Abstract
With the development of functional neuroimaging tools, the past two decades have witnessed an explosion of work examining functional brain maps, mostly in the adult brain. Against this backdrop of work in adults, developmental research begins to gather a substantial body of knowledge about brain maturation. The purpose of this review is to present some of these findings from the perspective of functional neuroimaging. First, a brief survey of available neuroimaging techniques (i.e., fMRI, MRS, MEG, PET, SPECT, and infrared techniques) is provided. Next, the key cognitive, emotional, and social changes taking place during adolescence are outlined. The third section gives examples of how these behavioral changes can be understood from a neuroscience perspective. The conclusion places this functional neuroimaging research in relation to clinical and molecular work, and shows how answers will ultimately come from the combined efforts of these disciplines.
Introduction
The study of human brain development across the lifespan has now become feasible with the advent of functional neuroimaging tools. The past two decades have witnessed an explosion of work examining functional brain maps, mostly of the adult brain. This experience has brought expertise and experimental standards in the methodology and interpretation of findings, paving the way for developmental brain research.
A number of functional neuroimaging tools, including electromagnetic (fMRI, MRS, MEG), and nuclear medicine (PET, SPECT), and infrared techniques are available. These techniques offer different advantages and limitations. The choice of the imaging modality mostly depends on the scientific question, and, of course, on the availability of the equipment at the research facility. A major limitation in the use of nuclear medicine techniques in the healthy pediatric population concerns radiation exposure. For this reason, most of the developmental work comes from fMRI research. However, imaging children poses unique challenges, relatively to those associated with research in adults. First, children tend to be more anxious in unfamiliar medical environments. Second, they have more difficulty remaining still in the scanner (Thomas et al., 1999; Poldrack et al., 2002). Both issues can be minimized with practice in a mock scanner, which familiarizes children with the environment, and helps them learn how to remain immobile (Rosenberg et al., 1997). In addition, the use of head restraint systems or motion training paired with feedback systems in the mock scanner have shown promising findings on motion artifacts (Slifer et al., 1993; Temple et al., 2001). A third issue concerns the validity of applying data acquisition algorithms and analytic tools used in adults to the pediatric population. While not significant in adolescents, this concern becomes substantial in younger children, particularly those younger than 6 years of age (Muzik et al., 2000). The main reasons are the age-related changes in brain sizes overall and individual structure sizes (Tien et al., 1992; Giedd et al., 1996; Paus et al., 1999; Durston et al., 2001; Giedd, 2004; Gogtay et al., 2004), and water content (Sakuma et al., 1991; Nomura et al., 1994), which is the basic substrate of the MRI signal (Paus et al., 2001). Adjustment in scanning parameters for data acquisition (Saunders et al., 2007), and use of age-appropriate structural brains for group analyses (Muzik et al., 2000) become critical. Much work needs to be done in this area. These issues, in addition to the status of vulnerable population as a whole, explain the scarcity of studies in this age group.
Here, we will first provide an overview of the functional neuroimaging techniques that have contributed to an understanding of brain development. This section will be followed by a review of the key cognitive, emotional, and social changes taking place during adolescence. The third section will give examples of how these behavioral changes can be understood from a neuroscience perspective. We will conclude by placing this research in relation to clinical and molecular work, and show how answers will ultimately come from the combined efforts of these disciplines.
Of note, this review is focused on functional neuroimaging research, and structural neuroimaging techniques and studies will not be addressed specifically.
Functional neuroimaging techniques
Comprehensive reviews of the functional neuroimaging techniques can be found in the volume, “Functional Neuroimaging in Child Psychiatry” (Ernst, and Rumsey, 2000) and its upcoming new edition “Neuroimaging in Developmental Clinical Neuroscience” (Rumsey and Ernst, in press). In brief, these techniques fall into two categories, those which utilize magnetic fields (functional magnetic resonance imaging [fMRI], magnetic resonance spectroscopy [MRS], and magnetoencephalography [MEG]) and those which use ionizing radiation (positron emission tomography [PET] and single photon emission computed tomography [SPECT]).
The physics underlying these two technologies are fundamentally different. PET and SPECT measure radioactive counts in the brain that accumulate over a certain amount of time (Herscovitch and Ernst, 2000), and MR measures distortions in electromagnetic fields proportional to changes in the concentration of deoxyhemoglobin (Blood Oxygen Level Dependent signal [BOLD]) (Eden and Zeffiro, 2000). PET and SPECT require that the radioactive compounds be prepared in a radiochemistry laboratory. Because of the short half-lives of these compounds for PET use, this laboratory needs to be in short proximity of the PET scanner. This is not so much the case in SPECT, which typically uses compounds with longer radioactive half-life. The success of these techniques is based on the development of radioligands that give valid and reliable measurements of the in vivo behavior of biological molecules of interest (see Table 1). MR requires electromagnetic isolation of the scanner room. Both technologies depend on the collaboration of physicists, mathematicians and clinicians. PET/SPECT requires the additional contribution of radiochemists.
Table 1.
Partial list of PET radiotracers
| Target system or process | Radiotracer | |
|---|---|---|
| Neuroreceptor Systems | ||
| Dopaminergic | Pre-synaptic dopamine pool | 18F-fluoro-L-dopa, 18F-fluoro-L-m-tyrosine |
| D2 Dopamine receptors | 11C-N-methylspiperone, 11C-raclopride | |
| 18F-spiperone, 18F-N-methylspiperone | ||
| D1 Dopamine receptors | 11C-SCH23390 | |
| Dopamine reuptake sites | 11C-nomifensine, 11C-cocaine, 18F-GBR | |
| 11C-carfentanil, 11C-diprenorphine | ||
| Opiate | 18F-cyclofoxy | |
| Benzodiazepine | 11C-flumazenil | |
| Serotonergic | Pre-synaptic serotonin pool | 11C-α-methyl-tryptophan |
| 5-HT1A receptors | 11C-WAY100,635 | |
| 5-HT2A receptors | 11C-MDL100,907, 18F-altanserin, 18F- setoperone | |
| 5-HT reuptake sites | 11C-McN5652 | |
| MAO-B enzyme | 11C-deprenyl | |
| Amino acid transport, protein synthesis | ||
| 11C-methionine, 11C-leucine, 11C-tyrosine | ||
| Tissue pH | 11CO2, 11C-DMO | |
| Tissue drug kinetics | 11C-phenytoin, 11C-valproate, 13N-BCNU |
PET and SPECT are similar techniques, with the practical difference that SPECT is easier to use in terms of both radiochemistry and instrumentation, but provides lower quantitative accuracy and is more limited by the relatively narrower range of available radioligands (Tables 1–2). For simplicity, we will focus mainly on PET technology.
Table 2.
Partial list of SPECT radiotracers
| Target system or process | SPECT radiotracers | |
|---|---|---|
| Cerebral Blood Flow | 123I-iodoamphetamine (123I-IMP) | |
| 99mTc-exametazime (99mTc-HMPAO) | ||
| 99mTc-ethyl cysteinate dimer (99mTc-ECD) | ||
| Cerebral plasma and red cell volume | 99mTc human serum albumin | |
| 99mTc red blood cells | ||
| Neuroreceptor Systems | ||
| Dopaminergic | D2 Dopamine receptors | 123I-IBZM, 123I-epidepride |
| D1 Dopamine receptors | 123I-SCH23982 | |
| Dopamine reuptake sites | 123I-β-CIT | |
| Benzodiazepine | 123I-iomazenil | |
| Cholinergic | muscarinic | 123I-QNB, 123I-iododexetimide |
| Serotonergic | 5-HT2A receptors | 123I-R93274 |
| Amino acid transport | 123I-iodo-α-methyl tyrosine |
PET paired with the tracer H215O (radioactive water) and fMRI both provide measures of cerebral blood flow that index neural activity. These techniques differ in their temporal resolution, i.e., approximately 60 seconds with PET and 2 to 5 seconds in fMRI (Figure 1). Spatial resolution is fairly equivalent, except that PET permits a better delineation of medial temporal and orbital frontal structures than fMRI. Indeed, the fMRI signal is affected by the proximity of air in the frontal sinuses. FMRI instrumentation and sequences of acquisition continue to be refined, with the goal of providing better spatial and temporal resolution and of minimizing physiological artifacts. MEG records electrical activity directly without the use of ionizing radiation and provides high temporal resolution, but moderate spatial resolution, particularly with respect to subcortical structures (Rojas et al., 2000). MEG is less available than either PET or fMRI, and thus fewer studies have been completed using this method. Figure 1 provides a comparison of the spatial and temporal resolution among imaging neuroscience tools.
Figure 1.
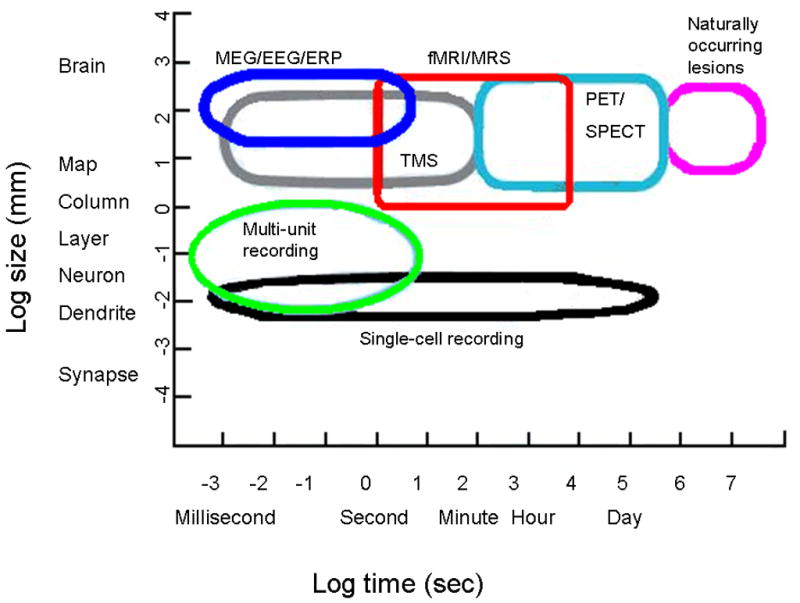
Figure illustrates some of the currently available cognitive neuroscience methods and sites of action on both temporal and spatial dimensions. Figure adapted from Churchland and Sejnowski, 1988.
The major drawback with PET is radiation exposure. It restricts the number of possible repeated measures, which is a serious limitation for developmental studies. Limits on radiation exposure to research subjects are set by regulatory bodies such as the US Department of Health and Human Services (e.g., http://www.hhs.gov, http://www.nrc.gov) and institutional radiation safety committees. In contrast, fMRI imposes no limit on repeated scanning. On the one hand, the PET signal (radioactive counts) is substantially stronger than the fMRI signal, and does not require large number of repeated measurements. On the other hand, repeated measures are required in the design of experiments that compare different experimental conditions, or for longitudinal studies. Regarding fMRI, it is important to stress that this technique is far from being without risk. Failure to carefully follow fMRI precaution guidelines, i.e., banning ferromagnetic material from the MR scanner room, can be lethal.
PET paired with radioligands and MRS provide neurochemical measures (Tables 1–2). Whereas PET and SPECT track the physiological behavior of molecules (e.g., neurotransmitter receptor binding potential, rate of neurotransmitter synthesis, synaptic concentration changes of neurotransmitter), MRS indexes the relative concentration of metabolites, whose functions are not as well-delineated (Table 3). PET and SPECT can detect very small concentrations (nanomolar range) of the compounds under study, whereas MRS is limited to larger amounts (millimolar range). For this reason, only compounds present in large quantities in the brain can be imaged. The main limitation in the advancement of PET and SPECT methods lies with the development of radioligands. In MRS, the sensitivity and specificity of the signals are the critical limiting factors. Tables 1–3 are providing exemplars of the measures that can be obtained with MRS, PET and SPECT.
Table 3. MRS Signal and functional significance.
Tables adapted from Herscovitch et al., 2000, and Yurgelun-Todd and Renshaw, 2000
| Functional significance | Signals |
|---|---|
| 1H-MRS | |
| Neuronal viability | N-acetyl-aspartate (NAA) |
| Levels increase with brain maturation | |
| Phospholipid metabolism | Choline (CHO) |
| Cellular energy metabolism | Creatinine + phospho creatine (CRE) |
| Anaerobic metabolism | Lactate (LAC) |
| 31P-MRS | |
| Information on the energy status of the brain | High energy phosphates, phosphocreatine (PCr) peak and polyphosphate regions of the spectrum (primarily ATP) |
| Building blocks of membrane phospholipids | Phosphomonoesters (PME) peak phosphoethanolamine(GPE), phosphocholine (PC), α-glycerol phosphate (GP) (sugar phosphate) |
| Major catabolic products of membrane phospholipid degradation and phospholipids | Phosphodiester (PDE) peak, GPE, GPC, mobile phospholipids |
| Final end-product of all of the phosphorus metabolites | Pi peak |
Potentially exciting future techniques are optical imaging (see chapter…) and the use of transcranial magnetic stimulation (TMS) in combination with other imaging tools. As a caveat, TMS’s potential adverse effects still need to be better characterized before being used experimentally in children.
Research in neurodevelopment is based principally on the study of healthy children. Ethical considerations mandate that studies with this vulnerable population be of minimal risk (http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm#subpartd), and as such, preclude the use of techniques such as PET or SPECT (Arnold et al., 2000; Munson et al., 2006). Although PET/SPECT studies have been conducted in healthy minors (Chugani et al., 1987; Ernst et al., 1997; Ernst et al., 1999; Takahashi et al., 1999), they will not be covered herein, since the majority of developmental work has been done with fMRI. Finally, because of the difficulty in imaging younger children and the paucity of studies in this age group, this chapter is dedicated to the late childhood/adolescence period.
Cognitive, affective, and social changes in adolescence
Adolescence is defined operationally as the transition period that characterizes the passage from childhood to adulthood. During this period, individuals acquire the skills that permit them to move away from the familial cell and start an independent and sexually active life. In humans, the skills necessary to make this transition successfully occur at multiple levels, within the cognitive, emotional and social spheres. These refinements result from synergistic changes in biology and environment. One of the most significant changes occurring in adolescence is puberty.
Puberty refers to sexual maturation, which affects both primary and secondary sexual characteristics, and results from adrenal and gonadic hormonal changes (for review see (Sisk and Foster, 2004). Besides physical development, pubertal maturation influences metabolism, and behavior through its modulation of brain function (Romeo et al., 2001; Romeo and Sisk, 2001; Nunez et al., 2002; Hebbard et al., 2003). The specificity and extent of hormonal-related cerebral changes are unclear. Studies of individuals with early or late puberty suggest that hormonal changes may not significantly affect cognitive development, in contrast to their influence on emotional development. Although the regional distribution of sex hormone receptors in the brain, i.e., sites of direct hormonal action, is fairly well documented (Yuan et al., 1995; Gu et al., 1999; Rosas-Arellano et al., 1999; Gundlah et al., 2000; Pareto et al., 2004; Catalano et al., 2005; Clark et al., 2006), the precise contribution of pubertal hormonal changes on the structural and functional development of the brain still needs to be determined. Research on the contribution of puberty to the shaping of brain function is wide open. Because of the scarcity of data using functional neuroimaging in this area, puberty will not be addressed in the remainder of this review.
Although cognitive function improves dramatically, qualitatively as well as quantitatively, from infancy into childhood, when the basic scaffold of adult cognition is in place, it continues to be refined across adolescence. Reaction time shortens (Williams et al., 1999; Bedard et al., 2002), motoric and cognitive inhibition improves (Munoz et al., 1998; Williams et al., 1999; Bedard et al., 2002), working memory capacity increases (Gathercole et al., 2004; Bayliss et al., 2005), computation processing expands as the number of items successfully manipulated increases (Bayliss et al., 2005), and the ability to set up rules for adaptive behavior that takes into account time estimation rises (McCormack et al., 1999). These elemental components of cognition contribute to permit adaptive goal-directed behavior.
However, observations suggest enhanced mood lability and emotional intensity in adolescence (Buchanan et al., 1992). Adolescence represents the peak onset of mood disorders (Pine et al., 1998; Angold et al., 1999), which supports the notion of alterations of affective regulation during this period. In addition, affective processes and response to reward/punishments, which contribute substantially to decision-making, may present in adolescence specific features that facilitate risk-taking behavior in this age group (Gerrard et al., 1996; Dahl, 2001; Martin et al., 2002).
Although also influenced by affective processing, social behavior presents unique characteristics in adolescence. This period is known for the primacy of peer relationships which tend to replace familial ties (Steinberg, 1989; Steinberg and Morris, 2001; Gardner and Steinberg, 2005). It is also a time for intense romantic involvement, as well as idealistic beliefs and extreme behaviors (Bouchey and Furman, 2003; Brown, 2004).
Neuroimaging in development
Structural neuroimaging has provided fundamental information regarding brain maturation. In adolescence, these structural changes vary by regions and include changes in the ratio of gray and white matter. Principally, white matter, an index of myelination, increases, while gray matter, an index of cellular and unmyelinated fiber density, decreases (Paus et al., 1999; Gogtay et al., 2006; Mabbott et al., 2006). Although the histological significance of these white and gray matter changes are not known at present, these changes could reflect, at least partially, the combination of processes of myelination, and pruning. Myelination provides faster communication between regions, and pruning more efficient neural coding. These two processes could contribute to faster reaction time and greater capacity for cognitive manipulation.
Of most interest, these structural changes are protracted and occur at different points in time according to regions. Changes seem to progress from posterior to anterior dorsal regions, such that parietal gray matter loss is most prominent from childhood to adolescence and frontal gray matter decrease is most salient from adolescence to adulthood (Sowell et al., 2004; Gogtay et al., 2006). Much less is known about ontogenic changes of subcortical structures. Reduced striatal volume with age has been reported (Giedd et al., 1996), whereas no systematic changes in hippocampal volume could be detected (Suzuki et al., 2005).
The significance of these changes at the behavioral level is not clear. The contribution of learning and experience vs. genetically determined alterations need to be elucidated. It is also critical to obtain information on connectivity. Diffusion tensor imaging can contribute to this question but improvements to the technique are still needed to interpret the findings (Neil et al., 2002; Schmithorst et al., 2002; Bengtsson et al., 2005). Functional neuroimaging can also help translate the functional significance of these structural changes.
The regional heterogeneity of maturation rates may have critical implications for the neural basis of the adolescent behavior. An attractive hypothesis is that of an imbalance between cognitive and emotional processing networks, mediated by a developmental shift in the balance between mesolimbic/striatal and mesocortical dopamine systems during adolescence (Spear, 2000; Andersen, 2003). A related hypothesis proposes an adolescent-typical pattern of equilibrium among three systems, approach, avoidance and supervisory systems (Ernst et al., 2006). Adolescent behavior suggests enhanced recruitment of limbic structures associated with the coding of emotions, and reduced or less efficient recruitment of cognitive inhibitory structures such as the ventrolateral prefrontal cortex. Approach-related structures, such as ventral striatum, that preferentially translate sensory inputs into appetitive stimuli to be approached, might be engaged more easily than are avoidance-related structures, such as amygdala, which preferentially translate sensory inputs into aversive stimuli to be avoided. Both scenarios would explain a propensity for intense emotions, novelty-seeking and risk-taking behavior, and diminished control over emotional processing.
At present, neurodevelopmental research using functional neuroimaging has been limited to cross-over studies that compare different age groups. Longitudinal studies, the gold-standard for examining development, are difficult to conduct, not only because of the research cost, but also in view of the rapid changes in instrumentation which often compromise the reliability of repeated measures. As will be seen below, three basic potential group differences can be noted in the immature brain: (1) lesser engagement of the mature network; (2) greater engagement of the mature network; (3) engagement of a different network, or any combination of the above. The interpretation of such findings continues to be speculative, and will need to be corroborated by additional judiciously designed experiments.
Developmental functional neuroimaging
Cognitive processes: Attention, working memory and response inhibition
The general principle underlying fMRI studies relies on the subtraction rule (Owen et al., 2001). This rule posits that task performance can engage several elemental cognitive processes, each of them relying on specific neural circuits. By manipulating the tasks and the type of cognitive processes being engaged, the specific circuit recruited by a given cognitive process can be isolated through subtraction of the different activation maps. For example, the activation map of a decision-making task associated with monetary rewards subtracted from the activation map of the same task without monetary rewards would provide information on the neural processing of rewards. Although simplistic, this theoretical framework seems to be adequate for most research questions.
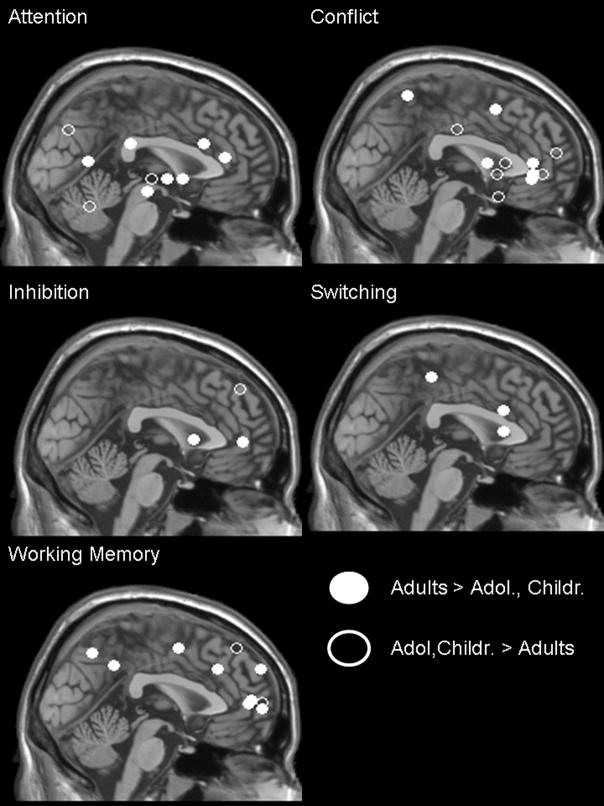
A number of processes have been studied this way, including attention, working memory, response inhibition, and reward-related performance (Figure 2). These normative developmental studies are still in their infancy and need not only to be further validated but also to be examined from different perspectives to better understand their functional and developmental significance.
Figure 2.
| Function | Reference | Talairach Coordinates (x, y, z) | ||
|---|---|---|---|---|
| Attention | Konrad, 2005 | −30 | 27 | −47 |
| −9 | −18 | −12 | ||
| 33 | −15 | −2 | ||
| 54 | −60 | 12 | ||
| Rubia, 2006 | −50 | −30 | 26 | |
| −32 | 7 | −2 | ||
| −29 | 37 | 15 | ||
| 3 | −74 | 37 | ||
| 25 | −4 | −2 | ||
| 40 | −59 | −24 | ||
| Conflict | Adleman, 2002 | −28 | 12 | 52 |
| Konrad, 2005 | −27 | 56 | 17 | |
| −18 | −52 | 63 | ||
| 45 | 38 | −4 | ||
| 53 | 14 | −18 | ||
| Marsh, 2006 | 7 | 46 | 0 | |
| 10 | 19 | 9 | ||
| 21 | −16 | 36 | ||
| −36 | 39 | 0 | ||
| −31 | 6 | 9 | ||
| −39 | 39 | 9 | ||
| 36 | 13 | 0 | ||
| Inhibition | Rubia, 2006 | 4 | 48 | 4 |
| Tamm, 2002 | −34 | 12 | 6 | |
| −14 | 46 | 46 | ||
| Switching | Rubia, 2006 | 14 | 19 | 26 |
| 32 | 19 | 9 | ||
| −40 | −33 | 53 | ||
| Working Memory | Klingberg, 2002 | 22 | −64 | 48 |
| 30 | 0 | 52 | ||
| Kwon, 2002 | −38 | 54 | 10 | |
| 48 | −48 | 38 | ||
| Schweinsburg, 2005 | 2 | 52 | 7 | |
| 30 | 62 | 3 | ||
| 26 | 61 | 35 | ||
| 44 | 29 | 35 | ||
| 12 | 62 | 8 | ||
| 44 | 43 | 52 | ||
Attention and Working Memory
Only a few studies have examined the development of attention using functional neuroimaging (Konrad et al., 2005). Konrad et al (2005) showed in children between 8–12 yo (N = 16) significantly reduced activity in brain areas associated with attention such as the frontal midbrain or temporo-parietal areas, relative to older controls (ages 20–34, N = 16). However, more areas outside the expected networks were activated in children relative to adults, which suggested that additional neural networks were required to support the underdeveloped attentional networks.
Developmental studies of working memory have focused mostly on visuospatial information rather than object or verbal stimuli. Most studies found enhanced activation with age (8 or 9 yo through 18 yo) in the circuits already identified in adults for supporting this process: dorsal or lateral prefrontal cortex and intra- or posterior parietal cortex (Klingberg et al., 2002; Kwon et al., 2002; Olesen et al., 2003).
In addition, sex differences in working memory performance seemed to emerge in adolescence. Schweinsburg et al (Schweinsburg et al., 2005) reported that males showed greater activity in frontopolar cortex during a spatial working memory task, while females exhibited reduced activity in the anterior cingulate. This suggested that males and females recruited different brain regions to perform a spatial working memory task.
Response Inhibition
A combination of increased and decreased regional activations with age has been reported in studies of response inhibition. A number of paradigms have been used, including antisaccade (Luna et al., 2001), go/no-go (Tamm et al., 2002; Durston and Casey, 2006; Rubia et al., 2006), stop signal (Rubia et al., 2006), and Stroop tasks (Adleman et al., 2002; Marsh et al., 2006).
Luna et al. (2001) contrasted fMRI results among children (7–12 yo), adolescents (13–17 yo) and young adults (18–22 yo) during the performance of an antisaccade task, and found decreased activation in the superior middle gyrus but increased activation in the striatum, intra-parietal sulcus, frontal eye field and lateral cerebellum with age. Activation of the dorsolateral prefrontal cortex was found to peak in adolescents relative to children and adults. How these qualitatively different developmental trajectories influence each other, and are integrated into patterns that subserve age-related typical cognitive control, is critical to understand.
Using the go/no-go task, Tamm et al (2002) reported age-related increased activation in left inferior frontal gyrus and reduced activation in superior and middle frontal gyri between 8 years and 20 years of age. Based on the notion that findings in adults converge towards the inferior prefrontal cortex as the seat for inhibitory control, the findings in adolescents suggest a strengthening of the mature substrates of inhibition, while relying less on the more immature neural correlates of inhibition. Similar activations in inferior frontal and anterior cingulate area have been reported in another developmental sample (8 to 20 years) (Durston, Mulder et al., 2006). Rubia et al. (2006) found increased prefrontal, temporo-parietal and striatal activation with age (10–17 yo vs. 20–43 yo).
Using the Stroop task, Adleman et al (2002) found increased activation in parietal cortex between children (7–11 yo) and adolescents (12–16 yo), and then increase in left middle frontal gyrus between adolescents and young adults (18–22 yo). This finding suggests qualitative differences in neural maturation in children and adolescents. Marsh et al. (2006) compared 20 children (mean age 13.5 years) with 50 adults (mean age 31.9 years), and reported greater activation in adults than in children in brain areas typically activated by the Stroop task (right frontostriatal circuits) and known to subserve self-regulatory function.
Overall, adults seem to be more able to recruit a specific circuit supporting response inhibition. Because of the differences among studies, including task constructs, age ranges, and sample sizes, it is difficult to clearly delineate the neural network responsible for response inhibition per se. The significance of decreased activation with age suggests disengagement of alternative neural circuits used in younger populations. However, the recruitment of these alternative neural networks may represent reliance on different cognitive strategies employed by younger individuals. It may also signal ongoing learning processes, or nonspecific age-group differences in their efforts to complete the task. Each of these interpretations can be tested in future work.
Affective and social processes
To date, much neuroimaging research directed toward the study of emotion has used paradigms involving the perception of facial expressions (Nelson et al., 2003; Lieberman et al., 2005; Meyer-Lindenberg et al., 2005; Noesselt et al., 2005; Pessoa et al., 2005; Fitzgerald et al., 2006; Rich et al., 2006; Roberson-Nay et al., 2006; Williams et al., 2006). These studies have focused primarily on the amygdala, which has been associated with fear processing (LeDoux, 2000) and social coding (Adolphs, 2003). Furthermore, negative emotions have been assessed more consistently, primarily because these emotions are easier to evoke and to control experimentally than positive emotions.
Davidson and Slagter (2000) have pointed out potential issues regarding developmental studies of emotion. For example, the perception and the production of emotion may be more difficult to separate in children than in adults, because stimuli used to probe emotion (e.g., facial expressions) may more readily evoke emotion in children than in adults. For this reason, the evaluation of behavioral performance and physiological reactions become important adjuncts in neuroimaging studies of emotion.
The largest neuroimaging developmental study of emotion to date compared 30 healthy adults (21–40 yo) with 31 healthy adolescents (9–17 yo) on a facial emotion task (Figure 3) (Guyer et al., in press). Findings replicated previous work (Monk et al., 2003), showing greater activation of the amygdala in adolescents compared to adults during passive viewing of fearful vs. neutral faces. In addition, adults showed greater amygdala-hippocampal functional connectivity than adolescents across the whole task. This finding may suggest that maturational changes strengthen amygdala/hippocampus interactions in forming memories of faces (Kilpatrick and Cahill, 2003; Dolcos et al., 2005). This interpretation would be in line with the continuing development of the relationship between cognitive and affective systems during adolescence (Casey et al., 2000; Nelson et al., 2005; Ernst et al., 2006). Of note, sex and age were not correlated with amygdala activation in neither the adolescent group nor the adult group (Guyer et al in press).
Figure 3.
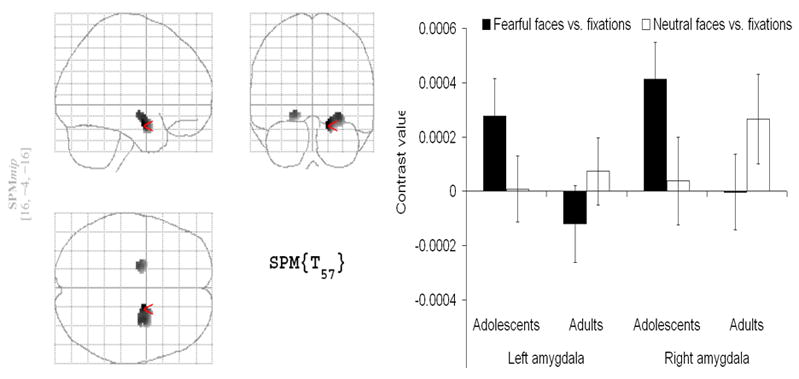
SPM99 glass brain indicating greater amygdala activation for adolescents relative to adults in response to an emotional face viewing task (Guyer et al., in press).
The main finding of greater amygdala activation in adolescents than in adults in response to fearful faces was consistent with prior work (Monk et al., 2003; Killgore and Yurgelun-Todd, 2004), but in opposite direction to the finding by Thomas et al. (Thomas et al., 2001), which used a sample of 12 adolescents (9–13 yo) and 6 adults (18–30 yo) as well as a different experimental design.
These findings are based on passive viewing, which, by nature, prevents the collection of self-reports. Consequently, their interpretation is limited. However, Guyer et al. (in press) also collected fear ratings in a different condition, and showed that these ratings did not differ between adults and adolescents. In addition, they included independently obtained eye movement data during the viewing of the faces. These data showed no differences in patterns of eye movements between adults and adolescents, perhaps indexing similar level of attention directed to the faces.
The field of affective developmental neuroscience research is wide open. The study of response to evocative face stimuli, which combines basic features of affective processing with features of social processing is only one way to probe the coding of emotions. Work is currently being conducted on social processes per se, using economic exchange paradigms and other tasks targeting issues of affiliations, fairness, trust, and theory of mind. Models of the neural networks involved in social processing are beginning to be proposed (Nelson et al., 2005), generating a priori hypotheses that can be tested in future work. It will be important to control for differences in emotion processing in these social paradigms, and, reciprocally, control for differences in social processing in affective paradigms, when appropriate.
Reward circuitry
Reward-related processes are at the heart of motivated behavior. As such, they have been the object of a huge body of research. Within neuroscience, they have been probed within three major disciplines: addiction (Pecina et al., 2006; Mahler et al., 2007), learning (Martin-Soelch et al., 2007), and decision-making (Ernst and Paulus, 2005). A third field, neuroeconomics, is emerging (Sanfey et al., 2003; Huettel et al., 2006). The situation at a cross road of converging research domains presents, on the one hand, the advantage of generating a large amount of information to understand these processes, yet, on the other hand, the drawback of the potential for mixing terminology that carries slightly different meaning (e.g., reward can refer to positive reinforcement in learning theories, induction of positive valence state in affective neuroscience, or positive utility value in economics).
With this caveat in mind, developmental “reward” neuroscience work is starting to raise considerable interest.
Much of this work has been reviewed in the recent description of the “triadic model” (Ernst et al., 2006). Since then, a few studies have been published (Galvan et al., 2006; van Leijenhorst et al., 2006), supporting the basic tenets of Ernst et al’s model (Figure 4). This developmental neurophysiological framework of reward processes originally proposed that the balance among three behavioral control systems differs between adults and adolescents. These three systems include an approach behavioral system, centered on the ventral striatum, an avoidance behavioral system, centered on the amygdala, and a supervisory system, centered on the medial prefrontal cortex.
Figure 4.
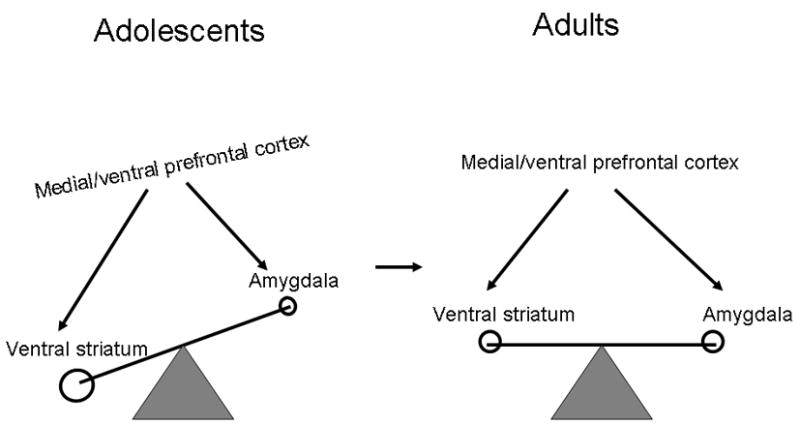
The triadic model from Ernst et al (2006) shows a functional imbalance among medial/ventral prefrontal cortex, ventral striatum and amygdala circuits in adolescents (left half) relative to the adult equilibrium (right half).
The triadic model is based on a priori hypotheses that find their origins in evolutionary theories. We speculate that the adolescent brain undergoes unique changes that promote behaviors supporting the evolutionary fitness theme of optimal species reproduction. Adolescence is the time when individuals begin to move away from the familial cell and acquire independence in order to fulfill adult roles in terms of reproductively mature and socially competent behavior. This impetus to move away from the protective nest is necessary for preventing genetic inbreeding and for enabling genetic diversity, which serves the overall evolutionary fitness goal. Behaviors of novelty-seeking and risk-taking facilitate this transition. As such, neural circuits that code approach behavior, such as ventral striatum, are likely to be more dominant during adolescence, whereas neural circuits that code avoidance, such as amygdala, are likely to be less powerful during adolescence than at other life periods.
A number of functional neuroimaging findings are consistent with this model. First, adolescents generally recruited a similar reward circuitry (May et al., 2004) as that identified in adults (Delgado et al., 2000) when responding to rewards or punishments. However, direct comparison of adults and adolescents showed that adolescents activated the ventral striatum more strongly than adults in response to rewarding stimuli (Ernst et al., 2005; Galvan et al., 2006), and deactivated the amygdala less strongly than adults in response to penalties (Ernst et al., 2005). In addition, during choice-making, adolescents engaged the medial prefrontal cortex, including the anterior cingulate gyrus, and orbital frontal cortex to a lesser extent than did adults (Figure 5)(Eshel et al., 2007).
Figure 5.
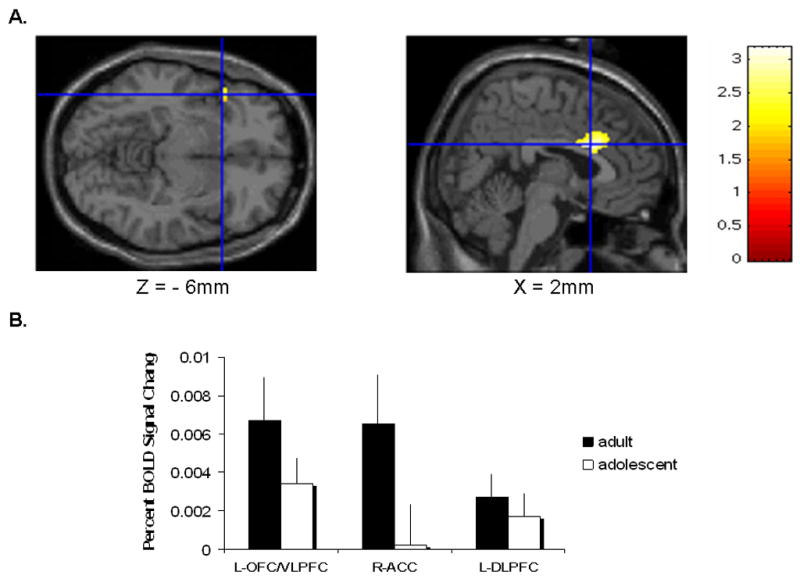
Coronal and sagittal views of the SPM99 T1 MRI brain, which shows activation loci that are greater for adults than adolescents in a decision making task. These foci are located in the left orbitofrontal/ventrolateral prefrontal cortex and anterior cingulate cortex (Eshel et al. 2007).
Adolescents may differ from pre-adolescents in the function of the reward circuitry (Galvan et al., 2006). Indeed, pre-adolescents (mean age 11.3 years) were found to show greater modulation of the medial prefrontal cortex as a function of the likelihood of receiving a reward, during the anticipation of this reward, relative to adults (mean age 21 years) (van Leijenhorst et al., 2006). This is in contrast to Eshel’s findings comparing response selection between adolescents (13.3 years old) and adults (see above). The discrepancy between both sets of findings might also relate to the different paradigms employed, rather than maturational changes between pre-adolescence and adolescence. Van Leijenhorst et al. (2006) manipulated the probability rather than the magnitude of the reward, and choice selection did not involve competing options, whereas Eshel et al. (2007) used a paradigm involving decision-making between two competing options that carried different risks and reward magnitudes. Thus, differences in age groups (13.3 yo vs. 11.3 yo) and/or in paradigms may account for the opposite findings of these studies with regards to the recruitment of medial prefrontal cortex in reward-processes.
Finally, Bjork et al. (2004) employed a monetary reaction-time task to directly compare adults and adolescents, and found that adolescents recruited less strongly the ventral striatum when anticipating possible gains. This finding, at odds with the studies by Ernst et al. (2005) and Galvan et al. (2006), highlights the need for more work.
Future Direction
The present overview provides a window into the promises of developmental cognitive neuroscience research. Among these promises, three major themes stand out.
First, from a methodological standpoint, more knowledge is warranted to understand how developing brain physiology affects functional imaging parameters, to formulate standardized procedures and to create age-appropriate structural brain templates.
Second, the increased use of neuroimaging techniques in young populations will help to interpret findings from the traditional developmental psychology perspective and generate neuroanatomical models of cognitive, affective and social processes of behaviour. For instance, neuroimaging data could be used to support cognitive theories of memory development (Reyna and Brainerd, 1998), or elucidate the brain mechanisms that underlie cognitive (Gardner and Steinberg, 2005; Steinberg, 2005) or evolutionary (Ernst et al., 2006) models that seek to explain adolescence as an increased period of vulnerability for psychiatric disorders.
Third, based on the recognition that most adult psychiatric disorders have their biological roots anchored in the developing brain, it appears paramount to be able to identify and understand the mechanism of these vulnerability factors across development. Normative data, including the role of sex and puberty, are clearly a requisite for this agenda and research is being under way to address this question using populations at risk by virtue of temperament or family history of psychiatric disorders (Guyer et al., 2006; Perez-Edgar et al., 2007; Monk et al., in press).
Finally, although not addressed in this chapter, a critical task of developmental cognitive neuroscience research will be to integrate molecular genetic advances with functional neuroimaging studies.
Footnotes
Invited contribution to special edition entitled “Dynamic imaging of the developing nervous system” (J. Glover, Ed.)
References
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Is the human amygdala specialized for processing social information? Ann N Y Acad Sci. 2003;985:326–340. doi: 10.1111/j.1749-6632.2003.tb07091.x. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychol Med. 1999;29:1043–1053. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- Arnold LE, Zametkin A, Caravella L, Korbly N. Ethical issues in neuroimaging research with children. In: Ernst M, Rumsey JM, editors. Functional neuroimaging in child psychiatry. Cambridge, UK: Cambridge University Press; 2000. pp. 99–109. [Google Scholar]
- Bayliss DM, Jarrold C, Baddeley AD, Gunn DM, Leigh E. Mapping the developmental constraints on working memory span performance. Dev Psychol. 2005;41:579–597. doi: 10.1037/0012-1649.41.4.579. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Nichols S, Barbosa JA, Schachar R, Logan GD, Tannock R. The development of selective inhibitory control across the life span. Dev Neuropsychol. 2002;21:93–111. doi: 10.1207/S15326942DN2101_5. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchey HA, Furman W. Dating and romantic experience during adolescence. In: Adams GR, Berzonsky MD, editors. Blackwell Handbook of Adolescence. Oxford: Blackwell Sci; 2003. pp. 313–329. [Google Scholar]
- Brown BB. Adolescents’ relationships with peers. In: Lerner RM, Steinberg L, editors. Handbook of Adolescent Psychology. New York: Wiley; 2004. pp. 363–394. [Google Scholar]
- Buchanan CM, Eccles JS, Becker JB. Are adolescents the victims of raging hormones: evidence for activational effects of hormones on moods and behavior at adolescence. Psychol Bull. 1992;111:62–107. doi: 10.1037/0033-2909.111.1.62. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Catalano PN, Bonaventura MM, Silveyra P, Bettler B, Libertun C, Lux-Lantos VA. GABA(B1) knockout mice reveal alterations in prolactin levels, gonadotropic axis, and reproductive function. Neuroendocrinology. 2005;82:294–305. doi: 10.1159/000093128. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- Clark AS, Costine BA, Jones BL, Kelton-Rehkopf MC, Meerts SH, Nutbrown-Greene LL, Penatti CA, Porter DM, Yang P, Henderson LP. Sex- and age-specific effects of anabolic androgenic steroids on reproductive behaviors and on GABAergic transmission in neuroendocrine control regions. Brain Res. 2006;1126:122–138. doi: 10.1016/j.brainres.2006.08.081. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Affect regulation, brain development, and behavioral/emotional health in adolescence. CNS Spectr. 2001;6:60–72. doi: 10.1017/s1092852900022884. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Slagter HA. Probing emotion in the developing brain: functional neuroimaging in the assessment of the neural substrates of emotion in normal and disordered children and adolescents. Ment Retard Dev Disabil Res Rev. 2000;6:166–170. doi: 10.1002/1098-2779(2000)6:3<166::AID-MRDD3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci U S A. 2005;102:2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Casey BJ. What have we learned about cognitive development from neuroimaging? Neuropsychologia. 2006;44:2149–2157. doi: 10.1016/j.neuropsychologia.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adolesc Psychiatry. 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Eden GF, Zeffiro TA. Functional magnetic resonance imaging. In: Ernst M, Rumsey JM, editors. Functional neuroimaging in child psychiatry. Cambridge, UK: Cambridge University Press; 2000. pp. 45–58. [Google Scholar]
- Ernst M, Cohen RM, Liebenauer LL, Jons PH, Zametkin A. Cerebral Glucose Metabolism In Adolescent Girls with Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. 1997;36:1399–1406. doi: 10.1097/00004583-199710000-00022. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Zametkin A, Matochik JA, Pascualvaca D, Jons P, Cohen RM. High midbrain DOPA decarboxylase activity in children in ADHD. Am J Psychiatry. 1999;156:1209–1215. doi: 10.1176/ajp.156.8.1209. [DOI] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Dev Psychol. 2005;41:625–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H. The structure of working memory from 4 to 15 years of age. Dev Psychol. 2004;40:177–190. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Gerrard M, Gibbons FX, Benthin A, Hessling RM. A longitudinal study of the reciprocal nature of risk behaviors and cognitions in adolescents: what you do shapes what you think, and vice versa. Health Psychology. 1996;15:344–354. doi: 10.1037//0278-6133.15.5.344. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF, 3rd, Herman DH, Ordonez A, Greenstein D, Hayashi KM, Clasen L, Toga AW, Giedd JN, Rapoport JL, Thompson PM. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Gu G, Varoqueaux F, Simerly RB. Hormonal regulation of glutamate receptor gene expression in the anteroventral periventricular nucleus of the hypothalamus. J Neurosci. 1999;19:3213–3222. doi: 10.1523/JNEUROSCI.19-08-03213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res Mol Brain Res. 2000;76:191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure EB, Nelson EE, Roberson-Nay R, Adler A, Leibenluft E, Pine DS, Ernst M. Developmental differences in amygdala response to fearful facial expressions in press. [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, Bjork JM, Henderson HA, Pine DS, Fox NA, Ernst M. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J Neurosci. 2006;26:6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbard PC, King RR, Malsbury CW, Harley CW. Two organizational effects of pubertal testosterone in male rats: transient social memory and a shift away from long-term potentiation following a tetanus in hippocampal CA1. Exp Neurol. 2003;182:470–475. doi: 10.1016/s0014-4886(03)00119-5. [DOI] [PubMed] [Google Scholar]
- Herscovitch P, Ernst M. Functional brain imaging with PET and SPECT. In: Ernst M, Rumsey JM, editors. Functional neuroimaging in child psychiatry. Cambridge, UK: Cambridge University Press; 2000. pp. 3–26. [Google Scholar]
- Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML. Neural signatures of economic preferences for risk and ambiguity. Neuron. 2006;49:765–775. doi: 10.1016/j.neuron.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. Neuroimage. 2004;21:1215–1223. doi: 10.1016/j.neuroimage.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage. 2003;20:2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J Cogn Neurosci. 2002;14:1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Thiel CM, Specht K, Hanisch C, Fan J, Herpertz-Dahlmann B, Fink GR. Development of attentional networks: an fMRI study with children and adults. Neuroimage. 2005;28:429–439. doi: 10.1016/j.neuroimage.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc Natl Acad Sci U S A. 2002;99:13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Hariri A, Jarcho JM, Eisenberger NI, Bookheimer SY. An fMRI investigation of race-related amygdala activity in African-American and Caucasian-American individuals. Nat Neurosci. 2005;8:720–722. doi: 10.1038/nn1465. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Mabbott DJ, Noseworthy M, Bouffet E, Laughlin S, Rockel C. White matter growth as a mechanism of cognitive development in children. Neuroimage. 2006;33:936–946. doi: 10.1016/j.neuroimage.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC. Endocannabinoid Hedonic Hotspot for Sensory Pleasure: Anandamide in Nucleus Accumbens Shell Enhances ‘Liking’ of a Sweet Reward. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, Peterson BS. A developmental fMRI study of self-regulatory control. Hum Brain Mapp. 2006;27:848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Soelch C, Linthicum J, Ernst M. Appetitive conditioning: Neural bases and implications for psychopathology. Neurosci Biobehav Rev. 2007;31:426–440. doi: 10.1016/j.neubiorev.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, Omar HA. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. J Am Acad Child Adolesc Psychiatry. 2002;41:1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, Carter CS. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biol Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- McCormack T, Brown GD, Maylor EA, Darby RJ, Green D. Developmental changes in time estimation: comparing childhood and old age. Dev Psychol. 1999;35:1143–1155. doi: 10.1037//0012-1649.35.4.1143. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, Morris CA, Berman KF. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat Neurosci. 2005;8:991–993. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, III, Guardino M, Masten CL, McClure EB, Fromm S, Blair RJR, Pine DS, Ernst M. Amygdala and Nucleus Accumbens Activation to Emotional Facial Expressions in Children and Adolescents at Risk for Major Depression. American Journal of Psychiatry. doi: 10.1176/appi.ajp.2007.06111917. in press. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Broughton JR, Goldring JE, Armstrong IT. Age-related performance of human subjects on saccadic eye movement tasks. Exp Brain Res. 1998;121:391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- Munson S, Eshel N, Ernst M. Ethics of PET Research in Children. In: Charron M, editor. Pediatric PET Imaging. New York: Springer; 2006. [Google Scholar]
- Muzik O, Chugani DC, Juhasz C, Shen C, Chugani HT. Statistical parametric mapping: assessment of application in children. Neuroimage. 2000;12:538–549. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- Neil J, Miller J, Mukherjee P, Huppi PS. Diffusion tensor imaging of normal and injured developing human brain - a technical review. NMR Biomed. 2002;15:543–552. doi: 10.1002/nbm.784. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nelson EE, McClure EB, Monk CS, Zarahn E, Leibenluft E, Pine DS, Ernst M. Developmental differences in neuronal engagement during implicit encoding of emotional faces: an event-related fMRI study. J Child Psychol Psychiatry. 2003;44:1015–1024. doi: 10.1111/1469-7610.00186. [DOI] [PubMed] [Google Scholar]
- Noesselt T, Driver J, Heinze HJ, Dolan R. Asymmetrical activation in the human brain during processing of fearful faces. Curr Biol. 2005;15:424–429. doi: 10.1016/j.cub.2004.12.075. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Sakuma H, Takeda K, Tagami T, Okuda Y, Nakagawa T. Diffusional anisotropy of the human brain assessed with diffusion-weighted MR: relation with normal brain development and aging. AJNR Am J Neuroradiol. 1994;15:231–238. [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, Sodhi J, Juraska JM. Ovarian hormones after postnatal day 20 reduce neuron number in the rat primary visual cortex. J Neurobiol. 2002;52:312–321. doi: 10.1002/neu.10092. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Brain Res Cogn Brain Res. 2003;18:48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Owen AM, Epstein R, Johnsrude IS. fMRI: applications to cognitive neuroscience. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI. New York: Oxford University Press; 2001. pp. 311–327. [Google Scholar]
- Pareto D, Alvarado M, Hanrahan SM, Biegon A. In vivo occupancy of female rat brain estrogen receptors by 17beta-estradiol and tamoxifen. Neuroimage. 2004;23:1161–1167. doi: 10.1016/j.neuroimage.2004.07.036. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pecina S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12:500–511. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- Perez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, McClure EB, Henderson HA, Fox NA, Pine DS, Ernst M. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35:1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Padmala S, Morland T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. Neuroimage. 2005;28:249–255. doi: 10.1016/j.neuroimage.2005.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Pare-Blagoev EJ, Grant PE. Pediatric functional magnetic resonance imaging: progress and challenges. Top Magn Reson Imaging. 2002;13:61–70. doi: 10.1097/00002142-200202000-00005. [DOI] [PubMed] [Google Scholar]
- Reyna VF, Brainerd CJ. Fuzzy-trace theory and false memory: new frontiers. J Exp Child Psychol. 1998;71:194–209. doi: 10.1006/jecp.1998.2472. [DOI] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson-Nay R, McClure EB, Monk CS, Nelson EE, Guyer AE, Fromm SJ, Charney DS, Leibenluft E, Blair J, Ernst M, Pine DS. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: An FMRI study. Biol Psychiatry. 2006;60:966–973. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Teale PD, Reite ML. Magnetoencephalography. In: Ernst M, Rumsey JM, editors. Functional neuroimaging in child psychiatry. Cambridge, UK: Cambridge University Press; 2000. pp. 77–95. [Google Scholar]
- Romeo RD, Cook-Wiens E, Richardson HN, Sisk CL. Dihydrotestosterone activates sexual behavior in adult male hamsters but not in juveniles. Physiol Behav. 2001;73:579–584. doi: 10.1016/s0031-9384(01)00499-1. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Sisk CL. Pubertal and seasonal plasticity in the amygdala. Brain Res. 2001;889:71–77. doi: 10.1016/s0006-8993(00)03111-5. [DOI] [PubMed] [Google Scholar]
- Rosas-Arellano MP, Solano-Flores LP, Ciriello J. Co-localization of estrogen and angiotensin receptors within subfornical organ neurons. Brain Res. 1999;837:254–262. doi: 10.1016/s0006-8993(99)01672-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Sweeney JA, Gillen JS, Kim J, Varanelli MJ, O’Hearn KM, Erb PA, Davis D, Thulborn KR. Magnetic resonance imaging of children without sedation: preparation with simulation. J Am Acad Child Adolesc Psychiatry. 1997;36:853–859. doi: 10.1097/00004583-199706000-00024. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma H, Nomura Y, Takeda K, Tagami T, Nakagawa T, Tamagawa Y, Ishii Y, Tsukamoto T. Adult and neonatal human brain: diffusional anisotropy and myelination with diffusion-weighted MR imaging. Radiology. 1991;180:229–233. doi: 10.1148/radiology.180.1.2052700. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Saunders DE, Thompson C, Gunny R, Jones R, Cox T, Chong WK. Magnetic resonance imaging protocols for paediatric neuroradiology. Pediatr Radiol. 2007;37:789–797. doi: 10.1007/s00247-007-0462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Tapert SF. fMRI reveals alteration of spatial working memory networks across adolescence. J Int Neuropsychol Soc. 2005;11:631–644. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Slifer KJ, Cataldo MF, Cataldo MD, Llorente AM, Gerson AC. Behavior analysis of motion control for pediatric neuroimaging. J Appl Behav Anal. 1993;26:469–470. doi: 10.1901/jaba.1993.26-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Pubertal maturation and parent-adolescent distance: an evoluntionary perspective. In: Adams GR, Montemayor R, Gullotta TP, editors. Advances in adolescent behavior and development. Newbury Park: Sage; 1989. pp. 71–97. [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Morris AS. Adolescent development. Annu Rev Psychol. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Hagino H, Nohara S, Zhou SY, Kawasaki Y, Takahashi T, Matsui M, Seto H, Ono T, Kurachi M. Male-specific volume expansion of the human hippocampus during adolescence. Cereb Cortex. 2005;15:187–193. doi: 10.1093/cercor/bhh121. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Shirane R, Sato S, Yoshimoto T. Developmental changes of cerebral blood flow and oxygen metabolism in children. AJNR Am J Neuroradiol. 1999;20:917–922. [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Salidis J, Deutsch GK, Tallal P, Merzenich MM, Gabrieli JD. Disrupted neural responses to phonological and orthographic processing in dyslexic children: an fMRI study. Neuroreport. 2001;12:299–307. doi: 10.1097/00001756-200102120-00024. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, Birmaher V, Casey BJ. A developmental functional MRI study of spatial working memory. Neuroimage. 1999;10:327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Tien RD, Kucharczyk J, Bessette J, Middleton M. MR imaging of the pituitary gland in infants and children: changes in size, shape, and MR signal with growth and development. AJR Am J Roentgenol. 1992;158:1151–1154. doi: 10.2214/ajr.158.5.1566682. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L, Crone EA, Bunge SA. Neural correlates of developmental differences in risk estimation and feedback processing. Neuropsychologia. 2006;44:2158–2170. doi: 10.1016/j.neuropsychologia.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Dev Psychol. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Williams LM, Liddell BJ, Kemp AH, Bryant RA, Meares RA, Peduto AS, Gordon E. Amygdala-prefrontal dissociation of subliminal and supraliminal fear. Hum Brain Mapp. 2006;27:652–661. doi: 10.1002/hbm.20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Bowlby DA, Brown TJ, Hochberg RB, MacLusky NJ. Distribution of occupied and unoccupied estrogen receptors in the rat brain: effects of physiological gonadal steroid exposure. Endocrinology. 1995;136:96–105. doi: 10.1210/endo.136.1.7828562. [DOI] [PubMed] [Google Scholar]