Abstract
Tlymphocytes recognize antigens as peptide fragments associated with molecules encoded by the major histocompatibility complex (MHC) and expressed on the surface of antigen-presenting cells1. In the thymus, T cells bearing αβ receptors that react with the MHC molecules expressed by radioresistant stromal elements are positively selected for maturation2–5. In (A × B → A) bone marrow chimaeras, T cells restricted to the MHC-A haplotype are positively selected, whereas MHC-B-reactive thymocytes are not. We investigated whether the introduction of particular thymic stromal elements bearing MHC-B molecules could alter the fate of B-reactive T cells in these (A × B → A) chimaeras. Thymic epithelial cell (TEC) lines expressing H-2b were introduced by intrathymic injection into (H-2b/s → H2S) bone marrow chimaeras and we measured their ability to generate H-2b-restricted cytotoxic T-lymphocytes (CTLs). We report here that one TEC line, 427.1, was able positively to select CTLs specific for influenza and vesicular stomatitis virus antigens in association with class I H–2b molecules. In addition, line 427.1 can process cytoplasmic proteins for presentation to H–2Kb- and H-2Db-restricted CTLs. Thus, a TEC line capable of normal class I MHC antigen processing and presentation in vitro can induce positive selection after intrathymic injection.
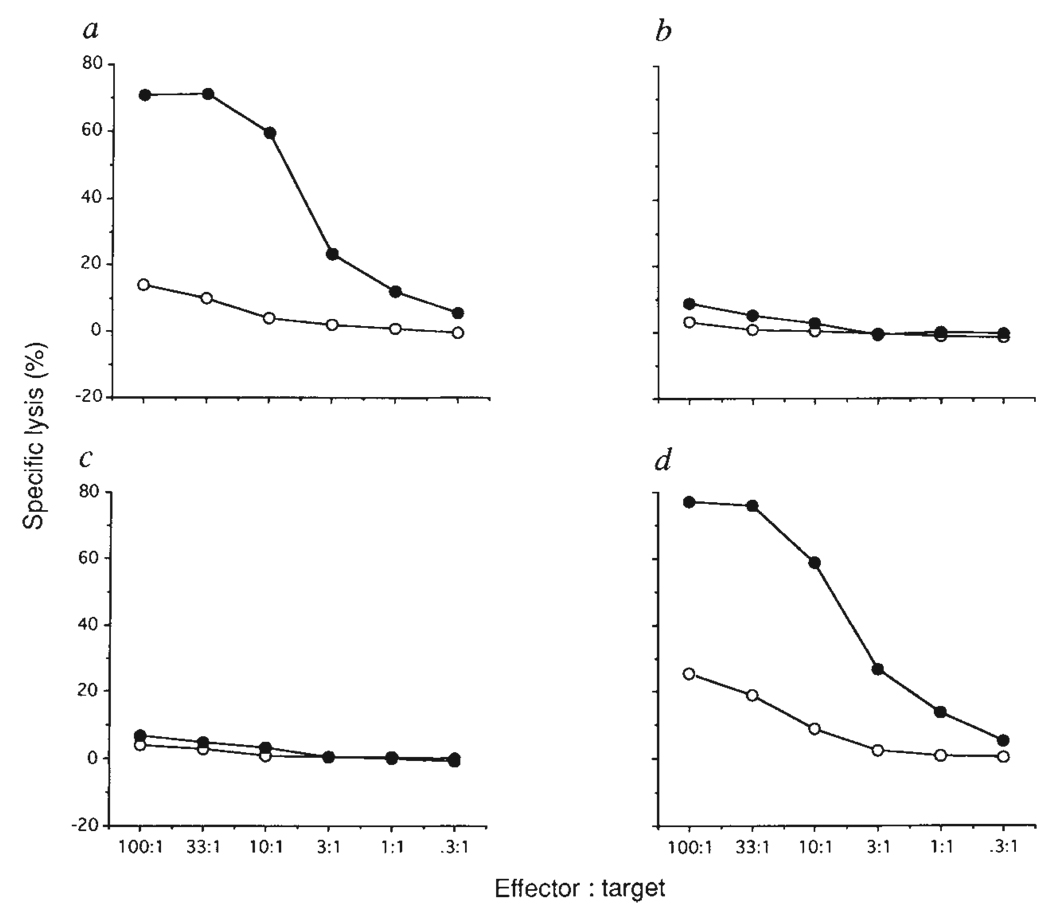
TEC lines 427.1 and 1308.1 were derived from Simian Virus 40 (SV40) T antigen transgenic (B6 × SJL)F2 mice (ref. 6 and S.J.F., J. L. Rothstein and B.B.K., manuscript in preparation). Both cell lines express H-2Kb and H-2Db class I MHC molecules (data not shown). The ability of these cell lines positively to select H-2b-restricted CTLs was tested by intrathymic injection into ((B6 × SJL)F1 → SJL) chimaeras 11 days after bone marrow reconstitution. Some weeks after intrathymic injection, the chimaeras were immunized with influenza virus and, after in vitro restimulation with infected B6 spleen cells, H-2b-restricted CTL responses were measured. In (F1 → F1) chimaeras a strong influenza-specific cytotoxic response was observed (Fig. 1a), whereas (F1 → SJL) chimaeras were unresponsive (Fig. 1b). (F1→ SJL) chimaeras injected intrathymically with 1308.1 TECs were similarly unresponsive (Fig. 1c). In contrast, T cells from (F1 → SJL) chimaeras injected intrathymically with 427.1 TECs generated a strong CTL response to influenza-infected H-2b targets (Fig. 1d). The MHC restriction and peptide specificity of the anti-influenza response is shown in Table 1, experiment 1. In H-2b mice, influenza-specific CTLs recognize the nucleoprotein-derived nonamer peptide 366–374 presented by H-2Db (refs 7, 8). Cytotoxic effectors generated both from F1 mice and from (F1 → SJL) chimaeras injected intrathymically with 427.1 TECs were able to kill influenza-infected H-2b targets but not influenza-infected H-2d or H-2S targets. Furthermore, H-2b target cells were killed if the synthetic peptide corresponding to nucleoprotein 366–374 was added to the CTL assay.
FIG. 1.
The effect of intrathymic injection of H-2b-expressing TEC lines into (F1→SJL) bone marrow chimaeras on positive selection of CTLs recognizing influenza virus plus H-2b. (B6 × SJL/J)F1 (a) or SJL/J (b–d) female mice were lethally irradiated (850 rads), and reconstituted with intravenous injection of 5 × 106 (B6 × SJL/J)F1 T-cell depleted bone marrow cells. After reconstitution (11 days) mice were left untreated (a, b) or injected intrathymically with l × 106 1308.1 (c) or 427.1 (d) epithelial cells. Five weeks after this the mice were immunized by intraperitoneal injection of 250 haemagglutinating units (HAU) influenza A/PR8/34 virus. Ten days after immunization spleen cells were restimulated in vitro for 5 days with irradiated (2,000 rads) influenza-infected B6 spleen cells. Cytotoxic activity against untreated (open symbols) or influenza-infected (filled symbols) EL4 (H-2b), 51Cr-labelled target cells was tested in a standard 4-h 51Cr-release assay.
METHODS. The isolation, and characterization of TEC lines 427.1 and 1308.1, that express markers found on subcapsular and cortical epithelium, respectively, will be described elsewhere (S.J.F., J. L Rothstein and B.B.K., manuscript in preparation). Bone marrow cells were depleted of Thy-1+ cells by T24 antibody29 and complement treatment. Intrathymic injections were done as in ref. 30. Some 5 × 105 TECs were injected per lobe. For the in vitro stimulation, 1 × 108 B6 spleen cells were infected with 1 × 103 HAU A/PR8/34. For CTL assays, 1 × 106 EL4 cells were simultaneously infected with 1 × 102 HAU A/PR8/34 and labelled with 51Cr.
Table 1.
Intrathymic injection of the H-2b/s TEC line 427.1 into (F1 → SJL) bone marrow chimaeras (H-2b/s → H-2S) induces positive selection of H-2b-restricted, influenza and VSV-specific cytotoxic T-cells
| Specific lysis (%) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell line | EL4 | P815 | B11 | P815* | ||||||||
| Experiment | injected | A/PR8/34- | PR8 NP | OVA | A/PR8/34- | A/PR8/34- | ||||||
| number | Chimaera | i.t. | immunization | — | infected | 366–374 | 257–264 | — | infected | — | infected | |
| 1 | (B6 × SJL/J)F1 | None | A/PR/8/34 | 22/7 | 66/45 | 48/32 | 0/−2 | 6/3 | 8/1 | 19/4 | 22/7 | 84/23 |
| F1 → SJL/J | None | A/PR/8/34 | 4/1 | 8/2 | 7/0 | 8/−1 | 5/1 | 8/1 | 7/1 | 7/5 | 64/14 | |
| F1 → SJL/J | 427.1 | A/PR/8/34 | 6/1 | 44/14 | 24/9 | −2/−2 | 13/3 | 12/3 | 12/7 | 16/10 | 79/20 | |
| VSV- | VSV N | OVA | VSV- | VSV- | ||||||||
| — | infected | 52–59 | 257–264 | — | infected | — | infected | |||||
| 2 | (B6 × SJL/J)F1 | None | VSV | 10/2 | 31/20 | 28/20 | 6/1 | 13/3 | 10/3 | 23/11 | 17/8 | 79/43 |
| F1 → SJL/J | None | VSV | 6/2 | 7/3 | 10/10 | 10/1 | 5/1 | 3/3 | 7/4 | 13/5 | 74/42 | |
| F1 → SJL/J | 427.1 | VSV | 7/1 | 27/11 | 27/12 | 5/0 | 7/3 | 5/2 | 14/2 | 10/9 | 68/34 | |
Chimaeras were made as in Fig.1 legend. Three to four weeks after TEC-injection mice were immunized by intraperitoneal injection with 250 HAU influenza A/PR8/34 virus or 5 × 106 plaque-forming units VSV. After immunization (9–13 days) spleen cells were restimulated in vitro for 5 days with irradiated (2,000 rads) influenza- or VSV-infected B6 spleen cells. Cytotoxic activity against untreated, influenza- or VSV-infected EL4 (H-2b), P815 (H-2d) or B11 (H-2S) 51Cr-labelled target cells was tested in a 4 h 51Cr-release assay. Synthetic peptides corresponding to A/PR8/34 nucleoprotein 366–3748, VSV N 52–599, or chicken ovalbumin (OVA) 257–26431 sequences were added to the CTL assay at 50 nM. Per cent specific lysis at effector: target ratios of 50:1/5:1 are shown, i.t., intrathymic; NP, nucleoprotein.
Spleen cells were stimulated in vitro with irradiated allogeneic DBA/2 (H-2d) spleen cells.
To investigate whether the TEC line 427.1 can positively select T cells restricted by another class 1 molecule, chimaeras were immunized with vesicular stomatitis virus (VSV). The CTL response to VSV is directed to the nucleocapsid (N) protein 52–59 peptide presented by H-2Kb (ref. 9). F1 mice and (F1 → SJL) chimaeras injected intrathymically with 427.1 cells, but not uninjected (F1 → SJL) chimaeras, could kill VSV-infected H-2b cells, but not VSV-infected H-2d or H-2S targets (Table 1, experiment 2). The addition of VSV N 52–59 synthetic peptide, but not chicken ovalbumin (OVA) 257–264, to the CTL assay rendered H-2b targets sensitive to these anti-VSV effectors.
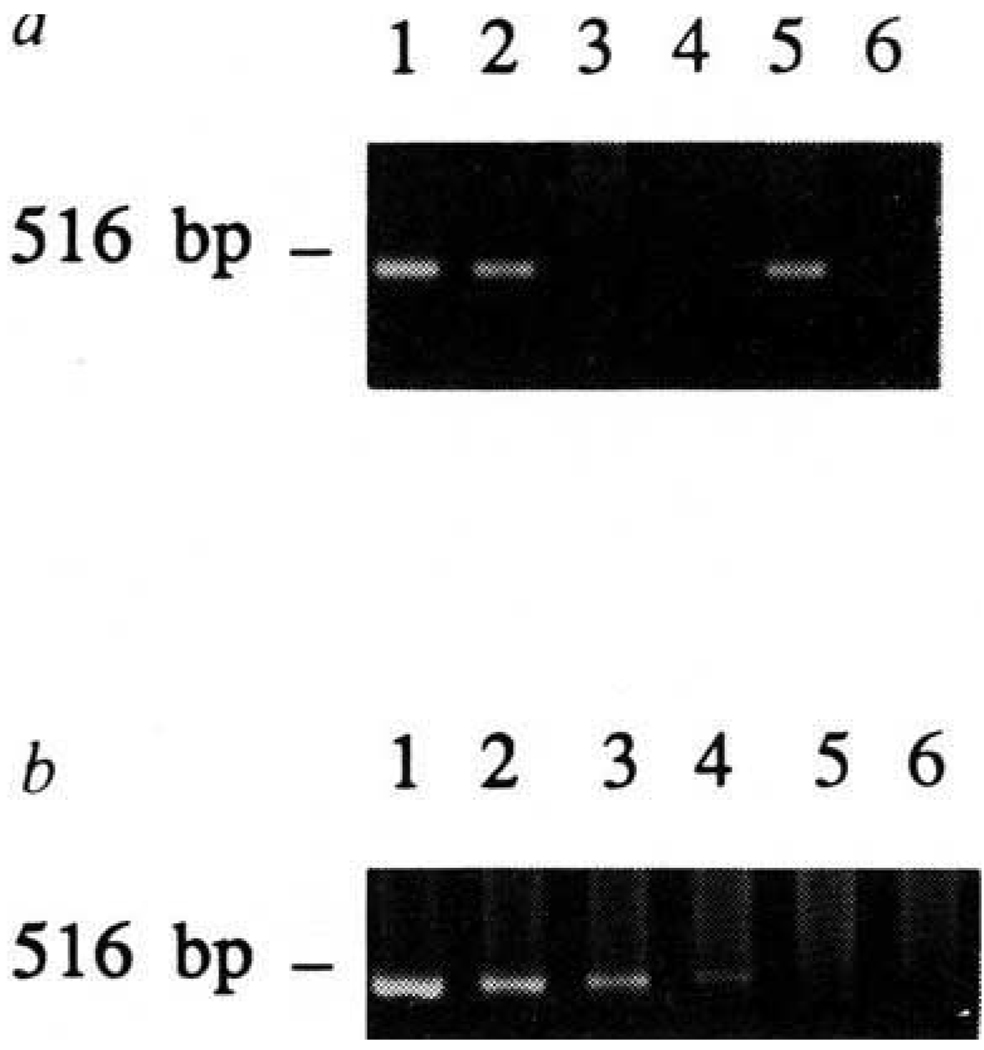
To assay for the presence of TECs in the thymus of injected mice, thymic DNA was isolated from mice immunized with influenza or VSV. A 0.5-kilobase (kb) fragment of the sequence encoding SV40 T antigen10 was amplified using polymerase chain reaction (PCR) from the thymus of chimaeras injected with line 427.1 (lane 5, Fig. 2a) but not from the thymus of (B6 × SJL)F1 mice, uninjected or 1308.1-injected (F1 → SJL) chimaeras (lanes 3, 4 and 6, respectively). To assess the sensitivity of detection, decreasing amounts of DNA isolated from line 427.1 were mixed with DNA isolated from the normal (B6 × SJL)F1 thymus, and SV40 T antigen sequences were amplified (Fig. 2b). The amount of DNA isolated from the equivalent of 800 427.1 cells provided sufficient template to generate visible PCR product (lane 4). Because we have routinely analysed 1% of the DNA from the thymus of injected mice, we can estimate that 8 × 104 cells was the minimum number of 427.1 cells present in the thymus (8% of the injected number). Therefore, TEC line 427.1 had the capacity to remain in the thymus weeks after injection, whereas line 1308.1 was not detectable. In other experiments, two other TEC lines, as well as macrophage cell line IC-21 also failed to remain detectable in the thymus (data not shown).
FIG. 2.
Detection of TEC-derived SV40 T antigen DNA in the thymi of injected mice, a, Chimaeras were made, injected with TECs, and immunized as described in Fig. 1. Thymi were taken from mice killed 9–13 days after immunization. DNA isolated from TEC line 427.1 (lane 1) or 1308.1 (lane 2), and thymic DNA from (B6 × SJL)F1 (lane 3), (F1→SJL) chimaera (lane 4), (F1→SJL) chimaera injected with 427.1 cells intrathymically (lane 5), (F1→SJL) chimaera injected with 1308.1 cells intrathymically (lane 6), was subjected to PCR using primers derived from SV40 T antigen sequence. Using primers derived from the genomic sequence of H-2Kb MHC class 1 molecule, a 0.5-kb fragment could be amplified from all samples (data not shown). b, DNA was isolated from line 427.1 and F1(B6 × SJL/J) thymus. DNA equivalents of 105 (lane 1), 2 ×104 (lane 2), 4 × 103 (lane 3), 8 × 102 (lane 4), or 1.6 × 102 (lane 5), or 0 427.1 cells (lane 6) were mixed with thymus DNA. DNA (2.5 µg) in each reaction was subjected to PCR reaction using SV40 T antigen primers.
METHODS. Thymi were homogenized and lysed with 0.5% SDS, and DNA was isolated by phenol-chloroform extraction of Proteinase K/RNase A-treated lysates. PCR was done in 100 µl 10 mM Tris, pH 8.3, 50 mM KCl, 2 mM MgCl2. Primers (5′-GTGCCCTTTACATCCTC-3′ and 5′-CAGCCAC-TATAAGTACC-3′ derived from the SV40 T antigen sequence10) were used at 20 µg ml−1, each dNTP at 240 µM, Taq DNA polymerase at 25 U ml−1. DNA (2–2.5 (µg) was used as template. Samples were subjected to 30 cycles of amplification, each consisting of denaturation at 94 °C for 60 s, annealing at 48 °C for 120 s, and polymerization at 72°C for 90 s. One tenth of the reaction was analysed by 1% agarose gel electrophoresis.
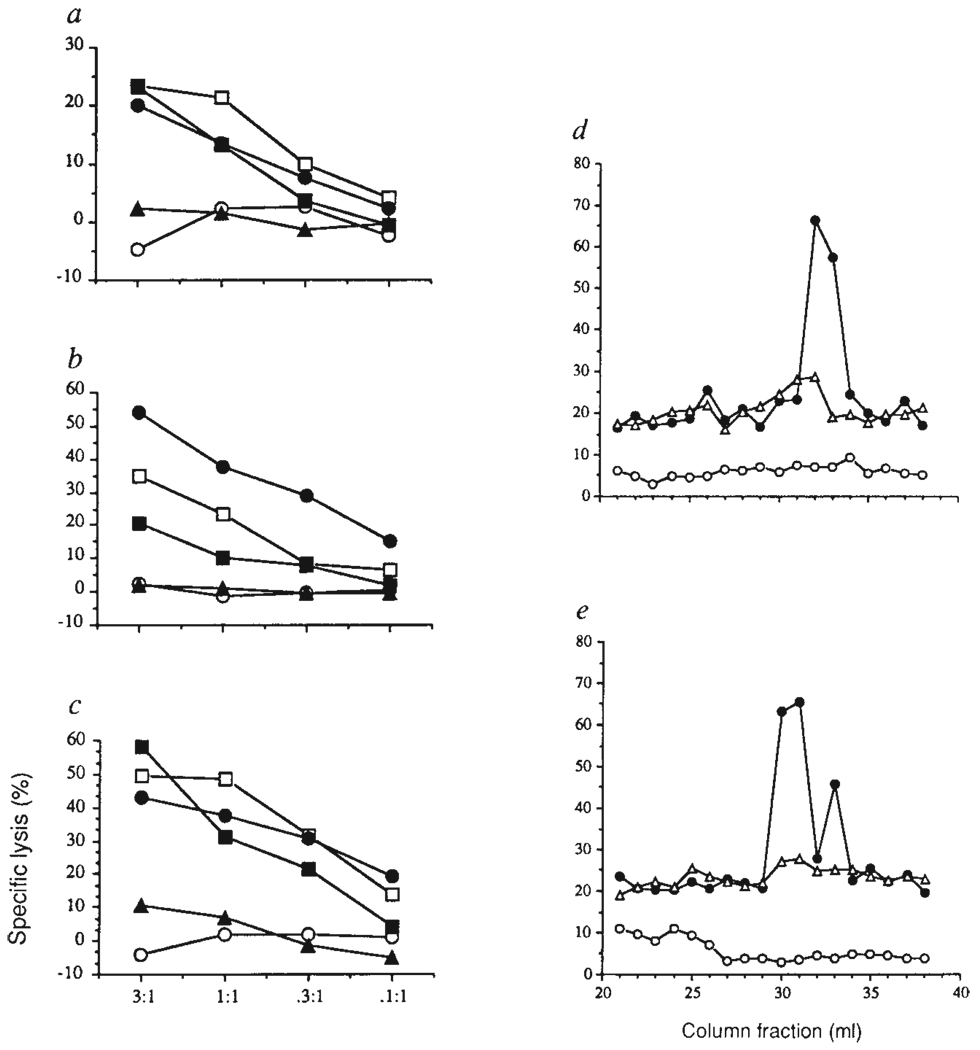
It has been suggested that the MHC molecules on the cells that induce positive and negative selection express different sets of peptides, so that positive and negative selection depend on different ligands for the TCR 11–14. Some mature T cells reactive with self-MHC plus a foreign peptide also recognize unmodified syngeneic thymic epithelium12. Furthermore, a peptide-MHC complex expressed abundantly in the periphery is hardly detectable in thymus cortical epithelium13. The peptides presented by MHC molecules influence positive selection15–17. In the case of MHC class I, the range of peptides bound to a given class I molecule can be substantially different dependent on whether the MHC-encoded transporter molecules are operative18–20. In cell lines with defects in the peptide transporter, only a few peptides can enter the endoplasmic reticulum lumen and associate with class I18,19, and thus the presentation of cytoplasmic antigens is less efficient21–23. We investigated whether there was a defect in normal class I presentation in the positively selecting cell line 427.1. Cells were infected with influenza virus or VSV and tested for sensitivity to the appropriate CTL lines. Also, OVA was introduced into the cytoplasm of 427.1 cells by osmotic loading24 and assayed for recognition by OVA-specific H-2Kb-restricted CTLs. All three effectors could lyse infected/loaded 427.1 targets (Fig. 3a–c). In addition, Brefeldin A, an agent that blocks the normal class I antigen-processing pathway25, 26, completely abrogated antigen presentation of the three endogenous antigens by 427.1 cells, while allowing the presentation of exogenously added synthetic peptides (Fig. 3a–c).
FIG. 3.
TEC line 427.1 can process antigens for presentation to class I MHC-restricted CTLs. a–c, Influenza virus-specific CTL line (a), VSV-specific CTL line (b), or ovalbumin-specific CTL clone GA4 (c) were tested for the ability to lyse 427.1 cells in the absence (open circles) or presence (open squares) of the appropriate synthetic peptide, and 427.1 cells infected with influenza (a), VSV (b) or loaded with OVA (c) (filled circles). Infected/loaded 427.1 cells were treated with Brefeldin A in the absence (filled triangles) or presence (filled squares) of the appropriate synthetic peptide. In preliminary experiments, 427.1 cells showed slightly but consistently lower targeting activity compared with infected/loaded EL4 cells (data not shown), d, e, Reversed-phase HPLC-separated fractions from anti-H-2Kb (circles) and anti-H-2Db (triangles) immunoprecipitates of 427.1 cell lysates were analysed for targeting activity for CTL clones d3.1 (d) and d3.3 (e) using 51Cr-RMA-S (open circles) or D65-transfected RMA-S (filled circles and open triangles) as targets.
METHODS. The anti-Hk/68 CTL line was established by priming F1(B6 × SJL) mice with 250 HAU of influenza Hk/68 virus. Two weeks later, spleen cells were restimulated with Hk/68-infected, irradiated (2,000 rads) B6 spleen cells. The line was maintained by weekly restimulation with Hk/68-infected, irradiated B6 spleen cells in the presence of 10% rat concanavalin A supernatant. The establishment and maintenance of the anti-VSV CTL line and the CTL clone GA4 were described previously22'32. Cells (1 × 106) from line 427.1 were infected by incubation in the presence of 100 HAU influenza Hk/68 or 250 × 106 p.f.u. VSV. Osmotic loading of OVA was done as described24. Cells were labelled with 51Cr during infection, or after osmotic loading. Brefeldin A was used at 10 µg ml−1 during infection and/or labelling with 51Cr, and at 2.5 µg ml−1 during CTL assay. Synthetic peptides from influenza nucleoprotein, VSV N or OVA, as in Table 1, were added to the CTL assay at 50 nM. To make peptide extracts of class I molecules, l × 108 427.1 cells were lysed and the H-2Kb and H-2Db were immunprecipitated as described27. Acid extraction of the washed precipitates, fractionation of the low molecular mass material on C18 reversed phase HPLC, and the targeting assay of the fractions has been described27. Derivation of the RMA-S cell line expressing the D65 mutant H-2Kb molecule and the CTL clones d3.1 and d3.3 has been described27.
To test whether self-peptides associated with class I molecules in other tissues are also present in line 427.1, peptide-specific alloreactive CTL clones were used. These clones are specific for H-2Kb binding peptides from spleen recognized in the context of a mutant H-2Kb molecule (Gln 65→ Asp 65), designated D65 (ref. 27). Targeting peptides were contained in reversed-phase high-performance liquid chromatography (HPLC) fractions 32 (for clone d3.1) and 30 and 33 (for clone d3.3) in acid extracts of H-2Kb isolated from B6 spleen cells27. Immunoprecipitated H-2Kb molecules from line 427.1 also yielded material with the same HPLC elution profile able to sensitize D65 transfected RMA-S cells for recognition by these CTL clones (Fig. 3d, e). Thus, the 427.1 cell line seems to have a similar representation of self-peptides in its MHC class I grooves, compared to peripheral cells.
We have shown that the TEC line 427.1, which can ‘conventionally’ process and present endogenous antigens to CTLs in vitro, can induce positive selection of H-2b-restricted CTLs in vivo when injected intrathymically. One implication of this is that the MHC-peptide ligands involved in positive and negative selection are the same. If this is the case, the range of T-cell receptors that are positively selected is broader than that deleted during negative selection11,28. These findings do not necessarily imply that all class I MHC-associated peptides of this cell line are same as those on bone marrow-derived cells, there are probably tissue-specific peptides expressed. Using 427.1 cells with controlled expression of genes it may be possible to investigate further the requirements for positive selection.
ACKNOWLEDGEMENTS
We thank J. Nikolić-Žugić and P. Fink for advice on intrathymic injections, J. Thorbecke for the B11 cell line, D. Debelak for technical assistance and J. Harty, S. Jameson, K. Hogquist and E. Pamer for discussion and comments. Supported by the NIAID and the HHMI.
References
- 1.Yewdell JW, Bennink JR. Adv. Immun. doi: 10.1016/s0065-2776(08)60875-5. (in the press) [DOI] [PubMed] [Google Scholar]
- 2.Bevan MJ. Nature. 1977;269:417–418. doi: 10.4049/jimmunol.176.1.677. [DOI] [PubMed] [Google Scholar]
- 3.Zinkermagel RM, et al. J. exp. Med. 1978;147:882–896. doi: 10.1084/jem.147.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sprent J. J exp. Med. 1978;146:1838–1842. doi: 10.1084/jem.147.6.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kisielow P, Teh HS, Bluthmann H, von Boehmer H. Nature. 1988;335:730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 6.Brinster RL, et al. Cell. 1984;37:367–379. doi: 10.1016/0092-8674(84)90367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Townsend ARM, et al. Cell. 1986;44:959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- 8.Rotzschke O, et al. Nature. 1990;348:252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- 9.Van Bleek GM, Nathenson SG. Nature. 1990;348:213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- 10.Fiers W, et al. Nature. 1978;273:113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- 11.Marrack P, Kappler J. Immun. Today. 1988;9:308–315. doi: 10.1016/0167-5699(88)91324-2. [DOI] [PubMed] [Google Scholar]
- 12.Marrack P, McCormack J, Kappler J. Nature. 1989;338:503–505. doi: 10.1038/338503a0. [DOI] [PubMed] [Google Scholar]
- 13.Murphy DB, et al. Nature. 1989;338:765–768. doi: 10.1038/338765a0. [DOI] [PubMed] [Google Scholar]
- 14.Mizuochi T, Kasai M, Kokuho T, Kakiuchi T, Hirokawa K. J. exp. Med. 1992;175:1601–1605. doi: 10.1084/jem.175.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikolic-Zugic J, Bevan M. J. Nature. 1990;344:65–67. doi: 10.1038/344065a0. [DOI] [PubMed] [Google Scholar]
- 16.Sha WC, et al. Proc. natn. Acad. Sci. U.S.A. 1990;87:6186–6190. doi: 10.1073/pnas.87.16.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berg LJ, Frank GD, Davis MM. Cell. 1990;60:1043–1053. doi: 10.1016/0092-8674(90)90352-f. [DOI] [PubMed] [Google Scholar]
- 18.Henderson RA, et al. Science. 1992;255:1264–1266. doi: 10.1126/science.1546329. [DOI] [PubMed] [Google Scholar]
- 19.Wei M, Cresswell P. Nature. 1992;356:443–446. doi: 10.1038/356443a0. [DOI] [PubMed] [Google Scholar]
- 20.Powis SJ, et al. Nature. 1992;357:211–215. doi: 10.1038/357211a0. [DOI] [PubMed] [Google Scholar]
- 21.Townsend A, et al. Nature. 1989;340:443–448. [Google Scholar]
- 22.Hosken NA, Bevan MJ. Science. 1990;248:367–370. doi: 10.1126/science.2326647. [DOI] [PubMed] [Google Scholar]
- 23.Spies T. Nature. 1992;355:644–646. doi: 10.1038/355644a0. [DOI] [PubMed] [Google Scholar]
- 24.Moore MW, Carbone FR, Bevan MJ. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 25.Nuchtern JG, Bonifacino JS, Biddison WE, Klausner RD. Nature. 1989;339:223–226. doi: 10.1038/339223a0. [DOI] [PubMed] [Google Scholar]
- 26.Yewdell JW, Bennink JR. Science. 1989;244:1072–1075. doi: 10.1126/science.2471266. [DOI] [PubMed] [Google Scholar]
- 27.Grandea AG, Bevan MJ. Proc. natn. Acad. Sci. U.S.A. 1992;89:2794–2798. doi: 10.1073/pnas.89.7.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fink P. Immun. Today. 1988;9:377–380. doi: 10.1016/0167-5699(88)91238-8. [DOI] [PubMed] [Google Scholar]
- 29.Dennert G, Hyman R, Lesley J, Trowbridge I. Cell Immun. 1980;53:350–364. doi: 10.1016/0008-8749(80)90335-4. [DOI] [PubMed] [Google Scholar]
- 30.Goldschneider I, Komschlies KL, Greiner DL. J. exp. Med. 1986;163:1–17. doi: 10.1084/jem.163.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rotzschke O, et al. Eur. J. Immun. 1991;21:2891–2894. doi: 10.1002/eji.1830211136. [DOI] [PubMed] [Google Scholar]
- 32.Nikolic-Zugic J, Carbone FR. Eur. J. Immun. 1990;20:2431–2437. doi: 10.1002/eji.1830201111. [DOI] [PubMed] [Google Scholar]