Abstract
Objective:
To cross-sectionally compare the regional white matter fractional anisotropy (FA) of cognitively normal (CN) older individuals and patients with mild cognitive impairment (MCI) and Alzheimer disease (AD), separately focusing on the normal-appearing white matter (NAWM) and white matter hyperintensities (WMH), and to test the independent effects of presumed degenerative and vascular process on FA differences.
Methods:
Forty-seven patients with AD, 73 patients with MCI, and 95 CN subjects received diffusion tensor imaging and vascular risk evaluation. To properly control normal regional variability of FA, we divided cerebral white matter into 4 strata as measured from a series of young healthy individuals (H1 = highest; H2 = intermediate high; H3 = intermediate low; H4 = lowest anisotropy stratum).
Results:
For overall cerebral white matter, patients with AD had significantly lower FA than CN individuals or patients with MCI in the regions with higher baseline anisotropy (H1, H2, and H3), corresponding to long corticocortical association fibers, but not in H4, which mostly includes heterogeneously oriented fibers. Vascular risk showed significant independent effects on FA in all strata except H1, which corresponds to the genu and splenium of the corpus callosum. Similar results were found within NAWM. FA in WMH was significantly lower than NAWM across all strata but was not associated with diagnosis or vascular risk.
Conclusions:
Both vascular and Alzheimer disease degenerative process contribute to microstructural injury of cerebral white matter across the spectrum of cognitive ability and have different region-specific injury patterns.
GLOSSARY
- AD
= Alzheimer disease;
- ANCOVA
= analysis of covariance;
- ANOVA
= analysis of variance;
- CC
= corpus callosum;
- CN
= cognitively normal;
- DTI
= diffusion tensor imaging;
- FA
= fractional anisotropy;
- MCI
= mild cognitive impairment;
- MDT
= minimal deformation template;
- MMSE
= Mini-Mental State Examination;
- NAWM
= normal-appearing white matter;
- ROI
= region of interest;
- SLF
= superior longitudinal fasciculus;
- UCD
= University of California, Davis;
- WMH
= white matter hyperintensities.
Fractional anisotropy (FA), a quantitative measure derived from diffusion tensor imaging (DTI), sensitively reflects the microstructural integrity of white matter fibers.1 Decrease in FA of cerebral white matter is known to occur with mild cognitive impairment (MCI) and Alzheimer disease (AD),2 as well as cognitively normal aging3 and cerebrovascular vascular disease.4
Little is known, however, about the FA changes within white matter hyperintensities (WMH) occurring in patients with AD or MCI, even though WMH are commonly associated with MCI5,6 and AD.5,7 Most previous DTI studies8–14 of patients with AD and MCI typically compared their white matter with that of cognitively normal (CN) older individuals, without proper consideration of WMH, or focused solely on normal-appearing white matter (NAWM).
Moreover, the separate and independent contributions of vascular and degenerative processes on FA alteration are not clear in older individuals, including patients with AD or MCI. Wallerian degeneration secondary to neuronal loss has been suggested as a causal factor for FA change in certain white matter tracts in AD and MCI.2,13 Evidence from epidemiologic, neuroimaging, pathologic, and clinical studies, however, indicates a significant contribution of vascular process to the clinical syndrome of AD.5,15
In this study, we first cross-sectionally compared the regional FA in the cerebral white matter of patients with MCI and AD versus CN individuals, separately focusing on the whole white matter, NAWM, and WMH. Second, we investigated the independent etiologic contribution of vascular risk, as well as degenerative process, to the regional FA changes in CN older individuals and those with MCI and AD.
METHODS
Forty-seven patients with AD, 73 patients with MCI, and 95 CN subjects received vascular risk evaluation and MRI with DTI sequence. The diagnosis of AD was made according to the National Institute of Neurological and Communication Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association criteria.16 MCI was diagnosed according to current consensus criteria.17 Subjects were recruited from the Alzheimer’s Disease Center at the University of California, Davis (UCD) from January 2002 to November 2007. The UCD institutional review board approved this study, and subjects or their legal representatives gave written informed consent.
The presence or absence of stroke, diabetes, hyperlipidemia, TIA, hypertension, and coronary artery disease was systematically assessed to create a composite score for vascular risks that was the sum of the factors present, ranging from 0 to 618 and reported as a percentage.
FA was calculated at each DTI image, and segmentation of WMH was performed by a semiautomated procedure previously described.19 All MRI modalities including FA and WMH map were coregistered to a minimal deformation template (MDT)20 to use group statistical methods for FA values over common regions of interest (ROIs).
Regional white matter FA values are highly variable even in normal healthy brain, depending on the baseline structure of the region, i.e., the innate homogeneity of fiber orientation within the region.21 Therefore, to properly detect FA alteration by pathologic processes beyond normal regional variability, we applied a baseline anisotropy level–based approach in which the whole cerebral white matter on the MDT was divided into 4 strata (H1: FA ≥0.600; H2: FA = 0.500∼0.599; H3: FA = 0.400∼0.499, and H4: FA = 0.300∼0.399) according to the FA values of the average normal young adult brain (figure e-1 on the Neurology® Web site at www.neurology.org). A traditional anatomic region–based approach was also used.
The details of the study methods, including subject recruitment and evaluation, image acquisition and processing, and statistical analysis, are described in appendix e-1.
RESULTS
Subject characteristics.
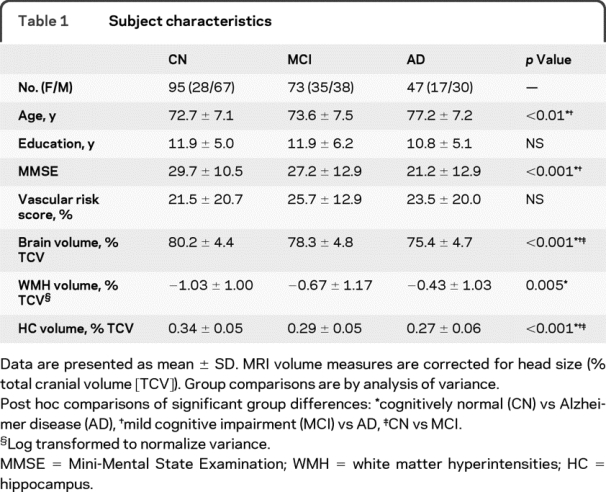
Demographic, clinical, and MRI volume description of all the subjects are summarized in table 1.
Table 1 Subject characteristics
Whole cerebral white matter FA.
Before regional analyses, we first examined the relationship of FA with age, gender, ethnicity, vascular risk, and diagnostic group for the whole cerebral white matter. The full multiple regression models for predicting FA of overall white matter and NAWM were highly significant (R2 = 0.16, p < 0.001 for overall white matter; R2 = 0.18, p < 0.001 for NAWM; figure e-2). The same variables were significantly associated with both overall white matter FA and FA in NAWM and included age, gender, and vascular risk. Multivariate analysis for WMH FA resulted neither in a significant full model nor in any significant associations with the individual independent variables (figure e-2). Importantly, mean FA values within NAWM were significantly higher than FA values within WMH for the same individuals.
Lobar white matter and CC FA.
Because mean FA values of cerebral white matter were correlated with age among the CN subjects (r = −0.37, p < 0.001), as suggested from previous studies,3 we controlled age as a covariate for all the following analyses for FA.
There were no significant group differences of mean FA in any of 4 lobar overall white matters. There was also no group difference of FA in the body of the corpus callosum (CC). In contrast, group differences in FA values were found in the genu and splenium of the CC (F = 4.75, p = 0.001 for the genu; F = 4.01, p = 0.002 for the splenium). Post hoc analyses showed that patients with AD had significantly lower FA in both the genu and splenium than CN individuals. Patients with AD also showed lower FA in the genu, but not in the splenium, than patients with MCI.
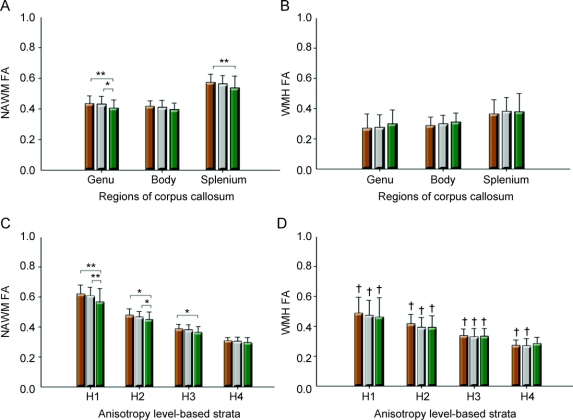
As for NAWM, group differences were also found only for FA of the genu and splenium of the CC (F = 5.02, p = 0.007 for the genu; F = 3.82, p = 0.024 for the splenium; figure, A), but not for any lobar regions and the body of the CC. There were no significant diagnostic group differences of FA in WMH for any lobar or CC regions (figure, B).
Figure Graphic displays of mean regional fractional anisotropy according to diagnostic group
(A) Normal-appearing white matter (NAWM) and (B) white matter hyperintensities (WMH) of subregions of the corpus callosum, and (C) NAWM and (D) WMH of baseline anisotropy level–based strata. Brown columns = cognitively normal older individuals; gray columns = patients with mild cognitive impairment; green columns = patients with Alzheimer disease. FA = fractional anisotropy; H1 = highest anisotropy stratum; H2 = high intermediate anisotropy stratum; H3 = low intermediate anisotropy stratum; H4 = lowest anisotropy stratum. Error bars indicates SDs. *p < 0.05, **p < 0.01 by Tukey post hoc diagnostic group comparison. †p < 0.001 by paired t test for the difference between WMH and NAWM.
Baseline anisotropy level–based white matter stratum FA.
Stratal overall white matter FA.
Diagnostic group differences.
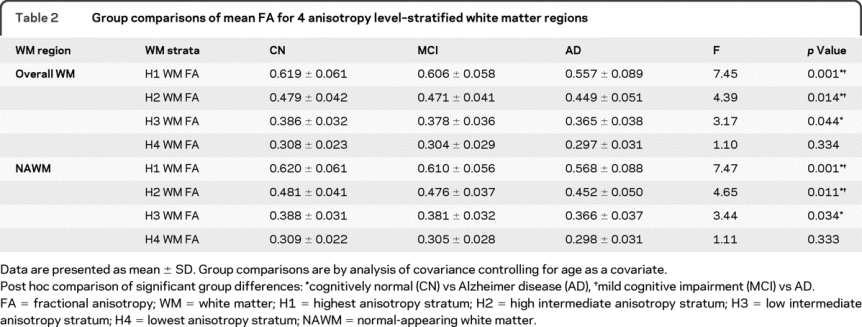
As shown in table 2, there were significant diagnostic group differences for H1, H2, and H3 stratum, but not for H4 stratum. Post hoc analyses showed that patients with AD had significantly lower FA than CN subjects in H1, H2, and H3 stratum. The AD group also showed lower FA compared with the MCI group in H1 and H2 stratum.
Table 2 Group comparisons of mean FA for 4 anisotropy level–stratified white matter regions
Independent effect of diagnosis, vascular risk, and age.
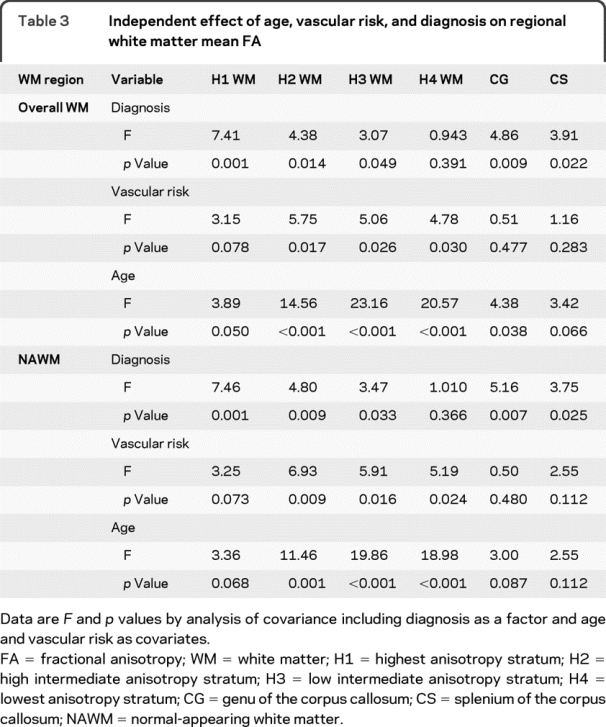
Table 3 shows analysis of covariance (ANCOVA) results for independent effect of diagnostic category, vascular risk, and age on the mean FA of each anisotropy stratum, and the genu and splenium of the CC. Significant diagnosis effects were found for the FA of H1, H2, and H3 stratum, but not for that of H4. In contrast, vascular risk showed significant effects on the FA of H2, H3, and H4 stratum, but not on that of H1 stratum. Age effects were also highly significant for H2, H3, and H4 stratum, but marginal for H1. The independent effect pattern of diagnosis, vascular risk, and age in the genu and splenium was similar to that in H1 stratum, i.e., strong diagnosis effect, no vascular effect, and weak or marginal age effect. All significant effects of diagnosis, vascular risk, and age on FA had a negative direction.
Table 3 Independent effect of age, vascular risk, and diagnosis on regional white matter mean FA
Interaction between stratum, diagnosis, and vascular risk.
To test the interactions between stratum and diagnosis or vascular risk, repeated-measures analysis of variance (ANOVA) with the mean FA values of 4 anisotropy strata as the repeated measures was performed controlling for age. When only 2-way interactions were considered in a model, there was a main effect of stratum (F = 49.12, p < 0.001), a stratum-by-diagnosis interaction (F = 3.68, p = 0.001), and a stratum-by-age interaction (F = 9.21, p < 0.001), but not a stratum-by-vascular risk interaction. In a model with a 3-way interaction between stratum, diagnosis, and vascular risk, as well as all the possible 2-way interaction, there was a main effect of stratum (F = 49.59, p < 0.001), a stratum-by-age interaction (F = 10.20, p < 0.001), and the 3-way interaction (F = 2.13, p = 0.049), but not a stratum-by-diagnosis interaction or a stratum-by-vascular risk interaction.
Stratal NAWM FA.
The same analyses were performed for stratal NAWM and WMH areas. The analysis results for strata NAWM were similar to those for strata overall white matter.
Diagnostic group differences.
There were significant diagnostic group differences for H1, H2, and H3 stratum, but not for H4 stratum. Post hoc analyses showed that patients with AD had significantly lower FA than CN subjects in H1, H2, and H3 stratum. The AD group also showed lower FA compared with the MCI group in H1 and H2 stratum (table 2 and figure, C).
Independent effect of diagnosis, vascular risk, and age.
ANCOVA was performed to evaluate the independent effect of diagnostic category, vascular risk, and age on the mean FA of each anisotropy stratum, and the genu and splenium of the CC. As shown in table 3, significant diagnosis effects were found for the FA of H1, H2, and H3 stratum, but not for that of H4. In contrast, vascular risk showed significant effects on the FA of H2, H3, and H4 stratum, but not on that of H1 stratum. Age effects were also highly significant for H2, H3, and H4 stratum, but marginal for H1. The independent effect pattern of diagnosis, vascular risk, and age in the genu and splenium was similar to that in H1 stratum, i.e., strong diagnosis effect, no vascular effect, and marginal or no age effect. All significant effects of diagnosis, vascular risk, and age on FA had a negative direction.
Interaction between stratum, diagnosis, and vascular risk.
When only 2-way interactions were considered in a repeated-measures ANOVA, there was a main effect of stratum (F = 47.69, p < 0.001), a stratum-by-diagnosis interaction (F = 4.16, p < 0.001), and a stratum-by-age interaction (F = 7.01, p < 0.001), but not a stratum-by-vascular risk interaction. In a model with a 3-way interaction between stratum, diagnosis, and vascular risk, as well as all the possible 2-way interaction, there was a main effect of stratum (F = 48.78, p < 0.001), a stratum-by-age interaction (F = 8.13, p < 0.001) and the 3-way interaction (F = 2.50, p = 0.022), but not a stratum-by-diagnosis or a stratum-by-vascular risk interaction.
Stratal WMH FA.
Significant group differences of any stratal WMH FA were not found (figure, D). There was also no significant independent diagnosis, vascular risk, or their interaction effect for any stratal WMH FA.
Difference of FA between stratal NAWM and WMH.
In both the CN and MCI groups, the mean FA of WMH was significantly lower than that of NAWM for every anisotropy level–based stratum (figure, C and D). In the case of AD, FA of WMH was significantly lower than that of NAWM for H1, H2, and H3, but not for H4 (figure, C and D).
Stratal white matter FA vs global cognition.
There was a correlation between the FA of overall white matter or NAWM in H1 and H2 stratum and Mini-Mental State Examination (MMSE) after controlling for age and education (overall white matter: partial correlation coefficient (rp) = 0.161, p = 0.036 for H1 and rp = 0.160, p = 0.036 for H2; NAWM: rp = 0.156, p = 0.042 and rp = 0.160, p = 0.037), but not for H3 and H4 stratum. No significant correlation was found between the FA of any stratal WMH and MMSE.
DISCUSSION
Our stratified anisotropy level–based approach showed that significant differences in the mean FA values between CN and AD exists in white matter regions with relatively higher baseline anisotropy (H1–H3), but not in the region with the lowest anisotropy (H4). Furthermore, the effect of diagnosis was greatest for the region with the highest baseline anisotropy (H1), suggesting that the most highly organized fibers are affected by the degenerative process. Conversely, the impact of vascular risk factors was greatest on for the less organized strata (H2–H4). These results suggest regional differences in the effect of these 2 processes on cerebral white matter similar to that previously described for WMH.5
Previous DTI studies of AD have reported reduced FA in white matter tracts with homogeneously oriented fibers, i.e., white matter tracts with high anisotropy, such as the genu or splenium of the CC,9,10 superior longitudinal fasciculus (SLF),9 and cingulum.11–13 Because we excluded the internal capsule defining the sets of ROI masks, the highest anisotropy region (H1) consisted almost entirely of the genu and splenium of the CC, which represent the interhemispheric corticocortical association tracts, as illustrated in figure e-1. A considerable proportion of the intermediate anisotropy regions (H2, H3) correspond to the body of the CC and the homogeneously oriented part of intrahemispheric long corticocortical association fibers, such as the SLF and cingulum. In contrast, the lowest anisotropy stratum (H4) covers mostly the corona radiata, where many heterogeneously oriented fibers occur. Although the corona radiate encompasses diffusely fanning portion of the CC and short association fibers (U fibers), the main part of the corona radiata consists of projection fibers, such as thalamic radiations (the corticothalamic and thalamocortical fibers) and long corticofugal fibers (the corticopontine, corticoreticular, corticobulbar, and corticospinal tracts).22
Interhemispheric and intrahemispheric corticocortical association fibers originate primarily from large pyramidal neurons in layers III and V of the neocortex.23,24 These neurons are known to be selectively affected by AD pathology, especially neurofibrillary tangles.25–27 In contrast, corticothalamic fibers originate from pyramidal cells in layer VI,23,24 where AD pathologies are less commonly found.25 Although corticofugal fibers originate from pyramidal neurons in layer V,23,24 the corresponding neocortical areas are rarely involved by AD pathology.25–27 The topographically specific neocortical AD involvement and secondary wallerian degeneration, therefore, may explain the associations between baseline anisotropy level and diagnostic group differences.
Although previous studies found FA changes in the frontal,8 parietal,8–10 and temporal9,10 white matter, we did not find differences in lobar FA among the diagnostic groups. This discrepancy seems to be related to the size and location of ROIs.28 As indicated above, if a small ROI is put primarily on a high baseline anisotropy region, FA differences due to the AD process are likely to be detected, whereas if an ROI encompasses a large low baseline anisotropy region, such FA differences would be less obvious. Our ROIs covered the entire white matter. Although both high and low anisotropy regions are included in the ROIs, the volume of the lowest anisotropy stratum (H4) covered around 50% of the cerebral white matter, with other strata covering less (i.e., H1: 5.5%; H2: 13.3%; H3: 32.3%; and H4: 48.9%). This may explain why we could not find any significant lobar and entire white matter differences of FA between diagnostic groups.
Our results revealed that, independently of diagnosis and aging effect, vascular risk significantly affects FA differences of not only overall white matter, but also NAWM. In addition, the white matter regions affected by vascular risk on FA were quite different from those affected by diagnosis. In contrast to the effect of clinical diagnosis, vascular risk factors were significantly associated with FA among lesser organized strata (e.g., H2, H3, H4), but not H1. Among the strata showing significant vascular effect, there was no increasing trend of effect size according to baseline anisotropy level of the strata. This distinctive difference between H1 and other strata is probably associated with anatomy of the vascular supply. The vascular supply to the central zone of the CC (most of H1 and a small part of H2), via short penetrating arterioles, is similar to that of the cerebral cortex, whereas the vascular supply to the extreme lateral CC and centrum semiovale (most of H2, H3, and H4) is substantially carried by long end arteries.19,29 The CC is known to be relatively resistant to lacunar infarct, hypoperfusion, and Binswanger disease.29 Our finding of evident vascular influence on NAWM FA decline is also in line with observations of ischemic brain disease.4,28
In contrast to the results for overall white matter and NAWM, no significant effects of diagnosis and vascular risk on FA were found in most WMH regions regardless of baseline regional anisotropy. When considered together with the significantly reduced FA of WMH, these findings indicate the possibility that microstructural changes within WMH, such as demyelization and axonal loss, by degenerative, vascular, or aging processes are the extreme consequence of white matter injury diffusely affecting cerebral white matter. The results of correlation analysis between FA and a global cognitive measure, MMSE, also support this possibility. MMSE scores were not correlated with FA values of any WMH region, whereas significant correlation was found with FA of NAWM region with higher baseline anisotropy. However, this notion of a floor effect of FA inside WMH contrasts with the existing knowledge for WMH, which gradually increases in volume and extent with aging, vascular risk, and degenerative processes.5–7,30 This also suggests that FA is not an appropriate parameter for monitoring the stage of more severe white matter injury when WMH are manifest, whereas it is a very sensitive tool for detecting and monitoring the early stage of alteration.
Although the baseline anisotropy–level based approach gives relatively limited information for a specific white matter location, this approach allowed us to test regional patterns of FA alterations by different pathologic processes while controlling for normal regional variability of FA.21 Moreover, in contrast with voxel-based approaches, this approach makes it possible to investigate WMH and NAWM FA separately.
AUTHOR CONTRIBUTIONS
Statistical analysis was performed by D.Y. Lee.
DISCLOSURE
Dr. Lee serves as a consultant to Wyeth Pharmaceuticals; and receives research support from H. Lundbeck A/S. Dr. Fletcher has received honoraria from Unilever (Foods For Health Initiative). Mr. Martinez, Mr. Ortega, Ms. Zozulya, Ms. Kim, and Ms. Tran report no disclosures. Dr. Buonocore serves as Associate Editor of IEEE Transactions on Medical Imaging and on the editorial board of Magnetic Resonance in Medicine; and receives research support from the NIH, NIMH, and the UC Davis M.I.N.D. Institute. Dr. Carmichael served on an external advisory board for the University of Southern California Alzheimer’s Disease Center and as a consultant to Unilever (Foods For Health Initiative); serves as Associate Editor of Alzheimer Disease and Associated Disorders; and receives research support from the NIH [K01 AG 030514 (PI)], the Dana Foundation, and the Hillblom Foundation. Dr. DeCarli serves as Editor-in-Chief of Alzheimer Disease and Associated Disorders; has received honoraria for lectures not funded by industry; serves/has served as a consultant to Eisai Inc. and Merck Serono; and receives research support from to Eisai Inc., Merck Serono, the Hillblom Foundation, and the Network for Cognitive Neuroscience of Diabetes and Aging.
Supplementary Material
Address correspondence and reprint requests to Dr. D.Y. Lee, Imaging of Dementia and Aging (IDeA) Laboratory, Department of Neurology and Center for Neuroscience, University of California at Davis, 1544 Newton Ct., Davis, CA 95616 selfpsy@snu.ac.kr
Editorial, page 1718
Supplemental data at www.neurology.org
e-Pub ahead of print on October 21, 2009, at www.neurology.org.
Supported by NIH P30 AG 10129, R01 AG010220, and R01 AG021028.
Disclosure: Author disclosures are provided at the end of the article.
Received June 3, 2009. Accepted in final form September 2, 2009.
REFERENCES
- 1.Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci 2008;34:51–61. [DOI] [PubMed] [Google Scholar]
- 2.Chua TC, Wen W, Slavin MJ, et al. Diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease: a review. Curr Opin Neurol 2008;21:83–92. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan EV, Pfefferbaum A. Neuroradiological characterization of normal adult ageing. Br J Radiol 2007;80:S99–S108. [DOI] [PubMed] [Google Scholar]
- 4.O’Sullivan M, Summers PE, Jones DK, et al. Normal-appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology 2001;57:2307–2310. [DOI] [PubMed] [Google Scholar]
- 5.Yoshita M, Fletcher E, Harvey D, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology 2006;67:2192–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeCarli C, Miller BL, Swan CE, et al. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol 2001;58:643–647. [DOI] [PubMed] [Google Scholar]
- 7.Scheltens P, Barkhof F, Valk J, et al. White matter lesions on magnetic resonance imaging in clinically diagnosed Alzheimer’s disease: evidence for heterogeneity. Brain 1992;115:735–748. [DOI] [PubMed] [Google Scholar]
- 8.Medina D, de Toledo-Morrell Leyla, Urresta Fabio, et al. White matter changes in mild cognitive impairment and AD: a diffusion tensor imaging study. Neurobiol Aging 2006;27:663–672. [DOI] [PubMed] [Google Scholar]
- 9.Xie S, Xiao JS, Gong GL, et al. Voxel-based detection of white matter abnormalities in mild Alzheimer’s disease. Neurology 2006;66:1845–1849. [DOI] [PubMed] [Google Scholar]
- 10.Naggara O, Oppenheim C, Rieu D, et al. Diffusion tensor imaging in early Alzheimer’s disease. Psychiatry Res 2006;146:243–249. [DOI] [PubMed] [Google Scholar]
- 11.Choo IH, Lee DY, Oh JS, et al. Posterior cingulated cortex atrophy and regional cingulum disruption in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging Epub 2008 Aug 5. [DOI] [PubMed]
- 12.Fellgiebel A, Müller MJ, Wille P, et al. Color-coded diffusion-tensor-imaging of posterior cingulate fiber tracts in mild cognitive impairment. Neurobiol Aging 2005;26:1193–1198. [DOI] [PubMed] [Google Scholar]
- 13.Rose SE, McMahon KL, Janke AL, et al. Diffusion indices on magnetic resonance imaging and neuropsychological performance in amnestic mild cognitive impairment. J Neurol Neurosurg Psychiatry 2006;77:1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Schuff N, Jahng GH, et al. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology 2007;68:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milionis HJ, Florentin M, Giannopoulos S. Metabolic syndrome and Alzheimer’s disease: a link to a vascular hypothesis? CNS Spectr 2008;13:606–613. [DOI] [PubMed] [Google Scholar]
- 16.Mckhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 17.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment: beyond controversies, towards a consensus—report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004;256:240–246. [DOI] [PubMed] [Google Scholar]
- 18.DeCarli C, Mungas D, Harvey D, et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology 2004;63:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeCarli C, Fletcher E, Ramey V, et al. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationship s between periventricular WMH, deep WMH, and total WMH burden. Stroke 2005;36:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kochunov P, Lancaster J, Thompson P, et al. Regional spatial normalization: toward an optimal target. J Comput Assist Tomogr 2001;25:805–816. [DOI] [PubMed] [Google Scholar]
- 21.Wiegell MR, Larsson HBW, Wedeen VJ. Fiber crossing in human brain depicted with diffusion tensor MR imaging. Radiology 2000;217:897–903. [DOI] [PubMed] [Google Scholar]
- 22.Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based diffusion tensor imaging in an ICBM template. Neuroimage 2008;40:570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nieuwenhuys R. The neocortex: an overview of its evolutionary development, structural organization and synaptology. Anat Embryol 1994;190:307–337. [DOI] [PubMed] [Google Scholar]
- 24.Jones EG. Laminar distribution of cortical efferent cells. In: Peters A, Jones EG, editors. Cerebral Cortex: Cellular Components of the Cerebral Cortex. New York: Plenum Publishing Corp.; 1984:1:521–553. [Google Scholar]
- 25.Giannakopoulos P, Hof PR, Bouras C. Selective vulnerability of neocortical association areas in Alzheimer’s disease. Microsc Res Tech 1998;43:16–23. [DOI] [PubMed] [Google Scholar]
- 26.Pearson RCA, Esiri MM, Hiorns RW, et al. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer’s disease. Proc Natl Acad Sci USA 1985;82:4531–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis DA, Campbell MJ, Terry RD, et al. Laminar and regional distribution of neurofibrillary tangles and neuritic plaques in Alzheimer’s disease: a quantitative study of visual and auditory cortices. J Neurosci 1987;7:1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Sullivan M, Morris RG, Huckstep B, et al. Diffusion tensor MRI correlates with executive dysfunction in patients with ischemic leukoaraiosis. J Neurol Neurosurg Psychiatry 2004;75:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moody DM, Bell MA, Challa VR. The corpus callosum, a unique white-matter tract: anatomic features that may explain sparing Binswanger disease and resistance to flow of fluid masses. Am J Neuroradiol 1988;9:1051–1059. [PMC free article] [PubMed] [Google Scholar]
- 30.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.