Abstract
In contrast to eukaryotes, most cyanobacteria contain several isoforms of the Rieske iron-sulfur protein, PetC, resulting in heterogeneity in the composition of the cytochrome b6f complexes. Of three isoforms in the mesophilic cyanobacterium Synechocystis PCC 6803, PetC1 is the major Rieske protein in the cytochrome b6f complex, whereas the physiological function of PetC2 and PetC3 is still uncertain. Comparison of wild type and various petC-deficient strains under selected light conditions revealed distinct functional differences: high-light exposure of wild type cells resulted in a significantly enhanced petC2 transcript level, whereas a ΔpetC1 mutant showed a low cytochrome b6f content, low electron flux, and a considerably increased accumulation of cytochrome-bd oxidase. In contrast to wild type and ΔpetC1, ΔpetC2 and ΔpetC3 strains still grew fast under high-light conditions although all three Rieske proteins are required for maximal electron transport rates. Although the presence of PetC3 appears to be required for activation of the cyclic electron transport, state transitions were more effective in the absence of PetC2 and/or PetC3. In summary, our data suggest defined roles of the various PetC proteins in short- and long-term light adaptation.
In cyanobacteria the cytochrome b6f complex is a shared component of the linear electron transport from water to NADP+, of all types of cyclic electron transfer around photosystem 1 (PS1),3 and of most types of pseudocyclic electron flows from water to the molecular oxygen (1–3). Therefore, this complex has a central and essential role in the complex cyanobacterial electron transport network (2). In general, the cytochrome b6f complex catalyzes plastoquinol (PQH2) oxidation and plastocyanin/cytochrome c6 reduction and translocates protons across the thylakoid membrane (for recent reviews see Refs. 4–8).
Although linear photosynthetic electron transport from PS2 via cytochrome b6f to PS1 results in both NADPH production and generation of a proton gradient (ΔpH) across the thylakoid membranes, cyclic electron flow(s) around PS1 establish(es) only a ΔpH, which is subsequently used for ATP generation. Thus, depending on the environmental (stress) and/or physiological conditions, the ATP:NADPH ratio can be adjusted to the required levels by tuning the cyclic-to-linear electron transport ratio. Beyond this, a redox poise of the PQ-pool via the cyclic/linear electron transport ratio adjustment is essential for both maximal light energy utilization and protection against photodamage or other types of stress (9–14). The dominant short-term (minutes) light-adaptation process in chloroplasts and cyanobacteria, by which the distribution of excitation energy transfer between the two photosystems is controlled, are the so-called state transitions (15, 16). By definition, in state I and state II the photosynthetic apparatus gives an optimal quantum yield of photosynthesis in light having a composition favoring its absorption by PS1 or PS2, respectively (17, 18). In the green alga Chlamydomonas reinhardtii state transitions are associated with a switching mechanism between linear and cyclic electron flow. Although state I, the light harvesting complex II (LHCII) is associated mainly with PS2 and is dominated by linear electron transport, state II with PS1-associated LHCII is governed by the cyclic one (18–20). In chloroplasts, state I to state II transition correlates with the activation of a kinase, which phosphorylates the LHCII leading to its lateral redistribution in favor of PS1. This activation is initiated by PQ-pool reduction and PQH2 binding to the Qo site of the cytochrome b6f complex (21–23), which is suggested to result in a conformational change of the Rieske protein, a cytochrome b6f core subunit, and finally leading to kinase activation (18, 23–25). Despite analogies to higher plants, the exact mechanism of state transitions in cyanobacteria is still under debate (for reviews, see Refs. 26 and 27). One major difference between chloroplasts and cyanobacteria is that instead of the membrane integral LHCII, the soluble light-harvesting phycobilisomes (PBS) capture light in cyanobacteria. They are located on the cytoplasmic surface of thylakoid membranes (28, 29) and transfer energy directly to the photosystems (30). Different from higher plants and green algae, cyanobacteria are in state I upon illumination and in state II in the dark or in very low light due to the respiratory electron flow reducing the PQ-pool (31, 32). According to available data, in cyanobacteria both the relative energy transfer from phycobilisomes to photosystems and the distribution of the absorbed light energy are regulated by state transitions, although the components of the regulatory signal cascade are essentially not known (26). Although no Rieske protein-mediated kinase activation has been described in cyanobacteria, it is assumed that the triggering mechanism is similar in cyanobacteria (26). Thus, besides its above described central function in electron transport and energy transduction processes, the cytochrome b6f complex can also be involved in regulating excitation energy distribution between PS1 and PS2 (4, 18, 20–26), as well as in an assembly mediated control of protein synthesis (25). Experimental data indicate that the Rieske iron-sulfur protein plays a key role in both processes.
Rieske iron-sulfur proteins are essential functional subunits of the cytochrome b6f complex. The Rieske protein binds a redox-active [2Fe-2S] center close to the p-side of the membranes and carries electrons in the high-potential branch of the bifurcated electron transport chain of the complex (4, 5, 7, 8). The cytochrome b6f monomer consist of four large (17.5–32 kDa) core subunits, cytochrome f (PetA), cytochrome b6 (PetB), the Rieske iron-sulfur protein (PetC), and subunit IV (SU IV, PetD), as well as four additional small (3.3–4.1 kDa) subunits, PetG, PetL, PetM, and PetN (7, 33). Except for PetL and PetM all subunits are required for a functional cytochrome b6f complex (34, 35). Besides these bona fide subunits additional weakly associated subunits are also reported (36, 37). The PetC (Rieske) protein has a single N-terminal located transmembrane helix followed by a mobile, water-soluble domain with the [2Fe-2S] cofactor binding regions, which extends into the thylakoid lumen. The α-helix and the soluble domain are connected by a flexible “hinge” region.
In the genome of eukaryotes and in some cyanobacteria the cytochrome b6f complex Rieske protein is encoded by a single petC gene. In contrast, in most cyanobacteria several Rieske isoforms exist, which are encoded by a petC gene family (for a review, see Ref. 38). The Synechocystis PCC 6803 genome contains three petC genes, petC1 (sll1316), petC2 (slr1185), and petC3 (sll1182) (39), which encode corresponding Rieske proteins PetC1, PetC2, and PetC3, respectively. Although PetC1 and PetC2 show high sequence identity, PetC3 differs significantly. In vivo analysis of petC gene-deficient mutant strains have indicated that PetC1 is the major Rieske iron-sulfur protein in Synechocystis PCC 6803 (40). Moreover, PetC3 has a much lower midpoint potential (+135 mV) than PetC1 and PetC2 (300–320 mV), suggesting that different electron donors have to reduce PetC3 (41). In Nostoc sp. PCC 7120, the PetC4 protein, which has a high sequence similarity to PetC1 and PetC2, has a role in nitrogen fixation and/or heterocyst formation and is also proposed to be a redox sensor (38). However, despite these suggestions, the physiological significance of petC multiplicity in cyanobacteria remains unclear.
In the present report we analyze low- and high-light-treated wild type (WT) and petC-deficient Synechocystis PCC 6803 strains on the transcript, the protein, and on a functional level to assign specific functions to the three different PetC proteins in light acclimation and electron transport processes. We observed alterations in cell growth of different ΔpetC mutant strains under high-light conditions. Our experiments also revealed various effects of the individual Rieske proteins on photosynthetic electron transport and the distribution of excitation energy between the two photosystems. Furthermore, accumulation of petC2 mRNA in WT cells as response to high light was shown by real time quantitative PCR (qPCR). Based on these results, we discuss the potential roles of the Synechocystis PetC1, PetC2, and PetC3 in regulation and light adaptation and propose a functional model with regulatory pathways.
EXPERIMENTAL PROCEDURES
Growth Conditions
Construction of single and double petC deletion (ΔpetC) strains from Synechocystis PCC 6803 is described in detail in Ref. 40. Synechocystis WT and mutant strains were grown in BG-11 medium (42) at 30 °C in glass tubes bubbled with 5% CO2-enriched air. Liquid medium for culturing ΔpetC1, ΔpetC2, and ΔpetC3 strains as well as agar plates were supplemented with 30 μg/ml of chloramphenicol, 100 μg/ml of spectinomycin, or 50 μg/ml of kanamycin, respectively. The culture medium for double mutants contained both corresponding antibiotics. Low-light (LL, 40 μmol photons m−2 s−1) and medium to high-light (HL) illumination (100–650 μmol photons m−2 s−1) were provided by white fluorescent and halogen lamps, respectively. Photon flux densities were determined with a QRT1 light meter (Hansatech).
Pigment Analysis
Pigment concentrations of the cultures are given per cell number with cell densities determined with a Z2 Coulter counter (Beckman Coulter, Fullerton, CA). Absorption spectra were recorded by a Hitachi U-3000 dual-beam spectrophotometer equipped with a 60-mm integrating sphere. Phycocyanin and chlorophyll concentrations were determined according to Ref. 43, and the phycocyanin/allophycocyanin ratio as in Ref. 44.
RNA Preparation and Quantitative PCR
10-ml cultures of WT cells were harvested at A730 = 0.2 by centrifugation (6000 × g, 4 °C, 10 min), suspended in 1 ml of diluted 1:4 RNA Protect Bacteria Reagent (Qiagen) in Tris-EDTA buffer (TE), and subsequently incubated on ice. Upon washing with TE buffer cells were suspended in 1 ml of saturated sodium iodide solution and subsequently washed twice with TE buffer. The cells were disrupted with glass beads in 350 μl of RNeasy lysis buffer (Qiagen) using a Mini-Beadbeater (Biospec). Isolation of total RNA was accomplished using the RNeasy mini kit (Qiagen) according to the manufacturer's instructions. The obtained RNA was treated with 2 units of TURBO DNase (Ambion) for 20 min at 37 °C to remove DNA contamination, and the reaction was subsequently terminated by addition of the DNase inactivation reagent (Ambion). 1 μg of purified RNA was used for cDNA synthesis using a Quantitect reverse transcription kit (Qiagen) according to the manufacturer's instructions.
cDNA synthesis was followed by real time qPCR in a DNA Engine Opticon 2 Real-time PCR Detection System (Bio-Rad), using 20 μl of reaction mixture supplied by the qPCR Core kit for SYBR® Green I (Eurogentec) containing 250 nm primers. The following primers were used for amplification: petC1F (5′-CCAGTACAACGCTGAAGGT-3′) and petC1R (5′-CCAGGTGCTGAGCACTAAT-3′) for petC1; petC2Fneu2 (5′-CCGTCGTCAATTACTCAACT-3′) and petC2Rneu2 (5′-TTGGCAATAATGCCTCCTC-3′) for petC2; petC3F (5′-AGTAACGGCCAAGGACAA-3′) and petC3R (5′-CGCACTAATCTGGCCACTA-3′) for petC3; rnpBF (5′-GAGAGTTAGGGAGGGAGTTG-3′) and rnpBR (5′-GTCAGTTACCAGTTAGTCAGC-3′) for rnpB. 40 cycles with a 15-s incubation at 95 °C and a 1-min incubation at 60 °C were applied. Changes in the mRNA levels were determined by relative quantification (45, 46). Threshold cycle numbers (Ct), real time PCR efficiency (E), and relative expression ratios were calculated using Opticon Monitor 2.02, LinRegPCR (47), and the Relative Expression Software Tool (REST®) (48), respectively.
SDS-PAGE and Western Blot Analysis
45 ml of WT and ΔpetC cells were harvested at A730 = 0.2 and dispersed in 75 μl of SDS-PAGE loading buffer. Cells were broken by vortexing with glass beads and a subsequent 15-min sonication (Brandelin Sonorex, Berlin, Germany) at 4 °C, followed by a 5-min incubation at 95 °C. After centrifugation, the supernatant was collected and stored at −20 °C until use. 5 μl of supernatant was separated by SDS-PAGE on a 12% SDS gel (49) and blotted on a polyvinylidene difluoride membrane. PetC1/2/3, cytochrome f, cytochrome b6, SU IV, as well as the CydA subunit of the cytochrome-bd oxidase were detected by immunoblotting using polyclonal antibodies (37, 41). The PetC2 and CydA antibodies was raised against synthesized peptide fragments DQIFISPWTDLDPRTGEKP (Iwaki, Tokyo, Japan) and KLAAMEALWETVPA (Davids Biotechnologie, Regensburg, Germany), respectively. The rabbit primary antibodies and the goat anti-rabbit IgG alkaline phosphatase-conjugated secondary antibody (Sigma) were used at dilutions of 1:2500 and 1:25000, respectively. Membranes were incubated with nitro blue tetrazolium chloride and 5-bromo-4-chloro-3′-indolyl phosphatase p-toluidine salt (Roche) at room temperature to visualize cross-reacting protein bands. Band intensities for protein quantification were established using ImageJ.
Measurement of State Transitions
State transitions in WT and mutant cells were monitored by two distinct methods. Although low temperature fluorescence emission spectra were recorded after PBS excitation as described previously (37), chlorophyll fluorescence transients at room temperature were measured in a Dual-PAM-100 measuring system (Heinz Walz GmbH) by adapting the method of Kovács and co-workers (50) to cyanobacteria. State I to state II and state II to state I transitions were induced by switching off and on PS1 light, respectively. PS1 was selectively excited by far red (FR) light using 730 nm LEDs of the DUAL-E measuring head set to their maximal light intensity (∼40 relative units). 300-ms saturating pulses (I = 10000 μmol photons m−2 s−1) were applied in state I and state II to probe the FmI and FmII fluorescence levels, respectively.
P700+ Reduction Kinetics and Quantification of the Linear and Cyclic Electron Transport Rates
P700+ reduction kinetic decays in LL- and HL-adapted cells were recorded with a dual PAM-100 measuring system at a chlorophyll concentration of 5 μg/ml as described in Ref. 51. Complete P700 oxidation was achieved by 100-ms saturating pulses (I = 10000 μmol photons m−2 s−1). Averages of three individual traces were taken. Rate constants (k) in the absence or presence of linear and/or (direct) cyclic electron transport inhibitors, 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea (DCMU) and methyl viologen (MV), respectively, were determined by fitting the decays by single exponential functions. The linear and cyclic electron transport activities were quantified as the differences of certain rate constant combinations. Although the rate constant of the linear electron transport was considered to be the mathematical difference of the rate constants determined in non-treated and DCMU-treated samples, the direct cyclic electron transport was calculated as difference of the corresponding k values of non-treated and MV-treated samples. Note that the direct cyclic electron transport may consist of several parallel individual routes (3), which were not distinguished.
RESULTS
Pigment and Protein Composition
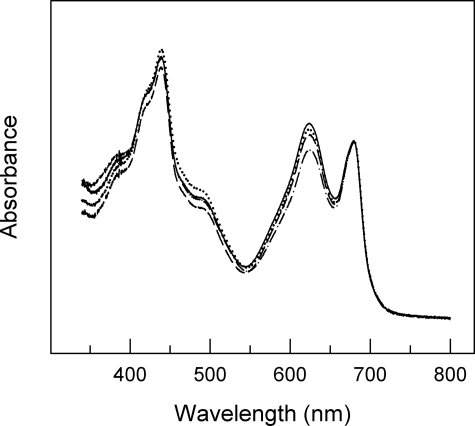
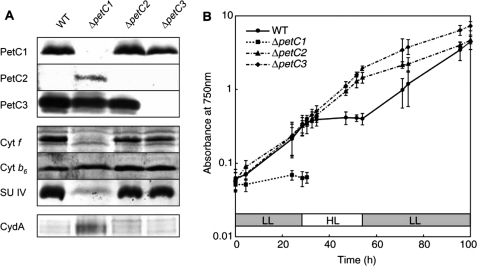
Up to now the physiological function of petC heterogeneity in cyanobacteria is essentially not understood. Usually, the construction of knock-out strains is a highly valuable standard method to elucidate the potential in vivo function of such proteins. The generation of ΔpetC Synechocystis strains has been reported recently (40), and it has already been shown that electron transfer is modified at least in the ΔpetC1 strain and that PetC2 can partly replace the major Rieske protein PetC1. However, it is unknown whether the absence of PetC1 (or the other PetC isoforms) influences pigment compositions in the cells or affects the expression, accumulation, or stability of the cytochrome b6f complex. To test these issues, we analyzed the chlorophyll, phycocyanin (PC), and allophycocyanin (APC) contents as well as the accumulation of the four large cytochrome b6f complex core subunits (including all three PetC isoforms) in WT and various ΔpetC strains (Figs. 1 and 2A). Absorption spectra of WT and the three petC single deletion mutants are shown in Fig. 1. All strains have the typical major chlorophyll absorption peaks at 439 and 679 nm and a PBS absorption peak at 625 nm. The PC/chlorophyll ratio is very similar in WT, ΔpetC1, and ΔpetC3 strains (Table 1), whereas it is significantly lowered in the ΔpetC2 strain. Noteworthy, by light microscopy we observed that the ΔpetC3 cells are considerably bigger (3.4 versus 2.7 μm) when compared with the WT or to the other deletion strains. This increased cell size results in increased chlorophyll and PC content per cell (Table 1), although the PC/chlorophyll ratio is similar to that for WT cells. Furthermore, the ratio of PC to APC is about the same in all but the ΔpetC1 strain (Table 1). This indicates an unchanged protein composition of the light harvesting PBSs in the absence of petC2 or petC3 genes and a decrease in size of the PBS rod complexes upon petC1 deletion.
FIGURE 1.
Absorption spectra of WT (solid line) and petC-deficient Synechocystis PCC 6803 strains (ΔpetC1, dashed; ΔpetC2, dash-dotted; ΔpetC3, dotted). Spectra are normalized to the absorption peak at 679 nm.
FIGURE 2.
A, immunological analysis of WT and petC-deficient Synechocystis PCC 6803 strains using antibodies against the major subunits of the cytochrome b6f complex (cytochrome f, cytochrome b6, and SU IV) and CydA. B, effects of HL on growth rates of WT and ΔpetC mutants. Cultures with identical initial optical densities have been exposed to LL for 28 h, followed by HL illumination for 30 h, and then by LL again. Error bars indicate the S.D. of three to five independent experiments.
TABLE 1.
Pigment composition of Synechocystis PCC 6803 WT and petC single deletion strains
Cells were grown under LL. Data are expressed as mean ± S.D. (n = 3). Determination of the various parameters and ratios is described under “Experimental Procedures.”
| Strain | Chlorophyll/10 cells | PC/10 cells | PC/chlorophyll | PC/APC |
|---|---|---|---|---|
| μg | μg | μg | μg | |
| WT | 3.8 ± 0.5 | 25 ± 4 | 6.6 ± 0.3 | 3.6 |
| ΔpetC1 | 4.5 ± 0.8 | 26 ± 7 | 5.6 ± 0.6 | 1.4 |
| ΔpetC2 | 4.0 ± 1.3 | 20 ± 8 | 4.8 ± 0.5 | 3.4 |
| ΔpetC3 | 7.4 ± 1.4 | 46 ± 10 | 6.2 ± 0.3 | 3.0 |
Expression of the individual PetC isoforms in the various strains as well as expression of the other three cytochrome b6f complex core subunits was tested by immunoblot analysis (Fig. 2A). Although all three PetC isoforms are expressed in the WT strain (see below), only PetC1 and PetC3 were detected by immunoblot analysis. The cellular content of PetC1 and PetC3 is similar in the WT and mutant strains where the corresponding genes are not deleted (Fig. 2A). Because the amount of the other three large cytochrome b6f subunits in ΔpetC2 and ΔpetC3 strains were similar to WT cells (Fig. 2A) a comparable cytochrome b6f complex population size can be assumed in these mutant strains. However, the protein profile in the ΔpetC1 strain is quite different when compared with WT cells and other mutant strains. First, expression of the PetC2 protein was only detected in this strain by immunoblot analysis. Second, this mutant also showed a drastic decrease of accumulated cytochrome f and SU IV (Fig. 2A) indicating a defect in formation of functional cytochrome b6f complexes upon deletion of petC1. As an increase in the cytochrome-bd oxidase activity in the ΔpetC1 strain had already been observed (35, 40), we also tested the expression of the major cytochrome-bd oxidase subunit I (CydA) both in WT and ΔpetC strains (Fig. 2A): whereas CydA was essentially undetectable by immunoblot analysis in WT, ΔpetC2, and ΔpetC3 strains, its expression was dramatically increased in the ΔpetC1 strain. This is in good agreement with earlier observations of a significantly increased cytochrome-bd oxidase activity in the ΔpetC1 strain (40). Most likely, due to cytochrome b6f complex deficiency in the ΔpetC1 strain, cytochrome-bd oxidase has to partly take over the role of the cytochrome b6f complex in PQH2 re-oxidation to keep electron transport and energy transduction efficient, i.e. to keep the cells alive.
Effect of Light Conditions on Growth Rates of WT and petC-deficient Synechocystis Strains
Although Synechocystis ΔpetC single and double deletion strains have already been initially characterized (40), these results reflect only cells grown under LL growth conditions. To investigate the effect of light intensity, these mutant cells were grown under LL for 1 day followed by HL illumination for 30 h and a subsequent reset to the LL regime. In agreement with our previous results (40), WT, ΔpetC2, and ΔpetC3 strains showed approximately the same growth rates, whereas the ΔpetC1 strain grew significantly slower under LL growth conditions. Upon switching from LL to HL, the ΔpetC1 cells died and the cultivation was discontinued (Fig. 2B). HL also had a severe impact on the viability of WT cells (Fig. 2B), slowing down its growth rate gradually to zero during the first 6 h of HL illumination. These cultures returned to normal growth under LL conditions. In contrast, ΔpetC2 and ΔpetC3 strains showed constant growth during the continuous 30-h HL, similar to the ΔpetC2/C3 double deletion strain (data not shown). A WT strain with an intact genome should typically not be less resistant against light stress than knock-out strains, these results could indicate that the petC2 and petC3 gene products are involved in regulatory processes, which might protect the organism against photo-damage.
High Light Induces an Elevated petC2 Gene Expression
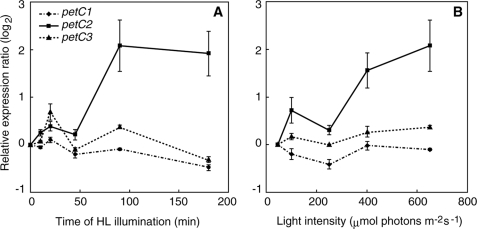
To further elucidate the role of the various petC gene products in light acclimation we examined the effect of high light on the mRNA expression level of the three petC genes in Synechocystis PCC 6803 WT by qPCR. For these experiments, the expression of the rnpB gene, which is independent of light intensity (52), was used as the internal control. In qPCR analysis, the threshold cycle number (Ct) is the fractional PCR cycle number, at which the emitted fluorescence exceeds the fixed threshold (background) fluorescence, i.e. a higher initial amount of a specific mRNA in a given sample results in an earlier detection in the PCR as fluorescence increase. Therefore, a lower Ct value reflects a higher expression of a certain gene. Ct values for the petC1, petC2, petC3, and rnpB genes in LL-adapted samples were 21.25 ± 0.90, 31.34 ± 1.85, 24.83 ± 1.01, and 17.12 ± 1.15, respectively, whereas the corresponding values in cells illuminated by HL for 90 min were 19.70 ± 0.74, 26.73 ± 0.87, 23.71 ± 0.64, and 16.12 ± 0.75, respectively (n = 9). Calculated PCR efficiencies (E) for petC1, petC2, petC3, and rnpB were 1.76, 1.52, 1.70, and 1.96, respectively. These results clearly show that under both LL and HL conditions the expression level of petC2 was lower than petC1 and petC3 in Synechocystis, which was confirmed with different primer sets for the petC2 transcript amplification (data not shown). This significant decrease of the petC2 Ct value under HL conditions clearly indicates an induction of the petC2 expression. The time course of petC transcript accumulation during HL is shown in Fig. 3A, whereas Fig. 3B shows the dependence of petC expression after a 90-min exposure to different light intensities. It is apparent from both panels that the expression levels of petC1 and petC3 are quite independent from the illumination time and light intensities, whereas petC2 expression increases with both light intensity and time. A significant increase in the petC2 transcript level was observed after a 90-min HL incubation (Fig. 3A) and above a light intensity of 350–400 μmol photons m−2 s−1 (Fig. 3B).
FIGURE 3.
Accumulation of petC transcripts in Synechocystis PCC 6803 WT cells as determined by qPCR analysis. The y axis shows the relative expression ratio (log2 scale), calculated by REST (see “Experimental Procedures”), relative to cells grown under LL conditions. The error bars represent the S.D. of three to nine independent measurements. A, cells at A750 = 0.2 were incubated under HL for 10, 20, 45, 90, or 180 min. B, accumulation of petC transcripts after incubation of Synechocystis cells at various light intensities. Cells were incubated for 180 min at elevated light intensities (100, 250, 400, and 650 μmol photons m−2 s−1).
Linear and Cyclic Electron Transport Activities
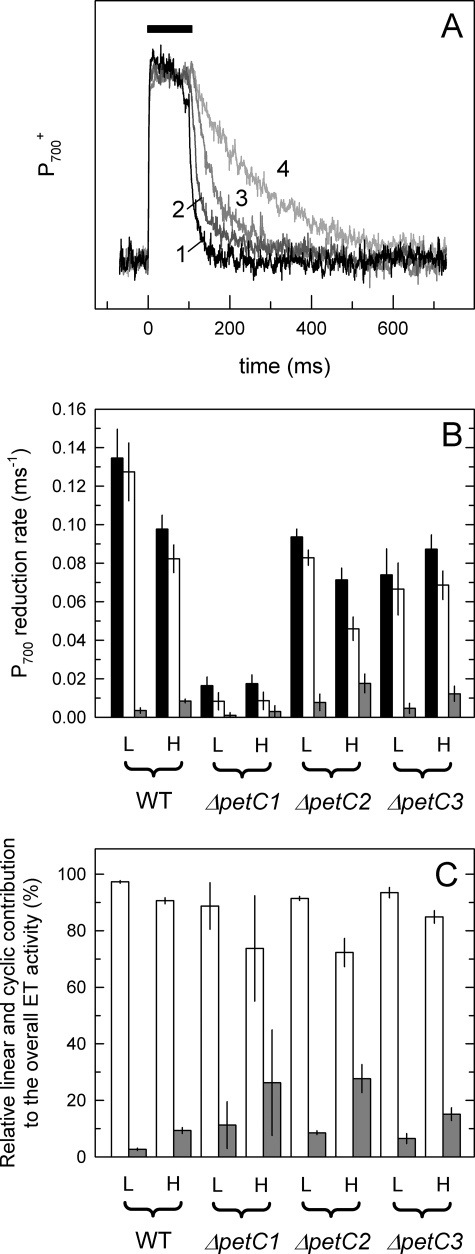
The petC2 transcript level is significantly enhanced in Synechocystis WT grown under HL conditions (Fig. 3), and growth of the ΔpetC2 and ΔpetC3 mutants was not suppressed by HL, in contrast to WT cells (Fig. 2B). To investigate the impact of petC deletions on electron transport activities under HL illumination, overall, linear and cyclic electron flow were quantified in LL- and HL-adapted Synechocystis cells using P700+ reduction kinetics. Fig. 4A illustrates the principles of this quantification: as an example, curves of WT Synechocystis cells adapted to LL conditions are shown. The time interval of the saturating pulse (100 ms, indicated as black rectangle over the traces) is followed by the (dark) reduction of P700+, which shows the fastest kinetics in untreated cells (curve 1). Addition of MV (curve 2), which blocks cyclic electron transport, DCMU (curve 3), which blocks linear electron transport, or a combination of them (curve 4), slowed down the reduction kinetics. The corresponding rate constants for linear and cyclic electron transport were determined as the difference of the two rate constants obtained by fitting the decays with single exponential functions in an appropriate combination (for details see “Experimental Procedures”). Fig. 4B summarizes the determined rates for Synechocystis WT and the various ΔpetC strains under LL and HL conditions and in Fig. 4C the relative contribution of linear and cyclic electron transport activities are shown. Although linear electron transport is the dominant form in all photoautotrophic cultures (Fig. 4B, white columns) both electron transport activities and their response to HL showed large variations between individual strains. First of all, the highest overall and linear electron transport activity was detected in WT cells with all three PetC proteins expressed. This indicates that all PetC isoforms are needed for maintaining maximal electron transport rate. Electron transport activities of LL cultures were considerably lower in the absence of PetC2 and/or PetC3. The observed very low electron transport activity in the ΔpetC1 strain (and also in the ΔpetC1/3 strain, data not shown) was most likely caused by the rather low population size of active cytochrome b6f complexes upon deletion of petC1 (compare Fig. 2A). Although in WT and ΔpetC2 strains the overall and linear electron transport rates are reduced under HL conditions in comparison to LL conditions, changes in ΔpetC1 and ΔpetC3 strains are within the range of error (Fig. 4B). In all strains an up-regulation of the cyclic electron transport was observed under HL conditions (Fig. 4C). Notably, the relative contribution of the cyclic electron transport was significantly higher in the two strains containing PetC3 (ΔpetC1 (26%) and ΔpetC2 (27%)) than in the ΔpetC3 deletion strains (14%) and WT cells (9%) (Fig. 4C).
FIGURE 4.
Quantification of linear and cyclic electron transport by P700 redox kinetic measurements. A, kinetic traces of LL-adapted WT cells. The black rectangle symbolizes the time interval of the saturating light pulse (100 ms), which is followed by the reduction of P700+ (in the dark). The fastest kinetics observed in the untreated sample (curve 1), which is slowed down in the presence of MV (curve 2) and/or DCMU (curves 3 and 4) due to blocked cyclic and/or linear route(s), respectively. Curves correspond to three averaged traces of the same sample. Rate constants were determined by fitting. the curves with single exponential functions. Individual rate constants of linear and cyclic electron transfer (B) were determined as described under “Experimental Procedures” and in Ref. 51, and for comparison normalized to the overall electron transport activities (C). Black, white, and gray columns represent the determined overall, linear, and cyclic activities, respectively. Electron transport rates are averaged from measurements with three to five independent cultures. Activities of HL samples have been determined after 180 min of HL illumination.
Energy Transfer and Short-term Light Adaptation (State Transitions)
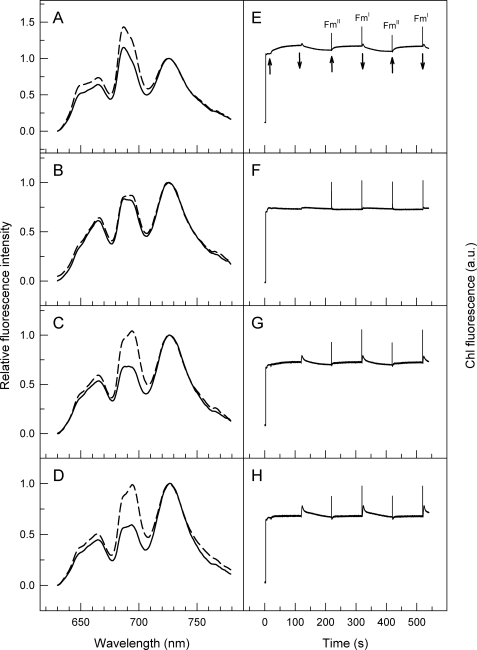
Upon excitation at 580 nm, low temperature (77 K) fluorescence emission spectra enable tracking of energy transfer from PBS antenna to photosystems in cyanobacteria. Although peaks at 685/695 and 725 nm (Fig. 5A) indicate energy transfer from PBS to PS2 and PS1, respectively, emission peak and the shoulder at 650 and 665 nm indicate functionally unconnected PC and APC, respectively. The relatively high PBS to PS1 energy transfer (Fig. 5A, solid line) confirms that dark adaptation induces state II in Synechocystis WT. In contrast, selective illumination of PS1 with far-red light oxidizes the PQ pool and induces state I with increased energy transfer from PBS to PS2 (Fig. 5A, dashed line). Comparison of WT with various petC mutants (Fig. 5, A–D) shows major differences in the capability of Synechocystis to perform state transitions: different from ΔpetC2 and ΔpetC3 strains (Fig. 5, C and D, respectively), ΔpetC1 cells seem to have essentially lost the capability for state transitions (Fig. 5B). Also, the “state transition efficiency” was higher in the ΔpetC2 and ΔpetC3 mutants than in WT (with the positions of PBS and photosystem emission peaks being identical). The spectral characteristics of double deletion strains ΔpetC1/3 and ΔpetC2/3 were similar to single deletion mutants ΔpetC1 and ΔpetC2 or ΔpetC3, respectively (data not shown). The high APC/PC ratio in the ΔpetC1 mutant deduced from absorption measurements (Table 1) was also seen in the fluorescence spectra of this strain (i.e. higher ratio of the fluorescence emission at 665 and 650 nm; Fig. 5B).
FIGURE 5.
State transitions followed by low temperature (77 K) fluorescence spectroscopy (A–D) or room temperature chlorophyll fluorescence (E – F) in WT (A and E), ΔpetC1 (B and F), ΔpetC2 (C and G), and ΔpetC3 (D and H) cells of Synechocystis PCC 6803. Left panels, excitation wavelength of 580 nm; solid and dashed curves represent two parallel samples that have been frozen after a 15-min dark incubation or after a subsequent FR illumination, respectively. All curves were normalized to the emission peak at 725 nm. Right panels, upward and downward arrows indicate “on” and “off” of FR illumination, respectively. Saturation pulses were given to record Fm in states I and II.
In addition to low temperature fluorescence emission spectra, state transitions can also be followed by room temperature chlorophyll fluorescence transients. According to the method of Ruban and co-workers (50), state transitions can be induced by switching on and off PS1 light in the presence of continuously applied PS2 light. Also, maximal fluorescence levels that should be different in states I and II (denoted as FmI and FmII, respectively) can be probed by saturating light pulses (50). Although the transients upon PS1-light on/off reflect the capacity of state transitions to balance the PQ redox state, FmI–FmII oscillations provide information on changes in the apparent (PS2) antenna cross-section.4 Fig. 5, E–H, show the results upon adaptation of this method for cyanobacteria. As in contrast to chloroplasts the cyanobacterial PQ-pool was significantly reduced in the dark (due to respiratory electron transfer), a pre-reduction of the PQ-pool by PS2 light was not required. PS1 light (Fig. 5E, left arrow) induces a gradual increase of chlorophyll fluorescence, reflecting an increasing PS2 antenna cross-section during state II to state I transition. Light off (downward arrow) causes a rapid transient rise that was followed by decay to the original dark level. This indicates a temporary increase in the PQ reduction level (absence of PS1 oxidation power), which was followed by PQ re-oxidation and a decrease of PS2 antenna cross-section (state I to II transition). Changes in the apparent antenna cross-section can be visualized by saturating light pulses after dark and FR periods, revealing FmII and FmI, respectively. Results presented in Fig. 5, F–H, agree well with low temperature fluorescence spectra (Fig. 5, B–D) in showing big differences in the petC-mutants to perform state transitions. In contrast to ΔpetC2 and ΔpetC3 strains (Fig. 5, G and H, in comparison with C and D, respectively), ΔpetC1 cells have lost the capability to perform state transitions (Fig. 5F): neither significant transient changes in the steady-state fluorescence level nor changes in the antenna cross-section were induced by switching on or off FR illumination. In agreement with the results of P700+ reduction kinetic experiments (Fig. 4) this shows an impaired electron flow through the cytochrome b6f complex, and in turn, a small impact of the PS1 on the plastoquinol oxidation. On the other hand, the FmI–FmII oscillation and fluorescence changes (with FR on and off) were more pronounced in ΔpetC2 and ΔpetC3 mutants than in WT (Fig. 5, G and H). This indicates significant transient changes in the PQ redox level that in turn induce a more robust change in the PS2 antenna cross-section. These dynamic changes in distribution of absorbed light energy between the two photosystems during state transitions can be followed by fluorescence measurements both at low (left panels) and at room temperature (right panels).
DISCUSSION
PetC1 Is the Major Rieske Protein
Although in higher plants and green algae the Rieske [2Fe-2S] protein of the cytochrome b6f complex is encoded by a single petC gene, in Synechocystis PCC 6803 and other cyanobacteria three (or even more) different petC genes code for a group of putative Rieske isoforms. On the phylogenetic tree of Rieske iron-sulfur proteins, only PetC1 of Synechocystis forms a branch with other cyanobacteria containing merely one petC gene, and this branch is phylogenetically close to the group of chloroplast Rieske proteins (38, 41). Based on an initial characterization of Synechocystis ΔpetC strains it has already been suggested that PetC1 is the major Rieske iron-sulfur protein in Synechocystis (40). Deletion of petC1 results in a highly reduced PQ-pool when respiratory electron transport is inhibited as well as in an increased PS1 to PS2 ratio (40), although this ratio was not altered in the ΔpetC2 or ΔpetC3 strains (40). In line with this, deletion of petC1 also results in diminished PS2 activity (40). Our present work further refines this predominant and crucial role of PetC1 in electron transport of Synechocystis. As evident from Fig. 4B (linear and cyclic), electron flow via the cytochrome b6f complex is severely affected in the ΔpetC1 strain, resulting in incomplete oxidation of the PQ-pool by PS1 (Fig. 5F) and in a light-sensitive growth phenotype (Fig. 2B). The diminished electron flux through the cytochrome b6f complex and, as a consequence, the easy reduction of the PQ-pool also severely affects the Synechocystis growth rate even under low-light conditions (Fig. 2B).
Due to the diminished electron transport through the cytochrome b6f complex, the PQ-pool appears never to be sufficiently oxidized by PS1 to induce a state transition in the ΔpetC1 strain, i.e. the cells remain in state II under all conditions (Fig. 5, B and F). A similar effect was recently reported for Synechocystis with the deleted ssr2998 gene (37). In these mutants the limited capabilities to control the PQ redox level by state transitions is compensated by adjusting the PS2 to PS1 ratio (37, 40). The lowered PC/APC ratio in the ΔpetC1 strain (Table 1, Fig. 5B) also decreases electron input into the PQ-pool and may help in maintaining the redox state of the PQ pool.
Deletion of the petC1 gene is apparently accompanied by increased cydA gene expression as shown by immunoblot analysis of CydA accumulation (Fig. 2A). Although an increased cytochrome-bd oxidase activity has already been suggested based on fluorescence induction measurements of mutants with impaired cytochrome b6f complexes (35, 40), this is the first direct proof for the presence of the cytochrome-bd oxidase in a cyanobacterial thylakoid membrane on the protein level. (For the original paper on the cytochrome-bd oxidase as a component of the cyanobacterial electron transport network, see Ref. 53; for two recent ones, see Refs. 54 and 55.) Thus, reduction of the cytochrome b6f complex activity and the resulting easily reducable PQ-pool do not only influence the regulation of the PS2 to PS1 and PC/APC stoichiometry but also seem to induce expression of cytochrome-bd oxidase.
Because the cytochrome b6f complex is a central and essential component of the energy-transducing system in chloroplasts and cyanobacteria deletion of petC should not be possible if there is only a single petC gene in the genome. The green alga C. reinhardtii is the only known example for a viable ΔpetC mutant (56), being able to grow heterotrophically and to generate ATP and NAD(P)H solely by oxidative phosphorylation in mitochondria. Even the cyanobacterium Synechococcus PCC 7002, with a genome containing three petC gene copies, was unable to survive petC1 deletion (57). In contrast, in Synechocystis PCC 6803 petC1 can be deleted and partially substituted by PetC2 (40), and deletion of petC1 results in an increased expression of PetC2 (Fig. 2A). However, despite redox and functional similarities the PetC2-b6f complex in this strain shows a significantly lower activity than could be expected on the basis of the amino acid sequence differences between PetC1 and PetC2 (Fig. 4B). The main reason for this is the small amount of functional cytochrome b6f in this strain (as evidenced by immunoblot analysis, Fig. 2A), resulting in a constantly low electron transport rate (Fig. 4B).
The exact reason for this small PetC2-b6f population size is unclear. However, because petC1 and petA are organized in the petCA operon (58) it appears likely that petC1 deletion also compromises expression of petA and thus accumulation of cytochrome f. Furthermore, the presence of PetC1 and/or cytochrome f appears to be critical for accumulation of SU IV but not for cytochrome b6 (Fig. 2A). As these subunits are encoded by co-transcribed genes organized in the petBD operon (58) this reflects different stabilities of SU IV and cytochrome b6 (59) in Synechocystis membranes. Although expression of petC2 is clearly up-regulated upon deletion of petC1 (Fig. 2A), this increased expression cannot fully compensate for PetC1 deficiency.
PetC2 and PetC3 Are Involved in Long-term Light Adaptation and Regulation of Cytochrome b6f Complex Activity
Despite sequence differences between the two Rieske proteins, PetC2 can partially substitute PetC1 and mediate electron transfer from PQH2 to cytochrome f (Fig. 4). However, due to its low abundance in WT thylakoid membranes (Fig. 2A) and the significant transcript accumulation under HL (Fig. 3), PetC2 is most likely mainly involved in regulatory processes. Furthermore, the ΔpetC2 strain has an unusually high growth rate under HL conditions in comparison to WT (Fig. 2B) suggesting a role of PetC2 in long-term light adaptation. As PetC1 and PetC2 are exclusively located in the thylakoid membrane (see below) and as the cytochrome b6f complex only functions as a dimer, it would be interesting to know whether PetC1-b6f and PetC2-b6f form hybrid dimers or exclusively homodimers. Although important for understanding the molecular mechanism, the answer for this question is difficult and beyond the scope of this contribution.
PetC3 shows several unique features different from the other two Rieske isoforms in Synechocystis. First, the primary structure of PetC3 is quite different from PetC1 and PetC2 (38, 41). Its N-terminal region is shorter by more than 10 residues, the sequence of its putative transmembrane helix is poorly conserved, and the connected hinge region is lacking. All this might indicate a function of PetC3 that is different from the other two isoforms. In agreement with this, PetC3 was recently reported to be located exclusively in the cytoplasmic membrane of Synechocystis (55, 60). Also, as the ΔpetC1/2 double deletion strain does not survive (40), PetC3 apparently cannot functionally substitute PetC1, and the determined midpoint redox potential (+135 mV) is too negative to allow PetC3 to be a component of the linear electron transport chain (41). Although all these observations indicate that PetC3 is not a component of the “classical” cytochrome b6f complex, our data show an impact on its function. A role of PetC3 in long-term light adaptation is supported by (a) the high growth rate of the ΔpetC3 mutant(s) under HL conditions (Fig. 2B) and (b) the observation that the maximal overall and linear electron transport rate is only achieved when all three PetC isoforms are present (Fig. 4B). The inability for an effective up-regulation of the cyclic electron transport relative to the linear one under HL in the ΔpetC3 strain (Fig. 4C) may also support this view. In summary, our data suggest defined roles of the various PetC proteins in short- and long-term light adaptation as a molecular basis for metabolic flexibility, which enables survival in an ever changing environment.
Acknowledgments
We are grateful for the excellent technical support of Ursula Altenfeld, Claudia König, Erdmut Thomas, and Dieter Wunsch, and for fruitful discussions with Jens Appel, Giovanni Finazzi, Yukako Hihara, Navassard Karapetyan, Teruo Ogawa, Thomas Pfannschmidt, and Alexander Ruban. We also acknowledge Friederike Koenig for providing access to spectroscopic equipment.
This work was supported by German Research Foundation Deutsche Forschungsgemeinschaft Grant SFB 480, project C1 (to M. R.) and SCHN 690/3-1 (to D. S.), grants from the Federal Ministry of Education and Research (BMBF) project Bio-H2 (to G. B.), the Kyoto University Foundation (to Y. T.), and the EU/NEST project Solar-H (to M. R.).
Dedicated to Prof. Dr. Mult. Achim Trebst on the occasion of his 80th birthday.
A. V. Ruban, personal communication.
- PS1 and PS2
- photosystem 1 and 2, respectively
- Ct
- threshold cycle numbers
- CydA
- cytochrome-bd oxidase subunit I
- DCMU
- 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea
- FmI and FmII
- maximal chlorophyll fluorescence yield in states I and II, respectively
- HL and LL
- high- and low-light, respectively
- MV
- methyl viologen
- P700
- the primary electron donor of PS1
- PBS
- phycobilisome
- PC
- phycocyanin
- PetC
- Rieske iron-sulfur protein of cytochrome b6f
- PQ and PQH2
- plastoquinone and plastoquinol, respectively
- SU IV
- subunit IV of cytochrome b6f
- WT
- wild type
- LHCII
- light harvesting complex II
- qPCR
- quantitative PCR
- APC
- allophycocyanin
- FR
- far red.
REFERENCES
- 1.Berry S., Schneider D., Vermaas W. F., Rögner M. (2002) Biochemistry 41, 3422–3429 [DOI] [PubMed] [Google Scholar]
- 2.Gutthann F., Egert M., Marques A., Appel J. (2007) Biochim. Biophys. Acta 1767, 161–169 [DOI] [PubMed] [Google Scholar]
- 3.Yeremenko N., Jeanjean R., Prommeenate P., Krasikov V., Nixon P. J., Vermaas W. F. J., Havaux M., Matthijs H. C. P. (2005) Plant Cell Phys. 46, 1433–1436 [DOI] [PubMed] [Google Scholar]
- 4.Allen J. F. (2004) Trends Plant Sci. 9, 130–137 [DOI] [PubMed] [Google Scholar]
- 5.Cape J. L., Bowman M. K., Kramer D. M. (2006) Trends Plant Sci. 11, 46–55 [DOI] [PubMed] [Google Scholar]
- 6.Cramer W. A., Zhang H. M. (2006) Biochim. Biophys. Acta 1757, 339–345 [DOI] [PubMed] [Google Scholar]
- 7.Cramer W. A., Zhang H., Yan J., Kurisu G., Smith J. L. (2006) Annu. Rev. Biochem. 75, 769–790 [DOI] [PubMed] [Google Scholar]
- 8.Cramer W. A., Baniulis D., Yamashita E., Zhang H., Zatsman A. I., Hendrich M. P. (2008) in Photosynthetic Protein Complexes, A Structural Approach (Fromme P. ed) pp. 155–179, Wiley-VCH, Weinheim [Google Scholar]
- 9.Bukhov N., Carpentier R. (2004) Photosynth. Res. 82, 17–33 [DOI] [PubMed] [Google Scholar]
- 10.Fork D. C., Herbert S. K. (1993) Photosynth. Res. 36, 149–168 [DOI] [PubMed] [Google Scholar]
- 11.Joliot P., Joliot A. (2006) Biochim. Biophys. Acta 1757, 362–368 [DOI] [PubMed] [Google Scholar]
- 12.Shikanai T. (2007) Annu. Rev. Plant Biol. 58, 199–217 [DOI] [PubMed] [Google Scholar]
- 13.Munekage Y., Hashimoto M., Miyake C., Tomizawa K., Endo T., Tasaka M., Shikanai T. (2004) Nature 429, 579–582 [DOI] [PubMed] [Google Scholar]
- 14.Rumeau D., Peltier G., Cournac L. (2007) Plant Cell Environ. 30, 1041–1051 [DOI] [PubMed] [Google Scholar]
- 15.Murata N. (1969) Biochim. Biophys. Acta 172, 242–251 [DOI] [PubMed] [Google Scholar]
- 16.Bonaventura C., Myers J. (1969) Biochim. Biophys. Acta 189, 366–383 [DOI] [PubMed] [Google Scholar]
- 17.Allen J. F. (2003) Science 299, 1530–1532 [DOI] [PubMed] [Google Scholar]
- 18.Wollman F. A. (2001) EMBO J. 20, 3623–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finazzi G., Rappaport F., Furia A., Fleischmann M., Rochaix J. D., Zito F., Forti G. (2002) EMBO Rep. 3, 280–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finazzi G., Forti G. (2004) Photosynth. Res. 82, 327–338 [DOI] [PubMed] [Google Scholar]
- 21.Vener A. V., Van Kan P. J., Gal A., Andersson B., Ohad I. (1995) J. Biol. Chem. 270, 25225–25232 [DOI] [PubMed] [Google Scholar]
- 22.Vener A. V., van Kan P. J., Rich P. R., Ohad I., Andersson B. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 1585–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zito F., Finazzi G., Delosme R., Nitschke W., Picot D., Wollman F. A. (1999) EMBO J. 18, 2961–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vener A. V., Ohad I., Andersson B. (1998) Curr. Opin. Plant Biol. 1, 217–223 [DOI] [PubMed] [Google Scholar]
- 25.de Vitry C., Ouyang Y., Finazzi G., Wollman F. A., Kallas T. (2004) J. Biol. Chem. 279, 44621–44627 [DOI] [PubMed] [Google Scholar]
- 26.Mullineaux C. W., Emlyn-Jones D. (2005) J. Exp. Bot. 56, 389–393 [DOI] [PubMed] [Google Scholar]
- 27.van Thor J. J., Mullineaux C. W., Matthijs H. C. P., Hellingwerf K. J. (1998) Bot. Acta 111, 430–443 [Google Scholar]
- 28.Grossman A. R., Schaefer M. R., Chiang G. G., Collier J. L. (1993) Microbiol. Rev. 57, 725–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacColl R. (1998) J. Struct. Biol. 124, 311–334 [DOI] [PubMed] [Google Scholar]
- 30.Mullineaux C. W. (2008) Photosynth. Res. 95, 175–182 [DOI] [PubMed] [Google Scholar]
- 31.Mao H. B., Li G. F., Ruan X., Wu Q. Y., Gong Y. D., Zhang X. F., Zhao N. M. (2002) FEBS Lett. 519, 82–86 [DOI] [PubMed] [Google Scholar]
- 32.Mullineaux C. W., Allen J. F. (1986) FEBS Lett. 205, 155–160 [Google Scholar]
- 33.Whitelegge J. P., Zhang H., Aguilera R., Taylor R. M., Cramer W. A. (2002) Mol. Cell Proteomics 1, 816–827 [DOI] [PubMed] [Google Scholar]
- 34.Schneider D., Volkmer T., Rögner M. (2007) Res. Microbiol. 158, 45–50 [DOI] [PubMed] [Google Scholar]
- 35.Schneider D., Berry S., Rich P., Seidler A., Rögner M. (2001) J. Biol. Chem. 276, 16780–16785 [DOI] [PubMed] [Google Scholar]
- 36.Zhang H., Whitelegge J. P., Cramer W. A. (2001) J. Biol. Chem. 276, 38159–38165 [DOI] [PubMed] [Google Scholar]
- 37.Volkmer T., Schneider D., Bernát G., Kirchhoff H., Wenk S. O., Rögner M. (2007) J. Biol. Chem. 282, 3730–3737 [DOI] [PubMed] [Google Scholar]
- 38.Schneider D., Schmidt C. L. (2005) Biochim. Biophys. Acta 1710, 1–12 [DOI] [PubMed] [Google Scholar]
- 39.Kaneko T., Sato S., Kotani H., Tanaka A., Asamizu E., Nakamura Y., Miyajima N., Hirosawa M., Sugiura M., Sasamoto S., Kimura T., Hosouchi T., Matsuno A., Muraki A., Nakazaki N., Naruo K., Okumura S., Shimpo S., Takeuchi C., Wada T., Watanabe A., Yamada M., Yasuda M., Tabata S. (1996) DNA Res. 3, 109–136 [DOI] [PubMed] [Google Scholar]
- 40.Schneider D., Berry S., Volkmer T., Seidler A., Rögner M. (2004) J. Biol. Chem. 279, 39383–39388 [DOI] [PubMed] [Google Scholar]
- 41.Schneider D., Skrzypczak S., Anemüller S., Schmidt C. L., Seidler A., Rögner M. (2002) J. Biol. Chem. 277, 10949–10954 [DOI] [PubMed] [Google Scholar]
- 42.Rippka R., Deruelles J., Waterbury J. B., Herdman M., Stanier R. Y. (1979) J. Gen. Microbiol. 111, 1–61 [Google Scholar]
- 43.Myers J., Graham J. R., Wang R. T. (1978) J. Phycol. 14, 513–518 [Google Scholar]
- 44.Bennett A., Bogorad L. (1973) J. Cell Biol. 58, 419–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bustin S. A. (2002) J. Mol. Endocrinol. 29, 23–39 [DOI] [PubMed] [Google Scholar]
- 46.Pfaffl M. W. (2001) Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramakers C., Ruijter J. M., Deprez R. H., Moorman A. F. (2003) Neurosci. Lett. 339, 62–66 [DOI] [PubMed] [Google Scholar]
- 48.Pfaffl M. W., Horgan G. W., Dempfle L. (2002) Nucleic Acids Res. 30, e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laemmli U. K. (1970) Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 50.Kovács L., Damkjaer J., Kereïche S., Ilioaia C., Ruban A. V., Boekema E. J., Jansson S., Horton P. (2006) Plant Cell 18, 3106–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernát G., Waschewski N., Rögner M. (2009) Photosynth. Res. 99, 205–216 [DOI] [PubMed] [Google Scholar]
- 52.Alfonso M., Perewoska I., Kirilovsky D. (2000) Plant Physiol. 122, 505–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howitt C. A., Vermaas W. F. J. (1998) Biochemistry 37, 17944–17951 [DOI] [PubMed] [Google Scholar]
- 54.Mogi T., Miyoshi H. (2009) J. Biochem. 145, 395–401 [DOI] [PubMed] [Google Scholar]
- 55.Schultze M., Forberich B., Rexroth S., Dyczmons N. G., Roegner M., Appel J. (2009) Biochim. Biophys. Acta, in press [DOI] [PubMed] [Google Scholar]
- 56.de Vitry C., Finazzi G., Baymann F., Kallas T. (1999) Plant Cell 11, 2031–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan J., Cramer W. A. (2003) J. Biol. Chem. 278, 20925–20933 [DOI] [PubMed] [Google Scholar]
- 58.Kallas T., Spiller S., Malkin R. (1988) J. Biol. Chem. 263, 14334–14342 [PubMed] [Google Scholar]
- 59.Kuras R., Wollman F. A. (1994) EMBO J. 13, 1019–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aldridge C., Spence E., Kirkilionis M. A., Frigerio L., Robinson C. (2008) Mol. Microbiol. 70, 140–150 [DOI] [PubMed] [Google Scholar]