes is critical for proper cell cycle regulation, multiple signals can integrate to control the activity of cyclin/CDK complexes. Activity of cyclin/CDK complexes is regulated by the accumulation of cyclins and by phosphorylation/dephosphorylation of specific complex components (Norbury and Nurse, 1992; Sherr et al., 1993; Malumbres and Barbacid, 2005; De Clercq and Inzé, 2006). Another important regulation of G1 cyclin/CDK complexes lies in their association with cyclin dependent kinase inhibitors (CKIs), such as p27KIP1. Cyclin dependent kinase inhibitors are often associated with diseases when mutated or deregulated. The p27KIP1 protein binds to various cyclin/CDK complexes throughout the cell cycle, and is one exemplary CDK inhibitor whose misregulation, and not genetic mutation, is found in diverse cancer types (Tsihilias et al., 1999; Besson et al., 2008; Chu et al., 2008).
The p27KIP1 protein was first identified as an inhibitor of cyclin E/CDK2 complexes during TGFβ-induced G1 arrest (Sheaff et al., 1997). The p27KIP1 protein inhibits cyclin/CDK activity by binding cyclin/CDK complexes through its N-terminal, blocking ATP binding, and physically occluding the catalytic cleft of the CDK (Russo et al., 1996; Hong et al., 2009; Zhang et al., 2009). A major regulatory mechanism of controlling the p27KIP1 inhibitory function is to regulate p27KIP1 protein levels through transcriptional, translational, and post-translational mechanisms (Hengst and Reed, 1996; Carrano et al., 1999; Boehm et al., 2002; Bagui et al., 2009; Shin et al., 2009; Trabosh et al., 2009). Recent study revealed that a novel regulatory mechanism during postphosphorylation and polyubiquitination of proteins could be another regulatory mechanism for p27KIP1. Pin1, peptidyl-prolyl isomerase, recognizes and stabilizes p27KIP1 when phosphorylated on Thr187 by inducing its conformational change (Zhou et al., 2009). Misregulation that results in increased degradation of p27KIP1 can be related to cancer development (Hershko, 2008; Mishra et al., 2009). In addition to TGFβ, other environmental factors such as serum starvation or contact inhibition can increase p27KIP1 protein whose role becomes essential during cell cycle arrest and differentiation. For instance, serum deprivation increases the amount of p27 phosphorylation during muscle differentiation or quiescence approacing G0 state of colon carcinoma cells and mitogen stimulation then causes cells to enter G1 with the translocation of p27 to the cytoplasm (Rodier et al., 2001; Boehm et al., 2002; Deng et al., 2003; 2004; Jin et al., 2009). Phosphorylation, as such, is the mechanism primarily used for regulating p27KIP1 activity. The p27KIP1 protein possesses multiple tyrosine, serine, or threonine phosphorylation sites (Figure 1). The inhibitory actions of p27KIP1 on cyclin/CDK complexes are weakened by phosphorylations directed by some signal transduction pathways (Larrea et al., 2008; Morishita et al., 2008; Tossidou et al., 2008; Jin et al., 2009). It appears that different signalling pathways direct the fate of the protein through differential phosphorylation patterns as Thr187 phosphorylation leads p27KIP1 to a SCFSkp2 ubiquitin ligase complex and promotes the polyubiquitination and degradation while T198 phosphorylation of p27KIP1 by ribosomal S6 kinase 1 (RSK1) promotes cell motility (Grimmler et al., 2007; Larrea et al., 2009). This suggests that signal transduction pathway utilizes regulatory mechanisms of p27KIP1 by phosphorylation to control the activity of cyclin/CDK complexes. Moreover, recent studies suggest that post-translational modification of p27KIP1 by phosphorylation can play diverse roles for p27KIP1 in regulations on its half life and subcellular localization. While it is evident that nuclear p27KIP1 through inactivating cyclinE/CDK2 complex acts as a tumor suppressor, tumorigenic properties of p27KIP1 have also been proposed in recent years especially when located in cytoplasm. (Blagosklonny, 2002; Sicinski et al., 2007; Hidaka et al., 2009). Pro-tumorigenic activities include enhancing cell mobility and assisting assembly of cyclin D/CDK4 complexes, thereby promoting metastasis and cell cycle progression (Besson et al., 2004; Jeon et al., 2008; Kim et al., 2008; Vervoorts and Lüscher 2008). Since cytoplasmic localization can be associated with cell migration promoting activity of p27KIP1, removal of p27KIP1 from nucleus to degradation is less oncogenic than translocalization of nuclear p27KIP1 to the cytoplasm. The puzzling dual function of p27KIP1 is intriguing and worth additional attention because it answers some mysteries surrounding p27KIP1, such as existence of the p27KIP1 protein in malignant cancers. While potential pro-oncogenic activity of p27KIP1 awaits further investigation, several research groups have independently generated null mutants in order to better understand biological function of p27KIP1 and to provide better evidence of tumor suppressor activity of the protein.
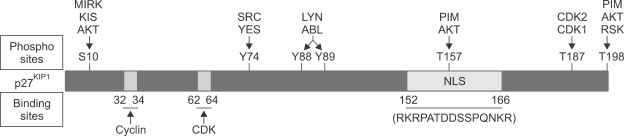
Figure 1.
Schematic diagram of p27KIP1 phosphorylation and binding sites. Phosphorylation sites (upper) and binding sites (lower) of full length p27KIP1 protein are illustrated with the specific site numbers for kinases, or with the binding regions for cyclin/CDK proteins. Nuclear localization sequences (NLS) are also depicted. Note that the threonine subject to AKT phosphorylation, T157, is located within the NLS.
The p27KIP1 null (-/-) mouse shows an overall increase in cell proliferation, resulting in approximately 30% increase in body size, multiple organ hyperplasia, and disorganization of sensory epithelia in the retina and inner ear. In addition, the null mice also display female sterility due to defective ovarian and uterine function (Fero et al., 1996; Chien et al., 2007; Kim et al., 2007). The tumor suppressive activity of p27KIP1 using null mice was first demonstrated by development of adenomas in the intermediate lobe of the pituitary gland, and by their inclination to develop tumors more easily when challenged with chemical carcinogens or irradiation (Nakayama et al., 1996). It is likely that combined loss of p27KIP1 with other tumor suppressor genes further enhances tumorigenesis (Di Cristofano et al., 2001; Glover et al., 2009). A 50% reduction in p27KIP1 protein level predisposes p27KIP1 heterozygous (+/-) mice to tumors in multiple organs, when combined with administration of carcinogens, or when genetically combined with additional oncogenes or tumor suppressors. In humans, p27KIP1 deficiency has also been found to be associated with sporadic tumorigenesis. The first human cases reported to have abnormally low amounts of nuclear p27KIP1 were associated with increased tumor aggressiveness and a relatively poor clinical outcome for breast and colon cancer (Loda et al., 1997).
Recent findings from knock-out models also show data supporting the pro-oncogenic property of p27KIP1 protein. Two groups reported that p27KIP1 heterozygous (+/-) mice were more susceptible to mammary and prostate tumors than p27KIP1 null (-/-) mice (Muraoka et al., 2002; Gao et al., 2004). Analysis of knock-in mice with CDK mutant p27KIP1, p27KIP1 (CK-), that lacks CDK inhibitory function revealed that p27KIP1 (CK-/ CK-) mice not only displayed the tumor development phenotype of p27KIP1 null (-/-) mice, but also developed a whole range of hyperplasia and neoplasia, suggesting that the p27KIP1 (CK-) protein function as an oncogenic protein (Besson et al., 2007). In human cancer cells, homozygous inactivation of the p27KIP1 gene in sporadic tumors is extremely rare, but the correlation of cytoplasmic localization of p27KIP1 protein with high tumor grade and poor prognosis was discovered (Slingerland and Pagano, 2000). The p27KIP1 (CK-) protein was also found in cytoplasm, which correlated with the hypothesis of the p27KIP inhibitory action of RhoA pathway.
Benefited from many clinical reports, we now understand that the p27KIP1 protein can be a prognostic indicator for breast, colon, prostate, lung, esophageal, and gastric cancers. Tumorigenic activity due to abated p27KIP1 is dose-dependent, meaning lower doses of the protein are associated with increased malignancy. Also, it is now understood that deregulation of p27KIP1 protein, not loss of its gene, is the cause of reduced p27KIP1 protein levels. Orderly regulation of p27KIP1 includes proper activation of the oncogenic signal proteins, deregulation of which in turn is commonly found in human cancers. Therefore, better understanding of the regulatory mechanisms of p27KIP1 function may provide good clinical value with prognostic and therapeutic applications. This fascinating protein has drawn the attention of clinicians as well as scientists, and as a result, understanding the exact cellular functions and mechanisms is currently under active investigation. This report will focus on the regulatory mechanisms and dual roles of p27KIP1 in cancer biology.
Interaction of p27KIP1 with cyclin/CDK complexes and its inhibitory function
Different from INK4 family proteins' binding to a CDK alone, CDK4 or CDK6, CIP/KIP proteins like p27KIP1 interact with the cyclin E/CDK2 complex. The first α-helical loop of a CIP/KIP protein interacts with the cyclin, and the second helix binds to the catalytic cleft of the CDK subunit, thereby blocking ATP loading (Sherr and Roberts, 1999; Yil et al., 2007; Jung et al., 2008). The inhibition of ATP loading blocks activation of the cyclin E/CDK2 complex, and prevents progression through the cell cycle. The p27KIP1 protein binds not only to the cyclin E/CDK2 complex, but also to the cyclin D/CDK4,6 complexes. However, the interaction with the cyclin D/CDK complexes is more complicated. It has become consensus that p27KIP1 is a required assembly factor for the complex, but whether the binding is inhibitory is still questionable. Moreover, how it might switch between the two modes of inhibitory and non-inhibitory needs to be answered.
Arguing for a two state mechanism, Dr. Blain's group has assiduously sought-after, and shown that p27KIP1 can be both a CDK4 bound inhibitor, and a bound non-inhibitor, depending on the growth state of the cell (Ray et al., 2009). They also have discovered that p27KIP1 associates with cyclin D/CDK4 constitutively, and that a specific tyrosine phosphorylation converts p27KIP1 from a bound inhibitor to a bound non-inhibitor under different growth conditions. To further support this, they showed that in vitro tyrosine phosphorylation, by the tyrosine kinase Abl, converts the bound inhibitor to a bound non-inhibitor. Larrea et al. reported a similar finding, demonstrating that phosphorylations at Thr157 and Thr198 are required for binding to cyclin D1 and CDK4, but are not sufficient to activate the cyclin D/CDK4 complex (Larrea et al., 2008). In addition, Larrea et al. showed that tyrosine phosphorylation by SRC activates the p27KIP1 bound cyclin D1/CDK4 complex, but tyrosine phosphorylated p27KIP1 does not affect assembly of the complex.
The p27KIP1 protein can also bind to the nuclear pore-associated protein (mNPAP60), and interact with the nuclear export protein chromosome region maintenance 1 protein (CRM1) (Shin et al., 2005). Since CRM1 mediates nuclear export, CRM1 interaction with p27KIP1 causes trans-localization into the cytoplasm and out of nucleus. Furthermore, interaction of p27KIP1 with CRM1 also causes displacement of cyclin D1 from CRM1, leading to increased cyclin D1 levels in the nucleus, and progression through the cell cycle.
p27KIP1 is phosphorylated at multiple sites by various signaling pathways
An ability of p27KIP1, together with other cell cycle molecules, is to respond to diverse extracellular demands. In doing so it helps cells adjust to the new environment through proper cell cycle regulation, which is pivotal to maintaining normal cellular homeostasis. The ability to properly respond to different signaling pathways comes from accordingly regulating the p27KIP1 protein by intricate, but unmistaken phosphorylations. Misregulation or functional inactivation of p27KIP1 protein is caused when oncogenic kinases such as PKB and SRC are over-activated, leading to the development of malignancies. If these oncogenic signaling pathways can be inhibited, it is possible to restore the tumor suppressive functions of misregulated p27KIP1 protein. Hence, understanding mechanisms of p27KIP1 phosphorylation will provide additional perspectives for finding potential targets for cancer prevention and therapy.
The p27KIP1 protein is short-lived, and activity of this protein largely depends on protein levels that are regulated mainly through proteasome-dependent degradation and/or transcriptional control. Recent studies show that potentially oncogenic serine/threonine kinases, PIM kinases, promote cell cycle progression by phosphorylating Thr157 and Thr198 of p27KIP1 leading to its nuclear export and proteasome-dependent degradation. However, while the mechanisms involved are under active investigation, p27KIP1 possesses seemingly contradictory actions in facilitating cell motility by interacting with proteins involved in functions aside from cell cycle regulations while located in the cytoplasm. The role of p27KIP1 in facilitating cell motility through inhibition of RhoA activation in the cytosol, implies that its cytosolic localization does more than free cyclin/CDK complexes from inhibition. It also does more than subject this inhibitor to proteasomal degradation (Figure 2). The significance of multiple biological functions for p27KIP1 protein is generally acknowledged. The onset of environmental cues delivers a new role to this cell cycle inhibitor as a result of post-translational modifications like phosphorylation or ubiquitination. Extensive studies have informed us that p27KIP1 protein remodels itself predominantly through phosphorylation and consequently alters its interacting protein partners, changes its cellular function, localization, as well as protein expression levels.
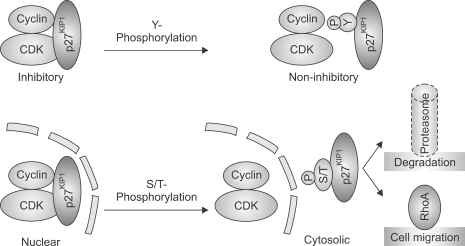
Figure 2.
The p27KIP1 protein is regulated by phosphorylation on multiple sites. Hypo-phosphorylated or unphosphorylated p27KIP1 acts as a cyclin/CDK inhibitor. Multiple phosphorylated forms of the protein, through activation of various mitogenic signals, are present in cells. Phospho-p27KIP1 diverges into discrete fates according to the location of phosphate groups. Respective functions of multifarious combinations of individual phospho sites are beyond comprehension at present. However, tyrosine phosphorylation in general renders phopho-p27KIP1 non-inhibitory and serine/threonine phosphorylation results in cytosolic localization. Some phospho forms of the cytosolic p27KIP1 are subject to proteasomal degradation, while others inhibit RhoA activation and facilitate cell migration.
Important roles for p27KIP1 in guarding cells against breast cancer have advanced our understanding of the relationship between p27KIP1 phosphorylation and its role in CDK inhibition. Phosphorylation of serine or threonine on p27KIP1 by ERK1 targets the protein for ubiquitination, and phosphorylation on more than one threonine site by different kinases is involved in cytoplasmic localization. Cyclin E/CDK2 phosphorylates p27KIP1 on Thr187 and leads to ubiquitin-dependent degradation. The p27KIP1 protein is phosphorylated by AKT at Thr157 and Thr198 to become a better assembler of cyclin D/CDK4 complexes, and the binding of p27KIP1 to cyclin D/CDK4 facilitates activation of cyclin E/CDK2 through sequestration of the inhibitory protein. Therefore, the differential binding of p27KIP1 to distinct CDKs during G1 cell cycle can also be attributed to the phosphorylation status of p27KIP1. As p27KIP1 phosphorylations are cell cycle dependent, the cyclin E/CDK2 inhibitory activity of p27KIP1 is maximal during G0, and falls as cells move through G1 into S phase. At the same time, the cyclin D/CDK4 bound p27KIP1 is maximal in early G1. All of these findings suggest that anti-mitogenic signaling, which can alter phosphorylation status of p27KIP1, can switch p27KIP1 binding from CDK4/6 complexes to cyclin E/CDK2 complexes, promoting restoration of cell cycle control.
p27KIP1 exerts anti- and pro-tumorigenic activities
Decreases in p27KIP1 expression levels have been implicated in the genesis and progression of many human malignancies. Just as a mouse knock-out system was used to determine anti-tumorigenic activities of p27KIP1, a mouse knock-in model was used to insert one allele of p27KIP1 that cannot bind cyclins and CDKs. This knock-in model has been generated to address the question of the tumor-prone phenotype with CDK inhibitory function. The knock-in mouse model shows an increase in spontaneous tumorigenesis in many tissues when compared with either wild-type or p27KIP1 null animals (Kim et al., 2005; Susaki et al., 2009). However, many studies indicate that the function of p27KIP1 cannot be solely attributed to its CDK inhibitory action. The critical function of p27KIP1 in tumorigenesis therefore may extend beyond its ability to regulate CDKs. Indeed, the fact that p27KIP1 levels in tumors do not always correlate with proliferative index and that the subcellular localization of p27KIP1 is a negative prognostic factor in some tumors, indicates that p27KIP1 may not function as a CDK inhibitor in these cases. Another study shows that the haploinsufficient p27KIP1 heterozygous mouse is more susceptible to tumor formation than the p27KIP1 null animals in mammary and prostate tumor models, suggesting an active contribution of the remaining p27KIP1 allele to tumor development (Gao et al., 2004). The group interpreted the enhanced susceptibility to be a result of partial down-regulation of the cyclin/CDK inhibitory activity but maintenance of other pro-tumorigenic roles. Mice carrying the p27KIP1 S10A allele, which renders p27KIP1 to nuclear localization, show a partial resistance to urethane-induced tumorigenesis, despite reduced overall abundance of the p27KIP1 protein (Besson et al., 2007). This study further supports the correlation between cytosolic localization and tumorigenic activity of p27KIP1.
The current model generally accepted is that p27KIP1 suppresses tumorigenesis by inhibiting cyclin/CDK activity in the nucleus, but exerts other functions in the cytoplasm that are potentially oncogenic. While both roles may be important for homeostasis, inactivation of the nuclear function and/or exaggeration of the cytoplasmic functions may promote tumor progression. The cytosolic functions include the regulation of the actin cytoskeleton and cell migration through modulation of RhoA activity. Cytosolic activities are generated by the C-terminal portion of the p27KIP1 protein (Besson et al., 2004; Batsi et al., 2009). During mouse embryonic development, this function is critical for the migration of cortical neurons (Nguyen et al., 2006). Interestingly, cyclin/CDK binding is through the N-terminal portion of p27KIP1, and this same region modulates differentiation of neuronal progenitors in vivo via stabilization of neurogenin-2, independent of cyclin/CDK interaction (Nguyen et al., 2006).
Conclusion
The p27KIP1 cyclin dependent kinase inhibitor displays apparently contradicting roles by acting as a classic tumor suppressor in one instance, and a pro-tumorigenic oncogene in the other. As its primary function as a CKI is to bind and inhibit cyclin/CDK complexes, the p27KIP1 protein functions throughout all cell cycle phases by interacting with different cyclin/CDK complexes. However, this cell cycle inhibitor has emerged to play important roles in other cellular functions, such as cell migration. Being a substrate of several important mitogenic signaling kinases, the p27KIP1 protein is post-translationally modified by phosphorylation. Mitogen-induced phosphorylation causes alterations in expression levels, intracellular localization, and induction of p27KIP1's diverse functions. The proper regulation of p27KIP1 expression levels, localization, and differential functions, must be important in maintaining homeostasis and preventing cells from forming tumors. Understanding the diverse roles of p27KIP1 protein during normal cell cycle progression and tumor development provides new insights into the field of tumor prognosis and therapeutics.
Acknowledgements
This work was supported by a grant from the Korea Science and Engineering Foundation (No. R13-2002-020-02001-0, 2007).
Abbreviations
- CDKs
cycle dependent kinases
- CKIs
cyclin dependent kinase inhibitors
- CRM1
chromosome region maintenance 1 protein
- Pin1
peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
- RSK1
ribosomal S6 kinase 1
- SCF
skp1-cullin1-F-box protein
- Skp2
S-phase kinase-associated protein 2
References
- 1.Bagui TK, Cui D, Roy S, Mohapatra S, Shor AC, Ma L, Pledger WJ. Inhibition of p27Kip1 gene transcription by mitogens. Cell Cycle. 2009;8:115–124. doi: 10.4161/cc.8.1.7527. [DOI] [PubMed] [Google Scholar]
- 2.Batsi C, Markopoulou S, Kontargiris E, Charalambous C, Thomas C, Christoforidis S, Kanavaros P, Constantinou AI, Marcu KB, Kolettas E. Bcl-2 blocks 2-methoxyestradiol induced leukemia cell apoptosis by a p27(Kip1)-dependent G1/S cell cycle arrest in conjunction with NF-kappaB activation. Biochem Pharmacol. 2009;78:33–44. doi: 10.1016/j.bcp.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004;18:862–876. doi: 10.1101/gad.1185504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besson A, Hwang HC, Cicero S, Donovan SL, Gurian-West M, Johnson D, Clurman BE, Dyer MA, Roberts JM. Discovery of an oncogenic activity in p27Kip1 that causes stem cell expansion and a multiple tumor phenotype. Genes Dev. 2007;21:1731–1746. doi: 10.1101/gad.1556607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besson A, Dowdy SF, Roberts JM. CDK inhibitors: Cell cycle regulators and beyond. Dev Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Blagosklonny MV. Are p27 and p21 cytoplasmic oncoproteins? Cell Cycle. 2002;1:391–393. doi: 10.4161/cc.1.6.262. [DOI] [PubMed] [Google Scholar]
- 7.Boehm M, Yoshimoto T, Crook MF, Nallamshetty S, True A, Nabel GJ, Nabel EG. A growth factor-dependent nuclear kinase phosphorylates p27(Kip1) and regulates cell cycle progression. EMBO J. 2002;21:3390–3401. doi: 10.1093/emboj/cdf343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrano AC, Eytan E, Hershko A, Pagano M. Skp2 is required for ubiquitin-mediated degradation of the Cdk inhibitor p27. Nat Cell Biol. 1999;1:193–197. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 9.Chien WM, Garrison K, Caufield E, Orthel J, Dill J, Fero ML. Differential gene expression of p27Kip1 and Rb knockout pituitary tumors associated with altered growth and angiogenesis. Cell Cycle. 2007;15:750–757. doi: 10.4161/cc.6.6.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 11.De Clercq A, Inze D. Cyclin-dependent kinase inhibitors in yeast, animals, and plants: A functional comparison. Crit Rev Biochem Mol Biol. 2006;41:293–313. doi: 10.1080/10409230600856685. [DOI] [PubMed] [Google Scholar]
- 12.Deng X, Ewton DZ, Pawlikowski B, Maimone M, Friedman E. Mirk/dyrk1B is a Rho-induced kinase active in skeletal muscle differentiation. J Biol Chem. 2003;278:41347–41354. doi: 10.1074/jbc.M306780200. [DOI] [PubMed] [Google Scholar]
- 13.Deng X, Mercer SE, Shah S, Ewton DZ, Friedman E. The cyclin-dependent kinase inhibitor p27Kip1 is stabilized in G(0) by Mirk/dyrk1B kinase. J Biol Chem. 2004;279:22498–22504. doi: 10.1074/jbc.M400479200. [DOI] [PubMed] [Google Scholar]
- 14.Di Cristofano A, De Acetis M, Koff A, Cordon-Cardo C, Pandolfi PP. Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nat Genet. 2001;27:222–224. doi: 10.1038/84879. [DOI] [PubMed] [Google Scholar]
- 15.Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai LH, Broudy V, Perlmutter RM, Kaushansky K, Roberts JM. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27Kip1(Kip1)-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 16.Gao H, Ouyang X, Banach-Petrosky W, Borowsky AD, Lin Y, Kim M, Lee H, Shih WJ, Cardiff RD, Shen MM, Abate-Shen C. A critical role for p27Kip1kip1 gene dosage in a mouse model of prostate carcinogenesis. Proc Natl Acad Sci USA. 2004;101:17204–17209. doi: 10.1073/pnas.0407693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glover CE, Gurley KE, Kim KH, Storer B, Fero ML, Kemp CJ. Endocrine dysfunction in p27Kip1 deficient mice and susceptibility to Wnt-1 driven breast cancer. Carcinogenesis. 2009;30:1058–1063. doi: 10.1093/carcin/bgp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimmler M, Wang Y, Mund T, Cilensek Z, Keidel EM, Waddell MB, Jäkel H, Kullmann M, Kriwacki RW, Hengst L. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell. 2007;128:269–280. doi: 10.1016/j.cell.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 19.Hengst L, Reed SI. Translational control of p27Kip accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- 20.Hershko DD. Oncogenic properties and prognostic implications of the ubiquitin ligase Skp2 in cancer. Cancer. 2008;112:1415–1424. doi: 10.1002/cncr.23317. [DOI] [PubMed] [Google Scholar]
- 21.Hidaka T, Hama S, Shrestha P, Saito T, Kajiwara Y, Yamasaki F, Sugiyama K, Kurisu K. The combination of low cytoplasmic and high nuclear expression of p27 predicts a better prognosis in high-grade astrocytoma. Anticancer Res. 2009;29:597–603. [PubMed] [Google Scholar]
- 22.Hong K, Choi Y, Lee J, Kim H, Kwon H, Seong Y, Kim HT, Park J, Bae C, Hong KM. Transient phosphorylation of tumor associated microtubule associated protein (TMAP)/cytoskeleton associated protein 2 (CKAP2) at Thr-596 during early phases of mitosis. Exp Mol Med. 2008;40:377–386. doi: 10.3858/emm.2008.40.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong YB, Kang HJ, Kim HJ, Rosen EM, Dakshanamurthy S, Rondanin R, Baruchello R, Grisolia G, Daniele S, Bae I. Inhibition of cell proliferation by a resveratrol analog in human pancreatic and breast cancer cells. Exp Mol Med. 2009;41:151–160. doi: 10.3858/emm.2009.41.3.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeon JY, An JH, Kim SU, Park HG, Lee MA. Migration of human neural stem cells toward an intracranial glioma. Exp Mol Med. 2008;40:84–91. doi: 10.3858/emm.2008.40.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin K, Ewton DZ, Park S, Hu J, Friedman E. Mirk regulates the exit of colon cancer cells from quiescence. J Biol Chem. 2009;284:22916–22925. doi: 10.1074/jbc.M109.035519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung JE, Kim HS, Lee CS, Shin YJ, Kim YN, Kang GH, Kim TY, Juhnn YS, Kim SJ, Park JW, Ye SK, Chung MH. STAT3 inhibits the degradation of HIF-1α by pVHL-mediated ubiquitination. Exp Mol Med. 2008;40:479–485. doi: 10.3858/emm.2008.40.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 28.Kim SM, Kim N, Lee S, Kim DK, Lee YM, Ahn SH, Song JH, Choi BK, Wu C, Jung KY. TGF-β1-induced PINCH-1-ILK-α-parvin complex formation regulates mesangial cell proliferation and hypertrophy. Exp Mol Med. 2007;39:514–523. doi: 10.1038/emm.2007.57. [DOI] [PubMed] [Google Scholar]
- 29.Kim EK, Yun SJ, Do KH, Kim MS, Cho M, Suh DS, Kim CD, Kim JH, Birnbaum Morris J, Bae SS. Lysophosphatidic acid induces cell migration through the selective activation of Akt1. Exp Mol Med. 2008;40:445–452. doi: 10.3858/emm.2008.40.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larrea MD, Liang J, Da Silva T, Hong F, Shao SH, Han K, Dumont D, Slingerland JM. Phosphorylation of p27Kip1 regulates assembly and activation of cyclin D1-Cdk4. Mol Cell Biol. 2008;28:6462–6472. doi: 10.1128/MCB.02300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larrea MD, Hong F, Wander SA, da Silva TG, Helfman D, Lannigan D, Smith JA, Slingerland JM. RSK1 drives p27Kip1 phosphorylation at T198 to promote RhoA inhibition and increase cell motility. Proc Natl Acad Sci USA. 2009;106:9268–9273. doi: 10.1073/pnas.0805057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 33.Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30:630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Mishra A, Godavarthi SK, Jana NR. UBE3A/E6-AP regulates cell proliferation by promoting proteasomal degradation of p27. Neurobiol Dis. 2009 doi: 10.1016/j.nbd.2009.06.010. In press. [DOI] [PubMed] [Google Scholar]
- 35.Morishita D, Katayama R, Sekimizu K, Tsuruo T, Fujita N. Pim kinases promote cell cycle progression by phosphorylating and down-regulating p27Kip1 at the transcriptional and posttranscriptional levels. Cancer Res. 2008;68:5076–5085. doi: 10.1158/0008-5472.CAN-08-0634. [DOI] [PubMed] [Google Scholar]
- 36.Muraoka RS, Lenferink AE, Law B, Hamilton E, Brantley DM, Roebuck L, Arteaga CL. ErbB2/Neu-induced, cyclin D1-dependent transformation is accelerated in p27Kip1-haploin-sufficient mammary epithelial cells but impaired in p27Kip1-null cells. Mol Cell Biol. 2002;22:2204–2219. doi: 10.1128/MCB.22.7.2204-2219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY, Nakayama K. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen L, Besson A, Heng JI, Schuurmans C, Teboul L, Parras C, Philpott A, Roberts JM, Guillemot F. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 2006;20:1511–1524. doi: 10.1101/gad.377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norbury C, Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- 40.Ray A, James MK, Larochelle S, Fisher RP, Blain SW. p27Kip1 inhibits cyclin D-cyclin-dependent kinase 4 by two independent modes. Mol Cell Biol. 2009;29:986–999. doi: 10.1128/MCB.00898-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodier G, Montagnoli A, Di Marcotullio L, Coulombe P, Draetta GF, Pagano M, Meloche S. p27 cytoplasmic localization is regulated by phosphorylation on Ser10 and is not a prerequisite for its proteolysis. EMBO J. 2001;20:6672–6682. doi: 10.1093/emboj/20.23.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russo AA, Jeffrey PD, Patten AK, Massague J, Pavletich NP. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature. 1996;382:325–331. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- 43.Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 44.Sherr CJ. Mammalian G1 cyclins. Cell. 1993;48:981–983. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- 45.Sherr CJ, Roberts JM. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 46.Shin I, Rotty J, Wu FY, Arteaga CL. Phosphorylation of p27Kip1 at Thr-157 interferes with its association with importin alpha during G1 and prevents nuclear re-entry. J Biol Chem. 2005;18:6055–6063. doi: 10.1074/jbc.M412367200. [DOI] [PubMed] [Google Scholar]
- 47.Shin MH, Mavila N, Wang WH, Vega Alvarez S, Hall MC, Andrisani OM. Time-dependent activation of Phox2a by the cAMP pathway modulates onset and duration of p27Kip1 transcription. Mol Cell Biol. 2009 doi: 10.1128/MCB.01928-08. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sicinski P, Zacharek S, Kim C. Duality of p27Kip1 function in tumorigenesis. Genes Dev. 2007;21:1731–1746. doi: 10.1101/gad.1583207. [DOI] [PubMed] [Google Scholar]
- 49.Slingerland J, Pagano M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol. 2000;183:10–17. doi: 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 50.Susaki E, Nakayama K, Yamasaki L, Nakayama KI. Common and specific roles of the related CDK inhibitors p27 and p57 revealed by a knock-in mouse model. Proc Natl Acad Sci USA. 2009;31:5192–5197. doi: 10.1073/pnas.0811712106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tossidou I, Dangers M, Koch A, Brandt DT, Schiffer M, Kardinal C. Tyrosine phosphatase SHP-2 is a regulator of p27(Kip1) tyrosine phosphorylation. Cell Cycle. 2008;7:3858–3868. doi: 10.4161/cc.7.24.7260. [DOI] [PubMed] [Google Scholar]
- 52.Trabosh VA, Divito KA, D Aguda B, Simbulan-Rosenthal CM, Rosenthal DS. Sequestration of E12/E47 and suppression of p27KIP1 play a role in Id2-induced proliferation and tumorigenesis. Carcinogenesis. 2009;30:1252–1259. doi: 10.1093/carcin/bgp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsihlias J, Kapusta L, Slingerland J. The prognostic significance of altered cyclin-dependent kinase inhibitors in human cancers. Annu Rev Med. 1999;50:401–423. doi: 10.1146/annurev.med.50.1.401. [DOI] [PubMed] [Google Scholar]
- 54.Vervoorts J, Lüscher B. Post-translational regulation of the tumor suppressor p27(KIP1) Cell Mol Life Sci. 2008;65:3255–3264. doi: 10.1007/s00018-008-8296-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yil TG, Baek JH, Kim HJ, Choi MH, Seo SB, Ryoo HM, Kim GS, Woo KM. Trichostatin A-mediated upregulation of p21WAF1 contributes to osteoclast apoptosis. Exp Mol Med. 2007;39:213–221. doi: 10.1038/emm.2007.24. [DOI] [PubMed] [Google Scholar]
- 56.Zhang HY, Du ZX, Liu BQ, Gao YY, Meng X, Guan Y, Deng WW, Wang HQ. Tunicamycin enhances TRAIL-induced apoptosis by inhibition of cyclin D1 and the subsequent downregulation of surviving. Exp Mol Med. 2009;41:362–369. doi: 10.3858/emm.2009.41.5.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou W, Yang Q, Low CB, Karthik BC, Wang Y, Ryo A, Yao SQ, Yang D, Liou YC. Pin1 catalyzes conformational changes of Thr187 in p27Kip1 and mediates its stability through a poly-ubiquitination process. J Biol Chem. 2009 doi: 10.1074/jbc.M109.022814. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]