Summary
Female vertebrates are endowed during development with a stockpile of oocytes that is gradually depleted over the organism’s lifetime through the process of apoptosis. The timer that triggers this cell death has yet to be identified. Here, using the ease of biochemical manipulation afforded by Xenopus eggs and oocytes, we examine the hypothesis that nutrient stores of the oocyte can regulate oocyte viability. We show that pentose phosphate pathway generation of NADPH is critical for oocyte survival and that the target of this regulation is caspase 2, previously shown to regulate oocyte death in mice. Pentose phosphate pathway-mediated inhibition of cell death resulted from the inhibitory phosphorylation of caspase 2 by Calcium calmodulin-dependent kinase II (CaMKII). A mutant of caspase 2 able to escape CaMKII phosphorylation overrode this inhibition of apoptosis. These data suggest that exhaustion of oocyte nutrients, resulting in an inability to generate NADPH, may contribute to ooctye depletion. These data also provide unexpected links between oocyte metabolism, CaMKII and caspase 2.
Introduction
Vertebrate female reproduction is limited by the oocyte stockpiles acquired during embryonic development. Apoptotic loss of oocytes over a lifetime leads ultimately to loss of fertility (Tilly, 2001). In addition, pathological insults or chemotherapeutic treatment can accelerate oocyte death, resulting in premature oocyte depletion and sterility (Perez et al., 1997). Despite the importance of oocyte survival in determining female fertility, the molecular pathways governing the timing of apoptosis in these cells are not fully understood.
Genetic analyses in mice and in vitro experiments using murine oocytes have provided a framework in which to understand the molecular underpinnings of oocyte apoptosis. Death of these cells can be prevented by caspase inhibitors or anti-apoptotic Bcl-2 proteins and induced by overexpression of either caspases or pro-apoptotic Bcl-2 family members (Morita et al., 1999; Morita and Tilly, 1999; Kim and Tilly, 2004; Morita et al., 2000). Conclusions from such studies have been borne out by analyses of knock-out mice in which Bax-deficient animals exhibited a reduction in oocyte apoptosis, with a consequent delay in oocyte depletion (Perez et al., 1999), while mice lacking Bcl-2 or Bcl-xL had a decreased oocyte reserve (Ratts et al., 1995; Watanabe et al., 1997).
Insight into oocyte apoptosis has come from studies of knock-out mice deficient in caspase 2. Surprisingly, these mice developed normally, with one striking exception: female mice were endowed with an excess of oocytes. Moreover, caspase 2-deficient oocytes were markedly resistant to cell death induced by chemotherapeutics (Bergeron et al., 1998). The precise role of caspase 2 in cell death has been controversial, but recent studies demonstrate that it can act upstream of the mitochondria in a number of settings to trigger cytochrome c release (Guo et al., 2002; Lassus et al., 2002; Robertson et al., 2002). Caspase 2 is activated by binding to oligomerizing adaptor proteins. In response to various stressors, caspase 2 is recruited into high molecular weight complexes reminiscent of, though distinct from, the Apaf-1/caspase 9 apoptosome (Read et al., 2002). The caspase 2 prodomain can recruit an adaptor protein, RAIDD, which, when overexpressed, induces caspase 2 activation (Duan and Dixit, 1997). Recently, it was reported that p53 induces expression of PIDD, a protein which promotes formation of a caspase 2 activation complex containing PIDD, caspase 2 and RAIDD (Tinel and Tschopp, 2004). It is not known whether PIDD or RAIDD regulate caspase 2 in the oocyte.
Although genetic analyses and microinjection studies have provided critical information for understanding oocyte apoptosis, most vertebrate oocytes are not amenable to biochemical analysis due to their small size and limited abundance. However, over a decade ago, Newmeyer et al. reported that extracts prepared from eggs of the frog, Xenopus laevis, could, upon prolonged incubation at room temperature, spontaneously recapitulate many events of apoptosis, including mitochondrial cytochrome c release, caspase activation, and nuclear fragmentation (Newmeyer et al., 1994). Importantly, this in vitro apoptosis could be inhibited by anti-apoptotic Bcl-2 proteins and caspase inhibitors, suggesting that at least some aspects of germ cell apoptosis are faithfully recapitulated in this system, thereby providing a biochemically manipulable setting in which to understand germ cell apoptosis (Kluck et al., 1997).
Despite the ease of manipulation of Xenopus eggs and oocytes and the manifestation of apoptotic markers, it was not clear what might be driving apoptosis in this system. Although initial reports suggested that the timing of hormone administration used to elicit egg-laying might determine the susceptibility of eggs to apoptosis, research in the intervening years has failed to establish a firm correlation between the rates of apoptosis in Xenopus eggs and the hormonal regimen used to obtain the eggs. Hence, we were driven to consider other features of the oocyte/egg that might contribute to cell death regulation. In this regard, we were interested in the fact that oocytes are uniquely endowed with a large stockpile of nutrients in the form of both yolk proteins and glycogen stores, which are used to sustain early embryonic development.
Numerous links have been established between metabolism and apoptosis. For example, the Bcl-2 family protein Bad associates with and regulates glucokinase/hexokinase 4 (Danial et al., 2003). Moreover, AKT requires glucose uptake to promote cell survival and this effect is exerted in part through targeting of hexokinase to the mitochondria (Majewski et al., 2004; Rathmell et al., 2003). In lymphocytes and neurons, growth factor withdrawal leads to a drop in glucose uptake and glycolytic rates prior to commitment to cell death (Deckwerth and Johnson, 1993; Deshmukh et al., 1996; Rathmell et al., 2000; Vander Heiden et al., 2001). Moreover, artificial maintenance of glycolysis confers some resistance to cell death induced by cytokine withdrawal, suggesting that active growth factor signaling is required to maintain sufficient metabolism to prevent death (Rathmell et al., 2001; Rathmell et al., 2003). In addition, glucose-6-phosphate dehydrogenase activity, which promotes the metabolism of glucose through the pentose phosphate pathway, can protect CHO cells from death induced by ionizing radiation (Tuttle et al., 2000). Conversely, inhibition of both glycolytic and pentose phosphate pathways can promote the apoptotic death of some cultured cells (Comin-Anduix et al., 2002; Tian et al., 1999; Tuttle et al., 2000). Thus, it was attractive to hypothesize that nutrient stores in the oocyte might support cell survival through modulation of metabolic pathways. According to such a scenario, depletion of energy stores within oocytes over time would result either in loss of a critical survival pathway or the de novo engagement of a cell death pathway.
Here, using the facile biochemistry provided by the Xenopus system, we report the identification and characterization of a novel caspase 2 regulatory pathway responsive to the metabolic state of the oocyte/egg. We demonstrate that glucose-6-phosphate (G6P) sufficient to drive continual operation of the pentose phosphate pathway can greatly prolong germ cell survival. Moreover, our data indicate that NADPH generation by this pathway is critical for promoting survival and that a surfeit of NADPH promotes a calcium/calmodulin-dependent protein kinase II (CaMK II)-dependent inhibitory phosphorylation of caspase 2. Mutant caspase 2 resistant to CaMKII phosphorylation can circumvent this metabolism-dependent suppression of apoptosis, both in egg extracts and intact oocytes. These data, which are fully consistent with the reported requirement for caspase 2 in mouse oocyte apoptosis, link the operation of a specific metabolic pathway to the direct CaMKII-mediated regulation of caspase 2, thereby providing insight into the control of germ cell life and death.
Results
G6P inhibits apoptosis in Xenopus egg extracts
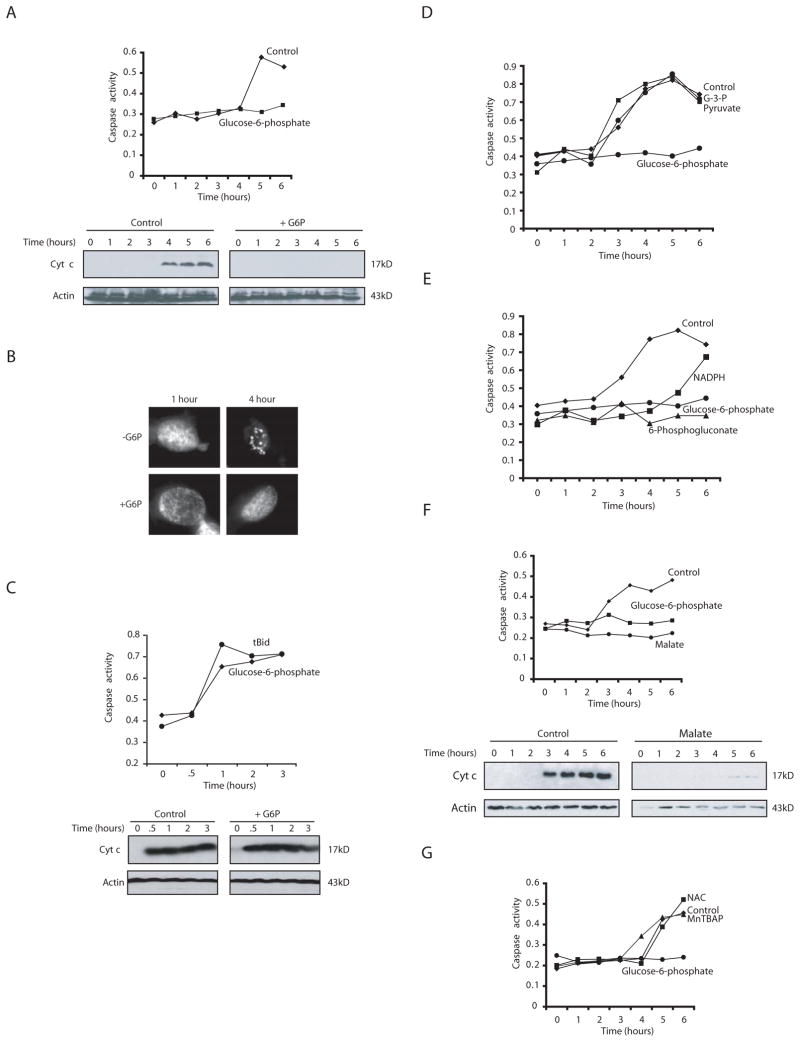
If nutrient depletion contributed to apoptotic induction in Xenopus eggs and oocytes, we hypothesized that boosting glucose-utilizing metabolic pathways might forestall apoptosis in this system. Accordingly, we added G6P directly to the in vitro egg extract system and monitored cleavage of the model substrate, DEVD-pNA, as a measure of caspase 3 activity. As shown in Fig. 1A, the caspase 3 activation observed following incubation of the extract at room temperature was entirely suppressed by G6P addition. Note that G6P, which in oocytes is derived largely from gluconeogenesis or glycogenolysis, was used in these experiments rather than glucose due to the presence of very low hexokinase activity in these extracts (C. Herman and L. Nutt, unpublished). We also found that G6P could inhibit apoptotic fragmentation of sperm nuclei added to egg extracts (Fig. 1B). Consistent with the critical role for mitochondria in the induction of Xenopus egg apoptosis, the suppressive effects of G6P could be seen at the level of cytochrome c release (Fig. 1A, lower panel). Moreover, the inhibitory effect of G6P on caspase activation and cytochrome c release could be overridden by addition of truncated Bid (tBid) to forcibly promote cytochrome c release (Fig. 1C). These data indicate that G6P-containing extracts were still capable of activating caspases and that the observed block to apoptosis following G6P treatment occurred upstream of or at the level of the mitochondria.
Fig. 1. NADPH is a potent inhibitor of egg apoptosis.
A) Xenopus egg extracts supplemented with G6P or buffer were analyzed for caspase 3 activity at various time points using the caspase substrate Ac-DEVD-pNA. Substrate cleavage was measured spectrophotometrically at 405nm. Cytochrome c release was measured in parallel by filtering extract through a 0.1μm microfilter. The filtrate, lacking mitochondria, was analyzed by SDS-PAGE and immunoblotting with anti-cytochrome c antibody. Blots were re-probed with anti-Actin antibody. B) Extracts treated as in A were supplemented with sperm nuclei and stained with Hoechst dye to detect DNA by fluorescence microscopy. Shown are representative nuclei at the indicated times. C) Truncated Bid (tBid) was added to extract treated with G6P. Caspase 3 activity and cytochrome c release were measured as in (A). D) Buffer, glyceraldehyde-3-phosphate (G-3-P), pyruvate, or G6P were added to extract and caspase 3 activity was measured. E) Pentose phosphate intermediates (NADPH, 6-phosphogluconate) or G6P were added to extract and caspase 3 activity measured. F) Malate was added to extracts and caspase 3 activity and cytochrome c release measured. G) A superoxide dismutase mimetic Mn(III)tetrakis(4–Benzoic acid)porphyrin Chloride (MnTBAP) or a precursor to glutathione, N-Acetyl-L-cysteine (NAC) was added to extracts and caspase activity was measured.
Pentose phosphate pathway intermediates suppress cell death
In Xenopus eggs/oocytes, G6P is used for glycogen deposition or is metabolized through the pentose phosphate pathway. Indeed, the fate of radiolabeled G6P has been followed in amphibian oocytes and little is metabolized by glycolysis (Dworkin and Dworkin-Rastl, 1989). Consistent with this, we found that glycolytic intermediates, including glyceraldehyde-3-phosphate and pyruvate, had no effect on caspase 3 activation in egg extracts (Fig. 1D). In contrast, 6-phosphogluconate, a pentose phosphate pathway intermediate, could mirror the ability of G6P to inhibit apoptosis (Fig. 1E). These data suggested that some feature of pentose phosphate pathway operation could suppress apoptosis. A key metabolic by-product of the pentose phosphate pathway is NADPH, produced when G6P is metabolized to 6-phosphogluconolactone and when 6-phosphogluconate is converted to ribulose-5-phosphate. Hypothesizing that production of NADPH might be the relevant consequence of G6P addition for apoptotic inhibition, we sought to generate NADPH using an alternative route and to examine the effects of direct NADPH addition to extracts. Addition of malate, which promotes NADPH production in concert with malic enzyme, potently inhibited mitochondrial cytochrome c release and caspase activation in the egg extract (Fig. 1F). Moreover, despite its relative instability in solution, NADPH could markedly suppress caspase activation (Fig. 1E). These data suggest that G6P flux through the pentose phosphate pathway, leading to NADPH production, can potently suppress egg extract apoptosis. Although NADPH can act as a reducing agent, we do not believe that it is this feature of NADPH function that is responsible for apoptotic suppression as neither the SOD mimetic MnTBAP, the electron scavenger, NAC, nor other reducing agents (TEMPO or reduced glutathione) could suppress extract apoptosis (Fig. 1G and data not shown).
Pentose phosphate pathway inhibition induces apoptosis in egg extracts and oocytes
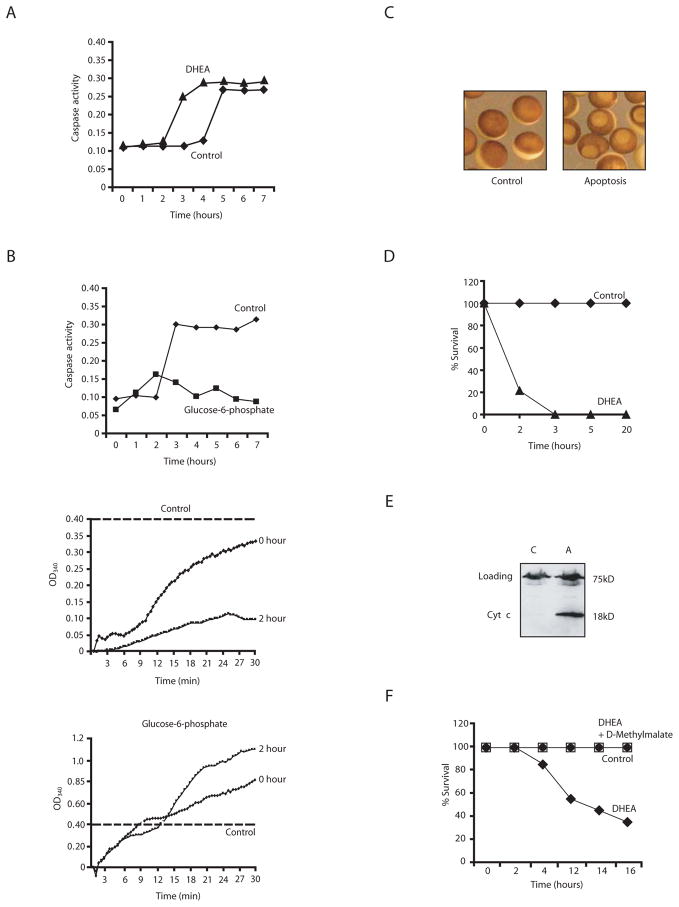
Since hyperstimulation of the pentose phosphate pathway or NADPH addition inhibited caspase activation, we speculated that inhibition of the endogenous pentose phosphate pathway in egg extracts should accelerate apoptosis, particularly if the spontaneous extract apoptosis relied upon nutrient depletion during incubation. To test this, we treated extracts with the G6P dehydrogenase inhibitor, dehydroisoandrosterone (DHEA), to prevent entry of endogenous G6P into the pentose phosphate pathway (Schwartz and Pashko, 2004). This treatment accelerated apoptosis, largely eliminating the lag normally seen before caspase activation, as would be expected if pentose phosphate pathway function were important for extract “survival” (Fig. 2A). We also examined extracts to determine whether endogenous G6P was depleted over time upon incubation. For this purpose, extracts were incubated at room temperature and assayed for caspase activation (Fig. 2B, top panel). Aliquots of extract withdrawn at the start of the incubation (0 hour) or just prior to caspase activation (2 hour) were incubated with a vast excess of G6P dehydrogenase and NADP and assayed over time for generation of NADPH, which, under these circumstances, depended solely on the available G6P. As shown in Fig. 2B (middle panel), G6P levels (as measured by the rate of NADPH generation) dropped substantially within the two hours of incubation. Note also that NADPH generation remained high in extracts supplemented with excess G6P (Fig. 2B, lower panel).
Fig. 2. Inhibition of G6P dehydrogenase induces apoptosis.
A) Extracts supplemented with either dehydroisoandrosterone (DHEA) or buffer were analyzed for caspase 3 activity. Shown is a representative experiment repeated on 3 separate batches of oocytes with similar results. B) (upper): Extract was incubated at room temp and assayed for caspase activity. (middle): Samples withdrawn at 0 and 2 h of incubation were assayed for G6P levels by monitoring NADPH production over time (absorbance at 340 nm) in the presence of excess G6P dehydrogenase and NADP. (lower): Addition of excess G6P maintained NADPH production at all times tested. Dotted line indicates the fact that the scales are different in the extracts +/− G6P as NADPH was produced at much higher rates in the presence of excess G6P. C) Oocytes treated with DHEA or buffer are shown in representative micrographs. D) Percent survival of oocytes treated with buffer or DHEA. E) Cytochrome c release was measured in parallel by filtering aliquots of lysed oocytes through a 0.1μm microfilter and analyzing the filtrate by anti-cytochrome c immunoblotting. F) Percent survival of oocytes treated with buffer, DHEA or DHEA and cell permeable D-methyl malate.
We wished to extend these observations to whole oocytes to determine if the pentose phosphate pathway was important for suppressing death in these intact cells. For this, we developed a visual assay for oocyte death. Normally, oocytes contain a light-colored vegetal half and a dark uniformly pigmented animal half. When induced to undergo maturation with progesterone, oocytes exhibit a white spot resulting from nuclear envelope breakdown and clearance of surrounding pigment granules. We noted that a similar, but considerably larger white spot appeared at the animal poles of oocytes injected with cytochrome c to induce caspase activation (C. Holley, unpublished). These oocytes did not exhibit elevated Cdc2/Cyclin kinase activity characteristic of maturing oocytes, but rather showed high levels of caspase 3 activity (data not shown). Morphological events of apoptosis have been observed in oocytes by others, both in Xenopus and in other species (Bagowski et al., 2001; Bhuyan et al., 2001; Braun et al., 2003; Demirci et al., 2001; Demirci et al., 2003; Demirci et al., 2002; Voronina and Wessel, 2001). Interestingly, we had observed that oocytes taken freshly out of a virgin female frog are remarkably refractory to apoptosis. With the idea that this resistance might stem from continual functioning of the pentose phosphate pathway, we soaked fresh oocytes in a DHEA-containing solution and monitored the appearance of large white spots at the animal poles. As shown in the micrographs in Fig. 2C and the graph in Fig. 2D, 200 μM DHEA rapidly killed oocytes, while control treated oocytes remained healthy. These data were corroborated in experiments where cytochrome c was released from mitochondria in DHEA-treated, but not control oocytes (Fig. 2E). Importantly, this acceleration of oocyte death occurred at even lower doses of DHEA (100 μM) and could be reversed by simultaneous treatment with the cell-permeant D-methylmalate, which would be expected to promote NADPH generation within oocytes (Fig. 2F). These data demonstrate that pentose phosphate pathway inhibition promotes rapid oocyte death, suggesting that this pathway is normally critical for oocyte viability.
Caspase 2 is a target of NADPH-mediated apoptotic inhibition
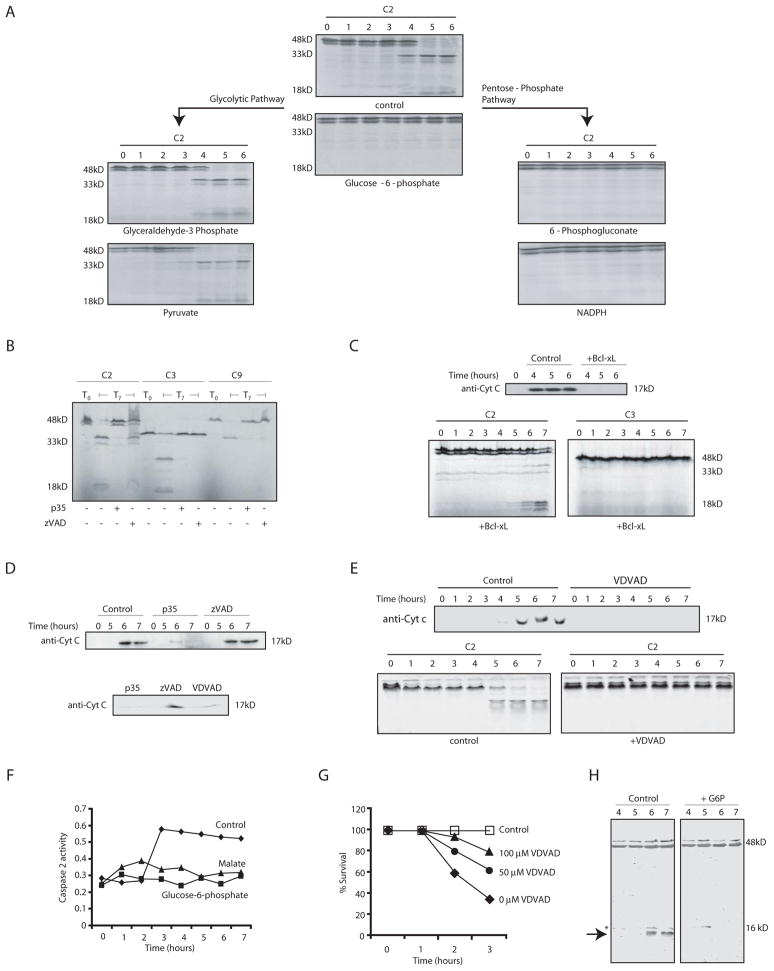
Since G6P (or NADPH) could inhibit apoptosis, we wished to identify the target of this inhibition. As mentioned above, genetic analyses in mice had implicated caspase 2 as an important constituent of oocyte apoptotic pathways (Bergeron et al., 1998). To determine if caspase 2 was affected by G6P, we first examined processing of radiolabeled caspase 2 during spontaneous apoptosis. As shown in Fig. 3A, addition of G6P, pentose phosphate pathway intermediates, malate, or NADPH all prevented caspase 2 processing, while glycolytic intermediates had no effect. Moreover, G6P inhibited endogenous caspase 2 processing, as detected by immunoblotting (Figure 3H). Caspase 2 processing in untreated extracts was inhibitable by the baculoviral caspase inhibitor p35 and by the peptide inhibitor of caspase 2, VDVAD-fmk, but was not inhibited by Bcl-xL, which prevented cytochrome c release and caspase 3 activation (Fig. 3B,C, and E). Interestingly, treatment with p35 or VDVAD-fmk also inhibited cytochrome c release, consistent with the involvement of a caspase (potentially caspase 2) upstream of mitochondria in this system (Fig. 3D, 3E). Moreover, neither caspase 2 processing nor cytochrome c release could be inhibited by the pan caspase inhibitor, z-VAD-fmk, consistent with previous reports that caspase 2 is markedly less sensitive to zVAD than other caspases (Garcia-Calvo et al., 1998)(Figs. 3B, D). Thus, G6P could inhibit the z-VAD-insensitive, p35 and VDVAD-sensitive processing of caspase 2, most likely catalyzed by caspase 2 itself. Consistent with these observations, G6P blocked cleavage of the peptide substrate VDVAD-pNA (Fig. 3F). In addition, VDVAD, which inhibited both caspase 2 processing and cytochrome c release in egg extracts, could block the DHEA-induced apoptosis of oocytes (Fig. 3G). These data agree with the idea that caspase 2 is a critical inhibitory target of pentose phosphate pathway metabolites.
Fig. 3. Pentose phosphate metabolites act upstream of cytochrome c release.
A) Egg extract was incubated with metabolic substrates and samples were analyzed for processing of in vitro translated caspase 2. B) Extract was incubated at room temp in the presence of caspase inhibitors (zVAD, 100 μM, VDVAD 100μM, or p35) and radiolabeled pro-caspases. Samples from time 0 (T0) and 7 hours (T7) were analyzed by SDS-page for processing of in vitro translated caspases 2 (C2), 3 (C3), and 9 (C9). C) Extract was incubated at room temp +/− Bcl-xL, and samples were analyzed every hour for in vitro translated C2 and C3 processing by SDS-PAGE and for cytochrome c release. D) (upper panel): Egg extract was incubated at room temp and treated with or without p35 and zVAD-fmk. Samples were taken every hour, mitochondria removed by filtration and the remaining cytosol immunoblotted with anti-cytochrome c antibody. (lower panel) same as above except a 7 h timepoint is shown also in the presence of VDVAD. E) (Upper panel): Samples were treated as in D, but in the presence of VDVAD (lower panel): Processing of radiolabeled caspase 2 was monitored +/− VDVAD. F) Cleavage of VDVAD-pNA was monitored in extracts containing buffer, malate or G6P. G) Oocytes were treated with DHEA as in 2A, with or without prior soaking in buffer containing the indicated concentrations of VDVAD. H) Cleavage of endogenous caspase 2 was monitored +/− G6P by anti-caspase 2 immunoblotting using a mix of C20 and H119 antibodies from Santa Cruz, which recognize only the full length 48 kD protein and the 12 kD cleavage fragment, indicated by the arrow. The asterisk indicates a background band present in all lanes.
G6P and NADPH prevent activation of caspase 2 by RAIDD
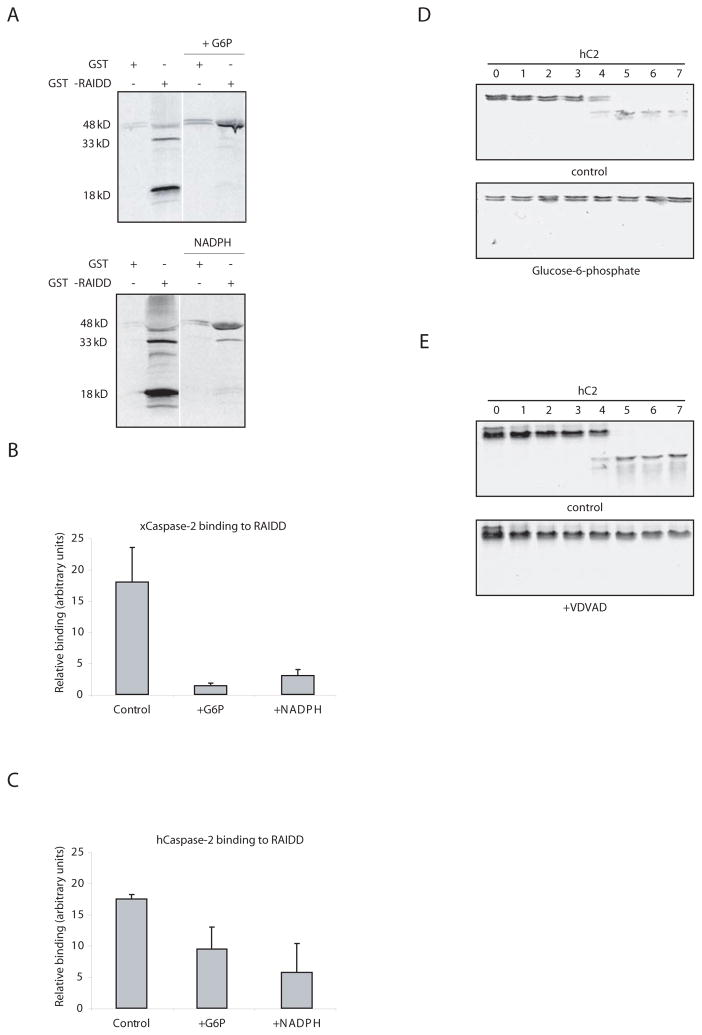
Caspase 2 is activated by oligomerization through binding of its CARD to adaptors (Duan and Dixit, 1997). Although our data suggested that G6P and NADPH could inhibit caspase 2, it was not clear that this inhibition was exerted at the level of caspase 2, rather than indirectly through inhibition of an upstream signaling component. To address this, we wished to promote direct caspase 2 oligomerization and determine if this, too, could be inhibited by G6P. The adaptor RAIDD binds to the caspase 2 CARD and has been implicated in caspase 2 activation in several settings. We have found that GST-RAIDD bound to glutathione sepharose can, upon addition to egg extracts, directly promote caspase 2 processing (Fig. 4A). Even this caspase 2 auto-processing was inhibited by G6P or NADPH, suggesting that NADPH can interfere with direct RAIDD-induced caspase 2 activation (Fig. 4A). Moreover, G6P or NADPH decreased the total amount of caspase 2 that could be retrieved from extracts in association with sepharose-RAIDD, suggesting that these metabolites could interfere with adaptor-caspase 2 complex formation (Fig. 4B). Similar results were obtained using in vitro translated human caspase 2, whose processing was also inhibited by G6P and VDVAD in the egg extract (Fig. 4C, D, and E). These data suggest that metabolic inhibition of caspase 2 can be exerted directly at the level of oligomerization/activation.
Fig. 4. Anti-apoptotic effects of NADPH are exerted at the level of caspase 2.
A) GST-RAIDD or GST bound to glutathione sepharose was dipped into extract +/− NADPH or G6P in the presence of radiolabeled pro-caspase 2. Beads were retrieved by centrifugation, boiled in SDS sample buffer and resolved by SDS-PAGE for autoradiography. Pro-caspase 2 not bound to beads was also unprocessed in the presence of G6P or NADPH (data not shown). B) GST-RAIDD sepharose was dipped into extracts with in vitro translated caspase 2 in the presence and absence of NADPH. Beads were washed extensively and radiolabeled caspase 2 binding was measured by phosphoimager. C) The same experiment in B was repeated using in vitro translated human, rather than Xenopus, caspase 2. D) Processing of radiolabeled human pro-caspase 2 supplemented into egg extracts was monitored in the presence and absence of G6P. E) The same as in D, but +/− VDVAD.
G6P and NADPH promote caspase 2 phosphorylation by CaMKII
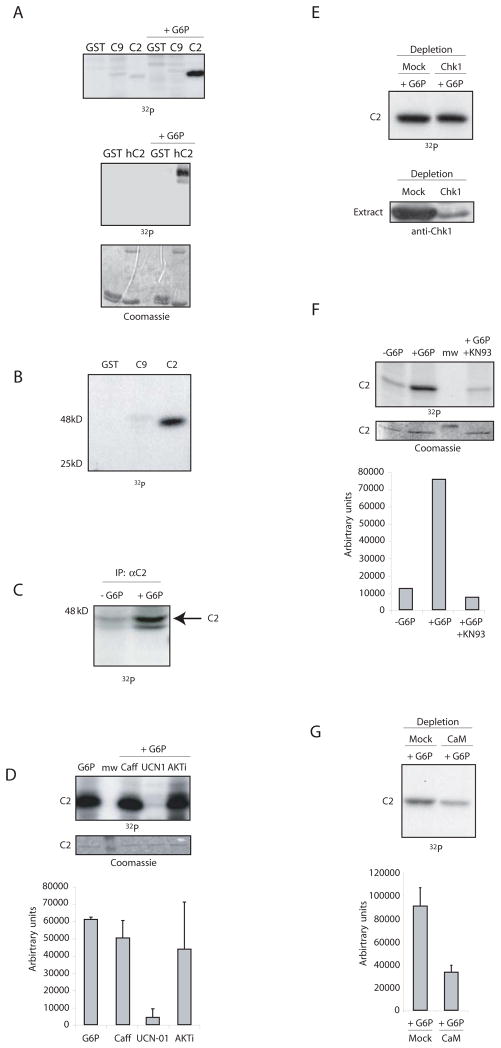
Since G6P and NADPH blocked activation of caspase 2 by RAIDD, we suspected either that these proteins were post-translationally modified to prevent their interaction or that a CARD-binding inhibitor was recruited in the presence of G6P. Experiments to detect proteins bound to either GST-RAIDD or the GST-caspase 2 prodomain did not reveal any potential inhibitors bound only in the presence of G6P (data not shown). Therefore, we speculated that the CARD-containing regions of these proteins might be post-translationally modified. Thus we looked to see whether GST-RAIDD or the GST-caspase 2 prodomain was phosphorylated in G6P-treated extracts. Although we saw no evidence of RAIDD phosphorylation, G6P addition greatly stimulated phosphorylation of the caspase 2 prodomain, derived from either human or Xenopus caspase 2 (Fig. 5A). The caspase 9 prodomain was not phosphorylated under these conditions (Fig. 5A). Note that prolonged exposure of kinase assay gels revealed basal phosphorylation of the caspase 2 prodomain in untreated extracts, but this was markedly stimulated by G6P (Fig. 5A and 5B). Moreover, endogenous pro-caspase 2 immunoprecipitated from egg extracts incubated with γ-32P-ATP was also phosphorylated and this phosphorylation was stimulated by G6P (Fig. 5C).
Fig. 5. G6P/NADPH induces phosphorylation and inactivation of caspase 2.
A) (upper) The GST-prodomain of Xenopus caspase 2 (C2) or 9 (C9) was bound to glutathione sepharose and dipped into cytosolic extracts +/− G6P, and γ-32P-ATP. Bead-bound proteins were washed and analyzed by SDS-PAGE and phosphorimaging. (lower): Similar results were obtained with the human caspase 2 prodomain. B) The experiment in A) was repeated minus G6P and over-exposed to detect basal caspase 2 phosphorylation. C) Anti-Xenopus caspase 2 prepared against a C-terminal 20 amino acid peptide or preimmune antibodies were bound to protein A beads and dipped into extract +/− G6P and γ-32P-ATP. Samples were resolved by SDS-PAGE and detected by autoradiography. D) The experiment in A) was repeated with 5 mM Caffeine, UCN-01 (1 μM), or Akt inhibitor (20 μM). Sepharose-bound GST proteins were washed and analyzed by SDS-PAGE and quantitated by phosphorimaging. E) Egg extracts were depleted with anti-Chk1 or control antibody and incubated with G6P. Samples were washed and analyzed by SDS-PAGE and autoradiography (upper panel) or by anti-Chk1 immunoblotting (lower panel). F) The experiment in A) was repeated with 5μM KN-93. Sepharose-bound GST proteins were washed and analyzed by SDS-PAGE and quantitated by phosphorimaging. G) Egg extracts were depleted with calmodulin sepharose or control sepharose and then incubated with G6P. Caspase 2 prodomain phosphorylation was monitored as in A.
To identify the kinase(s) phosphorylating the caspase 2 prodomain, we repeated the experiment in Fig. 5B in the presence of a battery of kinase inhibitors. Inhibition of several kinases previously implicated in apoptotic regulation, including PKC, PKA, AKT and MEK had no effect on G6P-stimulated caspase 2 phosphorylation (Fig. 5D and data not shown). However, UCN-01 blocked phosphorylation of the caspase 2 prodomain (Fig. 5D). Although UCN-01 is a broad-spectrum kinase inhibitor, it has been used extensively in Xenopus egg extracts to inhibit both Chk1 and CaMKII (Graves et al., 2000; Hutchins et al., 2003). The inability of caffeine, an inhibitor of Chk1-activating kinases, to override G6P suggested Chk1 might not play a role in this pathway (Fig. 5D). Indeed, Chk1 immunodepletion produced no loss of G6P-stimulated prodomain phosphorylation (Fig. 5E). Conversely, treatment of egg extracts with the general CaMK inhibitor, KN93, led to a substantial loss of G6P-stimulated caspase 2 phosphorylation (Fig. 5F). Moreover, depletion of extracts using calmodulin sepharose or treatment of extracts with the calmodulin inhibitors W7 or W13 markedly diminished G6P-triggered phosphorylation of caspase 2, consistent with the involvement of a calmodulin-binding protein in this pathway (Fig. 5G and 6D). Moreover, excess calcium stimulated and EGTA inhibited phosphorylation of the caspase 2 prodomain, consistent with the involvement of a calcium regulated kinase (Fig. 6C).
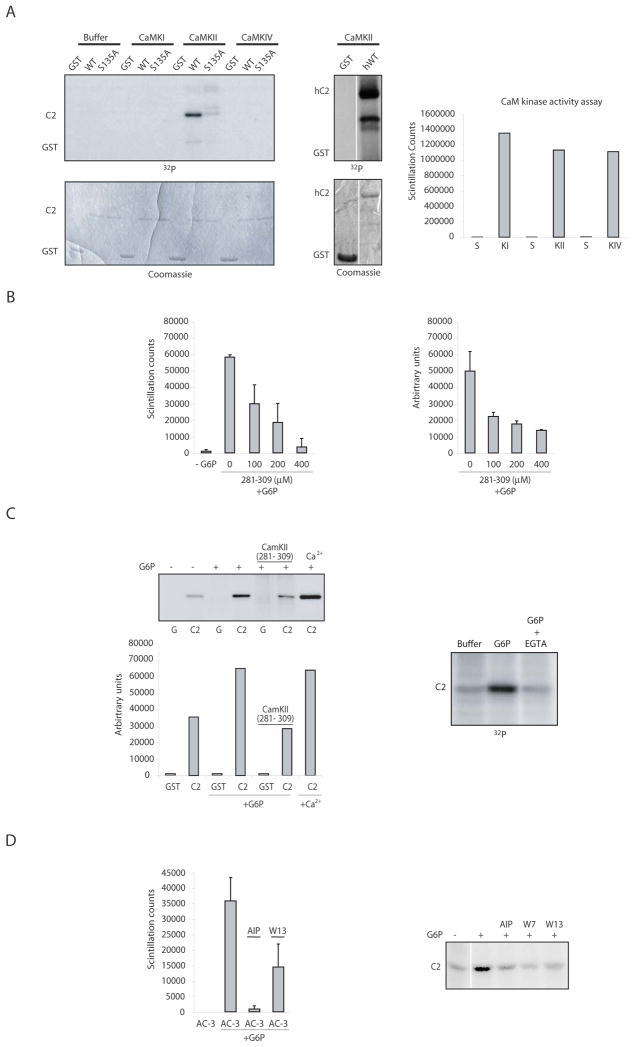
Fig 6. CaMKII phosphorylates the prodomain of caspase 2.
A) (left): In vitro kinase assays were performed with either buffer, CaMKI, CaMKII or CaMKIV and the GST-fused prodomain of caspase 2 (WT or S135A). Prodomain phosphorylation was analyzed by SDS-PAGE, and the gel was stained with coomassie blue to show equal loading. (middle): The same assay was performed using CaMKII and the human caspase 2 prodomain. (right): The activity of each kinase was tested against its cognate peptide substrate and compared to substrate (S) alone in the absence of kinase (right panel). B) (Left): Extract was incubated +/− G6P, γ-32P-ATP, and the peptide substrate Syntide-2 (Syn2) +/− various doses of a CaMKII peptide inhibitor (CaMKII 281–309). Substrate trapped on filter paper was washed extensively and scintillation counted. (right): The same extracts were tested using GST (G) or GST-caspase 2 prodomain (C2) as substrates by the same protocol as in Fig. 5B (right panel shows quantiation of GST-prodomain phosphorylation from 3 such gels exposed to the indicated inhibitor doses). C) (left upper panel): A represenative gel for the experiment shown in the right hand panel of B, with quantitation of the gel, below. (right): The assay in Fig. 5B was repeated in the presence of EGTA. D) (right): Using the same conditions as in B), another CaMKII ινηιβιτoρ (5 μM AIP) was tested using its peptide substrate AC- 3 (left) or the caspase 2 prodomain. (right): Calmodulin inhibitors (10 μM W13 and 10 μM W7) were tested in the same manner.
To determine if CaMKs could phosphorylate the caspase 2 prodomain, we incubated the GST-prodomain in vitro with γ-32P-ATP and either CaMKI, CaMKII, or CaMKIV. Only CaMKII could phosphorylate the recombinant caspase 2 prodomain (CaMKII could also phosphorylate the human caspase 2 prodomain), although all kinases were active against their model peptide substrates (Fig. 6A, right). More importantly, two specific peptide inhibitors of CaMKII (Ca2+/Calmodulin Kinase II Inhibitor 281–309 and autocamtide-2 related inhibitory peptide) could prevent G6P-induced phosphorylation of both a model CaMKII substrate and the caspase 2 prodomain (Fig. 6B, C, and D). These data strongly suggest that CaMKII mediates G6P-stimulated caspase 2 phosphorylation.
Abrogation of caspase 2 phosphorylation prevents metabolite-mediated survival
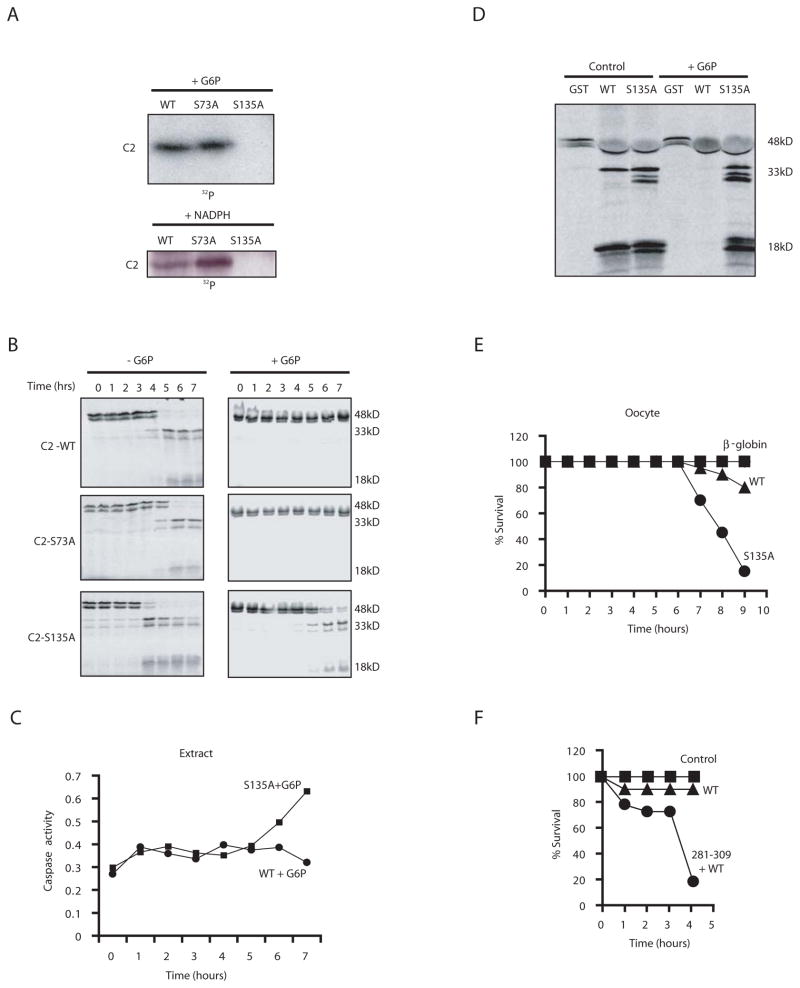
Given the involvement of CaMKII in caspase 2 phosphorylation, we scanned the caspase 2 prodomain for sequence motifs characteristic of CaMK phosphorylation sites and identified Ser 73 and Ser 135 as candidate sites; these were individually changed to Ala. When we assayed these mutants in egg extracts, we found that both G6P and NADPH-stimulated prodomain phosphorylation were abrogated by the S135A mutation, but were unaffected by Ser 73 alteration (Fig. 7A). Ser 135 mutation also prevented in vitro phosphorylation by CaMKII (Fig. 6A). To determine if this site was critical for metabolic suppression of caspase 2, we produced mRNA and in vitro-translated proteins for full-length wild type, S135A and S73A caspase 2. As shown in Fig. 7B, the S135A protein was processed even in the presence of G6P. More importantly, mutation of Ser 135 to Ala not only overrode the G6P-mediated block to spontaneous caspase 3 activation in egg extracts, but also overrode the inhibition of RAIDD-induced processing and binding to caspase 2 (Fig. 7C, D and data not shown). Strikingly, the S135A mutant also induced apoptosis in oocytes (Fig. 7E). Consistent with a role for CaMKII in suppressing caspase 2-mediated death of intact oocytes, prior injection of the CaMKII peptide inhibitor allowed efficient cell death induction by subsequent injection of the WT pro-caspase 2 mRNA (Fig. 7F). These data demonstrate that metabolic suppression of caspase 2 in eggs and oocytes is exerted through CaMKII-mediated phosphorylation of the caspase 2 prodomain.
Fig. 7. Mutation of S135 of caspase 2 to Ala abrogates the protective effects of NADPH.
A) The WT, S73A and S135A caspase 2 prodomains fused to GST were bound to glutathione sepharose and dipped into cytosolic extracts +/− G6P, +/− NADPH, and γ-32P-ATP. Sepharose-bound proteins were washed, eluted with sample buffer and resolved by SDS-PAGE. B) Extracts supplemented with WT, S135A, or S73A radiolabeled pro-caspase 2 were incubated at room temp and treated with or without G6P. Samples were taken at the indicated times and analyzed by SDS-PAGE. C) Pro-Caspase 2 S135A or WT mRNA and G6P were added to a translationally competent egg extract and samples were taken to measure cleavage of Ac-DEVD-pNA. D) GST-RAIDD was added to extracts with in vitro translated WT or S135A pro-caspase 2 +/− G6P for 1 h. Samples were taken to measure caspase 2 processing by SDS-PAGE. E) Oocytes were injected with mRNA encoding WT or S135A pro-caspase 2, or β-globin. Cell death was quantitated as in Fig. 2. Shown is a representative experiment repeated on 3 oocyte batches with similar results. F) Oocytes were injected with the 281–309 CaMKII inhibitory peptide and subsequently injected with WT pro-caspase 2-encoding mRNA and monitored as in E.
Discussion
Although it has long been appreciated that oocytes die by apoptosis, the signaling pathways governing death of these cells have not yet been fully described (Tilly, 2001). Here we have provided evidence for a molecular pathway linking metabolic processes with the survival of oocytes/eggs. The target of this pathway, caspase 2, has been previously implicated in oocyte apoptosis (Bergeron et al., 1998). We have found that stimulation of the pentose phosphate pathway, leading to NADPH generation, can restrain caspase 2 via the action of CaMKII, thereby promoting oocyte/egg survival.
Oocyte/egg metabolic pathways and apoptosis
Studies on metabolic flux in Xenopus oocytes have demonstrated that glycolysis is low in these cells and that the majority of carbon that could enter glycolytic pools is, instead, rapidly converted to glycogen (Dworkin and Dworkin-Rastl, 1989). However, this glycogen is not metabolized until after gastrulation and does not serve as an energy source to support early embryogenesis (Cohen, 1954; Jaeger, 1945). Rather, it has been proposed that amino acids derived from yolk protein breakdown can be partially oxidized to malate in mitochondria which is then oxidized to oxaloacetate and converted to phosphoenolpyruvate to generate G6P (Dworkin and Dworkin-Rastl, 1989; Kovacevic and Morris, 1972; Maller, 1985; Reitzer et al., 1979). According to this scenario, yolk proteins serve as the source of energy for the oocyte and early embryo. Our observations suggest that maintenance of G6P levels from adequate yolk protein stores can prevent death of oocytes and eggs. It is worth noting that the amount of G6P required to achieve full inhibition of apoptosis in egg extracts is quite high (i.e. greater than 2.5 mM). Consistent with previous measurements of radiolabeled G6P flux in amphibian oocytes, we believe that the vast majority of the added G6P is shunted directly into glycogen synthesis and that only a small amount is available to enter the pentose phosphate pathway (Dworkin and Dworkin-Rastl, 1989; Ureta et al., 2000). Within the animal, such levels could be maintained as long as yolk protein stores were continually feeding G6P production.
Because stimulation of pentose phosphate metabolism, malate, or NADPH could substitute for G6P to inhibit apoptosis, our data suggest that it is NADPH generation that transmits the anti-apoptotic signal. Addition of NADH or stimulation of NADH production did not have similar effects, pointing to the specificity of our results (Fig. 1A, 3A, and data not shown). Also consistent with the involvement of pentose phosphate pathway operation are our observations that inhibition of G6P dehydrogenase could accelerate caspase activation in both egg extracts and oocytes. Although the initiator of spontaneous death in the Xenopus egg system has not been previously identified, our data suggest that an active signaling system is engaged to keep these cells alive and that the default pathway, when key metabolites cannot be maintained, is cell death.
Although it is attractive to speculate that nutrient stockpiles serve as a timer for oocyte survival, changes in pentose phosphate pathway flux might also result from age- or hormonally-related alterations in enzyme activities. Indeed, age-related decreases in G6P dehydrogenase activity have been observed in mouse oocytes and it has been suggested that even if such oocytes survive, they may develop poorly post-fertilization (de Schepper et al., 1987).
Caspase 2 is a target for the anti-apoptotic action of NADPH
A previous report that caspase 2 was required for oocyte apoptosis led us to investigate caspase 2 as a potential target of metabolic regulation (Bergeron et al., 1998). In addition to our observation that caspase 2 processing was inhibited by G6P or NADPH, the fact that a caspase 2 mutant resistant to phosphorylation could override the effects of G6P in egg extracts as well as the natural resistance of intact oocytes to apoptosis, strongly suggests that caspase 2 is an important target of metabolic survival pathways.
It is not clear how caspase 2 is activated in oocytes. However, based on the ability of RAIDD-mediated activation to be regulated by NADPH and G6P, we hypothesize that an endogenous adaptor protein binding to the caspase 2 prodomain promotes activation; phosphorylation of the pro-domain could block access of the adaptor, preventing activation. Consistent with such a possibility, caspase 2 binding to GST-RAIDD was substantially reduced by G6P and NADPH addition.
CaMKII phosphorylates and inhibits caspase 2
Based on calmodulin sepharose depletion, kinase assays, and inhibitor studies we conclude that CaMKII maintains caspase 2 phosphorylation in response to pentose phosphate pathway operation. Although this phosphorylation was stimulated by G6P, we note that basal phosphorylation could be observed upon longer exposure of kinase assays. We suggest that basal phosphorylation can keep caspase 2 suppressed, and that dephosphorylation occurs prior to entry of extracts into apoptosis. Consistent with this, the GST-caspase 2 prodomain pre-phosphorylated with CaMKII loses its radioactive labeling immediately preceding caspase 3 activation in the extract (Nutt and Margolis, unpublished). We were able to maintain this phosphorylation through the simple provision of metabolites.
Although our data demonstrate that CaMKII activity is required for maintaining caspase 2 suppression in response to nutrients, it is not yet clear how NADPH levels are translated into sustained phosphorylation. It is possible that a caspase 2 -directed phosphatase is regulated to antagonize constitutive CaMKII-mediated caspase 2 phosphorylation supported by ambient calcium levels in the egg/oocyte. However, the fact that CaMKII phosphorylation of model peptides (which we might not expect to be targeted for dephosphorylation by the same phosphatases as the prodomain) was increased following G6P/NADPH treatment suggests that these metabolites may increase CaMKII activation or activity. It is possible that a phosphatase controlling CaMKII activity (through regulation of the CaMKII-activating phosphorylation at Thr 286 as observed in other settings) is regulated by NADPH (Blitzer et al., 1998). It is not clear whether pathways regulating calcium storage or utilization may also be regulated by NADPH to control CaMKII activation. In any event, the spontaneous activation of the S135A mutant caspase 2 (even in cytosol lacking mitochondria; data not shown) demonstrates that maintenance of this phosphorylation is critical to keep eggs/oocytes alive. Because processing of the S135A mutant was slightly slower in the presence of G6P (see Fig. 7B), it may be that additional modes of caspase 2 regulation (or targets other than caspase 2) contribute to G6P-mediated apoptotic suppression. However, S135 is clearly a major target of this regulatory pathway. Moreover, because there is still a lag time until apoptosis in the presence of the S135A mutant, pathways in addition to the CaMKII-caspase 2 pathway may contribute to the normal suppression of apoptosis in oocytes/eggs. Although further experiments will be required to fill in the precise regulatory details, this work has revealed key elements of a previously unknown pathway linking oocyte metabolism to caspase 2 through the action of CaMKII. This work may help to explain why oocytes are lost through apoptosis as females age, thereby providing potential therapeutic targets for the maintenance of oocyte viability and fertility.
Experimental procedures
Preparation of Xenopus oocytes and extracts
Egg extracts were prepared as in (Smythe and Newport, 1991). Stage VI oocytes were prepared from ovaries manually excised from PMSG-primed frogs, digested with 2.8 units of liberase in OR-2 buffer [82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES (pH 7.5)] for 1.5h at room temp, washed extensively with OR-2 buffer and stored in OR-2 buffer + 1% fetal bovine serum + 0.2% gentamicin at 18°C.
Caspase Assays and metabolite addition
To measure caspase activity, 3-μl extract aliquots were incubated with 90 μl DEVDase buffer (50 mM Hepes, pH 7.5, 100 mM NaCl, 0.1% CHAPS, 10 mM DTT, 1 mM EDTA, 10% glycerol) and the substrate Ac-DEVD-pNA (200 μM) at 37 °C for 30–60 min. Absorbance was measured at 405 nm. For some experiments, extracts were treated with: G6P (2.5–10mM), NADPH (5–10 mM), or other metabolites (all 10 mM). Other reagents were used at 100 μM (zVAD), 500 nM (Bcl-xL), and 25 nM (tBid).
Xenopus oocyte apoptosis assays
For pentose-phosphate pathway inhibition studies, ~ 50 Stage VI Xenopus oocytes were incubated with vehicle, 100–200 μM DHEA and/or 2.5 mM methyl-malate in OR-2 buffer at 16 °C. For caspase-2 potency studies, 40 ng of WT, S73A and S135A mutant Caspase-2 or β-globin-encoding mRNA was injected into oocytes and incubated in OR-2 buffer at 16 °C. To measure cytochrome c release, oocytes were manually lysed in 10 μl lysis buffer/oocyte (20 mM Hepes pH 7.5, 20 mM β-glycerophosphate, 15 mM MgCl2, 20 mM EGTA, 1 mM PMSF, 10 μg aprotenin/leupeptin) and spun at 14,000 g for 5 min. Samples were filtered through a 0.1 μm ultrafree-MC filter and immunoblotted for cytochrome c.
Protein Expression and Reagents
Xenopus caspase 2 was amplified by PCR using the primers 5′-CGCGGATCCATGCTGGGAGGCATGCAGCAAC-3′ and 5′-GACGGATCCTCACTTGGGGAGCCCGTTGCTTGG-3′. Purified PCR products were digested and cloned into the BglII site of pSP64T, an expression vector with flanking 5′ and 3′ β-globin UTRs. The Quikchange site-directed mutagenesis kit (Stratagene) was used to generate point mutants in caspase 2 in pSP64T. The S73A primers were 5′-GCAGAGCATT GCAGAATGCTGCAAAAGCACGTGGACCCC-3′ and its complement. The S135A primers were 5′-GCAGAGAGTACAGGGAAGAGGCTATTGATGATGGAGATGG -3′ and its complement.
To produce radiolabeled proteins, pSP64T-caspase 2, 3, and 9 templates were translated in TNT reticulocyte lysates (Promega) containing 1 μCi/μl 35S-Translabel according to manufacturer’s instructions. mRNA for microinjection was generated using the Stratagene mRNA capping kit.
The WT, S73A, and S135A caspase 2 prodomains were amplified by PCR using the pSP64T-caspase 2 clones as templates with the primers 5′-CGCGGATCCATGCTGGGAGGCATGCAGCAAC- 3′ and 5′-TGCTCTAGATCATGGACCATCTCCATCATCAATAG - 3′. PCR products were cloned into BamHI and XbaI sites of pGEXKG. pGEXKG Caspase-2 and RAIDD proteins were expressed in BL21 E.coli as in (Evans et al., 1997). GST-BCL-xL was a gift from M. Hardwick. GST-human Caspase-9 prodomain was expressed as in (Deming et al., 2004).
His-p35-encoding baculovirus was produced using the Invitrogen baculovirus expression system and proteins were purified on a nickel chelate column, eluted with 200 mM imidizole and dialyzed overnight into egg lysis buffer (250 mM sucrose, 2.5 mM MgCl, 1 mM DTT, 50 mM KCl, 10 mM Hepes, pH 7.7, ELB).
Kinase assays and depletions
1–2 μg recombinant GST-prodomain fusion proteins were incubated in egg extract with 20 μCi γ-32P-ATP, and various metabolites in the presence or absence of CaMK inhibitors for 1 h at room temp and retrieved on glutathione sepharose. Similar extracts were treated with caspase 2 antibody bound to protein-A sepharose to precipitate endogenous phosphorylated caspase 2. Samples were washed, eluted with SDS-PAGE sample buffer, and resolved by SDS-PAGE for autoradiography and quantitation by phosphorimager.
For in vitro phosphorylation, recombinant CaM kinases and 1–2 μg GST-prodomains were incubated in kinase buffer (25 mM Hepes, pH 7.5, .5 mM dithiothreitol, 10 mM MgCl2, 200 μM ATP, 20 μCi/reaction γ-32P-ATP, 1 μM A. nidulans CaM, 1 mM CaCl2, 0.1% Tween 20) for 1 h at room temp. Reactions were stopped with SDS-PAGE sample buffer, and resolved by SDS-PAGE for quantitation by phosphorimager.
CaM kinase activity was tested in 50-μl kinase buffer (as above) for 1 h at room temp. CaMKI and CaMKIV were assayed using 200 μM ADR1G (LKKLTRRASFSGQ) as a substrate; 60 μM syntide 2 (PLARTLSVAGLPGKK) was used for CaMKII. To measure CaMKII activity in the egg extract 60 μM Syntide-2 and 50 μM autocamtide was added to extract in the presence or absence of CaMK inhibitors with 20 μCi/reaction γ-32P-ATP. Reactions were terminated by spotting 20 μl onto p81 phosphocellulose filters followed by washing in 75 mM phosphoric acid. Dried filters were scintillation counted.
Egg extracts were depleted of CaM kinase by three 30 min rounds of incubation with calmodulin sepharose or control protein-A sepharose at 4°C.
RAIDD Assays
GST or GST-RAIDD proteins linked to glutathione-sepharose were added to 100 μl egg extract supplemented with energy mix, various metabolites or drugs and 35S-labeled WT or S135A caspase 2. Samples were incubated at room temp for 1 h. GST or GST-RAIDD beads were pelleted and washed three times with ELB supplemented with 300 mM NaCl and 0.1% Tween-20. Samples were eluted with sample buffer and resolved by SDS-PAGE for autoradiography.
Acknowledgments
We thank A.R. Means, C. Newgard, J. Chandra, F. Chow, and T. Ribar for helpful discussions. We thank D. Ribar for excellent technical assistance, D. Vandré and D. Weitzel for UCN-01, and V. Dixit for RAIDD. This work was supported by NIH RO1s GM 56518 and CA 102727 to SK. LKN was supported by an individual NRSA. JCR is the recipient of a Howard Temin Career Development award, a Sidney Kimmel Foundation Scholar award, and a V Foundation Scholarship. SSM is a recipient of the George H. Hitchings Fund for Health Research and Science Education of the Triangle Community Foundation.
References
- Bagowski CP, Xiong W, Ferrell JE., Jr c-Jun N-terminal kinase activation in Xenopus laevis eggs and embryos. A possible non-genomic role for the JNK signaling pathway. J Biol Chem. 2001;276:1459–1465. doi: 10.1074/jbc.M008050200. [DOI] [PubMed] [Google Scholar]
- Bergeron L, Perez GI, Macdonald G, Shi L, Sun Y, Jurisicova A, Varmuza S, Latham KE, Flaws JA, Salter JC, et al. Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev. 1998;12:1304–1314. doi: 10.1101/gad.12.9.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuyan AK, Varshney A, Mathew MK. Resting membrane potential as a marker of apoptosis: studies on Xenopus oocytes microinjected with cytochrome c. Cell Death Differ. 2001;8:63–69. doi: 10.1038/sj.cdd.4400773. [DOI] [PubMed] [Google Scholar]
- Blitzer RD, Connor JH, Brown GP, Wong T, Shenolikar S, Iyengar R, Landau EM. Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science. 1998;280:1940–1942. doi: 10.1126/science.280.5371.1940. [DOI] [PubMed] [Google Scholar]
- Braun T, Dar S, Vorobiov D, Lindenboim L, Dascal N, Stein R. Expression of Bcl-x(S) in Xenopus oocytes induces BH3-dependent and caspase-dependent cytochrome c release and apoptosis. Mol Cancer Res. 2003;1:186–194. [PubMed] [Google Scholar]
- Cohen AI. Studies on glycolysis during the early development of the Rana pipiens embryo. Physiol Zool. 1954;27:128–141. [Google Scholar]
- Comin-Anduix B, Boros LG, Marin S, Boren J, Callol-Massot C, Centelles JJ, Torres JL, Agell N, Bassilian S, Cascante M. Fermented wheat germ extract inhibits glycolysis/pentose cycle enzymes and induces apoptosis through poly(ADP-ribose) polymerase activation in Jurkat T-cell leukemia tumor cells. J Biol Chem. 2002;277:46408–46414. doi: 10.1074/jbc.M206150200. [DOI] [PubMed] [Google Scholar]
- Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- Demirci B, Salle B, Frappart L, Franck M, Guerin JF, Lornage J. Morphological alterations and DNA fragmentation in oocytes from primordial and primary follicles after freezing-thawing of ovarian cortex in sheep. Fertil Steril. 2002;77:595–600. doi: 10.1016/s0015-0282(01)03205-8. [DOI] [PubMed] [Google Scholar]
- de Schepper GG, van Noorden CJ, Houtkooper JM. Age-related changes of glucose-6-phosphate dehydrogenase activity in mouse oocytes. Histochem J. 1987;19:467–470. doi: 10.1007/BF01675415. [DOI] [PubMed] [Google Scholar]
- Deckwerth TL, Johnson EM., Jr Temporal analysis of events associated with programmed cell death (apoptosis) of sympathetic neurons deprived of nerve growth factor. J Cell Biol. 1993;123:1207–1222. doi: 10.1083/jcb.123.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deming PB, Schafer ZT, Tashker JS, Potts MB, Deshmukh M, Kornbluth S. Bcr-Abl-mediated protection from apoptosis downstream of mitochondrial cytochrome c release. Mol Cell Biol. 2004;24:10289–10299. doi: 10.1128/MCB.24.23.10289-10299.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh M, Vasilakos J, Deckwerth TL, Lampe PA, Shivers BD, Johnson EM., Jr Genetic and metabolic status of NGF-deprived sympathetic neurons saved by an inhibitor of ICE family proteases. J Cell Biol. 1996;135:1341–1354. doi: 10.1083/jcb.135.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Dixit VM. RAIDD is a new ‘death’ adaptor molecule. Nature. 1997;385:86–89. doi: 10.1038/385086a0. [DOI] [PubMed] [Google Scholar]
- Dworkin MB, Dworkin-Rastl E. Metabolic regulation during early frog development: flow of glycolytic carbon into phospholipids in Xenopus oocytes and fertilized eggs. Dev Biol. 1989;132:524–528. doi: 10.1016/0012-1606(89)90247-9. [DOI] [PubMed] [Google Scholar]
- Evans EK, Kuwana T, Strum SL, Smith JJ, Newmeyer DD, Kornbluth S. Reaper-induced apoptosis in a vertebrate system. Embo J. 1997;16:7372–7381. doi: 10.1093/emboj/16.24.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- Graves PR, Yu L, Schwarz JK, Gales J, Sausville EA, O’Connor PM, Piwnica-Worms H. The Chk1 protein kinase and the Cdc25C regulatory pathways are targets of the anticancer agent UCN-01. J Biol Chem. 2000;275:5600–5605. doi: 10.1074/jbc.275.8.5600. [DOI] [PubMed] [Google Scholar]
- Guo Y, Srinivasula SM, Druilhe A, Fernandes-Alnemri T, Alnemri ES. Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria. J Biol Chem. 2002;277:13430–13437. doi: 10.1074/jbc.M108029200. [DOI] [PubMed] [Google Scholar]
- Hutchins JR, Dikovskaya D, Clarke PR. Regulation of Cdc2/cyclin B activation in Xenopus egg extracts via inhibitory phosphorylation of Cdc25C phosphatase by Ca(2+)/calmodulin-dependent protein [corrected] kinase II. Mol Biol Cell. 2003;14:4003–4014. doi: 10.1091/mbc.E03-02-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger L. Glycogen utilization by the amphibian gastrulain relation to invagination and induction. J Cell Comp Physiol. 1945;25:97–120. [Google Scholar]
- Kim MR, Tilly JL. Current concepts in Bcl-2 family member regulation of female germ cell development and survival. Biochim Biophys Acta. 2004;1644:205–210. doi: 10.1016/j.bbamcr.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Kovacevic Z, Morris HP. The role of glutamine in the oxidative metabolism of malignant cells. Cancer Res. 1972;32:326–333. [PubMed] [Google Scholar]
- Lassus P, Opitz-Araya X, Lazebnik Y. Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science. 2002;297:1352–1354. doi: 10.1126/science.1074721. [DOI] [PubMed] [Google Scholar]
- Majewski N, Nogueira V, Bhaskar P, Coy PE, Skeen JE, Gottlob K, Chandel NS, Thompson CB, Robey RB, Hay N. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Maller JL. Regulation of amphibian oocyte maturation. Cell Differ. 1985;16:211–221. doi: 10.1016/0045-6039(85)90570-6. [DOI] [PubMed] [Google Scholar]
- Morita Y, Perez GI, Maravei DV, Tilly KI, Tilly JL. Targeted expression of Bcl-2 in mouse oocytes inhibits ovarian follicle atresia and prevents spontaneous and chemotherapy-induced oocyte apoptosis in vitro. Mol Endocrinol. 1999;13:841–850. doi: 10.1210/mend.13.6.0306. [DOI] [PubMed] [Google Scholar]
- Morita Y, Perez GI, Paris F, Miranda SR, Ehleiter D, Haimovitz-Friedman A, Fuks Z, Xie Z, Reed JC, Schuchman EH, et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000;6:1109–1114. doi: 10.1038/80442. [DOI] [PubMed] [Google Scholar]
- Morita Y, Tilly JL. Oocyte apoptosis: like sand through an hourglass. Dev Biol. 1999;213:1–17. doi: 10.1006/dbio.1999.9344. [DOI] [PubMed] [Google Scholar]
- Newmeyer DD, Farschon DM, Reed JC. Cell-free apoptosis in Xenopus egg extracts: inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria. Cell. 1994;79:353–364. doi: 10.1016/0092-8674(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Perez GI, Knudson CM, Leykin L, Korsmeyer SJ, Tilly JL. Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat Med. 1997;3:1228–1232. doi: 10.1038/nm1197-1228. [DOI] [PubMed] [Google Scholar]
- Perez GI, Robles R, Knudson CM, Flaws JA, Korsmeyer SJ, Tilly JL. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet. 1999;21:200–203. doi: 10.1038/5985. [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol. 2003;23:7315–7328. doi: 10.1128/MCB.23.20.7315-7328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, Thompson CB. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol Cell. 2000;6:683–692. doi: 10.1016/s1097-2765(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Ratts VS, Flaws JA, Kolp R, Sorenson CM, Tilly JL. Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology. 1995;136:3665–3668. doi: 10.1210/endo.136.8.7628407. [DOI] [PubMed] [Google Scholar]
- Read SH, Baliga BC, Ekert PG, Vaux DL, Kumar S. A novel Apaf-1-independent putative caspase-2 activation complex. J Cell Biol. 2002;159:739–745. doi: 10.1083/jcb.200209004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzer LJ, Wice BM, Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979;254:2669–2676. [PubMed] [Google Scholar]
- Robertson JD, Enoksson M, Suomela M, Zhivotovsky B, Orrenius S. Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptosis. J Biol Chem. 2002;277:29803–29809. doi: 10.1074/jbc.M204185200. [DOI] [PubMed] [Google Scholar]
- Schwartz AG, Pashko LL. Dehydroepiandrosterone, glucose-6-phosphate dehydrogenase, and longevity. Ageing Res Rev. 2004;3:171–187. doi: 10.1016/j.arr.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Smythe C, Newport JW. Systems for the study of nuclear assembly, DNA replication, and nuclear breakdown in Xenopus laevis egg extracts. Methods Cell Biol. 1991;35:449–468. doi: 10.1016/s0091-679x(08)60583-x. [DOI] [PubMed] [Google Scholar]
- Tian WN, Braunstein LD, Apse K, Pang J, Rose M, Tian X, Stanton RC. Importance of glucose-6-phosphate dehydrogenase activity in cell death. Am J Physiol. 1999;276:C1121–1131. doi: 10.1152/ajpcell.1999.276.5.C1121. [DOI] [PubMed] [Google Scholar]
- Tilly JL. Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol. 2001;2:838–848. doi: 10.1038/35099086. [DOI] [PubMed] [Google Scholar]
- Tinel A, Tschopp J. The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science. 2004;304:843–846. doi: 10.1126/science.1095432. [DOI] [PubMed] [Google Scholar]
- Tuttle S, Stamato T, Perez ML, Biaglow J. Glucose-6-phosphate dehydrogenase and the oxidative pentose phosphate cycle protect cells against apoptosis induced by low doses of ionizing radiation. Radiat Res. 2000;153:781–787. doi: 10.1667/0033-7587(2000)153[0781:gpdato]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ureta T, Fernandez WY, Centelles JJ, Cascante M. In vivo measurements of control coefficients for hexokinase and glucose-6-phosphate dehydrogenase in Xenopus laevis oocytes. FEBS Lett. 2000;475:145–149. doi: 10.1016/s0014-5793(00)01646-x. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol. 2001;21:5899–5912. doi: 10.1128/MCB.21.17.5899-5912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronina E, Wessel GM. Apoptosis in sea urchin oocytes, eggs, and early embryos. Mol Reprod Dev. 2001;60:553–561. doi: 10.1002/mrd.1120. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Shirayoshi Y, Koshimizu U, Hashimoto S, Yonehara S, Eguchi Y, Tsujimoto Y, Nakatsuji N. Gene transfection of mouse primordial germ cells in vitro and analysis of their survival and growth control. Exp Cell Res. 1997;230:76–83. doi: 10.1006/excr.1996.3366. [DOI] [PubMed] [Google Scholar]