Abstract
Objectives:
A 2003 evidence-based review of exogenous risk factors for sporadic amyotrophic lateral sclerosis (ALS) identified smoking as the only risk factor that attained “probable” (more likely than not) status, based on 2 class II studies. The purpose of the current, evidence-based, update was to see if the conclusion of the previous review needed to be modified, based on studies published since.
Methods:
A Medline literature search was conducted for the period between 2003 and April 2009 using the search terms smoking and (ALS or “amyotrophic lateral sclerosis” or MND or “motor neuron disease”). The references of primary articles and reviews were checked to assure completeness of the search. Primary articles published since the previous review were classified as before.
Results:
Twenty-eight titles were identified, but only 7 articles met inclusion criteria. Of these, 1 provided class II evidence, and 1 class III evidence: both showed increased risk of ALS with smoking. The class II study showed a dose-response effect, and risk decreasing with number of years since quitting smoking. Five articles provided class IV or V evidence, which may not be relied upon to draw conclusions.
Conclusions:
Smoking may be considered an established risk factor for sporadic amyotrophic lateral sclerosis (ALS) (level A rating; 3 class II studies, 1 class III study). Evidence-based analysis of epidemiologic data shows concordance among results of better-designed studies linking smoking to ALS, and lets those results drive the conclusions.
GLOSSARY
- ALS
= amyotrophic lateral sclerosis;
- HRT
= hormonal replacement therapy.
Amyotrophic lateral sclerosis (ALS) is the most common neurodegenerative disorder of motor neurons. Loss of pyramidal, brainstem, and spinal motor neurons affecting multiple regions of the body leads to progressive motor dysfunction, disability, and death. In a previous evidence-based review of the role of exogenous risk factors in sporadic ALS,1 smoking was identified as the only “probable” (“more likely than not”) risk factor for developing ALS based on 2 class II studies.2,3 Since publication of the initial review, additional reports have addressed the association between smoking and sporadic ALS, with conflicting conclusions. This evidence-based update was undertaken in order to see whether the new reports might necessitate revision of the conclusion of the original review with regard to the role of smoking as a risk factor for ALS. Since the conclusion of the original review was based on 2 class II studies,2,3 for this conclusion to be changed new evidence would need to be class II or higher.
METHODS
A Medline search was conducted by the author on the search terms smoking and (ALS or “amyotrophic lateral sclerosis” or MND or “motor neuron disease”). Titles and abstracts of articles published since the 2003 review1 were evaluated looking for articles with primary data and reference groups. The reference lists of primary articles and of review articles were examined in order to verify the completeness of the search. The full text of articles with primary data and reference groups published since the previous review was reviewed, and the articles were classified using the same method.1 This evidence-based approach first assigns a class of evidence to the information provided by each article reviewed, ranging from class I for information that is most reliable to class V, for information that cannot be relied on (appendix e-1 on the Neurology® Web site at www.neurology.org). Next, conclusions are drawn, based on the available evidence, and a level of certainty is assigned to each conclusion. Level A conclusions (established risk factor) and level B conclusions (probable, or “more likely than not” risk factor) require at least two concordant class I or class II studies with data which lend themselves to the application of criteria for inferring causation from association. Level C conclusions (possible risk factor) may be based on at most 1 class II and several class III studies overall pointing in one direction. Class U (unknown if this is a risk factor) is assigned otherwise (appendix e-2). Conclusions were based on the results of this and the previous1 review. This approach is concordant with the MOOSE criteria,4 except that the methods of drawing conclusions from data using evidence-based methods are different from those used in meta-analyses. Meta-analysis is not appropriate when the quality of the data is variable,1 and was not undertaken.
Appendices e-1 and e-2 are reproduced from Armon C. An evidence-based medicine approach to the evaluation of the role of exogenous risk factors in sporadic amyotrophic lateral sclerosis. Neuroepidemiology 2003;22:217–228, with permission from S. Karger AG, Basel, Switzerland.
RESULTS
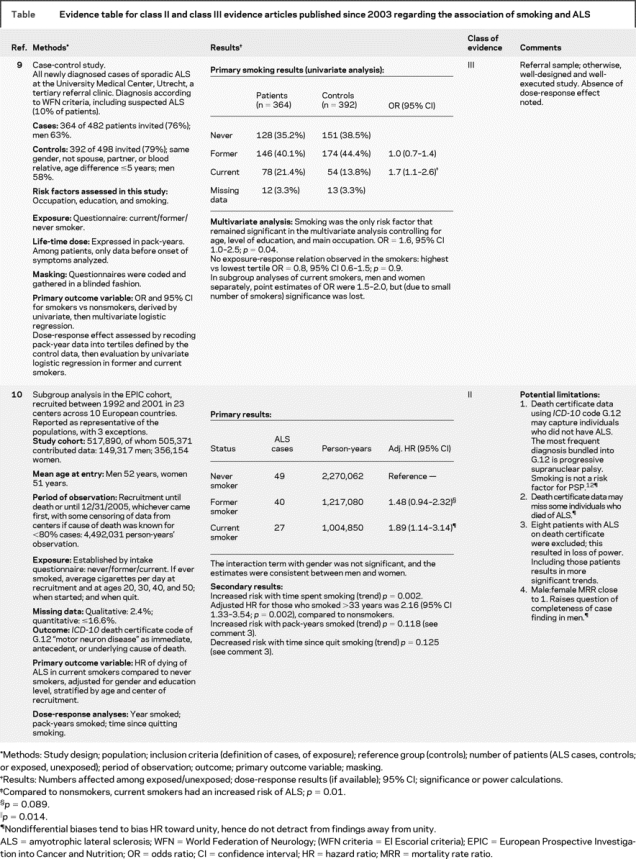
Twenty-eight titles published since the 2003 review were identified through the literature search strategy. Of these, 7 titles were of primary articles with reference groups.5–11 Review of reference lists of these articles as well as of review articles did not yield additional articles. One article provided class II evidence10 and 1 class III9 evidence: both showed increased risk of ALS with smoking. Their methods, principal findings, and limitations are presented in an evidence table (see table). The class II article10 showed a dose response effect, with an adjusted hazard ratio for those who smoked more than 33 years of 2.16 (95% confidence interval 1.33–3.54, p = 0.002) compared to nonsmokers. This article also showed trends to increased risk with number of pack-years smoked, and decreased risk with number of years since quitting smoking. These trends did not attain significance according to the authors’ original plan of analysis that excluded 8 individuals in whom the diagnosis of ALS appeared on the death certificates, but not as immediate, antecedent, or underlying cause of death. This exclusion is at variance from other studies using death certificates to determine ALS frequency, and is overly restrictive when death certificate data are used as a surrogate for disease incidence. When the authors performed sensitivity analysis that included those cases, the trends attained significance. Five articles5–8,11 provided class IV–V evidence. Their methods, principal findings, and limitations are summarized in table e-1 (on the Neurology® Web site at www.neurology.org). These five articles may not be relied upon to draw conclusions.
Table Evidence table for class II and class III evidence articles published since 2003 regarding the association of smoking and ALS
Conclusion.
Smoking may be considered an established risk factor for sporadic ALS (level A; 3 class II studies,2,3,10 1 class III study9).
DISCUSSION
A few general clarifications about the methods used in this review may preempt readers’ questions. Studies prior to 1990 were not listed in the previous evidence-based review1 and in this update because of the limitations of the methods used in epidemiologic studies prior to 1990, when judged by contemporary criteria, making them all class IV or V evidence. To exemplify this point, one article from 198113 was subjected to evidence-based classification. This article was selected because it was cited, uncritically, in a recent literature review of exogenous risk factors of ALS that did not use evidence-based methodology.14 Applying the criteria of the previous review1 to the article13 shows that the class of evidence it provides with regard to the association of smoking and ALS is class IV (table e-2). Hence no conclusions may be drawn from it. Attention to articles with inherent limitations burdens a review unnecessarily, detracts from the attention due to the high-quality articles, and blurs their findings. This is avoided when evidence-based methods are used, and makes evidence-based reviews stand apart from reviews that do not utilize this method.
Three longitudinal observational studies were evaluated in this update5,8,10 but the findings of 25,8 were considered class IV evidence, even though they described themselves as “prospective.” However, these studies are retrospective reviews of existing cohorts, where both exposures and outcomes have occurred before the hypotheses were generated.15 One particular retrospective aspect of these cohorts is echoed in the following statement: “In principle, a prospective cohort should be defined starting from exposure rather than from information on exposure collected after several years.16” These views inform the evidence-based approach applied here1 (appendix e-1, third paragraph under class III evidence) that considers retrospective reviews, or data-mining, in existing observational cohorts, as “exploratory” studies, which may generate hypotheses but not confirm them. There are theoretical and experiential reasons for adopting a conservative approach to evaluating results obtained in “cohorts of convenience.” On theoretical grounds, there is a high risk that such studies may identify spurious associations because 1) nonrandom assignment to exposure groups increases the risk for involuntary biases (that cannot be accounted for because they are unknown); 2) quantification of exposure in these studies, even if done at cohort inception, is often retrospective, and subject to bias,16 and there is limited ability to verify the quality of the information when a study is done many years after the cohort was formed; 3) the cohort is often not representative of the general population, due to its composition of self-selected individuals17–19; and 4) the multiple comparisons conducted in such studies increase the likelihood that chance associations will occur, be found significant and considered, erroneously, as nonrandom. The limitations of longitudinal studies analyzing health outcomes in postmenopausal women receiving hormonal replacement therapy (HRT) reinforce the appropriateness of this approach. A randomized, placebo-controlled trial of HRT in postmenopausal women showed that such treatment resulted in adverse health outcomes in multiple domains.20–22 Its findings contributed to changing the practice of prescribing HRT to postmenopausal women, based on observations of apparent beneficial health effects in numerous nonrandomized cohorts. A recent editorial attempted to reconcile the results of the randomized controlled trials and the observational studies on HRT23 showing that the observational studies were correct some of the time (identifying the risk of breast cancer), but incorrect by showing an apparent reduced risk of coronary heart disease. The RCTs were correct 100% of the time when evaluating combined estrogen/progestin therapy, but missed an increased risk of breast cancer incurred when initiating estrogen-only HRT close to menopause, because most of the women were enrolled into the RCT several years after menopause. Evaluating the reasons for the discrepancies led to important insights as to the time-dependencies of the risks of HRT (on time of start of treatment, on time of menopause). The evidence-worthiness of observational studies needs to be evaluated on a case-by-case basis (appendix e-1). Observations in exploratory studies may be considered class III evidence if the studies do not have additional limitations that downgrade the class of evidence to class IV. Such was the case for 2 of the 3 cohort studies identified5,8; hence no conclusions may be drawn from them.
In contrast, the design of the third cohort study10 was rigorous. It was established between 1992 and 2000 and drew on lessons learned from previous cohort studies. In particular, it used validated tools to collect precise exposure data, including on smoking, which are superior to those used in the earlier cohort studies.5,8 Smoking was the first risk factor for ALS reported from this cohort.10 The authors indicated they were doing so as smoking had emerged as the only “probable” risk factor for ALS in my previous review.1 Thus, adjustment for the risks of multiple comparisons is not required automatically, even though the authors do not state explicitly that this was their first intended analysis or the first analysis they performed. There was no gap in case finding, and the enrollees were in the right age groups to develop ALS. Of the sources of bias considered, they would tend to decrease the likelihood of identifying smoking as a risk factor; thus, they do not detract from the findings. This study qualified as class II evidence.
There is a general limitation in all epidemiologic studies, because individuals participating in them may be healthier than the general population from which they are drawn. In case-control studies, if individuals agreeing to be controls were healthier and smoked less than the general population, and the patients smoked as much as the general population, then the apparent excess smoking in patients might be due to the way the controls were chosen. It is possible to evaluate the extent to which this concern might apply by considering the percentage of individuals who agreed to participate from those asked to do so, from among patients and controls. In the 3 case-control studies relied on,2,3,9 the response rates among controls ranged from 76% to 79%, which is better than the median reported rate (74%) for case control studies published in early 2003.24 In one of the studies,3 36 of 84 controls who declined to participate fully in the study provided smoking history, and were found to have smoked less than the 256 controls who participated fully. The authors concluded that that study may have underestimated the magnitude of risk conferred by smoking. This limitation cannot confound a dose-response effect among patients, or decreasing risk with time since quitting smoking among patients. However, a dose-response effect among patients can be a result of self-selection bias if patients with greater degrees of exposure to a putative risk factor are more motivated to participate in a study evaluating a possible role for that risk factor than those with less or no exposure to it. However, smoking was not considered a risk factor for ALS at the time of the first 2 studies in which it was identified,2,3 so there is no reason to suspect that smokers preferentially chose to participate in them. To the extent that health-conscious (nonsmoking) patients with ALS are similar to the general population and were more likely to participate in a study than less health-conscious individuals (smokers), that would decrease the likelihood of identifying smoking as a risk factor in patients with ALS. In cohort studies, if a convenience cohort is healthier than the general population, then some risk factors may be missed (biasing RR toward unity), and others may emerge spuriously, for example, as a result of survivor bias. Hence, the effects of possible selection bias that need to be considered if a risk factor is found in a cohort study are different from those that need to be considered if a risk factor is not found. All the reported studies did not account for the effects of passive smoking. This biases the observed risk ratios toward unity. The risk of smoking may be missed entirely in some studies for this reason. Where an increased risk was found, the observed risk is likely an underestimate of the true risk.
Evidence-based analysis of epidemiologic data shows concordance among results of better-designed studies linking smoking to ALS, and lets those results drive the conclusions. An earlier article6 also demonstrated how higher class studies provided concordant findings at variance with those obtained in lower class studies. It showed how an apparent association of physical activity with ALS in lower class studies was not supported by the findings in studies providing a higher class of evidence, showing no such association. Less rigorous approaches to the evaluation of the epidemiologic literature are likely to lead to erroneous conclusions.
The role of reliable identification of risk factors is twofold. First, if a risk factor has no redeeming features, then its identification may lead to its avoidance, with future reduction of disease burden. Second, identification of an established risk factor for ALS can stimulate the generation of hypotheses about the biologic processes that trigger disease initiation.25,26 It is intriguing that the incidence of ALS continues to rise according to some, if not all reports, even as the prevalence of smoking is declining. There is more than one way to account for this observation. First, if at least part of the deleterious effect of smoking has a long latency, then the 25- to 35-year-olds who smoked in 1975 who will cross the 65-year median age at onset of ALS between 2005 and 2015 have still to manifest the full effect of the exposure. Second, since the effects of passive smoking are not reflected in any of the epidemiologic data on ALS, there is a risk that individuals exposed to passive smoking in 1975, including the children of smokers, have many more years to express fully the consequences of the exposure. Finally, 3 groups of investigators have shown, independently, that the observed rise in ALS incidence may be accounted for by loss of competing sources of mortality (Gompertzian considerations) rather than by deleterious changes to the environment.27–32 It is encouraging that the most recent of these reports32 found that “the ‘environmental’ factor showed a decreasing trend in the most recent birth cohorts.” While it might be overly optimistic to give too much weight to one report, this may reflect, in part, the decline in the prevalence of smoking. Another way of looking at this interpretation is that we may have been witnessing an even greater rise in the incidence of ALS if there had not been a decline in the prevalence of smoking. Hopefully, incidence studies of ALS in future cohorts, controlling for loss of competing sources of mortality will start to show a decline, reflecting the reduced prevalence of smoking.
NOTE ADDED IN PROOF
At press time, 2 studies examining the role of smoking as a risk factor for ALS had been presented in abstract form, but the full reports had not yet been published. The first33 found no association between smoking and the risk of developing ALS in a predominantly male sample of veterans. The second34 found an increased risk of ALS in female smokers, and no association between smoking and ALS in men. Both studies are class IV evidence due to methodologic limitations (details available from the author). Their results do not change the conclusions of this review.
DISCLOSURE
Dr. Armon serves on the editorial board of Neurology®; receives royalties from emedicine.com for updating electronic chapters; received research support from the NIH [R01 NS 048125 (Contributor) and N01-NS-2-2349 (Contributor)]; serves as a consultant to the Massachusetts Department for Public Health; and has given expert testimony on behalf of a group of current and former manufacturers of welding consumables related to risk factors and causation in ALS.
Supplementary Material
Address correspondence and reprint requests to Dr. Carmel Armon, Division of Neurology, Baystate Medical Center S4648, 759 Chestnut Street, Springfield, MA 01199 carmel.armon@bhs.org
Supplemental data at www.neurology.org
Presented in part at the 61st annual meeting of the American Academy of Neurology, Seattle, WA, April 2009.
Disclosure: Author disclosures are provided at the end of the article.
Received May 24, 2009. Accepted in final form August 18, 2009.
REFERENCES
- 1.Armon C. An evidence-based medicine approach to the evaluation of the role of exogenous risk factors in sporadic amyotrophic lateral sclerosis. Neuroepidemiology 2003;22:217–228. [DOI] [PubMed] [Google Scholar]
- 2.Nelson LM, McGuire V, Longstreth WT, Jr., Matkin C. Population-based case-control study of amyotrophic lateral sclerosis in western Washington state: I: cigarette smoking and alcohol consumption. Am J Epidemiol 2000;151:156–163. [DOI] [PubMed] [Google Scholar]
- 3.Kamel F, Umbach DM, Munsat TL, Shefner JM, Sandler DP. Association of cigarette smoking with amyotrophic lateral sclerosis. Neuroepidemiology 1999;18:194–202. [DOI] [PubMed] [Google Scholar]
- 4.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 5.Weisskopf G, McCullough ML, Calle EE, Thun MJ, Cudkowicz M, Ascherio A. Prospective study of cigarette smoking and amyotrophic lateral sclerosis. J Epidemiol 2004;160:26–33. [DOI] [PubMed] [Google Scholar]
- 6.Veldink JH, Kalmijn S, Groeneveld GJ, Titulaer MJ, Wokke JHJ, van den Berg LH. Physical activity and the association with sporadic ALS. Neurology 2005;64:241–245. [DOI] [PubMed] [Google Scholar]
- 7.Qureshi MM, Hayden D, Urbinelli L, et al. Analysis of factors that modify susceptibility and rate of progression in amyotrophic lateral sclerosis (ALS). Amyotroph Lateral Scler 2006;7:173–182. [DOI] [PubMed] [Google Scholar]
- 8.Fang F, Bellocco R, Hernán MA, Ye W. Smoking, snuff dipping and the risk of amyotrophic lateral sclerosis–a prospective cohort study. Neuroepidemiology 2006;27:217–221. [DOI] [PubMed] [Google Scholar]
- 9.Sutedja NA, Veldink JH, Fischer K, et al. Lifetime occupation, education, smoking, and risk of ALS. Neurology 2007;69:1508–1514. [DOI] [PubMed] [Google Scholar]
- 10.Gallo V, Bueno-De-Mesquita HB, Vermeulen R, et al. Smoking and risk for amyotrophic lateral sclerosis: analysis of the EPIC cohort. Ann Neurol 2009;65:378–385. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto K, Kihira T, Kondo T, et al. Lifestyle factors and risk of amyotrophic lateral sclerosis: a case-control study in Japan. Ann Epidemiol 2009;19:359–364. [DOI] [PubMed] [Google Scholar]
- 12.Golbe LI. The epidemiology of progressive supranuclear palsy. In: Duyckaerts C, Litvan I, eds. Dementias: Handbook of Clinical Neurology. Philadelphia: Elsevier Health Sciences; 2008:458. [DOI] [PubMed] [Google Scholar]
- 13.Kondo K, Tsubaki T. Case-control studies of motor neuron disease: association with mechanical injuries. Arch Neurol 1981;38:220–226. [DOI] [PubMed] [Google Scholar]
- 14.Gil J, Funalot B, Torny F, Lacoste M, Couratier P. [Exogenous risk factors in sporadic ALS: a review of the literature] (French). Rev Neurol 2007;163:1021–1030. [DOI] [PubMed] [Google Scholar]
- 15.Hennekens CH, Buring JE. Epidemiology in Medicine. Boston: Little, Brown; 1987. [Google Scholar]
- 16.Beghi E, Morrison KE. ALS and military service. Neurology 2005;65:972. Response to letter. [DOI] [PubMed]
- 17.Garfinkel L. Selection, follow-up, and analysis in the American Cancer Society Prospective Studies. Natl Cancer Inst Monogr 1985;67:49–52. [PubMed] [Google Scholar]
- 18.Stellman SD, Garfinkel L. Smoking habits and tar levels in a new American Cancer Society prospective study of 1.2 million men and women. JNCI 1986;76:1067–1083. [PubMed] [Google Scholar]
- 19.Riboli E, Hunt KJ, Slimani N, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study population and data collection. Public Health Nutrition 2002;5:1113–1124. [DOI] [PubMed] [Google Scholar]
- 20.Rossouw JE, Anderson GL, Prentice RL, et al, Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- 21.Anderson GL, Judd HL, Kaunitz AM, et al, Women’s Health Initiative Investigators. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women’s Health Initiative randomized trial. JAMA 2003;290:1739–1748. [DOI] [PubMed] [Google Scholar]
- 22.Anderson GL, Limacher M, Assaf AR, et al, The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative Randomized Controlled Trial. JAMA 2004;291:1701–1712. [DOI] [PubMed] [Google Scholar]
- 23.Vandenbroucke JP. The HRT controversy: observational studies and RCTs fall in line. Lancet 2009;373:1233–1235. [DOI] [PubMed] [Google Scholar]
- 24.Morton LM, Cahill J, Hartge P. Reporting participation in epidemiologic studies: a survey of practice. Am J Epidemiol 2006;163:197–203. [DOI] [PubMed] [Google Scholar]
- 25.Armon C. Acquired nucleic acid changes may trigger sporadic amyotrophic lateral sclerosis. Muscle Nerve 2005;32:373–377. [DOI] [PubMed] [Google Scholar]
- 26.Armon C. From clues to mechanisms: understanding ALS initiation and spread. Neurology 2008;71:872–873. [DOI] [PubMed] [Google Scholar]
- 27.Riggs JE. Longitudinal Gompertzian analysis of amyotrophic lateral sclerosis mortality in the U.S., 1977–1986: evidence for an inherently susceptible population subset. Mech Ageing Dev 1990;55:207–220. [DOI] [PubMed] [Google Scholar]
- 28.Riggs JE, Schochet SS, Jr. Rising mortality due to Parkinson’s disease and amyotrophic lateral sclerosis: a manifestation of the competitive nature of human mortality. J Clin Epidemiol 1992;45:1007–1012. [DOI] [PubMed] [Google Scholar]
- 29.Neilson S, Robinson I, Hunter M. Longitudinal Gompertzian analysis of ALS mortality in England and Wales, 1963–1989: estimates of susceptibility in the general population. Mech Ageing Dev 1992;64:201–216. [DOI] [PubMed] [Google Scholar]
- 30.Neilson S, Robinson I. Cross-sectional Gompertzian analysis: the development of a Gompertz mortality ratio (GMR) and its applicability. Mech Ageing Dev 1993;68:137–149. [DOI] [PubMed] [Google Scholar]
- 31.Neilson S, Robinson I, Hunter M. Static and dynamic models of interdisease competition: past and projected mortality from amyotrophic lateral sclerosis and multiple sclerosis. Mech Ageing Dev 1993;66:223–224. [DOI] [PubMed] [Google Scholar]
- 32.Chiò A, Magnani C, Schiffer D. Gompertzian analysis of amyotrophic lateral sclerosis mortality in Italy, 1957–1987; application to birth cohorts. Neuroepidemiology 1995;14:269–277. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt S, Allen K, Rimmler J, et al. Do head injury or cigarette smoking contribute to the increased risk of amyotrophic lateral sclerosis in US veterans? Neuroepidemiology 2008;30:134. [Google Scholar]
- 34.Alonso A, Logroscino G, Jick S, Hernán M. Smoking and amyotrophic lateral sclerosis: a prospective study. Neuroepidemiology 2009;33:72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


