Abstract
Vaccines represent a significant potential means of decreasing global morbidity and mortality due to malaria. Clinical trials in the U.S. with Plasmodium falciparum Apical Membrane Antigen 1 (AMA1) showed that the vaccine induced biologically active antibodies judged by an in vitro parasite Growth Inhibition Assay (GIA). However, the same vaccine in Malian adults did not increase biological activity although it elevated ELISA titers. As GIA has been used to evaluate the biological activity of antibodies induced by blood-stage malarial vaccine candidates, we explored this discrepancy in this study. We affinity purified AMA1-specific antibodies from both US vaccinees and from non-vaccinated individuals living in a malaria-endemic area of Mali, and performed ELISA and GIA. Both AMA1-specifc antibodies induced by vaccination (US) and by natural infection (Mali) have comparable biological activity in GIA when the ELISA titer is normalized. However, a fraction of Malians’ IgG which did not bind to AMA1 protein (Mali-non-AMA1 IgG) reduced the biological activity of the AMA1 antibodies from US vaccinees; in contrast, US-non-AMA1 IgGs did not show a reduction of the biological activity. Further investigation revealed that the reduction was due to malaria-specific IgGs in the Mali-non-AMA1 IgGs. The fact that both US- and Mali-AMA1-specific antibodies showed comparable biological activity supports further development of AMA1-based vaccines. However, the reduction of biological activity of AMA1-specific antibody by other malaria-specific IgGs likely explains the limited effect on growth-inhibitory activity of antibodies induced by AMA1 vaccination in Malian adults and may complicate efforts to develop a blood-stage malaria vaccine.
Introduction
The malarial parasite remains a scourge on human civilization. Snow and colleagues estimate that theremay be 300 to 500 million clinical cases of malaria annually (1), and WHO Commission on Macroeconomics and Healthhas found that malaria reduces economic growth in sub-SaharanAfrica by over 1% per year (2).As the burden of disease and death due directly and indirectlyto malaria has increased, the need for an effective vaccinehas also assumed greater importance (3).
Of the major vaccine candidates directed against blood-stagemalaria parasites which are responsible for the pathology associatedwith this disease, Plasmodium falciparum Apical Membrane Antigen1 (AMA1) is one of the best studied (4, 5). AMA1 appears to play a pivotal role in erythrocyte invasion (6), participating in the attachment and reorientation of the merozoite to the host red cell surface (7, 8). Various pieces of evidence from both non-human primate models (9–11) and human epidemiologic studies which have shown that a high AMA1 antibody level is associated with a reduced risk of clinical malaria (12, 13), support AMA1 as a promising blood-stagemalarial vaccine candidate. In addition, AMA1 antibodies from individuals who live in malaria endemic areas can inhibit the invasion of erythrocytes by P. falciparum merozoitesin vitro (14, 15).
Our previous studies (16, 17) have shown that in clinical trials conducted in malaria-naive individuals in the United States, the AMA1-C1 vaccine (a mixture of two recombinant proteins based on the 3D7 and FVO allelic forms of AMA1) induces antibodies which can inhibit in vitro parasite invasion and/or growth, as judged by the Growth Inhibition Assay (GIA). In these studies, there was a strong correlation between the antibody level as measured by ELISA and the biological activity as measured by GIA, in agreement with previous studies in animals (18–20). However, this was not the case in a phase 1 clinical trial of the same vaccine conducted in Malian adults (21). Prior to vaccination, the Malian volunteers already had measurable AMA1 antibodies, presumably due to previous infections with P. falciparum, and the total IgGs isolated from their baseline sera showed growth-inhibitory activity of the parasite in vitro. Although levels of AMA1 antibodies increased significantly from baseline after two immunizations as judged by ELISA, the biological activity of the total IgGs remained unchanged for most of the volunteers and there was no significant correlation between change in AMA1 antibody level and in degree of growth inhibition (21).
At present, no in vitro assay result has been shown to predict clinical protection against blood-stage malaria, as no effective blood-stage vaccine has been developed. However, the GIA (also referred to as the Invasion Inhibition Assay, or IIA) is currently one of very few biological assays that are widely used to evaluate the potential of vaccine candidates in both animal and human studies; this is because the assay evaluates whether antibodies induced by vaccination can bind to native antigen on the surface of parasites (or infected erythrocytes) and display an effector function in vitro. Since a number of vaccine trials with AMA1 have been or soon will be conducted in malaria endemic areas (5), it is important to determine why the GIA results from vaccinated adult Malians were different from those of malaria-naive US volunteers. There are two possible explanations for this phenomenon: 1) the AMA1 antibodies induced by vaccination are qualitatively different from the antibodies induced by natural infection, and/or 2) some fraction(s) of IgG from the Malians (naturally-exposed individuals) interferes with the growth-inhibitory activity of AMA1 antibodies induced by the AMA1-C1 vaccine. With regard to the latter possibility, in the case of Merozoite Surface Protein 1 (MSP1), another blood-stage vaccine candidate, it has been shown that a proportion of anti-MSP1 antibodies called “blocking” antibodies, which are found in people living in malaria endemic areas, competes with an anti-MSP1 monoclonal antibody capable of inhibiting merozoite invasion of erythrocytes in vitro, as judged by a competition ELISA and by a MSP1 processing assay (22–24). It has been proposed that these “blocking” antibodies interfere with the activity of anti-MSP1 antibodies which actually inhibit invasion. Like MSP1, the AMA1 protein is initially synthesized as a precursor, which then undergoes proteolytic cleavage (25, 26). It is possible that a portion of AMA1-specific antibodies may interfere with this proteolytic cleavage, thereby inhibiting invasion to erythrocytes(27). It may also be possible that there are other AMA1-specific antibody specificities that interfere with this activity.
To address these possibilities, we affinity purified AMA1-specific antibodies from both US vaccinees and non-vaccinated individuals living in a malaria-endemic area of Mali, and performed ELISA and GIA with the purified antibodies. We chose to study the anti-AMA1 antibodies prevalent in non-vaccinated Malian individuals, because if we purified the antibody from vaccinated Malians, we would be unable to separate or distinguish the AMA1-specific antibodies elicited by infection and later vaccination. We are not aware of any previous study that directly compares anti-malaria antibodies to blood-stage antigens that are induced by vaccination with those induced by natural infection. Importantly, we have shown that AMA1-specific antibodies from both of these study populations have similar biological activity in GIA at comparable concentrations. However, when non-AMA1 IgGs isolated from the sera of the Malian study participants, in particular, malaria-specific IgGs isolated from the non-AMA1 IgGs, were mixed with AMA1 antibodies obtained either from vaccinated US volunteers or from non-vaccinated Malian adults, growth-inhibitory activity was substantially reduced. These results likely account for our previous observations in AMA1-immunized Malians and also have implications for interpretation of blood-stage vaccine trials in malaria-endemic areas.
Materials and Methods
Specimens from AMA1-C1 clinical trial in the US
Details of the trial will be described elsewhere (17). In brief, a single-blind, randomized, dose-escalating phase 1 clinical trial was conducted in 75 healthy volunteers immunized with AMA1-C1 malaria vaccine formulated on Alhydrogel® with or without CPG 7909 (Coley Pharmaceuticals). The study was reviewed and approved by the Institutional Review Board of the National Institute of Allergy and Infectious Disease (NIAID), and by the University of Rochester Research Subjects Review Board. Written informed consent was obtained from all volunteers. Participants were healthy volunteers between 18 and 45 years old. Vaccinations were administered on Days 0, 28, and 56. Post-vaccination sera from this study were shown to recognize both the AMA1(3D7) and AMA1(FVO) proteins, as judged by ELISA (Mullen et al, manuscript submitted).
Plasma samples from five individuals with high levels of anti-AMA1 antibody (as determined by ELISA) on day 70 were collected 3 months after the last immunization. To perform this study, from these five plasma samples, a pool was made using three of the samples, whereas the other two were tested individually. Furthermore, two more pools were made from sera collected on days 42 (two weeks post-vaccination #2) and 70 (two weeks post-vaccination #3): 1) out of 75 volunteers, day 42 and 70 sera from 34 volunteers with high anti-AMA1 antibody levels were pooled, and 2) a pool was made using day 70 antisera from all 75 volunteers.
Specimens from Malian epidemiological study
Specimens were collected from participants of a longitudinal epidemiological study conducted in the village of Donéguébougou, Mali, where malaria transmission is seasonal and high (29). Individuals between the ages of 6 months and 45 years were randomly selected from a village census and invited to participate in the study until a cohort of approximately 200 study participants was assembled. Serum samples were collected from the volunteers at 4 cross-sectional visits between July 2002 and April 2003. Community permission was obtained from village elders, as well as approval from the ethical review committees of the Faculty of Medicine, Pharmacy, and Dentistry at the University of Bamako (Mali) and NIAID. Individual written informed consent was obtained from all participants or their guardians. Previous analysis of sera from the participants showed that both AMA1-3D7 and anti-AMA1-FVO were recognized to a comparable extent by ELISA.
To perform this study, approximately 800 serum samples (~200 participants, 4 visits) were ranked based on their anti-AMA1 antibody level as judged by ELISA (regardless of age, bleed day, etc.) and were divided into 4 serum pools. “Mali-1” was a pool of sera with the lowest anti-AMA1 antibody level, and “Mali-4” having the highest.
IgG preparation
From both the U.S. and Malian serum/plasma samples, total IgGs were purified as described previously (16). The protein concentration of total IgG was adjusted to 40 mg/ml. The AMA1-specific antibodies in the total IgG preparation were isolated by affinity adsorption to AMA1(3D7) or AMA1(FVO) protein immobilized on NHS-activated Sepharose 4 Fast Flow (GH Healthcare, Piscataway, NJ) according to the manufacturer’s instructions. The AMA1 protein used was the same lot of cGMP-grade material as contained in the vaccine formulation. Total IgGs (1 ml of each) were loaded onto the column and the IgG fraction which did not bind to the column was collected [non-AMA1(3D7) or non-AMA1(FVO) IgG]. AMA1(3D7)- or AMA1(FVO)-specific antibodies were eluted using elution buffer (0.1M glycine, pH 2.7). Both non-AMA1 IgG and AMA1-specific antibodies were dialyzed against RPMI 1640 and concentrated to 40 mg/ml (non-AMA1 IgG) or 150~300 μl of final product (AMA1 antibodies). As a negative control, IgGs were purified from a serum pool collected from malaria-naive and unvaccinated US volunteers, in the same way as described above.
ELISA, Growth Inhibition Assay (GIA) and avidity test
The standardized methodology for performing the ELISA (for both total IgG and IgG subclasses) and the GIA has been described previously (16, 28). ELISA unit value of a standard was assigned as the reciprocal of the dilution giving an O.D. 405 = 1 in a standardized assay. The absorbance of individual test samples was converted into ELISA units using a standard curve generated by serially diluting the standard in the same plate; ELISA units of the standard were fixed once assigned, regardless of actual O.D. 405 value of a standard curve in a plate. For clarity, only GIA data with anti-AMA1(3D7) and/or non-AMA1(3D7) IgGs against P. falciparum 3D7 parasites are presented in this paper. Experiments were also conducted using anti-AMA1(FVO) and/or non-AMA1(FVO) IgGs against P. falciparum FVO parasites, but since the results were comparable to those for P. falciparum 3D7, they are not presented here.
To determine the avidity of anti-AMA1 antibodies, total IgGs (i.e., primary antibodies) were diluted to 2.5 ELISA units. A 15 minute incubation step with varying concentrations of urea (from 0 to 5 M) in Tris-buffered saline (BioFluids, Camarillo, CA) was performed between the primary and secondary antibody incubation steps. The remainder of the ELISA procedure was the same as described previously. The concentration of urea resulting in 50% of the original ELISA units (EC50) was calculated using linear regression. All of the data sets fit the linear regression models well (r2>0.94, data not shown).
Experiments with Malaria-specific IgG
To determine the impact of malaria-specific IgGs from Mali non-AMA1(3D7) IgGs on anti-AMA1 antibody in GIA, three Mali non-AMA1(3D7) IgGs were prepared again from the Mali serum pools following the procedure mentioned above. A malaria extract of late trophozoite/schizont P. falciparum 3D7 was prepared by layering culture blood on a 60% Percoll gradient, centrifuging, freeze thawing and then sonicating the cells several times. The protein concentration of the extract was measured by BCA protein assay kit (PIERCE, Rockford, IL). The malaria extract-specific antibodies were isolated by affinity adsorption to the extract protein immobilized on NHS-activated Sepharose 4 Fast Flow. The Mali non-AMA1(3D7) IgGs (40 mg of each) were loaded onto the column and malaria-specific antibodies were eluted, dialyzed against RPMI 1640 and concentrated to 160 μl of final product. As a negative control, 40 mg of total IgGs from a serum pool collected from malaria-naive and unvaccinated US volunteers was loaded onto the malaria-extract affinity column and treated in the same way as described above.
The activity of these malaria-specific IgGs was tested either alone or with US-total IgG by GIA.
Statistics
Linear and non-linear regression analyses were performed using Prism 5 (GraphPad Software, Inc. San Diego, CA). Best-fit formulations of the GIA data – for both the dose-response curve and EC50 – were calculated using logarithm-transformed ELISA units or antibody protein concentration. To compare multiple slopes of best-fit lines, Analysis of Covariance (ANCOVA) was performed followed by a post hoc Bonferroni test. Comparisons between EC50’s from the avidity test were performed using the Mann-Whitney test. Correlations between antibody concentration (either ELISA units or μg/ml) and growth-inhibitory activity were assessed using a Spearman rank correlation test. All statistical tests were performed using Prism 5 and probability values less than 0.05 were considered significant.
Results
Characteristics of anti-AMA1 IgG induced by vaccination (US-anti-AMA1 IgG) and by natural infection (Mali-anti-AMA1 IgG) as judged by ELISA
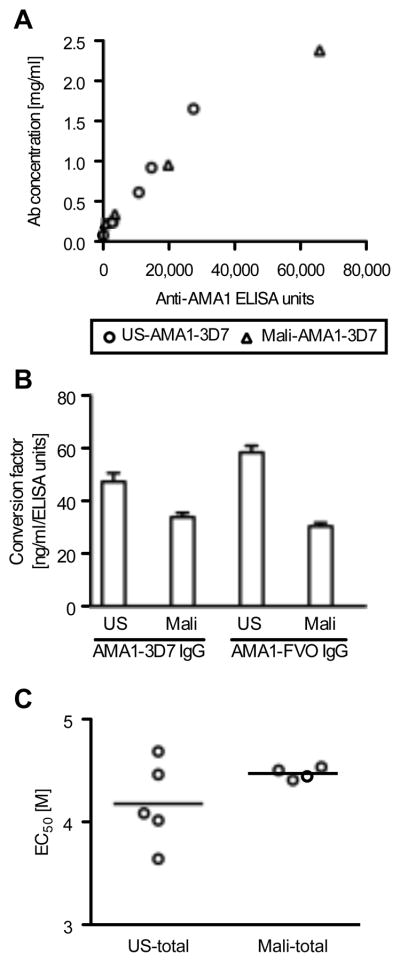
AMA1-specific antibodies were purified from total IgG fractions obtained from the participants in the US and Malian studies in order to compare the characteristics of these two types of antibodies. To express the concentration of AMA1-specific antibody in a more general way, we first converted the antibody concentration as expressed in standardized ELISA units (OD based antibody amount calculated from a standard curve run in duplicate on each plate), which we have used in previous publications, to mass values (i.e., μg/ml). When the ELISA units of the US-AMA1(3D7)-specific antibodies were plotted against the protein concentrations in μg/ml (Fig. 1A), a linear relationship was observed (r2=0.98). Likewise, Mali-AMA1(3D7) antibodies also showed a linear relationship between ELISA units and mass values (r2=0.99).
FIGURE 1.
Characteristic differences between US- and Mali-anti-AMA1 IgGs as measured by ELISA. A, The ELISA units to AMA1-3D7 plates (x-axis) and protein concentrations (y-axis) of five US-AMA1(3D7) antibody preparations and four Mali-AMA1(3D7) antibodies. B, Conversion factor (CF, the protein concentration of anti-AMA antibody equivalent to 1 ELISA unit) as calculated by linear regression. Error bars represent 95% confidence intervals. There is a significant difference between the four CFs (p<0.0001 by ANCOVA test). C, The avidity of anti-AMA1 IgG was tested using total IgGs by incubating ELISA plates with increasing concentrations of urea. EC50 of individual samples and the mean of the group are shown for US and Malian samples.
Based on the best-fit lines, a conversion factor (CF, the protein concentration of antibody equivalent to 1 ELISA unit) was calculated for both the 3D7 and FVO allelic forms of AMA1 (Fig. 1B). The 4 CFs were significantly different (p<0.0001 by ANCOVA test) and the CFs for US-AMA1 antibodies were significantly higher than that of Mali-AMA1 antibodies for both antigens (p<0.001 for both AMA1(3D7) and AMA1(FVO), post hoc Bonferroni test). These results indicate that US-AMA1 antibodies require a higher mass concentration of antibody to produce the same level of ELISA units as Mali-AMA1 antibodies tested against the same antigen.
Consequently, the two sets of antibodies were compared further by analyzing the avidity and subclasses of antigen-specific IgG. Mean antibody avidity of US-total IgG was slightly lower than the avidity of the Mali-total IgG, although there was greater variance in the avidities of the US-total IgG samples (Fig. 1C). As a result, there was no statistically significant difference between the mean avidities of US- and Mali-total IgGs (Mann-Whitney test).
When anti-AMA1 IgG subclasses were measured, both US and Malian samples were predominantly of the IgG1 subclass, with a small proportion of IgG3 and virtually no antigen-specific IgG2 or IgG4. Therefore, no differences in the distribution of antigen-specific IgG subclasses were observed between the US and Malian volunteers (data not shown).
Biological activity of US- and Mali-anti-AMA1 IgGs as judged by GIA
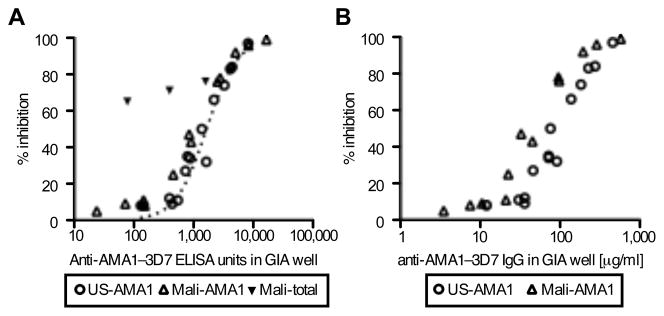
The biological activity of US- and Mali-AMA1(3D7) antibodies was evaluated by a standardized in vitro GIA (Fig. 2A) tested against P. falciparum 3D7 parasites. Similar to our previous observation with anti-AMA1 total IgG from volunteers in a phase 1 study of AMA1-C1 in the US (16), when the anti-AMA1 ELISA units of individual samples were plotted against the % inhibition as measured by GIA, there was a strong correlation between ELISA units and % inhibition for both US-AMA1(3D7) (Spearman rank correlation; rs=0.97, 95% CI 0.92–0.99, p<0.0001) and Mali-AMA1(3D7) antibodies (rs=0.97, 95% CI 0.90–0.99, p<0.0001). When the ELISA units were log transformed, the relationship followed a symmetrical sigmoid curve (r2=0.97 and 0.99 for the US- and Mali-AMA1 antibodies, respectively). The EC50 (effective concentration of antibody giving 50% inhibition in GIA) of the US-AMA1(3D7) antibodies (1570 ELISA units; 95% CI 1368–1802) was 1.5-times higher than that of Mali-AMA1(3D7) antibodies (1002 ELISA units; 95% CI 895–1122). The EC50 of US-AMA1(3D7) antibodies overlapped with the EC50 of US-total IgG (1647 ELISA units; 95% CI 1566–1732), but the Mali-total IgG had higher inhibitory activity at the same level of anti-AMA1 ELISA units (Fig. 2A). The latter finding presumably reflects the presence of non-AMA1 IgGs with biological activity against P. falciparum.
FIGURE 2.
Biological activity of US- and Mali-AMA1(3D7) antibodies against P. falciparum 3D7 parasites judged by in vitro GIA. A, Five US-AMA1(3D7) antibodies (US-1~5) and four Mali-AMA1(3D7) antibodies (Mali-1~4) were tested at 5, 15 and 30% dilutions (v/v) by GIA. The anti-AMA1(3D7) ELISA units in the GIA well (x-axis) are plotted against % inhibition (y-axis) to P. falciparum 3D7 parasites. Four Mali-total IgGs were also tested at 30% (v/v). The dotted line represents the best-fit of the data from a study where 205 total IgGs of the US vaccinees in the same AMA1-C1 human trial were tested by GIA (17). B, The antibody concentrations of US- and Mali- AMA1(3D7) antibodies in the GIA well are plotted on the x-axis, instead of ELISA units.
The GIA data were re-plotted using the protein concentration of antibody in mass units (i.e., μg/ml) in the GIA well on the x-axis (Fig. 2B), instead of ELISA units. Similar to Fig. 2A, both US- and Mali-AMA1(3D7) antibodies showed strong correlations between protein concentration and % inhibition (US-AMA1(3D7) antibodies; rs=0.97, 95% CI 0.91–0.99, p<0.0001 and Mali-AMA1(3D7) antibodies; rs=0.99, 95% CI 0.95–1.00, p<0.0001). However, the difference between the EC50 of US-AMA1(3D7) antibodies (100.3 μg/ml; 95%CI 88.9–113.2) and the EC50 of Mali-AMA1(3D7) antibodies (46.3 μg/ml; 95%CI 40.0–53.7) was greater than the difference between the EC50s of the two when calculated using ELISA units.
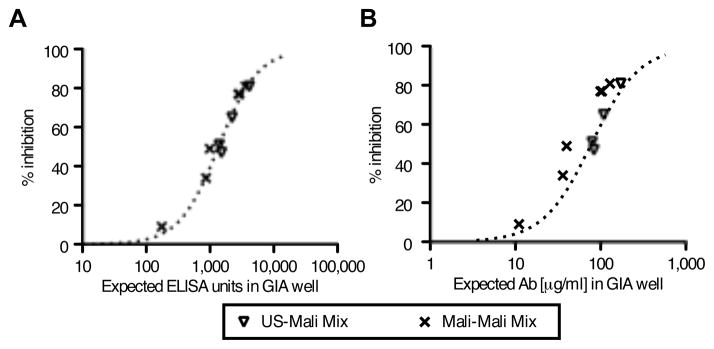
To investigate whether Mali-AMA1(3D7) antibodies induced by natural infection include “blocking” antibodies, similar to what has been proposed for MSP1 (22, 23), we performed GIA experiments with mixtures of two different IgG samples: (1) a mixture of US-AMA1(3D7) antibodies with each of the four Mali-AMA1(3D7) antibodies; and, (2) a mixture of two of the Mali-AMA1(3D7) antibodies (Fig. 3). As shown in Fig 3A, when the expected ELISA units of the antibody mixtures were plotted on the x-axis (e.g., when an antibody sample of 2,000 ELISA units was mixed with a sample of 1,000 ELISA units, the expected ELISA units of the mixture was 3,000, etc.), the % inhibition of the mixture followed the same dose-response curve (dotted line) generated by the GIA result when individual (i.e., unmixed) samples were tested. The same data were re-plotted using the protein concentration of the mixtures in mass units (i.e., μg/ml) on the x-axis (Fig. 3B). While the data of the US-Mali mixtures overlapped with the best-fit curve calculated from GIA results of the individual samples, the data of the Mali-Mali mixture in Fig 3B was less congruent with the best-fit curve compared to the same Mali-Mali mixture data in Fig. 3A.
FIGURE 3.
Biological activity of mixtures of US- and Mali-AMA1(3D7) antibodies against P. falciparum 3D7 parasites as judged by GIA. A, Mixtures of US-AMA1(3D7) (5% v/v) and Mali-AMA1(3D7) antibody (5% v/v) or of different Mali-AMA1(3D7) antibodies (5% v/v each) were tested by GIA. The sum of anti-AMA1-3D7 ELISA units for the two mixed IgGs (i.e., the estimated number of units for the mixture) are plotted on the x-axis. The dotted line represents the best-fit of the same US- and Mali-AMA1 (3D7) IgG data sets as in Fig. 2A. B, The antibody concentrations (μg/ml) of antibodies in the GIA well are plotted on the x-axis, instead of ELISA units. The dotted line represents the best-fit of the US- and Mali-AMA1 (3D7) IgG data sets as in Fig. 2B.
As US- and Mali-AMA1(3D7) antibodies showed similar biological activity in vitro when they displayed similar ELISA units and did not interfere with each other in the GIA, we next tested the fraction of total IgG which did not bind to AMA1-3D7 (non-AMA1(3D7) IgG) for its effects on growth-inhibitory activity.
Biological activity of non-AMA1 IgGs
The US-total IgG depleted of antibodies to AMA1-3D7 (US-non-AMA1(3D7) IgG) contained less than 6% of the ELISA units when tested on ELISA plates coated with AMA1(3D7) as compared to the original US-total IgG (Table I) and showed less than 3% inhibition to P. falciparum 3D7 parasites. The Mali-non-AMA1(3D7) IgGs showed approximately the same level of residual anti-AMA1(3D7) antibodies as US-non-AMA1(3D7) IgG, as judged by ELISA. However, when the growth-inhibitory activity was measured using the Mali-non-AMA1(3D7) IgGs against 3D7 parasites, the IgGs showed comparable levels of growth-inhibitory activity as the original (i.e., pre-depletion) Mali-total IgGs. That is, no major loss of growth-inhibitory activity was observed after removal of nearly all the Malian AMA1-3D7 antibody specificities, based on the anti-AMA1(3D7) ELISA units of the IgGs.
Table I.
ELISA units and growth-inhibitory activity of total and non-AMA1(3D7) IgGs
| Total IgG |
Non-AMA1(3D7) IgG |
|||
|---|---|---|---|---|
| ELISAa | GIAb | ELISAa (%c) | GIAb (%c) | |
| US IgG | ||||
| US-1 | 1,775 | 8 | 54 (3) | 0 (0) |
| US-2 | 1,504 | 0 | <30d (NDe) | 0 (NDe) |
| US-3 | 4,591 | 35 | 226 (5) | 0 (0) |
| US-4 | 6,082 | 58 | 376 (6) | 1 (2) |
| US-5 | 14,032 | 69 | 856 (6) | 3 (4) |
| Mali IgG | ||||
| Mali-1 | 259 | 65 | <30d (NDe) | 65 (100) |
| Mali-2 | 1,328 | 71 | 39 (3) | 64 (90) |
| Mali-3 | 5,328 | 76 | 198 (4) | 70 (92) |
| Mali-4 | 14,313 | 82 | 432 (3) | 80 (98) |
ELISA units to AMA1(3D7) ELISA plates are shown.
GIA was performed with 12 mg/ml of total or non-AMA1(3D7) IgG in the test wells and percent inhibition against P. falciparum 3D7 parasites is shown.
Percent relative to the total IgG.
Less than the minimal detection level of ELISA units.
Percent cannot be determined.
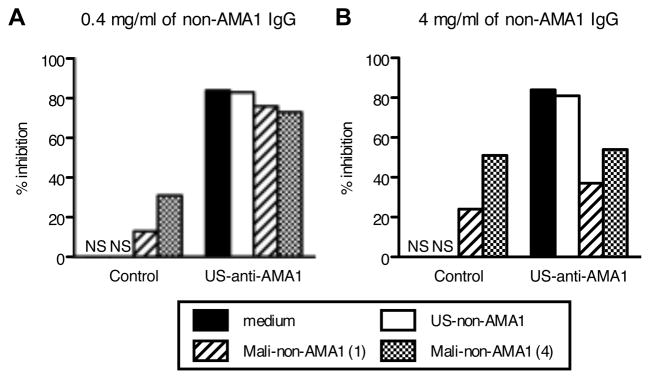
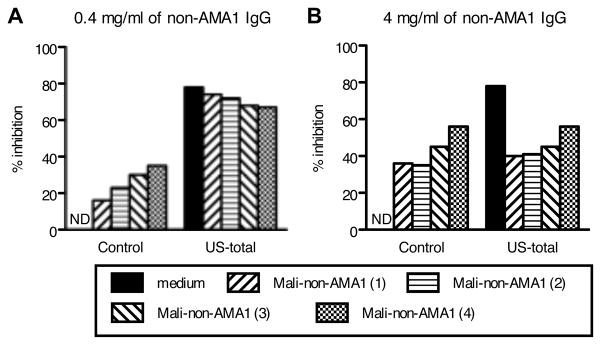
To assess the potential interference of US- and Mali-non-AMA1(3D7) IgGs on the biological activity of US-anti-AMA1(3D7) antibody, GIA was performed using 3D7 parasites (Fig. 4). US-non-AMA1(3D7) IgG did not show growth-inhibitory activity either at 0.4 mg/ml (Fig. 4A) or 4 mg/ml (Fig. 4B), while the Mali-non-AMA1(3D7) IgGs showed biological activity at both concentrations. When US-non-AMA1(3D7) IgGs were mixed with US-AMA1(3D7) antibodies, the US-non-AMA1(3D7) IgG (at either 0.4 mg/ml or 4 mg/ml) did not change the growth-inhibitory activity of US-AMA1(3D7) antibodies. When 0.4 mg/ml of Mali-non-AMA1(3D7) IgGs were mixed with US-AMA1(3D7) antibodies (Fig. 4A), the mixture showed an 8% (with non-AMA1 IgG from the Mali-1) and 11% (Mali-4) reduction of growth-inhibitory activity compared to US-AMA1(3D7) antibodies alone. When US-AMA1(3D7) antibodies were mixed with 4.0 mg/ml of Mali-non-AMA1(3D7) IgGs (Fig. 4B), there was a clear reduction in the % inhibition of P. falciparum 3D7 growth (47% reduction for Mali-1 and 30% for Mali-4). Similar to the mixture experiment with US-AMA1(3D7) antibodies, 4.0 mg/ml of Mali-non-AMA1(3D7) also showed a clear reduction in the % inhibition of Mali-AMA1(3D7) antibodies (46% reduction), while 4.0 mg/ml of US-non-AMA1(3D7) IgG did not change the % inhibition of Mali-AMA1 (3D7) antibody (2% reduction).
FIGURE 4.
Mali-non-AMA1(3D7) IgG reduces growth-inhibitory activity of US-AMA1(3D7) antibody against P. falciparum 3D7 parasites. Negative control IgG (30% v/v), which were purified from a pool of malaria-naive US normal serum, or US-AMA1(3D7) antibody (30% v/v), were mixed with either medium (positive control) or non-AMA1(3D7) IgGs at 0.4 mg/ml (A) or 4 mg/ml (B). The growth-inhibitory activity of the mixtures was tested against P. falciparum 3D7 parasites. US-AMA1(3D7) antibody and US-non-AMA1(3D7) IgGs were purified from US-4 total IgG and Mali-non-AMA1(3D7) IgGs were from Mali-1 or Mali-4 total IgGs. NS: % inhibition was in the range of ±5%.
To address whether Mali-non-AMA1(3D7) IgGs could reduce the growth-inhibitory activity of anti-AMA1 antibodies in the presence of other specificities of IgG and whether all four Mali-non-AMA1(3D7) IgG preparations had such modulating activity, we conducted an independent study where US-total IgG was incubated separately with each of the four Mali-non-AMA1(3D7) IgGs (Fig. 5). As shown in Fig. 5A, addition of 0.4 mg/ml of Mali-non-AMA1(3D7) IgGs did not markedly change the growth-inhibitory activity of US-total IgG (less than 11% reduction). On the other hand, when 4 mg/ml of Mali-non-AMA1(3D7) IgGs were mixed with US-total IgG, all of the four IgGs reduced the growth-inhibitory activity by more than 22% when compared to the US-total IgG alone (Fig. 5B). To confirm that the inhibitory effect of the non-AMA1(3D7) IgG was specific to the IgGs from Malians, a mixture of US- (or Mali-) AMA1(3D7) antibodies with US-non-AMA1(3D7) IgG at 10.0 mg/ml was tested. The US-non-AMA1(3D7) IgG did not result in a reduction of the growth-inhibitory activity of either US or Malian anti-AMA1(3D7) antibodies, even when tested at a 2.5-fold higher protein concentration than the tests using Mali-non-AMA1(3D7) IgGs in Fig. 4B and 5B (data not shown).
FIGURE 5.
Mali-non-AMA1(3D7) IgGs reduce growth-inhibitory activity of US-total IgG against P. falciparum 3D7 parasites. US-total IgG (12 mg/ml) were mixed with medium (positive control) or with Mali-non-AMA1(3D7) IgGs at 0.4 (A) or 4 mg/ml (B), and the growth-inhibitory activity of the mixtures was tested. ND: % inhibition was 0 by definition.
Biological activity of malaria-specific Mali-non-AMA1(3D7) IgGs
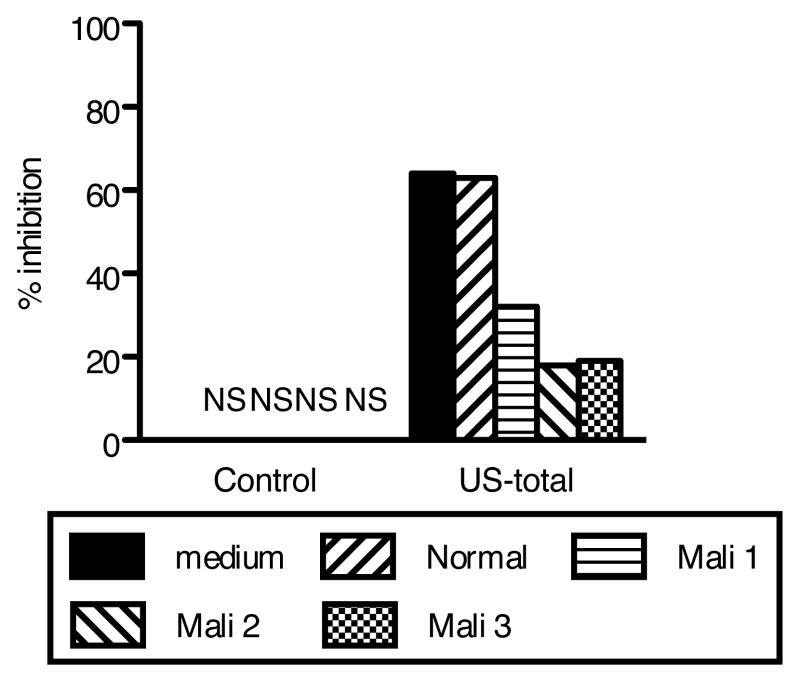
To determine whether the interference in the growth-inhibitory activity of Mali non-AMA1(3D7) IgG was due to antibodies specific for other malaria antigens, malaria-specific IgGs were affinity purified from three Mali non-AMA1(3D7) IgGs and tested by GIA either alone or with US-total IgG (Fig. 6). The malaria-specific IgGs by themselves showed less than 5% inhibition at the concentration tested. However, the same concentration of malaria-specific IgGs from Mali non-AMA1(3D7) IgG reduced the % inhibition of US-total IgG (32~46% reduction), while IgG preparations from malaria-naïve total IgG (Normal) did not change the growth-inhibitory activity of US-total IgG.
FIGURE 6.
Malaria-specific IgGs from Mali-non-AMA1(3D7) IgGs reduce growth-inhibitory activity of US-total IgG against P. falciparum 3D7 parasites. Malaria-specific IgGs from Mali-non-AMA1(3D7) IgGs (Mali 1~3) and from negative control IgG (Normal IgG purified from a pool of malaria-naive US normal serum), were tested (50%v/v in GIA well) either alone (left side of figure) or with US-total IgG (10 mg/ml, right side of figure). As a positive control, the US-total IgG was also tested alone (medium, solid black bar). NS: % inhibition was in the range of ± 5%.
Discussion
In this study, we compared the in vitro P. falciparum growth-inhibitory activity of human antibodies induced by natural infection (using samples from individuals living in Mali) and by vaccination with the AMA1-C1 (in malaria-naive US volunteers), and made two important findings. The first is that AMA1-specific antibodies either induced by natural infection or by vaccination show comparable levels of growth-inhibitory activity in vitro at the same level of ELISA units. The second is that malaria-specific, but non-AMA1, IgGs from naturally-exposed Malian individuals reduced the biological activity of AMA1-specific antibodies in vitro. For clarity, while GIA results are presented here for anti-AMA1(3D7) and/or non-AMA1(3D7) IgGs against P. falciparum 3D7, we have conducted identical studies with anti-AMA1(FVO) and/or non-AMA1(FVO) IgGs against FVO parasites, and obtained similar results, establishing that this is not limited to a single AMA1 sequence or parasite strain.
We affinity purified AMA1-specific antibodies from the total IgG fraction from US and Malian volunteers, particularly since Malian sera contain a variety of antibodies against different malaria proteins in addition to AMA1, making it difficult to compare the characteristics of the two AMA1-specific antibodies. For both total IgGs and AMA1-specific antibodies, there is a correlation between the level of anti-AMA1 ELISA units of the sample in the GIA well and its growth-inhibitory activity. However, as shown in Fig. 1A, the Mali-total IgG had higher parasite inhibitory activity than US-total IgG at equivalent levels of anti-AMA1 ELISA units, which is similar to previous observations in phase 1 trials of the AMA1-C1 vaccine in Malian and US adults (16, 21). The higher growth-inhibitory activity of the Malian total IgG is likely due to anti-malarial antibodies with specificities other than AMA1. The data suggest that Malians with high levels of anti-AMA1 antibodies are likely to have high levels of antibodies to other malarial antigens, as has been reported in other epidemiological studies (30, 31), and as a consequence, the total IgG with higher anti-AMA1 ELISA units showed higher growth-inhibitory activity in vitro.
Our findings also show the importance of affinity purification of specific IgGs to compare the activity of two types of antibodies, as shown in both Table I and Fig. 1. Both US- and Mali-non-AMA1 IgGs contained less than 6% of anti-AMA1(3D7) ELISA units as compared to the original total IgGs, and US-non-AMA1 IgG showed a negligible level of biological activity to the parasites, indicating that the affinity purification was highly effective. Surprisingly, however, the Mali-non-AMA1 IgGs showed comparable levels of growth-inhibitory activity as the original Mali-total IgGs prior to affinity-purification. Thus, despite the removal of nearly all the anti-AMA1(3D7) antibodies, the GIA result against 3D7 parasites was not markedly affected. This data is in agreement with our previous observation that pre-incubation with AMA1 protein did not reverse the growth-inhibitory activity of total IgG samples taken from Malian volunteers even though the IgG preparation included significant levels of anti-AMA1 antibodies as judged by ELISA (21). These data clearly show that non-AMA1 IgGs from Malians have biological activity in vitro, because the very limited remaining anti-AMA1-3D7 IgGs were too low in concentration to inhibit the growth of homologous P. falciparum 3D7 parasites. In addition, the data also suggest that the growth-inhibitory activity of antibodies directed to two (or more) different parasite antigens are not necessarily additive, although mixing of AMA1 antibodies from two different sources did show additive effects in a predictable manner (Fig. 3). In other words, even though pre-incubation of IgGs from people living in an endemic area with an antigen does not change the growth-inhibitory activity in the GIA, such a finding does not prove that antibodies against that particular antigen have no biological activity. GIA experiments we have performed using rabbit anti-AMA1 and anti-MSP1 antibodies have also shown non-additive effects (unpublished data).
Although we have shown that growth-inhibitory activity is a function of antibody level (measured in ELISA units) in both preclinical studies in animals and in a clinical trial in US vaccinees (16, 18–20), to express the concentration of specific antibody in a more generalizable way, we converted the arbitrary ELISA units to actual protein concentration (i.e., μg/ml) using the affinity purified AMA1-specific antibodies. As shown in Fig. 1B, there were significant differences in the CF (the protein concentration of antibody equivalent to 1 ELISA unit) between US- and Mali-AMA1 antibodies against both AMA1(3D7) and AMA1(FVO). Although it is possible that the affinity purification method used in this study damaged the antibodies and resulted in the difference observed in the CF, we do not believe that is the case. As shown in Fig. 2A, US-AMA1 antibodies and US-total IgG showed the same level of growth-inhibitory activity at the same level of ELISA units. Similarly, we purified AMA1-specific antibody from animals vaccinated with AMA1 (mice, rabbits and monkeys) and did not find a reduction in growth-inhibitory activity when comparing AMA1-specific antibodies to total IgGs (Miura et al, manuscript in preparation). Taken together, this indicates that the affinity purification method utilized did not significantly diminish the binding activity of the antibody. No significant differences in the avidity of anti-AMA1 IgGs or the distribution of anti-AMA1 IgG subclasses were detected between samples collected from US and Malian volunteers. However, the mean value of EC50 in US-total antibodies was lower than that of Mali-total antibodies and the small sample size in this study may have made it difficult to show a significant difference. While an additional study (e.g., with more test samples) is needed to clarify whether the difference in CF between US and Mali-AMA1 antibodies is due to differences in avidity and/or other factor(s), the results of the CF calculations show that US-AMA1 antibodies requires a greater mass concentration of antibody to give the same level of ELISA units compared to Malian antibodies tested using the same antigen. Since it is well known that repeated infection induces antibody maturation, the difference in CF might reflect the fact that Malian AMA1 antibodies elicited by multiple infections over a long period of time were of higher affinity than US-AMA1 antibodies which were elicited by only 3 immunizations with the AMA1-C1 vaccine.
Establishing the CF for the anti-AMA1 antibodies allowed us to compare our results with those of other investigators. Hodder and colleagues have previously shown that human affinity-purified AMA1-3D7-specific IgG from pooled plasma from people living in Papua New Guinea displayed approximately 75% inhibition to P. falciparum 3D7 parasites when tested at a concentration of 100 μg/ml (14). Based on the best-fit curves of our data, we estimate that 75% growth inhibition of our US-AMA1(3D7) antibodies would occur at a concentration of 188.4 μg/ml (95% CI, 158.1–231.2), whereas for Mali-AMA1(3D7) antibodies the corresponding figure would be 90.6 μg/ml (95% CI, 74.0–114.0). By expressing the antibody level in μg/ml, we can show that Mali-AMA1 antibodies and Papua New Guinea-anti-AMA1 antibodies have similar biological activities in vitro when tested at the same antibody concentration. However, our data (Fig. 2 and 3) show that the growth-inhibitory activities of US- and Mali-AMA1 antibodies overlap somewhat better when we used ELISA units to express the amount of anti-AMA1 antibody. Further studies are required to determine whether or not the level of antibody as measured by ELISA is a better predictor of the biological activity of anti-AMA1 antibody judged by GIA.
As noted previously, Guevara Patino and colleagues have reported that a fraction of human anti-MSP1 antibodies blocks the invasion-inhibitory activity of mouse anti-MSP1 monoclonal antibody mAb 12.8 (22). However, the same human antibodies did not block the biological activity of another inhibitory anti-MSP1 monoclonal antibody, mAb 12.10. Therefore, there was no clear evidence that such “blocking” antibodies interfere with the activity of human polyclonal anti-malarial antibodies (including anti-MSP1 antibodies) in a biological assay, such as GIA. As shown in Fig. 3A, the additive effects observed between the two types of anti-AMA1 antibodies (US- and Mali-AMA1 antibodies) demonstrate that there is no clear biological interference between anti-AMA1-specific antibodies induced by vaccination and by natural infection. However, when anti-AMA1 antibodies, either from US vaccinees and non-vaccinated Malian adult, were mixed with Mali-non-AMA1 IgGs, consistent reductions in growth-inhibitory activity of anti-AMA1 antibodies were observed. These mixing experiments (Fig. 3, 4, 5 and 6) suggest that the discrepancy in GIA results between our previous AMA1-C1 vaccine trials in a malaria endemic area of Africa (Mali) and a non-endemic naive population in the US was likely due to the effects of Mali-non-AMA1 IgGs, and not from qualitative differences in the AMA1-specific antibodies obtained from the two study groups.
To our knowledge, this is the first report showing that IgGs from people who live in malaria-endemic areas can reduce the growth-inhibitory activity of human anti-malarial polyclonal antibodies directed to an antigen expressed during the erythrocytic stage of parasites. Since non-AMA1 IgGs from Malians living in a malaria-endemic area themselves have growth-inhibitory activity, reduction of inhibition can be observed only when a sufficient quantity of AMA1 antibody, which shows more growth-inhibitory activity than Mali-non-AMA1 IgGs, is used in the GIA experiment. Actually, when 0.4 mg/ml of Mali-non-AMA1 IgGs were tested, the reduction in growth-inhibitory activity (less than 11% inhibition differences) was within the range of error of the GIA (+/− ~10% inhibition, unpublished data), so it is difficult to conclude whether the reduction was real or not. On the other hand, when 4 mg/ml of Mali-non-AMA1 IgG was used for the mixture experiments (Fig. 4 and 5), a clear reduction (i.e., more than 20%) was observed, despite 4 mg/ml of non-AMA1 IgGs itself showing higher growth-inhibitory activity than when tested at 0.4 mg/ml. Thus, showing the reduction effect of Mali-non-AMA1 IgGs experimentally was not straightforward. However, the levels of anti-AMA1 antibody used in this study (2000 to 3000 ELISA units in each GIA well) are comparable to those induced by the AMA1-C1 vaccine in human trials and the concentration of non-AMA1 IgG tested (4 mg/ml) was one-half to one-fifth of the concentration of total IgG found in human serum. Therefore, it is possible that Mali-non-AMA1 IgGs interfere with growth-inhibitory activity of AMA1 antibody at physiological concentrations.
We further investigated whether the “interfering” activity of Mali-non-AMA1(3D7) IgGs against anti-AMA1 antibodies is due to antibodies to other malaria antigens or to non-malaria antibodies. As shown in Fig 6, malaria-specific IgGs from Mali-non-AMA1(3D7) IgGs interfered with the growth-inhibitory activity of anti-AMA1 antibodies in GIA. While all Mali-non-AMA1(3D7) IgG and also Mali-malaria-specific IgG samples tested in this study interfered with the biological activity of the US-total IgG (Fig. 5 and 6), it is not clear whether all individuals have antibodies capable of this activity since all four fractions were obtained by pooling different samples. We also do not know whether IgGs from young children or infants who have not had many malaria episodes have similar capability. Although AMA1 vaccination by itself did not induced “interfering” antibodies in the US trial (Fig. 4), it is possible that the vaccination of people who live in malaria endemic areas changes the effect and/or prevalence of “interfering” antibody in vaccinees. Since we had not expected to see this interference effect on GIA when we designed the epidemiology study (from which we made the serum pools) and the AMA1 trial in Malian adults, we didn’t collect enough individual serum/plasma to perform this type of study. We plan to conduct other epidemiology studies and vaccine trials in Mali, and once additional serum/plasma will be available, we will attempt to answer the questions mentioned above. At present we do not know what anti-malarial antibody specificities interfere with the growth-inhibitory activity of anti-AMA1 antibody or how this interference is mediated. We can speculate that malaria infection induces an array of antibody specificities, some of which reduce the effector function of other anti-malarial antibodies. Such “interfering” antibodies may allow the malaria parasite to maintain persistent levels of parasitemia in the human host.
There are currently no accepted in vitro correlations of protective immunity to malaria, so that it is unknown whether the biological activity that we are measuring in vitro reflects such immunity in vivo. However, the GIA (or IIA) is one assay which allows evaluation of antibody effector activity against viable parasites and it has been used to evaluate the biological activity of antibodies induced by vaccine candidates in phase 1 trials. Therefore, further investigation into the possible existence of antibodies that interfere with other, as in the study presented here, is extremely important for future malaria blood-stage vaccine development, and blood-stage vaccine trials in endemic areas need to take these findings into account in evaluating potential in vitro correlates of protective immune responses as well as vaccine efficacy.
Acknowledgments
We are very grateful to all volunteers who participated in the clinical trials. We also appreciate Drs. John Treanor, Ruth Ellis and Allan Saul for contributions to the human trials.
Grant support: This study was funded in part bythe PATH/Malaria Vaccine Initiative and the Intramural Program of the National Institute of Allergy and Infectious Diseases/National Institutesof Health.
Abbreviations
- GIA
Growth Inhibition Assay
- AMA1
Apical Membrane Antigen1
- MSP1
Merozoite Surface Protein1
- CF
conversion factor
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415:680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 3.Mahanty S, Saul A, Miller LH. Progress in the development of recombinant and synthetic blood-stage malaria vaccines. J Exp Biol. 2003;206:3781–3788. doi: 10.1242/jeb.00646. [DOI] [PubMed] [Google Scholar]
- 4.Peterson MG, V, Marshall M, Smythe JA, Crewther PE, Lew A, Silva A, Anders RF, Kemp DJ. Integral membrane protein located in the apical complex of Plasmodium falciparum. Mol Cell Biol. 1989;9:3151–3154. doi: 10.1128/mcb.9.7.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remarque EJ, Faber BW, Kocken CH, Thomas AW. Apical membrane antigen 1: a malaria vaccine candidate in review. Trends Parasitol. 2008;24:74–84. doi: 10.1016/j.pt.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Triglia T, Healer J, Caruana SR, Hodder AN, Anders RF, Crabb BS, Cowman AF. Apical membrane antigen 1 plays a central role in erythrocyte invasion by Plasmodium species. Mol Microbiol. 2000;38:706–718. doi: 10.1046/j.1365-2958.2000.02175.x. [DOI] [PubMed] [Google Scholar]
- 7.Kato K, Mayer DC, Singh S, Reid M, Miller LH. Domain III of Plasmodium falciparum apical membrane antigen 1 binds to the erythrocyte membrane protein Kx. Proc Natl Acad Sci U S A. 2005;102:5552–5557. doi: 10.1073/pnas.0501594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell GH, Thomas AW, Margos G, Dluzewski AR, Bannister LH. Apical membrane antigen 1, a major malaria vaccine candidate, mediates the close attachment of invasive merozoites to host red blood cells. Infect Immun. 2004;72:154–158. doi: 10.1128/IAI.72.1.154-158.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins WE, Pye D, Crewther PE, Vandenberg KL, Galland GG, Sulzer AJ, Kemp DJ, Edwards SJ, Coppel RL, Sullivan JS, Morris CL, Anders RF. Protective immunity induced in squirrel monkeys with recombinant apical membrane antigen-1 of Plasmodium fragile. Am J Trop Med Hyg. 1994;51:711–719. doi: 10.4269/ajtmh.1994.51.711. [DOI] [PubMed] [Google Scholar]
- 10.Deans JA, Knight AM, Jean WC, Waters AP, Cohen S, Mitchell GH. Vaccination trials in rhesus monkeys with a minor, invariant, Plasmodium knowlesi 66 kD merozoite antigen. Parasite Immunol. 1988;10:535–552. doi: 10.1111/j.1365-3024.1988.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 11.Stowers AW, Kennedy MC, Keegan BP, Saul A, Long CA, Miller LH. Vaccination of monkeys with recombinant Plasmodium falciparum apical membrane antigen 1 confers protection against blood-stage malaria. Infect Immun. 2002;70:6961–6967. doi: 10.1128/IAI.70.12.6961-6967.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polley SD, Mwangi T, Kocken CH, Thomas AW, Dutta S, Lanar DE, Remarque E, Ross A, Williams TN, Mwambingu G, Lowe B, Conway DJ, Marsh K. Human antibodies to recombinant protein constructs of Plasmodium falciparum Apical Membrane Antigen 1 (AMA1) and their associations with protection from malaria. Vaccine. 2004;23:718–728. doi: 10.1016/j.vaccine.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 13.Nebie I, Diarra A, Ouedraogo A, Soulama I, Bougouma EC, Tiono AB, Konate AT, Chilengi R, Theisen M, Dodoo D, Remarque E, Bosomprah S, Milligan P, Sirima SB. Humoral responses to Plasmodium falciparum blood stage antigens and association with incidence of clinical malaria in children living in an area of seasonal malaria transmission in Burkina Faso, West Africa. Infect Immun. 2007;76:759–766. doi: 10.1128/IAI.01147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodder AN, Crewther PE, Anders RF. Specificity of the protective antibody response to apical membrane antigen 1. Infect Immun. 2001;69:3286–3294. doi: 10.1128/IAI.69.5.3286-3294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nair M, Hinds MG, Coley AM, Hodder AN, Foley M, Anders RF, Norton RS. Structure of domain III of the blood-stage malaria vaccine candidate, Plasmodium falciparum apical membrane antigen 1 (AMA1) J Mol Biol. 2002;322:741–753. doi: 10.1016/s0022-2836(02)00806-9. [DOI] [PubMed] [Google Scholar]
- 16.Malkin EM, Diemert DJ, McArthur JH, Perreault JR, Miles AP, Giersing BK, Mullen GE, Orcutt A, Muratova O, Awkal M, Zhou H, Wang J, Stowers A, Long CA, Mahanty S, Miller LH, Saul A, Durbin AP. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect Immun. 2005;73:3677–3685. doi: 10.1128/IAI.73.6.3677-3685.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullen GE, Ellis RD, Miura K, Malkin E, Nolan C, Hay M, Fay MP, Saul A, Zhu D, Rausch K, Moretz S, Zhou H, Long CA, Miller LH, Treanor J. Phase 1 trial of AMA1-C1/Alhydrogel plus CPG 7909: an asexual blood-stage vaccine for Plasmodium falciparum malaria. PLoS ONE. 2008;3:e2940. doi: 10.1371/journal.pone.0002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giersing B, Miura K, Shimp R, Wang J, Zhou H, Orcutt A, Stowers A, Saul A, Miller LH, Long C, Singh S. Posttranslational modification of recombinant Plasmodium falciparum apical membrane antigen 1: impact on functional immune responses to a malaria vaccine candidate. Infect Immun. 2005;73:3963–3970. doi: 10.1128/IAI.73.7.3963-3970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullen GE, Giersing BK, Ajose-Popoola O, Davis HL, Kothe C, Zhou H, Aebig J, Dobrescu G, Saul A, Long CA. Enhancement of functional antibody responses to AMA1-C1/Alhydrogel®, a Plasmodium falciparum malaria vaccine, with CpG oligodeoxynucleotide. Vaccine. 2006;24:2497–2505. doi: 10.1016/j.vaccine.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 20.Miura K, Zhou H, Muratova OV, Orcutt AC, Giersing B, Miller LH, Long CA. In immunization with Plasmodium falciparum apical membrane antigen 1, the specificity of antibodies depends on the species immunized. Infect Immun. 2007;75:5827–5836. doi: 10.1128/IAI.00593-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dicko A, Diemert DJ, Sagara I, Sogoba M, Niambele MB, Assadou MH, Guindo O, Kamate B, Baby M, Sissoko M, Malkin EM, Fay MP, Thera MA, Miura K, Dolo A, Diallo DA, Mullen GE, Long CA, Saul A, Doumbo O, Miller LH. Impact of a Plasmodium falciparum AMA1 Vaccine on Antibody Responses in Adult Malians. PLoS ONE. 2007;2:e1045. doi: 10.1371/journal.pone.0001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guevara Patino JA, Holder AA, McBride JS, Blackman MJ. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J Exp Med. 1997;186:1689–1699. doi: 10.1084/jem.186.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nwuba RI, Sodeinde O, Anumudu CI, Omosun YO, Odaibo AB, Holder AA, Nwagwu M. The human immune response to Plasmodium falciparum includes both antibodies that inhibit merozoite surface protein 1 secondary processing and blocking antibodies. Infect Immun. 2002;70:5328–5331. doi: 10.1128/IAI.70.9.5328-5331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okech BA, Corran PH, Todd J, Joynson-Hicks A, Uthaipibull C, Egwang TG, Holder AA, Riley EM. Fine specificity of serum antibodies to Plasmodium falciparum merozoite surface protein, PfMSP-119, predicts protection from malaria infection and high-density parasitemia. Infect Immun. 2004;72:1557–1567. doi: 10.1128/IAI.72.3.1557-1567.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howell SA, Withers-Martinez C, Kocken CH, Thomas AW, Blackman MJ. Proteolytic processing and primary structure of Plasmodium falciparum apical membrane antigen-1. J Biol Chem. 2001;276:31311–31320. doi: 10.1074/jbc.M103076200. [DOI] [PubMed] [Google Scholar]
- 26.Narum DL, Thomas AW. Differential localization of full-length and processed forms of PF83/AMA-1 an apical membrane antigen of Plasmodium falciparum merozoites. Mol Biochem Parasitol. 1994;67:59–68. doi: 10.1016/0166-6851(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 27.Dutta S, Haynes JD, Moch JK, Barbosa A, Lanar DE. Invasion-inhibitory antibodies inhibit proteolytic processing of apical membrane antigen 1 of Plasmodium falciparum merozoites. Proc Natl Acad Sci U S A. 2003;100:12295–12300. doi: 10.1073/pnas.2032858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. 2008;26:193–200. doi: 10.1016/j.vaccine.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dicko A, Sagara I, Diemert D, Sogoba M, Niambele MB, Dao A, Dolo G, Yalcouye D, Diallo DA, Saul A, Miller LH, Toure YT, Klion AD, Doumbo OK. Year-to-year variation in the age-specific incidence of clinical malaria in two potential vaccine testing sites in Mali with different levels of malaria transmission intensity. Am J Trop Med Hyg. 2007;77:1028–1033. [PubMed] [Google Scholar]
- 30.Johnson AH, Leke RG, Mendell NR, Shon D, Suh YJ, Bomba-Nkolo D, Tchinda V, Kouontchou S, Thuita LW, van der Wel AM, Thomas A, Stowers A, Saul A, Zhou A, Taylor DW, Quakyi IA. Human leukocyte antigen class II alleles influence levels of antibodies to the Plasmodium falciparum asexual-stage apical membrane antigen 1 but not to merozoite surface antigen 2 and merozoite surface protein 1. Infect Immun. 2004;72:2762–2771. doi: 10.1128/IAI.72.5.2762-2771.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biswas S, Seth RK, Tyagi PK, Sharma SK, Dash AP. Naturally acquired immunity and reduced susceptibility to falciparum malaria in two subpopulations of endemic eastern India. Scand J Immunol. 2008;67:177–184. doi: 10.1111/j.1365-3083.2007.02047.x. [DOI] [PubMed] [Google Scholar]