Abstract
Phagocytosis prevents the release of potentially harmful or immunogenic materials from dying cells. Milk fat globule EGF-factor VIII (MFG-E8) mediates the clearance of apoptotic cells. We have previously shown that the administration of MFG-E8-rich exosomes from immature dendritic cells promotes the phagocytosis of apoptotic cells and improves survival in sepsis. Since endotoxin is elevated in polymicrobial sepsis, we hypothesized that downregulation of MFG-E8 is mediated via the LPS-CD14 pathway, eventually leading to the accruement of apoptotic cells. Polymicrobial sepsis was induced by cecal ligation and puncture (CLP) in CD14-deficient (CD14−/−), TLR4-mutated and wild-type (WT) mice. In addition, endotoxemia was elicited by intraperitoneal injection of LPS. LPS was also neutralized by pre-treating CLP-induced WT mice with polymyxin B. Splenic MFG-E8 expression, phagocytic activity and apoptosis were assessed 5 h and 20 h after CLP or 5 h after LPS administration. In septic WT mice, MFG-E8 mRNA and protein levels were suppressed by 49% and 33%, respectively. Endotoxemia reduced MFG-E8 mRNA expression in a dose dependent manner and the downregulation of MFG-E8 mRNA expression in CLP-induced sepsis was attenuated by polymyxin B. This CLP-induced suppression was not observed in both CD14−/− and TLR4-mutated mice. CLP significantly decreased phagocytic activity of peritoneal macrophages in WT (by 30%), but not in CD14−/− mice. CLP also induced significant apoptosis in the spleen of WT (by 61%), but less in CD14−/− mice. Thus, MFG-E8 production is downregulated in sepsis by LPS-CD14 dependent fashion, leading to a reduction of phagocytosis of apoptotic cells.
Keywords: Monocytes/Macrophages, Spleen, Lipopolysaccharide, Phagocytosis, and Apoptosis
Introduction
Sepsis, defined as systemic inflammatory response syndrome (SIRS), which is caused by infection (1), is the most common case of mortality in the intensive care units (2). Sepsis triggers the secretion of proinflammatory cytokines (3), activation of leukocytes (4), and collapse of coagulation and fibrinolysis (5). Sepsis also leads to an immunocompromised state with decreasing B and CD4+ T cells due to apoptosis (6), and increasing anti-inflammatory cytokines (7). Recently, Hotchkiss et al. have shown that pretreatment of animals with apoptotic splenocytes worsens the outcome of sepsis (8) and that overexpression of Bcl-2 or administration of caspase inhibitors protects lymphocyte apoptosis and improves survival in polymicrobial sepsis (9). Hence, immediate removal of apoptotic cells is required for producing beneficial effects in sepsis (10, 11).
Milk fat globule epidermal growth factor-factor VIII (MFG-E8) is a membrane-associated glycoprotein and is identical to lactadherin in humans (12). It is a major constituent of milk fat globules and is considered to be a marker of breast cancer (12). It is detectable in various tissues and has also been identified in macrophages (13) and dendritic cells (14). MFG-E8 has been shown to bridge apoptotic cells and phagocytes by binding to phosphatidylserine (PS) exposed on apoptotic cells via its C-terminal coagulation factor VIII homologous domain (15), and to αvβ3 integrin on phagocytes via its N-terminal epidermal growth factor (EGF)-like domain (16). MFG-E8 deficiency causes autoimmune diseases through impaired uptake of apoptotic B cells (16). We have recently shown that administration of immature dendritic cell-derived exosomes, which are abundant in MFG-E8, increases phagocytic activity and improves survival in septic rats, and that lipopolysaccharide (LPS) downregulates MFG-E8 expression in macrophages in vitro (17). However, the mechanism responsible for MFG-E8 downregulation in sepsis remains unclear. LPS plays an important role in CLP-induced polymicrobial sepsis and LPS signaling is mainly CD14 dependent (18). We therefore hypothesized that bacterial LPS directly inhibits MFG-E8 production via the LPS-CD14 pathway and therefore impairs phagocytosis of apoptotic cells.
Materials and Methods
Cecal ligation and puncture
Male weight-matched (20–25g) wild-type (C3H/HeN, Taconic, Albany, NY), CD14-deficient mice (C3H/HeN), and TLR4-mutated mice (C3H/HeJ, Jackson Laboratories, Bar Harbor, ME) were housed in a temperature-controlled room on a 12-h light/dark cycle and fed purina chow diet. Before the induction of sepsis, the mice were fasted overnight but allowed water ad libitum. The mice were anesthetized by isoflurane inhalation, and the abdomen was shaved and washed with 10% povidone iodine. The animals were randomly assigned to various groups, and the cecum was ligated and double-punctured with a 22-gauge needle. Sham operated animals underwent the same procedure with the exception that the cecum was neither ligated nor punctured. The animals were resuscitated with 1 ml/25 g body weight (BW) isotonic sodium chloride solution (subcutaneous injection) immediately after surgery. The animals were anesthetized at 5 h and 20 h after cecal ligation and puncture (CLP) or sham operation (Sham) for the collection of tissue samples. All experiments were performed in accordance with the National Institutes of Health’s Guidelines for Use of Experimental Animals. This project was approved by the Institutional Animal Care and Use Committee of The Feinstein Institute for Medical Research.
Administration of endotoxin in mice
Endotoxemia was induced by intraperitoneal injection of 15 or 45 mg/kg body weight (BW) LPS (E. coli 055:B5; Difco Laboratories) dissolved in 1 ml of saline. 1 ml of normal saline (0 mg/kg BW) was injected to the control group. Samples were collected at 5 h after LPS injection.
Administration of polymyxin B
At 1 h before CLP, as well as at 10 h after CLP, polymyxin B (Sigma, St Louis, MO), at a dose of 2000 U/kg BW, or 0.2 ml of normal saline was administered intramuscularly. Polymyxin B is an antibiotic, which binds and detoxifies lipid A. In vitro studies have confirmed that polymyxin B neutralizes LPS activity (19). Our previous study has shown that administration of polymyxin B markedly decreased plasma levels of LPS after CLP in vivo (20).
Determination of splenic MFG-E8 gene expression by quantitative PCR
Quantitative PCR was performed to detect splenic MFG-E8 mRNA expression levels in mice. Total RNA was prepared using TRIzol reagent (Invitrogen, Carlsbad, CA). 25 mg of tissue was homogenized in 1 ml TRIzol and the homogenate was separated into aqueous and organic phases. The aqueous liquid was isolated and followed by chloroform addition and centrifugation. RNA was precipitated from the aqueous phase by addition of isopropanol, and washed with ethanol. The pellet was dissolved in 0.1% DEPC-treated, deionized, and distilled water. RNA concentration and purity were determined by measuring the absorbance at 260 and 280 nm. Reverse transcription was performed on total RNA (4 μg) using Oligo(dT)12–18 primer (Invitrogen, Carlsbad, CA). The resulting samples from individual mice were diluted and divided into aliquots for separate PCRs for MFG-E8 and the housekeeping gene β-actin. Mouse MFG-E8 primers were forward (5′-3′)-CGC ACA GGG ATC GTC AAT G and reverse (5′-3′)-CGC AGA AGG TTC ACC TGG AT. β-actin primer sequences were forward (5′-3′)-TGT TAC CAA CTG GGA CGA CA and reverse (5′-3′)-GGG GTG TTG AAG GTC TCA AA. Quantitative PCR was performed by 7300 Real Time PCR system (Applied Biosystems, Foster City, CA) with SYBR Green as detection dye. The reaction was carried in a 25 μl final reaction volume containing 0.2 μM concentration of each forward and reverse primer, 2.5 μl cDNA, 7.5 μl H2O and 12.5 μl SYBR Green PCR Mast Mix (Applied Biosystems, Foster City, CA). The thermal profile for the real-time PCR was 50°C for 2 min, 95°C for 10 min and followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 min. Relative quantification was analyzed according to ΔΔCt method (User Bulletin #2: Relative Quantitation of Gene Expression, Applied Biosystems). In addition, dissociative curve analysis was performed to confirm the specificity of PCR product in this experiment.
Determination of splenic MFG-E8 protein level by Western blotting
Tissue samples (25 mg) were lysed and homogenized in 300 μl of lysis buffer (10 mmol/L Tris-buffered saline, 1 mmol/L EDTA, 1 mmol/L EGTA, 2 mmol/L sodium orthovanadate, 0.2 mmol/L phenylmethanesulfonyl fluoride, 2 μg/mL leupepcin, 2 μg/mL Aprotinin, and 1 % Triton X-100) for 30 min on ice, and tissue sample lysate was cleared by centrifugation at 14000 rpm for 15 min at 4°C. Samples were dissolved in 1% sodium dodecyl sulfate and quantified using the DC protein assay (Bio-Rad, Hercules, CA). The protein (15 μg) was fractionated on a 4% to 12% Bis-Tris gel and transferred to a 0.2-μm nitrocellulose membrane. Nitrocellulose membranes were blocked by incubation in TBST (10 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, and 0.1% Tween 20) containing 5% BSA for 1 h. Membranes were incubated with goat anti-mouse MFG-E8 antibody (AF2805, R&D systems, Minneapolis, MN) overnight at 4°C, washed three times in TBST for 15 min and incubated subsequently with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Specific proteins were visualized using an ECL system (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK). Band densities were determined using a Bio-Rad image system. Bands corresponded to splenic MFG-E8 protein were normalized by splenic β-actin expression.
Ex vivo phagocytosis assay
This novel phagocytosis assay was performed following the method as recently developed in our laboratory. In brief, freshly collected cells by peritoneal lavage with cold HBSS from Sham and 20 h CLP animals were washed twice with PBS and red blood cells were lysed with ammonia-chloride potassium (ACK)-buffer, and then, cells were plated at a density of 1×106 cells in 6 well plate and cultured in DMEM containing 10% heat-inactivated FBS, 10 mmol/L HEPES, 100 U/mL penicillin and 100 mg/mL streptomycin for 2 h at 37°C in a humidified atmosphere containing 5% CO2.
Autologous thymocytes were cultured in Roswell Park Memorial Institute (RPMI) medium substituted with 10% heat-inactivated FBS, 10 mmol/L HEPES, 100 U/mL penicillin and 100 mg/mL streptomycin and treated with 10 μM dexamethasone for 24 hrs (> 99% apoptotic CD 90+ cells assessed by annexin V) at 37°C in a humidified atmosphere containing 5% CO2. After washing with PBS twice, apoptotic thymocytes were stained with 20 ng/ml pHrodo™ succinimidyl ester (SE) (Invitrogen, Eugene, OR) for 30 min at RT, which is a pH-sensitive fluorescent dye that emits light in the red range (approximate fluorescence excitation and emission maxima, 560/585nm) at an increased intensity with decreasing environmental pH. After washing with PBS, cells were used as targets for cultured macrophages at a ratio of 4:1 (apoptotic cells/peritoneal cells) in DMEM containing 1% FBS for 1 h. After co-incubating, cells were washed with PBS twice thoroughly to remove unengulfed thymocytes, and collected by gentle scraping and stained with both allophycocyanin (APC)-labeled anti-CD11b antibody and Alexa Fluor® 700-labeled anti-GR-1 antibody (BD Pharmingen, San Jose, CA). Analysis was performed by flow cytometry (LSRII, BD Biosciences, San Jose, CA).
Detection of apoptotic cells
Fresh spleens from sham-operated and septic animals were collected and whole splenocytes were obtained by gentle grinding of spleens between frosted glass slides, lysing red blood cells with ACK-buffer, passing cells through a mesh. Collected splenocytes were washed twice in PBS, counted, and reconstituted in Ca2+-rich annexin V binding buffer (BD Pharmingen, San Diego, CA) at a concentration of 107 cells per milliliter. Cell suspension (100 μl) was stained with 2.5 μl annexin V-FITC and/or 1μl propidium iodine (PI) for 15 min and adjusted to a total volume of 500 μl with binding buffer. Cells were then analyzed by flow cytometry with FACSCalibur (BD Biosciences, San Jose, CA), and annexin V+-PI− cells were considered as apoptotic cells.
Statistical analysis
All data are expressed as means ± SEM and compared by one-way ANOVA and Student-Newman-Keuls test, or Student’s t-test. Differences in value were considered significant if P < 0.05.
Results
Suppression of MFG-E8 in the spleen in late phase of sepsis
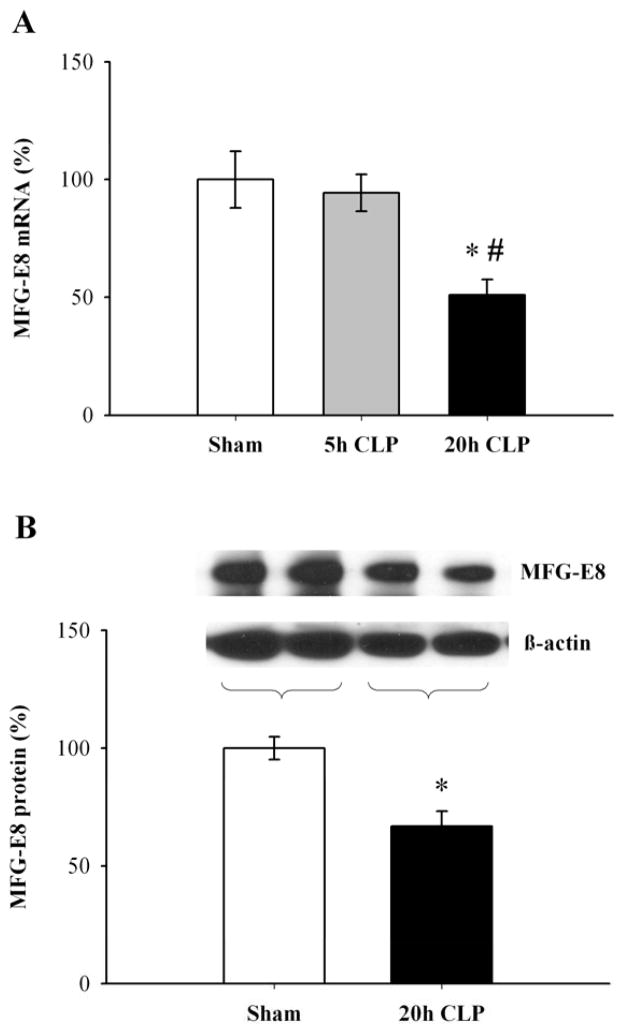
As shown in Figure 1A, MFG-E8 mRNA expression in the spleen did not decrease at 5 h after CLP (5h CLP), but significantly decreased by 49% at 20 h after CLP (20h CLP) as compared to sham-operated animals, and by 46% as compared to 5h CLP in wild type (WT) mice (P < 0.05). Splenic MFG-E8 protein levels also significantly decreased by 33% at 20 h after CLP as compared to sham-operated animals (P < 0.05, Fig. 1B), similar to what we have previously shown in rats (17).
Figure 1.
Suppression of MFG-E8 production in the spleen in septic WT mice. Splenic MFG-E8 mRNA expression (A, percentage of Sham), and splenic MFG-E8 protein level (B, percentage of Sham) in WT mice were assessed by quantitative PCR at 5 h and 20 h after cecal ligation and puncture (5h CLP and 20h CLP) or sham operation (Sham), or Western blotting at 20 h CLP or Sham. Data are expressed as means ± SEM (n = 4–6/group) and compared by one-way ANOVA and Student-Newman-Keuls test or by Student’s t-test: * P < 0.05 versus sham-operated animals, # P < 0.05 versus 5h CLP animals.
Effect of LPS on MFG-E8 expression in the spleen
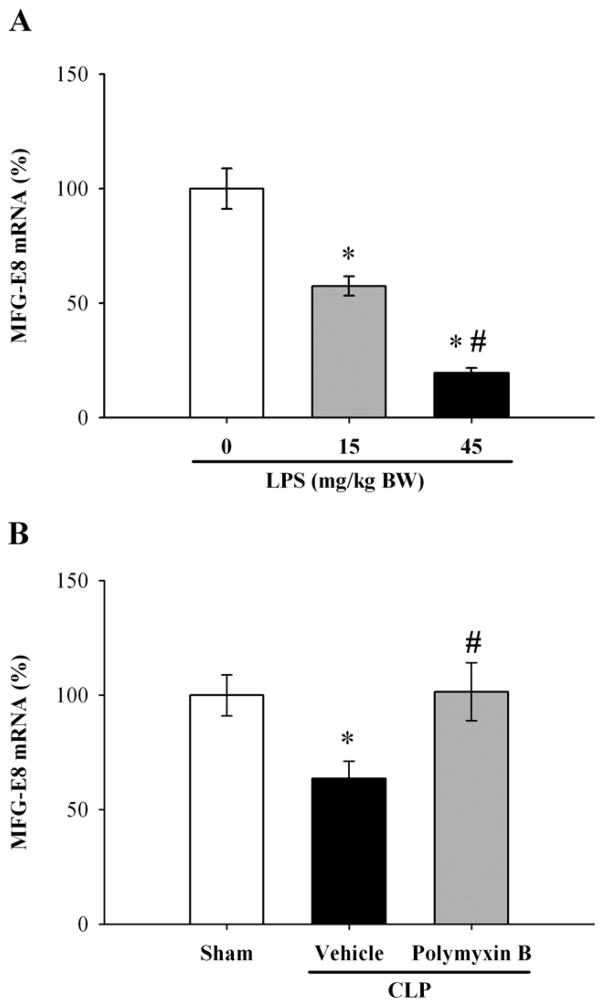
We have already reported that LPS downregulates MFG-E8 production from cultured RAW 264.7 cells (macrophage cell line) in vitro (17). To elucidate whether LPS is responsible for MFG-E8 downregulation in vivo, we used an animal model of endotoxemia produced by LPS injection. Our result showed that mRNA expression of MFG-E8 significantly decreased, in a dose dependent manner, by 43% at a dose of 15 mg/kg BW and by 80% at that of 45 mg/kg BW at 5 h after LPS injection (P < 0.05, Fig. 2A). To further confirm the downregulatory effect of LPS on MFG-E8 expression in sepsis, polymyxin B was administered intramuscularly to septic mice to inhibit LPS activity. With administration of polymyxin B, downregulation of MFG-E8 gene expression in the spleen was attenuated as compared with vehicle-treated septic animals (P < 0.05, Fig. 2B).
Figure 2.
Effect of LPS on MFG-E8 mRNA expression in the spleen. MFG-E8 mRNA expression in the spleen was assessed by quantitative PCR at 5 h after administration of 0 (normal saline), 15, and 45 mg/kg BW of LPS (A, percentage of control). In the LPS-inhibition study, alteration in MFG-E8 mRNA expression in the spleen was assessed by quantitative PCR at 20 h after CLP with normal saline (Vehicle) or polymyxin B (polymyxin B) treatment (B, percentage of control). Data are expressed as means ± SEM (n = 6/group) and compared by one-way ANOVA and Student-Newman-Keuls test: * P < 0.05 versus 0 mg/kg BW LPS, # P < 0.05 versus 15 mg/kg BW LPS in Fig. 2A; * P < 0.05 versus sham-operated animals, # P < 0.05 versus CLP + Vehicle in Fig. 2B.
Lack of MFG-E8 suppression in septic CD14 deficient mice and in septic TLR4 mutated mice
To further verify that LPS is responsible for the MFG-E8 suppression in polymicrobial sepsis, CD14-deficient (CD14−/−) mice were subjected to CLP. CD14 is an LPS receptor (21) and CD14−/− mice are resistant to LPS (18). As seen in Figs. 3A and B, both mRNA expression and protein levels of MFG-E8 in the spleen did not significantly change in sepsis. Commercial LPS can stimulate not only TLR4, but also TLR2 (22). TLR4-mutated mice (C3H/HeJ) mice were also subjected to CLP. As seen in Figs. 3C and D, both mRNA expression and protein levels of MFG-E8 in the spleen did not significantly change in sepsis. These results indicate that the LPS plays an important role in the MFG-E8 suppression in sepsis.
Figure 3.
Lack of MFG-E8 suppression in the spleen in septic (CLP) CD14−/− and septic (CLP) TLR4 mutated mice. Splenic MFG-E8 mRNA expression (A, percentage of Sham), and splenic MFG-E8 protein level (B, percentage of Sham) in CD14−/− mice were assessed by quantitative PCR or Western blotting at 20 h after CLP or Sham. Splenic MFG-E8 mRNA expression (C, percentage of Sham), and splenic MFG-E8 protein level (D, percentage of Sham) in TLR4 mutated mice were assessed by quantitative PCR or Western blotting at 20 h after CLP or Sham. Data are expressed as means ± SEM (n = 4–6/group in A and B, n = 6/group in C and D) and compared by Student’s t-test. No statistical differences were found.
Effect of sepsis on phagocytic activity of peritoneal macrophages against apoptotic cells
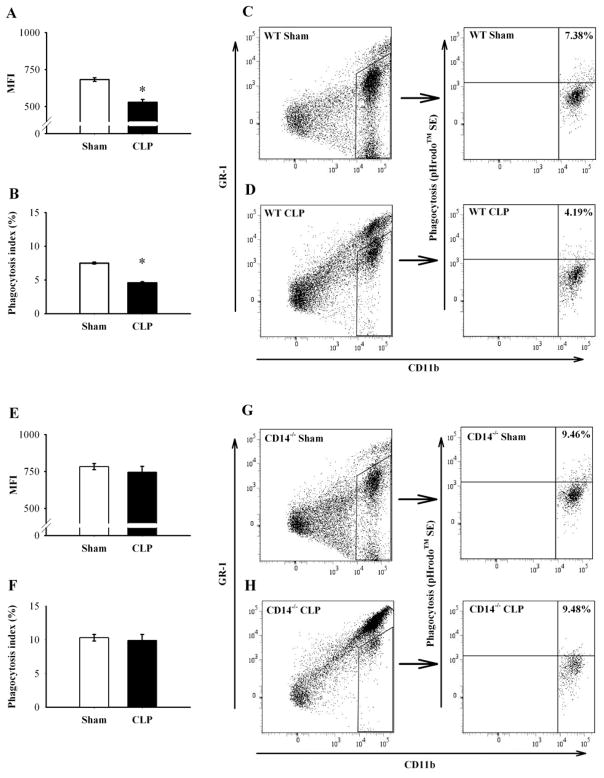
MFG-E8 is necessary for the phagocytosis of apoptotic cells (16), and its activity is impaired in sepsis (17). We collected peritoneal cells from both septic WT and CD14−/− mice. Autologous apoptotic thymocytes stained with pHrodo™ SE, and peritoneal cells tagged by CD11b and GR-1 were used to determine the effect of sepsis on phagocytic activity ex vivo. Macrophages are identified by the combination of CD11b and GR-1 expression. CD11bhigh and GR-1int-low cells were considered as macrophages and thus, 60 analyzed for phagocytic activity. CD11bhigh and GR-1high cells were considered as granulocytes (neutrophils) (23) and excluded from the analysis. Mean fluorescence intensity (MFI) and phagocytic activity of peritoneal macrophages (phagocytosis index) were significantly decreased in septic WT mice (683.4 ± 12.6 to 528.3 ± 19.4 in MFI, Fig. 4A and 7.50 ± 0.15% to 4.59 ± 0.15% in phagocytosis index, P < 0.05, Figs. 4B–D), but was not altered in septic CD14−/− mice compared with Sham (783.1 ± 20.1 to 745.7 ± 38.4 in MFI, Fig. 4E and 10.32 ± 0.49% to 9.91 ± 0.90% in phagocytosis index, Fig. 4F–H).
Figure 4.
Effect of sepsis on phagocytic activity of peritoneal macrophages against apoptotic cells. Peritoneal cells were collected by peritoneal lavage from septic (CLP) or non-septic (Sham) WT or CD14−/− mice, cultured with pHrodo-stained apoptotic thymocytes for 1 h, and analyzed by flow cytometry. Mean fluorescence intensity (MFI) and phagocytic activity of peritoneal macrophages (phagocytosis index) were assessed in WT (MFI in A, phagocytosis index in B), or CD14−/− (MFI in E, phagocytosis index in F). Representative flow cytometric analysis of CD11b and GR-1 (WT in C left, CD14−/− in H left panels), and phagocytosis assay in the gated area by CD11b and pHrodo™ SE (WT in C right, CD14−/− in H right panels) are shown. Data are expressed as means ± SEM (n = 4/group) and compared by Student’s t-test: * P < 0.05 versus sham-operated animals.
Effect of sepsis on the clearance of apoptotic cells in the spleen
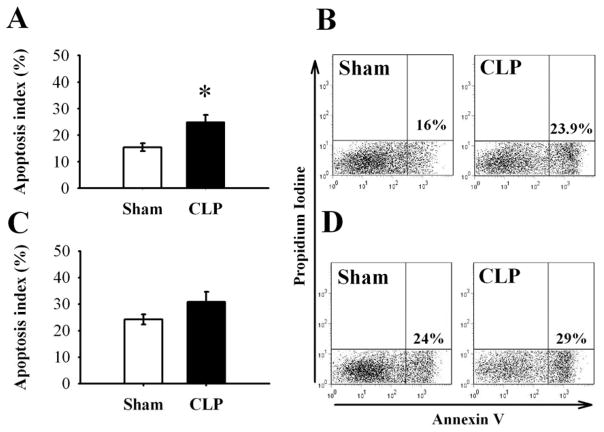
We have previously shown that MFG-E8 by itself is not anti-apoptotic, but increases the clearance of apoptotic cells by enhancing phagocytosis (17). This results in a reduction of apoptotic cells, and confers beneficial effects in sepsis. Our current results indicate that splenic apoptotic cells were significantly increased by 61% in septic WT mice (15.4 ± 1.5% in Sham, 24.9 ± 2.8% in CLP, P < 0.05, Fig. 5A, B), but increased only slightly in septic CD14−/− mice (24.2 ± 1.9% in Sham, 30.8 ± 3.8% in CLP, Fig. 5C, D). This indicates a preserved clearance of apoptotic cells in the spleen of septic CD14−/− mice via normal MFG-E8 levels.
Figure 5.
Effect of sepsis on the clearance of apoptotic cells in the spleen in WT and CD14−/− mice. Freshly collected splenocytes were stained with annexin V and propidium iodine (PI). Annexin V+-PI− cells were considered apoptotic cells and analyzed by flow cytometry in WT (A, B, percentage of cells) or CD14−/− (C, D, percentage of cells). Representative data are shown in B and D. Data are expressed as means ± SEM (n = 5/group) and compared by Student’s t-test in Fig 5A, C: * P < 0.05 versus sham-operated animals.
Discussion
Apoptosis is one of the biologically necessary functions for homeostasis (24). However in sepsis, excessive apoptosis exists which induces both immunosuppression (6, 7) and proinflammatory cytokine upregulations (25). Thus, immediate elimination of apoptotic cells is required to avoid further tissue injury (10, 11). To reduce harmful apoptotic cells, many studies focus on the suppression of apoptosis, for example, by overexpressing Bcl-2, administering caspase inhibitors (9), or blocking complement factor C5a (26). However, promotion of the removal of apoptotic cells by phagocytes before the secondary necrotic cell development, which leads to the release of a variety of proinflammatory cytokines, is critical to reduce apoptotic cells.
Recently, Hanayama et al. have shown that MFG-E8 is one of the bridging molecules between apoptotic cells and phagocytes (13). This 64 kDa molecule has a unique structure, which is composed of two EGF-like domains (EGF-1 and EGF-2) containing the RGD motif (the amino acid sequence Arg-Gly-Asp) in its N-terminal (13), and is also composed of two coagulation factor VIII-like domains (C1 and C2, discoidin domain) in its C-terminal domain (15). MFG-E8 has been shown to bridge apoptotic cells and phagocytes by binding to phosphatidylserine (PS) exposed on apoptotic cells via its coagulation factor VIII homologous domain, and to αvβ3 integrin on phagocytes via its RGD motif domain (13), and plays a critical role in the clearance of apoptotic cells (16). We recently have shown that immature dendritic cell-derived exosomes, which are abundant in MFG-E8, confer beneficial effects in sepsis (17). Furthermore, our recent unpublished study has also shown that the MFG-E8 deficiency increased apoptotic cells because of less phagocytic activity, and worsened the survival in CLP-induced septic mice. Thus, it can be concluded that MFG-E8 played a crucial role in sepsis (unpublished observation). Previous studies have revealed that the spleen is a major immunological organ to produce MFG-E8 (16), notably, follicular dendritic cells are responsible for the production of MFG-E8 in the spleen rather than tringible-body macrophages (27).
In the present study, we demonstrated that splenic MFG-E8 production decreased in a time dependent manner in septic WT mice, and endotoxemia suppressed MFG-E8 mRNA expression in the spleen in a dose dependent manner. The effect of LPS on MFG-E8 suppression in polymicrobial sepsis was attenuated by the administration of polymyxin B, which neutralizes LPS activities. These results strongly suggest that LPS is responsible for the suppression of MFG-E8 production in polymicrobial sepsis. It still remains the possibility that Polymyxin B did not neutralize LPS activities, but weakened the severity of sepsis by working as an antibiotic. To further define this, CD14−/− mice and TLR4-mutated mice were subjected to CLP. CD14-TLR4 receptor complex is one of the most important signaling components in gram negative bacterial infections. LPS binds with LPS-binding protein (LBP) (28) and transduces its signal via the CD14 (29), TLR4 (30), and MD-2 (31) complex. CD14−/− mice are resistant to LPS-mediated SIRS (18), but not to trauma injury-mediated SIRS, for which TLR4 is responsible (32). Our results suggest that the absence of either CD14 or TLR4 prevents the decrease in MFG-E8 mRNA expression and protein levels in the spleen in polymicrobial sepsis. These results strongly support that the LPS-CD14-TLR4 pathway plays an important role in the expression of MFG-E8 in the spleen in polymicrobial sepsis. Our results also exhibited that the phagocytic activity of peritoneal macrophages against autologous apoptotic thymocytes decreased in septic WT, but not in septic CD14−/− mice. Additionally, apoptosis of splenocytes was increased in WT, but not in CD14−/− mice after CLP. This lack of the increase in splenic apoptotic cells was possibly due to the maintenance of phagocytic activity in septic CD14−/− mice. In a recent study, Chung et al. have shown the role of TLR4 and Fas/FasL in sepsis-induced apoptosis. CD14-TLR4 signaling is not involved in the induction of apoptosis in sepsis (33). Thus, the apoptosis pathway in sepsis is not dependent on CD14-TLR4, and that the decrease in splenic apoptotic cells observed in septic CD14−/− mice is most likely caused by maintaining phagocytic activity.
There are certain limitations of our investigations for phagocytosis assay ex vivo. We used dexamethasone-induced, rather than CLP or LPS-induced, apoptotic thymocytes to measure the phagocytosis of peritoneal macrophages from septic mice. Second, this method measures the phagocytic activity of CLP-treated peritoneal macrophages, which might be different from that of the peritoneal cavity in vivo. In addition, peritoneal cells are dramatically increased after CLP in both WT and CD14−/− mice (approximately 7–10 times, data not shown) and the cell population has also been dramatically changed. It has been reported that CD11bhigh and GR-1low cells are considered as resident macrophages, CD11bhigh and GR-1int cells as recruited (possibly activated) macrophages, and CD11bhigh and GR-1high cells as granulocytes (neutrophils) (23). In our experiments, even in sham mice, there were more CD11bhigh and GR-1int cells than CD11bhigh and GR-1low cells, and CD11bhigh and GR-1low cells have been decreased in both septic mice. As expected, these phenomena indicate that most peritoneal cells are activated and recruited in sepsis. In addition, some peritoneal resident macrophages might also be activated after sham operation alone. As such, we could only compare the phagocytic activity of the mixture of resident and activated macrophages between sham and sepsis. Furthermore, sham CD14−/− mice have less amount of macrophages in the peritoneal cavity than sham WT mice and most peritoneal cells are neutrophils in septic CD14−/− mice in which the phagocytic activity of macrophages is not impaired, so that the total amount of phagocytosis might decrease in septic CD14−/− mice. Haziot et al. have also shown a similar result that there is early infiltration of neutrophils in peritoneal cavity after E. coli injection in CD14−/− mice (34). The decrease in macrophages in septic CD14−/− mice may possibly cause similar mortality to WT mice (35).
CD14 has also been described as a molecule that tethers apoptotic cells to phagocytes and CD14 deficiency leads to persisting apoptotic cells because of impaired phagocytic activity of CD14 deficient macrophages (36). They conclude that the persistence of apoptotic cells in CD14−/− mice is the result of impaired clearance, rather than of increased apoptosis. In our study, the number of basal apoptotic cells was higher in CD14−/− mice (Fig. 5), but basal phagocytic activity was not different from WT mice. These different results might be caused by different animal strains, experimental design, and/or the use of the mixture of resident and activated macrophages which were activated by sham operation.
With regard to the relationship between MFG-E8 and mortality, we have shown that MFG-E8 deficiency worsens the survival in septic mice in our unpublished study. Therefore, adequate MFG-E8 levels lead to the maintenance of the phagocytic activity, which could cause the reduction of the mortality in sepsis. With regard to the relationship between LPS-CD14-TLR4 and mortality, further investigations are required. In spite of resistance to LPS and lower cytokine levels, it has been reported that the mortality of CD14−/− mice in polymicrobial sepsis is similar to in WT mice (35). There are some reports that TLR4-mutated (C3H/HeJ) mice have a survival benefit over WT (C3H/HePas or C3H/HeN) mice in sepsis (37, 38), and that the phagocytosis of apoptotic cells is not affected in the absence of TLR4 (39). On the other hand, other investigators have shown that there is no significant difference of mortality between TLR4-mutated (C3H/HeJ) mice and WT (C3H/HeSnJ or C3H/OuJ) mice (40, 41). This discrepancy could be due to the difference in strain and/or the severity of CLP. Furthermore, Scott et al. recently have shown that CD14 and TLR4 are required for LPS uptake by hepatocytes (42). CD14 and TLR4 are important not only for LPS signaling but also for LPS uptake which is possibly leading to LPS clearance.
Our results show that sepsis downregulates the production of MFG-E8 in the spleen and the blockade of LPS attenuates that alteration. That downregulation of MFG-E8 in polymicrobial sepsis has also been attenuated in both CD14−/− and TLR4-mutated mice. Especially, in septic CD14−/− mice, phagocytic activity is maintained, preventing the increase of apoptotic cells in the spleen. Sepsis-induced downregulation of splenic MFG-E8 production is mainly LPS-CD14-TLR4 pathway dependent, and the phagocytosis of apoptotic cells in sepsis is associated with the LPS-mediated downregulation of MFG-E8. These findings help to further understand the pathophysiological mechanisms in sepsis and further demonstrate the complexity of SIRS. Maintenance of MFG-E8 levels in sepsis may need to be considered for the management of septic patients in the future.
Acknowledgments
This work was supported by National Institutes of Health grants, R01 GM057468, R01 GM053008, and R01 AG028352 (P. Wang).
We sincerely thank Weifeng Dong for his excellent technical assistance, Herb Borrero and Stella Stefanova for their help with inputting and performing flow cytometry analysis for the phagocytosis assay and detection of apoptotic cells, and Dr. Asha Varghese for critical reading of the manuscript.
Abbreviations in this paper
- MFG-E8
milk fat globule EGF-factor VIII
- CLP
cecal ligation and puncture
- SIRS
systemic inflammatory response syndrome
- CD14−/−
CD14-deficient
- WT
wild-type
- BW
body weight
- MFI
mean fluorescence intensity
References
- 1.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Ulloa L, Tracey KJ. The "cytokine profile": a code for sepsis. Trends Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Muller WA. Leukocyte-endothelial cell interactions in the inflammatory response. Lab Invest. 2002;82:521–533. doi: 10.1038/labinvest.3780446. [DOI] [PubMed] [Google Scholar]
- 5.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 6.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 7.Ayala A, Deol ZK, Lehman DL, Herdon CD, Chaudry IH. Polymicrobial sepsis but not low-dose endotoxin infusion causes decreased splenocyte IL-2/IFN-gamma release while increasing IL-4/IL-10 production. J Surg Res. 1994;56:579–585. doi: 10.1006/jsre.1994.1092. [DOI] [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Chang KC, Grayson MH, Tinsley KW, Dunne BS, Davis CG, Osborne DF, Karl IE. Adoptive transfer of apoptotic splenocytes worsens survival, whereas adoptive transfer of necrotic splenocytes improves survival in sepsis. Proc Natl Acad Sci U S A. 2003;100:6724–6729. doi: 10.1073/pnas.1031788100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, Korsmeyer SJ, Karl IE. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci U S A. 1999;96:14541–14546. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 12.Taylor MR, Couto JR, Scallan CD, Ceriani RL, Peterson JA. Lactadherin (formerly BA46), a membrane-associated glycoprotein expressed in human milk and breast carcinomas, promotes Arg-Gly-Asp (RGD)-dependent cell adhesion. DNA Cell Biol. 1997;16:861–869. doi: 10.1089/dna.1997.16.861. [DOI] [PubMed] [Google Scholar]
- 13.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 14.Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mather IH, Banghart LR, Lane WS. The major fat-globule membrane proteins, bovine components 15/16 and guinea-pig GP 55, are homologous to MGF-E8, a murine glycoprotein containing epidermal growth factor-like and factor V/VIII-like sequences. Biochem Mol Biol Int. 1993;29:545–554. [PubMed] [Google Scholar]
- 16.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 17.Miksa M, Wu R, Dong W, Das P, Yang D, Wang P. Dendritic cell-derived exosomes containing milk fat globule epidermal growth factor-factor VIII attenuate proinflammatory responses in sepsis. Shock. 2006;25:586–593. doi: 10.1097/01.shk.0000209533.22941.d0. [DOI] [PubMed] [Google Scholar]
- 18.Haziot A, Ferrero E, Kontgen F, Hijiya N, Yamamoto S, Silver J, Stewart CL, Goyert SM. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407–414. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 19.Morrison DC, Jacobs DM. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976;13:813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- 20.Yang S, Zhou M, Chaudry IH, Wang P. The role of lipopolysaccharide in stimulating adrenomedullin production during polymicrobial sepsis. Biochim Biophys Acta. 2001;1537:167–174. doi: 10.1016/s0925-4439(01)00069-2. [DOI] [PubMed] [Google Scholar]
- 21.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 22.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 23.Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 2004;4:432–444. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 25.Chung CS, Xu YX, Chaudry IH, Ayala A. Sepsis induces increased apoptosis in lamina propria mononuclear cells which is associated with altered cytokine gene expression. J Surg Res. 1998;77:63–70. doi: 10.1006/jsre.1998.5339. [DOI] [PubMed] [Google Scholar]
- 26.Guo RF, Huber-Lang M, Wang X, Sarma V, Padgaonkar VA, Craig RA, Riedemann NC, McClintock SD, Hlaing T, Shi MM, Ward PA. Protective effects of anti-C5a in sepsis-induced thymocyte apoptosis. J Clin Invest. 2000;106:1271–1280. doi: 10.1172/JCI10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kranich J, Krautler NJ, Heinen E, Polymenidou M, Bridel C, Schildknecht A, Huber C, Kosco-Vilbois MH, Zinkernagel R, Miele G, Aguzzi A. Follicular dendritic cells control engulfment of apoptotic bodies by secreting Mfge8. J Exp Med. 2008;205:1293–1302. doi: 10.1084/jem.20071019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jack RS, Fan X, Bernheiden M, Rune G, Ehlers M, Weber A, Kirsch G, Mentel R, Furll B, Freudenberg M, Schmitz G, Stelter F, Schutt C. Lipopolysaccharide-binding protein is required to combat a murine gram-negative bacterial infection. Nature. 1997;389:742–745. doi: 10.1038/39622. [DOI] [PubMed] [Google Scholar]
- 29.Schumann RR, Leong SR, Flaggs GW, Gray PW, Wright SD, Mathison JC, Tobias PS, Ulevitch RJ. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 30.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 31.Schromm AB, Lien E, Henneke P, Chow JC, Yoshimura A, Heine H, Latz E, Monks BG, Schwartz DA, Miyake K, Golenbock DT. Molecular genetic analysis of an endotoxin nonresponder mutant cell line: a point mutation in a conserved region of MD-2 abolishes endotoxin-induced signaling. J Exp Med. 2001;194:79–88. doi: 10.1084/jem.194.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy RM, Prince JM, Yang R, Mollen KP, Liao H, Watson GA, Fink MP, Vodovotz Y, Billiar TR. Systemic inflammation and remote organ damage following bilateral femur fracture requires Toll-like receptor 4. Am J Physiol Regul Integr Comp Physiol. 2006;291:R970–976. doi: 10.1152/ajpregu.00793.2005. [DOI] [PubMed] [Google Scholar]
- 33.Chung CS, Song GY, Moldawer LL, Chaudry IH, Ayala A. Neither Fas ligand nor endotoxin is responsible for inducible peritoneal phagocyte apoptosis during sepsis/peritonitis. J Surg Res. 2000;91:147–153. doi: 10.1006/jsre.2000.5929. [DOI] [PubMed] [Google Scholar]
- 34.Haziot A, Hijiya N, Gangloff SC, Silver J, Goyert SM. Induction of a novel mechanism of accelerated bacterial clearance by lipopolysaccharide in CD14-deficient and Toll-like receptor 4-deficient mice. J Immunol. 2001;166:1075–1078. doi: 10.4049/jimmunol.166.2.1075. [DOI] [PubMed] [Google Scholar]
- 35.Ebong SJ, Goyert SM, Nemzek JA, Kim J, Bolgos GL, Remick DG. Critical role of CD14 for production of proinflammatory cytokines and cytokine inhibitors during sepsis with failure to alter morbidity or mortality. Infect Immun. 2001;69:2099–2106. doi: 10.1128/IAI.69.4.2099-2106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devitt A, Parker KG, Ogden CA, Oldreive C, Clay MF, Melville LA, Bellamy CO, Lacy-Hulbert A, Gangloff SC, Goyert SM, Gregory CD. Persistence of apoptotic cells without autoimmune disease or inflammation in CD14−/− mice. J Cell Biol. 2004;167:1161–1170. doi: 10.1083/jcb.200410057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alves-Filho JC, de Freitas A, Russo M, Cunha FQ. Toll-like receptor 4 signaling leads to neutrophil migration impairment in polymicrobial sepsis. Crit Care Med. 2006;34:461–470. doi: 10.1097/01.ccm.0000198527.71819.e1. [DOI] [PubMed] [Google Scholar]
- 38.Baker CC, Niven-Fairchild T, Caragnano C, Kupper TS. Outcome following femur fracture and subsequent cecal ligation and puncture in endotoxin-sensitive (C3H/HeN) and endotoxin-resistant (C3H/HeJ) mice. J Surg Res. 1991;50:170–174. doi: 10.1016/0022-4804(91)90242-e. [DOI] [PubMed] [Google Scholar]
- 39.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 40.McMasters KM, Peyton JC, Hadjiminas DJ, Cheadle WG. Endotoxin and tumour necrosis factor do not cause mortality from caecal ligation and puncture. Cytokine. 1994;6:530–536. doi: 10.1016/1043-4666(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 41.Mercer-Jones MA, Heinzelmann M, Peyton JC, Wickel DJ, Cook M, Cheadle WG. The pulmonary inflammatory response to experimental fecal peritonitis: relative roles of tumor necrosis factor-alpha and endotoxin. Inflammation. 1997;21:401–417. doi: 10.1023/a:1027366403913. [DOI] [PubMed] [Google Scholar]
- 42.Scott MJ, Billiar TR. beta 2-integrin induced p38MAPK activation is a key mediator in the CD14/TLR4/MD2-dependent uptake of LPS by hepatocytes. J Biol Chem. 2008 doi: 10.1074/jbc.M803905200. [DOI] [PMC free article] [PubMed] [Google Scholar]