Abstract
Background
Previous theories implicate hippocampal dysfunction in anxiety disorders. Most of the data supporting these theories stem from animal research, particularly lesion studies. The generalisation of findings from rodent models to human function is hampered by fundamental inter-species differences. The present work uses a task of spatial orientation, which is known to rely on hippocampal function. Deficits in spatial navigation in anxious children suggest that the hippocampal network involved in spatial orientation is also implicated in anxiety disorders.
Methods
34 treatment-naive children with an anxiety disorder (mean 11.00 years ± 2.54) are compared to 35 healthy age- and IQ-matched healthy children (mean 11.95 years ± 2.36) on a virtual, computer-based equivalent of the Morris Water Maze task.
Results
Results indicate that children with anxiety disorder exhibit overall impaired performance relative to the comparison group. Anxious children made more heading direction errors and had worse accuracy in completing trials relative to controls.
Conclusions
The results present novel evidence that spatial orientation deficits occur in pediatric anxiety.
Keywords: pediatric, anxiety, hippocampus, water maze, spatial navigation
Introduction
Anxiety disorders impair daily functioning (Mendlowicz & Stein, 2000), and, in pediatric populations, carry high risk for later adult psychopathology (Beesdo et al., 2007; Pine et al., 1998). Consequently, considerable research is devoted to the understanding of the mechanisms underlying these disorders and their treatment (Vasa et al., 2007). Yet, the characterisation of the behavioural profile of anxiety and its relation to the underlying affected neurocircuitry remains poorly understood.
Cognitive research on anxiety has traditionally focused on emotion-related biases in attention processing (Bar-Haim et al., 2007; Mogg et al., 2000). These studies show that individuals with an anxiety disorder exhibit a bias toward processing threat-related stimuli. Such bias has been hypothesized to reflect deficits in amygdala function, whose role in emotion regulation is well established (LeDoux, 2000). Other researchers, however, have suggested a critical role of the septo-hippocampal system (SHS) in anxiety (Gray & McNaughton, 2000). Most of the evidence for this hypothesis stems from research in animals. For instance, pharmacological (McNamara et al., 1993; McNaughton & Morris, 1992) and lesion studies (Deacon et al., 2002) in rodents have implicated the SHS in the behavioural manifestations of anxiety. In humans, neuroimaging studies have reported hippocampal abnormalities in some forms of anxiety (Bonne et al., 2008; Schneider et al., 1999; Tillfors et al., 2002). Adults with social phobia show increased hippocampal activation to faces associated with negative odor after conditioning (Schneider et al., 1999), and higher cerebral blood flow in the amygdaloid/hippocampal complex during public speaking (Tillfors et al., 2002) relative to healthy controls.
The hippocampal formation is known primarily for its role in learning and memory (Alvarez et al., 2008; Moscovitch et al., 2006; Scoville & Milner, 1957), and in spatial navigation (Cornwell et al., 2008; Maguire et al., 1998; O'Keefe & Nadel, 1978). Based on rodent-work, a subregional functional specialization of the hippocampus has been proposed, with the ventral region implicated in anxiety-related behaviour and the dorsal region implicated in spatial-related behaviour (Bannerman et al., 2004). However, recent studies of dorsal hippocampal lesions in mice have shown reduced extinction of aversive memories in conjunction with impairments in spatial navigation (Heldt et al., 2007). This would suggest that reduced output in one component of hippocampal function, related to navigation, can be paralleled by increased output in another component, related to fear and anxiety. Other work implicates both dorsal and ventral hippocampus in spatial learning, which might suggest that impaired hippocampal function predicts both reduced navigation and reduced anxiety (Ferbinteanu et al., 2003). Given cross-species differences in brain complexity and experimental methodology, research in humans is critically needed to examine the role of hippocampal function in anxiety.
Available data in humans are consistent with rodent-based studies suggesting that hippocampal disruptions of cognitive function are paralleled by increases in anxiety-related behaviors. For example, available behavioural studies in humans report impaired visual memory in adults (Cohen et al., 1996) and youth (Vasa et al., 2007) with anxiety disorders relative to unaffected controls. Functional imaging studies suggest that both the anterior and posterior hippocampus may be critical for intact spatial navigation (Iaria et al., 2007; Maguire et al., 1998). Thus, functional (Iaria et al., 2007; Schneider et al., 1999) and cognitive (Vasa et al., 2007) studies in humans support the hypothesis that increased levels of anxiety will be associated with disrupted hippocampal involvement in spatial navigation. While studies of visuo-spatial memory in adults and youth support this anxiety-hippocampus link, no studies in any age group have assessed spatial navigation in anxiety disorders. The goal of the present work was to address this gap.
The water maze task is one of the best validated paradigms of spatial navigation in animals (D'Hooge & De Deyn, 2001; Morris, 1984). Virtual equivalents of this task have been developed for human studies. Consistent with data in rodents, virtual equivalents of water-maze navigation in humans have been shown to be sensitive to sex in both healthy adults (Astur et al., 2004; Ross et al., 2006) and pre-pubertal children (Newhouse et al., 2007), and to sex steroid imbalance in adolescents (Mueller, Temple et al., 2008). In addition, they have been found to engage the SHS (Cornwell et al., 2008; Shipman & Astur, 2008). The present study used a task modelled after the Morris water maze task to compare spatial navigation performance in healthy adolescents and in treatment-naive youths suffering from an anxiety disorder. We predicted that the anxious group would perform worse on the water maze task relative to the healthy control group. Futhermore, based on reports of sex differences in spatial navigation, we also expected boys to perform better than girls (Newhouse et al., 2007).
Methods
Participants
Thirty-four patients diagnosed with an anxiety disorder (18 female; 11.00 ± 2.54 years old) and 35 healthy children (19 female; 11.95 ± 2.36 years old) participated in the study (Table 1). Both groups were similar on age (t(67)=1.61, ns), IQ (64)=-0.54, ns) and sex distribution (x2(1)=0.37, ns). In addition, anxious patients did not differ from controls on the IQ subscale scores of vocabulary (t(63)=-1.33, p=.19) or matrix reasoning (t(52.98)=0.84, p=.41), suggesting that anxiety did not affect performance on cognitive tasks that are not directly dependent on hippocampal function.
Table 1.
Demographic characteristics of the anxious and the control group including the type and percentage of comorbid disorders. * Note that a patient could meet criteria for more than one anxiety disorder, such that the sum of subjects with a given diagnosis is larger than the number of patients in the anxious group. GAD = generalised anxiety disorder, SAD = separation anxiety disorder, CDI = Children's Depression Inventory.
| Anxious (N=34) | Control (N=35) | Statistic (P) | |
|---|---|---|---|
| Age (Mean, StDev) | 11.00 (2.54) | 11.95 (2.36) | ns |
| IQ (Mean, StDev) | 113.42 (14.85) | 111.75 (9.41) | ns |
| CDI (Mean, StDev) | 48.67 (9.55) | 40.38 (5.06) | <.001 |
| Scared (Mean, StDev) | 27.75 (13.02) | 12.57 (9.98) | <.001 |
| * Comorbid Dx (number, %) | |||
| GAD | 21 (62%) | - | |
| SAD | 15 (44%) | - | |
| Phobia | 13 (38%) | - | |
| Panic | 1 (3%) | - |
The parents of participants provided written consent, and the children provided written assent to participate in protocols approved by the Institutional Review Board of the National Institute of Mental Health (NIMH). Children in the anxious group were recruited when they sought treatment at the NIMH for an impairing anxiety disorder. Children from the comparison group were recruited via newspaper advertisements and word-of-mouth. Inclusion criteria for children from the comparison group consisted of an absence of medical or psychiatric problems, as determined by physical examination and structured interviews (Kiddie-Schedule-for-Affective-Disorders–Present-and-Lifetime-version (Kaufman et al., 1997)). Inclusion criteria for children from the anxious group consisted of a primary diagnosis of anxiety disorder; score >9 on the Pediatric Anxiety Rating Scale (Rupp, 2001), and a score < 60 on the Children Global Assessment Scale (CGAS (Shaffer et al., 1983)). Criteria for exclusion were current use of any psychoactive substances, Obsessive-Compulsive Disorder (OCD), Major Depressive Disorder (MDD), Tourette's Syndrome, Post-Traumatic Stress Disorder, Conduct Disorder, Suicidal Ideation, a lifetime history of Mania or Psychosis, ADHD, a Pervasive Developmental Disorder, or an IQ < 70. Self-report measures of anxiety and depression were collected with the Screen for Child Anxiety Related Emotional Disorders (SCARED (Birmaher et al., 1997)), and the Child Depression Inventory (CDI (Helsel & Matson, 1984) questionnaires. Children with anxiety reported higher scores on the SCARED (t(61)=-5.26, p<.001) and the CDI (t(48.96)=-4.35, p<.001) relative to healthy comparisons. The psychiatric interviews were conducted by experienced clinicians with excellent inter-rater reliability (k>0.75).
Materials
A virtual version of the Morris Water Maze (NeuroInvestigations Inc.) was used. It consisted of the display of a square room containing a circular pool of water. Four equally sized abstract rectangular paintings that were distinguishable by their shapes, colours and placements on the walls surrounding the pool, served as navigational cues to aid orientation. Each of these cues was placed on a different wall of the room and stretched from the ceiling to the pool wall (see Figure 1). Participants navigated in the pool from a first-person perspective and moved around using the ‘up’, ‘left’ and ‘right’ arrow curser keys of the keyboard. They were told not to back up, and the ‘back’ arrow key was disabled. Instead, if they wanted to turn around, they had to spin on their left or right axis using the left or right arrow keys.
Figure 1.
Sample screenshot of a typical trial in which the person must navigate through the water maze from a first-person perspective with the distal cue (the painting) visible in the background.
Procedure
Participants completed the experiment on a laptop with a 17 inch monitor in a windowless room of the pediatric clinic of the Clinical Center at the NIH. The experiment was completed in one session without breaks and lasted about 15 minutes. The task consisted of 18 trials, including 2 initial practice trials and 16 experimental trials. On the first practice trial, participants had 30 seconds to explore the room and to learn to navigate comfortably in this environment. No platform was present during this first trial. The platform was introduced to the participants on the second practice trial. For this trial, participants were asked to simply “swim” towards the visible platform. Over the next 16 experimental trials, the platform was hidden. Participants were dropped randomly across trials at four locations one the side of the pool wall. For each trial, the task consisted of “swimming” directly to the hidden platform. Once participants successfully reached the platform, a sound occurred. Participants remained on the platform for 2 seconds before the onset of the next trial. On each trial, participants were given 60 seconds to find the platform, after which the platform became visible and a written message appeared on the screen indicating the visibility of the platform and encouraged participants to move towards it.
Analysis
The location of each subject in the pool was recorded every 100 ms. Five performance variables were used: (1) overall accuracy, i.e., number of failed attempts, in which the latency exceeded 60 sec; (2) heading error (in deg), i.e., the angle between optimal heading direction and participant's heading direction; (3) latency, i.e., the time (sec) spent to reach the platform; (4) path length, i.e., the distance (relative to the pool diameter) covered to reach the platform, and 5) first-move latency, an indicator how long subjects remained at the wall edge at the beginning of a trial before they started moving towards the platform. To examine learning patterns, the 16 experimental trials were binned into four blocks of four trials each, i.e. block 1: trials 1-4, block 2: trials 5-8 (2), block 3: trials 9-12 and block 4: trials 13-16. A repeated measures ANOVA was conducted for each variable using a Block (1-4) by Sex (male vs. female) by Group (anxious vs. control) design. Pearson product-moment coefficients were calculated to examine correlations of performance variables with anxiety and depression scores. Effect sizes were calculated using Cohen's d (Cohen, 1988).
Results
Overall accuracy of performance
The number of incomplete trials decreased over the experiment indicating a significant learning effect as seen in a main effect of block: F(3,195)=14.95, p<.001 and a significant linear trend: F(1,65)=28.73, p<.001. However, as hypothesized, patients performed worse than controls (interaction of group-by-block: F(3,195)=3.51, p=.016). This effect reflected a larger number of incomplete trials at the beginning of the experiment for patients relative to healthy comparisons, while both groups had similar failure rates towards the end of the experiment (Figure 3A). There was no significant influence of sex as a main effect (F(1,65)=1.69, p=.19) or in interaction with other factors.
Figure 3.
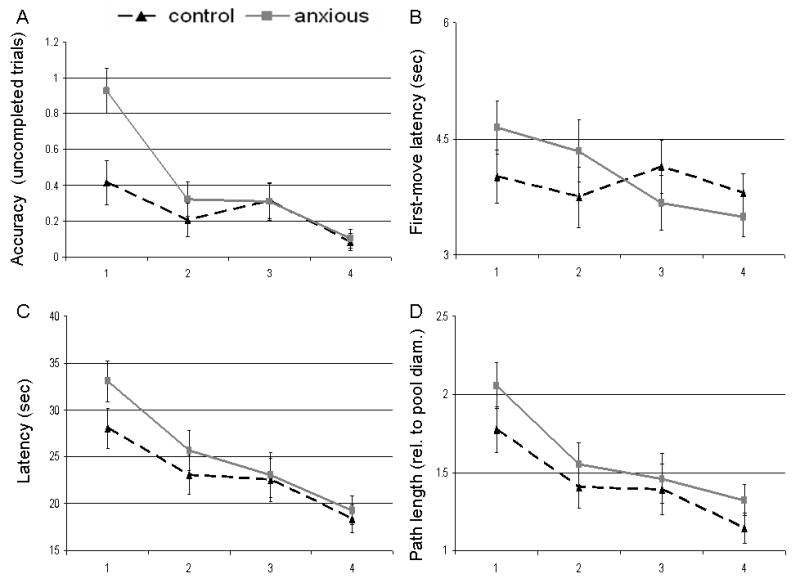
Means (standard errors) for the anxious (dashed black lines) and control (solid grey lines) groups across task parameters. A. Average number of trials in which trial was not completed successfully. B.First-move latency (in sec). C. Latencies in seconds for participants to reach the platform. D. Distance traversed in the environment to reach the platform.
Heading Error
Heading direction error decreased over the course of the experiment as seen in a main effect of block (F(3,195)=2.94, p<.05) and a significant linear trend (F(1,65)=5.57, p<.05), again consistent with a learning effect. Importantly, a significant main effect of group emerged with a large effect size (F(1,65)=11.40, p=.001, d=0.81). This effect reveals that, on average, the anxious group exhibited more difficulty finding the platform (28.48 deg ± 1.88 SEM) relative to the comparison group (19.58 deg ± 1.84 SEM) (Figure 2). The interaction of group by block was not significant, indicating that both groups had similar learning trajectories (F(3,195)=0.43, p=.73). Although, sex had no influence as a main effect (F(1,65)<.001, ns), the interaction between heading error and sex was significant (F(3,195)=2.68, p<.05) indicating that females exhibited initially worse heading error than males on the first block but then improved to the level of the males. However, the three way interaction between sex, group and heading error was not significant (F(3,195)=1.53, p=.21).
Figure 2.
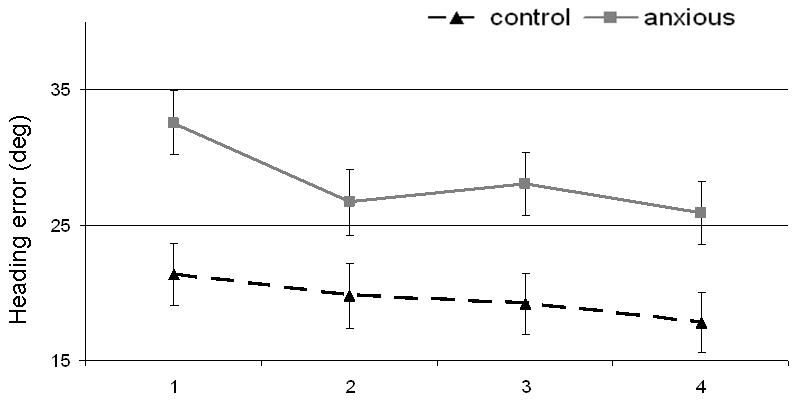
Means (standard errors) for the anxious (dashed black lines) and control (solid grey lines) groups on heading errors (in degrees).
First move latency
Subjects started to move faster once they were being placed on the platform as the experiment progressed (F(3,195)=3.39, p=.019). This was documented by a significant linear trend (F(1,65)=8.61, p<.01). However, there was a significant block by group interaction (F(3,195)=3.51, p=.016). This interaction indicated that anxiety patients moved initially slower for the first two blocks, but then shifted and moved faster than the controls during the last two blocks of trials (Figure 3B). There was no significant effect of sex (F(1,65)=.87, p=.36) as a main effect or in interaction with other factors.
Latency and Path length
Group had no influence on both variables as a main effect, or in interaction with block or sex. However, across both groups, a main effect of Block was present for latency: F(3,195)=19.83, p<.001, and path length: F(3,195)=13.42, p<.001 (Figure 3C/D). Both measures decreased significantly over the course of the experiment, which was also characterized by significant linear trends (F(1,65)=59.15, p<.001 and F(1,65)=36.27, p<.001, respectively). This effect of block confirmed the learning effect already detected on both overall accuracy and heading errors. Sex had no main effect on either latency (F(1,65)=2.00, p=.16, d = 0.36) or path length (F(1,65)=0.45, p=.51, d=0.19).
Correlations of mood and anxiety symptom severity with performance variables
Anxiety scores from the SCARED questionnaire correlated significantly with heading error (r2(63)=.34, p<.01), indicating that higher anxiety ratings in the pooled sample of controls and patients were associated with worse directional performance throughout the task. SCARED, however, did not correlate with any other task performance variable (all p > .10) or IQ (full and subscales, all p > .27). No correlations between CDI and performance variables were significant.
Discussion
Anxiety has been linked to dysfunction within the septo-hippocampal system (Gray & McNaughton, 2000), which is also known to play a critical role in spatial navigation (Maguire et al., 1998; O'Keefe & Nadel, 1978). The present study provides indirect evidence of a link between anxiety and the SHS by showing, for the first time, spatial navigation deficits in children with an anxiety disorder.
The pattern of group differences on the water maze task suggests that a combination of navigation-related deficits occurs in pediatric anxiety disorders. Children with an anxiety disorder were impaired on initial attempts to navigate relative to control children but they were not impaired on the learning aspect of the task. Group differences in accuracy emerged in the first performance block. Subsequently, accuracy of the anxious children improved to the level of the control group, suggesting that patients were able to correct their initial deficit with experience. In contrast, deficits on another measure of navigation in the anxiety group did not normalize with task exposure. Indeed, patients showed excessive heading errors throughout the experiment, not only at the beginning of the task. The normalization of accuracy with time, in the face of persistent deficit in heading errors, may indicate that patients learned to compensate for their spatial deficit by adopting a different, but not as efficient, strategy to find the platform (Kallai et al., 2005; Sutherland et al., 1983). This hypothesis is further supported by the findings of group differences in the latency-to-first-move variable: while taking longer to initiate moving towards the platform at the beginning of the experiment, anxious patients were found to move faster in the last two blocks relative to controls. Speculations about the nature of the different strategies adopted by the anxious children are premature. Indeed, the current task did not examine orientation strategy (e.g., egocentric vs. allocentric). Previous neuroimaging studies have documented right hippocampal involvement when a spatial (vs. non-spatial) strategy was used (Iaria et al., 2003) and other studies have highlighted potential differences of left and right hippocampus to spatial memory (Cornwell et al., 2008; de Toledo-Morrell et al., 2000). Thus, future behavioural work could employ orientation-type tasks (Livingstone & Skelton, 2007) to further qualify strategic differences as potential contributor to group differences. Moreover, future imaging (fMRI) or electrophysiological (EEG/ERP) studies could begin to 1) examine use of spatial strategies in anxious populations and 2) investigate the potential contribution of the hemispheres to the present findings.
The design of this study raises the question of the differential role of state vs. trait anxiety in the observed navigation deficits. Severity of ongoing anxiety, as measured by the SCARED, correlated with heading error, indicating that higher levels of anxiety were associated with worse directional performance throughout the task. It could be argued that worse anxiety could implicate a general pattern of state-related perturbations in cognition that would affect any cognitive task. However, measures of anxiety severity did not correlate with other task parameters or cognitive performance scores on the IQ subscales of vocabulary and matrix reasoning. The data are consistent with previous research that could not find differences between pediatric anxiety and healthy controls on measures of IQ (Monk et al., 2008), verbal learning (Toren et al., 2000), and attention and inhibition (Gunther et al., 2004). Consequently, the complex pattern of associations between anxiety severity and cognition generates insights for future work, given that distinct cognitive measures seem to be influenced by different neural systems.
Finally, differences between males and females emerged only in a significant block-by-sex interaction in heading errors, indicating worse performance on the first task block for females relative to males. Against predictions, there was no significant main effect of faster performance of males relative to females. However, the direction of the means on task parameters were as expected (e.g., healthy males found the platform on average 5 seconds faster than healthy females, 20.79 sec vs. 25.36 sec, which corresponded to a medium effect d=0.50, p=.15). Overall, the absence of sex differences might not be too surprising. Indeed, findings of sex differences on spatial memory tasks have been mixed in pre-pubertal populations (Newhouse et al., 2007; Voyer et al., 1995), in contrast to the more consistent reports in adults (Astur et al., 1998; Ross et al., 2006).
A number of strengths and limitations of the study need to be discussed. A major strength of the study is that the present findings could not be accounted for by effects of psychotropic medication since none of the participants were or had ever been medicated for anxiety. All participants were studied during the screening period, prior to starting treatment for their anxiety. Moreover, all patients suffered from relatively severe anxiety disorders, occurring in the absence of other disorders, such as major depression, that frequently complicate anxiety. With regards to limitations, the relatively small numbers of patients in any given DSM-IV anxiety diagnosis and the frequent co-morbidity among anxiety disorders did not enable us to carry out analyses of specific anxiety disorders. However, given that anxiety disorders are commonly characterised by co-morbidity (Wittchen & Jacobi, 2005), our sample might provide appropriate generalizable findings to the population of anxiety disorders at large. Nevertheless, this argument does not diminish the need to understand disorder-specific mechanisms and to carry forward disorder-specific research. Second, no information was collected on subjects' experience with computer games, which can influence level of dexterity and motor control ability. Previous studies in similar (Newhouse et al., 2007) and older (Moffat et al., 1998; Mueller, Jackson et al., 2008) age groups have not found prior computer experience to modulate performance on the virtual water maze task. In a related fashion, our findings also could be influenced by dyspraxia, given that prior work does link pediatric anxiety to deficits in motor performance (Pine et al., 1997). On the other hand, all of the children in our study presented with primary complaints of anxiety, and none reported dyspraxia or showed frank signs of motor deficit on physical exam. Regardless, future studies might conduct more comprehensive assessments of motor function to evaluate this possibility. Finally, future studies need to further characterize neuropsychological performance of pediatric anxiety groups by incorporating tasks that do not rely on hippocampal function. The absence of group differences on the IQ subscales of matrix reasoning and vocabulary consistent with prior findings of selective cognitive deficits in anxiety (Monk et al., 2008; Toren et al., 2000; Vasa et al., 2007) suggests that our findings do not reflect a more general cognitive dysfunction in these patients.
In summary, this is the first study to document abnormalities of spatial navigation in a group of clinically anxious children. Deficits in spatial navigation may point to an underlying dysfunction of the SHS, which has been suggested to play a role in anxiety. This preliminary finding offers a promising avenue for furthering our understanding of the pathophysiology of anxiety disorders and bears clinical relevance for the testing of novel therapeutic approaches incorporating a spatial orientation component. Future studies will need to employ functional imaging techniques to examine the precise neural substrate of spatial orientation deficits in anxiety.
Text box
The hippocampus is known to be critical to human and rodent spatial navigation
In rodent studies, the hippocampus has also been related to clinical manifestations of anxiety
However, evidence in humans for such an effect are scarce
Using a spatial navigation task known to require intact hippocampal function in rodents, we tested youth with first-onset clinical anxiety and show impairment of spatial navigation in this group relative to a healthy control group
The findings bears clinical relevance for development of novel therapies for anxiety such as learning orientation techniques
Findings also broaden the characterisation of cognitive deficits in youth with anxiety
Acknowledgments
This research was supported, in part, by the Intramural Research Programme of the NIMH, NIH. We would like to thank the three anonymous reviewers for their helpful comments in improving this manuscript.
Footnotes
Financial Disclosure: None of the authors has a conflict of interest to declare.
References
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. J Neurosci. 2008;28(24):6211–6219. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behav Brain Res. 1998;93(12):185–190. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Astur RS, Tropp J, Sava S, Constable RT, Markus EJ. Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behav Brain Res. 2004;151(12):103–115. doi: 10.1016/j.bbr.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28(3):273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IMH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Bittner A, Pine DS, Stein MB, Hofler M, Lieb R, et al. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch Gen Psychiatry. 2007;64(8):903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36(4):545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Bonne O, Vythilingam M, Inagaki M, Wood S, Neumeister A, Nugent AC, et al. Reduced Posterior Hippocampal Volume in Posttraumatic Stress Disorder. J Clin Psychiatry. 2008:e1–e5. doi: 10.4088/jcp.v69n0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LJ, Hollander E, DeCaria CM, Stein DJ, Simeon D, Liebowitz MR, et al. Specificity of neuropsychological impairment in obsessive-compulsive disorder: a comparison with social phobic and normal control subjects. J Neuropsychiatry Clin Neurosci. 1996;8(1):82–85. doi: 10.1176/jnp.8.1.82. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Johnson LL, Holroyd T, Carver FW, Grillon C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. J Neurosci. 2008;28(23):5983–5990. doi: 10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36(1):60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- de Toledo-Morrell L, Dickerson B, Sullivan MP, Spanovic C, Wilson R, Bennett DA. Hemispheric differences in hippocampal volume predict verbal and spatial memory performance in patients with Alzheimer's disease. Hippocampus. 2000;10(2):136–142. doi: 10.1002/(SICI)1098-1063(2000)10:2<136::AID-HIPO2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Bannerman DM, Rawlins JN. Anxiolytic effects of cytotoxic hippocampal lesions in rats. Behav Neurosci. 2002;116(3):494–497. doi: 10.1037//0735-7044.116.3.494. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, Ray C, McDonald RJ. Both dorsal and ventral hippocampus contribute to spatial learning in Long-Evans rats. Neurosci Lett. 2003;345(2):131–135. doi: 10.1016/s0304-3940(03)00473-7. [DOI] [PubMed] [Google Scholar]
- Gray J, McNaughton N. The Neuropsychology of Anxiety. Oxford; Oxford University Press; 2000. [Google Scholar]
- Gunther T, Holtkamp K, Jolles J, Herpertz-Dahlmann B, Konrad K. Verbal memory and aspects of attentional control in children and adolescents with anxiety disorders or depressive disorders. J Affect Disord. 2004;82(2):265–269. doi: 10.1016/j.jad.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12(7):656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsel WJ, Matson JL. The assessment of depression in children: the internal structure of the Child Depression Inventory (CDI) Behav Res Ther. 1984;22(3):289–298. doi: 10.1016/0005-7967(84)90009-3. [DOI] [PubMed] [Google Scholar]
- Iaria G, Chen JK, Guariglia C, Ptito A, Petrides M. Retrosplenial and hippocampal brain regions in human navigation: complementary functional contributions to the formation and use of cognitive maps. Eur J Neurosci. 2007;25(3):890–899. doi: 10.1111/j.1460-9568.2007.05371.x. [DOI] [PubMed] [Google Scholar]
- Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: variability and change with practice. J Neurosci. 2003;23(13):5945–5952. doi: 10.1523/JNEUROSCI.23-13-05945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallai J, Makany T, Karadi K, Jacobs WJ. Spatial orientation strategies in Morris-type virtual water task for humans. Behav Brain Res. 2005;159(2):187–196. doi: 10.1016/j.bbr.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Livingstone SA, Skelton RW. Virtual environment navigation tasks and the assessment of cognitive deficits in individuals with brain injury. Behav Brain Res. 2007;185(1):21–31. doi: 10.1016/j.bbr.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O'Keefe J. Knowing where and getting there: a human navigation network. Science. 1998;280(5365):921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- McNamara RK, dePape GE, Skelton RW. Differential effects of benzodiazepine receptor agonists on hippocampal long-term potentiation and spatial learning in the Morris water maze. Brain Res. 1993;626(12):63–70. doi: 10.1016/0006-8993(93)90563-3. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Morris RG. Buspirone produces a dose-related impairment in spatial navigation. Pharmacol Biochem Behav. 1992;43(1):167–171. doi: 10.1016/0091-3057(92)90653-w. [DOI] [PubMed] [Google Scholar]
- Mendlowicz MV, Stein MB. Quality of life in individuals with anxiety disorders. Am J Psychiatry. 2000;157(5):669–682. doi: 10.1176/appi.ajp.157.5.669. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Hampson E, Hatzipantelis M. Navigation in a “Virtual” Maze: Sex Differences and Correlation With Psychometric Measures of Spatial Ability in Humans. Evol Hum Behav. 1998;19:73–87. [Google Scholar]
- Mogg K, Millar N, Bradley BP. Biases in eye movements to threatening facial expressions in generalized anxiety disorder and depressive disorder. J Abnorm Psychol. 2000;109(4):695–704. doi: 10.1037//0021-843x.109.4.695. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65(5):568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr Opin Neurobiol. 2006;16(2):179–190. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Jackson CP, Skelton RW. Sex differences in a virtual water maze: an eye tracking and pupillometry study. Behavioural Brain Research. 2008;193:209–215. doi: 10.1016/j.bbr.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Temple V, Oh E, VanRyzin C, Williams A, Cornwell B, et al. Early androgen exposure modulates spatial cognition in congenital adrenal hyperplasia (CAH) Psychoneuroendocrinology. 2008;33:973–980. doi: 10.1016/j.psyneuen.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse P, Newhouse C, Astur RS. Sex differences in visual-spatial learning using a virtual water maze in pre-pubertal children. Behav Brain Res. 2007;183(1):1–7. doi: 10.1016/j.bbr.2007.05.011. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford; Oxford University Press; 1978. [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55(1):56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Pine DS, Wasserman GA, Fried JE, Parides M, Shaffer D. Neurological soft signs: one-year stability and relationship to psychiatric symptoms in boys. J Am Acad Child Adolesc Psychiatry. 1997;36(11):1579–1586. doi: 10.1016/S0890-8567(09)66568-0. [DOI] [PubMed] [Google Scholar]
- Ross SP, Skelton RW, Mueller SC. Gender differences in spatial navigation in virtual space: implications when using virtual environments in instruction and assessment. Virtual Reality. 2006;10:175–184. [Google Scholar]
- Rupp. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. The Research Unit on Pediatric Psychopharmacology Anxiety Study Group. N Engl J Med. 2001;344(17):1279–1285. doi: 10.1056/NEJM200104263441703. [DOI] [PubMed] [Google Scholar]
- Schneider F, Weiss U, Kessler C, Muller-Gartner HW, Posse S, Salloum JB, et al. Subcortical correlates of differential classical conditioning of aversive emotional reactions in social phobia. Biol Psychiatry. 1999;45(7):863–871. doi: 10.1016/s0006-3223(98)00269-8. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, et al. A children's global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40(11):1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Shipman SL, Astur RS. Factors affecting the hippocampal BOLD response during spatial memory. Behav Brain Res. 2008;187(2):433–441. doi: 10.1016/j.bbr.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Whishaw IQ, Kolb B. A behavioural analysis of spatial localization following electrolytic, kainate- or colchicine-induced damage to the hippocampal formation in the rat. Behav Brain Res. 1983;7(2):133–153. doi: 10.1016/0166-4328(83)90188-2. [DOI] [PubMed] [Google Scholar]
- Tillfors M, Furmark T, Marteinsdottir I, Fredrikson M. Cerebral blood flow during anticipation of public speaking in social phobia: a PET study. Biol Psychiatry. 2002;52(11):1113–1119. doi: 10.1016/s0006-3223(02)01396-3. [DOI] [PubMed] [Google Scholar]
- Toren P, Sadeh M, Wolmer L, Eldar S, Koren S, Weizman R, et al. Neurocognitive correlates of anxiety disorders in children: a preliminary report. J Anxiety Disord. 2000;14(3):239–247. doi: 10.1016/s0887-6185(99)00036-5. [DOI] [PubMed] [Google Scholar]
- Vasa RA, Roberson-Nay R, Klein RG, Mannuzza S, Moulton JL, 3rd, Guardino M, et al. Memory deficits in children with and at risk for anxiety disorders. Depress Anxiety. 2007;24(2):85–94. doi: 10.1002/da.20193. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol Bull. 1995;117(2):250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Jacobi F. Size and burden of mental disorders in Europe--a critical review and appraisal of 27 studies. Eur Neuropsychopharmacol. 2005;15(4):357–376. doi: 10.1016/j.euroneuro.2005.04.012. [DOI] [PubMed] [Google Scholar]


