Abstract
Purpose
Metronomic chemotherapy is a minimally toxic and frequently effective new treatment strategy that is beginning to show promising phase II clinical trial results, particularly for metastatic breast cancer when combined with various molecularly targeted antitumor agents. Here, we assessed a treatment strategy that uses trastuzumab plus daily oral metronomic cyclophosphamide on metastatic Her-2–positive human breast cancer models.
Experimental Design
Treatments were initiated on orthotopic transplanted primary tumors as well as established visceral metastatic disease of two independent Her-2–positive breast cancer models, both independently derived from the human MDA-MB-231 breast cancer cell line. Outcome was assessed by noninvasive measurements of tumor cell–secreted human choriogonadotropin in the urine as a surrogate marker of relative tumor burden, or by whole body bioluminescent imaging, in addition to prolongation of survival.
Results
Orthotopic primary tumors responded to trastuzumab monotherapy with significant growth delays, whereas minimal antitumor effect was observed when mice with metastatic disease were treated. Nevertheless, trastuzumab showed a benefit in this latter setting when combined with metronomic low-dose cyclophosphamide as assessed by prolongation of survival. This benefit was similar to trastuzumab plus maximum tolerated dose cyclophosphamide, but was associated with lesser toxicity.
Conclusions
Trastuzumab combined with metronomic cyclophosphamide may be an effective long-term maintenance strategy for the treatment of Her-2–positive metastatic breast cancer.
One significant advance in breast cancer medical oncology over the last decade has been the development and approval of drugs that target the erbB-2/Her-2 (Her-2) oncogene (1, 2). These drugs include trastuzumab (Herceptin), the humanized monoclonal antibody (2–5), and lapatinib (Tykerb), a small molecule receptor tyrosine kinase inhibitor that targets both the epidermal growth factor receptor (erbB-1) and Her-2 (6). Trastuzumab has shown benefit in a number of randomized clinical trials involving breast cancer patients whose tumors overexpress Her-2, in both the metastatic and adjuvant treatment settings, especially the latter (2, 7, 8). With respect to treatment of metastatic disease, the clinical results have highlighted the importance of both intrinsic and acquired resistance, because more than half of breast cancer patients whose tumors overexpress Her-2 do not respond to trastuzumab, and among those that do, acquired resistance to the drug inevitably develops, generally within 1 year of treatment initiation (2, 9). Moreover, trastuzumab monotherapy for the treatment of advanced metastatic disease in patients is associated with minimal, if any, activity; its benefit is derived by integration with chemotherapy. In contrast, trastuzumab monotherapy is effective in preclinical models, but these invariably involve treatment of localized transplanted primary tumors, not visceral metastatic disease.
Recently, we have developed models of metastatic disease using either the parental MDA-MB-231 human breast cancer cell line (10) or a Her-2+ variant of MDA-MB-231 called H2N (11, 12). These models are associated with extensive visceral metastases that can become established in the lungs, liver, and lymph nodes of most mice within one or several months of resection of the primary tumor (10, 11).
Given the development of these metastatic models of Her-2–positive breast cancer, we decided to initiate a preclinical analysis of trastuzumab plus chemotherapy using either a conventional-type maximum tolerated dose (MTD) chemotherapy regimen or continuous low-dose metronomic chemotherapy, using the same drug, cyclophosphamide (CTX). Our results, which constitute one of the first reports of experimental therapy of metastatic Her-2–positive xenografts, illustrate the possible benefits of using trastuzumab in combination with metronomic chemotherapy for metastatic breast cancer, for which preliminary clinical evidence is also beginning to emerge (13).
Materials and Methods
Cell lines
The parent MDA-MB-231 human breast cancer cell line (14) was used to derive the erbB2-transduced 231-H2N (12), the metastatic variants met2 (11), and LM2-4H2N (erbB2-transduced LM2-4 cells; ref. 10). LM2-4 cells are highly metastatic from a primary orthotopic transplant, after surgical resection of the tumor, as previously described (10). The LM2-4 cells were cotransfected with the firefly luciferase vector pGL3 (Promega Corporation) and pSV2neo, and selected using G418, from which a luciferase-expressing clone was isolated; these cells were then used to generate the LM2-4H2N (erbB2-transduced LM2-4 cells) as previously described (12). The met2 cells were obtained as described in the Results section.
β-hCG measurements
β-hCG in the mouse urine was measured as previously detailed (11), and normalized to urine creatinine levels (using QuantiChrom TM Creatinine assay kit from BioAssay Systems) as described by Shih et al. (15).
Orthotopic tumor implantation
Female 4-wk-old CB17 severe combined immunodeficient (SCID) mice were purchased from Charles River Canada (Saint-Constant) and allowed to acclimatize for 2 wk. MDA-MB-231 cell variants were harvested by trypsin treatment, washed thrice in ice-cold PBS, and resuspended in serum-free DMEM. Cells (2 × 106) were injected in 50-μL volumes into the inguinal mammary fat pad.
Spontaneous (orthotopic) metastasis assays
LM2-4H2N (Her-2 positive) and met2 (Her-2 positive) cells were injected into the mammary fat pad of female CB17 SCID mice. LM2-4H2N and met2 tumors were measured using calipers and removed when they reached an average size of 500 mm3. Mice were monitored for body weight, β-hCG urine levels, or luciferase expression. End points were determined according to institutional guidelines; mice were sacrificed when cachexia (>15% body weight loss) was observed, or when mice showed evidence of lymph node metastases, or when they exhibited moribund symptoms (lethargy and/or reduced mobility). Statistical analysis (log-rank test for survival or ANOVA with Newman-Keuls comparison test for weight loss) was done using Prism software (GraphPad).
Trastuzumab administration
Treatments for primary tumors were initiated when the average tumor size was ~250 mm3. Treatment of metastatic disease was initiated 3 wk after resection of the primary tumor. This time point was empirically determined as optimal in view of the sporadic rate of appearance of spontaneous metastases (data not shown). If treatment was initiated beyond 3 wk after surgery (when up to 50% of the mice would show detectable hCG/luciferin luminescence), we observed that mice with the most advanced disease would have to be sacrificed before the end of the 5th week, i.e., before any treatment could start to have an impact. Trastuzumab was given twice weekly at 20 mg/kg i.p. (12).
MTD and metronomic CTX administration
CTX treatment was initiated concomitant with trastuzumab therapy. Metronomic CTX was administered at 20 mg/kg (cumulative dose, 420 mg/kg/21 d) via the drinking water (16). A conventional MTD CTX cycle was given as 70 mg/kg i.p. on day 1, then again on days 3 and 5; mice were then allowed a break period to recover until day 21 when a subsequent MTD CTX cycle would begin again (cumulative dose, 210 mg/kg/21 d).
Bioluminescent (luciferin) imaging
Mice were injected (i.p.) with 150 mg/kg of luciferin (Caliper Life Sciences) made up in PBS, and 10 min, later they were anaesthetized and imaged for 1 to 60 s. Imaging was done using a IVIS200 Xenogen system (Xenogen) following the manufacturer’s recommendations. Luminescence was detected via the IVIS camera and analyzed using the Living Image software (Xenogen).
Results
Impact of trastuzumab plus metronomic or MTD CTX on orthotopic H2N tumors
Previously, we described the combination of trastuzumab plus metronomic CTX as an effective treatment for primary H2N tumors when compared with trastuzumab plus MTD CTX treatment (12). However, in that study, a Kaplan-Meier survival analysis showed that the principal reason for the difference was the extreme toxicity that developed following a third round of MTD CTX (12). Thus, MTD CTX plus trastuzumab was not a less effective antitumor therapy, but rather one that eventually became too toxic (12). Therefore, to compare over a long-term period metronomic-based schedule with a more conventional-type regimen (i.e., several near MTD rounds of chemotherapy), we chose an empirical 30% reduction “MTD” CTX group. We reasoned that this would compensate for the multiple MTD CTX rounds (as opposed to the one or two rounds commonly used in most preclinical studies) in our regimen. Thus, instead of 300 mg/kg (12), we chose 210 mg/kg of CTX for each round of MTD. H2N tumors were grown orthotopically and treated with saline (control) or with one of two combinations of CTX plus trastuzumab. One combination involved low-dose metronomic CTX at a daily dose of 20 mg/kg administered in the drinking water, plus trastuzumab. The other combination was near MTD CTX (210 mg/kg/21-day cycle) plus trastuzumab. Figure 1 shows that the tumor response was virtually identical when comparing the two combination therapies. However, the MTD-based regimen did show toxicity, particularly after the fifth MTD dose (around days 100–110). Around day 192, as both therapies were beginning to fail, we noticed a drop in weight in some of the mice of both groups that did not seem to follow any particular CTX administration. Autopsy on these mice revealed the presence of metastases to the lungs, bone marrow, and brain (data not shown). Thus, long-term treatment of trastuzumab plus CTX is effective, but distant metastases eventually develop, which seem resistant to the same therapy that continues to inhibit primary tumor growth.
Fig. 1.
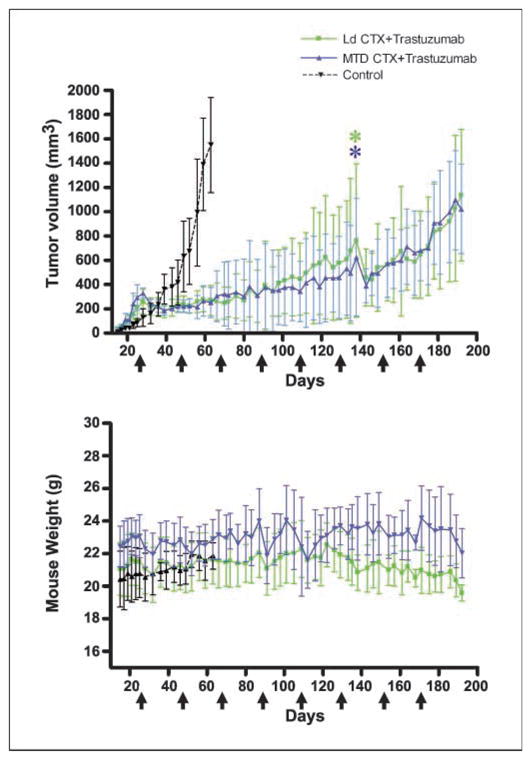
Impact of concurrent combination of trastuzumab-chemotherapy (CTX) therapy regimens on orthotopic primary H2N tumors in SCID mice. When tumors reached 250 mm3, mice were treated with saline (control, n = 3) or metronomic low-dose (Ld) CTX plus trastuzumab (Ld CTX + trastuzumab, n = 8), or with a MTD CTX plus trastuzumab (MTD CTX+ trastuzumab, where MTD CTX is 210 mg/kg every 21 d, n = 5). Top, tumor volume; bottom, mouse weights. Arrows, each MTD cycle (i.e., every 21 d). *, one mouse in each group had to be sacrificed at the indicated point. Trastuzumab was given twice weekly at 20 mg/kg i.p., and metronomic low-dose CTX was ~20 mg/kg/d.
Efficacy of trastuzumab-based chemotherapy regimens on spontaneous metastatic disease
The aforementioned results raise the question of whether our previous analysis (12) overestimated the benefits of our combination therapies, because they were only tested on single, localized primary tumors, and not on metastatic disease. Furthermore, because it is extremely rare for H2N tumors to metastasize (12), it was not an appropriate model to address this question. Indeed, this probably explains why it took over 192 days for even a small fraction of the mice to show evidence of H2N metastatic disease.
We therefore “tagged” the H2N line with hCG, to monitor growth of metastatic disease by measuring hCG levels in the mouse urine (11, 15), and then used two rounds of in vivo selection of H2N.hCG cells, as detailed elsewhere (11). This selection protocol, which took ~12 months, resulted in the establishment of the metastatic cell line called met2 (11). Next, having developed the model, we repeated the experiment described in Fig. 1—using the met2 line, i.e., we assessed its response to therapy as a primary tumor. This was undertaken to confirm that the met2 line is responsive to the combination therapies. In parallel, and at the same time using the same batch of met2 cells, a spontaneous metastasis model was evaluated to test the effect of the therapies on metastatic disease. An outline of this experimental plan is shown schematically in Fig. 2. For the primary tumors, we noted the expected response pattern (Fig. 3A) as was originally observed with the parental H2N (Fig. 1). Thus met2 cells gave rise to primary tumors that were highly responsive to trastuzumab plus low-dose metronomic CTX, a finding independently confirmed by monitoring hCG urine levels (Fig. 3A). Regression of established tumors was induced, followed by 120 days of suppressed growth during continuous therapy. Regrowth (relapse) became apparent around day 170.
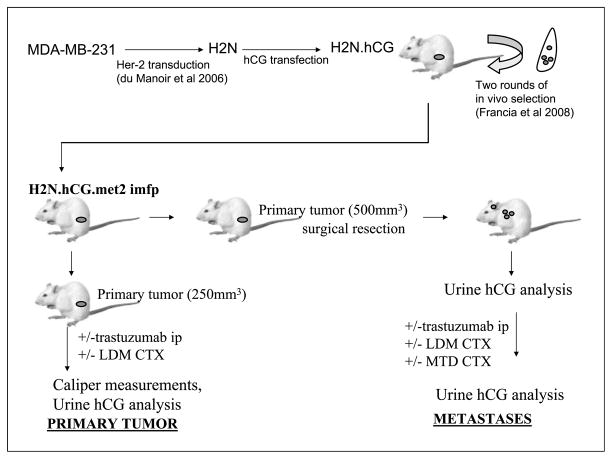
Fig. 2.
Schematic of met2 metastatic tumor therapy experiment. Human breast cancer MDA-MB231 cells were modified to express Her-2 and secrete hCG, and subsequently selected for high metastatic potential through two rounds of in vivo selection, as described previously (10). The resulting met2 metastatic variant was generated, and subsequently injected into female SCID mice. The growth of this tumor can be monitored via hCG levels in the mouse urine. Mice were then used to assess the impact of therapies on the growth of primary tumors and, in parallel, in mice with metastatic disease.
Fig. 3.
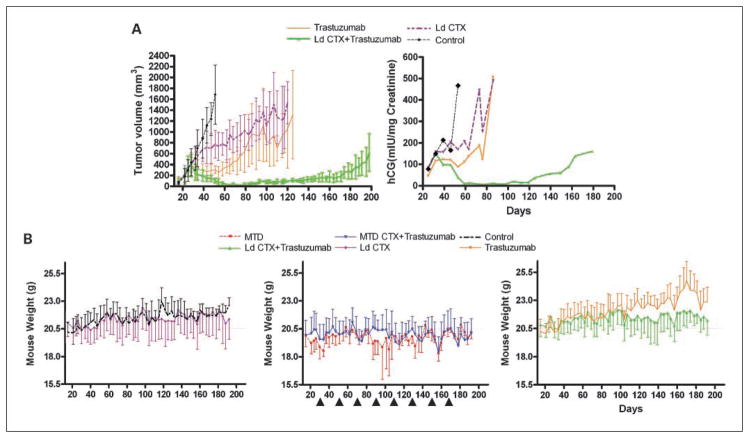
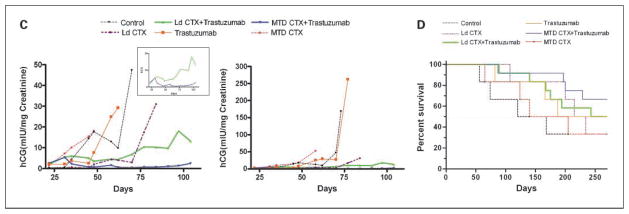
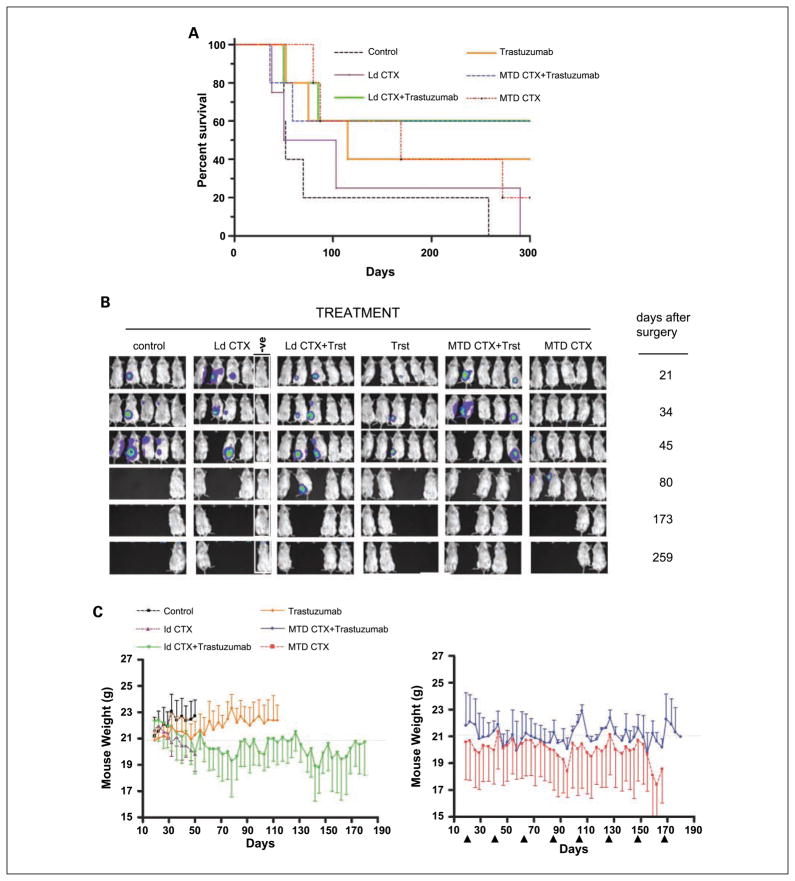
A, effect on tumor volume (top) and hCG levels (bottom) of the different therapies on met2 orthotopically implanted primary tumors. When tumors reached 250 mm3, mice were treated with saline (control, n = 5), or trastuzumab alone (n = 6), or low-dose metronomic CTX alone (Ld CTX, n = 6) or the combination of trastuzumab plus low-dose metronomic CTX (Ld CTX+ trastuzumab, n = 6). The curves show that, similar to H2N tumors (see Fig. 1), the met2 line gave rise to primary tumors that are highly responsive to the low-dose metronomic CTX + trastuzumab combination therapy. The urine hCG values (corresponding to pooled urine hCG for each group, normalized to urine Creatinine levels) were found to be concordant with tumor volume measurements. B, treatment of met2 metastases and assessment of mouse weights. Orthotopically implanted met2 tumors were surgically removed, and 3 wk later, various therapies were initiated. Mice were treated with MTD CTX alone (MTD, n = 6) or low-dose CTX alone (n = 6) or control saline (n = 6), or trastuzumab alone (n = 6). In addition, other mice were given trastuzumab plus metronomic CTX (n = 12), or trastuzumab plus MTD CTX (n = 12; arrows, MTD dosing). Weight loss on day 115 for MTD therapies was significantly different (P < 0.05) versus the other regimens. Trastuzumab was given twice weekly at 20 mg/kg i.p., and low-dose metronomic CTX alone was ~20 mg/kg/d. C, top, hCG curves of met2 metastases treated with the regimens indicated. Note at the start of therapy (day 24), the low hCG levels made it difficult to accurately normalize the groups. As a consequence, some groups (e.g., low-dose metronomic CTX alone, see days 25–50) subsequently turned out to have relatively low hCG levels. Nonetheless, sequential hCG measurements showed controls and the monotherapies to rapidly increase after a lag phase (particularly long for the low-dose metronomic CTX group). In contrast, mice treated with the combination therapies did not show increases in hCG readings for 3 mo after treatment was initiated. The combination of MTD CTX + trastuzumab showed a decrease in hCG levels suggesting this approach to be the most effective therapy. Low-dose metronomic CTX + trastuzumab showed unchanged hCG levels for the first 3 mo of treatment, followed by an increase thereafter. Boxed graph, the same data with expanded Y-axis and only showing the hCG curves for the combination therapies for ease of comparison. Bottom, graph showing the same data on the full range of detected hCG values (i.e., 0–500), indicating that at the time of sacrifice monotherapies had 100-fold higher hCG burden than the ongoing combination therapies. Note that the data are of pooled urine hCG, normalized to creatinine levels. D, corresponding survival curve of therapy experiment for met2 metastases (see C) showing the impact of MTD CTX+ trastuzumab (P = 0.03) in this model, relative to the other regimens tested. MTD CTX is 210 mg/kg every 21 d. Trastuzumab was given twice weekly at 20 mg/kg i.p., and Ld CTX was ~20 mg/kg/d.
In the parallel experiment involving metastatic disease, therapy was initiated 3 weeks after surgery. Mouse weights were then monitored (Fig. 3B). For the control groups, we observed a slow but steady increase in average mouse weight. In contrast, MTD CTX caused cycles of weight loss that coincided with rounds of MTD dosing. Interestingly, and consistent with previous studies (12), in between MTD rounds the mice maintained a steady average weight (around 20 grams; Fig. 3B). The steady weight increase seen in the control group was also observed in the trastuzumab monotherapy groups, but this increase was reduced in the trastuzumab plus low-dose CTX group. Thus, although the trastuzumab plus low-dose CTX regimen did not result in any significant toxicity, the length (i.e., >200 days) of this experiment allowed us to appreciate that this regimen may impair aging-related increase (albeit modest) in body weight.
Although the mice were monitored to evaluate the therapies by a traditional survival curve, we could also obtain interim data on the relative metastatic burden in each treatment group (Fig. 3C) by monitoring urine hCG levels. These measurements indicated that both controls and MTD CTX monotherapy were ineffective at preventing a surge in hCG levels. The same apparent lack of activity was observed with trastuzumab monotherapy, which is consistent with earlier observations using this model (11). In contrast, the combination of MTD CTX plus trastuzumab caused hCG levels to drop to barely detectable levels. Metronomic CTX plus trastuzumab also had a suppressive impact on hCG levels, although the kinetics of the hCG suppression were less dramatic. Therefore, trastuzumab can be effectively combined with either mode of CTX administration (i.e., MTD or metronomic) to treat Her-2–positive breast cancer metastases. However, the results also indicate that trastuzumab plus MTD CTX is the most effective treatment, at least initially (in the debulking of metastatic disease, as assessed by hCG levels). On the other hand, the trastuzumab plus low-dose CTX combination was not as toxic as the MTD CTX combination treatment (Fig. 3B). Thus, determining the preferred treatment option between these two regimens could depend on the relative importance of antitumor efficacy versus acceptable levels of toxicity. This can be assessed in the resulting survival curve for this experiment (Fig. 3D). The data showed that both combination regimens were effective in prolonging survival, and that the superior survival curve was actually obtained with the trastuzumab plus MTD CTX regimen (consistent with the hCG data; Fig. 3C). However, it should be stressed that 2 of 12 mice in this group had to be sacrificed (between days 190 and 220) due to extreme weight loss, and that at autopsy, no evidence of metastatic disease was found. Thus, these mice most likely succumbed to toxicity caused by the treatment. Importantly, we never observed any such toxicity in the trastuzumab plus low-dose CTX group in the 200-day therapy period.
Evaluation of an independent model of metastasis
To exclude the possibility that the above observations were unique to the met2 model, we developed an independent spontaneously metastatic and Her-2–positive breast cancer model. The LM2-4 line was previously generated as a highly metastatic variant of the MDA-231 human breast cancer cell line, following in vivo selection (10), and later transfected with luciferase to generate the luc14+line (Table 1). Subsequently, the luc14+ line was transduced with H2N/YFP, resulting in the generation of the LM2-4luc+H2N cell line (Fig. 4) that has stable and high Her-2 expression and expresses luciferase. This tumor line was implanted orthotopically into female SCID mice, and 3 weeks after surgery, treatments were initiated. The survival curve (Fig. 5A) shows that the monotherapies were less effective than the combination regimens. Trastuzumab plus MTD CTX proved equivalent to trastuzumab plus low-dose CTX. All mice that showed detectable luciferase luminescence after surgery (Fig. 5B) eventually succumbed to metastatic disease. Indeed, in this model, we did not observe a debulking of metastatic disease, as was observed in Fig. 3C with hCG levels for the groups treated with trastuzumab plus MTD CTX.
Table 1.
MDA-MB231 generated Her-positive Variants
| Variant line | Details | Metastatic potential | Reference |
|---|---|---|---|
| H2N | Her-2 transduced (retrovirus)/YFP | Low | du Manoir et al. 2006 |
| met2 (H2NhCGmet2) | Her-2 transduced (retrovirus)/YFP hCG transfected | High | Francia et al. 2008 |
| LM2-4H2N (LM2-4H2Nluc+) | Her-2 transduced (retrovirus)/YFP Luciferase transfected | Very high | Munoz et al. 2006 |
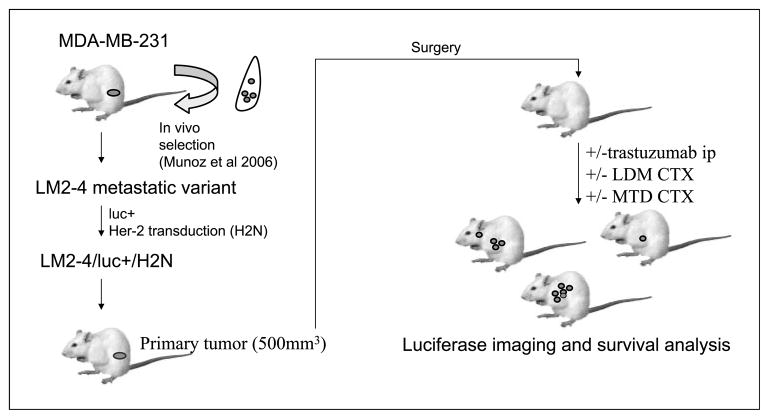
Fig. 4.
Schematic of LM2-4H2N therapy experiment. The human breast cancer cell line MDA-MB231 was selected in vivo for high metastatic potential (10) and thereafter transfected to express luciferase, and then transduced to express Her-2. The resulting LM2-4H2N line was injected orthotopically into female SCID mice. A spontaneous metastasis assay was carried out, with therapies beginning 3 wk after resection of the primary tumors.
Fig. 5.
A, survival of mice with LM2-4H2N metastatic disease treated with therapies indicated (for all groups, n = 5). Note that 60% of mice treated with Low-dose metronomic CTX + trastuzumab were still alive by day 300 (P = 0.05), as was observed for the MTD CTX + trastuzumab group, whereas survival was below 50% for all monotherapies by day 170. MTD CTX is 210 mg/kg every 21 d. Trastuzumab was given twice weekly at 20 mg/kg i.p., and low-dose metronomic CTX was ~20 mg/kg/d. B, luciferase imaging of mice with LM2-4H2N metastases treated with the therapies indicated. Mice that developed detectable metastatic disease had evidence of lymph node or lung metastases (e.g., two leftmost mice on MTD CTX, by day 80 after surgery) and/or developed ascites and disease near the primary tumor site (e.g., the two mice with luciferase signal on day 21 after surgery in the low-dose metronomic CTX only group). Some mice (at autopsy) were subsequently found to have brain metastases, although this was not detected by the routine luciferase imaging. One mouse (rightmost in the low-dose metronomic CTX group) did not give a primary tumor take and therefore had no subsequent surgery, and was used as a negative (−ve) control for luciferase imaging throughout this experiment. C, mouse weights for the therapy experiment of LM2-4H2N metastases. Note that both groups that included MTD CTX showed a cyclical loss of weight (e.g., days 110–120) that reflect the 21-day dosing cycle of this drug, although some cycles had more drastic effect than others on the relative mouse weights.
Analysis of mouse weights (Fig. 5C) showed that controls, metronomic CTX, and trastuzumab monotherapies exhibited an aging-related increase in weight. In contrast, MTD CTX and trastuzumab plus MTD CTX led to cycles of weight-loss that correlated with the timing of the MTD dosing (particularly at days 100 and 140 after surgery). Thus, in this model, trastuzumab plus low-dose CTX is superior to trastuzumab plus MTD CTX in that equivalent survival curves were generated, yet the metronomic-based regimen lacked the repeating cycles of weight loss. We conclude that both are valid combination treatment regimens, and that evaluating the most effective regimens depends on the balance of the need to target the tumor population with the desire of achieving such effects with minimal associated toxicity.
Discussion
This study represents the latest in a series of investigations, including from our laboratory, evaluating therapeutic outcomes after therapy is initiated in mice with established visceral metastatic disease, and comparing the results with those obtained using the more traditional primary tumor models, including orthotopic transplants (10, 11, 17–19). This particular study represents the first detailed preclinical study of Her-2–positive spontaneous metastatic breast cancer (14), of which we are aware, which uses mice with established metastatic disease. Previous studies have used Her-2–transfected MDA-MB-231 cells injected into the arterial circulation as a means of generating “artificial” metastases, e.g., brain metastases, as a model for therapy of Her-2 metastatic breast cancer using drugs such as lapatinib (20). These studies involved initiation of therapy within one or a few days of tumor cell injection and thus did not involve macroscopic established metastasis. In our studies with orthotopically implanted and intact primary H2N tumors, we observed that such “primary” tumors would eventually start to relapse on treatment using the combination of trastuzumab plus metronomic CTX; in some cases, this was accompanied by the development of metastatic disease. However the minimal metastatic capacity of the H2N tumor model made it initially difficult to ascertain whether this result was due to the ineffectiveness of the therapy against late-developing metastatic disease or simply to metastases that had also developed resistance to the treatment. We therefore developed the metastatic met2 model (11), with the hCG “tag” allowing us to generate a “metastases”-growth curve (by plotting the relative weekly pooled urine hCG levels).
We have used the met2 model to test the effectiveness of trastuzumab plus CTX regimens on established spontaneous metastatic disease after primary tumor resection. Therapies were also tested, in parallel, using the intact primary orthotopic tumor model. This is a fundamental aspect of our study, because, as discussed above, the majority of preclinical therapies reported in the literature are routinely assessed using only primary tumor models, either ectopic or orthotopic. Furthermore, in the few cases where therapies were initiated against spontaneous metastatic disease, there was no (control) equivalent test on a primary tumor using the same tumor cell populations. We found trastuzumab combinations (i.e., with either MTD or metronomic CTX) to show superimposable tumor regressions followed by growth delays. Yet hCG analysis of metastatic growth (which is another fundamental aspect of our study) showed that the impact of these therapies was not equivalent when applied to the metastatic setting. This result indicates that growth behavior and kinetics at a secondary (metastatic) site are unique and distinct phenomena from those at the primary site.
Of considerable interest, especially from a clinical perspective, is the contrast in the results we observed with the drug combinations tested to those obtained against orthotopically implanted primary tumors. Thus, we observed less impressive treatment benefits when treating established metastatic disease. We would argue that the experiments described here, although more time consuming, may be necessary to avoid or minimize the over interpretations that could be drawn from results obtained based only on treating primary tumors. In this respect, we note that the hCG methodology permitted an evaluation of the effectiveness of the different regimens on metastatic disease within 50 days from the start of treatment.
We also monitored the relative toxicity of long-term therapies particularly in view of the overt toxicity associated with MTD protocols. This also applies in the clinical setting, as dose-limiting toxicities of chemotherapeutic agents are sometimes implicated in the deaths of a small percentage of patients in clinical trials (21). Indeed, although in one of our metastatic models (met2) we observed that trastuzumab plus MTD chemotherapy was most effective in increasing survival, this was accompanied by a 16% rate of death of mice that showed no evidence of metastases. These mice seem to have succumbed to the overt toxicity of the long-term MTD regimen. In contrast, no such severe toxicity was observed in the trastuzumab plus low-dose CTX group. These results suggest that after an initial MTD-based treatment regimen, it may be beneficial to switch to a minimally toxic maintenance metronomic-type regimen—as we and others have already suggested for CTX (22) or vinblastine regimens (23–25). Thus, an interesting possible strategy (which we are currently assessing) could be to combine trastuzumab with several rounds of MTD CTX followed by a maintenance regimen of trastuzumab plus metronomic CTX. From a clinical perspective, the lesser toxicity and increased convenience of certain oral low-dose chemotherapy regimens, e.g., metronomic CTX, may be especially suitable for elderly patients who might be less able or willing to withstand MTD chemotherapy regimens. An example of this is the randomized phase II study of metronomic CTX plus the aromatase inhibitor letrozole in elderly women with metastatic breast cancer (26).
Our analysis using the LM2-4H2N spontaneous metastasis model confirmed that trastuzumab plus low-dose CTX is as effective in delaying the appearance and growth of metastasis. The combination of trastuzumab plus MTD CTX was also found to be effective, although it was associated with cycles of weight loss following MTD administration. Thus, the minimal toxicity of metronomic chemotherapy regimens may also make them especially suitable for long term adjuvant therapy of early stage disease, including breast cancer. Such trials are now under way, including a randomized phase III trial using daily low-dose CTX and twice a week methotrexate maintenance therapy in early stage breast cancer after surgery and initial conventional chemotherapy treatment, i.e., the International Breast Cancer Study Group (IBCSG-00-22) trial.4 The metronomic CTX/methotrexate protocol being used in the trial was based on initial results obtained in phase II trials involving metastatic breast cancer patients (27). Antimetabolic drugs such as UFT, a 5-fluorouracil oral prodrug composed of uracil and tegafur that has been used in a daily low-dose metronomic-like fashion for 2 years for the adjuvant treatment of early-stage cancers including non–small cell lung cancer (28) and breast cancer (29), might also be particularly suitable for metronomic chemotherapy based regimens.
In summary, we have shown that trastuzumab plus low-dose metronomic chemotherapy is effective in preclinical models of highly aggressive Her-2–positive spontaneously metastatic human breast cancer. These results suggest that such a treatment strategy could be effective if applied as maintenance regimens following MTD-based protocols. They also serve as an additional reminder of the potential benefit of studying more advanced stage and clinically relevant tumor models when evaluating new therapeutic strategies.
Translational Relevance
The Her-2–targeting antibody, trastuzumab, in combination with chemotherapy has proved effective against Her-2–overexpressing breast cancers in the clinic, both in the metastatic as well as in the adjuvant setting. Here, we used models of preclinical Her-2–positive metastatic breast cancer to test the efficacy of trastuzumab combined with one of two forms of administration of chemotherapy (using cyclophosphamide): either pulsatile maximum tolerated dose or a daily “low-dose” extended metronomic delivery. Combinations of trastuzumab with either mode of chemotherapy administration proved effective in suppressing the progression of Her-positive metastatic disease. Furthermore, the combination involving metronomic chemotherapy did not exhibit the severe toxicity associated with the maximum tolerated dose regimen. These results suggest that trastuzumab plus metronomic chemotherapy may be an effective long-term treatment regimen for metastatic Her-2–positive breast cancer.
Acknowledgments
We thank Cassandra Cheng for her excellent secretarial assistance, and Isaiah J. Fidler, Ian Hart (Cancer UK), and Yuval Shaked for critical review of the manuscript.
Grant support: NIH, the National Cancer Institute of Canada, and Canadian Institutes for Health Research (R.S. Kerbel). R.S. Kerbel holds a Tier I Canada Research Chair in Tumor Biology, Angiogenesis and Antiangiogenic Therapy. U. Emmenegger was funded by OICR (Clinician-Scientist II Investigator Award). Trastuzumab was a generous gift from Genentech.
Footnotes
Disclosure of Potential Conflicts of Interest
R.S. Kerbel, commercial research grant, Glaxo-Smith-Kline; consultant, Taiho (Japan). The other authors disclosed no potential conflicts of interest.
References
- 1.Dahabreh IJ, Linardou H, Siannis F, Fountzilas G, Murray S. Trastuzumab in the adjuvant treatment of early-stage breast cancer: a systematic review and meta-analysis of randomized controlled trials. Oncologist. 2008;13:620–30. doi: 10.1634/theoncologist.2008-0001. [DOI] [PubMed] [Google Scholar]
- 2.Hudis CA. Trastuzumab-mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 3.Robert N, Leyland-Jones B, Asmar L, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2006;24:2786–92. doi: 10.1200/JCO.2005.04.1764. [DOI] [PubMed] [Google Scholar]
- 4.Pegram MD, Konecny GE, O’Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst. 2004;96:739–49. doi: 10.1093/jnci/djh131. [DOI] [PubMed] [Google Scholar]
- 5.Arteaga CL, O’neill A, Moulder SL, et al. A phase I–II study of combined blockade of the ErbB receptor network with trastuzumab and gefitinib in patients with HER2 (ErbB2)-overexpressing metastatic breast cancer. Clin Cancer Res. 2008;14:6277–83. doi: 10.1158/1078-0432.CCR-08-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron DA, Stein S. Drug Insight: intracellular inhibitors of HER2-clinical development of lapatinib in breast cancer. Nat Clin Pract Oncol. 2008;5:512–20. doi: 10.1038/ncponc1156. [DOI] [PubMed] [Google Scholar]
- 7.Dinh P, de Azambuja E, Cardoso F, Piccart- Gebhart MJ. Facts and controversies in the use of trastuzumab in the adjuvant setting. Nat Clin Pract Oncol. 2008;5:645–54. doi: 10.1038/ncponc1219. [DOI] [PubMed] [Google Scholar]
- 8.Metro G, Mottolese M, Fabi A. HER-2-positive metastatic breast cancer: trastuzumab and beyond. Expert Opin Pharmacother. 2008;9:2583–601. doi: 10.1517/14656566.9.15.2583. [DOI] [PubMed] [Google Scholar]
- 9.Piccart M. Circumventing de novo and acquired resistance to trastuzumab: new hope for the care of ErbB2-positive breast cancer. Clin Breast Cancer. 2008;8:S100–113. doi: 10.3816/cbc.2008.s.006. [DOI] [PubMed] [Google Scholar]
- 10.Munoz R, Man S, Shaked Y, et al. Highly efficacious non-toxic treatment for advanced metastatic breast cancer using combination UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 2006;66:3386–91. doi: 10.1158/0008-5472.CAN-05-4411. [DOI] [PubMed] [Google Scholar]
- 11.Francia G, Emmenegger U, Lee CR, et al. Long term progression and therapeutic response of visceral metastatic disease non-invasively monitored in mouse urine using β-hCG choriogonadotropin secreting tumor cell lines. Mol Cancer Ther. 2008;7:3452–9. doi: 10.1158/1535-7163.MCT-08-0200. [DOI] [PubMed] [Google Scholar]
- 12.du Manoir JM, Francia G, Man S, et al. Strategies for delaying or treating in vivo acquired resistance to trastuzumab (Herceptin®) in human breast cancer xenografts. Clin Cancer Res. 2006;12:904–16. doi: 10.1158/1078-0432.CCR-05-1109. [DOI] [PubMed] [Google Scholar]
- 13.Orlando L, Cardillo A, Ghisini R, et al. Trastuzumab in combination with metronomic cyclophosphamide and methotrexate in patients with HER-2 positive metastatic breast cancer. BMC Cancer. 2006;6:225. doi: 10.1186/1471-2407-6-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price JE, Polyzos A, Zhang RD, Daniels LM. Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 1990;50:717–21. [PubMed] [Google Scholar]
- 15.Shih IM, Torrance C, Sokoll LJ, Chan DW, Kinzler KW, Vogelstein B. Assessing tumors in living animals through measurement of urinary β-human chorionic gonadotropin. Nat Med. 2000;6:711–4. doi: 10.1038/76299. [DOI] [PubMed] [Google Scholar]
- 16.Man S, Bocci G, Francia G, et al. Antitumor and anti-angiogenic effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res. 2002;62:2731–5. [PubMed] [Google Scholar]
- 17.Cruz-Munoz W, Man S, Xu P, Kerbel RS. Development of a preclinical model of spontaneous human melanoma CNS metastasis. Cancer Res. 2008;68:4500–5. doi: 10.1158/0008-5472.CAN-08-0041. [DOI] [PubMed] [Google Scholar]
- 18.Kerbel RS. What is the optimal rodent model for anti-tumor drug testing? Cancer Metastasis Rev. 1998;17:301–4. doi: 10.1023/a:1006152915959. [DOI] [PubMed] [Google Scholar]
- 19.Francia G, Emmenegger U, Kerbel RS. Tumor-associated fibroblasts as “Trojan Horse” mediators of resistance to anti-VEGF therapy. Cancer Cell. 2009;15:3–5. doi: 10.1016/j.ccr.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Gril B, Palmieri D, Bronder JL, et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst. 2008;100:1092–103. doi: 10.1093/jnci/djn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 22.Kerbel RS, Kamen BA. Antiangiogenic basis of low-dose metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–36. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 23.Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol. 2005;23:939–52. doi: 10.1200/JCO.2005.07.093. [DOI] [PubMed] [Google Scholar]
- 24.Klement G, Baruchel S, Rak J, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaked Y, Emmenegger U, Francia G, et al. Low-dose metronomic combined with intermittent bolus-dose cyclophosphamide is an effective long-term chemotherapy treatment strategy. Cancer Res. 2005;65:7045–51. doi: 10.1158/0008-5472.CAN-05-0765. [DOI] [PubMed] [Google Scholar]
- 26.Bottini A, Generali D, Brizzi MP, et al. Randomized phase II trial of letrozole and letrozole plus low-dose metronomic oral cyclophosphamide as primary systemic treatment in elderly breast cancer patients. J Clin Oncol. 2006;24:3623–8. doi: 10.1200/JCO.2005.04.5773. [DOI] [PubMed] [Google Scholar]
- 27.Colleoni M, Rocca A, Sandri MT, et al. Low dose oral methotrexate and cyclophosphamide in metastatic breast cancer: anti-tumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol. 2002;13:73–80. doi: 10.1093/annonc/mdf013. [DOI] [PubMed] [Google Scholar]
- 28.Kato H, Ichinose Y, Ohta M, et al. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med. 2004;350:1713–21. doi: 10.1056/NEJMoa032792. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe T, Sano M, Takashima S, et al. Oral uracil and tegafur compared with classic cyclophosphamide, methotrexate, fluorouracil as postoperative chemotherapy in patients with node-negative, high-risk breast cancer: National Surgical Adjuvant Study for Breast Cancer 01 Trial. J Clin Oncol. 2009;27:1368–74. doi: 10.1200/JCO.2008.18.3939. [DOI] [PubMed] [Google Scholar]