Abstract
The expression of the Syk protein-tyrosine kinase in breast cancer cells is inversely correlated with invasive growth and metastasis. The expression of Syk inhibits cell motility while supporting the formation of cell clusters by enhancing cell-cell contacts and promoting the redistribution of the adhesion proteins cortactin and vinculin to these contacts. Syk associates physically with cortactin and catalyzes its phosphorylation on tyrosine. The clustering of integrins leads to the phosphorylation of Syk and of numerous cellular proteins in a manner dependent on the activity of the kinase and on the presence of tyrosine-342 located in the linker B region. The ability of Syk to participate in integrin-mediated, protein-tyrosine phosphorylation correlates well with its ability to inhibit cell motility.
Keywords: Syk, tyrosine kinase, breast cancer, cortactin, adhesion, integrins
Introduction
Syk is a 72 kDa, cytoplasmic protein-tyrosine kinase that has been studied extensively in hematopoietic cells where it is best characterized as an essential element in the signal transduction pathways operating downstream from a variety of immune recognition receptors (1). Recently, the expression of Syk has been reported also in several nonhematopoietic cells (2). Interestingly, in both immune and nonimmune cells, Syk has been described as either a positive or a negative regulator of tumorigenesis depending on the cell type. In breast cancer and melanoma cells, the loss of Syk correlates with enhanced malignancy while the opposite appears to be true in squamous cell carcinomas and in many lymphomas (3–15). Thus, there is considerable interest in determining the ways in which Syk modifies cell behavior.
Several observations indicate an important role for Syk as a negative regulator of tumor cell progression in breast cancer. Syk mRNA and protein are present in numerous breast carcinoma cell lines of low tumorigenic potential, but are absent from highly tumorigenic cell lines due to methylation-induced gene silencing (3, 4). In human breast tissue samples, Syk is present in normal epithelial cells and benign breast lesions, but is absent from many highly malignant breast cancer cells (5, 6). This reduced expression correlates with an increased incidence of distant metastases (7, 8). The re-expression of Syk in highly malignant breast cancer cells reduces anchorage-independent growth, invasive growth in basement membrane matrices and the formation of tumors and lung metastases in mice (3, 9). Likewise, the introduction of a dominant negative Syk into cells of low tumorigenic potential enhances their ability to form tumors (3). This altered metastatic potential may involve changes in cell migration since the inhibition of Syk expression in MCF7 breast cancer cells increases their motility (10). Thus, in breast cancer cells, Syk has the properties of a tumor suppressor, a function not frequently associated with a protein-tyrosine kinase.
In many cells of the immune system, Syk is physically and functionally coupled to cell adhesion molecules whose function is modulated by Syk and whose engagement in turn regulates the activity of the kinase (16–24). In monocytes and neutrophils, integrin engagement leads to the activation of Syk, which is required for activities such as cell spreading on extracellular matrix (ECM) proteins, induction of a respiratory burst, degranulation and, under some conditions, enhanced motility (22–24). The activation of Syk following integrin ligation also has been described in airway epithelial cells (25). Since integrins are components of both cell-matrix and cell-cell adhesion complexes, we were prompted to examine a role for Syk in both motility and cell-cell interactions in breast epithelial cells. A hallmark of the progression of carcinoma cells of epithelial origin to a tumorigenic phenotype is a loss of cell-cell contacts. We find that the expression of Syk not only inhibits the motility of breast cancer cells, but also promotes the formation of cell-cell contacts. Syk physically associates with and phosphorylates cortactin, which, along with vinculin, is recruited to cell-cell junctions in a Syk-dependent manner. We also find that integrin ligation in breast epithelial cells is coupled to the activation of Syk and influences its localization within the cell, causing it to become excluded from the nucleus. The ability of Syk to enhance cell-cell contacts and to decrease cell motility may underlie its functions as a tumor suppressor in breast cancer.
Materials and Methods
Cells and plasmids
MCF7 cells were obtained from ATCC (MCF7(ATCC)) while MCF7 cells expressing the rTetR plasmid were purchased from BD Biosciences (MCF7(BD)). All MCF7 cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FCS, 100 U/ml penicillin and 100μg/ml streptomycin. To prepare stably transfected lines, MCF7(BD) cells were transfected with vectors coding for Syk-EGFP or Syk-EGFP(K396R) (26) together with a puromycin resistance gene, selected in media containing puromycin, expanded and screened for kinase expression by fluorescence microscopy and Western blotting. Tet-inducible MCF7(BD) and MDA-MB-231 cells (ATCC) expressing Syk-EGFP were constructed using the T-REX™ system (Invitrogen). MCF10A cells were cultured in DMEM-F12 medium supplemented with 5% horse serum, 1 mM L-glutamine, 20 ng/ml epidermal growth factor, 10 μg/ml insulin, 0.5 μg/ml hydrocortisone and 1 × anti-mycotic antibiotic (Gibco). cDNA’s for the expression of site-directed mutants were generated using the transformer mutagenesis kit (Clontech). Ras-transformed MCF10A cells were as described previously (27).
Cell aggregation assays
MCF10A or MCF7-derived cells were harvested, suspended in complete medium and allowed to form aggregates in droplets placed on the lid of a petri dish at a cell density of 2.5 × 104 cells/15 μl in the presence or absence of 4 mM EGTA, various concentrations of the Syk inhibitors piceatannol or 3-(1-methyl-1H-indol-3-yl-methylene)-2-oxo-2,3-dihydro-1H-indole-5-sulfonamide (Calbiochem) or DMSO carrier (1% final concentration). Droplets were harvested at timed intervals and examined under a hemocytometer to count particle numbers.
Cell motility assays
Stable cell lines or Tet-responsive cells were suspended in serum-free DMEM in the upper chamber of a transwell apparatus with medium containing 20% FCS in the lower chamber as a chemoattractant. Cells on the upper surface were removed and those on the lower surface of the filter were stained with DAPI, observed using fluorescence microscopy and counted. For MDA-MB-231 cells transiently transfected with EGFP-tagged proteins, the cells at the lower surface of the filter were stained with DAPI and then observed by fluorescence microscopy to calculate the ratio of green to blue cells, which was normalized to that of the original cell population.
Immunofluorescence
Cells cultured on coverslips were fixed with 3.7% formaldehyde for 5 min, permeabilized with 1% Triton X-100 in PBS, blocked in PBS containing 1 mg/ml BSA, 0.05% Tween 20 and 10% goat serum and stained with antibodies against E-cadherin (DECMA1, Sigma; or 4A2C7, Invitrogen), cortactin (4F11, Upstate) or vinculin (hVIN-1, Sigma). Bound primary antibodies were detected using a Texas red-conjugated donkey anti-mouse antibody (Jackson Immunoresearch). For some experiments, cells were plated on coverslips coated with fibronectin (20 ng/ml) prior to fixation and staining. For the calcium switch experiments, MCF10A cells transiently transfected with the plasmid coding for Syk-EGFP were treated with 4 mM EGTA in media for 20 min and then switched to complete media for the times indicated. F-actin was detected by staining with rhodamine-phalloidin (Invitrogen).
Paraffin-embedded breast sections (Spring Bioscience) were deparaffinized and rehydrated. Antigens were retrieved overnight using the 2100-Retriever (PickCell Laboratories) in antigen unmasking solution (H-3300, Vector Laboratories). Samples were washed with PBS, and blocked using the MOM blocking reagent (BMK-2202, Vector Laboratories). Primary antibodies (N19) were added to sections overnight at 4°C. Bound primary antibodies were detected using goat-anti-rabbit biotinylated secondary antibody (Vector Laboratories) followed by avidin-Alexa 594 (Molecular Probes). Coverslips were mounted with Fluorosave (Calbiochem) and examined by fluorescence microscopy.
Coimmunoprecipitations
Cells were lysed in 50 mM Tris/HCl (pH 7.4),150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 1mM EGTA, 10% glycerol and protease inhibitor cocktail (Sigma). The lysateswere centrifuged at 14,000 × g for 1 min. Proteins in pre-cleared supernatants were adsorbed onto protein G Plus-agarose beads containing immobilized anti-cortactin (4F11) or anti-Syk (4D10; Santa Cruz) antibodies at 4°C for 2 h. Samples were washed 4times with lysis buffer and bound proteins analyzed by Western blotting with anti-Syk (N19; Santa Cruz) or antiphosphotyrosine (4G10; Millipore/Upstate Biotechnology). Antibodies to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and GFP were obtained from Santa Cruz Biotechnology and BD Biosciences, respectively.
In vitro phosphorylation
GST-Syk was isolated from lysates of Sf9 cells that had been infected with a baculovirus (28) by adsorption to and then elution with glutathione from glutathione-Sepharose. GST was purified from bacteria transformed with the GST-expressing vector pGEX-4T2. Anti-cortactin immune complexes were incubated with purified GST-Syk or GST in 25 mM Hepes, pH 7.5, 2mM MnCl2, 1 mM Na3VO4, 10 μg/ml each aprotinin and bleupeptin, 10 μM ATP and 10 μCi [γ-32P]ATP at 30°C for 30 min.
Integrin cross-linking
Cells (1.67 × 106 cells/ml) suspended in serum-free DMEM were incubated with monoclonal anti-β1 integrin (2.5 μg/ml, Chemicon) on ice for 30 min, washed twice with serum-free DMEM, incubated with goat-anti-mouse IgG (2.4 μg/ml, Sigma) on ice for 15 min and then quickly transferred to 37 °C for the indicated times. For some experiments, the serum-starved cells were plated on coverslips pre-coated with fibronectin (20 μg/ml; Sigma) for 1 h at room temperature and then stored at 4°C overnight.
Results
Syk enhances cell-cell interactions
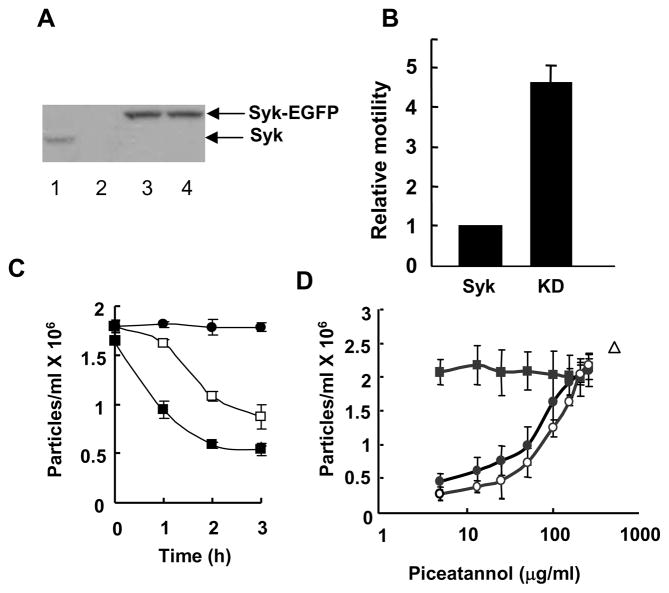
While MCF7 cells generally express Syk (3), we identified one clone purchased from BD Biosciences that lacked detectable levels of the kinase (Fig. 1A). These cells originated from ATCC and, as is characteristic of MCF7 cells, lacked endogenous caspase 3 (data not shown). The lack of Syk in these cells provided a unique opportunity to examine the effects of its expression on the adhesive properties of these breast cancer cells. We generated two stable lines, one expressing Syk with an enhanced green fluorescent protein tag at the C-terminus (Syk-EGFP) and a second expressing a catalytically inactive version (Syk-EGFP(K396R)). Western blotting analyses indicated that each cell line expressed comparable amounts of expressed fusion protein (Fig. 1A). A comparison of the abilities of these two cell lines to migrate through the pores of a polycarbonate transwell insert in response to a gradient of growth factors confirmed the expected differences in motility as the cells expressing Syk-EGFP exhibited a considerably reduced motility as compared to cells expressing the catalytically inactive kinase (Fig. 1B).
Figure 1.
Syk alters cell aggregation and motility. A, lysates from MCF10A (lane1), Syk-deficient MCF7 (lane 2) or MCF7 cells stably expressing Syk-EGFP (lane 3) or Syk-EGFP(K396R) (lane 4) were analyzed by Western blotting with an antibody against Syk (N19). Arrows mark the migration positions of the Syk-EGFP and Syk-EGFP(K396R) fusion proteins (upper arrow) and endogenous Syk (lower arrow). B, MCF7 cells stably expressing Syk-EGFP (Syk) or Syk-EGFP(K396R) (KD) were analyzed in the transwell migration assay. The cells at the lower surface of the filter were stained with DAPI and observed using fluorescence microscopy. Motility was normalized to that of cells expressing Syk-EGFP. The data indicate the mean and standard deviations of three independent experiments (P < 0.01). C, stable MCF7 cell lines expressing Syk-EGFP (■) or Syk-EGFP(K396R) (□) were allowed to aggregate for the indicated periods of time. MCF7 cells did not aggregate in the presence of EGTA (●). The number of particles formed in four individual samples was counted. The averages and standard deviations for three experiments are shown. D, MCF10A cells were allowed to form cell-cell contacts for a period of 0 (■), 1 (●) or 2 (○) h in the presence of increasing concentrations of piceatannol. The number of particles formed in 2 h in the absence of piceatannol, but in the presence of EGTA is indicated (△). The number of particles is defined as a single cell or a single cluster of cells. The data indicate the mean and standard deviations of three independent experiments.
To explore a possible connection between Syk and cell-cell adhesion, we monitored the rate at which each of these two cell lines formed aggregates in suspension. In this assay, detached cells were suspended in a droplet from the lid of a cell culture plate and the number of particles, defined as a single cell or a single cluster of cells, was counted as a function of time. Cells competent for forming cell-cell contacts aggregate over time, resulting in a decrease in total particle number. We found that the rate of formation of cellular aggregates was significantly slower in cells expressing inactive Syk-EGFP(K396R) as compared to active Syk(EGFP) (Fig. 1C). This observation suggests that Syk also has a role in promoting cell-cell adhesion.
To further explore this possibility, we examined the effect of a Syk inhibitor on the aggregation of MCF10A cells, which are immortalized, but nontransformed cells that express endogenous Syk (Fig. 1A). For this assay, the aggregation of cells was examined in the presence or absence of the Syk selective inhibitor, piceatannol (29). As shown in Fig. 1D, the formation of cell clusters was inhibited in a dose-dependent manner by piceatannol. This occurred without any change in the total cell number. At the concentrations used, DMSO, the solvent carrier for piceatannol, had no effect on cell aggregation while treatment with EGTA, which blocks the formation of E-cadherin-mediated interactions, completely blocked aggregation. Cell viability, as monitored by exclusion of Trypan blue, was not affected by any of the treatment protocols. Similar effects were seen in MCF7(ATCC) cells, which also express endogenous Syk and with a different Syk inhibitor (supplemental Fig. 1). These data are consistent with a role for Syk in promoting the formation of cell-cell contacts.
Syk promotes the redistribution of vinculin
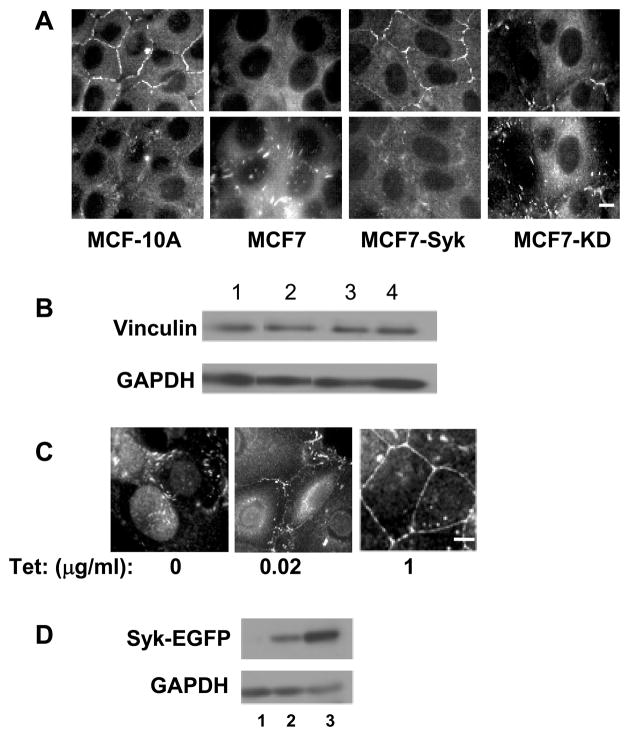
The structural protein, vinculin, is a component of both adherens junctions and focal adhesions and thus plays a role in both cell-cell and cell-matrix interactions (30, 31). To determine if the expression of Syk altered the localization of vinculin, MCF-10A and stable MCF-7 cell lines expressing either Syk-EGFP or Syk-EGFP(K396R) were examined by indirect immunofluorescence using antibodies against vinculin (Fig. 2A). Vinculin staining was prominent at sites of cell-cell contacts in MCF-10A cells, but was largely absent from these regions in the Syk-deficient MCF7(BD) cells. However, the re-expression of active Syk-EGFP, but not inactive Syk-EGFP(K396R), in the MCF7(BD) cells enhanced the localization of vinculin to cell-cell junctions (Fig. 2A). This occurred without a change in the overall level of the protein (Fig. 2B). In cells lacking Syk or expressing a catalytically inactive form of Syk, there was a corresponding increase in the staining of vinculin within punctate structures that could be visualized by focusing the microscope on the basolateral surface of the cells (Fig. 2A).
Figure 2.
The expression of Syk causes a redistribution of vinculin. A, MCF10A, MCF7, and MCF7 cells expressing Syk-EGFP (MCF7-Syk) or Syk-EGFP(K396R) (MCF7-KD) were grown on glass coverslips, fixed, stained for vinculin and examined by fluorescence microscopy. Two focal planes including cell-cell contacts (upper panels) or focal adhesions (lower panels) are shown. B, Western blotting analysis of vinculin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in lysates from MCF10A (lane 1), MCF7 (lane 2), MCF7-Syk (lane 3) and MCF7-KD (lane 4) cells. The images are derived from the same gel and blot, but, with the exception of lanes 3 and 4, are from nonadjacent lanes. C, Tet-response MCF7 cells were untreated (0) or treated with 20 ng/ml (0.02) or 1 μg/ml (1) tetracycline to induce expression of Syk-EGFP. Cells grown on untreated coverslips were fixed, stained for vinculin and examined by fluorescence microscopy. D, Western blotting analysis of Syk-EGFP and GAPDH in lysates of Tet-responsive MCF7 cells that were untreated (lane 1) or treated with 20 ng/ml (lane 2) or 1 μg/ml (land 3) tetracycline to induce expression of Syk-EGFP. Bars = 10μm.
To confirm this observation, we generated an MCF7(BD) cell line in which the expression of Syk-EGFP could be induced by incubation with tetracycline (Tet) and monitored the localization of vinculin as a function of the level of expressed Syk-EGFP. In the absence of Tet, vinculin was localized primarily to punctate structures (Fig. 2C). A low level expression of Syk-EGFP resulted in a partial relocalization of vinculin to sites of cell-cell interactions while a higher level of expressed Syk-EGFP resulted in a more dramatic relocalization. This also occurred without a measurable change in the total level of vinculin in the cells (data not shown). Thus, Syk promotes an apparent change in the localization of vinculin, most likely from focal adhesions to adherens junctions. This may account for Syk’s ability to enhance the strength of intercellular interactions and inhibit motility in MCF7 cells.
We then asked if any Syk could be visualized at sites of cell-cell interactions. In MCF-7(BD) cells induced to express Syk-EGFP, the tagged protein was found in multiple locations including the nucleus, cytoplasm, and perinuclear region. In addition, a small portion of the protein appeared at the plasma membrane at sites of cell-cell interactions (Fig. 3A). To investigate this localization further, we transiently transfected vectors for the expression of Syk-EGFP into MCF10A cells and observed a similar localization of the tagged protein (Fig. 3B). When cells were treated with EGTA to disrupt cell-cell contacts by chelating the calcium required for E-cadherin-mediated interactions, the cells rounded up and neither Syk-EGFP nor E-cadherin could be visualized at the plasma membrane. Following the restoration of calcium to the medium and the re-formation of adherens junctions, both Syk-EGFP and E-cadherin reappeared at the plasma membrane at sites of cell-cell contacts.
Figure 3.
Partial localization of Syk to cell-cell contacts. A, Tet-inducible MCF7 cells plated on glass coverslips were treated with tetracycline to induce Syk-EGFP and examined by fluorescence microscopy. B, MCF10A cells transiently expressing Syk-EGFP were treated with EGTA for 20 min, washed and placed in calcium-containing medium for 0, 20 or 45 min. Cells were fixed, permeabilized and stained for E-cadherin using DECMA-1 and a fluorescently tagged secondary antibody. Cells were examined for localization of Syk-EGFP (green) and E-cadherin (red) by fluorescence microscopy. Images were overlayed to produce the pictures in the bottom row of panels. C, MCF7 cells expressing Syk-EGFP(K396R) were fixed and examined by fluorescence microscopy. The arrowhead illustrates the localization of a subset of Syk-EGFP(K396R) to the plasma membrane. D, Syk-EGFP was transiently expressed in Ras-transformed MCF10A cells, which were fixed and examined by fluorescence microscopy. The arrowhead illustrates the weak localization of a subset of Syk-EGFP to the plasma membrane. E, Western blotting of lysates from Ras-MCF10A cells (lanes 1 and 2) or MCF10A cells (lane 3) with an anti-Syk antibody. Bars = 10 μm.
To determine if the localization of Syk-EGFP to sites of cell-cell contacts required its catalytic activity, we examined the Syk-deficient MCF7(BD) cells expressing Syk-EGFP(K396R) by fluorescence microscopy. The catalytically inactive enzyme displayed a similar localization to that of the active Syk-EGFP (Fig. 3C). We also examined the localization of Syk-EGFP expressed in MCF10A cells transformed by the expression of activated H-Ras since these cells have weak adherens junctions (27, 32). In these Ras-MCF10A cells, the localization of Syk-EGFP to the plasma membrane was reduced as compared to normal MCF10A cells (Fig. 3D). Western blotting analyses indicated that endogenous Syk was expressed in these cells at a level comparable to that in MCF10A cells (Fig. 3E).
Syk interacts with cortactin
Cortactin is a structural protein known to be recruited to E-cadherin adhesion assemblies in epithelial cells where it plays a critical role in stabilizing the adherens junction (33). Cortactin also has been shown to physically associate with Syk in platelets and in myeloid leukemia cells (34, 35). Consequently, we examined a possible interaction between the two proteins in breast epithelial cells. Cortactin was localized in diffuse puncta located throughout the cytoplasm in Syk-deficient MCF7(BD) cells, but was partially relocalized to sites of cell-cell contacts in Syk-expressing cells (Fig. 4A). To look for an interaction between the two proteins, cortactin was immunoprecipitated from lysates of MCF7(BD) cells stably expressing either Syk-EGFP or Syk-EGFP(K396R) and the resulting immune complexes were examined by Western blotting for the presence of either fusion protein (Fig. 4B). Both the active and inactive forms of ectopically expressed Syk-EGFP could be detected in anti-cortactin immune complexes. To confirm this interaction, cortactin also was immunoprecipitated from lysates of MCF-10A cells and these immune complexes were examined for associated wild-type, endogenous Syk. Western blotting of the anti-cortactin immune complex again revealed Syk as an associated protein (Fig. 4C). Interestingly, E-cadherin also was found in the anti-cortactin immune complexes, consistent with a previous report (33). Cortactin also could be detected as a protein present in anti-Syk immune complexes (Fig. 4D). These results indicate that cortactin is a Syk-binding protein in epithelial cells.
Figure 4.
Syk interacts with cortactin. A, MCF7 cells or MCF7 cells expressing Syk-EGFP were grown on glass coverslips, fixed, stained for cortactin and examined by fluorescence microscopy. B, Lysates (lysate) or anti-cortactin immune complexes (cortactin IP) prepared from MCF7 (7), or MCF7 cells expressing Syk-EGFP (Syk) or Syk-EGFP(K396R) (KD) were analyzed by Western blotting using anti-cortactin or anti-Syk antibodies. C, lysates from MCF10A cells were adsorbed to protein G plus-agarose beads (Ctrl IP) or protein G plus-agarose beads containing immobilized anti-cortactin antibodies (Cort IP). Proteins in the lysate (lysate) or bound to the beads were immunoblotted using antibodies against cortactin (upper panel), Syk (middle panel) or E-cadherin (lower panel). D, lysates from MCF10A cells were adsorbed to protein G plus-agarose beads containing immobilized anti-Syk antibodies (Syk IP) or to protein G plus-agarose beads (Ctrl IP). Proteins in the lysate (lysate) or bound to the beads were immunoblotted using antibodies against Syk (upper panel) or cortactin (lower panel). E, GST or GST-Syk was incubated with a cortactin immune-complex isolated from MCF10A cells in a kinase reaction buffer containing [γ-32P]ATP. Protein G beads without antibody were used as a control. Samples were resolved by SDS-PAGE, transferred to a PVDF membrane and detected by autoradiography (32P) or Western blotting (WB) with anti-cortactin antibodies. F, lysates from MCF10A cells transiently transfected to express EGFP (lane 1) or Syk-EGFP (lane 2) were analyzed by Western blotting with anti-Syk antibodies (left panel). Confluent cultures of each were treated with EGTA to disrupt cell-cell contacts and then calcium was restored for a period of 25 min. Anti-cortactin immune complexes isolated from cell lysates were analyzed by Western blotting using anti-cortactin or anti-phosphotyrosine (pY) antibodies.
To determine if cortactin might serve as a substrate for Syk, we incubated cortactin in vitro with GST-Syk isolated from insect cells in a kinase reaction buffer containing [γ-32P]ATP. Phosphorylation of cortactin was observed in reactions containing GST-Syk (Fig. 4E). We also examined the possible phosphorylation of cortactin in intact cells. Cortactin from MCF10A cells, which form tight cell-cell junctions, contained phosphotyrosine as detected by Western blotting of anti-cortactin immune complexes. The extent of phosphorylation of cortactin was increased in cells transfected to express Syk-EGFP (Fig. 4F).
Syk is activated by integrin-crosslinking
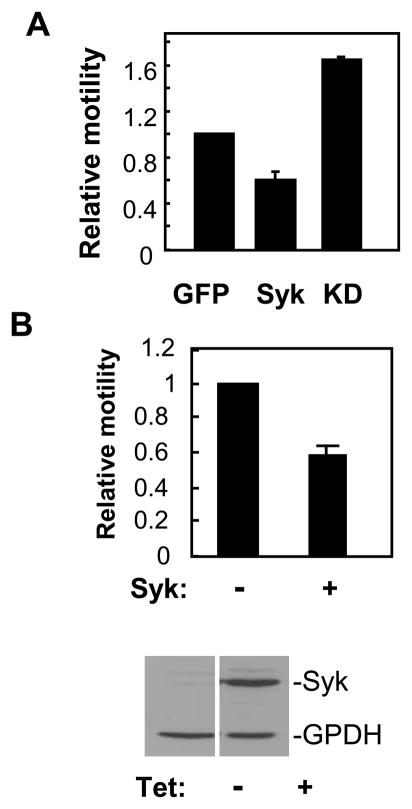
Integrins are important modulators of both cell-cell and cell-matrix interactions. Furthermore, Syk is activated following integrin crosslinking and is required for integrin-mediated signaling in hematopoietic cells (16–24). To examine a possible effect of Syk on integrin function, we examined MDA-MB-231 breast cancer cells. These cells lack E-cadherin and so are unable to form adherens junctions, lack endogenous Syk and express high levels of β1-integrin (36, 37). To confirm that Syk affected the motility of these cells, we transfected them with plasmids coding for EGFP, Syk-EGFP or Syk-EGFP(K396R) and examined these in the transwell migration assay. The expression of Syk-EGFP, but not Syk-EGFP(K396R), inhibited the motility of the MDA-MB-231 cells (Fig. 5A). To further confirm this observation, we generated a stable line of MDA-MB-231 cells in which the expression of Syk-EGFP was under the control of a tetracycline-inducible promoter. Motility was reduced in cells that were induced to express Syk-EGFP by treatment with tetracycline (Fig. 5B). Thus, Syk inhibits the motility even of cells that are unable to form cell-cell contacts.
Figure 5.
Syk inhibits MDA-MB-231 cell migration. A, MDA-MB-231 cells were transfected with expression plasmids for EGFP (GFP), Syk-EGFP (Syk) or Syk-EGFP(K396R) (KD) and analyzed in the transwell motility assay. The ratio of the number of green to blue cells at the lower surface of the filter was compared to that of initial cell population. This number from each sample was further normalized to values obtained for the migration of cells expressing EGFP alone. The data illustrate the mean and standard deviations of three independent experiments. P < 0.01. B, Tet-responsive MDA-MB-231 cells were left untreated (−) or were treated (+) with tetracycline and individually plated onto a transwell insert for the migration assay. Migrated cells at the lower surface of the two samples were counted and then normalized to the number of Syk-deficient (−) migrating cells. The data indicate the mean and standard deviations of three independent experiments. P < 0.01. The expression of Syk-EGFP and GADPH in Tet-treated (+) or untreated (−) cells was determined by Western blotting (lower panel).
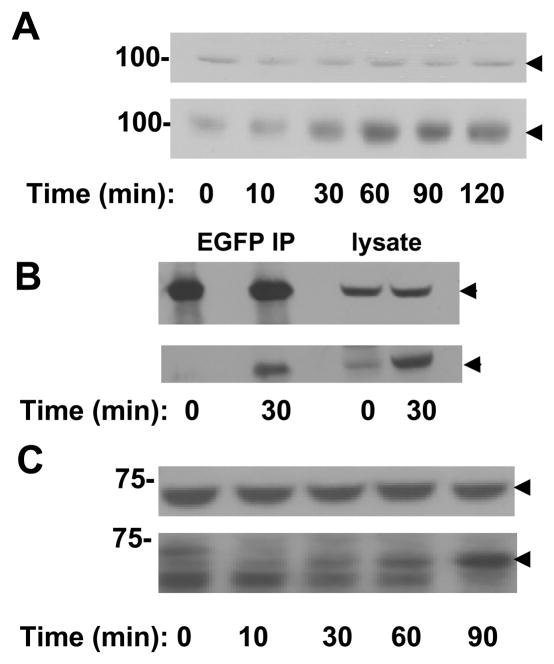
We then asked if the plating of these cells on fibronectin, a ligand for β1-integrins, could alter the activity of Syk-EGFP. The state of phosphorylation of Syk-EGFP on tyrosine as well as that of multiple cellular proteins was enhanced as a function of time following the adhesion of cells to fibronectin-coated surfaces (Fig. 6A). This effect required the catalytic activity of Syk since the tyrosine-phosphorylation of proteins in MDA-MB-231 cells expressing Syk-EGFP(K396R), which lacks a lysine required for catalytic activity, was not affected.
Figure 6.
Syk is activated by integrin-crosslinking in MDA-MB-231 cells. A, after being transiently transfected to express Syk-EGFP (Syk) or Syk-EGFP(K396R) (KD-Syk), MDA-MB-231 cells were removed and plated onto fibronectin-coated coverslips. After different time points (0, 10, 30, and 60 min), cell lysates were collected and analyzed by Western blotting with antiphosphotyrosine antibodies. The migration positions of Syk-EGFP or Syk-EGFP(K396R) are indicated by the arrowheads. B, integrins were crosslinked for the indicated times (in minutes) with anti-β1 integrin antibodies on MDA-MB-231 cells in suspension that were transiently expressing Syk-EGFP (Syk), Syk-EGFP(Y317F/Y342F/Y346F) (F3), EGFP (EGFP) or Syk-EGFP(K396R) (KD-Syk). Cell lysates were analyzed by Western blotting with antibodies against phosphotyrosine (pY), Syk or GAPDH. The migration positions of Syk-EGFP or Syk-EGFP mutants are indicated by the closed arrowheads and that of GAPDH by the open arrowheads. C, integrins were crosslinked for the indicated times (in minutes) with anti-β1 integrin antibodies on MDA-MB-231 cells in suspension that were transiently expressing Syk-EGFP(Y317F) (Y317F), Syk-EGFP(Y342F/Y346F) (Y342F/Y346F), Syk-EGFP (Syk), Syk-EGFP(Y342F) (Y342F) or Syk-EGFP(Y346F) (Y346F). Cell lysates were analyzed by Western blotting with antibodies against phosphotyrosine (pY), Syk or GAPDH. The migration positions of Syk-EGFP or Syk-EGFP mutants are indicated by the closed arrowheads and that of GAPDH by the open arrowheads. D, The ability of MDA-MB-231 cells transfected with expression plasmids for Syk-EGFP (Syk), Syk-EGFP(K396R) (KD-Syk), Syk-EGFP(Y317F/Y342F/Y346F) (F3), Syk-EGFP(Y317F) (Y317F), Syk-EGFP(Y342F/Y346F) (F2), Syk-EGFP(Y342F) (Y342F) or Syk-EGFP(Y346F) (Y346F) to migrate through the pores of a transwell filter was measured. Motility was normalized to that of cells expressing Syk-EGFP. The data shown represent the mean and standard deviations of three independent experiments. * P < 0.05.
To determine if the activity of Syk was regulated specifically by the clustering of integrins, we treated Syk-EFGP-expressing MDA-MB-231 cells in suspension with antibodies against β1 integrin. The antibody-mediated crosslinking of integrins led to the phosphorylation on tyrosine of the kinase and of many other cellular proteins (Fig. 6B). Integrin-mediated phosphorylation was not observed in cells expressing EGFP alone or catalytically inactive Syk-EGFP(K396R) (Fig. 6B), indicating that this is a phenomenon dependent on the presence of an active Syk kinase.
Syk tyrosine-342 is important for integrin-stimulated protein phosphorylation
The ability of Syk to couple receptors to intracellular pathways in hematopoietic cells is a function not only of its catalytic activity, but also of specific tyrosines whose phosphorylation serves to mediate its interactions with effector proteins bearing SH2 domains or other phosphotyrosine-binding motifs (38). To examine a role for these tyrosines in breast epithelial cells, we transfected cells with a plasmid expressing a mutant Syk-EGFP in which all three major linker B sites of tyrosine-phosphorylation (Y317, Y342 and Y346) (39) were replaced by phenylalanines (Syk-EGFP(Y317F/Y342F/Y346F) or (F3)). Replacement of these three tyrosines largely abrogated integrin-mediated phosphorylation of the kinase and of cellular proteins in general (Fig. 6B).
To determine which of these tyrosines was most important for signaling, we generated plasmids for the expression of mutants of Syk-EGFP in which specific tyrosines were replaced. The replacement of Y317, which in B cells is a negative regulatory site of phosphorylation (39–41), had no significant effect on the ability of Syk to couple integrins to protein-tyrosine phosphorylation (Fig. 6C). Little or no phosphorylation on tyrosine of either the fusion protein or of other cellular proteins was observed with a form of Syk-EGFP lacking both tyrosines 342 and 346 (Syk-EGFP(Y342F/Y346F)), indicating that one or both of these residues was important for signaling (Fig. 6C). To determine which of these two sites was most important, we generated additional mutants of Syk-EGFP lacking only tyrosine 342 (Syk-EGFP(Y342F)) or 346 (Syk-EGFP(Y346F)). As shown in Fig. 6C, elimination of Y346 had little effect on integrin-stimulated protein tyrosine-phosphorylation, while the elimination of Y342 substantially reduced phosphorylation. Thus, Y342 is particularly important for Syk-dependent protein phosphorylation in response to integrin ligation.
We then asked if there was a correlation between the ability of Syk to couple integrin ligation to protein tyrosine-phosphorylation and its ability to inhibit cell motility. For this assay, MDA-MB-231 cells were transiently transfected with an expression plasmid coding for Syk-EGFP or for one of the tyrosine-substituted mutants and then tested for mobility in the transwell assay. As shown in Fig. 6D, motility was lowest for cells expressing forms of Syk that were capable of efficiently coupling integrins to increases in protein-tyrosine phosphorylation (i.e., Syk-EGFP, Syk-EGFP(Y317F) and Syk-EGFP(Y346F)). Catalytically inactive Syk or forms of Syk lacking Y342 did not inhibit motility.
Integrin cross-linking leads to changes in the subcellular localization of Syk
To determine if the integrin-dependent phosphorylation and activation of Syk correlated with any changes in the location of the kinase within the cell, we examined the effects of integrin-crosslinking on MCF7(BD) cells expressing Syk-EGFP. As was observed in transfected MDA-MB-231 cells, Syk-EGFP also became phosphorylated on tyrosine after integrin-crosslinking when expressed in MCF7(BD) cells (Fig. 7A). The identification of this protein as Syk-EGFP was confirmed by immunoprecipitation of the fusion protein followed by Western blotting with phosphotyrosine antibodies (Fig. 7B). Similarly, the antibody-mediated crosslinking of β1 integrins on MCF-10A cells, which express wild-type Syk, led to the phosphorylation of the endogenous kinase on tyrosine (Fig. 7C).
Figure 7.
Syk is phosphorylated following integrin-crosslinking. A, integrins on MCF7 cells expressing Syk-EGFP in suspension were crosslinked with anti-β1 integrin antibodies for the times indicated. Cell lysates were analyzed by Western blotting with antibodies against Syk (upper panel) or phosphotyrosine (lower panel). The migration position of Syk-EGFP is indicated by the arrowheads. B, lysate and anti-GFP immune complexes from MCF7 cells expressing Syk-EGFP and stimulated in suspension with anti-β1 integrin antibodies for the indicated times were analyzed by Western blotting with antibodies against Syk (upper panel) or phosphotyrosine (lower panel). The migration position of Syk-EGFP is indicated by the arrowheads. C, integrins on MCF10A cells in suspension were crosslinked with anti-β1 integrin antibodies for the times indicated. Cell lysates were analyzed by Western blotting with antibodies against Syk (upper panel) or phosphotyrosine (lower panel). The migration position of Syk is indicated by the arrowheads.
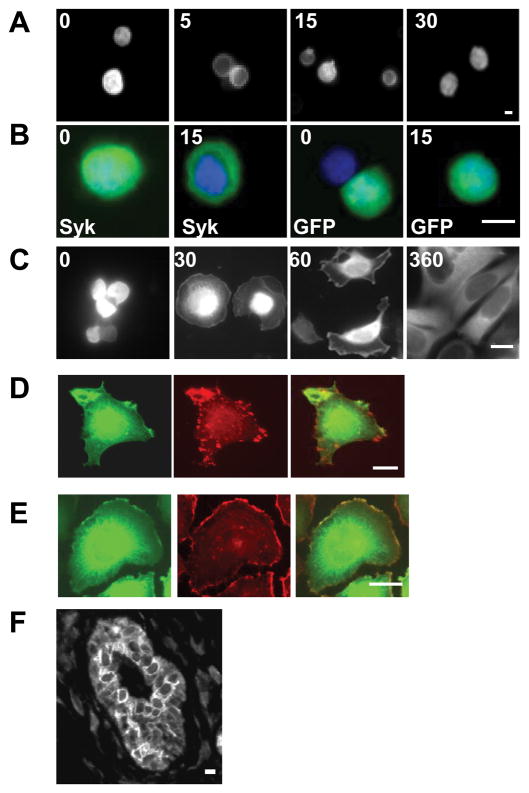
An examination of the Syk-EGFP-expressing MCF7(BD) cells in suspension by fluorescence microscopy showed that Syk-EGFP was located throughout the cell including both the cytoplasm and nucleus (Fig. 8A and B and Ref. 42). Interestingly, the antibody-mediated crosslinking of β1 integrin led to a change in the subcellular distribution of Syk-EGFP, which became excluded from the nucleus and confined to the cytoplasm shortly following addition of the anti-integrin antibody (Fig. 8A and B). At longer times following integrin-engagement, the fusion protein returned to its original distribution throughout the cell. No comparable changes were seen in cells transfected to express only EGFP.
Figure 8.
Integrin ligation alters the subcellular localization of Syk. A, MCF7 cells transfected with a Syk-EGFP-expression plasmid were stimulated by crosslinking β1 integrins for 0, 5, 15 or 30 min, fixed and examined by fluorescence microscopy. B, MCF7 cells transfected with plasmids expressing Syk-EGFP (Syk) or EGFP (GFP) were treated with β1 integrin antibodies for 0 or 15 min, fixed, stained with DAPI and examined by fluorescence microscopy. Merged images illustrating the location of Syk-EGFP or EGFP (green) and DAPI-stained nuclei (blue) are shown. C, Tet-inducible MCF7 cells treated with tetracycline to induce Syk-EGFP were plated on fibronectin-coated coverslips for the times indicated (in minutes) and then fixed and examined by fluorescence microscopy. D and E, MCF7 cells transfected with a Syk-EGFP-expression vector were plated on coverslips coated with fibronectin, allowed to spread for 60 min and then fixed, permeabilized and stained with monoclonal antibodies against vinculin (C) or with rhodamine phalloidin (D). The localization of Syk-EGFP (green, D, E), endogenous vinculin (red, D), and F-actin (red, E) were visualized with fluorescence microscopy. F, a human mammary tissue slice was stained with anti-Syk antibodies for immunohistochemical analysis. Bars = 10 μm.
To explore further a relationship between integrins and the localization of Syk-EGFP, we plated Syk-EGFP-expressing MCF7(BD) cells on coverslips that were coated with fibronectin. In cells in suspension or in freshly plated cells, Syk was distributed throughout the cell (Fig. 8C). As cells began to spread, a small fraction of the protein also appeared at the cell periphery. This fraction of Syk-EGFP did not localize to focal adhesions, marked by immunostaining with antibodies against vinculin (Fig. 8E). Syk-EGFP did, however, co-localize at the cell periphery with F-actin (Fig. 8F). With time, Syk-EGFP became distributed diffusely throughout the cytoplasm and was largely excluded from the nucleus except for a faint staining in nucleoli. Syk-EGFP also was excluded from sites of cell-cell contacts, which is in contrast to what was observed for cells plated on uncoated coverslips where a fraction of Syk-EGFP was found within the nucleus and at the plasma membrane (Fig. 3A).
To compare this subcellular localization to that of Syk in actual breast epithelial tissue, we stained a section of normal human mammary tissue using an anti-Syk antibody and a fluorescently tagged secondary antibody. The results, illustrated in Fig. 8D and supplemental Fig. 1, indicated that Syk, which was present primarily in luminal epithelial cells, where it was largely excluded from the nucleus.
Discussion
The progression of breast cancer cells from a benign to a malignant phenotype is accompanied by dramatic changes in their adhesive properties as all invasive carcinomas exhibit defects in the formation of cell-cell contacts (43, 44). These can arise from the downregulation of E-cadherin itself or of other components of the adherens junction complex through genetic or epigenetic mechanisms (45). Alternatively, changes in the phosphorylation of components of the adherens junction complex also affect the assembly or disassembly of cell-cell junctions (46, 47). While many tyrosine kinases including Src and the receptors for EGF, HGF and FGF promote the disruption of adhesion complexes (48, 49), our data suggest that Syk plays a role in enhancing the formation or stability of these intercellular contacts. This effect, coupled with the inhibitory effect of Syk on cell motility even in breast cancer cells lacking E-cadherin helps explain why a loss of Syk expression in breast epithelial cells could contribute to a transformed phenotype.
The protein or proteins with which Syk interacts to modulate cell-cell adhesions are of considerable interest. We have been unable to demonstrate a direct interaction between Syk and E-cadherin or vinculin by co-immunoprecipitation assays (data not shown). However, we do detect interactions between Syk and cortactin. Cortactin is an F-actin and Arp2/3-binding protein originally identified as a substrate for v-Src in RSV-transformed fibroblasts (50, 51). Cortactin exhibits a punctate cytoplasmic staining pattern, but is recruited to E-cadherin-containing adhesion complexes and can be co-immunoprecipitated with E-cadherin from monolayers of MDCK cells (33). It is also a protein that associates with cortical actin and is important for integrin-induced cell spreading (50, 52). An interaction between Syk and cortactin has been described previously in platelets and in K562 chronic myeloid leukemia cells (34, 35). Syk, in a complex with c-Src, also is found to co-localize with cortactin in membrane ruffles in CHO cells (53), which is consistent with the appearance of Syk-EGFP at the edges of breast epithelial cells spreading on fibronectin. In K562 cells, Syk is associated with cortactin in a manner independent of its activation (35) and in platelets, Syk’s association with cortactin does not require its phosphorylation (34). These findings are consistent with our observation that a catalytically inactive Syk mutant also can bind cortactin. Thus, cortactin is a candidate Syk-interacting protein in multiple cell types, which includes epithelial cells where this interaction may explain the co-localization of both proteins to cell-cell junctions. An enhanced recruitment of cortactin would be expected to increase the stability of adherens junction complexes as the downregulation of cortactin by siRNA or the expression of dominant-negative mutants of cortactin disrupts the formation of and recruitment of E-cadherin to cell-cell contacts (33). Similarly, cortactin associates with N-cadherin-adhesion complexes and also strengthens intercellular interactions in fibroblasts (54). Thus, the recruitment of cortactin to sites of cell-cell contacts would reasonably be expected to strengthen these interactions and may explain, in part, how Syk stabilizes intercellular adhesion. In conjunction with these observations, we also observe a decreased association of Syk with sites of cell-cell contacts in cells with weakened cell-cell interactions. In both Ras-transformed MCF10A cells and MCF7 cells plated on fibronectin, conditions that cause disassembly of adherens junctions (27, 32, 55), we observe a notable decrease in the amount of Syk at the membrane where cells interact.
In addition to cortactin, vinculin also is recruited to sites of cell-cell interactions by the expression of catalytically active Syk. Vinculin is a component of both focal adhesions and adherens junctions where it associates with components of the E-cadherin adhesion complex and restores adhesive interactions to vinculin-null cells (30, 31). Interestingly, the expression of Syk results in a loss of vinculin from focal adhesions concomitant with an increase of vinculin at adherens junctions, suggesting a Syk-dependent relocalization of vinculin within the cell. This contrasts with activated Src, which has the opposite effect of causing a redistribution of vinculin from adherens junctions to focal adhesions (56). Thus, activated Src disrupts while Syk enhances cell-cell contacts.
Numerous studies have demonstrated an involvement of β1 integrins in cancer invasion and metastasis. Tumor cells expressing β1 integrin form significantly larger primary tumors and have a dramatically increased metastasis into liver and lung (57). In addition, inhibition of β1 integrin function decreases the number and size of metastases in a mouse mammary carcinoma model (58) and decreases the malignancy of several breast cancer cell lines (59). Integrin-crosslinking initiates the formation of focal adhesions, which mediate interactions between the ECM and the cellular cytoskeletal network. Numerous signaling molecules are recruited to focal adhesions including Src and the focal adhesion kinase, FAK. These accelerate focal adhesion disassembly to enhance cell motility (60). Again, Syk has the opposite effect and instead inhibits motility.
Syk has been described as co-localizing with β1 integrin in focal adhesions in some non-hematopoietic cells (25), but not in others (61). While we were not able to demonstrate an obvious concentration of Syk-EGFP into mature focal adhesions in breast epithelial cells, we did observe a functional connection between Syk and integrins. The activity of Syk in hematopoietic cells has been known for some time to be regulated by the ligation of integrins (16–20). Syk, in turn, is required for a number of integrin-dependent signaling functions (22–24) and also plays a role in “inside-out” signaling to promote integrin-ECM interactions (21). The fact that integrin-crosslinking leads to the activation of Syk and to the Syk-dependent phosphorylation of other cellular proteins suggests a role for Syk in integrin-signaling in breast epithelial cells as well. This activation of Syk by integrin-crosslinking in breast epithelial cells is consistent with a previous report of the rapid activation of Syk through integrin clustering in airway epithelial cells (25).
The ability of Syk to inhibit cell motility correlates well with its ability to catalyze the phosphorylation of cellular proteins in response to integrin ligation. This suggests that the two processes might be related. The integrin-stimulated phosphorylation of proteins on tyrosine requires the catalytic activity of Syk as well as Y342, an amino acid residue located in the linker B region that separates the catalytic domain from the N-terminal, tandem pair of SH2 domains. The importance of Y342 may reflect either a general role for this residue in regulating the overall activity of the kinase (62), or a more selective and specific role as a docking site for effector molecules bearing SH2 domains. Interestingly, Y342, when phosphorylated, is the principal binding site on Syk for the SH2 domain of Vav-family proteins. These guanine nucleotide exchange factors for Rho-family GTPases serve as important regulators of the actin cytoskeleton and of cell motility (63). A Syk-Vav interaction requiring phosphorylation at this site has been reported previously to be important for promoting β2 integrin-mediated motility in neutrophils (24). Why this same interaction would inhibit motility in breast epithelial cells is uncertain, but it is known that Rho-family GTPases are involved in a variety of cytoskeletal functions including the assembly of focal adhesions and the promotion of cell-cell contacts (64). Y342 also is a docking site for the SH2 domains of other effector proteins including phospholipase C-γ and Src-family tyrosine kinases (38, 65). In monocytes, for example, the phosphorylation of Y342 generates a binding site for Fgr, which promotes an interaction that inhibits Syk’s activity and thus inhibits cell spreading (22). More work will be necessary to fully understand the critical interactions and protein substrates that mediate Syk’s effects on cell motility and cell-cell adhesion.
In B cells and cultured breast epithelial cells, Syk is found in both nuclear and cytoplasmic compartments (26, 42). Immunohistochemical analyses of Syk in tissue samples, however, have yielded variable results. In the airway, Syk is localized primarily to the cytoplasm of columnar epithelial cells, but in the nuclei of basal cells (25). Syk is primarily nuclear in gastric epithelial cells (66), but largely cytoplasmic in breast epithelial cells (5). Our results suggest that this differential localization of Syk may be a reflection of growth conditions and receptor engagement. We find that the clustering of integrins on MCF7 cells in suspension, for example, leads to a transient translocation of Syk out of the nucleus and that the plating of cells on fibronectin also leads to an exclusion of Syk from the nucleus. Crosslinking the B cell antigen receptor also leads to the transit of Syk from the nucleus (26). These differences in the localization of Syk are interesting in that specific nuclear functions for the kinase in the regulation of invasive growth, gene expression and stress responses have been reported (42, 67, 68).
These studies support the hypothesis that Syk’s functions as a tumor suppressor are related to its effects on cell adhesion and motility. However, in the immune system, Syk is known to couple the BCR to multiple downstream effectors, some which might reasonably be expected in enhance the survival or proliferation of cells including the Ras/Erk and the PI3K/Akt pathways (for a recent review, see 69). In addition, our recent studies on the expression of Syk in MCF7(BD) cells also reveal a role for Syk in enhancing cell survival in response to TNF through modulating the activation of NF-κB (70). Thus, the overall effect that Syk’s expression has on the transformed phenotype of a cell likely depends on multiple factors including the cell type and the repertoire of receptors and proteins that are expressed.
Supplementary Material
Acknowledgments
Grant support: This work was supported by United States Public Health Services grant CA115465 awarded by the National Cancer Institute and U.S. Army Breast Cancer Research Program award DAMD17-02-1-0554.
References
- 1.Turner M, Schweighoffer E, Colucci F, Di Santo JP, Tybulewicz VLJ. Tyrosine kinase SYK: essential functions for immunoreceptor signalling. Immunol Today. 2000;21:148–54. doi: 10.1016/s0167-5699(99)01574-1. [DOI] [PubMed] [Google Scholar]
- 2.Yanagi S, Inatome R, Takano T, Yamamura H. Syk expression and novel function in a wide variety of tissues. Biochem Biophys Res Commun. 2001;288:495–8. doi: 10.1006/bbrc.2001.5788. [DOI] [PubMed] [Google Scholar]
- 3.Coopman PJ, Do MT, Barth M, et al. The Syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature. 2000;406:742–7. doi: 10.1038/35021086. [DOI] [PubMed] [Google Scholar]
- 4.Yuan Y, Mendez R, Sahin A, Dai JL. Hypermethylation leads to silencing of the SYK gene in human breast cancer. Cancer Res. 2001;61:5558–61. [PubMed] [Google Scholar]
- 5.Moroni M, Soldatenkov V, Zhang L, et al. Progressive loss of Syk and abnormal proliferation in breast cancer cells. Cancer Res. 2004;64:7346–54. doi: 10.1158/0008-5472.CAN-03-3520. [DOI] [PubMed] [Google Scholar]
- 6.Repana K, Papazisis K, Foukas P, et al. Expression of Syk in invasive breast cancer: correlation to proliferation and invasiveness. Anticancer Res. 2006;26:4949–54. [PubMed] [Google Scholar]
- 7.Toyama T, Iwase H, Yamashita H, et al. Reduced expression of the Syk gene is correlated with poor prognosis in human breast cancer. Cancer Lett. 2003;189:97–102. doi: 10.1016/s0304-3835(02)00463-9. [DOI] [PubMed] [Google Scholar]
- 8.Dejmek J, Leandersson K, Manjer J, et al. Expression and signaling activity of Wnt-5a/discoidin domain receptor-1 and Syk plays distinct but decisive roles in breast cancer patient survival. Clin Cancer Res. 2005;11:520–8. [PubMed] [Google Scholar]
- 9.Yuan Y, Liu H, Sahin A, Dai JL. Reactivation of SYK expression by inhibition of DNA methylation suppresses breast cancer cell invasiveness. Int J Cancer. 2005;113:654–9. doi: 10.1002/ijc.20628. [DOI] [PubMed] [Google Scholar]
- 10.Mahabeleshwar GH, Kundu GC. Syk, a protein-tyrosine kinase, suppresses the cell motility and nuclear factor kappa B-mediated secretion of urokinase type plasminogen activator by inhibiting the phosphatidylinositol 3′-kinase activity in breast cancer cells. J Biol Chem. 2003;278:6209–21. doi: 10.1074/jbc.M208905200. [DOI] [PubMed] [Google Scholar]
- 11.Hoeller C, Thallinger C, Pratscher B, et al. The non-receptor-associated tyrosine kinase Syk is a regulator of metastatic behavior in human melanoma cells. J Invest Dermatol. 2005;124:1293–9. doi: 10.1111/j.0022-202X.2005.23685.x. [DOI] [PubMed] [Google Scholar]
- 12.Muthusamy V, Duraisamy S, Bradbury CM, et al. Epigenetic silencing of novel tumor suppressors in malignant melanoma. Cancer Res. 2006;66:11187–93. doi: 10.1158/0008-5472.CAN-06-1274. [DOI] [PubMed] [Google Scholar]
- 13.Luangdilok S, Box C, Patterson L, et al. Syk tyrosine kinase is linked to cell motility and progression in squamous cell carcinomas of the head and neck. Cancer Res. 2007;67:7907–16. doi: 10.1158/0008-5472.CAN-07-0331. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Monit S, Juszczynski P, et al. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood. 2008;111:2230–7. doi: 10.1182/blood-2007-07-100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman AL, Sun DX, Law ME, et al. Overexpression of Syk tyrosine kinase in peripheral T-cell lymphomas. Leukemia. 2008;22:1139–43. doi: 10.1038/leu.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark EA, Shattil SJ, Ginsberg MH, Bolen J, Brugge JS. Regulation of the protein tyrosine kinase pp72syk by platelet agonists and the integrin αIIbβ3. J Biol Chem. 1994;269:28859–64. [PubMed] [Google Scholar]
- 17.Lin TH, Rosales C, Mondal K, Bolen JB, Haskill S, Juliano RL. Integrin-mediated tyrosine phosphorylation and cytokine message induction in monocytic cells. A possible signaling role for the Syk tyrosine kinase. J Biol Chem. 1995;270:16189–97. doi: 10.1074/jbc.270.27.16189. [DOI] [PubMed] [Google Scholar]
- 18.Gao J, Zoller KE, Ginsberg MH, Brugge JS, Shattil SJ. Regulation of the pp72syk protein tyrosine kinase by platelet integrin αIIbβ3. EMBO J. 1997;16:6414–25. doi: 10.1093/emboj/16.21.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan SR, Huang M, Berton G. Signaling by adhesion in human neutrophils: activation of the p72syk tyrosine kinase and formation of protein complexes containing p72syk and Src family kinases in neutrophils spreading over fibrinogen. J Immunol. 1997;158:1902–10. [PubMed] [Google Scholar]
- 20.Gotoh A, Takahira H, Geahlen RL, Broxmeyer HE. Cross-linking of integrins induces tyrosine phosphorylation of the proto-oncogene product Vav and the protein tyrosine kinase Syk in human factor-dependent myeloid cells. Cell Growth Diff. 1997;8:721–9. [PubMed] [Google Scholar]
- 21.Stupack DG, Li E, Silletti SA, et al. Matrix valency regulates integrin-mediated lymphoid adhesion via Syk kinase. J Cell Biol. 1999;144:777–88. doi: 10.1083/jcb.144.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vines CM, Potter JW, Xu Y, et al. Inhibition of β2 integrin receptor and Syk kinase signaling in monocytes by the Src family kinase Fgr. Immunity. 2001;15:507–19. doi: 10.1016/s1074-7613(01)00221-7. [DOI] [PubMed] [Google Scholar]
- 23.Mocsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk Is required for integrin signaling in neutrophils. Immunity. 2002;16:547–58. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- 24.Schymeinsky J, Sindrilaru A, Frommhold D, et al. The Vav binding site of the non receptor tyrosine kinase Syk at Tyr 348 is critical for β2 integrin (CD11/CD18) mediated neutrophil migration. Blood. 2006;108:3919–27. doi: 10.1182/blood-2005-12-030387. [DOI] [PubMed] [Google Scholar]
- 25.Ulanova M, Puttagunta L, Marcet-Palacios M, et al. Syk tyrosine kinase participates in beta1-integrin signaling and inflammatory responses in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L497–L507. doi: 10.1152/ajplung.00246.2004. [DOI] [PubMed] [Google Scholar]
- 26.Ma H, Yankee TM, Hu J, Asai DJ, Harrison ML, Geahlen RL. Visualization of Syk-antigen receptor interactions using green fluorescent protein: differential roles for Syk and Lyn in the regulation of receptor capping and internalization. J Immunol. 2001;166:1507–16. doi: 10.4049/jimmunol.166.3.1507. [DOI] [PubMed] [Google Scholar]
- 27.Kinch M, Clark G, Der C, Burridge K. Tyrosine phosphorylation regulates the adhesions of Ras-transformed breast epithelia. J Cell Biol. 1995;130:461–71. doi: 10.1083/jcb.130.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters JD, Furlong MT, Asai DJ, Harrison ML, Geahlen RL. Syk, activated by cross-linking the B-cell antigen receptor, localizes to the cytosol where it interacts with and phosphorylates alpha-tubulin on tyrosine. J Biol Chem. 1996;271:4755–62. doi: 10.1074/jbc.271.9.4755. [DOI] [PubMed] [Google Scholar]
- 29.Oliver JM, Burg DL, Wilson BS, McLaughlin JL, Geahlen RL. Inhibition of mast cell Fc epsilon R1-mediated signaling and effector function by the Syk-selective inhibitor, piceatannol. J Biol Chem. 1994;269:29697–703. [PubMed] [Google Scholar]
- 30.Rüdiger M. Vinculin and α-catenin: shared and unique functions in adherens junctions. BioEssays. 1998;20:733–40. doi: 10.1002/(SICI)1521-1878(199809)20:9<733::AID-BIES6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 31.Xu W, Coll J-L, Adamson ED. Rescue of the mutant phenotype by reexpression of full-length vinculin in null F9 cells; effects on cell locomotion by domain deleted vinculin. J Cell Science. 1998;111:1535–44. doi: 10.1242/jcs.111.11.1535. [DOI] [PubMed] [Google Scholar]
- 32.Basolo F, Elliott J, Tait L, et al. Transformation of human breast epithelial cells by c-Ha-ras oncogene. Mol Carcinogen. 1991;4:25–35. doi: 10.1002/mc.2940040106. [DOI] [PubMed] [Google Scholar]
- 33.Helwani FM, Kovacs EM, Paterson AD, et al. Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J Cell Biol. 2004;164:899–910. doi: 10.1083/jcb.200309034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallet C, Rosa J-P, Habib A, Lebret M, Lévy-Tolédano S, Maclouf J. Tyrosine phosphorylation of cortactin associated with Syk accompanies thromboxane analogue-induced platelet shape change. J Biol Chem. 1999;274:23610–16. doi: 10.1074/jbc.274.33.23610. [DOI] [PubMed] [Google Scholar]
- 35.Maruyama S, Kurosaki T, Sada K, Yamanashi Y, Yamamoto T, Yamamura H. Physical and functional association of cortactin with Syk in human leukemic cell line K562. J Biol Chem. 1996;271:6631–35. doi: 10.1074/jbc.271.12.6631. [DOI] [PubMed] [Google Scholar]
- 36.Denhart BC, Guidi AJ, Tognazzi K, Dvorak HF, Brown LF. Attachment characteristics and involvement of integrins in adhesion of breast cancer cell lines to extracellular bone matrix components. Lab Invest. 1997;77:665–75. [PubMed] [Google Scholar]
- 37.Price EA, Coombe DR, Murray JC. Beta-1 integrins mediate tumour cell adhesion to quiescent endothelial cells in vitro. Br J Cancer. 1996;74:1762–6. doi: 10.1038/bjc.1996.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong JJ, Yankee TM, Harrison ML, Geahlen RL. Regulation of signaling in B cells through the phosphorylation of Syk on linker region tyrosines. J Biol Chem. 2002;277:31703–14. doi: 10.1074/jbc.M201362200. [DOI] [PubMed] [Google Scholar]
- 39.Keshvara LM, Isaacson CC, Yankee TM, Sarac R, Harrison ML, Geahlen RL. Syk- and Lyn-dependent phosphorylation of Syk on multiple tyrosines following B cell activation includes a site that negatively regulates signaling. J Immunol. 1998;161:5276–83. [PubMed] [Google Scholar]
- 40.Yankee TM, Keshvara LM, Sawasdikosol S, Harrison ML, Geahlen RL. Inhibition of signaling through the B cell antigen receptor by the protooncogene product, c-Cbl, requires Syk tyrosine 317 and the c-Cbl phosphotyrosine-binding domain. J Immunol. 1999;163:5827–35. [PubMed] [Google Scholar]
- 41.Lupher ML, Jr, Rao N, Lill NL, et al. Cbl-mediated negative regulation of the Syk tyrosine kinase. A critical role for Cbl phosphotyrosine-binding domain binding to Syk phosphotyrosine 323. J Biol Chem. 1998;273:35273–81. doi: 10.1074/jbc.273.52.35273. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Duke L, Zhang PS. Alternative splicing disrupts a nuclear localization signal in spleen tyrosine kinase that is required for invasion suppression in breast cancer. Cancer Res. 2003;63:4724–30. [PubMed] [Google Scholar]
- 43.Hajra KM, Fearon ER. Cadherin and catenin alterations in human cancer. Genes Chromosome Canc. 2002;34:255–68. doi: 10.1002/gcc.10083. [DOI] [PubMed] [Google Scholar]
- 44.Berx G, Van Roy F. The E-cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Cancer Res. 2001;3:289–93. doi: 10.1186/bcr309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Path. 1998;133:333–9. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinberg MS, McNutt PM. Cadherins and their connections: adhesion junctions have broader functions. Curr Opin Cell Biol. 1999;11:554–60. doi: 10.1016/s0955-0674(99)00027-7. [DOI] [PubMed] [Google Scholar]
- 47.Roura S, Miravet S, Piedra J, de Herreros AG, Dunach M. Regulation of E-cadherin/catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36724–40. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- 48.Steinberg MS, McNutt PM. Cadherins and their connections: adhesion junctions have broader functions. Curr Opin Cell Biol. 1999;11:554–60. doi: 10.1016/s0955-0674(99)00027-7. [DOI] [PubMed] [Google Scholar]
- 49.Roura S, Miravet S, Piedra J, de Herreros AG, Dunach M. Regulation of E-cadherin/catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36724–40. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- 50.Weed SA, Parsons JT. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene. 2001;20:6418–34. doi: 10.1038/sj.onc.1204783. [DOI] [PubMed] [Google Scholar]
- 51.Daly RJ. Cortactin signalling and dynamic actin networks. Biochem J. 2004;382:13–25. doi: 10.1042/BJ20040737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Illes A, Enyedi B, Tamas P, Balazs A, Bogel G, Lukacs M, Buday L. Cortactin is required for integrin-mediated cell spreading. Immunol Lett. 2006;104:124–30. doi: 10.1016/j.imlet.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 53.de Virgilio M, Kiosses WB, Shattil SJ. Proximal, selective, and dynamic interactions between integrin αIIbβ3 and protein tyrosine kinases in living cells. J Cell Biol. 2004;165:305–11. doi: 10.1083/jcb.200402064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El Sayegh TY, Arora PD, Laschinger CA, et al. Cortactin associates with N-cadherin adhesions and mediates intercellular adhesion strengthening in fibroblasts. J Cell Science. 2004;117:5117–31. doi: 10.1242/jcs.01385. [DOI] [PubMed] [Google Scholar]
- 55.Hu M, Carles-Kinch KL, Zelinski DP, Kinch MS. EphA2 induction of fibronectin creates a permissive microenvironment for malignant cells. Mol Can Res. 2004;2:533–40. [PubMed] [Google Scholar]
- 56.Avizienyte E, Wyke AW, Jones RJ, et al. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signaling. Nature Cell Biol. 2002;4:632–8. doi: 10.1038/ncb829. [DOI] [PubMed] [Google Scholar]
- 57.Brakebusch C, Wennerberg K, Krell HW, et al. Beta1 integrin promotes but is not essential for metastasis of ras-myc transformed fibroblasts. Oncogene. 1999;18:3852–61. doi: 10.1038/sj.onc.1202770. [DOI] [PubMed] [Google Scholar]
- 58.Elliott BE, Ekblom P, Pross H, Niemann A, Rubin K. Anti-beta 1 integrin IgG inhibits pulmonary macrometastasis and the size of micrometastases from a murine mammary carcinoma. Cell Adhes Commun. 1994;1:319–32. doi: 10.3109/15419069409097263. [DOI] [PubMed] [Google Scholar]
- 59.Park CC, Zhang H, Pallavicini M, et al. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66:1526–35. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Webb DJ, Donais K, Whitmore LA, et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–61. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 61.Luangdilok S, Box C, Patterson L, et al. Syk tyrosine kinase is linked to cell motility and progression in squamous cell carcinomas of the head and neck. Cancer Res. 2007;67:7907–16. doi: 10.1158/0008-5472.CAN-07-0331. [DOI] [PubMed] [Google Scholar]
- 62.Brdicka T, Kadlecek TA, Roose JP, Pastuszak AW, Weiss A. Intramolecular regulatory switch in ZAP-70: analogy with receptor tyrosine kinases. Mol Cell Biol. 2005;25:4924–33. doi: 10.1128/MCB.25.12.4924-4933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bustelo XR. Vav proteins, adaptors and cell signaling. Oncogene. 2001;20:6372–81. doi: 10.1038/sj.onc.1204780. [DOI] [PubMed] [Google Scholar]
- 64.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348:241–55. [PMC free article] [PubMed] [Google Scholar]
- 65.Groesch TD, Zhou F, Matilla S, Geahlen RL, Post CB. Structural basis for the requirement of two phosphotyrosine residues in signaling mediated by Syk tyrosine kinase. J Mol Biol. 2006;356:1222–36. doi: 10.1016/j.jmb.2005.11.095. [DOI] [PubMed] [Google Scholar]
- 66.Nakashima H, Natsugoe S, Ishigami S, et al. Clinical significance of nuclear expression of spleen tyrosine kinase (Syk) in gastric cancer. Cancer Lett. 2006;236:89–94. doi: 10.1016/j.canlet.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 67.Wang L, Devarajan E, He J, Reddy SP, Dai JL. Transcription repressor activity of spleen tyrosine kinase mediates breast tumor suppression. Can Res. 2005;65:10289–97. doi: 10.1158/0008-5472.CAN-05-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou F, Hu J, Ma H, Harrison ML, Geahlen RL. Nucleocytoplasmic trafficking of the Syk protein-tyrosine kinase. Mol Cell Biol. 2006;26:3478–91. doi: 10.1128/MCB.26.9.3478-3491.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Skaggs BJ, Clark MR. Proximal B cell receptor signaling pathways. Signal Trans. 2004;5–6:173–94. [Google Scholar]
- 70.Zhou Q, Geahlen RL. The protein-tyrosine kinase Syk interacts with TRAF-interacting protein TRIP in breast epithelial cells. Oncogene. 2009 doi: 10.1038/onc.2008.493. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.