Abstract
Objective:
Parkinson disease (PD) may affect the autonomic nervous system and may cause constipation; however, few studies have explored constipation preceding the motor onset of PD. We investigated constipation preceding PD using a case-control study design in a population-based sample.
Methods:
Using the medical records-linkage system of the Rochester Epidemiology Project, we identified 196 subjects who developed PD in Olmsted County, MN, from 1976 through 1995. Each incident case was matched by age (±1 year) and sex to a general population control. We reviewed the complete medical records of cases and controls in the medical records-linkage system to ascertain the occurrence of constipation preceding the onset of PD (or index year).
Results:
Constipation preceding PD or the index year was more common in cases than in controls (odds ratio [OR] 2.48; 95% confidence interval [CI] 1.49 to 4.11; p = 0.0005). This association remained significant after adjusting for smoking and coffee consumption (ever vs never), and after excluding constipation possibly induced by drugs. In addition, the association remained significant in analyses restricted to constipation documented 20 or more years before the onset of motor symptoms of PD. Although the association was stronger in women than in men and in patients with PD with rest tremor compared with patients with PD without rest tremor, these differences were not significant.
Conclusions:
Our findings suggest that constipation occurring as early as 20 or more years before the onset of motor symptoms is associated with an increased risk of Parkinson disease.
GLOSSARY
- CI
= confidence interval;
- OR
= odds ratio;
- PD
= Parkinson disease.
Dysautonomia is a component of the pathogenetic process underlying Parkinson disease (PD), and Lewy bodies are consistently found in the autonomic nervous system of patients who died with PD.1–3 Constipation is one of the most frequent clinical manifestations of this dysautonomia, and is commonly reported at the time of onset of motor symptoms of PD or during progression of the disease.4–6
In addition, it has been suggested that constipation may precede the appearance of motor symptoms of PD in some patients.7–9 In the only cohort study that addressed this question, the Honolulu-Asia Aging Study, men who reported less frequent bowel movements had a significantly higher risk of PD over a 24-year follow-up period.10,11 In subsequent publications from the same study, constipation was also associated with incidental Lewy body disease, which is thought to represent a preclinical phase of PD, and with a reduced neuronal density in the substantia nigra.12,13 Consistent with these clinical and pathologic findings, the Braak staging of the neuropathologic involvement in PD predicts that the autonomic system is involved early in the disease process.14
To further explore the hypothesis that constipation may be an early, premotor manifestation of PD, we conducted a population-based case-control study of incident PD cases. Because constipation was assessed through a medical records-linkage system, we were able to study the length of time between appearance of constipation and onset of motor symptoms without relying on the recall of historical events. In addition, we extended the scope of the Honolulu-Asia Aging Study by including both men and women in our study.
METHODS
Cases.
Using the medical records-linkage system of the Rochester Epidemiology Project, we identified all subjects residing in Olmsted County, MN, who developed PD from 1976 through 1995. Details about the study population and the identification of incident cases were reported elsewhere.15 Our diagnostic criteria included 2 steps: the definition of parkinsonism as a syndrome and the definition of PD within the syndrome. Parkinsonism was defined as the presence of at least 2 of 4 cardinal signs: rest tremor, bradykinesia, rigidity, and impaired postural reflexes. PD was defined as the presence of parkinsonism with all 3 of the following criteria. 1) No other cause (e.g., repeated stroke with stepwise progression; repeated head injury; history of encephalitis; neuroleptic treatment within 6 months before onset; hydrocephalus; brain tumor). 2) No documentation of unresponsiveness to levodopa at doses of at least 1 g/day in combination with carbidopa (applicable only to patients who were treated). 3) No prominent or early (within 1 year of onset) signs of more extensive nervous system involvement (e.g., dementia or dysautonomia) not explained otherwise.15 Our clinical classification of patients with PD through medical records review by a movement disorders specialist (J.H.B.) was found to be valid compared with a direct neurologic examination by a movement disorders specialist, as reported elsewhere.16 Onset of PD was defined as the year in which a cardinal sign of PD was first noted by the patient, by family members, or by a care provider (as recorded in the medical record).
Controls.
Each case was individually matched by age (±1 year) and sex to a general population control residing in Olmsted County and free of PD, other parkinsonism, or tremor of any type in the index year (year of onset of PD in the matched case). The list of all county residents from which potential controls were randomly drawn was provided by the medical records-linkage system.17 This list has been shown to be complete by comparison with a random-digit-dialing telephone sample and with the census.17 Records of potential controls were reviewed by a neurologist (D.M.M.) to exclude the presence of PD, other types of parkinsonism, or tremor of any type before or during the index year. The presence of dementia or other neurologic diseases was not an exclusion criterion. Our exclusion of parkinsonism in controls through medical record review was found to be valid compared with a direct examination by a movement disorders specialist, as reported elsewhere.16
Ascertainment of constipation.
The complete medical records of cases and controls, which are routinely linked and stored in the medical records-linkage system of the Rochester Epidemiology Project,17 were reviewed and abstracted by a physician (R.S.) to ascertain the occurrence of constipation. Constipation was defined by the presence of at least 1 of 2 criteria: 1) a diagnosis of constipation in the medical records; or 2) the use of drugs to treat constipation (laxatives) even in the absence of a recorded diagnosis. We abstracted data for constipation in chronological order starting from the first available record through the onset of PD (or the index year).
To assess severity, we collected additional information on the referral of patients to a gastroenterologist (need for specialist care) and on the use of laxatives (need for treatment). In addition, we abstracted data on the concomitant use of constipation-inducing drugs (calcium-containing antacids, medications with anticholinergic effects, antidepressants, calcium channel blockers, cholestyramine, clonidine, diuretics, levodopa, narcotics, nonsteroidal antiinflammatory drugs, psychotropics, and sympathomimetics). Only occurrences of constipation documented in the medical record before the index year were accepted as exposure. To avoid a possible bias in the definition of constipation (exposure suspicion bias),18 we included only subjects who were given a diagnosis of constipation by their caregiving physician (historically, at the time of medical evaluation) or were prescribed laxatives (in the absence of a diagnosis). We did not assign new diagnoses retrospectively and did not modify the historical diagnoses based on current criteria or practices.
To validate our abstracting procedure for constipation, a second physician (not a coauthor) who was kept unaware of the case or control classification of subjects reabstracted the complete medical records for a random sample of 20 study subjects (approximately 5% of all cases and controls). The interrater agreement on presence or absence of constipation was 90.0% (positive agreement for 5 pairs and negative agreement for 13 pairs) with a kappa value of 0.76 (95% confidence interval [CI] 0.46 to 1.00). This small study suggests that our abstracting procedure was reliable.
Data analysis.
Consistent with our design, matched-pairs analyses were performed using conditional logistic regression, and the odds ratio (OR) was used to estimate the relative risk. For each variable, we calculated an OR, a 95% CI, and a p value (two-tailed test, alpha = 0.05). We conducted a set of primary analyses without adjustment and an additional set of secondary analyses adjusted by smoking and coffee consumption (ever vs never). Smoking and coffee consumption were considered potential confounding variables because they have been found to be associated with impaired gastrointestinal function,19,20 and with a reduced risk of PD.21,22 Because of incomplete data on smoking and coffee consumption, these adjusted analyses were conducted ignoring the matching and including age (age at index year in quartiles) and sex in the regression models to remove residual confounding.
In a sensitivity analysis, we restricted the definition of constipation to patients who were not using constipation-inducing drugs. In addition, to investigate severity of constipation, we performed analyses restricted to those subjects who ever used laxatives or who were ever referred to a gastroenterologist. We also conducted analyses stratified by sex, age at onset of PD (≤71 vs >71 years; median cutoff), and for PD with or without rest tremor.23 To explore the effect of constipation experienced across life, we also conducted analyses stratified by lag time between onset of constipation (first diagnosis or first use of laxatives) and onset of PD (0 to 19 years vs 20 or more years before the onset of PD or index date) and analyses stratified by age at time of onset of constipation (0 to 49 years old vs 50 years or older). All analyses were performed using SAS® version 9 (SAS Institute, Cary, NC).
Standard protocol approvals, registrations, and patient consent.
The study was approved by the institutional review boards of the Mayo Clinic and of Olmsted Medical Center. Written informed consent was not required for passive medical record review.
RESULTS
We identified 202 patients who developed PD from 1976 through 1995 (incident cases). These patients were matched by age and sex with 202 controls. However, 6 individuals (5 cases and 1 control) did not authorize the use of their medical records for research and the corresponding pairs could not be studied. Therefore, we included 196 case-control pairs for a total of 392 individuals. Among the cases, 121 (61.7%) were men and 75 (38.3%) were women; the median age at time of onset of PD was 71 years (range 41 to 97 years). The distribution by age and sex was similar in controls due to the matched design. The median duration of enrollment in the medical records-linkage system preceding the index year was 38 years (range 2 to 73 years) for cases and 38 years (range <1 to 73 years) for controls (Wilcoxon signed rank test, p = 0.35).
The table shows the results of our case-control analyses without adjustment (primary models) and with adjustment for smoking and coffee consumption (fully adjusted models). Seventy-one (36.2%) cases and 40 (20.4%) controls had constipation (OR 2.48; 95% CI 1.49 to 4.11; p = 0.0005). The association was confirmed after excluding constipation in subjects who were using constipation-inducing drugs (OR 2.00; 95% CI 1.20 to 3.34; p = 0.008). In addition, the association was confirmed after restricting the definition of constipation to subjects who used laxatives (OR 2.07; 95% CI 1.09 to 3.92; p = 0.03). Results were consistent in all adjusted analyses including smoking and coffee consumption in the models (table, right columns). No significant differences were observed between strata by sex, age at onset of PD (≤71 vs >71 years), and for PD with or without rest tremor.
Table Association between Parkinson disease (PD) and preceding constipation (196 cases and 196 controls)
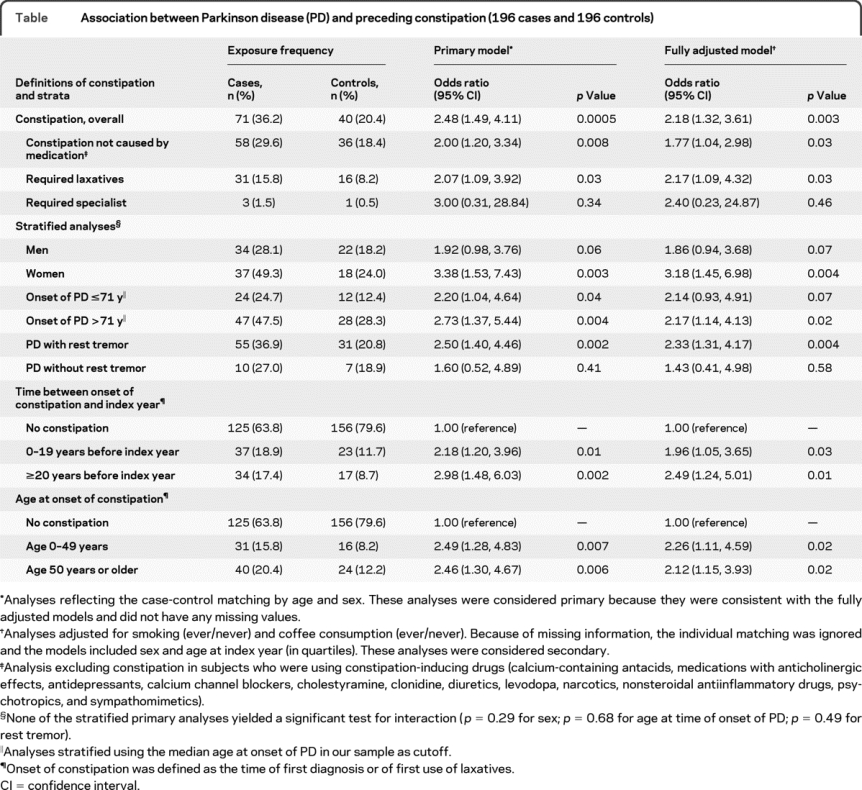
The association was stronger in women (OR 3.38; 95% CI 1.53 to 7.43; p = 0.003) than in men (OR 1.92; 95% CI 0.98 to 3.76; p = 0.06); however, the difference was not significant. In addition, the association did not differ significantly for early vs late onset of PD and for patients with or without rest tremor (see table, footnote §).
Our analyses showed similar associations between constipation and PD regardless of the time interval between the onset of constipation and the onset of PD (or index year). Indeed, we found an association both for constipation starting 20 or more years before PD onset (OR 2.98; 95% CI 1.48 to 6.03; p = 0.002) and for constipation starting within 20 years of PD onset or index year (OR 2.18; 95% CI 1.20 to 3.96; p = 0.01; p for interaction = 0.44). The figure shows that the distribution in cases was shifted toward longer lag times between onset of constipation and index year compared with controls; however, this difference was not significant. The association was also similar in 2 strata defined by age at the time of onset of constipation (table).
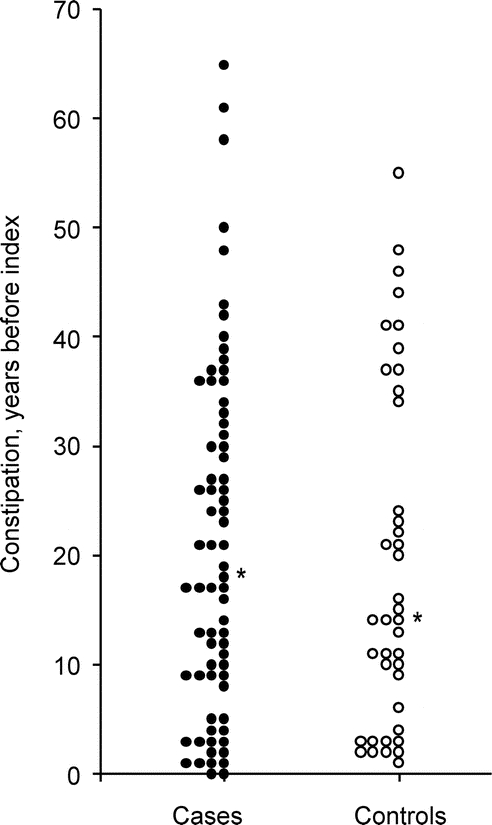
Figure Distribution of the lag time between onset of constipation and onset of motor symptoms of Parkinson disease (or index year for controls)
Each dot represents the lag time for a given case or control rounded to single years. The asterisk indicates the median. The distribution was shifted toward longer lag times in cases (median 17.9 years; range <1.0 to 65.2) compared with controls (median 14.0 years; range <1.0 to 54.9; Wilcoxon rank sum test, p = 0.63).
DISCUSSION
Our findings may suggest that constipation is an early manifestation of the neurodegenerative process underlying PD, and that it frequently precedes the classic motor signs of PD by several decades in both men and women. This is consistent with the findings restricted to men from the Honolulu-Asia Aging Study. In that cohort study, men who reported less frequent bowel movements experienced an increased risk of PD,10,11 of incidental Lewy body disease (presumed to reflect preclinical PD),12 and of reduced neuronal density in the substantia nigra.13
The early occurrence of constipation is biologically plausible and consistent with the predictions of the Braak staging.14 The earliest neuropathologic features of PD are found not only in the lower brainstem and olfactory bulbs, but also in the autonomic nervous system, including the gastrointestinal tract.24,25 Indeed, Lewy bodies have frequently been reported in the autonomic nervous system of persons with incidental Lewy body disease, which is thought to reflect preclinical PD (Braak stages 1–2).14,26–29 This contrasts with the later involvement of the substantia nigra, the substrate for the motor findings of PD, which becomes manifest only during more advanced Braak stages (Braak stages 3–4). In addition, the Honolulu-Asia Aging Study showed incidental Lewy bodies in the locus ceruleus and substantia nigra of subjects with constipation who died free of PD.12 Finally, the Honolulu-Asia Aging Study showed that subjects with constipation who did not smoke had a reduced neuronal density in the substantia nigra, independent of the presence of Lewy bodies (after adjustment for PD and incidental Lewy bodies).13
The association between constipation and PD was evident several decades before the onset of PD. Indeed, the association remained significant when restricted to constipation documented more than 20 years before the onset of motor symptoms. This extends the findings from the Honolulu-Asia Aging Study where there was a mean of 12 years between the documentation of constipation and the subsequent development of PD.10 This early link with constipation suggests that the neurodegenerative process underlying PD may begin more than 2 decades prior to the onset of motor symptoms of PD. A similar time frame has been reported for the association of anxiety disorders preceding PD.30 Similarly, REM sleep behavior disorder, another nonmotor manifestation of PD, was reported to precede PD by an average of nearly 13 years.31
These estimates of the duration of premotor PD are longer than the estimates derived from neuropathologic or imaging studies of the substantia nigra. In particular, neuropathologic extrapolation predicted a 4.6-year preclinical state,32 and dopaminergic imaging estimated an approximate 6-year premotor interval.33–36
Despite the biologic plausibility of the hypothesis that constipation is an early nonmotor manifestation of PD, there are alternative explanations for this association. For example, both constipation and PD could be independent manifestations of a third unknown risk factor (e.g., a certain dietary preference or physical activity) or of a genetic susceptibility (e.g., one or several genetic variants). In addition, constipation may have an indirect causal role in PD by increasing the intestinal absorption of some unidentified substances that are toxic for the substantia nigra.37 However, the evidence in support of these alternative interpretations remains limited.10
Our study has several strengths. First, it was based on a series of incident PD cases and on well-defined general population controls, thus reducing referral bias and incidence-prevalence bias.18 Second, we were able to avoid recall bias by considering episodes of constipation or of use of laxatives that were historically documented in medical records before the onset of PD (or the index year).18 Third, we carefully excluded from our case series patients affected by other types of parkinsonism with early autonomic failure, such as multiple system atrophy.38 Fourth, our study included men and women, thus providing the opportunity to explore differences across sex, and also to extend the Honolulu-Asia Aging Study results, which were restricted to men.10,11 Fifth, we conducted a set of secondary analyses to exclude 2 possible confounders, smoking and coffee consumption, which have been associated with PD and with constipation.19–22 However, neither of these 2 potential confounders modified the association. Similarly, smoking and coffee consumption also failed to modify the association between bowel movement frequency and PD in the Honolulu-Asia Aging Study.11 Finally, the use of historical medical records in the medical records-linkage system facilitated the study of episodes of constipation that occurred several decades before the motor onset of PD. This time frame was important to explore the possible lag time between onset of constipation and onset of motor symptoms of PD.
On the other hand, the study has several limitations. First, it is possible that some subjects did not bring constipation to the attention of medical personnel or were treated at a medical facility outside of the medical records-linkage system, and thus were not documented in the system. Second, we cannot exclude the possibility of underascertainment of constipation because the physicians did not use a systematic approach or a formal questionnaire to assess gastrointestinal symptoms. In particular, physicians may not have queried the patients about symptoms of defecatory dysfunction. By contrast, some subjects may have overreported constipation because constipation was not confirmed using, for example, a stool diary.9 Because this overreporting or underreporting occurred historically before we designed our study, it should be symmetric between cases and controls (nondifferential over- or underascertainment). In addition, the frequency of constipation observed in our controls (20.4%) was similar to or higher than the frequency observed in the overall population of Olmsted County (8.0%) or in the United States (12% to 19%),39,40 and our findings were consistent with a higher frequency of constipation in women than men, as reported by others.39 Third, it is possible that patients with constipation were under more intensive medical care than subjects of the same age but without constipation (surveillance bias). However, the significant association for constipation experienced 20 or more years before the onset of motor symptoms of PD cannot be easily explained by a short-term surveillance bias. Fourth, information about physical activity or diet at the time of onset of constipation was not available from the medical records, and could not be considered in adjusted analyses. Finally, as part of this case-control study, we also investigated erectile dysfunction as another autonomic symptom that could precede the motor onset of PD in men. However, we did not find an association (results not shown).
AUTHOR CONTRIBUTIONS
Statistical analyses were conducted by J.M. Carlin and B.R. Grossardt.
ACKNOWLEDGMENT
The authors thank Barbara J. Balgaard for typing the manuscript. Dr. Savica conducted this study while on leave from the Department of Neurosciences, Psychiatry, and Anesthesiology, University of Messina, Messina, Italy.
DISCLOSURE
The case-control sample on which the study was conducted was established with funding from the NIH grants R01 NS033978 and R01 AR030582 (Rochester Epidemiology Project). Dr. Savica, J.M. Carlin, and B.R. Grossardt report no disclosures. R. Bower has served as a consultant to Allergan, Inc. Dr. Ahlskog received a Fred Springer Award from the American Parkinson’s Disease Association; receives royalties from publishing The Parkinson’s Disease Treatment Book (Oxford University Press, 2005) and Parkinson’s Disease Treatment Guide for Physicians (Oxford University Press, 2009), Parkinson’s Disease and Movement Disorders (Humana Press, 2000), and Surgical Treatment of Parkinson’s Disease and Other Movement Disorders (Humana Press, 2003); has received honoraria for lectures or educational activities not funded by industry; and receives research support from NIH/NINDS [P50 NS 40256-R (Co-I)]. Dr. Maraganore may accrue revenue from pending patent applications related to the prediction of Parkinson disease and the treatment of neurodegenerative disease; has received license fee payments and royalty payments from Alnylam Pharmaceuticals (Method to treat Parkinson’s disease); and receives research support from the NIH [ES10751 (PI)]. Dr. Bharucha has served on scientific advisory boards for Novartis and Pfizer Inc.; serves on editorial advisory boards for the American Journal of Gastroenterology, the International Foundation for Functional Gastrointestinal Disorders, and Neurogastroenterology and Motility; has served as a consultant to Amylin Pharmaceuticals, American Medical Systems, Nordic Biotech, Helsinn Healthcare, and Merck Serono; and receives research support from Pfizer Inc., Novartis, Sucampo Pharmaceuticals, and the NIH/NIDDK [DK 78924 (PI), PO1 DK 68055 (PI of Project 3)]. Dr. Rocca receives research support from the NIH [AR030582 (PI), AG006786 (Co-I), and ES010751 (Co-I)].
Address correspondence and reprint requests to Dr. W.A. Rocca, Division of Epidemiology, Department of Health Sciences Research, Mayo Clinic, 200 First Street SW, Rochester, MN 55905 rocca@mayo.edu
Disclosure: Author disclosures are provided at the end of the article.
Received April 10, 2009. Accepted in final form August 20, 2009.
REFERENCES
- 1.Singaram C, Ashraf W, Gaumnitz EA, et al. Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation. Lancet 1995;346:861–864. [DOI] [PubMed] [Google Scholar]
- 2.Micieli G, Tosi P, Marcheselli S, Cavallini A. Autonomic dysfunction in Parkinson’s disease. Neurol Sci 2003;24 suppl 1:S32–S34. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui MF, Rast S, Lynn MJ, Auchus AP, Pfeiffer RF. Autonomic dysfunction in Parkinson’s disease: a comprehensive symptom survey. Parkinsonism Relat Disord 2002;8:277–284. [DOI] [PubMed] [Google Scholar]
- 4.Edwards LL, Quigley EM, Harned RK, Hofman R, Pfeiffer RF. Characterization of swallowing and defecation in Parkinson’s disease. Am J Gastroenterol 1994;89:15–25. [PubMed] [Google Scholar]
- 5.Edwards LL, Quigley EM, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease: frequency and pathophysiology. Neurology 1992;42:726–732. [DOI] [PubMed] [Google Scholar]
- 6.Byrne KG, Pfeiffer R, Quigley EM. Gastrointestinal dysfunction in Parkinson’s disease: a report of clinical experience at a single center J Clin Gastroenterol 1994;19:11–16. [DOI] [PubMed] [Google Scholar]
- 7.Ueki A, Otsuka M. Life style risks of Parkinson’s disease: association between decreased water intake and constipation. J Neurol 2004;251:18–23. [DOI] [PubMed] [Google Scholar]
- 8.Korczyn AD. Autonomic nervous system disturbances in Parkinson’s disease. Adv Neurol 1990;53:463–468. [PubMed] [Google Scholar]
- 9.Ashraf W, Pfeiffer RF, Park F, Lof J, Quigley EM. Constipation in Parkinson’s disease: objective assessment and response to psyllium. Mov Disord 1997;12:946–951. [DOI] [PubMed] [Google Scholar]
- 10.Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 2001;57:456–462. [DOI] [PubMed] [Google Scholar]
- 11.Abbott RD, Ross GW, White LR, et al. Environmental, life-style, and physical precursors of clinical Parkinson’s disease: recent findings from the Honolulu-Asia Aging Study. J Neurol 2003;250 suppl 3:III30–III39. [DOI] [PubMed] [Google Scholar]
- 12.Abbott RD, Ross GW, Petrovitch H, et al. Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord 2007;22:1581–1586. [DOI] [PubMed] [Google Scholar]
- 13.Petrovitch H, Abbott RD, Ross GW, et al. Bowel movement frequency in late-life and substantia nigra neuron density at death. Mov Disord 2009;24:371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 2006;396:67–72. [DOI] [PubMed] [Google Scholar]
- 15.Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976–1990. Neurology 1999;52:1214–1220. [DOI] [PubMed] [Google Scholar]
- 16.Elbaz A, Peterson BJ, Yang P, et al. Nonfatal cancer preceding Parkinson’s disease: a case-control study. Epidemiology 2002;13:157–164. [DOI] [PubMed] [Google Scholar]
- 17.Melton LJ, 3rd. History of the Rochester Epidemiology Project. Mayo Clin Proc 1996;71:266–274. [DOI] [PubMed] [Google Scholar]
- 18.Sackett DL. Bias in analytic research. J Chronic Dis 1979;32:51–63. [DOI] [PubMed] [Google Scholar]
- 19.Rausch T, Beglinger C, Alam N, Gyr K, Meier R. Effect of transdermal application of nicotine on colonic transit in healthy nonsmoking volunteers. Neurogastroenterol Motil 1998;10:263–270. [DOI] [PubMed] [Google Scholar]
- 20.Boekema PJ, Samsom M, van Berge Henegouwen GP, Smout AJ. Coffee and gastrointestinal function: facts and fiction: a review. Scand J Gastroenterol Suppl 1999;230:35–39. [DOI] [PubMed] [Google Scholar]
- 21.Benedetti MD, Bower JH, Maraganore DM, et al. Smoking, alcohol, and coffee consumption preceding Parkinson’s disease: a case-control study. Neurology 2000;55:1350–1358. [DOI] [PubMed] [Google Scholar]
- 22.Ritz B, Ascherio A, Checkoway H, et al. Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol 2007;64:990–997. [DOI] [PubMed] [Google Scholar]
- 23.Elbaz A, Bower JH, Peterson BJ, et al. Survival study of Parkinson disease in Olmsted County, Minnesota Arch Neurol 2003;60:91–96. [DOI] [PubMed] [Google Scholar]
- 24.Kupsky WJ, Grimes MM, Sweeting J, Bertsch R, Cote LJ. Parkinson’s disease and megacolon: concentric hyaline inclusions (Lewy bodies) in enteric ganglion cells. Neurology 1987;37:1253–1255. [DOI] [PubMed] [Google Scholar]
- 25.Cersosimo MG, Benarroch EE. Neural control of the gastrointestinal tract: implications for Parkinson disease. Mov Disord 2008;23:1065–1075. [DOI] [PubMed] [Google Scholar]
- 26.Klos KJ, Ahlskog JE, Josephs KA, et al. Alpha-synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology 2006;66:1100–1102. [DOI] [PubMed] [Google Scholar]
- 27.Bloch A, Probst A, Bissig H, Adams H, Tolnay M. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol 2006;32:284–295. [DOI] [PubMed] [Google Scholar]
- 28.Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K. Parkinson’s disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol 2007;113:421–429. [DOI] [PubMed] [Google Scholar]
- 29.Minguez-Castellanos A, Chamorro CE, Escamilla-Sevilla F, et al. Do α-synuclein aggregates in autonomic plexuses predate Lewy body disorders? A cohort study. Neurology 2007;68:2012–2018. [DOI] [PubMed] [Google Scholar]
- 30.Shiba M, Bower JH, Maraganore DM, et al. Anxiety disorders and depressive disorders preceding Parkinson’s disease: a case-control study. Mov Disord 2000;15:669–677. [DOI] [PubMed] [Google Scholar]
- 31.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology 1996;46:388–393. [DOI] [PubMed] [Google Scholar]
- 32.Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 1991;114:2283–2301. [DOI] [PubMed] [Google Scholar]
- 33.Morrish PK, Rakshi JS, Bailey DL, Sawle GV, Brooks DJ. Measuring the rate of progression and estimating the preclinical period of Parkinson’s disease with [18F]dopa PET. J Neurol Neurosurg Psychiatry 1998;64:314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrish PK, Sawle GV, Brooks DJ. An [18F]dopa-PET and clinical study of the rate of progression in Parkinson’s disease. Brain 1996;119:585–591. [DOI] [PubMed] [Google Scholar]
- 35.Morrish PK, Sawle GV, Brooks DJ. The rate of progression of Parkinson’s disease: a longitudinal [18F]DOPA PET study. Adv Neurol 1996;69:427–431. [PubMed] [Google Scholar]
- 36.Brooks DJ. Morphological and functional imaging studies on the diagnosis and progression of Parkinson’s disease. J Neurol 2000;247:II11–II18. [DOI] [PubMed] [Google Scholar]
- 37.Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol 2007;33:599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence of progressive supranuclear palsy and multiple system atrophy in Olmsted County, Minnesota, 1976 to 1990. Neurology 1997;49:1284– 1288. [DOI] [PubMed] [Google Scholar]
- 39.Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 2004;99:750–759. [DOI] [PubMed] [Google Scholar]
- 40.Bharucha AE, Locke GR, Zinsmeister AR, et al. Differences between painless and painful constipation among community women. Am J Gastroenterol 2006;101:604–612. [DOI] [PubMed] [Google Scholar]


