Abstract
Objective:
To investigate the relationships between history of falls and cholinergic vs dopaminergic denervation in patients with Parkinson disease (PD).
Background:
There is a need to explore nondopaminergic mechanisms of gait control as the majority of motor impairments associated with falls in PD are resistant to dopaminergic treatment. Alterations in cholinergic neurotransmission in PD may be implicated because of evidence that gait control depends on cholinergic system–mediated higher-level cortical and subcortical processing, including pedunculopontine nucleus (PPN) function.
Methods:
In this cross-sectional study, 44 patients with PD (Hoehn & Yahr stages I–III) without dementia and 15 control subjects underwent a clinical assessment and [11C]methyl-4-piperidinyl propionate (PMP) acetylcholinesterase (AChE) and [11C]dihydrotetrabenazine (DTBZ) vesicular monoamine transporter type 2 (VMAT2) brain PET imaging.
Results:
Seventeen patients (38.6%) reported a history of falls and 27 patients had no falls. Analysis of covariance of the cortical AChE hydrolysis rates demonstrated reduced cortical AChE in the PD fallers group (−12.3%) followed by the PD nonfallers (−6.6%) compared to control subjects (F = 7.22, p = 0.0004). Thalamic AChE activity was lower only in the PD fallers group (−11.8%; F = 4.36, p = 0.008). There was no significant difference in nigrostriatal dopaminergic activity between PD fallers and nonfallers.
Conclusions:
Unlike nigrostriatal dopaminergic denervation, cholinergic hypofunction is associated with fall status in Parkinson disease (PD). Thalamic AChE activity in part represents cholinergic output of the pedunculopontine nucleus (PPN), a key node for gait control. Our results are consistent with other data indicating that PPN degeneration is a major factor leading to impaired postural control and gait dysfunction in PD.
GLOSSARY
- AChE
= acetylcholinesterase;
- ANCOVA
= analysis of covariance;
- MMSE
= Mini-Mental State Examination;
- PD
= Parkinson disease;
- PPN
= pedunculopontine nucleus;
- PSP
= progressive supranuclear palsy;
- UPDRS
= Unified Parkinson’s Disease Rating Scale;
- VOI
= volume of interest.
Falls are common and disabling in Parkinson disease (PD).1 Because of nigrostriatal pathology in PD, it is asserted often that postural instability is attributable mainly to striatal dopaminergic denervation. However, balance-related deficits are least responsive to levodopa treatment.1,2 Therefore, there is a need to explore nondopaminergic mechanisms of gait control in PD. Until recently, gait was generally viewed as a largely automated motor task, requiring minimal cognitive input. Increasing evidence, however, links alterations in cognitive function to gait disturbances.3 Cortical cholinergic denervation in PD is associated with cognitive impairment4 but effects of alterations in cholinergic neurotransmission on mobility control in PD are poorly understood. There are 2 major sources of cholinergic projections in the brain. The nucleus basalis of Meynert (NBM) provides the principal cholinergic input of the entire cortical mantle and degenerates in PD.5 The pedunculopontine nucleus (PPN), a brainstem locomotor center, provides cholinergic inputs to the basal ganglia, thalamus, cerebellum, several brainstem nuclei, and the spinal cord,6 and also degenerates in PD.7
[11C]PMP PET imaging assesses cholinergic terminal integrity with cortical activity reflecting NBM integrity and thalamic uptake reflecting PPN integrity. It was the goal of the present study to investigate associations of fall status in PD with cortical-NBM and thalamic-PPN cholinergic function and to compare this to the degree of nigrostriatal dopaminergic denervation. We hypothesized that pathology within the NBM and/or PPN may be associated with fall status in PD.
METHODS
Subjects and clinical test battery.
This cross-sectional study involved 44 subjects with PD (34 male and 10 female) and 15 control subjects without PD (7 male and 8 female). Patients met the UK PD Society Brain Bank Research Center clinical diagnostic criteria for PD.8 In keeping with these criteria, none of the patients had clinical evidence of supranuclear gaze palsy, cervical dystonia, spastic bulbar symptoms, ataxia, prominent dysautonomia, or prominent postural instability within the first year of disease onset.
The diagnosis of PD was also confirmed by the presence of nigrostriatal dopaminergic denervation on DTBZ PET imaging. Patients had mild to moderate severity of disease: 2 patients in stage 1, 1 patient in stages 1.5, 11 patients in stage 2, 17 patients in stage 2.5, and 13 patients in stage 3 of the modified Hoehn & Yahr classification.9 The mean duration of disease was 7.1 ± 4.2 (SD) years (range 1–17). Subjects with a Mini-Mental State Examination (MMSE) score of 24 or less were not eligible for the study.10 The mean MMSE score was 29.0 ± 1.4 (range 25–30).
The Unified Parkinson’s Disease Rating Scale (UPDRS) was performed.11 Axial motor score was calculated as the summed score of the UPDRS items 27–30 (arising from chair, posture, gait, postural stability). Subjects on dopaminergic drugs were examined and imaged in the morning after withholding dopaminergic drugs overnight. The mean UPDRS motor score was 25.6 ± 8.2 (range 5–40). Patients also completed a 2-day motor “on-off” diary at home to estimate average daily time spent in “off” state.12 The UPDRS part II, item 13 on falling (unrelated to freezing) was used to determine fall status, with nonfallers having a score of 0 and fallers a score of 1 or higher.
Twenty-five patients were taking a combination of dopamine agonist and carbidopa-levodopa medications, 13 on carbidopa-levodopa alone, 4 on dopamine agonists alone, and 2 were not on dopaminergic drugs. No patients were on (anti-) cholinergic drugs. Patients with PD were recruited from the movement disorders clinic at the University of Michigan and Ann Arbor VA during the period 2006–2008.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Institutional Review Boards of the University of Michigan and Ann Arbor VA for studies involving human subjects. Written informed consent was obtained from all subjects. ClinicalTrials.gov identifiers: NCT00737217 and NCT00736671.
Imaging techniques.
DTBZ and PMP PET imaging was performed in 3-dimensional imaging mode using an ECAT HR+ tomograph (Siemens Molecular Imaging, Inc., Knoxville, TN), which acquires 63 transaxial slices (slice thickness: 2.4 mm; intrinsic in-plane resolution: 4.1 mm full-width at half maximum over a 15.2 cm axial field of view). A NeuroShield (Scanwell Systems, Montreal, Canada) head-holder/shielding unit was attached to the patient bed to reduce the contribution of detected photon events originating from the body outside the scanner field of view.13 Prior to the DTBZ and PMP injections, a 5-minute transmission scan was acquired using rotating 68Ge rods for attenuation correction of emission data using the standard vendor-supplied segmentation and reprojection routines.
DTBZ PET imaging.
No-carrier-added (+)-[11C]DTBZ (250 to 1000 Ci/mmol at the time of injection) was prepared as reported previously.14 Dynamic PET scanning was performed for 60 minutes immediately following a bolus injection of 55% of 666 MBq (18 mCi) of (+)-[11C]DTBZ dose (containing less than 50 μg of cold DTBZ mass) over the first 15 to 30 seconds of the study, while the remaining 45% of the dose was continuously infused over the next 60 minutes, resulting in stable arterial tracer levels and equilibrium with brain tracer levels after 30 minutes.15 A series of 15 frame sequence of scans over 60 minutes were obtained as following: 4 × 30 seconds; 3 × 1 minute; 2 × 2.5 minutes; 2 × 5 minutes; and 4 × 10 minutes.
[11C]PMP was prepared in high radiochemical purity (>95%) by N-[11C]methylation of piperidin-4-yl propionate using a previously described method.16 Dynamic PET scanning was performed for 70 minutes immediately following a bolus IV injection of 666 MBq (18 mCi) of [11C]PMP. The dose contained less than 200 μg cold PMP mass. Emission data were collected in 16 sequential emission scans (the DTBZ protocol plus an additional 10-minute frame). All subjects were studied supine, with eyes and ears unoccluded, resting quietly in a dimly lit room.
MRI.
All subjects underwent brain MRI on a 3 Tesla Philips Achieva system (Philips, Best, the Netherlands) utilizing an 8-channel head coil and the ISOVOX examination card protocol primarily designed to yield isotropic spatial resolution. A standard T1-weighted series of a 3-dimensional inversion recovery–prepared turbo-field echo was performed in the sagittal plane using repetition time/echo time/inversion time = 9.8/4.6/1041 msec; turbo factor = 200; single average; field of view = 240 × 200 × 160 mm; acquired matrix = 240 × 200. A total of 160 slices were reconstructed to 1 mm isotropic resolution. This sequence maximizes contrast among gray matter, white matter, and CSF and provides high-resolution delineation of cortical and subcortical structures. The brain MRI scans of the patients were also reviewed with specific attention to signs of atypical parkinsonism, such as midbrain atrophy (including the “hummingbird” sign), pontine atrophy (including the “hot cross bun” sign), cerebellar atrophy, or hyperintense signal at the posterolateral portion of the putamen. None of the scans demonstrated imaging evidence of either multiple system atrophy or progressive supranuclear palsy in the patients.17 Subjects with evidence of focal intracranial pathology or large vessel stroke were not eligible for the study.
Data analysis.
All image frames were spatially coregistered within subjects with a rigid-body transformation to reduce the effects of subject motion during the imaging session.18 IDL image analysis software (Research Systems, Inc., Boulder, CO) was used to manually trace volumes of interest (VOIs) on the MRI to include the thalamus, caudate nucleus, and putamen of each hemisphere. Total cortical VOI were defined using semiautomated thresholding delineation of cortical gray matter signal on the MRI. Left and right hemispheric VOIs were averaged because of absence of significant differences in left-to-right hemispheric AChE activity.
DTBZ images were analyzed using equilibrium modeling to estimate the nondisplaceable binding potential (BPND), which is equivalent to the ratio of specific (VS) to nondisplaceable (VND) binding in each imaged voxel or target VOI.15 The total volume of distribution (VT) in any voxel or VOI contains contributions of nondisplaceable ligand (VND) in addition to that of specifically bound ligand (VS).19 We estimated specific DTBZ binding by subtraction of the occipital cortex value (Vctx), a reference region very low in VMAT2 binding sites, with the assumption that the nondisplaceable distribution is uniform across the brain (VND = Vctx) at equilibrium:
Reporting specific binding relative to the nondisplaceable as measured in the occipital cortex yields the nondisplaceable binding potential measure BPND:
AChE hydrolysis rates (k3) were estimated using a method using the striatal VOI (defined by manual tracing on MRI) as the reference input tissue.20 The operational equation is:
where A(T) and R(T) are radioactivity concentrations in VOIs and the reference tissue, striatum, which is assumed to have complete trapping of PMP.
Standard pooled-variance t or Satterthwaite’s method of approximate t tests were used for group comparisons (SAS version 9.1, SAS institute, Cary, NC). Pearson correlation coefficients were calculated for correlation between clinical or PET variables. Analysis of covariance (ANCOVA) was performed to compare differences between groups while controlling for age and/or the degree of nigrostriatal denervation. Duncan multiple range post hoc test was used to determine differences between subgroups.
RESULTS
The mean age of the patients was 68.9 ± 9.5 years (range 51–83) and did not differ from the non-PD control subjects (64.4 ± 9.6 years; range 50–81; t = −1.57, p = 0.12). Cortical and thalamic AChE hydrolysis rates and striatal DTBZ BPND were significantly lower in the patients compared to the control subjects (table 1).
Table 1 Mean ± SD thalamic and cortical AChE hydrolysis rates (k3; min−1) and striatal VMAT2 (BPND) activity in the patients with PD and control subjects
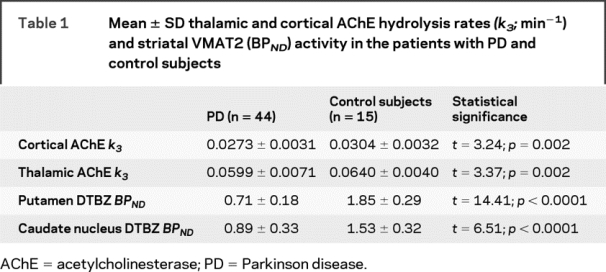
Longer duration of disease correlated with lower striatal DTBZ binding (R = −0.42, p = 0.005) and lower thalamic AChE hydrolysis rates (R = −0.33, p = 0.02). There was a borderline relationship between longer duration of disease and lower cortical AChE hydrolysis rates (R = −0.29, = 0.055). UPDRS motor scores correlated with striatal DTBZ binding (R = −0.35, p = 0.024) and cortical AChE hydrolysis rates (R = −0.43, p = 0.004). There was a borderline relationship between higher UPDRS motor scores and lower thalamic AChE hydrolysis rates (R = −0.25, p = 0.09). Higher UPDRS axial motor scores correlated with lower striatal DTBZ binding (R = −0.32, p = 0.04), lower cortical (R = −0.34, p = 0.03) but not with thalamic AChE hydrolysis rates (−0.10, p = 0.48).
Higher MMSE scores correlated with higher cortical AChE hydrolysis rates (R = 0.36, p = 0.02) but not with thalamic AChE activity (R = 0.13, p = 0.41) or striatal DTBZ binding (R = −0.03, p = 0.86). Higher scores on the UPDRS activities of daily living scale correlated with lower cortical AChE hydrolysis rates (R = −0.35, p = 0.02) but not with striatal DTBZ binding (R = −0.22, p = 0.14) and borderline with thalamic AChE activity (R = −0.27, p = 0.08).
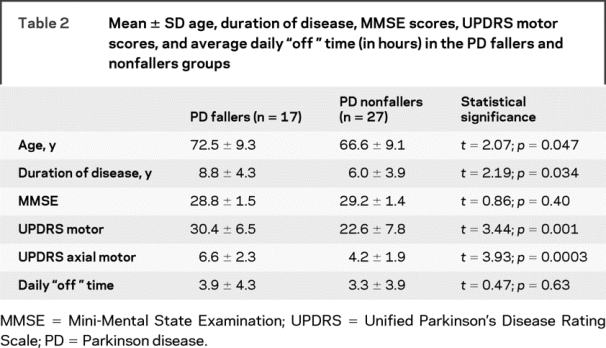
Seventeen patients (38.6%) reported a history of falls and 27 patients had no falls. PD fallers were slightly older, had longer duration of disease, and had higher motor UPDRS scores compared to the PD nonfallers (table 2). There were no significant differences in mean MMSE scores or average daily time spent in “off” states (table 2).
Table 2 Mean ± SD age, duration of disease, MMSE scores, UPDRS motor scores, and average daily “off” time (in hours) in the PD fallers and nonfallers groups
ANCOVA was used to compare striatal DTBZ and thalamic and cortical AChE activity in the PD fallers, PD nonfallers, and control subjects while adjusting for the effects of age. As expected, striatal DTBZ binding was significantly lower in the patients with PD compared to the control subjects. However, Duncan post hoc analysis did not demonstrate significant differences between PD fallers and PD nonfallers in either putamen or caudate nucleus DTBZ binding (table 3).
Table 3 Mean ± SD cortical and thalamic AChE hydrolysis rates (k3; min−1) and striatal VMAT2 BPND activity in the PD fallers, PD nonfallers, and control subjects

Age-adjusted ANCOVA of the cortical AChE hydrolysis rates demonstrated significant differences between all groups, with lowest rates seen in the PD fallers group (−12.3%) followed by the PD nonfallers (−6.6%), both significantly different from control subjects. Group differences between the PD fallers and PD nonfallers were also significant. ANCOVA of the thalamic AChE hydrolysis rates did demonstrate significantly lower rates in PD fallers (−11.8%) compared to both PD nonfallers and control subjects. However, there was no significant difference between the PD nonfallers and control subjects (table 3).
A post hoc ANCOVA was performed to evaluate group differences in AChE rates while controlling for the effects of age and the degree of nigrostriatal dopaminergic denervation (table 4). The addition of striatal DTBZ binding as a covariate into the model did not change the significantly decreased thalamic AChE hydrolysis rates in the PD fallers compared to the PD nonfallers and control subjects. However, the differences in cortical AChE activity between the PD fallers and control subjects trended but failed to achieve statistical significance (table 4).
Table 4 Mean ± SD cortical and thalamic AChE hydrolysis rates (k3; min−1) activity in the PD fallers, PD nonfallers, and control subjects

DISCUSSION
Our findings indicate that unlike nigrostriatal dopaminergic denervation, thalamic cholinergic denervation is associated with falls in PD. Although PD fallers had significantly lower cortical and thalamic AChE activity compared to nonfallers and control subjects, thalamic AChE enzyme hydrolysis rates remained significantly decreased even after adjusting for the degree of nigrostriatal dopaminergic denervation. Thalamic AChE activity derives mainly from terminals of brainstem PPN neurons that play a central role in the generation of movement.7 The PPN is located in the dorsolateral part of the pontomesencephalic tegmentum,21 and is composed of 2 groups of neurons: a pars compacta predominantly containing cholinergic projection neurons and a pars dissipata containing glutamatergic projections. The PPN sends profuse ascending cholinergic efferent fibers to several thalamic nuclei, particularly the intralaminar complex that is also reciprocally connected with the basal ganglia.7 PPN efferents appear to be highly collateralized and loss of thalamic AChE is likely to reflect PPN neuron dysfunction or degeneration. Our results are consistent with a key role for the PPN in the maintenance of balance in humans and with PPN dysfunction/degeneration as a cause of impaired postural control and gait in PD.
A previous cholinergic neuroimaging study found a nonsignificant reduction of thalamic AChE activity of about 13% in patients with PD compared to control subjects.22 The same authors reported a greater loss of thalamic AChE activity (−38%) in patients with progressive supranuclear palsy (PSP). The much higher incidence of falls in PSP compared to PD may reflect the more prominent degree of thalamic cholinergic denervation and PPN pathology in PSP. The importance of thalamic cholinergic denervation in PSP is further emphasized by the relatively preserved cortical cholinergic innervation in this disorder compared to PD.22 In PSP patients, experimental administration of the antimuscarinic drug scopolamine has been reported to worsen gait in a dose-dependent manner.23 Although scopolamine worsened gait functions, it did not negatively affect UPDRS motor ratings of bradykinesia or rigidity. Although anticholinergic drugs have been used to treat tremor or rigidity in PD we are unaware of formal studies on the effects of these drugs on falls in PD. However, a recent study on antimuscarinic anticholinergic drug effects in non-PD elderly found evidence of significant slowing in both gait speed and simple response time that may contribute to an increased risk of falls.24
Our findings and prior work raise the question as to whether cholinergic therapy may have a place in the management of mobility problems in PD. Currently approved cholinesterase inhibitors have been mainly evaluated for cognitive or behavioral benefits in patients with dementia. It is uncertain whether the current generations of cholinesterase inhibitors have sufficient brain penetrance to produce meaningful clinical benefits. We showed previously that about 60% of patients with AD treated with donepezil (10 mg/day) had limited cerebral enzyme inhibition in vivo.25 There is preliminary evidence that selective α4β2 nicotinic cholinergic receptor drugs may have a beneficial effect on gait. A recent case series reported improved gait functions in patients with ataxia taking varenicline.26 Varenicline is a selective partial agonist at the α4β2 nicotinic cholinergic receptor that is approved by the Food and Drug Administration for smoking cessation. These preliminary clinical observations suggest that α4β2 nicotinic cholinergic receptors may play a role in human mobility.
We previously determined in vivo cortical AChE activity with PET imaging in PD subjects with and without dementia. Reductions in cortical AChE levels were greater and more extensive in parkinsonian dementia than in PD without dementia.27 Studies have also shown an association between the postural instability and gait difficulty motor phenotype in PD and increased risk of dementia.28,29 These findings raise the question whether postural instability and gait difficulty and PD dementia share a common cholinergic mechanism contributing to these 2 clinical manifestations.
Gait is no longer considered as merely an automated motor activity and the multifaceted neuropsychological influences on walking are increasingly appreciated.3,30 Unlike cortical AChE activity, we did not find a significant correlation between thalamic AChE levels and MMSE scores. Therefore, our findings cannot be exclusively explained by pure cortical or cognitive mechanisms. A previous study found a correlation between lower cortical AChE activity and higher total UPDRS scores in patients with PD.22 These authors interpreted these findings that the ascending cholinergic system from the NBM to the cerebral cortex is impaired more severely as PD advances. We also found similar findings for cortical AChE activity. Although PD fallers had higher UPDRS motor scores, our findings of reduced thalamic cholinergic activity remained significant in this group while controlling for the degree of nigrostriatal dopaminergic denervation. Therefore, nigrostriatal dopaminergic denervation is insufficient to explain fall status in PD by itself. The pathophysiology of falls in PD may reflect multifactorial mechanisms and may relate both to motor functions, such as postural imbalance and gait, and nonmotor functions. Such multifactorial mechanisms are expected to reflect complicated disease processes to be caused by multiple transmitter deficiencies. Although our data provide supportive evidence for a role of cholinergic hypofunction and fall risk in PD, cholinergic denervation does not occur in isolation, but is associated with other neurodegenerative processes in this disorder.
A limitation of our study is that categorization of groups into fallers and nonfallers glosses over the multidimensional nature of falls in PD. Motor fluctuations, for example, have been identified as a significant cause of falls. We did not find significant differences between fallers and nonfallers in average daily time spent in the motor “off” state. There are also other limitations of our study. The [11C]PMP radioligand PET imaging technique cannot reliably assess striatal AChE hydrolysis assessed because high activity striatal AChE activity makes it flow dependent.31 Effects of striatal cholinergic neurons on mobility control in PD cannot be excluded. Another limitation of the PET technique is that accurate assessment of small brainstem nuclei, such as the PPN, is technically challenging because of partial volume effects.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Martijn Müller and Dr. Nicolaas I. Bohnen.
ACKNOWLEDGMENT
The authors thank Christine Minderovic, Virginia Rogers, the PET technologists, cyclotron operators, and chemists, for their assistance.
DISCLOSURE
Dr. Bohnen receives research support from the NIH [P01 NS15655 (Project Director), the Department of Veterans Affairs, and the Michael J. Fox Foundation. Dr. Müller receives research support from the NIH [5P30AG024824 (Project Director) and P01 NS15655 (Co-I)], the Department of Veterans Affairs, and the Michael J. Fox Foundation. Dr. Koeppe receives research support from Elan Corporation and the NIH [UO1 AG024904-01 (Co-I), PO1 NS15655 (Co-I), RO1 HL079540 (Co-I), RO1 DA022520 (Co-I), and RO1 DA016423 (Co-I)]. Dr. Studenski serves on scientific advisory boards for and/or serves as a consultant to Pfizer Inc., Merck Serono, Asubio Pharmaceuticals, Inc., and GlaxoSmithKline; serves as Associate Editor of the Journal of Gerontology Medical Sciences and on the editorial boards of the Journal of the American Geriatrics Society and the American Journal of Geriatric Pharmacotherapy; receives royalties from publishing Hazzard’s Geriatric Medicine & Gerontology, Sixth Edition (Principles of Geriatric Medicine & Gerontology) (McGraw-Hill Professional, 2009); and receives research support from Merck Serono, Humana, the NIH [NIA P30 AG024827 (PI), NIA/NCI P20 CA103730 (Co-PI), NIA #R01AG027017 (Co-I), NIA T32 AG 021885 (PI), NIA T35 AG026778 (PI), NIA R13 AG028230 (PI), and NIA R01 AG029232 (Co-I)], the John A. Hartford Foundation Center of Excellence in Geriatric Medicine, the Department of Veterans Affairs, and the Hartford Foundation. Dr. Kilbourn serves on a scientific advisory board for, holds stock options in, and may receive license fee payments and royalty payments (AV-133) from Avid Radiopharmaceuticals, Inc.; serves on the editorial board of Nuclear Medicine and Biology; may accrue revenue on patent WO 2007130365: Preparation of radiolabeled dihydrotetrabenazine derivatives and their use as imaging agents (Issued 2007); and receives research support from Biovail Laboratories, the NIH [NS15655 (Co-I), RR024986 (Co-I), and CA046592 (Co-I)], and the Department of Energy. Dr. Frey serves on the Board of Directors of the American Board of Nuclear Medicine; serves as a consultant to the NIH, Yale University, the Tourette Syndrome Association, and MIMvista Corp.; serves on the editorial board of the Quarterly Journal of Nuclear Medicine; receives research support from Avid Radiopharmaceuticals, Inc., the NIH [P01 NS15655 (PI), P50 AG008671 (Subproject Director), UL1 RR024986 (Core Director), U01 HL077150 (Co-I), P01 CA059827 (Co-I), R21 CA127057 (Co-I), R43 DK079416 (Co-I)], and the Dana Foundation; and has served as an expert consultant to Neurobehavioral Associates, Inc., Ann Arbor, MI, on several legal cases regarding alleged encephalopathy associated with low-level occupational solvent exposure. Dr. Albin serves on the editorial boards of Neurology®, Neurobiology of Disease, and Experimental Neurology; receives research support from the NIH [PO50 AG08671 (Project Director), R21 NS059537 (PI), and PO3 NS15655 (Project Director)], the Department of Veterans Affairs (Merit Review Grant), and the Michael J. Fox Foundation; and has given expert testimony in a proceeding involving Boehringer Ingelheim and Pfizer Inc.
Address correspondence and reprint requests to Dr. Nicolaas I. Bohnen, Functional Neuroimaging, Cognitive and Mobility Laboratory, Departments of Radiology and Neurology, University of Michigan, 24 Frank Lloyd Wright Drive, Box 362, Ann Arbor, MI 48105-9755 nbohnen@umich.edu
Disclosure: Author disclosures are provided at the end of the article.
Received March 25, 2009. Accepted in final form August 20, 2009.
REFERENCES
- 1.Bloem BR, Steijns JA, Smits-Engelsman BC. An update on falls. Curr Opin Neurol 2003;16:15–26. [DOI] [PubMed] [Google Scholar]
- 2.Bohnen NI, Cham R. Postural control, gait, and dopamine functions in parkinsonian movement disorders. Clin Geriatr Med 2006;22:797–812. [DOI] [PubMed] [Google Scholar]
- 3.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture 2002;16:1–14. [DOI] [PubMed] [Google Scholar]
- 4.Bohnen NI, Kaufer DI, Hendrickson R, et al. Cognitive correlates of cortical cholinergic denervation in Parkinson’s disease and parkinsonian dementia. J Neurol 2006;253:242–247. [DOI] [PubMed] [Google Scholar]
- 5.Mesulam MM, Geula C. Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetylcholinesterase and choline acetyltransferase. J Comp Neurol 1988;275:216–240. [DOI] [PubMed] [Google Scholar]
- 6.Heckers S, Geula C, Mesulam M. Cholinergic innervation of the human thalamus: dual origin and differential nuclear distribution. J Comp Neurol 1992;325:68–82. [DOI] [PubMed] [Google Scholar]
- 7.Lee MS, Rinne JO, Marsden CD. The pedunculopontine nucleus: its role in the genesis of movement disorders. Yonsei Med J 2000;41:167–184. [DOI] [PubMed] [Google Scholar]
- 8.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinicopathologic study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoehn M, Yahr M. Parkinsonism: onset, progression, and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 10.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatry Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 11.Fahn S, Elton R. Members of the UPDRS development committee: Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden C, Calne D, Goldstein M, eds. Recent Developments in Parkinson’s Disease. Florham Park, NJ: Macmillan Healthcare Information; 1987:153–164. [Google Scholar]
- 12.Hauser RA, Friedlander J, Zesiewicz TA, et al. A home diary to assess functional status in patients with Parkinson’s disease with motor fluctuations and dyskinesia. Clin Neuropharmacol 2000;23:75–81. [DOI] [PubMed] [Google Scholar]
- 13.Thompson CJ, Kecani S, Boelen S. Evaluation of a neck-shield for use during neurological studies with a whole-body PET scanner. IEEE Trans Nucl Sci 2001;48:1512–1517. [Google Scholar]
- 14.Jewett DM, Kilbourn MR, Lee LC. A simple synthesis of [11C]dihydrotetrabenazine (DTBZ). Nucl Med Biol 1997;24:197–199. [DOI] [PubMed] [Google Scholar]
- 15.Koeppe RA, Frey KA, Kume A, Albin R, Kilbourn MR, Kuhl DE. Equilibrium versus compartmental analysis for assessment of the vesicular monoamine transporter using (+)-alpha-[11C]dihydrotetrabenazine (DTBZ) and positron emission tomography. J Cereb Blood Flow Metab 1997;17:919–931. [DOI] [PubMed] [Google Scholar]
- 16.Snyder SE, Tluczek L, Jewett DM, Nguyen TB, Kuhl DE, Kilbourn MR. Synthesis of 1-[11C]methylpiperidin-4-yl propionate ([11C]PMP) for in vivo measurements of acetylcholinesterase activity. Nucl Med Biol 1998;25:751–754. [DOI] [PubMed] [Google Scholar]
- 17.Sitburana O, Ondo WG. Brain magnetic resonance imaging (MRI) in parkinsonian disorders. Parkinsonism Relat Disord 2009;15:165–174. [DOI] [PubMed] [Google Scholar]
- 18.Minoshima S, Koeppe RA, Fessler JA, et al. Integrated and automated data analysis method for neuronal activation studying using O15 water PET. Tokyo: Excerpta Medica; 1993:409–418. [Google Scholar]
- 19.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007;27:1533–1539. [DOI] [PubMed] [Google Scholar]
- 20.Nagatsuka S, Fukushi K, Shinotoh H, et al. Kinetic analysis of [(11)C]MP4A using a high-radioactivity brain region that represents an integrated input function for measurement of cerebral acetylcholinesterase activity without arterial blood sampling. J Cereb Blood Flow Metab 2001;21:1354–1366. [DOI] [PubMed] [Google Scholar]
- 21.Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson’s disease. Brain 2000;123:1767–1783. [DOI] [PubMed] [Google Scholar]
- 22.Shinotoh H, Namba H, Yamaguchi M, et al. Positron emission tomographic measurement of acetylcholinesterase activity reveals differential loss of ascending cholinergic systems in Parkinson’s disease and progressive supranuclear palsy. Ann Neurol 1999;46:62–69. [PubMed] [Google Scholar]
- 23.Litvan I, Blesa R, Clark K, et al. Pharmacological evaluation of the cholinergic system in progressive supranuclear palsy. Ann Neurol 1994;36:55–61. [DOI] [PubMed] [Google Scholar]
- 24.Nebes RD, Pollock BG, Halligan EM, Kirshner MA, Houck PR. Serum anticholinergic activity and motor performance in elderly persons. J Gerontol A Biol Sci Med Sci 2007;62:83–85. [DOI] [PubMed] [Google Scholar]
- 25.Bohnen NI, Kaufer DI, Hendrickson R, et al. Degree of inhibition of cortical acetylcholinesterase activity and cognitive effects by donepezil treatment in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2005;76:315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zesiewicz TA, Sullivan KL. Treatment of ataxia and imbalance with varenicline (Chantix): report of 2 patients with spinocerebellar ataxia (types 3 and 14). Clin Neuropharmacol 2008;31:363–365. [DOI] [PubMed] [Google Scholar]
- 27.Bohnen NI, Kaufer DI, Ivanco LS, et al. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol 2003;60:1745–1748. [DOI] [PubMed] [Google Scholar]
- 28.Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Mov Disord 2006;21:1123–1130. [DOI] [PubMed] [Google Scholar]
- 29.Taylor JP, Rowan EN, Lett D, O’Brien JT, McKeith IG, Burn DJ. Poor attentional function predicts cognitive decline in patients with non-demented Parkinson’s disease independent of motor phenotype. J Neurol Neurosurg Psychiatry 2008;79:1318–1323. [DOI] [PubMed] [Google Scholar]
- 30.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord 2008;23:329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Namba H, Iyo M, Fukushi K, et al. Human cerebral acetylcholinesterase activity measured with positron emission tomography: procedure, normal values and effect of age. Eur J Nucl Med 1999;26:135–143. [DOI] [PubMed] [Google Scholar]


