Abstract
Recent investigations have highlighted the importance of subcellular localization of mRNAs to cell function. While AKAP350A, a multifunctional scaffolding protein, localizes to the Golgi apparatus and centrosomes, we have now identified a cytosolic pool of AKAP350A. Analysis of AKAP350A scaffolded complexes revealed two novel interacting proteins, CCAR1 and caprin-1. CCAR1, caprin-1 and AKAP350A along with G3BP, a stress granule marker, relocate to RNA stress granules after arsenite treatment. Stress also caused loss of AKAP350 from the Golgi and fragmentation of the Golgi apparatus. Disruption of microtubules with nocodazole altered stress granule formation and changed their morphology by preventing fusion of stress granules. In the presence of nocodazole, arsenite induced smaller granules with the vast majority of AKAP350A and CCAR1 separated from G3BP-containing granules. Similar to nocodazole treatment, reduction of AKAP350A or CCAR1 expression also altered the size and number of G3BP-containing stress granules induced by arsenite treatment. A limited set of 69 mRNA transcripts was immunisolated with AKAP350A even in the absence of stress, suggesting the association of AKAP350A with mRNA transcripts. These results provide the first evidence for the microtubule dependent association of AKAP350A and CCAR1 with RNA stress granules.
Introduction
The generation of signals and regulation of specific responses are often determined by sequestration of both signal production and signal response to specific subcellular compartments. These mechanisms are well-established for scaffolded multi-protein complexes specific to membrane organelles or cytoskeletal elements. Nevertheless, over the past several years, a number of investigations have increasingly emphasized the importance of mRNA localization to cellular function. Thus, segregation of Oskar mRNA in embryos is critical for asymmetric division [1]. The importance of the transport of mRNAs into dendrites is now well-established in neurons, where segregation of particular mRNAs in dendrites and axons in part establishes the polarity of neurons [2]. The importance of the processes regulating mRNA localization is highlighted by mutations in a specific RNA binding protein in the Fragile X Syndrome, which leads to a number of neurological sequelae [3].
Reflective of transport of mRNA species within cells, a number of recent studies have defined populations RNA granules in a many cell types. Three major classes of RNA-containing granules have been identified. First, in neurons, mRNAs move into dendrites in RNA transport granules for translation of proteins within dendritic spines [4]. Second, P bodies are ubiquitous RNA–containing granules that serve as a sites for RNA degradation and storage [5]. Finally third, perhaps the most dynamic examples of mRNA containing granules are the RNA stress granules induced in many cell types in the response to various cellular stresses. Following stress exposure, subsets of mRNAs are relocated into RNA stress granules where they are sequestered in a translationally silenced state [6]. While these three classes of RNA granules contain some proteins in common, they each demonstrate proteins associated specifically with particular classes of RNA granules [7, 8]. A number of proteins are associated specifically with stress granules. The critical step in assembly of stress granules is phosphorylation of translation initiation factor eIF2α, which blocks initiation of translation, promotes polysome disassembly and leads to formation of stress granules. The Ras-GAP SH3 domain binding protein (G3BP) is also specifically associated with RNA stress granules [9-11] and is absent from P bodies. G3BP regulates RNA stability and self-aggregation of G3BP promotes assembly of stress granules [12]. Recent investigations have indicated that stress granule are functionally dynamic structures that also communicate with P bodies [8].
Control of mRNA stability is tightly connected with regulation of translation. The regulation of translation is central to the response of cells to various stressful scenarios. RNA stress granules are formed under a number of cell stresses including heat shock, oxidative stress (e.g. arsenite exposure) or mitochondrial stress (e.g. from exposure to clotrimazole). Prevailing concepts indicate that these stress granules sequester critical mRNAs in a translational arrested state for future re-expression following the relief of cellular stress [6, 7, 13]. Importantly, the stress granules sequester “housekeeping” transcripts from expression during stress, whereas transcripts that are vital for stress-response such as heat shock proteins messages do not enter stress granules and continue to be translated under the stress conditions [13]. While the formation of translationally inactive RNA complexes appears to be a ubiquitous response to stress, the diversity and specificity of pathways for delivery of RNAs to stress granules remains obscure.
Previous investigations have indicated that AKAP350A (also known as AKAP450 or CG-NAP), a multifunctional scaffolding protein, localizes to both the Golgi apparatus and centrosomes [14-16]. However, we have now identified the existence of a prominent pool of AKAP350A in cytosol. Analysis of AKAP350A scaffolded complexes by immunoprecipitation followed by mass spectrometry revealed two novel interacting proteins: CCAR1/CARP-1 (Cell Cycle and Apoptosis Regulatory Protein-1) and Caprin-1 (Cytoplasmic Activation/Proliferation-associated protein1). AKAP350A and CCAR1 along with G3BP and caprin-1 relocate to stress granules after arsenite treatment or heat-shock. Movement of these proteins to stress granules is dependent on intact microtubules and loss of either AKAP350A or CCAR1 alters stress granule formation. All of these findings suggest that AKAP350A and CCAR1 participate in the microtubule-dependent formation of RNA stress granules following the induction of cellular stress.
Material and methods
Cell culture
HeLa cells (American Type Culture Collection, ATCC) were maintained in RPMI media supplemented with 10% FBS at 5%CO2. HepG2 (ATCC) were maintained in D-MEM/F12 media supplemented with 10% FBS at 5%CO2. Transfections were performed with Effectine transfection reagent (Qiagen) according to manufacturer's instruction.
Cell treatments
For microtubule depolymerization, HeLa cells were preincubated at 4°C for 30 min, and nocodazole (Calbiochem) was then added to a final concentration of 33 μM and incubated for a further 30 min at 4°C. The cells were then shifted to 37°C and incubated a further 90 min prior inducing stress. Stress was induced by incubation of HeLa cells at 42°C for 15 min (heat shock) or addition of 0.5 mM sodium arsenite for 30 min at 37°C.
siRNA for AKAP350A knockdown was made as described previously [17, 18]. siRNA for CCAR1 knockdown were from Ambion. HeLa cells were transfected with 30 nM siRNA duplexes using NeoFlex transfection reagent (Ambion) and examined 72h after transfection.
Western blotting
Subcellular fractions of HepG2 cells were prepared by differential centrifugation [17]. For separation of AKAP350 as well as all other proteins of interest discontinuous step-gradient (4%-8%-12%) separating gels were used. Proteins were transferred to nitrocellulose membrane and blots were probed with the primary antibodies: mouse anti-AKAP350 14G2 [14], which recognizes all known splice variants of AKAP350, 1:500; rabbit anti-CCAR1 (Bethyl), 1:10,000; rabbit anti-caprin-1 (Abgent), 1:1,000; mouse anti-α-tubulin antibody, 1:10,000 (Sigma); mouse anti-G3BP (BD/Transduction Labs), 1:1,000; mouse gm130 (BD/Transduction Labs), 1:1,000 followed by incubation with horseradish-peroxidase conjugated secondary antibodies and detection with Supersignal substrate (Pierce).
Preparation of subcellular fractions of HepG2 by differential centrifugation
HepG2 (near confluent monolayer) cells were scraped with a rubber policeman into 0.25M sucrose, 20 mM Hepes-KOH, pH 7.4, containing 1 mM EDTA and cells were disrupted by 10-20 passes through 21gauge needle, resulted in greater than 90% of cells sheared open. The nuclear fraction was pelleted by centrifugation at 1,000g for 10 min, the heavy membrane fraction was prepared by centrifuging the post-nuclear supernatant at 3,000g for 10min, and the light membrane fraction was pelleted from the post-heavy membrane supernatant at 16,000g for 10 min. The microsomal fraction was pelleted by centrifuging the post-light membrane supernatant at 100,000g for 45 min. All pellets were re-suspended in lysis buffer to ensure complete dispersion of the pellets and all samples were subject to BCA protein assay (Pierce).
Immunoprecipitation
Cells grown in 100 mm plates were lysed in 1 ml of M-PER (Pierce) supplemented with mammalian protease inhibitors (Sigma) followed by centrifugation at 16,000g for 5 minutes. Protein lysates (0.5-1 mg) were pre-cleared with pre-immune serum and Dynabeads M-280 Sheep anti-Rabbit IgG (Dynal Biotech), incubated with 5 μg of specific anti-AKAP350A, anti-CCAR1 or with pre-immune rabbit serum for 1 h at 4°C followed by incubation with 100 μl of Dynabeads for 3h at 4°C. Beads were eluted with 1% SDS sample buffer. For mass spectrometry analysis, antibodies were covalently linked to beads to prevent sample contamination with IgG [19]. Covalent linking of the antibodies to bead did not alter the efficiency of immunoprecipitation of AKAP350A.
Mass spectrometry analysis
Dynabeads incubated with either affinity purified anti-AKAP350A polyclonal antibodies or pre-immune rabbit IgG were cross-linked, as previously described [19]. Immunoprecipitation of AKAP350A was performed as above. The eluted proteins were run into an 8% SDS-PAGE gel, the gel was stained with GelCode Blue colloidal Coomassie blue stain reagent (Pierce) and the stacked protein band at the interface of the stacking and resolving gel was excised and submitted for tryptic digest and analysis on LTQ linear ion trap mass spectrometer (Thermo, Vanderbilt Proteomics Laboratory) as previously described [19].
RNA extraction, gene microarray and expression analyses
AKAP350A was immunoprecipitated from HepG2 cells as above with addition of RNAse inhibitor. RNAs were recovered from beads using RNeasy micro kit (Qiagen). The quality of RNAs was analyzed by Agilent Bioanalysis. Five ng of RNA were linearly amplified and labeled using a NuGen Ovation kit. Affymetrix U133 2.0 gene microarrays were probed with labeled cDNAs (Vanderbilt Microarray Core Facility). RNAs associated with AKAP350A were identified in each sample as species with expression levels over 50 units and at least 8-fold enrichment in the specific AKAP350A isolation. For PCR confirmation of transcript expression, approximately 150 nt sequences were amplified from cDNAs using an Advantage 2 PCR kit (30 cycles).
Plasmid construction
Human G3BP was amplified from HepG2 cDNA using primers 5′-GAGCGTCGACATGGTGATGGAGAAGCCTAGT-3′ and 5′-GAGCGGATCCTCACTGCCGTGGCGCAAGCCCCCT-3′ and was ligated as a SalI/BamHI fragments into SalI/BamHI sites of pmCherry-C1 (a gift from Dr. Roger Tsien, University of California, San Diego).
Fluorescence microscopy and analysis
Cells were fixed and permeabilized as previously described [17] and incubated with rabbit anti-AKAP350A antibody (1:200) [15], which is specific for the major AKAP350A splice variant, rabbit anti-caprin-1 (1:1,000) [20], rabbit anti-CCAR1 (1:1,000)(Bethyl Laboratories), rabbit anti-PheIF2a (1:100)( Stressgen), mouse anti-G3BP (1:700), mouse anti-gm130 (1:300)(BD Transduction Laboratories), goat anti-TIA-1(1:100), goat anti-GW182(1:100)(Santa Cruz) antibodies for 1h at room temperature followed by incubation with species-specific fluorescent secondary antibodies (Invitrogen or Jackson Immunoresearch). The cells were imaged using an Olympus FV1000 confocal fluorescence microscope (Vanderbilt Cell Imaging Shared Resource) using 60x oil lens. The average size and number of stress granules before and after nocodazole treatment were determined using ImageJ software (NIH) for at least ten cells. Changes in size and number of stress granules were analyzed using a Students' t-test.
Live cell fluorescence videos images were acquired on Nikon TE2000E microscope equipped with Perfect Focus System using a 60x oil lens and back-illuminated EM-CCD camera Cascade 512B (Photometrics) driven by IPLab software (Scanalytics). 120 images were captured over a period of 40 min (Frames were taken 20 sec apart during 40 min), videos were prepared using ImageJ software; animation rate was 6 frames/sec.
Results
Identification of a cytosolic AKAP350A scaffolded complex
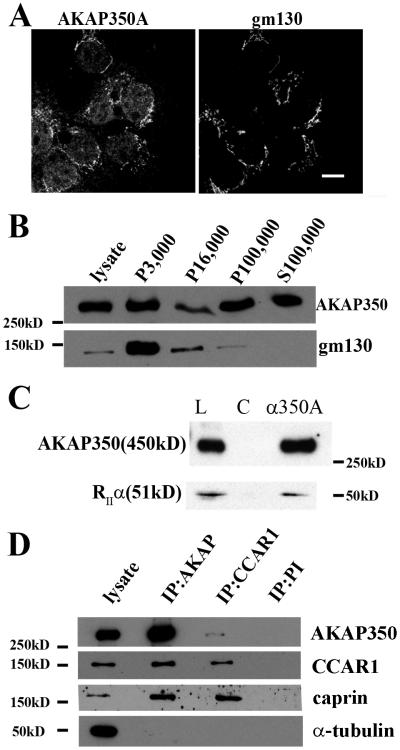
While the association of AKAP350A with the Golgi apparatus is well-established in a number of systems, we had previously noted that the majority of AKAP350 was found in the high speed supernatant after homogenization of rabbit parietal cells [14]. Staining with rabbit polyclonal anti-AKAP350A antibodies in HepG2 cells demonstrated prominent localization with the Golgi apparatus as well as in the cytosol (Fig. 1A). We therefore analyzed AKAP350A distribution in subcellular fractions of HepG2 cells prepared by homogenization in isotonic lysis medium in the absence of detergents [17]. AKAP350 was present in all fractions, including the 3,000g membranes, which were also enriched for Golgi markers (Fig. 1B). Nevertheless, a large fraction of the total AKAP350 in cell homogenates was found in the 100,000g supernatant (S100,000) fraction. These findings indicated that a prominent cytosolic pool of AKAP350 exists in cells.
Fig. 1.
A complex of AKAP350A with CCAR1 and caprin-1. (A) Immunostaining of HepG2 cells with rabbit polyclonal antibodies against AKAP350A show co-localization at the Golgi apparatus with marker of cis-Golgi gm130 as well as diffuse cytosolic staining. Bar = 10 μm. (B) AKAP350 in subcellular fractions of HepG2. Confluent HepG2 cells were homogenized and fractionated by serial spins and western blots were probed for AKAP350 and gm130. 3,000g membranes were enriched for gm130. (C) Cell lysates (L) were immunoisolated with non-specific rabbit IgG (C) or anti-AKAP350A (a350A) and preparations were blotted for both AKAP350 and RIIa. (D) Verification of proteomics data by immunoprecipitation followed by western blot. Lysates of HepG2 cells were immunoprecipitated with anti-AKAP350A and CCAR1 antibody or pre-immune rabbit serum. Eluates of immunoprecipitations (IPs) corresponding 50 mg of starting material and 10 mg of starting lysate were resolved on the same SDS-PAGE gel and probed for AKAP350A, CCAR1, caprin-1 and a-tubulin (as a control for specific immunoprecipitation). CCAR1 and caprin-1 were both immunoisolated with AKAP350A-specific antibody. Caprin-1 as well as a fraction of AKAP350A were recovered with anti-CCAR1 antibody. All immunoisolations were free of a-tubulin-contamination.
Given that AKAP350A is a potential scaffold for a number of proteins [14, 16, 17, 21-26], we sought to determine the composition of AKAP350A-scaffolded complexes. We utilized polyclonal antibodies against the AKAP350A carboxyl terminus to immunoisolate native protein complexes. Table S1 lists the proteins identified by mass-spectroscopy analysis of the immunoisolated AKAP350A complexes. Proteomic analysis of immunoisolated proteins revealed only five consistently identified proteins. As expected, we detected peptides for AKAP350 itself and the regulatory subunits of Type II A-kinase (both RIIα and RIIβ). Of interest, however, we also identified two previously unrecognized associated proteins: CCAR1 and caprin-1.
Relatively little is known about the functional roles of either caprin-1 or CCAR1, but they have both been implicated in processes related to cell proliferation and apoptosis. CCAR1 was originally identified in 2003 as a perinuclear phosphoprotein with putative bihelical DNA binding and cold-shock RNA binding domains [27]. CCAR1 is a regulator of diverse apoptosis signaling pathways and its overexpression stimulates apoptosis [27, 28]. CCAR1-induced apoptosis involves sequestration of 14-3-3 protein, as well as reduced expression cell cycle regulatory genes including c-myc and CyclinB1. Decreasing CCAR1 expression by either anti-sense or siRNA leads to a decrease in apoptotic indices.
Similarly, there is only limited knowledge of the function of caprin-1. Originally cloned as a putative GPI-anchored protein designated p137 [29], more recent studies have revealed that the GPI-linked assignment was a mistake in cloning. The protein was independently identified by Schrader and colleagues as caprin-1, a cytosolic pro-apoptotic protein, which was implicated in cytoskeletal dynamics and pro-apoptotic signaling [30]. The absence of caprin-1 prolongs the G1 phase of cell cycle and results in defective cellular proliferation [31]. Caprin-1 interacts with G3BP (Ras-GAP SH3 domain binding protein), and these two proteins were co-purified together as a tight complex by affinity chromatography [9] and co-immunoprecipitation [20, 31]. Caprin-1 also enters stress granules and selectively interacts with mRNAs transcripts for c-myc and CyclinD2 [20].
To validate the interactions found by mass-spectrometry, we examined the composition of immunoprecipitated AKAP350A complexes by western blot. Fig. 1C demonstrates that AKAP350A antibodies immunoisolated both AKAP350 and RIIα. Immunoisolation of AKAP350A from HepG2 cells also co-isolated both CCAR1 and caprin-1 (Fig. 1D). Immunoisolation with CCAR1-specific antibodies recovered caprin-1 as well as a population of AKAP350A (Fig. 1D). These results suggested that AKAP350A formed a complex with both caprin-1 and CCAR1.
Association of AKAP350A, CCAR1 and caprin-1 with stress granules
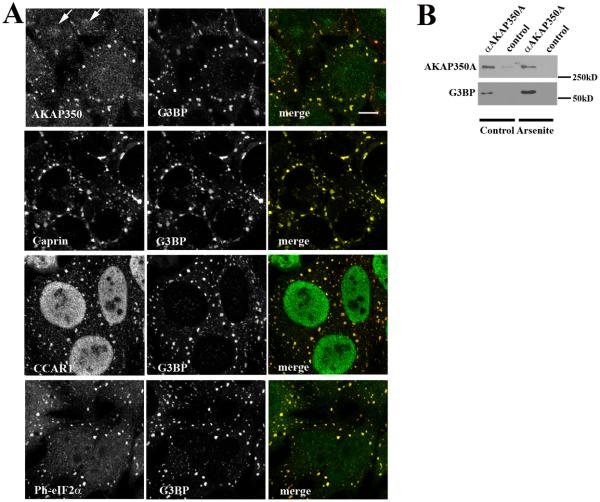
Previous investigations have indicated that caprin-1 can associate with G3BP and that both can relocate from the cytosol to RNA stress granules following induction of cellular stress [20]. Interestingly, examination of the proteomic profiles revealed that G3BP was isolated with AKAP350A antibodies in one out of four preparations. We therefore evaluated whether AKAP350A was also associated with stress granules. HeLa cells were treated with 0.5 mM arsenite for 30 min at 37°C (Fig. 2A) and then fixed and double immunostained for G3BP with antibodies against AKAP350A, caprin-1, CCAR1 or Phosphorylated-eIF2α. As previously reported, G3BP has a diffuse cytosolic distribution in untreated cells (see Supplemental Fig. S1), and stress caused relocation of G3BP to specific cytoplasmic foci in RNA stress granules (Fig. 2A). Prior to stress, also as noted previously, AKAP350A immunostaining was present in both the cytosol and the Golgi, and as previously reported, caprin-1 was cytosolic [20], and CCAR1 was present in both the nucleus and cytoplasm [27] (see Supplemental Fig. S1). Arsenite treatment (Fig. 2A) caused relocation of AKAP350, caprin-1 and CCAR1 to stress granules, where they colocalized with G3BP. We also observed similar relocation of AKAP350, caprin-1 and CCAR1 to stress granules following 43 C heat stress (data not shown). The G3BP-containing stress-induced granules also were stained with antibodies against the phosphorylated translation initiation factor eIF2α (Fig. 2A), which is specifically present in stress granules, and not in P bodies [7]. Since G3BP was detected by mass spectrometry analysis of the AKAP350 complex in one out of 4 preparations, we then examined immunoisolation of G3BP with AKAP350A antibodies before and after arsenite treatment. Fig. 2B demonstrates that some G3BP was immunoisolated with AKAP350A from untreated cells, but arsenite treatment markedly increased the recovery of G3BP with AKAP350A antibodies. All of these results indicated that AKAP350 and CCAR1 relocated to RNA stress granules following induction cellular stress. Interestingly, immunostaining for the RII regulatory subunit of A-kinase did not redistribute with AKAP350 into stress granules (data not shown), suggesting a dynamic organization of the AKAP350-scaffolding complex.
Fig. 2.
Association of AKAP350A, CCAR1 and caprin-1 with G3BP-containing stress granules. (A) HeLa cells were treated with 0.5 mM arsenite for 30 min, fixed and dual stained for G3BP (red in merged images) and with AKAP350A, caprin-1, CCAR1, or Phosphorylated-eIF2a (green in merged images). Arrows indicate partially displaced AKAP350A staining at Golgi. Bar = 10 μm. (B) Stress increases the association of AKAP350A with G3BP. AKAP350A immunoisolation was performed on lysates of untreated HepG2 (control) or arsenite-treated HepG2 cells. Blots were probed for AKAP350 (450 kD) or G3BP (68 kD).
To investigate the nature of granules containing AKAP350A we analyzed distribution of markers selective for stress granules (Phosphorylated-eIF2α, G3BP and TIA-1) and P-bodies (GW182) [8, 32, 33]. Previous investigations have shown that, in cells undergoing oxidative stress, Phosphorylated-eIF2α, G3BP and TIA-1 were associated exclusively with stress granules, whereas GW182 predominantly associated with P-bodies [6]. In arsenite treated cells, AKAP350A was predominantly associated with the stress granules markers G3BP (Supplemental Fig. S2) and TIA-1 (Supplemental Fig. S4), whereas the majority of the P-bodies labeled with the autoantibody GW182 were distributed in smaller granules that were not positive for AKAP350. Nevertheless, some of the biggest granules were positive for both G3BP and GW182, as well as for AKAP350. Similarly, CCAR1 and caprin-1 were mostly associated with G3BP, but some largest granules also included GW182. These results support a dynamic link between stress granules and P-bodies in the process of mRNA remodeling [8]. Previous studies have also shown that treatment of cells with emetine, a drug that inhibits elongation of translation, dispersed stress granules without affecting P-bodies [34]. We observed that emetine treatment eliminated association of AKAP350A, CCAR1 and caprin-1 with G3BP-positive stress granules and led to relocation of AKAP350A, CCAR1 and caprin-1 to the cytosol (Supplemental Fig. S2-S4). All of these results support the concept that AKAP350A and CCAR1 associate with stress granules.
Relocation of AKAP350A from Golgi to stress granules and Golgi fragmentation during stress
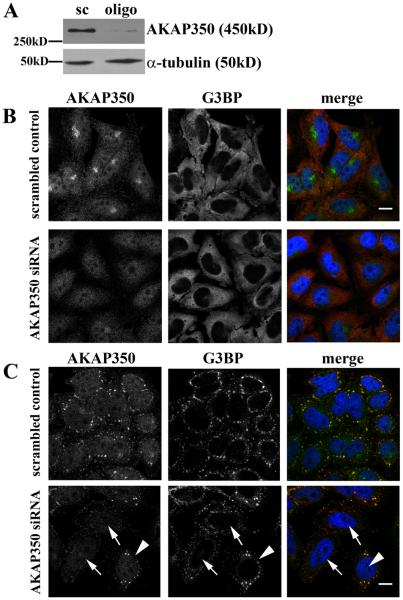
We have previously noted that reduction of AKAP350 expression by siRNA led to fragmentation of the Golgi apparatus [17]. Fig. 3A demonstrates that siRNA for AKAP350 caused dispersal of GM130-labeling Golgi membranes. In addition to the relocation of AKAP350A to stress granules after stress, we also noted that AKAP350A staining was diminished in the Golgi (Fig. 2A, arrows and Fig. 3B). We therefore examine the structure of the Golgi apparatus after induction of stress. Induction of stress with arsenite treatment led to fragmentation of the GM130 staining of the Golgi apparatus. The extent of Golgi fragmentation increased with greater loss of AKAP350 from the Golgi. While both arsenite treatment and AKAP350 siRNA altered Golgi structure, effects of arsenite were more dramatic, with greater dispersal into small membrane elements. Similar fragmentation of the Golgi and loss of AKAP350A staining from the Golgi was also observed following 43 C heat shock (data not shown). The results indicate that arsenite treatment, in addition to inducing translational arrest of mRNAs, also leads to alterations in Golgi structure.
Fig. 3.
Both knockdown of AKAP350 (A) and stress (B) alter Golgi structure. HeLa cells were transfected with either non-specific scrambled RNA duplexes or siRNA duplexes specific for AKAP350A (A) or treated with 0.5 mM arsenite for 30 min (B), fixed and dual stained for AKAP350A (green in merged images) and cis-Golgi marker gm130 (red in merged images). (A) Reduction of AKAP350A expression altered Golgi apparatus morphology (dispersal of Golgi elements). (B) Arrows indicate intact Golgi structure with AKAP350 co-localization; arrowheads indicate partially displaced AKAP350A staining from Golgi to stress granules and Golgi disassembly. Bar = 10 μm.
Microtubules regulate the association of AKAP350A and CCAR1, but not caprin-1, with stress granules
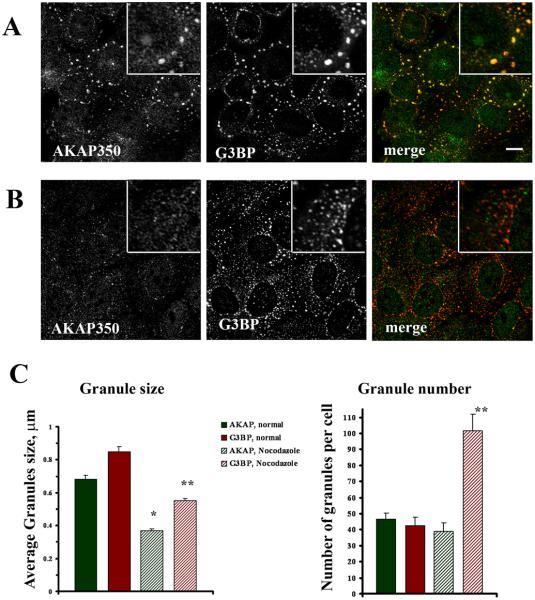
In our previous studies of AKAP350 and Golgi apparatus structure, we observed that there was a subtle but consistent loss of microtubules concentrating in the Golgi apparatus region in AKAP350A-depleted cells [17]. Depletion of AKAP350A by short interference RNA (siRNA) delayed microtubule regrowth after cessation of nocodazole treatment, while initial aster formation after withdrawal of nocodazole was not altered in AKAP350-depleted cells [18]. These results suggested a role for AKAP350A in microtubule elongation or stabilization of growing microtubules. We, therefore, asked whether AKAP350 function in stress is related to microtubule-dependent stress granule dynamics. Stress granules are dynamic structures with constant turnover of mRNAs shuttling between polysomes, stress granules and the processing bodies (P bodies), where degradation of mRNAs occurs [7, 8]. A previous study suggested that formation of stress granules is dependent on intact microtubules [35]. We examined how disruption of microtubules affects the composition of AKAP350A complexes and their association with stress granules. In contrast with the previous investigation [35], depolymerization of microtubules with nocodazole did not abolish formation of G3BP-positive arsenite-induced stress granules, but rather led to development of a greater number of smaller granules (Fig. 4A,B). Microtubule disassembly with nocodazole was confirmed with α–tubulin staining (see Supplemental Fig. S7). After nocodazole treatment, the number of arsenite-induced G3BP-staining stress granules increased approximately 2-fold, whereas the average size of G3BP-positive stress granules decreased by approximately 70% (Fig. 4C). In the presence of microtubule disruption, the majority of AKAP350A in the cytosol did show some aggregation into small granules, but AKAP350A did not localize with G3BP-containing granules in the cytosol. In arsenite-treated cells without nocodazole treatment stress granules formed larger circular structures surrounding the perinuclear region, which contained both AKAP350A and G3BP. In contrast, localization of G3BP-containing stress granules in nocodazole-treated cells was altered with granules randomly dispersed through cytosol (Fig. 4B). These results suggested that intact microtubules are not required for initial stress granule formation, but are essential for stress granule relocation and coalescence.
Fig. 4.
Nocodazole inhibits delivery of AKAP350A to stress granules. AKAP350A (green) and G3BP (red) localization in HeLa cells treated with arsenite in the absence (A) or presence (B) of nocodazole pre-treatment. Microtubule disassembly with nocodazole was confirmed with a–tubulin staining (see Supplemental Fig. S7). Bar = 10 μm. Enlarged images are shown in insets in the right corners. Note that nocodazole treatment resulted in a major separation of AKAP350A from G3BP-positive stress granules. (C) Quantification of AKAP350A and G3BP in stress granules based on size (μm) or the number of stress granules per cell demonstrates that nocodazole elicits greater amount of smaller G3BP-positive stress granules. Solid green bars: AKAP350 control; solid red bars: G3BP control; hatched green bars: AKAP50 nocodazole; hatched red bars: G3BP nocodazole. *p<0.001 vs AKAP350 staining in untreated; **p<0.001 vs G3BP staining in controls. The results are the mean±SE.
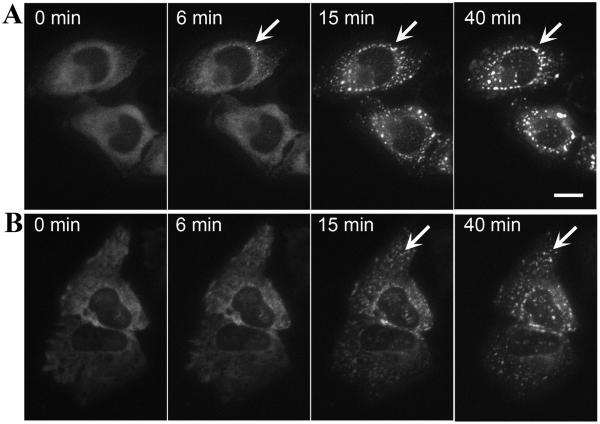
To test this hypothesis more directly, formation and movement of stress granules were monitored by live cell microscopy in HeLa cells expressing mCherry-G3BP (Fig. 5 A,B and Video S1, Supplemental Materials). Importantly, we examined only cells expressing a low amount of diffuse cytosolic G3BP for these experiments, since over-expression of G3BP causes spontaneous formation of stress granules [6]. In cells expressing low levels of mCherry-G3BP, arsenite induced formation and coalescence of G3BP-containing stress granules (Fig. 5 and Video S1A). In comparison, arsenite-induced formation of G3BP-positive stress granules was delayed in nocodazole-pretreated cells compared to control cells (Fig. 5 and Video S1B). Six minutes after stress induction, control cells had already started to develop stress granules (Fig. 5A), whereas microtubule-depleted cells did not demonstrate any stress granule formation at six minutes (Fig. 5B). Furthermore, movement of stress granules in nocodazole-treated cells was limited and granules did not fuse as much as in untreated cells. In addition, the patterns of movement were different in nocodazole-treated cells, with chaotic movement as opposed to directional centripetal movement of stress granules in untreated cells (see Video S1).
Fig. 5.
Formation and dynamics of stress granules. Video-frames showing development of G3BP-positive stress granules in control HeLa (A) and nocodazole-treated HeLa cells (B). HeLa cells were transfected with G3BP-mCherry and only cells with low expression were chosen for microscopy. Two hours before imaging, the cells were pre-treated with nocodazole to disperse microtubules. Development of stress granules (see arrows) after addition of 0.5 mM arsenite was delayed in nocodazole pre-treated cells and movement was chaotic, versus the well-organized distribution around nucleus in cells with intact microtubules (see Supplemental Materials, Video 1). Bar = 10 μm.
The dynamics of stress granules are properties associated with the movement and sorting of sequestered mRNA transcripts and proteins and their accumulation at particular cytoplasmic foci [7]. We reasoned that microtubule-dependent transport, although not essential for the formation of stress granules per se, might regulate transport and delivery of proper components of stress granules including mRNAs and proteins. In fact, as shown in Fig. 4 disruption of microtubules caused substantial displacement of AKAP350A from G3BP-containing stress granules (see enlarged areas in inserts). Nocodazole treatment also led to relocation of CCAR1 from stress granules into the cytosol (Fig. 6A). However, in nocodazole-treated cells, caprin-1 still associated with G3BP-containing stress granules after arsenite treatment (Fig. 6B). Since previous studies have noted a direct association of caprin-1 and G3BP [20], these results suggest that caprin-1 can still interact with G3BP in the absence of intact microtubules. Nevertheless, the smaller size and aberrant distribution of stress granules in nocodazole-treated cells suggest that the proper composition of stress granules requires microtubule-dependent transport. Finally, to determine whether small G3BP-positive foci observed in cells lacking microtubules are processing bodies rather than abortive stress granules, we evaluated both G3BP and GW182 composition of granules after nocodazole treatment (Supplemental Fig. S5-S7). We observed that all of the smaller granules labeling for AKAP350A, CCAR1, caprin or G3BP after arsenite treatment in absence of the microtubule network failed to label with GW182. Notably, nocodazole treatment also led to dissociation of majority of phosphorylated-eIF2α from stress granules into the cytosol. All these results support the concept that disruption of microtubules inhibits the complete assembly of stress granules without shunting components to P bodies.
Fig. 6.
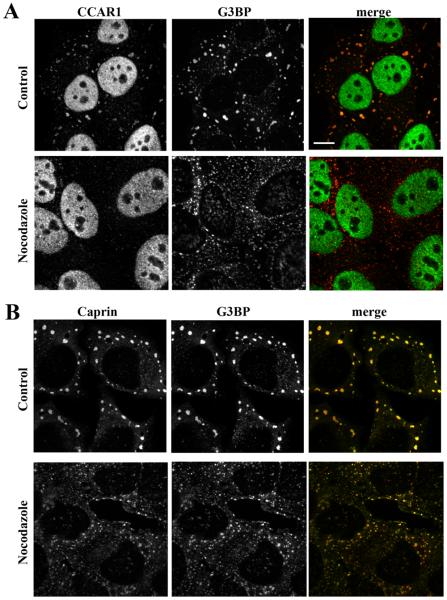
Disruption of microtubules by nocodazole treatment leads to substantial displacement of CCAR1 but not caprin-1 from G3BP-positive stress granules. Control or nocodazole-treated HeLa cells were fixed and immunostained for either CCAR1 (A) or caprin-1 (B) (green) and G3BP (red). Disruption of microtubules caused formation of a greater number of smaller G3BP-positive stress granules that contained caprin-1, but not CCAR1. Bar = 10 μm.
The apparent loss of AKAP350A from G3BP-containing stress granules after nocodazole treatment suggested that AKAP350A is involved in microtubule dependent delivery to stress granules. Therefore to evaluate the importance of AKAP350A in stress granule formation, we assessed the effect of a decrease in the expression of AKAP350A by siRNA on the formation of stress granules. The transfection of siRNA for AKAP350A elicited a marked reduction of protein expression (Fig. 7 A,B). In arsenite treated cells showing a loss of AKAP350A expression, we observed a pattern similar to that seen with nocodazole treatment: an increase in the number of G3BP-containing stress granules, which were generally smaller than those observed in cells maintaining AKAP350A expression (Fig. 7C). Quantitation of G3BP in stress granules based on size (μm) or the number of stress granules per cell demonstrated that reduction of AKAP350 expression elicited greater number of smaller G3BP-positive stress granules (see Supplemental Fig. S8). These small G3BP-containing granules also were positive for another stress granules marker TIA-1 (see Supplemental Fig. S9). Taken together, the foregoing results indicate that the complete formation and coalescence of stress granules requires both microtubule-dependent and AKAP350A-dependent processes.
Fig. 7.
Knockdown of AKAP350 alters stress granule formation. HeLa cells were transfected with non-specific scrambled RNA duplexes or siRNA duplexes specific for AKAP350A. Western blotting (A) was performed for AKAP350 and α-tubulin and transfected cells were immunostained (B) for AKAP350A (green) and G3BP (red) with DAPI (blue). Both western blots and immunostaining demonstrate a significant reduction of AKAP350A expression in cells transfected with siRNA. (C) HeLa cells with normal and reduced levels of AKAP350A expression were treated with 0.5 mM arsenite for 30 min. Arrows note the positions of cells with loss of AKAP350A expression, while arrowheads indicate the positions of non-transfected cells with normal levels of expression. In AKAP350A siRNA treated cells, G3BP-positive stress granules were smaller and showed irregular shapes. Bar = 10 μm.
We also examined the formation of G3BP-containing stress granules following siRNA-dependent reduction of CCAR1 expression. Similar to the findings following AKAP350A knockdown, reduction of the expression of CCAR1 led to formation greater number of smaller G3BP-labeled stress granules (Fig. 8).
Fig. 8.
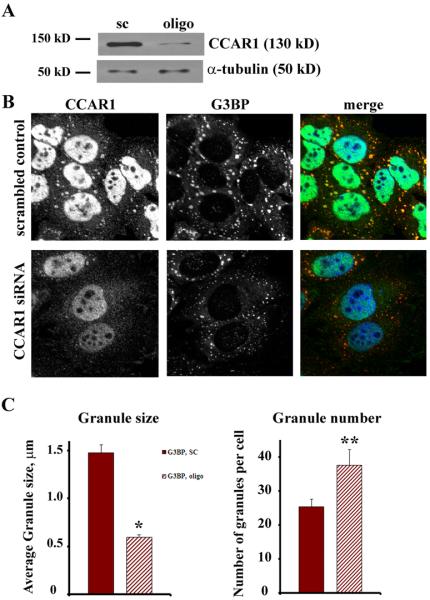
Knockdown of CCAR1 alters stress granule formation. HeLa cells were transfected with non-specific scrambled RNA duplexes or siRNA duplexes specific for CCAR1. Western blotting (A) and immunostaining (B) demonstrate a significant reduction of CCAR1 expression in cells transfected with siRNA. (B) HeLa cells with normal and reduced levels of CCAR1 expression were treated with 0.5 mM arsenite for 30 min. In CCAR1 siRNA treated cells, G3BP-positive stress granules were significantly smaller. Bar = 10 μm. (C) Quantitation of G3BP in stress granules based on size (μm) or the number of stress granules per cell demonstrates that reduction of level of CCAR1 expression elicits a greater number of smaller G3BP-positive stress granules. Solid bars: scrambled control; hatched bars: CCAR1 knockdown. *p<0.001, **p<0.05. The results are the mean±SE.
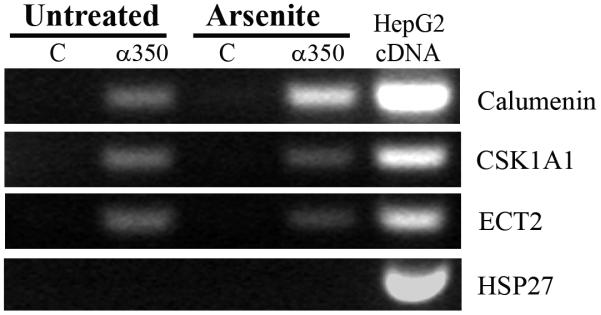
AKAP350A associates with a subset of mRNA transcripts
Stress granules are known to sequester poly-A mRNAs for protection during the stress followed by re-expression after stress is resolved [11]. We therefore sought to determine whether AKAP350A is associated with mRNA transcripts. AKAP350A was immunoprecipitated from untreated and arsenite-treated HepG2 cells as in the proteomic studies, and RNA transcripts were isolated, amplified and used to probe Affymetrix gene microarrays. We found 69 mRNA transcripts that were enriched greater than 8-fold in AKAP350A immunoisolates from both untreated and arsenite-treated cells (Database S1, Supplemental Materials). Only two transcripts were present in immunoisolates from untreated cells, but absent in arsenite-treated cells. Five transcripts were present in immunoisolates from arsenite-treated cells that were not observed in untreated cells. Thus, the vast majority of mRNA transcripts that associated with AKAP350A were complexed with the scaffolding protein in the absence of any stress. While pathway analysis did not reveal any distinct relationship among the immunoisolated transcripts, we noted that the majority of mRNAs possessed 3′-untranslated regions of greater than 1000 nt (Database S1). We validated selected transcripts by performing RT-PCR on immunoisolated RNAs from both untreated and arsenite-treated cells. Fig. 8 demonstrates that transcripts for calumenin, casein kinase 1A1 (CSNK1A1) and Epithelial Cell-Transforming 2 (ECT2) were detected only in AKAP350A immunoisolates. Previous studies have suggested that, during stress, cells repress translation of “house-keeping genes” until stress is resolved, whereas translation of messages coding heat-shock proteins responsible for stress response is active and thus mRNAs coding heat-shock proteins do not enter stress granules [13]. If mRNA trafficking and concomitant translational silencing not only occurs under stress conditions, but also serves as a regulatory mechanism for cells under normal physiology, then heat-shock protein mRNAs should be excluded from AKAP350A-associated complexes in both normal and stressed cells. Therefore we examined heat shock protein 27 (HSP27) transcripts as a control for evaluation of AKAP350A-mRNAs complex composition. As shown in Fig. 8, while transcripts for HSP27 were abundant in cell extracts, HSP27 mRNA was not associated with AKAP350A immunoisolates.
Discussion
Previous studies have noted that various cell stresses induce the formation of RNA stress granules. These granules contain a subset of total cell mRNAs, which appear to be protected against degradation and are sequestered in a translation-arrested state [13]. Prevailing concepts have suggested that these sequestered RNAs are necessary for re-establishment of normal cellular functions after the removal of various stressors. The results presented here indicate that AKAP350A associates with two RNA binding proteins, CCAR1 and caprin-1, to form a complex, which can be delivered to stress granules in a microtubule-dependent fashion. Thus, while previous studies have implicated the association of AKAP350A with the centrosomes and the Golgi apparatus, these findings indicate a novel role for this multifunctional scaffolding protein in another microtubule-dependent process, cytosolic relocation of mRNA transcripts and their association with RNA stress granules.
Over the past several years, a number of investigations have noted the association of AKAP350A with proteins associated with the centrosomes or the Golgi apparatus. AKAP350A can scaffold a variety of protein kinases and phosphatases as well as potential down-stream effectors of second messenger dependent signaling. Nevertheless, it is increasingly evident that AKAP350A–scaffolded complexes are likely distinct in different regions of cells. At the centrosome, previous studies have implicated AKAP350 association with calmodulin, TACC3, casein kinase 1, and the components of the α-tubulin ring complex [21, 26, 36, 37]. At the Golgi apparatus, AKAP350A interacts with CIP4 and CLIC5b [17, 22]. Still, the cytosolic AKAP350A-containing complex must contain scaffolded proteins distinct from those associated with the Golgi and the centrosome. We have confirmed that RII regulatory subunit of A-kinase is also present in immunoisolates. However, we have not been able to confirm the presence of phosphatases 1 or 2a in these complexes. Thus, the cytosolic AKAP350A complexes associating with RNA likely contain only a subset of potential AKAP350-scaffolded partners.
No previous investigations have addressed the role of the Golgi apparatus in stress response. The results presented here indicate that there may be a dynamic role for AKAP350A movement from the Golgi apparatus. We observed loss of AKAP350A staining in the Golgi in concert with fragmentation of the Golgi during arsenite stress. The patterns of Golgi fragmentation were similar to those seen during AKAP350A depletion with siRNA. While it is presently not clear whether AKAP350A lost from the Golgi can relocate to the stress granules, these findings do suggest that AKAP350A may participate in multiple steps in the cellular response to stress.
A number of investigations have indicated that AKAP350 may associate with microtubules and regulate their polymerization or stabilization. AKAP350 associates with the centrosome and can regulate microtubule out growth from the centrosomes [14-16, 18, 21, 26, 36-38]. AKAP350 scaffolds a number of potential regulators of microtubule polymerization including A-kinase itself, calmodulin [36], TACC3 [21], casein kinase 1 [26] and the α-tubulin ring complex [37]. The present results indicate that complexes containing AKAP350 are also involved in microtubule-dependent relocation of proteins into stress granules. Previous investigations had indicated that depolymerization of microtubules prevented formation of stress granules [35, 39]. However, the results presented here in both live and fixed cell studies indicate that microtubule-dependent transport is necessary for the fusion of smaller aggregates in the periphery into larger more centrally located stress granules. The reason for the discrepancy between the results in the present investigations and these previous studies may relate to the markers used for stress granule localization. While we have utilized G3BP as a stress granule marker, the previous investigation of Ivanov, et al. examined p170 and PABP as markers of stress granules [35] and Kwon, et al. followed HDAC6 [39]. Thus, it is possible that these three markers, like CCAR1, lose their association with stress granules after microtubule depolymerization. In general, the live cells studies here indicate that small granule aggregates form locally throughout the cell after initiation of stress and are then transported centripetally towards the perinuclear region, where they fuse into larger granules. In the presence of nocodazole, AKAP350A and G3BP-containing granules induced by arsenite treatment were markedly smaller. In addition, the smaller granules containing G3BP were substantially distinct from those containing AKAP350A. Thus, it is possible that specialized classes of smaller aggregates initially form based on their association with specific scaffolding proteins such as AKAP350A likely containing specific subsets of mRNA transcripts. These smaller aggregates would then be consolidated into larger granules following their microtubule-dependent transport from the cell periphery. While recent studies have suggested that P bodies also move along microtubules [40], the smaller G3BP-containing aggregates after nocodazole were not associated with GW182-stained P bodies. These findings are compatible with the models proposed by Anderson and Kedersha for sequential formation and maturation of stress granules [8].
The results presented here indicate that AKAP350A along with CCAR1 associates with a defined subset of mRNAs. The mRNAs associate with the AKAP350A scaffolded complex even before stress and relocate with AKAP350A to the stress granules following treatment with arsenite. While the associated mRNAs do not fall into particular groupings, they do all show long 3′-untranslated regions. Previous investigations examining aspects of RNA trafficking have noted the importance of elements in the 3′-untranslated regions of transcripts in providing specificity for interactions with trafficking elements and protein complexes. It is notable that another AKAP, AKAP121, also is involved in RNA binding complexes and the delivery of specific RNAs for import into mitochondria [41]. While AKAP350 does not contain any known RNA binding motifs, both CCAR1 and caprin-1 have RNA binding domains. Thus, the association of RNA transcripts with the AKAP350A complex is most likely indirect through these associated proteins. Association of mRNA transcripts with AKAP121 complexes is dictated by sequences in the 3′-untranslated regions. Furthermore, recognition of 3′-untranslated sequences is critical for transport of CaM kinase II mRNA sequences into neuronal dendrites [42]. Indeed, in preliminary studies, we have observed that AKAP350A is also associated with a subset of RNA transport granules in the dendrites of neurons (data not shown). Future studies will be required to determine the specific elements in mRNA sequences required for association with the AKAP350A scaffolded complex and processes that regulate their association with stress granules.
In summary, the present investigations have led to the recognition of a novel function for the large scaffolding protein AKAP350A in the coordination of a complex with CCAR1 and caprin-1, which associates with mRNA transcripts and relocates to RNA stress granules. The process of trafficking to stress granules is microtubule-dependent and the association of AKAP350A with a subset of transcripts also indicates that the AKAP350-coordinated complex likely participates in cytosolic movement and regulation of mRNA transcripts in non-stressed cells.
Supplemental Materials
Supplementary Material
Database of mRNA transcripts associated with AKAP350A complex. Inclusion required 8-fold enrichment of a transcript in the immunoisolate over IgG control immunoisolates. Information is provided for the 69 mRNA transcripts identified in complexes immunoisolated from both untreated and arsenite-treated cells. In addition, information is shown for transcripts that were present in the immunoisolates from untreated cells but not arsenite-treated cells, and transcripts present in immunoisolates from arsenite-treated cells but not in untreated cells.
Knockdown of AKAP350 alters TIA-1 labeled stress granules. HeLa cells were transfected with non-specific scrambled RNA duplexes or siRNA duplexes specific for AKAP350A. After fixation cells were stained for AKAP350 (red in merged images), G3BP (green in merged images) and TIA-1 (blue in merged images). HeLa cells with normal and reduced levels of AKAP350A expression were treated with 0.5 mM arsenite for 30 min. In AKAP350A siRNA treated cells, stress granules were smaller and showed positive staining for both G3BP and TIA-1. Bar = 10 μm.
Mass spectrometry identification of AKAP350A-complex composition. Sequences of peptides identified are shown for each protein.
Formation and dynamic of stress granules in normal HeLa cells (A) and nocodazole treated HeLa cells (B). HeLa cells were transfected with G3BP-mCherry, and only cells with low expression were chosen for microscopy. These videos correspond to still images shown in Fig. 4. Two hours before imaging, cells were pre-treated with nocodazole to disperse microtubules. Development of stress granules after addition of 0.5 mM arsenite in nocodazole pre-treated cells was delayed and movement was chaotic, versus well-organized distribution around nucleus in cells with intact microtubules. 120 images were captured over a period of 40 min (Frames were taken 20 sec apart during 40 min), animation rate was 6 frames/sec.
Formation and dynamic of stress granules in normal HeLa cells (A) and nocodazole treated HeLa cells (B). HeLa cells were transfected with G3BP-mCherry, and only cells with low expression were chosen for microscopy. These videos correspond to still images shown in Fig. 4. Two hours before imaging, cells were pre-treated with nocodazole to disperse microtubules. Development of stress granules after addition of 0.5 mM arsenite in nocodazole pre-treated cells was delayed and movement was chaotic, versus well-organized distribution around nucleus in cells with intact microtubules. 120 images were captured over a period of 40 min (Frames were taken 20 sec apart during 40 min), animation rate was 6 frames/sec.
Localization of AKAP350A, caprin-1, CCAR1 and Phospho-eIF2α prior to stress. HeLa cells growing in normal conditions were fixed and immunostained for AKAP350A, caprin-1, CCAR1 or Phospho-eIF2α (green in merged images) and G3BP (red in merged images). Merged images are shown at right. Bar = 10 μm.
Localization of AKAP350 in stress granules. HeLa cells were treated with 0.5 mM arsenite for 30 min followed by addition of 20 μg/ml of emetine for 1 h. After fixation cells were stained for AKAP350A or Phosphorylated-eIF2α (red in merged images), G3BP (green in merged images) and GW182 (blue in merged images). AKAP350 was predominantly associated with stress granules markers, although some of biggest granules were positive for both the stress granules marker (G3BP) and the P bodies marker (GW182). Emetine treatment eliminated association of AKAP350 and Phospho-eIF2α with G3BP-positive stress granules and led to relocation of AKAP350 and Phospho-eIF2α to the cytosol. Bar = 10 μm.
Localization of CCAR1 and caprin-1 in stress granules. HeLa cells were treated with 0.5 mM arsenite for 30 min followed by addition of 20 μg/ml of emetine for 1 h. After fixation cells were stained for CCAR1 or caprin-1 (red in merged images), G3BP (green in merged images) and GW182 (blue in merged images). Both CCAR1 and caprin-1 were predominantly associated with stress granules markers, although some of biggest granules were positive for both the stress granule marker (G3BP) and P body marker (GW182). Emetine treatment eliminated the association of CCAR1 and caprin-1 with G3BP-positive stress granules. Bar = 10 μm.
Localization of AKAP350A and TIA-1 in stress granules. HeLa cells were treated with 0.5 mM arsenite for 30 min followed by addition of 20 μg/ml of emetine for 1 h. After fixation cells were stained for AKAP350 (red in merged images), G3BP (green in merged images) and TIA-1 (blue in merged images). AKAP350 was associated with both G3BP and TIA-1 markers of stress granules. Emetine treatment led to relocation of all three proteins to cytosol. Bar = 10 μm.
Nocodazole inhibited delivery of AKAP350 to stress granules (A) and led to relocation of majority of phosphorylated-eIF2α from stress granules to the cytosol (B). HeLa cells were treated with arsenite in the absence (control) or presence of nocodazole pre-treatment. Microtubule disassembly with nocodazole was confirmed with α–tubulin staining (see Supplemental Fig. S7). AKAP350 or Phosphorylated-eIF2α (red in merged images), G3BP (green in merged images) and GW182 (blue in merged images). Enlarged images are shown in insets. Note that nocodazole treatment resulted in separation of AKAP350A from G3BP-positive stress granules. Bar = 10 μm.
Disruption of microtubules by nocodazole treatment led to displacement of CCAR1 (A), but not caprin-1 (B), from G3BP-positive stress granules. Control or nocodazole-treated HeLa cells were fixed and immunostained for either CCAR1 or caprin-1 (red in merged images), G3BP (green in merged images) and GW182 (blue in merged images). Disruption of microtubules caused formation of a greater number of smaller G3BP-positive stress granules that contained caprin-1, but not CCAR1. Bar = 10 μm.
Disruption of microtubules in Hela cells. Hela cells were treated with nocodazole as described in Material and methods and stained for α-tubulin. Treatment led to complete disruption of the microtubule network. Bar = 10 μm.
Knockdown of AKAP350 alters stress granule formation. HeLa cells were transfected with non-specific scrambled RNA duplexes or siRNA duplexes specific for AKAP350A. Quantitation of G3BP in stress granules based on size (μm) or the number of stress granules per cell demonstrates that reduction of level of AKAP350 expression elicits a greater number of smaller G3BP-positive stress granules. Solid bars: scrambled control; hatched bars: AKAP350 knockdown. *p<0.001. The results are the mean±SE.
Fig. 9.
RNA transcripts associated with stress granules. Enrichment of specific RNA transcripts in association with AKAP350A. RNAs were isolated from untreated and arsenite-treated HepG2 cells with either AKAP350A antibodies (α350) or preimmune rabbit serum (C). A random primed cDNA was prepared from each fraction and RNAs were detected by PCR amplification of 120 nt fragments with primers specific for calumenin, CSNK1A1 or ECT2 (30 cycles). The cDNA from HepG2 cell was used as a positive control. Calumenin, CSNK1A1 and ECT2 were only detected in the specific AKAP350A immunoisolates. Note that HSP27 mRNA was abundant in HepG2 cells, but was not associated with AKAP350A.
Acknowledgments
This work was supported by grants to J.R.G. from NIH (DK48370) and to A.K.R. from a VA Merit Review award. Proteomic studies in this work was supported the Vanderbilt Digestive Disease Research Center and the Vanderbilt Protein Mass Spectrometry Core Facility. We thank Dr. Lynne Lapierre for critical reading of manuscript.
References
- 1.Dollar G, Struckhoff E, Michaud J, Cohen RS. Rab11 polarization of the Drosophila oocyte: a novel link between membrane trafficking, microtubule organization, and oskar mRNA localization and translation. Development. 2002;129:517–526. doi: 10.1242/dev.129.2.517. [DOI] [PubMed] [Google Scholar]
- 2.Kiebler MA, Bassell GJ. Neuronal RNA granules: movers and makers. Neuron. 2006;51:685–690. doi: 10.1016/j.neuron.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 3.Bear MF, Dolen G, Osterweil E, Nagarajan N. Fragile X: translation in action. Neuropsychopharmacology. 2008;33:84–87. doi: 10.1038/sj.npp.1301610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macchi P, Hemraj I, Goetze B, Grunewald B, Mallardo M, Kiebler MA. A GFP-based system to uncouple mRNA transport from translation in a single living neuron. Mol Biol Cell. 2003;14:1570–1582. doi: 10.1091/mbc.E02-08-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu CY, Rana TM. Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS biology. 2006;4:e210. doi: 10.1371/journal.pbio.0040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson P, Kedersha N. RNA granules. J Cell Biol. 2006;172:803–808. doi: 10.1083/jcb.200512082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends in biochemical sciences. 2008;33:141–150. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Katsafanas GC, Moss B. Vaccinia virus intermediate stage transcription is complemented by Ras-GTPase-activating protein SH3 domain-binding protein (G3BP) and cytoplasmic activation/proliferation-associated protein (p137) individually or as a heterodimer. J Biol Chem. 2004;279:52210–52217. doi: 10.1074/jbc.M411033200. [DOI] [PubMed] [Google Scholar]
- 10.Irvine K, Stirling R, Hume D, Kennedy D. Rasputin, more promiscuous than ever: a review of G3BP. The International journal of developmental biology. 2004;48:1065–1077. doi: 10.1387/ijdb.041893ki. [DOI] [PubMed] [Google Scholar]
- 11.Cande C, Vahsen N, Metivier D, Tourriere H, Chebli K, Garrido C, Tazi J, Kroemer G. Regulation of cytoplasmic stress granules by apoptosis-inducing factor. J Cell Sci. 2004;117:4461–4468. doi: 10.1242/jcs.01356. [DOI] [PubMed] [Google Scholar]
- 12.Tourriere H, Chebli K, Zekri L, Courselaud B, Blanchard JM, Bertrand E, Tazi J. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J Cell Biol. 2003;160:823–831. doi: 10.1083/jcb.200212128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Kedersha N, Anderson P. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem Soc Trans. 2002;30:963–969. doi: 10.1042/bst0300963. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt PH, Dransfield DT, Claudio JO, Hawley RG, Trotter KW, Milgram SL, Goldenring JR. AKAP350, a multiply spliced protein kinase A-anchoring protein asociated with centrosomes. J.Biol.Chem. 1999;274:3055–3066. doi: 10.1074/jbc.274.5.3055. [DOI] [PubMed] [Google Scholar]
- 15.Shanks RA, Steadman BT, Schmidt PH, Goldenring JR. AKAP350 at the Golgi apparatus. I. Identification of a distinct Golgi apparatus targeting motif in AKAP350. J Biol Chem. 2002;277:40967–40972. doi: 10.1074/jbc.M203307200. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi M, Shibata H, Shimakawa M, Miyamoto M, Mukai H, Ono Y. Characterization of a novel giant scaffolding protein, CG-NAP, that anchors multiple signaling enzymes to centrosome and the Golgi apparatus. J.Biol.Chem. 1999;274:17267–17274. doi: 10.1074/jbc.274.24.17267. [DOI] [PubMed] [Google Scholar]
- 17.Larocca MC, Shanks RA, Tian L, Nelson DL, Stewart DM, Goldenring JR. AKAP350 interaction with cdc42 interacting protein 4 (CIP4) at the Golgi apparatus. Mol Biol Cell. 2004;15:2771–2781. doi: 10.1091/mbc.E03-10-0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larocca MC, Jin M, Goldenring JR. AKAP350 modulates microtubule dynamics. Eur J Cell Biol. 2006;85:611–619. doi: 10.1016/j.ejcb.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Lapierre LA, Avant KM, Caldwell CM, Ham AJ, Hill S, Williams JA, Smolka AJ, Goldenring JR. Characterization of immunoisolated human gastric parietal cells tubulovesicles: identification of regulators of apical recycling. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1249–1262. doi: 10.1152/ajpgi.00505.2006. [DOI] [PubMed] [Google Scholar]
- 20.Solomon S, Xu Y, Wang B, David MD, Schubert P, Kennedy D, Schrader JW. Distinct structural features of caprin-1 mediate its interaction with G3BP-1 and its induction of phosphorylation of eukaryotic translation initiation factor 2alpha, entry to cytoplasmic stress granules, and selective interaction with a subset of mRNAs. Mol Cell Biol. 2007;27:2324–2342. doi: 10.1128/MCB.02300-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steadman BT, Schmidt PH, Shanks RA, Lapierre LA, Goldenring JR. Transforming acidic coiled-coil-containing protein 4 interacts with centrosomal AKAP350 and the mitotic spindle apparatus. J Biol Chem. 2002;277:30165–30176. doi: 10.1074/jbc.M201914200. [DOI] [PubMed] [Google Scholar]
- 22.Shanks RA, Larocca MC, Berryman M, Edwards JC, Urushidani T, Navarre J, Goldenring JR. AKAP350 at the Golgi apparatus. II. Association of AKAP350 with a novel chloride intracellular channel (CLIC) family member. J Biol Chem. 2002;277:40973–40980. doi: 10.1074/jbc.M112277200. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi M, Mukai H, Oishi K, Isaawa T, Ono Y. Association of immature hypophosphorylated protein kinase Ce with an anchoring protein CG-NAP. J.Biol.Chem. 2000;275:34592–34596. doi: 10.1074/jbc.M005285200. [DOI] [PubMed] [Google Scholar]
- 24.Gillingham AK, Munro S. The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep. 2000;1:524–529. doi: 10.1093/embo-reports/kvd105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi M, Mukai H, Oishi K, Isagawa T, Ono Y. Association of immature hypophosphorylated protein kinase cepsilon with an anchoring protein CG-NAP. J Biol Chem. 2000;275:34592–34596. doi: 10.1074/jbc.M005285200. [DOI] [PubMed] [Google Scholar]
- 26.Sillibourne JE, Milne DM, Takahashi M, Ono Y, Meek DW. Centrosomal anchoring of the protein kinase CK1delta mediated by attachment to the large, coiled-coil scaffolding protein CG-NAP/AKAP450. J Mol Biol. 2002;322:785–797. doi: 10.1016/s0022-2836(02)00857-4. [DOI] [PubMed] [Google Scholar]
- 27.Rishi AK, Zhang L, Boyanapalli M, Wali A, Mohammad RM, Yu Y, Fontana JA, Hatfield JS, Dawson MI, Majumdar AP, Reichert U. Identification and characterization of a cell cycle and apoptosis regulatory protein-1 as a novel mediator of apoptosis signaling by retinoid CD437. J Biol Chem. 2003;278:33422–33435. doi: 10.1074/jbc.M303173200. [DOI] [PubMed] [Google Scholar]
- 28.Rishi AK, Zhang L, Yu Y, Jiang Y, Nautiyal J, Wali A, Fontana JA, Levi E, Majumdar AP. Cell cycle- and apoptosis-regulatory protein-1 is involved in apoptosis signaling by epidermal growth factor receptor. J Biol Chem. 2006;281:13188–13198. doi: 10.1074/jbc.M512279200. [DOI] [PubMed] [Google Scholar]
- 29.Ellis JA, Luzio JP. Identification and characterization of a novel protein (p137) which transcytoses bidirectionally in Caco-2 cells. J Biol Chem. 1995;270:20717–20723. doi: 10.1074/jbc.270.35.20717. [DOI] [PubMed] [Google Scholar]
- 30.Grill B, Wilson GM, Zhang KX, Wang B, Doyonnas R, Quadroni M, Schrader JW. Activation/division of lymphocytes results in increased levels of cytoplasmic activation/proliferation-associated protein-1: prototype of a new family of proteins. J Immunol. 2004;172:2389–2400. doi: 10.4049/jimmunol.172.4.2389. [DOI] [PubMed] [Google Scholar]
- 31.Wang B, David MD, Schrader JW. Absence of caprin-1 results in defects in cellular proliferation. J Immunol. 2005;175:4274–4282. doi: 10.4049/jimmunol.175.7.4274. [DOI] [PubMed] [Google Scholar]
- 32.Kedersha N, Anderson P. Mammalian stress granules and processing bodies. Methods Enzymol. 2007;431:61–81. doi: 10.1016/S0076-6879(07)31005-7. [DOI] [PubMed] [Google Scholar]
- 33.Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell. 2004;15:5383–5398. doi: 10.1091/mbc.E04-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kedersha N, Cho MR, Li W, Yacono PW, Chen S, Gilks N, Golan DE, Anderson P. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J Cell Biol. 2000;151:1257–1268. doi: 10.1083/jcb.151.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivanov PA, Chudinova EM, Nadezhdina ES. Disruption of microtubules inhibits cytoplasmic ribonucleoprotein stress granule formation. Exp Cell Res. 2003;290:227–233. doi: 10.1016/s0014-4827(03)00290-8. [DOI] [PubMed] [Google Scholar]
- 36.Gillingham A, Munro S. The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO reports. 2000;1:524–529. doi: 10.1093/embo-reports/kvd105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi M, Yamagiwa A, Nishimura T, Mukai H, Ono Y. Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring gamma-tubulin ring complex. Mol Biol Cell. 2002;13:3235–3245. doi: 10.1091/mbc.E02-02-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witczak O, Skalhegg BS, Keryer G, Bornens M, Tasken K, Jahnsen T, Orstavik S. Cloning and characterization of a cDNA encoding an A-kinase anchoring protein located in the cetrosome, AKAP450. EMBO J. 1999;18:1858–1868. doi: 10.1093/emboj/18.7.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwon S, Zhang Y, Matthias P. The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Genes Dev. 2007;21:3381–3394. doi: 10.1101/gad.461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aizer A, Brody Y, Ler LW, Sonenberg N, Singer RH, Shav-Tal Y. The Dynamics of Mammalian P Body Transport, Assembly and Disassembly In Vivo. Mol Biol Cell. 2008 doi: 10.1091/mbc.E08-05-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ginsberg MD, Feliciello A, Jones JK, Avvedimento EV, Gottesman ME. PKA-dependent binding of mRNA to the mitochondrial AKAP121 protein. J Mol Biol. 2003;327:885–897. doi: 10.1016/s0022-2836(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 42.Rook MS, Lu M, Kosik KS. CaMKIIalpha 3′ untranslated region-directed mRNA translocation in living neurons: visualization by GFP linkage. J Neurosci. 2000;20:6385–6393. doi: 10.1523/JNEUROSCI.20-17-06385.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Database of mRNA transcripts associated with AKAP350A complex. Inclusion required 8-fold enrichment of a transcript in the immunoisolate over IgG control immunoisolates. Information is provided for the 69 mRNA transcripts identified in complexes immunoisolated from both untreated and arsenite-treated cells. In addition, information is shown for transcripts that were present in the immunoisolates from untreated cells but not arsenite-treated cells, and transcripts present in immunoisolates from arsenite-treated cells but not in untreated cells.
Knockdown of AKAP350 alters TIA-1 labeled stress granules. HeLa cells were transfected with non-specific scrambled RNA duplexes or siRNA duplexes specific for AKAP350A. After fixation cells were stained for AKAP350 (red in merged images), G3BP (green in merged images) and TIA-1 (blue in merged images). HeLa cells with normal and reduced levels of AKAP350A expression were treated with 0.5 mM arsenite for 30 min. In AKAP350A siRNA treated cells, stress granules were smaller and showed positive staining for both G3BP and TIA-1. Bar = 10 μm.
Mass spectrometry identification of AKAP350A-complex composition. Sequences of peptides identified are shown for each protein.
Formation and dynamic of stress granules in normal HeLa cells (A) and nocodazole treated HeLa cells (B). HeLa cells were transfected with G3BP-mCherry, and only cells with low expression were chosen for microscopy. These videos correspond to still images shown in Fig. 4. Two hours before imaging, cells were pre-treated with nocodazole to disperse microtubules. Development of stress granules after addition of 0.5 mM arsenite in nocodazole pre-treated cells was delayed and movement was chaotic, versus well-organized distribution around nucleus in cells with intact microtubules. 120 images were captured over a period of 40 min (Frames were taken 20 sec apart during 40 min), animation rate was 6 frames/sec.
Formation and dynamic of stress granules in normal HeLa cells (A) and nocodazole treated HeLa cells (B). HeLa cells were transfected with G3BP-mCherry, and only cells with low expression were chosen for microscopy. These videos correspond to still images shown in Fig. 4. Two hours before imaging, cells were pre-treated with nocodazole to disperse microtubules. Development of stress granules after addition of 0.5 mM arsenite in nocodazole pre-treated cells was delayed and movement was chaotic, versus well-organized distribution around nucleus in cells with intact microtubules. 120 images were captured over a period of 40 min (Frames were taken 20 sec apart during 40 min), animation rate was 6 frames/sec.
Localization of AKAP350A, caprin-1, CCAR1 and Phospho-eIF2α prior to stress. HeLa cells growing in normal conditions were fixed and immunostained for AKAP350A, caprin-1, CCAR1 or Phospho-eIF2α (green in merged images) and G3BP (red in merged images). Merged images are shown at right. Bar = 10 μm.
Localization of AKAP350 in stress granules. HeLa cells were treated with 0.5 mM arsenite for 30 min followed by addition of 20 μg/ml of emetine for 1 h. After fixation cells were stained for AKAP350A or Phosphorylated-eIF2α (red in merged images), G3BP (green in merged images) and GW182 (blue in merged images). AKAP350 was predominantly associated with stress granules markers, although some of biggest granules were positive for both the stress granules marker (G3BP) and the P bodies marker (GW182). Emetine treatment eliminated association of AKAP350 and Phospho-eIF2α with G3BP-positive stress granules and led to relocation of AKAP350 and Phospho-eIF2α to the cytosol. Bar = 10 μm.
Localization of CCAR1 and caprin-1 in stress granules. HeLa cells were treated with 0.5 mM arsenite for 30 min followed by addition of 20 μg/ml of emetine for 1 h. After fixation cells were stained for CCAR1 or caprin-1 (red in merged images), G3BP (green in merged images) and GW182 (blue in merged images). Both CCAR1 and caprin-1 were predominantly associated with stress granules markers, although some of biggest granules were positive for both the stress granule marker (G3BP) and P body marker (GW182). Emetine treatment eliminated the association of CCAR1 and caprin-1 with G3BP-positive stress granules. Bar = 10 μm.
Localization of AKAP350A and TIA-1 in stress granules. HeLa cells were treated with 0.5 mM arsenite for 30 min followed by addition of 20 μg/ml of emetine for 1 h. After fixation cells were stained for AKAP350 (red in merged images), G3BP (green in merged images) and TIA-1 (blue in merged images). AKAP350 was associated with both G3BP and TIA-1 markers of stress granules. Emetine treatment led to relocation of all three proteins to cytosol. Bar = 10 μm.
Nocodazole inhibited delivery of AKAP350 to stress granules (A) and led to relocation of majority of phosphorylated-eIF2α from stress granules to the cytosol (B). HeLa cells were treated with arsenite in the absence (control) or presence of nocodazole pre-treatment. Microtubule disassembly with nocodazole was confirmed with α–tubulin staining (see Supplemental Fig. S7). AKAP350 or Phosphorylated-eIF2α (red in merged images), G3BP (green in merged images) and GW182 (blue in merged images). Enlarged images are shown in insets. Note that nocodazole treatment resulted in separation of AKAP350A from G3BP-positive stress granules. Bar = 10 μm.
Disruption of microtubules by nocodazole treatment led to displacement of CCAR1 (A), but not caprin-1 (B), from G3BP-positive stress granules. Control or nocodazole-treated HeLa cells were fixed and immunostained for either CCAR1 or caprin-1 (red in merged images), G3BP (green in merged images) and GW182 (blue in merged images). Disruption of microtubules caused formation of a greater number of smaller G3BP-positive stress granules that contained caprin-1, but not CCAR1. Bar = 10 μm.
Disruption of microtubules in Hela cells. Hela cells were treated with nocodazole as described in Material and methods and stained for α-tubulin. Treatment led to complete disruption of the microtubule network. Bar = 10 μm.
Knockdown of AKAP350 alters stress granule formation. HeLa cells were transfected with non-specific scrambled RNA duplexes or siRNA duplexes specific for AKAP350A. Quantitation of G3BP in stress granules based on size (μm) or the number of stress granules per cell demonstrates that reduction of level of AKAP350 expression elicits a greater number of smaller G3BP-positive stress granules. Solid bars: scrambled control; hatched bars: AKAP350 knockdown. *p<0.001. The results are the mean±SE.