Abstract
Objectives
Increased prevalence of deep white matter hyperintensities (DWMHs) has been consistently observed in patients with geriatric depression and bipolar disorder. DMWHs are associated with chronicity, disability, and poor quality of life. They are thought to be ischemic in their etiology and may be related to the underlying pathophysiology of mood disorders in the elderly. Notably, these lesions strikingly resemble radiological findings related to the cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephelopathy (CADASIL) syndrome. CADASIL arises from mutations in Notch3, resulting in impaired signaling via cellular Fas-associated death domain-like interleukin-1-beta-converting enzyme-inhibitory protein (c-FLIP) through an extracellular signal-regulated kinase (ERK)-dependent pathway. These signaling abnormalities have been postulated to underlie the progressive degeneration of vascular smooth muscle cells (VSMC). This study investigates the possibility that the anticonvulsant valproate (VPA), which robustly activates the ERK mitogen-activated protein kinase (MAPK) cascade, may exert cytoprotective effects on VSMC through the Notch3/c-FLIP pathway.
Methods
Human VSMC were treated with therapeutic concentrations of VPA subchronically. c-FLIP was knocked down via small interfering ribonucleic acid transfection. Cell survival, apoptosis, and protein levels were measured.
Results
VPA increased c-FLIP levels dose- and time-dependently and promoted VSMC survival in response to Fas ligand-induced apoptosis in VSMC. The anti-apoptotic effect of VPA was abolished by c-FLIP knockdown. VPA also produced similar in vivo effects in rat brain.
Conclusions
These results raise the intriguing possibility that VPA may be a novel therapeutic agent for the treatment of CADASIL and related disorders. They also suggest that VPA might decrease the liability of patients with late-life mood disorders to develop DWMHs.
Keywords: apoptosis, bipolar disorder, FLIP, hyperintensity, Notch3, VPA, VSMC
One of the most replicated magnetic resonance imaging (MRI) findings in patients with geriatric depression and bipolar disorder (BPD) is an increased prevalence of deep white matter hyper-intensities (DWMHs) compared to the general population (1, 2). White matter lesions in the fronto-limbic and fronto-striatal pathways are likely to confer vulnerability to late-life depression and to cognitive impairment in executive functions. Understanding the pathophysiology of DWMHs and studying treatments that reduce liability to develop DWMHs in patients with mood disorders might have a dramatic clinical impact on reducing the burden associated with geriatric depression and late-life BPD.
The pathophysiology of these lesions has yet to be fully elucidated, but recent neuropathological postmortem findings suggest that WMHs are most likely determined by focal cerebral ischemia (3). Other possible causes include dilated perivascular spaces and oligemic demyelinazation (4). Notably, the MRI findings described above are similar to those reported in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephelopathy (CADASIL) syndrome (5), whose genetic etiology has been recently described. CADASIL manifests itself mainly as a late-onset central nervous system degenerative disorder caused by diffuse angiopathy. However, patients with CADASIL often present predominantly with mood symptoms and migraine headaches prior to overt degenerative disorder. Together, these observations led to the investigation of MRI hyperintensities as a potential endophenotype for BPD and to the investigation of Notch3 (the gene etiologically implicated in CADASIL) as a candidate gene for BPD (6); this small, underpowered study yielded negative results.
The diffuse arteriopathy associated with CADASIL syndrome is characterized by prominent degeneration and eventual loss of vascular smooth muscle cells (VSMC) from the vessel wall (6, 7). Histopathologically, there is granular thickening of the cerebral arterioles. Patients with CADASIL syndrome also have an increased prevalence of mood disorders compared to the general population (8), and the presence of a comorbid psychiatric disorder is specifically associated with DWMHs rather than with other lesions (9).
The gene causing CADASIL, Notch3, has been mapped to chromosome 19 (10) and contains at least 29 exons spanning greater than 5,600 base pairs of DNA. Mutations in Notch3 in CADASIL patients cluster primarily in exons 3 and 4 (10, 11). Although the molecular mechanisms underlying the CADASIL-associated Notch3 receptor malfunction are still being elucidated, the pathogenetic mechanisms underlying the main vascular diseases appear to involve a series of activations and/or reactivations of cell programs that regulate growth, differentiation, and apoptosis. In this context, it has been demonstrated that Notch signaling clearly plays a central role in these cell functions (12, 13). Furthermore, it is now clear that Notch system components are expressed in the adult vascular system, and that their expression is related to events causing vascular damage (14).
Notch signaling begins when the Notch receptor binds ligands (Delta, Serrate/Jagged) and ends when the Notch intracellular domain enters the nucleus and activates transcription of target genes. In the classic model, in response to Notch signaling, RBP-Jk activates transcription of basic helix-loop- helix (bHLH) transcription factors such as hairy and enhancer of split (15) and the hairy-related transcription factor (HRT) genes (16), which regulate expression of other target genes. This core pathway is subject to a wide array of regulatory influences and protein-protein interactions, and is correlated with other signaling pathways. The Notch signaling pathway plays an important role in cell fate determination, vasculogenesis, and organogenesis. Mutations in the human Notch3 gene cause CADASIL, a vascular stroke and dementia syndrome characterized by degeneration of VSMC and multiple small infarcts in the white and deep gray matter of the brain (17). It has been suggested that Notch3 is necessary to generate functional arteries by regulating arterial differentiation and maturation of VSMC (18), and plays a critical role in determining VSMC growth (19). Furthermore, it has been demonstrated that Notch3 signaling is a critical determinant of VSMC survival and vascular structure by modulating the expression of cellular Fas-associated death domain-like interleukin-1-beta-converting enzyme-inhibitory protein (c-FLIP), a downstream mediator of apoptosis (20, 21).
Various studies have suggested that apoptosis can play an important role in atherogenic processes and vascular remodeling. Apoptosis is regulated by different systems, one of which depends on the Fas receptor from the tumor necrosis factor (TNF) family. Fas is expressed in many tissues, including the vascular system. Activation of Fas via its ligand induces apoptosis by caspase-8 recruitment. Fas-induced induction of apoptosis may be blocked by different intracellular proteins, including c-FLIP, which competitively inhibits binding between caspase- 8 and the Fas receptor–ligand complex, blocking the subsequent caspase activation cascade. It has been shown that c-FLIP is highly expressed in vessel walls under normal conditions, conferring resistance to apoptosis on VSMC (21, 22); in contrast, attenuation of c-FLIP makes VSMC susceptible to Fas-induced apoptosis.
Most relevant for the present study, Wang and associates (20) recently demonstrated that Notch3- mediated activation of the extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) cascade was key to increased c-FLIP expression and VSMC survival. Their studies demonstrated that Notch3 signaling may be a critical determinant of VSMC survival and vascular structure, and that these effects occur via signaling cross-talk with the ERK/MAPK pathway. Overall, the expression of c-FLIP appears to be highly regulated by the ERK and phosphoinositide- 3 kinase (PI-3K) pathways (20). Because the expression level of c-FLIP is a focal point for survival of VSMC, ERK- and/or PI-3K-dependent c-FLIP regulation represents an attractive target for therapeutic intervention with death receptor signaling.
Valproate (VPA) is an anticonvulsant extensively used in the treatment of both epilepsy and mood disorders (23, 24). Although VPA acutely affects gamma-aminobutyric acid (GABA) levels, recent studies have shown that chronic VPA robustly upregulates the ERK/MAPK cascade in neuronal cells (25, 26); this raises the intriguing possibility that VPA, via its effects on the ERK/MAPK cascade, may be able to regulate Notch3/c-FLIP-mediated cytoprotection. Based on these previous findings, we hypothesized that VPA might positively regulate VSMC apoptosis and cell survival, and that this ameliorative effect might have promising clinical implications such as WMH prevention (see Fig. 1). The present study aimed to: (i) investigate VPA’s effects on VSMC apoptosis and cell survival in vitro, as well as its actions on rat brain tissues in vivo; and (ii) elucidate the underlying molecular mechanism(s) of these effects. The findings raised important clinical implications, including WMH prevention, which are also discussed.
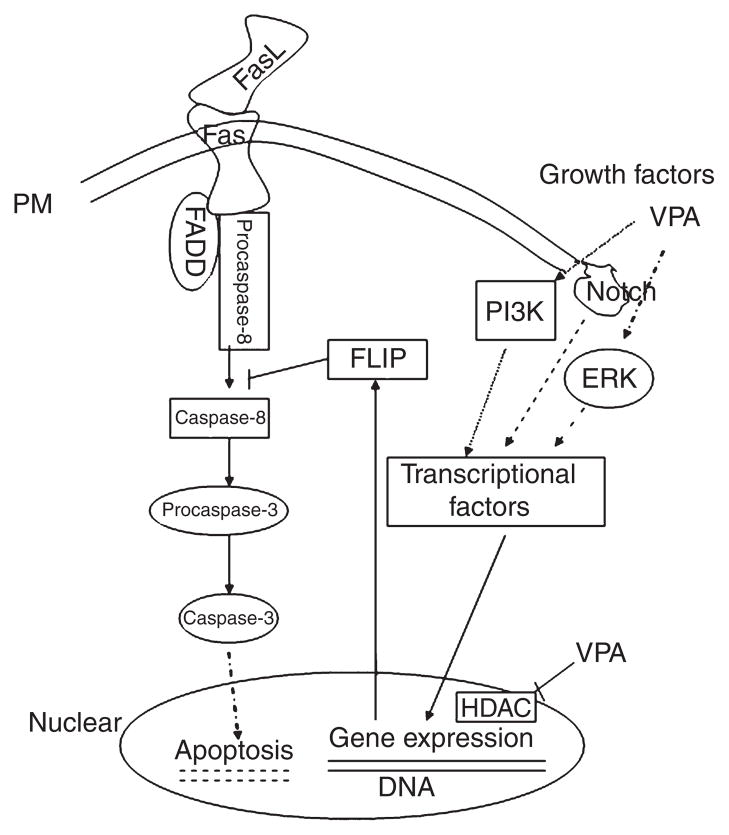
Fig. 1.
Schematic representation of valproate’s intracellular cytoprotective mechanisms. Fas ligand (FasL)/Fas interactions cause effector caspase activation. FasL-mediated apoptosis can be blocked by the inhibition of caspase-8 protease through cellular Fas-associated death domain-like interleukin- 1-beta-converting enzyme-inhibitory protein (c-FLIP). Valproate (VPA) activates mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and PI- 3K pathways, which upregulate Notch3, c-FLIP, and other proteins (Bcl-2, BDNF, etc.) via transcriptional factors. VPA also regulates gene expression by inhibiting histone deacetylase (HDAC). Hatched lines represent membrane; dotted lines represent indirect effects. FADD = Fas-associated death domain- containing protein.
Materials and methods
Cell culture and drug treatment
Human T/G HA-VSMC (CRL-1999) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were grown in DMEM/F12K media (Invitrogen, Carlsbad, CA, USA) with supplements per ATCC recommendations (15% FBS and ECGS) at 37°C with 5% CO2. The experiments were performed when the cells reached 80~90% confluence between passages 14~16. Cells were incubated in the serum-free medium 24 hours before exposure to VPA treatment, except for transfection experiments. ECGS and VPA were purchased from Sigma (St. Louis, MO, USA). Fas-ligand (FasL) and anti-Fas anti-body (clone C11) were obtained from Millipore (Billerica, MA, USA). MAPK kinase (MEK) inhibitor PD98059 and PI-3K inhibitor LY294002 were obtained from Calbiochem (Gibbstown, NJ, USA). All cell culture experiments were conducted with six wells or dishes per group, and independently replicated three times.
Small interfering ribonucleic acid (SiRNA) vector construct and transfection
c-FLIP-targeted siRNA (specific for nucleotides 472–492 and 908–928 of FLIP-L) was constructed to the pSilencer4.1-CMVneo vector (Ambion, Austin, TX, USA). Negative control siRNA, a 21-nucleotide RNA duplex with no known sequence homology, was also purchased from Ambion (Austin, TX, USA). Plasmid subcloning, amplification, and purification were done as previously described (27). Transfection was achieved by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s recommendation with minor modifications (28, 29). Briefly, the cells were seeded for transfection in medium without antibiotics 1 day before transfection so that they were ~90% confluent on the day of transfection. For transfection, regular growth medium was replaced with low serum media (1% FBS) without antibiotics. The cells were transfected with siRNA and Lipofectamine 2000 at a ratio of 1:3. The cells were incubated with the siRNA-Lipofectamine 2000 complexes overnight, and the medium was then replaced with fresh serum-free media without antibiotics and incubated for further studies.
Cell viability assay
Cell viability assay was performed in 96-well plate with Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies, Gaithersburg, MD, USA), which detects cellular dehydrogenase activity in living cells. Briefly, 10 μl CCK-8 solution was added directly to the cells and incubated at 37°C for 1 h to produce an orange formazan product that is soluble in tissue culture medium. Absorbance read-outs were measured in a 96-well plate format at 450 nm using the Wallac 1420 Victor2 Multilabel Counter (PerkinElmer, Inc., Waltham, MA, USA) to determine the cell viability in each well. The amount of formazan produced was directly proportional to the number of living cells. This assay is very stable and has little cytotoxicity, and its detection sensitivity is higher than assays using other tetrazolium salts such as MTT [3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]. Cell viability was expressed as a percentage of the value in untreated control culture (30).
Scratch wound motility assay
VSMC were plated at near confluence onto six-well plates. The following day, a uniform straight scratch was made in the monolayer using a yellow plastic pipette tip. Monolayer cells were washed gently, marked (for reference), and photographed on an inverted microscope. The cells were kept in a state of serum deprivation for the duration of the experiment, so that the results measure basal motility of the VSMC in the normally non-motile state. Calculations were based on the number of cells that moved into the wound area. Five counts were made along each wound. Cell numbers are derived from the average of these fields from triplicate samples (31, 32).
Detection of apoptosis
The Vybrant apoptosis assay Kit #7 (Invitrogen, Carlsbad, CA, USA) was used according to the manufacturer’s instructions to determine the amount of apoptosis and dead cells in VSMC cultures. YO-PRO-1 is a particularly effective method for distinguishing apoptotic cells; dead cells appear highly fluorescent, apoptotic cells appear moderately fluorescent, and live cells are dimly fluorescent or nonfluorescent. The commonly used blue fluorescent dye DAPI (4′, 6- diamidino-2-phenylindole dihydrochloride) stains the condensed chromatin of apoptotic cells more brightly than the chromatin of normal cells. To quantify apoptotic events, photographs of five random fields of each treated and stained culture were taken through a ×20 objective lens under fluorescent microscope. Apoptotic and total cell nuclei were counted, and the percentages of the apoptotic nuclei in the total nuclei in each treatment were calculated. This method of in situ DNA staining allows visualization of chromatin condensation and fragmentation as typical patterns of apoptosis (33, 34). Cells were treated with 0.5 μM staurosporine as a positive control for apoptosis.
Fluorescence-activated cell sorting (FACS) analysis of apoptosis
Cells were released from the culture dish by trypsinization after removing the culture medium, which also contained some floating cells. Floating and trypsinized cells from each treatment were collected and combined to ensure complete recovery of the cell population. After staining with fluorescent dye, 5,000 events from the gated subpopulation of each treatment were recorded separately with FACSVantage (BD Biosciences, Rockville, MD, USA). Cell Quest Acquisition and Analysis software (BD Biosciences, Rockville, MD, USA) was used to acquire and quantify the fluorescence signal distributions and intensities from individual cells (35). Data are presented as percentage of total cells.
Animals and animal treatments
All animal experiments were approved by the National Institutes of Health (NIH) Animal Care and Use Committee in accordance with NIH guidelines on the care and use of animals. Animal treatments were performed using a previously described protocol (36). In brief, male Wistar rats (150–200 gm) were housed four per cage with water and food available ad libitum and were maintained under a 12-hour light/dark cycle. After a 1-week accommodation period, the rats were fed either regular or VPA-supplemented (20 gm/kg) chow (n = 12 per group). Routine ingestion of VPA-supplemented chow yields blood levels of VPA close to or within the human therapeutic window (78.53 ± 7.15 μg/ml, equivalent to 0.54 ± 0.05 mM). Rats were decapitated between 8:00 a.m. and 12:00 noon after 4 weeks of treatment. Brain tissues were dissected on ice and frozen in dry ice immediately followed by freezer storage at −80°C. Trunk blood samples were collected to measure VPA levels as previously described (25).
Immunoblotting
Western blot was performed as previously described (25). Briefly, rat brain tissues or cultured cell samples from the various treatments described above were lysed with RIPA lysis buffer containing proteinase inhibitor and phosphatase inhibitor cocktails (Sigma, St. Louis, MO, USA). Lysates were rocked for 10 minutes and spun at 12,000 × g for 10 minutes to remove debris. All procedures were performed at 4°C or on ice. Supernatant was stored at −80°C until use. Protein concentrations were measured with the Pierce BCA kit adapted for microtiter plates (Pierce Biotechnology, Rockford, IL, USA). The same quantity of protein samples (10~40 μg) were boiled in SDS sample loading buffer, resolved by SDS-PAGE (4~20% tris-glycin gel), and blotted onto 0.2 μm pore size nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA, USA). Membranes were blocked for 1 h in TBS-T (TBS + 0.1% Tween 20) containing 5% milk, and incubated in primary antibodies (1:200~1:1000) and HRP-conjugated anti-mouse or anti-rabbit IgG (1:3000, GE Healthcare, Piscataway, NJ, USA) in TBST + 5% milk. Bands were visualized using GE Healthcare (Piscataway, NJ, USA) enhanced chemiluminescence detection reagents and autoradiography as described by the vendor. Prestained rainbow protein standard markers (GE Healthcare, Piscataway, NJ, USA) were used as molecular weight markers. Densitometry analysis of films was performed using the Kodak Image Station 440CF (Eastman Kodak Co., Rochester, NY, USA). Primary antibodies against c-FLIP, Notch3, caspase-8, and caspase-3 were purchased from Santa Cruz Biotechnology (diluted at 1:200; Santa Cruz, CA, USA); antibodies against p-ERK1/2, ERK1/2, p-GSK-3β, GSK-3, and β-actin were obtained from Cell Signaling Technology (diluted at 1:1000; Danvers, MA, USA). β-actin was used as a loading control within all of the immunoblots.
Statistical analysis
ANOVA with Fisher’s Projected Least Significant Difference test was used for multiple comparisons. Comparisons between two groups were analyzed via a Student’s t-test. A p-value < 0.05 was considered statistically significant. Data are expressed as mean ± SEM.
Results
VPA promoted human VSMC survival and motility in serum-free medium
The results of this study showed that VPA affects VSMC survival. As determined by the CCK-8 assay, VPA dose-dependently promoted the survival of the VSMC treated with FasL (25 ng/ml) in serum-free medium (Fig. 2A). The number of viable cells increased to 120~150% of the control group after VPA treatment for three days. VPA’s cytoprotective effects were evident in its therapeutically relevant concentration range of 0.25– 1.0 mM and were maximized after treatment with 0.5 mM [ANOVA: F(4,26) = 3.65, p = 0.038]. However, in cultures without FasL challenging, VPA had little effect on cell survival. Furthermore, FasL-treated groups showed much lower intracellular lactate dehydrogenase (LDH) activity compared to groups without FasL treatment, which confirmed that 25 ng/ml of FasL had significant cytotoxic in vitro effects on VSMC.
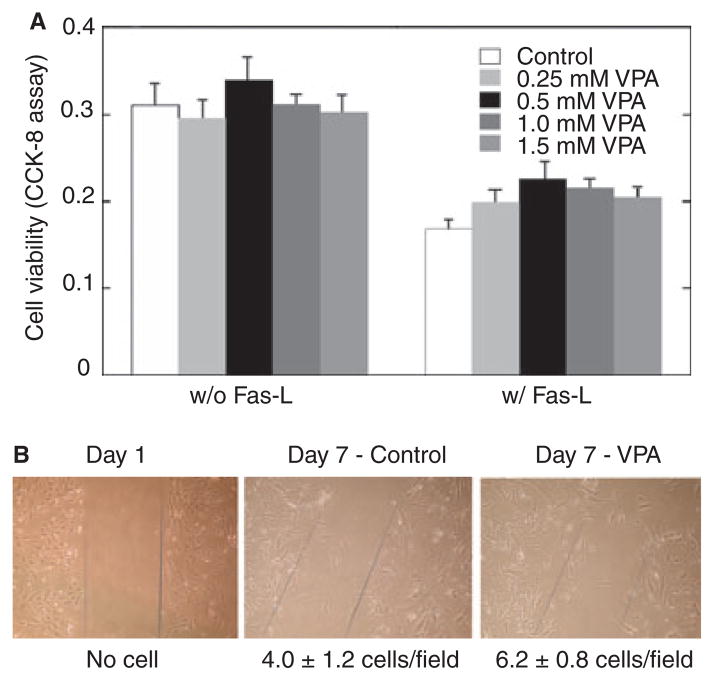
Fig. 2.
(A) Valproate (VPA) promotes vascular smooth muscle cell (VSMC) survival under Fas-ligand (FasL) challenge. Human VSMC were subcultured in 96-well plates, pretreated with (or without) VPA at the indicated concentrations in serum-free medium for two days, and then exposed to FasL (25 ng/ml) in the serum-free medium with VPA for 24 hours. Cell viability was measured with Cell Counting Kit-8 (CCK-8) as described in Materials and methods. Data represent means ± SEM, n = 6, p < 0.05.
(B) Representative photomicrographs of cells in each group show that VPA increased VSMC motility in serum-free medium. VSMC were cultured in six-well plates; the cell monolayer was disrupted and cells were treated with or without VPA (0.8 mM) in serum-free medium. Migration of cells into the wound was quantified under microscopy. The numbers below the panels represent the number of cells that migrated into the wound area. Results are representative of at least three separate experiments.
VSMC motility is a crucial event in angiogenesis and the development of atherosclerotic vascular lesions. To determine if VPA alters VSMC motility, we measured the migration of VSMC after disruption of the cell monolayer in serum-free medium. We observed that, after seven days, a few cells moved into the wound area in untreated control monolayers (4.0 ± 1.2 cells/field); however, seven days of VPA treatment significantly increased the migration of VSMC into the wound area [6.2 ± 0.8 cells/field; Student’s t-test: t(10) = 2.46, p = 0.041, Fig. 2B].
VPA inhibited caspase-8 and caspase-3 cleavage in VSMC
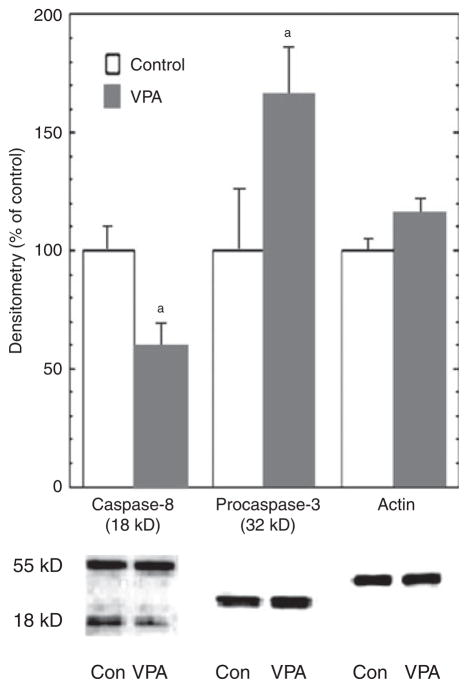
Cysteine proteases (caspases) play a major role in most forms of apoptotic cell death; notably, caspase-8 is a key enzyme in the extrinsic apoptosis pathway, and caspase-3 has been classified as a critical effector caspase (37). Our investigation of VPA’s effects on these two major caspases in FasL-treated VSMC showed that three days of treatment with VPA, at 0.8 mM (mimicking therapeutic levels), resulted in ~40% lower levels of cleaved (activated) caspase-8 [Student’s t-test: t(10) =2.57, p = 0.035], and ~70% higher levels of pro-caspase- 3 [Student’s t-test: t(10) = 3.16, p = 0.011, respectively, versus control; Fig. 3]; levels of pro-caspase-8 were not significantly different, and cleaved caspase-3 was not detectable with this caspase-3-specific antibody. These results are consistent with VPA-induced inhibition of caspase-8 and caspase-3 activation, although their activities were not measured directly in the study.
Fig. 3.
Valproate (VPA) inhibited caspase-8 and caspase-3 activation. Human vascular smooth muscle cells (VSMC) were treated with VPA (0.8 mM) in serum-free medium for three days, and Fas-ligand (25 ng/ml) was added to the medium during the last day of treatment. Immunoblot analyses were performed with anti-caspase-8, anti-caspase-3, and anti-β-actin antibodies. Pro-caspase and/or cleaved caspase bands were quantified with Kodak Image Station. VPA significantly inhibited caspase-8 and caspase-3 cleavage (n = 6). ap < 0.05.
VPA attenuated Fas-L induced apoptosis
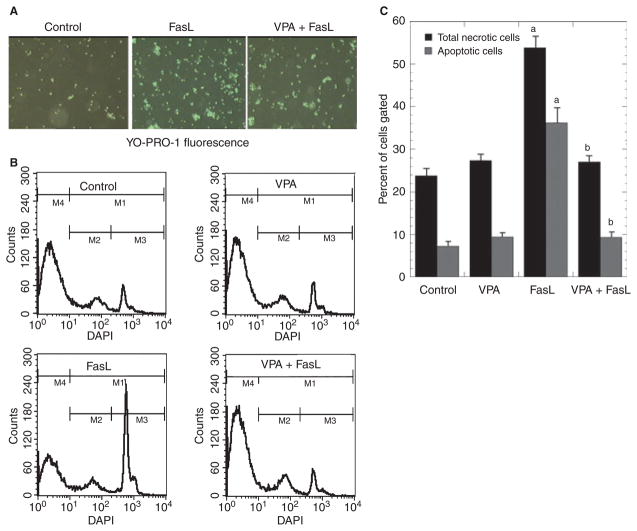
Binding of Fas ligand (FasL) to Fas (receptor; CD95) initiates a cascade of events that ultimately activate effector caspases and lead to cellular apoptosis. Apoptosis-related chromatin condensation is believed to be mediated primarily by the nuclear protein acinus (apoptotic chromatin condensation inducer in the nucleus) after its cleavage by activated caspase 3 (38). To examine the effects of VPA on FasL-induced apoptosis, cells were stained with YO-PRO-1 and visualized under an inverted fluorescent microscope (Fig. 4A). There were about 20% YO-PRO-1 positive cells (green) in control wells, ~80% bright green cells in FasL-treated wells, and ~40% positive cells in VPA + FasL treated wells. FACS analysis was used to quantify the percentage of apoptotic and dead cells in each culture condition. Figure 4B is a representative FACS histogram of three separate experiments. As shown in Figure 4C, exposure of human VSMC to 25 ng/ml FasL for 24 hours led to marked apoptosis and cell death in serum-free medium (~37% apoptotic and ~53% necrotic). In contrast, much less apoptosis and cell death were observed in VPA-pretreated (0.8 mM for 48 h) cultures [~10% apoptotic and ~28% necrotic; one-way ANOVA F(3, 16) = 9.036, p < 0.01]. Treatment of cells with the CH11 agonistic anti- Fas mAb (instead of FasL) yielded very similar results (data not shown).
Fig. 4.
Valproate (VPA) inhibits Fas-ligand (FasL)-induced vascular smooth muscle cells (VSMC) apoptosis. Human VSMC were pretreated with or without VPA (0.8 mM) for two days, and challenged with Fas-ligand (25 ng/ml) for 12 hours. (A) Representative photographs of VSMC stained with YO-PRO-1 fluoresence. (B) Fluorescence-activated cell sorting (FACS) analyses after staining with DAPI (4′,6-diamidino-2-phenylindole dihydrochloride) to detect apoptotic cells. (C) Data represent three independent experiments. FasL significantly induced apoptotic cells (ap < 0.01 versus control); pretreatment with VPA (two days) significantly blocked FasL’s proapoptotic effect in VSMC (bp < 0.01, VPA + FasL versus FasL).
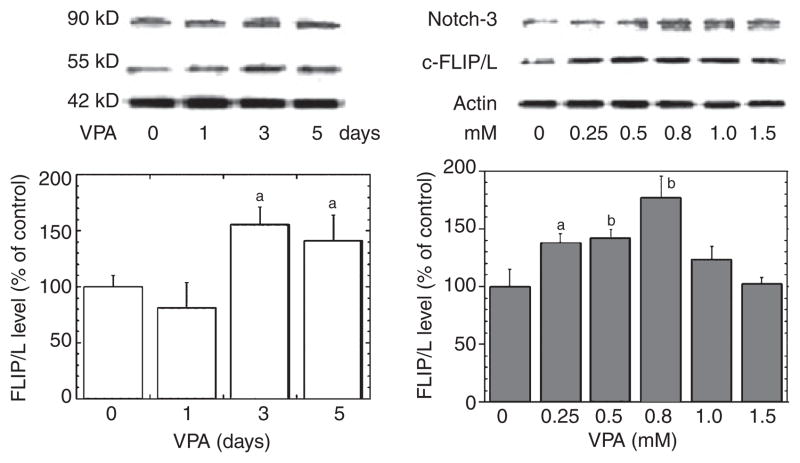
VPA time- and dose-dependently increased Notch3/c-FLIP expression in human VSMC
To further delineate the potential mechanisms by which VPA confers resistance to FasL-induced cell death, we investigated the expression level of c-FLIP, which is the primary cellular caspase-8 inhibitor and can block Fas-mediated apoptosis. Thus, upregulation of c-FLIP confers relative resistance to FasL-induced apoptosis. As shown in Figure 5, VPA-induced c-FLIP/L gene expression was both time- and dose-dependent, an effect that was maximized at 3–5 days and 0.8 mM of VPA treatment, and was accompanied by increased Notch3 levels; c-FLIP/s levels changed in a similar pattern (data not shown). Higher than therapeutically relevant concentrations of VPA were associated with relatively lower levels of c-FLIP, which might be related to VPA’s cytotoxic effect.
Fig. 5.
Valproate (VPA) increases Notch3 and cellular Fas-associated death domain-like interleukin-1-beta-converting enzyme-inhibitory protein (c-FLIP) levels dose- and time-dependently in vascular smooth muscle cells (VSMC). Human VSMC were cultured in growth media close to confluence. The media were then replaced with serum-free media, and the cells were treated with or without VPA at concentrations for the time period as indicated (same endpoint). Immunoblotting of Notch3, c-FLIP, or β-actin was conducted. VPA concentration (0~1.5 mM for 48 h) and time (1, 3, or 5 days at 0.8 mM) dependently increased Notch3 and c-FLIP levels. Bar graph densitometric results representing mean ± SEM of three or more sets of samples immunoblotted in duplicate. ap < 0.05; bp < 0.01 compared with control cells.
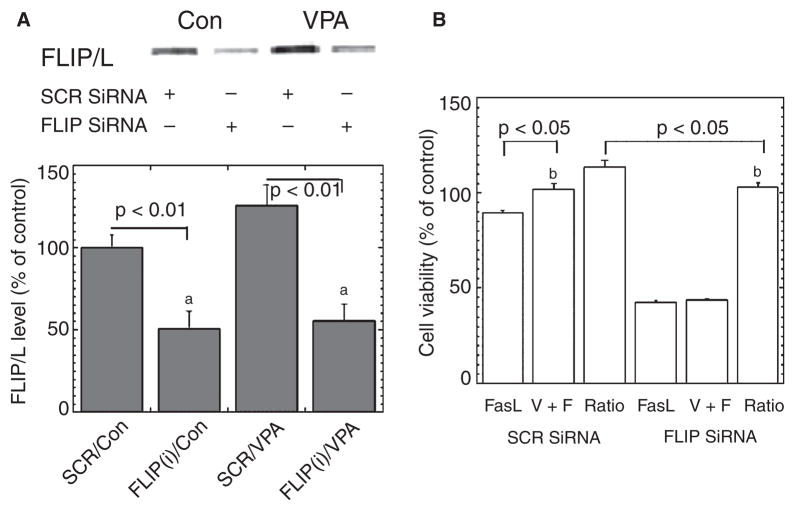
SiRNA-induced downregulation of c-FLIP attenuates VPA’s anti-apoptotic effects in VSMC
To ascertain whether c-FLIP plays a causal role in VPA’s ability to attenuate FasL-induced apoptosis of VSMC, a siRNA strategy was used. Of several siRNAs tested, FLIP-siRNA, a c-FLIP-targeted siRNA, potently downregulated expression of both c-FLIP splice variants: c-FLIP/s and c-FLIP/L. c-FLIP levels were markedly reduced (~50% of control) by FLIP-siRNA transfection, and VPA-induced c-FLIP gene expression was completely blocked by FLIP-siRNA (Fig. 6A). Notably, knockdown of c-FLIP by c-FLIP siRNA attenuated VPA’s cytoprotective effects against FasL-induced decreases in cell viability, as measured with CCK-8 assay (Fig. 6B). Collectively, these results indicate that downregulation of c-FLIP dramatically sensitizes VSMC cells to FasL-induced apoptosis, and that c-FLIP plays a critical role in VPA’s cytoprotective effects.
Fig. 6.
Cellular Fas-associated death domain-like interleukin-1-beta-converting enzyme-inhibitory protein (c-FLIP) small interfering ribonucleic acid (siRNA) abolishes valproate’s (VPA) cytoprotective effects. (A) Transfection with c-FLIP siRNA plasmid knocked down the expression of c-FLIP/L in human vascular smooth muscle cells. The single blot is representative of three separate experiments. ap < 0.01. (B) Cell viability determined with Cell Counting Kit-8 showed that c-FLIP siRNA transfection suppressed cell viability and partially eliminated VPA’s cytoprotective effect. Data are mean ± SEM and represent three separate experiments, bp < 0.05. Con = control medium without VPA; SCR = scramble siRNA plasmid, which served as negative control.
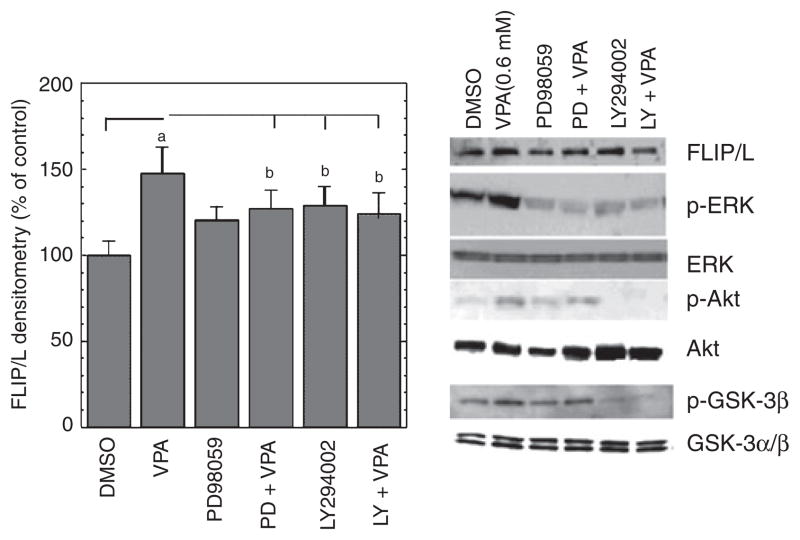
ERK and PI-3K/Akt pathways are involved in VPA-induced c-FLIP upregulation
Previous studies have suggested that VPA is capable of activating both the ERK and PI- 3K/Akt cascades (25, 26). We therefore next determined if these pathways play a role in VPA-induced c-FLIP upregulation. We quantitated both the total and phosphorylated forms of 42/44 ERK (which provides a readout for ERK activation). We also quantitated both the total and phosphorylated forms of GSK-3β (at Ser-9), which is a downstream target for Akt and is postulated to be partially responsible for Akt’s cytoprotective effects (39). VPA markedly increased levels of phospho-ERK1/2 (~twofold) and phospho-GSK-3β (~1.5-fold) in VSMC, indicating that VPA robustly activated both the ERK and P-I3K/Akt cascades (Fig. 7); in contrast, VPA did not affect total protein levels of ERK 1/2 or GSK-3β.
Fig. 7.
Valproate (VPA) activates extracellular signal-regulated kinase (ERK) and phosphoinositide-3 kinase (PI-3K) signal pathways in human vascular smooth muscle cells (VSMC), and specific inhibitors of MEK and PI-3K abolish VPA’s upregulation of c-FLIP. Human VSMC were cultured in growth media close to confluence. The media were then replaced with serum-free media, and the cells were treated with VPA (0.8 mM) in the absence or presence of indicated inhibitors for two days. Immunoblotting was conducted as described in Materials and Methods. MEK inhibitor PD98059 (50 μM) and PI-3K inhibitor LY294002 (20 μM) attenuated VPA-induced increases in c-FLIP/L, phospho-ERK1/2 (P-p44/42), phospho-Akt, and phospho-GSK-3β, ap < 0.01, bp < 0.05, compared with cells treated with dimethy sulfoxide (DMSO, final concentration 0.1%) alone. Total ERK1/2 (p44/42), Akt, and GSK-3 α/β levels did not change significantly.
Having established that VPA activates both the ERK and PI-3K/Akt cascades, we next sought to investigate their involvement in VPA’s effects on c-FLIP gene expression in VSMC. 50 μM PD98059 (a specific inhibitor of MEK) or 20 μM LY294002 (a specific inhibitor of PI-3K) had little effect on basal c-FLIP levels. VPA-induced increases in c-FLIP levels were partially attenuated (~25%) by pretreatment with PD98059 or LY294002 (Fig. 7). These results indicate that VPA upregulates c-FLIP gene expression at least partially through both the MAPK/ERK and PI-3K/Akt pathways.
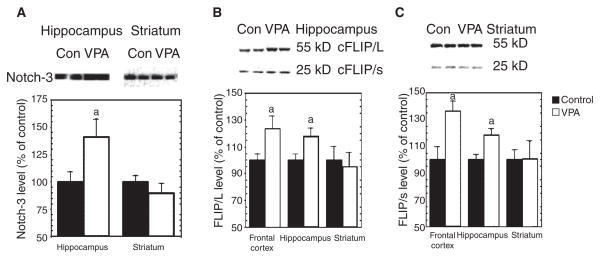
Chronic in vivo treatment of rats with VPA increases cleaved Notch3 and c-FLIP levels in the frontal cortex and hippocampus
There is increasing evidence that aberrant Notch signaling plays a role in the pathogenesis of a number of neurological disorders (40), and this evidence suggests that Notch may itself be a potential therapeutic target. Thus, we treated rats chronically with VPA to confirm that the effects of VPA on the Notch3/c-FLIP cascade were not simply isolated in vitro effects, but also occurred in the brain in vivo; in particular, we examined the in vivo effects of VPA on Notch3/c-FLIP pathway in rat brain regions related to mood disorders. Chronic treatment of rats with VPA chow (serum VPA concentration 78.53 ± 7.15 μg/ml) resulted in ~40% higher cleaved Notch3 in the hippocampus (Fig. 8A). c-FLIP/L and c-FLIP/s levels were also increased in the frontal cortex and hippocampus (Fig. 8B), although no differences were observed in the striatum. These results indicate that VPA does, indeed activate the Notch3 signaling pathway and upregulates c-FLIP expression in rat brain in vivo. Due to the technical limitations associated with dissecting white matter, it was not evaluated in this study. Further immunohistochemical studies are warranted.
Fig. 8.
Valproate (VPA) activates Notch3 signaling in vivo. Adult male Wistar rats received VPA chow for four weeks. Protein extracts from frontal cortex, hippocampus, or striatum were separated on SDS-PAGE, and then immunoblotted with anti-Notch3 or anti-cellular Fas-associated death domain-like interleukin-1-beta-converting enzyme-inhibitory protein (c-FLIP) antibody. The resultant bands were quantified and standardized by bands of β-actin. (A) VPA significantly increased cleaved Notch3 protein levels in hippocampus [t(22) = 2.51, p = 0.02], but not striatum. (B) VPA significantly increased c-FLIP/L and c-FLIP/s levels in rat frontal cortex and hippocampus (ap < 0.05 versus control), but not in striatum. (C) Representative blot with frontal cortex samples was similar to that of hippocampal samples.
Discussion
This study demonstrates that VPA, an anticonvulsant and mood stabilizer safely used in humans for decades, activates the Notch3/c-FLIP cascade, conferring cytoprotective effects on VSMC. It is not our contention that CADASIL and BPD are equivalent illnesses; however, understanding the cellular mechanisms by which WMHs develop in this model offers the possibility to investigate therapeutic strategies to attenuate their development and/or progression.
Notably, the effects of Notch3/c-FLIP on VSMC survival and vascular structure have been shown to occur via the ERK/MAPK pathway (20). Because VPA has previously been demonstrated to activate the ERK/MAPK pathway (26), we undertook the present study to determine VPA’s effects on the Notch3/c-FLIP cascade, and thus on its ability to regulate VSMC survival and vascular structure. In this study, we observed that long-term treatment with VPA upregulates Notch3 and c-FLIP expression in two rat brain regions: the frontal cortex and the hippocampus. These changes might be restricted to cerebral VSMC and/or progenitor cells. This finding suggests that VPA might be beneficial in the treatment of some neurological, immunological, or cardiovascular disorders, including CADASIL, because c-FLIP has been shown to be dysregulated in these disorders (41–43).
A genetic study that investigated Notch3 as a potential candidate gene for BPD found no significant association between Notch3 and BPD in subjects from a family with high genetic loading (6). Because CADASIL is a much more classic degenerative disorder, it would not be altogether surprising if patients with BPD might present more subtle downstream abnormalities in the Notch3 signaling pathway compared to CADASIL syndrome. Another intriguing possibility is that DWMHs in late-onset BPD might have a different etiology from DWMHs that arise very early in the course of the illness. For instance, pediatric patients with nonfamilial BPD might develop DWMHs through environmental mechanisms, such as prenatal or perinatal trauma, perhaps leading to hypoxia and ischemic events in deep white matter. In contrast, mutations in Notch3 might be responsible for the development of DWMHs only in subjects with late-onset mood disorders (44). In support of this hypothesis, De Asis and colleagues (45) found that DWMHs were more prevalent in geriatric patients with BPD than in a group of healthy controls, and that DWMHs were associated with later age of onset of mania. Furthermore, recent studies have demonstrated that DWMHs are increased in late-life depression compared to the general population, even when controlling for potential risk factors such as hypertension and vascular disease (46), suggesting that more subtle ischemic processes might be responsible for these lesions (3).
This study also demonstrated that VPA does indeed promote cell survival and resistance to FasL-induced apoptosis in VSMC. Increasing evidence suggests that Fas-mediated death plays a critical role in VSMC biology and pathobiology (47, 48). The phenotype of Fas resistance in VSMC may result from reduced expression of proapoptotic proteins involved in Fas signaling, including FasL, Fas receptor, and caspase-8, and increased expression of antiapoptotic proteins such as c-FLIP and Bcl-2 (21). Our results indicate that VPA upregulated c-FLIP expression time- and dose-dependently, and inhibited caspase-8 and caspase-3 activation in VSMC. Furthermore, specific knockdown of c-FLIP with siRNA abolished VPA’s cytoprotective effect, indicating that c-FLIP plays a crucial role in the prevention of extrinsic apoptosis by VPA.
Notably, our results demonstrate that both the ERK/MAPK and PI-3K/Akt pathways are involved in VPA’s upregulation of c-FLIP expression in human VSMC. It has previously been reported that chronic VPA treatment activates the ERK pathway in other cell types (26, 49). Here we demonstrate that a similar activation of the ERK (and indeed PI-3K/Akt) occurs in VSMC, and that this results in activation of Notch3/c-FLIP. VPA has multiple effects on diverse systems, and the targets responsible for these effects have not been fully defined. For instance, VPA is a direct inhibitor of several molecular targets, including histone deacetylase (HDAC), GABA transaminase, and succinate semialdehyde dehydrogenase; VPA can also indirectly affect the function of a number of molecules, including activator protein 1 (29), protein kinase C, MAPK/ERK (26), peroxisome proliferator-activated receptor (50), and GSK-3/beta-catenin (51).
HDAC and histone acetyltransferase can modify the structure and function of histones and proteins in transcription factor complexes, which are involved in the regulation of gene expression, as well as many nonhistone proteins involved in regulating cell proliferation and cell death. HDAC inhibitors selectively alter the expression of genes. It has been suggested that VPA’s teratogenic properties are due to HDAC inhibition, although its anticonvulsant properties are probably not. HDAC inhibitors can induce cancer cell death, whereas normal cells are relatively resistant to HDAC inhibitor-induced cell death (52, 53). The current study showed that therapeutically relevant levels of VPA promote VSMC survival against extrinsic challenge through Notch3/c-FLIP upregulation, while higher concentrations of VPA have the opposite effect. The inhibitory effect of VPA on HDAC might result in altered chromatin to a more open conformation (54), which might then favor Notch3/c-FLIP transcriptional initiation. This possibility needs to be further investigated in VSMC. Our in vivo study confirmed that the effect of chronic VPA treatment on Notch-3/c-FLIP gene expression occurred in the frontal cortex and hippocampus, rat brain regions related to mood modulation.
Similar alterations could also be expected to occur in brain white matter, because blood vessels are distributed throughout the brain as a vascular tree. White matter is composed mostly of fatty substance (myelin) surrounding the axon fibers, and the microvasculature density is lower in white matter than in gray matter. White matter was not examined directly in this study because of the technical challenge posed by the precise dissection of rat white matter, and the subsequent preparation of immunoblot proteins. However, further immunohistochemical studies are warranted.
Besides c-FLIP, there are several other down-stream targets of Notch3 signaling, including the RBP-Jk-HRT/Hes pathway (20). Additional studies are needed to better define the functional role of Notch receptor signaling and the gene activity of other downstream effectors in VSMC. Strategies to study Notch3 function include blocking the cleavage of Notch3 intracellular domain, inhibiting the transcriptional activity of this domain, siRNA knockdown, transfection overexpression, and mutation. To establish a clear cause-and-effect relationship, it would be interesting to compare VSMC from Notch3-knockout mice with those of wild-type mice after challenge.
In addition, it is significant that mutations in the Notch3 pathway are also associated with arteriopathy in humans that predisposes to early-onset stroke. We speculate that changes in the expression and activity of the Notch signaling pathway may also contribute to the pathogenesis of acquired arteriopathies. We anticipate that further investigation of the regulation of the Notch signaling pathway in VSMC will provide new insights into the molecular mechanisms of vascular complications.
From a clinical perspective, several studies have demonstrated that patients with late-onset BPD likely represent a distinct group from patients with classic BPD—whose onset is typically in late adolescence—both in terms of clinical characteristics and comorbidities (55, 56). Notably, the presence of cerebrovascular risk factors is increased in patients with late-onset BPD (56). How these risk factors are linked with the development of DWMHs is still poorly understood. A major limitation of the research in this area is the lack of adequately conducted longitudinal and postmortem studies. It is also poorly understood whether these lesions progress over time, remain stable, or diminish in size. Despite these limitations, there is evidence that DWMHs are correlated with poorer prognosis and worse psychosocial outcome, both in late-onset major depressive disorder and BPD (57, 58).
This study has several implications for future research, because the prevention of DWMHs via drugs with cytoprotective effects is undoubtedly an important goal. Our results suggest that VPA might prevent the development of these white matter abnormalities through the activation of the Notch3 signaling pathway and c-FLIP expression. However, this hypothesis needs to be verified in longitudinal studies with patients at risk for late-onset mood disorders; also, the presence of risk factors for DWMHs, such as hypertension and substance abuse (59), needs rigorous controlling.
Taken together, these results suggest a number of intriguing possibilities. VPA—a medication that has been used safely for decades—appears able to target some of the core pathophysiological processes related to the development of WMHs. Thus, VPA may be useful in the treatment of CADASIL and other similar neurological disorders associated with ischemia-induced hyperintensities. The data also suggest that VPA might be particularly helpful in these populations through its cytoprotective vascular effects, and might reduce disability and chronicity and ameliorate prognosis by decreasing the prevalence of DWMHs. Because VPA crosses the blood-brain barrier and has a well-established safety profile, clinical and longitudinal studies are clearly warranted.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Mental Health (NIMH). The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this manuscript. HKM is now at Johnson and Johnson Pharmaceutical Research and Development.
The authors would like to acknowledge the invaluable editorial assistance of Ioline Henter.
References
- 1.Videbech P. MRI findings in patients with affective disorder: a meta-analysis. Acta Psychiatr Scand. 1997;96:157–168. doi: 10.1111/j.1600-0447.1997.tb10146.x. [DOI] [PubMed] [Google Scholar]
- 2.Altshuler LL, Curran JG, Hauser P, Mintz J, Denico K, Post R. T-2 hyperintensities in bipolar disorder – magnetic-resonance- imaging comparison and literature metaanalysis. Am J Psychiatry. 1995;152:1139–1144. doi: 10.1176/ajp.152.8.1139. [DOI] [PubMed] [Google Scholar]
- 3.Thomas AJ, Perry R, Kalaria RN, Oakley A, McMeekin W, O’Brien JT. Pathologies and pathological mechanisms for white matter hyperintensities in depression. Ann N Y Acad Sci. 2002;977:333–339. doi: 10.1111/j.1749-6632.2002.tb04835.x. [DOI] [PubMed] [Google Scholar]
- 4.Thomas AJ, O’Brien JT, Davis S, et al. Ischemic basis for deep white matter hyperintensities in major depression – a neuropathological study. Arch Gen Psychiatry. 2002;59:785–792. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]
- 5.Ruchoux MM, Guerouaou D, Vandenhaute B, Pruvo JP, Vermersch P, Leys D. Systemic vascular smooth muscle cell impairment in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Acta Neuropathol (Berl) 1995;89:500–512. doi: 10.1007/BF00571504. [DOI] [PubMed] [Google Scholar]
- 6.Ahearn EP, Speer MC, Chen YT, et al. Investigation of Notch3 as a candidate gene for bipolar disorder using brain hyperintensities as an endophenotype. Am J Med Genet. 2002;114:652–658. doi: 10.1002/ajmg.10512. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann M, Ebke M, Yuan Y, Bruck W, Mugler M, Schwendemann G. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL): a morphological study of a German family. Acta Neuropathol (Berl) 1996;92:341–350. doi: 10.1007/s004010050528. [DOI] [PubMed] [Google Scholar]
- 8.Chabriat H, Vahedi K, Ibazizen MT, et al. Clinical spectrum of cadasil – a study of 7 families. Lancet. 1995;346:934–939. doi: 10.1016/s0140-6736(95)91557-5. [DOI] [PubMed] [Google Scholar]
- 9.Singhal S, Rich P, Markus HS. The spatial distribution of MR Imaging abnormalities in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy and their relationship to age and clinical features. Am J Neuroradiol. 2005;26:2481–2487. [PMC free article] [PubMed] [Google Scholar]
- 10.Joutel A, Corpechot C, Ducros A, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 11.Kalimo H, Ruchoux MM, Viitanen M, Kalaria RN. CADASIL: a common form of hereditary arteriopathy causing brain infarcts and dementia. Brain Pathol. 2002;12:371–384. doi: 10.1111/j.1750-3639.2002.tb00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianchi S, Dotti MT, Federico A. Physiology and pathology of notch signalling system. J Cell Physiol. 2006;207:300–308. doi: 10.1002/jcp.20542. [DOI] [PubMed] [Google Scholar]
- 13.Lasky JL, Wu H. Notch signaling, brain development, and human disease. Pediatr Res. 2005;57:104R–109R. doi: 10.1203/01.PDR.0000159632.70510.3D. [DOI] [PubMed] [Google Scholar]
- 14.Lindner V, Booth C, Prudovsky I, Small D, Maciag T, Liaw L. Members of the Jagged/Notch gene families are expressed in injured arteries and regulate cell phenotype via alterations in cell matrix and cell-cell interaction. Am J Pathol. 2001;159:875–883. doi: 10.1016/S0002-9440(10)61763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breeze JL, Hesdorffer DC, Hong X, Frazier JA, Renshaw PF. Clinical significance of brain white matter hyperintensities in young adults with psychiatric illness. Harv Rev Psychiatry. 2003;11:269–283. [PubMed] [Google Scholar]
- 16.Morrow D, Scheller A, Birney YA, et al. Notch-mediated CBF-1/RBP-J{kappa}-dependent regulation of human vascular smooth muscle cell phenotype in vitro. Am J Physiol Cell Physiol. 2005;289:C1188–C1196. doi: 10.1152/ajpcell.00198.2005. [DOI] [PubMed] [Google Scholar]
- 17.Ruchoux MM, Domenga V, Brulin P, et al. Transgenic mice expressing mutant Notch3 develop vascular alterations characteristic of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Am J Pathol. 2003;162:329–342. doi: 10.1016/S0002-9440(10)63824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domenga V, Fardoux P, Lacombe P, et al. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev. 2004;18:2730–2735. doi: 10.1101/gad.308904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campos AH, Wang W, Pollman MJ, Gibbons GH. Determinants of Notch-3 receptor expression and signaling in vascular smooth muscle cells: implications in cell-cycle regulation. Circ Res. 2002;91:999–1006. doi: 10.1161/01.res.0000044944.99984.25. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Prince CZ, Mou Y, Pollman MJ. Notch3 signaling in vascular smooth muscle cells induces c-FLIP expression via ERK/MAPK activation. Resistance to Fas ligand-induced apoptosis. J Biol Chem. 2002;277:21723–21729. doi: 10.1074/jbc.M202224200. [DOI] [PubMed] [Google Scholar]
- 21.Imanishi T, Hano T, Nishio I, Liles WC, Schwartz SM, Han DK. Transition of apoptotic resistant vascular smooth muscle cells to troptotic sensitive state is correlated with downregulation of c-FLIP. J Vasc Res. 2000;37:523–531. doi: 10.1159/000054085. [DOI] [PubMed] [Google Scholar]
- 22.Kataoka T, Schroter M, Hahne M, et al. FLIP prevents apoptosis induced by death receptors but not by perforin/granzyme B, chemotherapeutic drugs, and gamma irradiation. J Immunol. 1998;161:3936–3942. [PubMed] [Google Scholar]
- 23.Macritchie K, Geddes JR, Scott J, Haslam D, de Lima M, Goodwin G. Valproate for acute mood episodes in bipolar disorder. Cochrane Database Syst Rev. 2003:CD004052. doi: 10.1002/14651858.CD004052. [DOI] [PubMed] [Google Scholar]
- 24.Gidal BE, Pitterle ME, Spencer NW, Maly MM. Relationship between valproic acid dosage, plasma concentration and clearance in adult monotherapy patients with epilepsy. J Clin Pharm Ther. 1995;20:215–219. doi: 10.1111/j.1365-2710.1995.tb00651.x. [DOI] [PubMed] [Google Scholar]
- 25.Hao Y, Creson T, Zhang L, et al. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci. 2004;24:6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan PX, Huang LD, Jiang YM, Gutkind JS, Manji HK, Chen G. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J Biol Chem. 2001;276:31674–31683. doi: 10.1074/jbc.M104309200. [DOI] [PubMed] [Google Scholar]
- 27.Chen G, Yuan PX, Jiang YM, Huang LD, Manji HK. Valproate robustly enhances AP-1 mediated gene expression. Brain Res Mol Brain Res. 1999;64:52–58. doi: 10.1016/s0169-328x(98)00303-9. [DOI] [PubMed] [Google Scholar]
- 28.Chawla-Sarkar M, Bae SI, Reu FJ, Jacobs BS, Lindner DJ, Borden EC. Downregulation of Bcl-2, FLIP or IAPs (XIAP and survivin) by siRNAs sensitizes resistant melanoma cells to Apo2L/TRAIL-induced apoptosis. Cell Death Differ. 2004;11:915–923. doi: 10.1038/sj.cdd.4401416. [DOI] [PubMed] [Google Scholar]
- 29.Sharp DA, Lawrence DA, Ashkenazi A. Selective knock-down of the long variant of cellular FLICE inhibitory protein augments death receptor-mediated caspase-8 activation and apoptosis. J Biol Chem. 2005;280:19401–19419. doi: 10.1074/jbc.M413962200. [DOI] [PubMed] [Google Scholar]
- 30.Bae SN, Kim J, Lee YS, Kim JD, Kim MY, Park LO. Cytotoxic effect of zinc-citrate compound on choriocarcinoma cell lines. Placenta. 2007;28:22–30. doi: 10.1016/j.placenta.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Sung HJ, Eskin SG, Sakurai Y, Yee A, Kataoka N, McIntire LV. Oxidative stress produced with cell migration increases synthetic phenotype of vascular smooth muscle cells. Ann Biomed Eng. 2005;33:1546–1554. doi: 10.1007/s10439-005-7545-2. [DOI] [PubMed] [Google Scholar]
- 32.Zargham R, Thibault G. alpha8beta1 Integrin expression in the rat carotid artery: involvement in smooth muscle cell migration and neointima formation. Cardiovasc Res. 2005;65:813–822. doi: 10.1016/j.cardiores.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Blank M, Lerenthal Y, Mittelman L, Shiloh Y. Condensin I recruitment and uneven chromatin condensation precede mitotic cell death in response to DNA damage. J Cell Biol. 2006;174:195–206. doi: 10.1083/jcb.200604022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ekshyyan O, Aw TY. Decreased susceptibility of differentiated PC12 cells to oxidative challenge: relationship to cellular redox and expression of apoptotic protease activator factor-1. Cell Death Differ. 2005;12:1066–1077. doi: 10.1038/sj.cdd.4401650. [DOI] [PubMed] [Google Scholar]
- 35.Hillion JA, Takahashi K, Maric D, Ruetzler C, Barker JL, Hallenbeck JM. Development of an ischemic tolerance model in a PC12 cell line. J Cereb Blood Flow Metab. 2005;25:154–162. doi: 10.1038/sj.jcbfm.9600003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Einat H, Yuan P, Gould TD, et al. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci. 2003;23:7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aron JL, Parthun MR, Marcucci G, et al. Depsipeptide ( FR901228) induces histone acetylation and inhibition of histone deacetylase in chronic lymphocytic leukemia cells concurrent with activation of caspase 8-mediated apoptosis and down-regulation of c-FLIP protein. Blood. 2003;102:652–658. doi: 10.1182/blood-2002-12-3794. [DOI] [PubMed] [Google Scholar]
- 38.Sahara S, Aoto M, Eguchi Y, Imamoto N, Yoneda Y, Tsujimoto Y. Acinus is a caspase-3-activated protein required for apoptotic chromatin condensation. Nature. 1999;401:168–173. doi: 10.1038/43678. [DOI] [PubMed] [Google Scholar]
- 39.Koh SH, Kim SH, Kwon H, et al. Phosphatidylinositol-3 kinase/Akt and GSK-3 mediated cytoprotective effect of epigallocatechin gallate on oxidative stress-injured neuronal- differentiated N18D3 cells. Neurotoxicology. 2004;25:793–802. doi: 10.1016/j.neuro.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Joutel A, Tournier-Lasserve E. Notch signalling pathway and human diseases. Semin Cell Dev Biol. 1998;9:619–625. doi: 10.1006/scdb.1998.0261. [DOI] [PubMed] [Google Scholar]
- 41.Semra YK, Seidi OA, Sharief MK. Overexpression of the apoptosis inhibitor FLIP in T cells correlates with disease activity in multiple sclerosis. J Neuroimmunol. 2001;113:268–274. doi: 10.1016/s0165-5728(00)00443-4. [DOI] [PubMed] [Google Scholar]
- 42.Micheau O. Cellular FLICE-inhibitory protein: an attractive therapeutic target? Expert Opin Ther Targets. 2003;7:559–573. doi: 10.1517/14728222.7.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomes AC, Jonsson G, Mjornheim S, Olsson T, Hillert J, Grandien A. Upregulation of the apoptosis regulators cFLIP, CD95 and CD95 ligand in peripheral blood mononuclear cells in relapsing-remitting multiple sclerosis. J Neuroimmunol. 2003;135:126–134. doi: 10.1016/s0165-5728(02)00437-x. [DOI] [PubMed] [Google Scholar]
- 44.Chang K, Barnea-Goraly N, Karchemskiy A, et al. Cortical magnetic resonance imaging findings in familial pediatric bipolar disorder. Biol Psychiatry. 2005;58:197–203. doi: 10.1016/j.biopsych.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 45.de Asis JM, Greenwald BS, Alexopoulos GS, et al. Frontal signal hyperintensities in mania in old age. Am J Geriatr Psychiatry. 2006;14:598–604. doi: 10.1097/01.JGP.0000200603.70504.d5. [DOI] [PubMed] [Google Scholar]
- 46.Lenze E, Cross D, McKeel D, Neuman RJ, Sheline YI. White matter hyperintensities and gray matter lesions in physically healthy depressed subjects. Am J Psychiatry. 1999;156:1602–1607. doi: 10.1176/ajp.156.10.1602. [DOI] [PubMed] [Google Scholar]
- 47.Imanishi T, Hano T, Nishio I, Han DK, Schwartz SM, Karsan A. Apoptosis of vascular smooth muscle cells is induced by Fas ligand derived from endothelial cells. Jpn Circ J. 2001;65:556–560. doi: 10.1253/jcj.65.556. [DOI] [PubMed] [Google Scholar]
- 48.Apenberg S, Freyberg MA, Friedl P. Shear stress induces apoptosis in vascular smooth muscle cells via an autocrine Fas/FasL pathway. Biochem Biophys Res Commun. 2003;310:355–359. doi: 10.1016/j.bbrc.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 49.Michaelis M, Suhan T, Michaelis UR, et al. Valproic acid induces extracellular signal-regulated kinase 1/2 activation and inhibits apoptosis in endothelial cells. Cell Death Differ. 2006;13:446–453. doi: 10.1038/sj.cdd.4401759. [DOI] [PubMed] [Google Scholar]
- 50.Lagace DC, Nachtigal MW. Inhibition of histone deacetylase activity by valproic acid blocks adipogenesis. J Biol Chem. 2004;279:18851–18860. doi: 10.1074/jbc.M312795200. [DOI] [PubMed] [Google Scholar]
- 51.Jin N, Kovacs AD, Sui Z, Dewhurst S, Maggirwar SB. Opposite effects of lithium and valproic acid on trophic factor deprivation-induced glycogen synthase kinase-3 activation, c-Jun expression and neuronal cell death. Neuropharmacol. 2005;48:576–583. doi: 10.1016/j.neuropharm.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Gurvich N, Tsygankova OM, Meinkoth JL, Klein PS. Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res. 2004;64:1079–1086. doi: 10.1158/0008-5472.can-03-0799. [DOI] [PubMed] [Google Scholar]
- 53.Stockhausen MT, Sjolund J, Manetopoulos C, Axelson H. Effects of the histone deacetylase inhibitor valproic acid on Notch signalling in human neuroblastoma cells. Br J Cancer. 2005;92:751–759. doi: 10.1038/sj.bjc.6602309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sinn DI, Kim SJ, Chu K, et al. Valproic acid-mediated neuroprotection in intracerebral hemorrhage via histone deacetylase inhibition and transcriptional activation. Neurobiol Dis. 2007;26:464–472. doi: 10.1016/j.nbd.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Depp CA, Jeste DV. Bipolar disorder in older adults: a critical review. Bipolar Disord. 2004;6:343–367. doi: 10.1111/j.1399-5618.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 56.Wylie ME, Mulsant BH, Pollock BG, et al. Age at onset in geriatric bipolar disorder. Effects on clinical presentation and treatment outcomes in an inpatient sample. Am J Geriatr Psychiatry. 1999;7:77–83. [PubMed] [Google Scholar]
- 57.Moore PB, Shepherd DJ, Eccleston D, et al. Cerebral white matter lesions in bipolar affective disorder: relationship to outcome. Br J Psychiatry. 2001;178:172–176. doi: 10.1192/bjp.178.2.172. [DOI] [PubMed] [Google Scholar]
- 58.Taylor WD, Steffens DC, MacFall JR, et al. White matter hyperintensity progression and late-life depression outcomes. Arch Gen Psychiatry. 2003;60:1090–1096. doi: 10.1001/archpsyc.60.11.1090. [DOI] [PubMed] [Google Scholar]
- 59.Bae SC, Lyoo IK, Sung YH, et al. Increased white matter hyperintensities in male methamphetamine abusers. Drug Alcohol Depend. 2006;81:83–88. doi: 10.1016/j.drugalcdep.2005.05.016. [DOI] [PubMed] [Google Scholar]