Abstract
Background:
The utility of poststroke cognitive status, namely dementia, cognitive impairment no dementia (CIND), mild cognitive impairment (MCI), and no cognitive impairment (NCI), in predicting dementia has been previously examined. However, no studies to date have compared the ability of subtypes of MCI and CIND to predict dementia in a poststroke population.
Methods:
A cohort of ischemic stroke patients underwent neuropsychological assessment annually for up to 5 years. Dementia was defined using the DSM-IV criteria. Univariate and multivariable Cox proportional regression was performed to determine the ability of MCI subtypes, CIND severity, and individual domains of impairment to predict dementia.
Results:
A total of 362 patients without dementia were followed up for a mean of 3.4 years (17% drop out), with 24 developing incident dementia. Older age, previous and recurrent stroke, and CIND and MCI subtypes were significant predictors of dementia. In multivariable analysis controlling for treatment allocation, patients who were older, had previous or recurrent stroke, and had either CIND moderate or multiple domain MCI with amnestic component were at elevated risk for dementia. In multivariable domain analysis, recurrent strokes, age, and previous strokes, verbal memory, and visual memory were significant predictors of dementia. Receiver operating characteristic curve analysis showed that CIND moderate (area under the curve: 0.893) and multiple domain MCI with amnestic component (area under the curve: 0.832) were significant predictors of conversion to dementia. All other classifications of cognitive impairment had areas under the curve less than 0.7.
Conclusion:
Stroke patients with cognitive impairment no dementia (CIND) moderate are at higher risk of developing dementia, while CIND mild patients are not at increased risk of developing dementia.
GLOSSARY
- AD
= Alzheimer disease;
- AUC
= area under the curve;
- CI
= confidence interval;
- CIND
= cognitive impairment no dementia;
- DSM-IV
= Diagnostic and Statistical Manual of Mental Disorders, 4th edition;
- ESPRIT
= European Australasian Stroke Prevention in Reversible Ischemia Trial;
- ESPRIT-Cog
= European Australasian Stroke Prevention in Reversible Ischemia Trial, cognitive substudy;
- HR
= hazard ratio;
- LACI
= lacunar infarct;
- MCI
= mild cognitive impairment;
- mRS
= modified Rankin scale;
- NCI
= no cognitive impairment;
- OCSP
= Oxfordshire Community Stroke Project;
- PACI
= partial anterior circulation infarct;
- POCI
= posterior circulation infarct;
- ROC
= receiver operating curve;
- TACI
= total anterior circulation infarct;
- VaD
= vascular dementia;
- WAIS-R
= Wechsler Adult Intelligence Scale–Revised;
- WMS-R
= Wechsler Memory Scale–Revised.
Dementia, mild cognitive impairment (MCI), and cognitive impairment no dementia (CIND) are frequently underdiagnosed and their incidence is likely to increase in aging populations. CIND and MCI are concepts that are commonly used to define the transitional period between normal aging and dementia. CIND has a broad scope, and is used to define impairments in any objective cognitive domains in neuropsychological testing in the absence of dementia.1 MCI was originally identified as a precursor to Alzheimer disease (AD) and defined as a complaint of defective memory with an abnormal memory function for age, along with normal activities of daily living, normal general cognitive functioning, and absence of dementia.2 More recently, MCI definitions have been categorized into 4 subtypes: amnestic MCI, nonamnestic single domain MCI, multiple-domain MCI with amnestic component, and nonamnestic multiple domain MCI.3
While comparisons of the predictive ability of MCI and CIND have been conducted in epidemiologic settings,4 they have not been performed in poststroke patients, who are known to have a high risk of dementia.5 The broad definition of CIND has been shown to be unstable in a poststroke setting.6 We therefore aimed to determine which CIND subtype predicts for dementia among poststroke patients. We also aimed to compare CIND and MCI subtypes as predictors of dementia and assessed the ability of cognitive domains to predict dementia.
METHODS
Subjects.
All patients with recent TIAs or nondisabling ischemic stroke who were seen in the Singapore General Hospital between 1999 and 2005 were screened for eligibility for the European Australasian Stroke Prevention in Reversible Ischemia Trial (ESPRIT). Detailed methodology for the main study have been previously reported.7 Briefly, patients were eligible if they were within 6 months of a TIA (including transient monocular blindness) or nondisabling ischemic stroke (grade ≤3 on the modified Rankin scale8) (mRS) of presumed arterial origin. The exclusion criteria were a possible cardiac source of embolism, high-grade carotid stenosis for which carotid endarterectomy or endovascular treatment was planned, any blood coagulation disorder, any contraindication for aspirin or dipyridamole, and a limited life expectancy.
Patients recruited into ESPRIT were eligible to enter this cognitive substudy (ESPRIT-Cog) with the following additional exclusion criteria: confusion, severe aphasia (expressive or receptive), major psychoses diagnosed according to DSM-IV criteria,9 or dominant upper limb paralysis.
Standard protocol approvals, registrations, and patient consents.
The study protocol was approved by Singapore General Hospital’s Institutional Review Board and Ethics Committee. Written informed consent was obtained from all patients or legal guardians. The ESPRIT Trial was registered under clinicaltrials.gov with the identifier NCT00161070.
Neuropsychological test battery.
Patients who consented to ESPRIT-cog received their baseline cognitive assessment 3 to 4 months after their qualifying event and annually thereafter for up to 5 years. Trained research psychologists administered a neuropsychological test battery that has previously been validated for use in Singapore.10 The battery assessed 6 domains, 4 of which were nonmemory domains. Education-adjusted cutoffs of 1.5 standard deviations below established normal means were used on individual tests. Failure in at least half of the tests in a domain constituted failure in that domain. The assessment was administered in English, Malay, Mandarin, or Chinese dialects according to the subject’s habitual language. The entire battery took under an hour and a half to complete.
The nonmemory domains were Attention, as defined by Digit Span,11 Visual Span,11 and Auditory Detection; Language, as defined by Modified Boston Naming and Category Fluency (Animals and Food subtasks); Visuomotor speed, as defined by Symbol Digit Modality Test,12 Digit Cancellation,13 and Maze Task14; and Visuoconstruction, as defined by Wechsler Memory Scale–Revised (WMS-R)11 subtest Visual Reproduction Copy task, Clock Drawing, and Wechsler Adult Intelligence Scale–Revised (WAIS-R)15 Block Design subtest.
The memory domains were Verbal Memory, as defined by Word List Recall16 (Immediate, Delayed, and Delayed Recognition) and Story Recall (Immediate and Delayed); and Visual Memory, as defined by Picture Recall (Immediate, Delayed, and Delayed Recognition) and WMS-R Visual Reproduction11 (Immediate, Delayed, and Delayed Recognition).
Diagnosis of dementia.
Diagnoses of dementia were made at weekly consensus conferences that were attended by neurologists, neuropsychologists, research nurses, and research assistants. Diagnoses were made according to the DSM-IV9 criteria. CT, MRI, and magnetic resonance angiography were reviewed as part of the diagnostic process. The etiologic diagnoses followed the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria for AD17 and the National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche en l’Enseignement en Neurosciences criteria18 for vascular dementia (VaD). The sample without dementia included individuals with diagnoses of CIND, MCI, or no cognitive impairment (NCI). Patients with CIND were impaired in at least one domain of the neuropsychological test battery, but did not meet criteria for dementia.1 On the basis of the sample median, CIND was divided into CIND mild (1–2 domains impaired) and CIND moderate (3–6 domains impaired). Patients were also classified by MCI subtypes (amnestic MCI, nonamnestic single domain MCI, multiple domain MCI with an amnestic component, and nonamnestic multiple domain MCI) according to the revised MCI criteria.3
Baseline risk factors.
Risk factor information was collected at baseline. Stroke subtype was classified according to the Oxfordshire Community Stroke Project (OCSP)19 as total anterior circulation infarct (TACI), partial anterior circulation infarct (PACI), posterior circulation infarct (POCI), or lacunar infarct (LACI).19 Vascular risk factor data, such as age, diabetes mellitus status, hypertension, hyperlipidemia, smoking status, ischemic heart disease, peripheral artery disease, as well as past history of stroke, angina, and myocardial infarction were obtained verbally from the patient and confirmed with hospital records.
Outcome measures.
Patients were followed up annually for up to 5 years. Patients underwent full neuropsychological assessment at the outpatient clinic. If a recurrent event had occurred, detailed hospital records were obtained to verify the occurrence of the vascular event.
Statistical analysis.
Analysis of variance or χ2 analysis was used to test for significant differences among NCI, CIND mild, and CIND moderate patients. Analysis was done in 3 stages. In the first stage, univariate regressions were performed to determine which baseline characteristics were predictive of dementia. Univariate regression analyses were repeated 3 times, once with CIND severity as the indicator of baseline cognitive impairment, then with an indicator of 1 domain of impairment vs multiple domains of impairment, and again with MCI subtypes as the indicator of cognitive impairment. In the second stage of analyses, multivariable regression models controlling for treatment allocation were performed with significant predictors in the univariate stage being included in the models. Analyses were repeated again with CIND severity and MCI subtypes as indicators of baseline cognitive impairment. In the third stage of analysis, individual domains of cognition were analyzed for their ability to predict conversion to dementia in both univariate analyses, and multivariable analyses which adjusted for significant predictors of dementia from stage 1, and treatment allocation. Cox proportional hazards models were used in all stages of analyses. Analyses were performed in Stata 10.0,20 and significance was determined with a 2-tailed alpha of 0.05 in stages 1 and 2 of analyses while Bonferroni adjustment for multiple comparisons in stage 3 yielded an alpha of 0.008. Finally, uniform scores were derived for each domain and averaged across the patients in different MCI and CIND severity, after which receiver operating curves (ROC) were plotted to compare the area under the curve (AUC) of the different classifications.
RESULTS
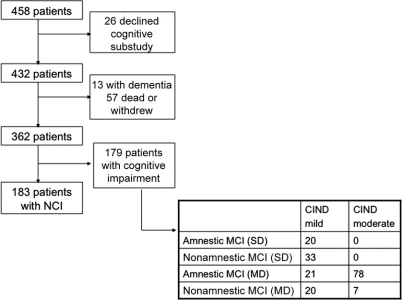
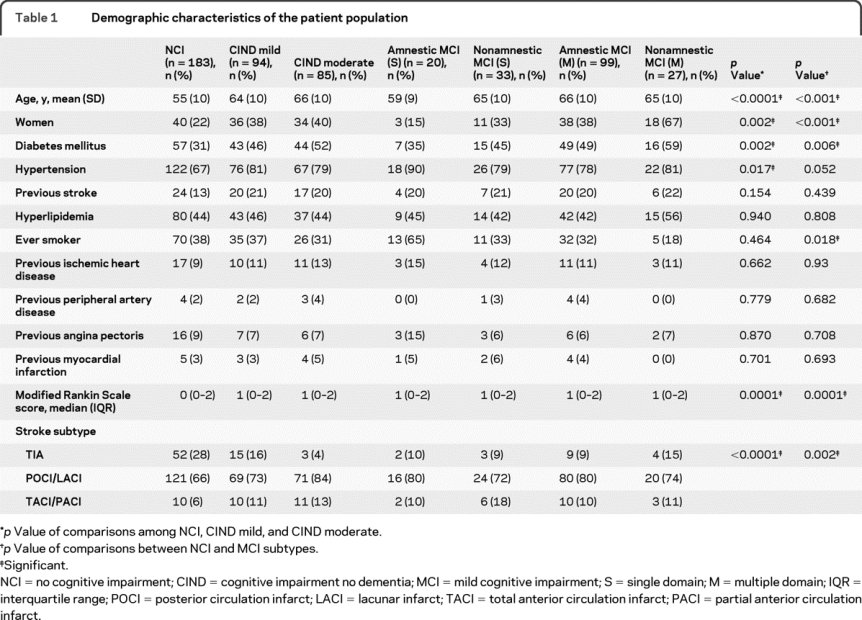
A total of 458 patients were recruited into ESPRIT at the Singapore General Hospital site, of which 432 consented to participate in the ESPRIT-cog substudy (figure). Of these 432 patients, 13 had dementia at baseline, and 57 died or withdrew from the study before undergoing follow-up neuropsychological evaluation. We thus present data of 362 patients (mean age 60 ± 11 years, 30% women) who were followed for an average of 3.2 years. There were 183 patients with NCI, 94 with CIND mild, and 85 with CIND moderate. The demographic characteristics of the study population stratified by baseline cognitive status are summarized in table 1. Patients with more severe cognitive impairment were significantly older; more likely to be women, diabetic, and hypertensive; and more likely to have had more severe stroke.
Figure. Study design.
Table 1 Demographic characteristics of the patient population
Among the 179 patients with MCI, 20 had amnestic MCI, 33 nonamnestic single domain MCI, 99 multiple-domain MCI with amnestic component, and 27 nonamnestic multiple domain MCI.
During the course of the study, 24 patients converted to dementia: 3 AD, 15 VaD, and 6 mixed dementia. The incidence of dementia was 11 per 1,000 in NCI patients, 42 per 1,000 in CIND mild patients, and 212 per thousand in CIND moderate patients. By MCI subtypes, the incidence of dementia for MCI subtypes was 11 in NCI, 50 in amnestic MCI, 30 in nonamnestic single-domain MCI, 181 in multiple-domain MCI with amnestic component, and 74 in nonamnestic multiple domain MCI patients.
In univariate analysis, older patients, patients with prior strokes, patients who experienced another stroke, as well as those with more severe baseline cognitive impairment (CIND moderate, hazard ratio [HR] = 22.5, confidence interval [CI] 5.22–97.2, in CIND severity; multiple domain MCI with amnestic component, HR = 19.3, CI 4.48–83.4; and nonamnestic multiple domain MCI, HR = 7.87, CI 1.11–55.9, in MCI subtypes, and MMSE, HR = 0.91, CI 0.83–0.99) were at higher risk of conversion to dementia (table 2 [condensed version], table e-1 on the Neurology® Web site at www.neurology.org [full version]).
Table 2 Results of univariate and multivariable Cox proportional hazards models for the prediction of dementia (condensed version)
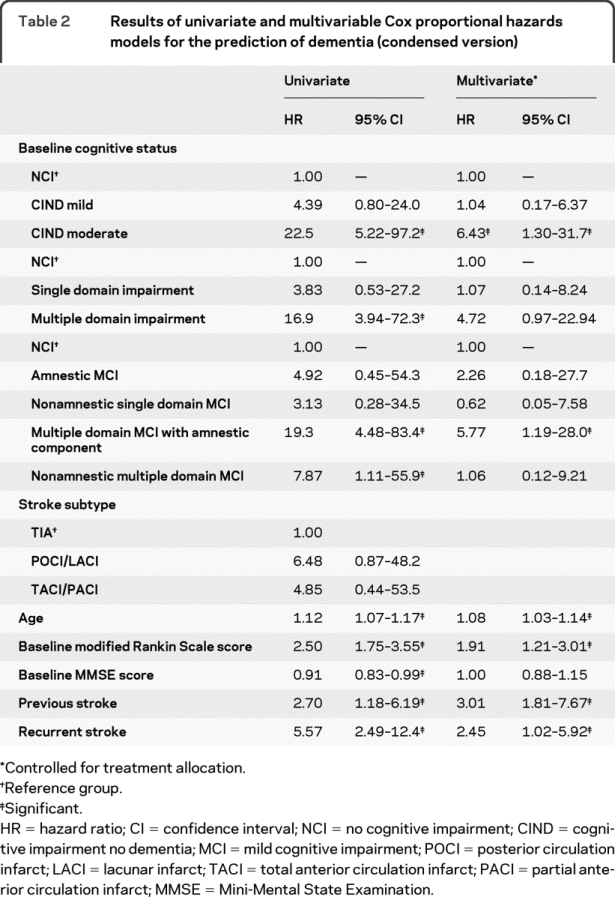
In multivariable analysis controlling for treatment allocation, age (HR = 1.08, CI 1.03–1.14), occurrence of a previous stroke (HR = 3.01, CI 1.18–7.67), occurrence of another stroke (HR = 2.45, CI 1.02–5.92), and baseline cognitive status as defined by either CIND moderate (HR = 6.43, CI 1.30–31.7) or multiple domain MCI with amnestic component (HR = 5.77, CI 1.19–28.0) were significant predictors of dementia (table 2 [condensed version], table e-1 [full version]).
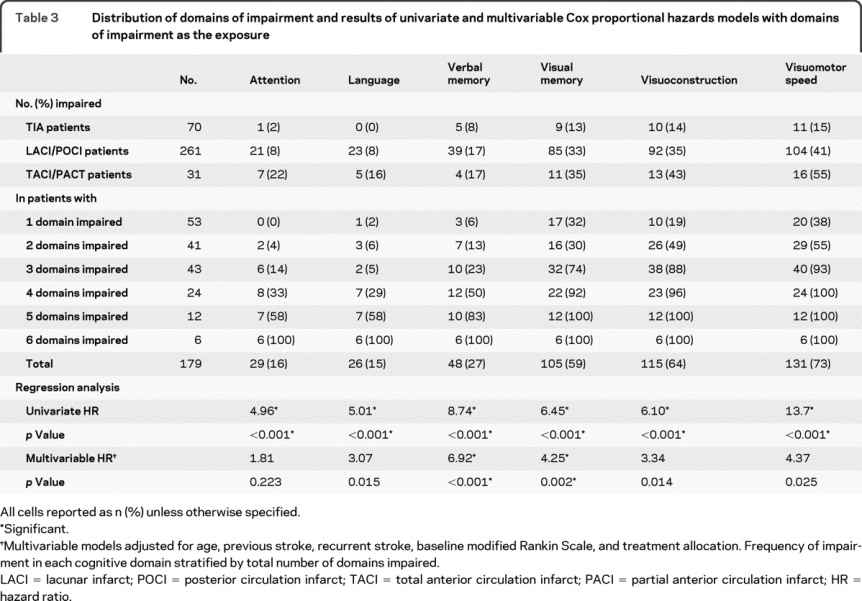
Table 3 summarizes impairment of cognitive domains stratified by the number of domains impaired. Visuomotor speed was the domain that was most commonly impaired, followed by visuoconstruction and visual memory. In univariate domain analysis, all domains were significant predictors of dementia (table 3). In multivariable domain analysis, while verbal memory, visual memory, visuoconstruction, and visuomotor speed were significant at an alpha of 0.05, only verbal memory (HR = 6.92, p < 0.001) and visual memory (HR = 4.25, p = 0.002) were significant predictors of dementia after Bonferroni adjustment (table 3).
Table 3 Distribution of domains of impairment and results of univariate and multivariable Cox proportional hazards models with domains of impairment as the exposure
ROC curve analysis showed that CIND moderate (AUC 0.893) was not significantly better than multiple domain MCI with amnestic component in predicting dementia (AUC 0.832) (p = 0.50). All other classifications of cognitive impairment had AUCs less than 0.7.
DISCUSSION
In this study, we evaluated the ability of CIND severity to predict dementia in a poststroke population. The CIND severity (CIND mild and CIND moderate) were able to differentiate patients who were at risk of conversion to dementia. CIND moderate patients had a sixfold increased risk of conversion to dementia compared to NCI patients while CIND mild patients’ risk was similar to that of NCI patients. Both multidomain MCI subtypes and CIND moderate subtypes were able to predict incident dementia. We confirmed findings from prior studies which showed that age, the occurrence of a prior stroke, and the occurrence of recurrent strokes were all significant predictors of dementia. We also found that impairments in the domains of verbal and visual memory were able to predict incident dementia.
While prestroke cognitive decline is associated with poststroke dementia,21 there have been only a few studies examining cognitive states after stroke and their association with incident dementia. One study22 assessed poststroke survivors without dementia at 3 and 15 months but found that none of the criteria utilized at baseline (MCI, age-associated cognitive decline, vascular CIND) identified patients at risk of incident dementia. Another study23 examined the predictive accuracy of MCI subtypes for dementia in a mixed cohort of memory clinic and poststroke patients, and showed that the multiple domain MCI subtype had a high sensitivity but did not investigate CIND severity. Finally, a more recent study24 determined the frequency of CIND in a poststroke population and found that it predicted for incident dementia. However, there was no attempt to differentiate between mild and moderate CIND.
Previous studies comparing patients with incident AD and patients with VaD have shown few differences in their preclinical cognitive profiles.25–28 However, the lack of difference could well be an artifact of the requirement of memory impairment for a diagnosis of dementia in the DSM-IV criteria. An early analysis of subjects with vascular CIND in the Canadian Study of Healthy Aging found that impairments in tests of memory and category fluency were associated with incidence of dementia.29 However, a later analysis of subjects with NCI from the same study,30 which investigated neuropsychological predictors, found that while abstract reasoning scores were lower in the incident vascular cognitive impairment group, memory test scores were lower in the incident AD group. Therefore, we suggest that in populations with cognitive impairment of predominantly vascular causes, CIND severity be used as opposed to MCI subtypes, which emphasize an amnestic component. Larger epidemiologic studies in populations at risk for cognitive impairment of predominantly vascular causes are needed to confirm our findings. Additionally, our proposed CIND subtype definitions may be supplemented with executive functioning and abstract reasoning tests.
In support of our findings that impairments in the domains of visual memory and verbal memory were associated with an increased risk of incident dementia, the Sydney Stroke Cohort has shown that verbal memory was more likely to deteriorate in ischemic stroke or TIA patients who converted to dementia.31 Cognitive impairment in stroke patients may lead to an increase of mortality and recurrent cerebrovascular events due to several causes. Cognitively impaired patients may be less compliant with medication, thereby reducing the effectiveness of secondary prevention therapies, or be less able to alter their lifestyle habits, which may lead to poorer control of vascular comorbidities.21 This is particularly important in stroke patients, who tend to have more vascular comorbidities than patients with AD.32 Therefore, while the initial magnitude of cognitive decline seen among poststroke patients is less than that of patients with prodromal AD,33 the subsequent effect on mortality and morbidity might be greater than in patients with AD.
Our study has several limitations. The inclusion/exclusion criteria limit recruitment to those without dominant upper limb paralysis and who had a baseline mRS ≤3. Hence this may limit the generalizability of our findings as these criteria have resulted in a younger population in ESPRIT than most stroke populations. With only 24 patients progressing to dementia, we were unable to perform separate analysis on patients who progressed to AD, VaD, or mixed dementia. Larger studies should endeavor to investigate the predictive ability of CIND mild and CIND moderate separately in patients who progress to AD and VaD. Another limitation of this study was that we were underpowered to examine the interaction of recurrent vascular event and CIND moderate status at baseline. However, as we controlled for the recurrence of stroke as well as the history of previous strokes, we believe that our sample size will not affect our conclusions. While prestroke dementia was excluded, we were unable to control for prestroke cognitive impairment. Furthermore, although the cognitive battery utilized was validated by administration to an elderly community-dwelling population in Singapore in order to elicit formal structural domains, identify items that may not be culturally relevant, and to replace those items with culturally appropriate items, more studies need to be performed using other cognitive instruments to confirm the predictive abilities of the CIND moderate classification. We recognize that our findings may be due to the definitions of CIND severity and MCI subgroups, which results in CIND moderate representing more global cognitive impairments than either form of multidomain MCI, and also results in CIND mild overlapping with multidomain MCI. As there are 4 MCI subclassifications compared to the two CIND subclassifications that we proposed, this may result in a loss of power in this study for the MCI subclassification, which could explain our results. Additionally, we recognize that our classifications of CIND did not adopt the typical threshold of less than 1 SD from the mean, but instead adopted the usual MCI threshold of <1.5 standard deviations from the mean so as to allow comparison. Hence, further studies are needed to validate the operationalized criteria for MCI and CIND in different populations that are at high risk of developing dementia.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Kaavya Narasimhalu.
ACKNOWLEDGMENT
The authors thank the psychologists and trial coordinators who collected data for this study.
DISCLOSURE
This study was funded by the National Medical Research Council (Singapore). K. Narasimhalu, S. Ang, Dr. De Silva, Dr. Wong, Dr. Chang, and Dr. Chia report no disclosures. Dr. Auchus has received funding for travel for lectures or educational activities not funded by industry; serves on the editorial advisory board of Alzheimer Disease and Associated Disorders; has served as a consultant to Myriad Genetics, Inc.; serves on a speakers’ bureau for Novartis; received research support from Pfizer Inc., Eisai Inc., and Elan Corporation, the NIH/NIA [U01AG10483 (Site PI)], R01AG030114 (Consultant), and the University of Tennessee Health Science Center Neuroscience Institute; and served as mentor for the Ellison Medical Foundation/American Federation for Aging Research Senior Postdoctoral Fellows Grant (E07097). Dr. Chen has received honoraria from Lundbeck Inc., SERVIER, Pfizer Inc., and Elan Corporation.
Supplementary Material
Address correspondence and reprint requests to Dr. Kaavya Narasimhalu, Duke-NUS Graduate Medical School, Khoo Teck Puat Building, 8 College Road, Singapore 169857 nkaavya@gmail.com
Supplemental data at www.neurology.org
Disclosure: Author disclosures are provided at the end of the article.
Received December 21, 2008. Accepted in final form September 15, 2009.
REFERENCES
- 1.Graham JE, Rockwood K, Beattie BL, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet 1997;349:1793–1796. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Kokmen E, Tangelos EG. Aging, memory, and mild cognitive impairment. Int Psychogeriatr 1997;9 suppl 1:65–69. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- 4.Di Carlo A, Lamassa M, Baldereschi M, et al. CIND and MCI in the Italian elderly: frequency, vascular risk factors, progression to dementia. Neurology 2007;68:1909–1916. [DOI] [PubMed] [Google Scholar]
- 5.Sachdev PS, Brodaty H, Valenzuela MJ, et al. Clinical determinants of dementia and mild cognitive impairment following ischaemic stroke: the Sydney Stroke Study. Dement Geriatr Cogn Disord 2006;21:275–283. [DOI] [PubMed] [Google Scholar]
- 6.Tham W, Auchus AP, Thong M, et al. Progression of cognitive impairment after stroke: one year results from a longitudinal study of Singaporean stroke patients. J Neurol Sci 2002;203–204:49–52. [DOI] [PubMed] [Google Scholar]
- 7.De Schryver EL. Design of ESPRIT: an international randomized trial for secondary prevention after non-disabling cerebral ischaemia of arterial origin: European/Australian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT) group. Cerebrovasc Dis 2000;10:147–150. [DOI] [PubMed] [Google Scholar]
- 8.Bonita R, Beaglehole R. Modification of Rankin Scale: recovery of motor function after stroke. Stroke 1988;19:1497–1500. [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 10.Yeo D, Gabriel C, Chen C, Lee S, Loenneker T, Wong M. Pilot validation of a customized neuropsychological battery in elderly Singaporeans. Neurol J South East Asia 1997;2:123. [Google Scholar]
- 11.Wechsler D. Wechsler Memory Scale: Revised (3rd ed). San Antonio, TX: Harcourt Brace Jovanovich; 1997. [Google Scholar]
- 12.Smith A. Symbol Digit Modalities Test. In: Services WP, ed. Symbol Digit Modalities Test. Los Angeles, CA: 1973. [Google Scholar]
- 13.Diller L, Ben-Yishay Y, Gerstman LJ. Studies in Cognition and Rehabilitation in Hemiplegia: Rehabilitation Monograph No. 50. New York: New York University Medical Center Institute of Rehabilitation Medicine; 1974. [Google Scholar]
- 14.Porteus SD. The Maze Test and clinical psychology. Palo Alto, CA: Pacific Books; 1959. [Google Scholar]
- 15.Wechsler D. Wechsler Adult Intelligence Scale: Revised. San Antonio, TX: Harcourt Brace Jovanovich; 1981. [Google Scholar]
- 16.Sahadevan S, Tan NJ, Tan TC, Tan S. Cognitive testing of elderly Chinese from selected community clubs in Singapore. Ann Acad Med Singapore 1997;26:271–277. [PubMed] [Google Scholar]
- 17.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 18.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993;43:250–260. [DOI] [PubMed] [Google Scholar]
- 19.Mead GE, Lewis SC, Wardlaw JM, Dennis MS, Warlow CP. How well does the Oxfordshire community stroke project classification predict the site and size of the infarct on brain imaging? J Neurol Neurosurg Psychiatry 2000;68:558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Statacorp. Stata (ed 10.0). College Station, TX: Statcorp; 2008. [Google Scholar]
- 21.Leys D, Henon H, Kowiak-Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol 2005;4:752–759. [DOI] [PubMed] [Google Scholar]
- 22.Ballard C, Rowan E, Stephens S, Kalaria R, Kenny R. Prospective follow-up study between 3 and 15 months after stroke: improvements and decline in cognitive function among dementia-free stroke survivors >75 years of age. Stroke 2003;34:2440–2444. [DOI] [PubMed] [Google Scholar]
- 23.Rasquin S, Lodder J, Visser P, Lousberg R, Verhey F. Predictive accuracy of MCI subtypes for Alzheimer’s disease and vascular dementia in subjects with mild cognitive impairment: a 2-year follow-up study. Dement Geriatr Cogn Disord 2005;19:113–119. [DOI] [PubMed] [Google Scholar]
- 24.Serrano S, Domingo J, Rodriguez-Garcia E, Castro MD, del Ser T. Frequency of cognitive impairment without dementia in patients with stroke: a two-year follow-up study. Stroke 2007;38:105–110. [DOI] [PubMed] [Google Scholar]
- 25.Meyer JS, Xu G, Thornby J, Chowdhury MH, Quach M. Is mild cognitive impairment prodromal for vascular dementia like Alzheimer’s disease? Stroke 2002;33:1981–1985. [DOI] [PubMed] [Google Scholar]
- 26.Laukka EJ, Jones S, Fratiglioni L, Backman L. Cognitive functioning in preclinical vascular dementia: a 6-year follow-up. Stroke 2004;35:1805–1809. [DOI] [PubMed] [Google Scholar]
- 27.Laukka EJ, Jones S, Small BJ, Fratiglioni L, Backman L. Similar patterns of cognitive deficits in the preclinical phases of vascular dementia and Alzheimer’s disease. J Int Neuropsychol Soc 2004;10:382–391. [DOI] [PubMed] [Google Scholar]
- 28.Sacuiu S, Sjogren M, Johansson B, Gustafson D, Skoog I. Prodromal cognitive signs of dementia in 85-year-olds using four sources of information. Neurology 2005;65: 1894–1900. [DOI] [PubMed] [Google Scholar]
- 29.Ingles JL, Wentzel C, Fisk JD, Rockwood K. Neuropsychological predictors of incident dementia in patients with vascular cognitive impairment, without dementia. Stroke 2002;33:1999–2002. [DOI] [PubMed] [Google Scholar]
- 30.Ingles JL, Boulton DC, Fisk JD, Rockwood K. Preclinical vascular cognitive impairment and Alzheimer disease: neuropsychological test performance 5 years before diagnosis. Stroke 2007;38:1148–1153. [DOI] [PubMed] [Google Scholar]
- 31.Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz LM, Koschera A. Progression of cognitive impairment in stroke patients. Neurology 2004;63:1618–1623. [DOI] [PubMed] [Google Scholar]
- 32.Cechetto DF, Hachinski V, Whitehead SN. Vascular risk factors and Alzheimer’s disease. Expert Rev Neurother 2008;8:743–750. [DOI] [PubMed] [Google Scholar]
- 33.Schneider LS. Galantamine for vascular dementia: some answers, some questions. Lancet 2002;359:1265–1266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.