Abstract
Interstrand cross-links (ICLs) are absolute blocks to transcription and replication and can provoke genomic instability and cell death. Studies in bacteria define a two-stage repair scheme, the first involving recognition and incision on either side of the cross-link on one strand (unhooking), followed by recombinational repair or lesion bypass synthesis. The resultant monoadduct is removed in a second stage by nucleotide excision repair. In mammalian cells, there are multiple, but poorly defined, pathways, with much current attention on repair in S phase. However, many questions remain, including the efficiency of repair in the absence of replication, the factors involved in cross-link recognition, and the timing and demarcation of the first and second repair cycles. We have followed the repair of laser-localized lesions formed by psoralen (cross-links/monoadducts) and angelicin (only monoadducts) in mammalian cells. Both were repaired in G1 phase by nucleotide excision repair-dependent pathways. Removal of psoralen adducts was blocked in XPC-deficient cells but occurred with wild type kinetics in cells deficient in DDB2 protein (XPE). XPC protein was rapidly recruited to psoralen adducts. However, accumulation of DDB2 was slow and XPC-dependent. Inhibition of repair DNA synthesis did not interfere with DDB2 recruitment to angelicin but eliminated recruitment to psoralen. Our results demonstrate an efficient ICL repair pathway in G1 phase cells dependent on XPC, with entry of DDB2 only after repair synthesis that completes the first repair cycle. DDB2 accumulation at sites of cross-link repair is a marker for the start of the second repair cycle.
Interstrand cross-links (ICLs)2 are among the most dangerous DNA lesions. They are absolute blocks to replication and transcription and, unlike monoadducts, cannot be carried through a proliferative cycle. Their accumulation over time is believed to contribute to genomic instability and aging in tissues and organs (1, 2). If not removed, they can provoke chromosomal breakage, rearrangements, or cell death (3, 4). Mice and humans deficient in genes associated with ICL repair, such as members of the ERCC1-XPF complex, display severe pathology and greatly reduced life span (2, 5–9). Given the challenge of repairing lesions that engage both strands of the duplex, it is understandable that multiple repair pathways are engaged (10–12) and that repair is more complex than for monoadducts.
In Escherichia coli, the NER apparatus incises one strand on either side of the ICL. This produces an “unhooked” substrate with the excised fragment still attached to the non-incised strand by the cross-linking agent. The immediate product of unhooking is typically depicted as a gapped structure with the cross-linked oligonucleotide flipped out of the duplex (although this structure may not actually form (13)). The incised/gapped strand is repaired by homology-directed repair using information from an undamaged homologous chromosome (14–17). The gap may also be filled by lesion bypass synthesis (18). The product of the first repair cycle is a novel monoadduct in which the cross-linked bases are still joined, although no longer in the context of the duplex. These are the essentials of a “two-cycle” repair pathway found in all organisms: recognition, unhooking, and gap repair by recombination or lesion bypass synthesis, followed by a second cycle of NER to remove the monoadduct produced by the first cycle (see Ref. 3) (Fig. 1A). However, despite the conceptual framework provided by the Cole model, there is considerable uncertainty as to the individual steps in the process in mammalian cells, beginning with the question of how cross-links are recognized.
FIGURE 1.
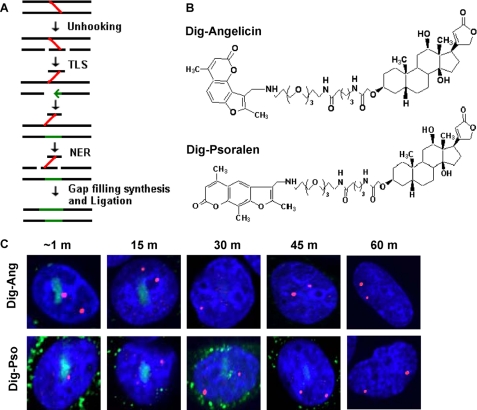
Repair of psoralen and angelicin adducts in G1 phase cells. A, schematic of ICL repair. In the first cycle, the ICL is recognized and then unhooked by incision on one strand on either side of the cross-linked base. Gap repair synthesis past the still adducted base on the non-incised strand restores the helix and presents the monoadducted base for the second cycle of repair by NER. B, the structure of digoxigenin-derivatized trimethyl psoralen (Dig-Pso) and angelicin (Dig-Ang). C, SW480 cells were incubated with the dig compounds and then laser-photoactivated in the subnuclear region in individual cells in a defined time sequence. Cells were fixed and immunostained for the dig tag (green) and for the NPAT cell cycle phase marker (red, two spots, G1 phase) cells.
Cells deficient in the ERCC1-XPF endonuclease complex are hypersensitive to cross-linking agents. In reactions with model substrates, the complex can incise on either side of a defined ICL placed adjacent to a fork (19), a structure that could occur during replication of cross-linked templates (20–22). Based in part on those results, it has been proposed that ICLs are recognized when they block or break replication forks (22–26). It has also been argued that another structure-specific endonuclease, MUS81-EME1, introduces a break at an arrested fork followed by cleavage by ERCC1-XPF to unhook the cross-link (24) (for a dissenting view, see Ref. 27). Although many details are uncertain, it is clear that there are effective repair pathways in S phase and that encounters with the replication apparatus can be a means of cross-link recognition.
Monoadducts repaired by NER are recognized either as blocks to transcription, triggering transcription coupled repair (28), or because they cause distortions in the helical structure of DNA (29–31). These can be bound by the proteins of the XPE complex (DDB1-DDB2) (32–34) and/or by the XPC complex (XPC-HR23B) (35, 36). In yeast, genetic evidence indicates a requirement for NER activities, including XPC, during ICL repair in G1 phase (37, 38). Additionally, unhooking has been shown in G1 phase mammalian cells (39) and is dependent on NER functions (40). Repair in mammalian cells of non-replicating plasmids carrying defined cross-links requires NER (41), as does incision in the vicinity of a cross-link in cell-free extracts (42). However, XPC-independent unhooking in cell-free extracts has also been reported (43).
There are other suggestions for early factors in ICL repair in the absence of replication. Based on experiments in cell extracts, it has been proposed that ICLs are recognized by the mismatch repair factor MutSβ, stimulated by proliferating cell nuclear antigen (44). In this experimental system, these proteins initiated formation of an unhooking complex additionally containing ERCC1-XPF, the Werner syndrome helicase, replication protein A, and the Pso4 pre-mRNA splicing complex (45). Finally, ICLs block transcription (46), and repair by transcription-coupled repair (47, 48) would be independent of early recognition functions.
The field of DNA repair has been greatly advanced by the development and application of techniques for introducing localized damage in subnuclear regions, followed by immunofluorescent visualization of repair protein recruitment. This approach is limited to DNA lesions that can be induced, directly or indirectly, by exposure to light/radiation. Damage localization results from restricting exposure of cells to light/radiation by masking with grates or micropore filters (49, 50) or by activation of defined nuclear regions by laser light (51). The lesions that have received the most attention are UV-induced pyrimidine photoproducts (52), which can be detected by specific antibodies, and single and double strand breaks, for which there are surrogate protein markers, such as XRCC1 (53) and phosphorylated histone H2AX (51).
Psoralens are cross-linking agents that are DNA-reactive only upon photoactivation and so would appear appropriate for experiments employing laser-directed targeting. However, there are no reagents, such as specific antibodies, generally available for detection of psoralen photoadducts in situ. We have overcome this limitation by synthesizing psoralen linked to an antigenic tag, digoxigenin (dig). This sterol has received extensive use as an immunotag, and reliable commercial antibodies are available. We also synthesized the equivalent derivative of angelicin, a photoreactive analogue of psoralen that can form only monoadducts, which are repaired by NER. Cells treated with the tagged psoralens are exposed to 365-nm laser photoactivation in a defined region of the nucleus of living cells (54). The location of adducts can be visualized by immunofluorescence following fixing and immunostaining. Using this approach, we have shown that psoralen adducts can be localized in the nucleus, within a defined volume. The psoralen adducts were removed over time in repair-proficient cells but were persistent in cells deficient in ERCC1-XPF. By performing experiments in parallel with dig-psoralen (dig-pso) and dig-angelicin (dig-ang), events specific to psoralen/UVA photoproducts can be distinguished from those provoked by a classical monoadduct, introduced by laser photoactivation at the same wavelength. In the experiments described here, we have employed the new approach, as well as more conventional experimental techniques, to ask if ICLs can be repaired in G1 phase mammalian cells, and which early recognition proteins are involved in the process.
EXPERIMENTAL PROCEDURES
Cell Lines, Synchronization, and Transfection
Repair-proficient human primary colorectal adenocarcinoma cells, SW480 (CCL-228), and mismatch repair-deficient human colorectal adenocarcinoma cells, LoVo (CCL-229), were obtained from the American Type Culture Collection (Manassas, VA). Human fibroblasts derived from xeroderma pigmentosum patients, complementation group XPC (GM16370) and XPE (GM01389), were obtained from Coriell Cell Repositories (Camden, NJ). XPC-deficient XP4PA-SV-EB and wild type XPC-complemented cells (XP4PA-SE2) were the kind gift of Dr. Ken Kraemer (NCI, National Institutes of Health). The XP4PA-SE2 is a fully corrected and stable clone constructed by transfecting the pXPC3 plasmid, expressing wild type XPC, into XP4PA-SV-EB cells (55). XPE cells were grown in Eagle's minimum essential medium, supplemented with penicillin, streptomycin, l-glutamine, non-essential amino acids, and 15% fetal bovine serum. All other cells were grown in Dulbecco's modified Eagle's medium, supplemented with penicillin, streptomycin, and 10% fetal bovine serum. SW480 and XPE cells were synchronized in G1 phase via serum deprivation for 48 h, resulting in 90% of the cells with G1 phase DNA content, as measured by fluorescence-activated cell sorting analysis. XP4PA-SV-EB cells were synchronized in G1 phase using double thymidine block. In brief, cells were incubated with 2 mm thymidine for 18 h. Cells were fed with fresh medium for 9 h before adding 2 mm thymidine for another 17 h. Cells were again fed with fresh medium for 16 h prior to experiments. In some experiments, cells in random culture were employed, and the cell cycle phase was determined by immunostaining with an antibody against the marker proteins NPAT (nuclear protein of ATM locus; two spots, G1 phase; four spots, S/G2 phase) (56), or cyclin A (strong nuclear signal in S phase). In DDB2 recruitment experiments, FLAG-tagged DDB2 plasmid, a generous gift from Dr. Vesna Rapič-Otrin, was transfected into SW480 cells using an Amaxa nucleoporator.
Cell Survival Assay
sCells were seeded into a 96-well plate a day before the experiment. On the day of the experiment, cells were incubated with variable concentrations of 8-methoxypsoralen for 30 min at 37 °C, followed by exposure to 1.8 J/cm2 UVA in a Rayonet chamber. Cells were fed with fresh medium and allowed to grow for 3 days before a WST-1 colorimetric assay was carried out according to the manufacturer's instructions (Clontech).
Alkaline Comet Assay
Cells synchronized in G1 were treated with 75 ng/ml 8-methoxypsoralen for 30 min, followed by UVA exposure. Cells were either harvested immediately or 6 h later. The alkaline comet assay and analysis were performed as described previously (40). One hundred cells were examined for each time point.
Digoxigenin-tagged Photoactive Compounds
Digoxigenin-tagged trimethylpsoralen and digoxigenin-tagged dimethylangelicin were synthesized as described (54).
Laser-localized Photoadducts
Cells were seeded in a 35-mm glass bottom culture dish (MatTekTM) 2 days before an experiment to achieve 50–60% confluence. They were incubated with the compound 20 μm dig-pso, 50 μm dig-ang, 5 μm trioxalen, or 40 μm angelicin at 37 °C for 20 min prior to laser treatment. In some experiments, cells were incubated with 2 μg/ml actinomycin D for 2 h or 100 μm arabinosylcytosine (Ara-C) for 1 h prior to the addition of compounds. Localized irradiation was performed using a Nikon Eclipse TE2000 confocal microscope equipped with an SRS NL100 nitrogen laser-pumped dye laser (Photonics Instruments, St. Charles, IL) that fires 3-ns pulses with a repetition rate of 10 Hz at 365 nm, with a power of 0.7 nanowatts, measured at the back aperture of the ×60 objective. The laser, controlled by Volocity-5 software (Improvision; PerkinElmer Life Sciences), was directed to deliver pulses to a specified rectangular region of interest within the nucleus of a cell (4 × 20 pixels, 0.16 μm/pixel) visualized with a Plan Fluor ×60/1.25 numerical aperture oil objective. The laser beam was oriented by galvanometer-driven beam displacers and fired randomly throughout the region until the entire region was exposed. The diffraction-limited spot size was ∼300 nm. Two firing cycles were applied to each region in order to drive monoadducts to ICLs (57). Throughout an experiment, cells were maintained at 37 °C, 5% CO2, and 80% humidity using a Live CellTM environmental chamber. At different time intervals, cells from different areas of the dish were treated with the laser to generate a time course on a single plate. After the final time point, cells were fixed immediately in freshly prepared 4% formaldehyde in PBS for 10 min at room temperature.
Antibodies and Immunofluorescence Staining
Fixed cells were permeabilized with 0.5% Triton X-100, 1% bovine serum albumin, 100 mm glycine, and 0.2 mg/ml EDTA in PBS on ice for 10 min. The cells were subsequently digested with RNase A in PBS-EDTA (5 mm) solution for 30 min at 37 °C. Cells were blocked in 10% goat serum in PBS and 0.01% sodium azide for 1 h at 37 °C or overnight at 4 °C. For immunofluorescence staining, cells were incubated with appropriate primary antibody diluted in blocking solution for 1 h at 37 °C. After three 10-min washes using 0.05% Tween 20 in PBS, cells were incubated with a corresponding fluorescence-tagged secondary antibody (Alexa Fluor goat anti-mouse or Alexa Fluor goat anti-rabbit; Molecular Probes). After another three 10-min washes, cells were mounted with ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole from Molecular Probes. Primary antibodies against XPB (TFIIH p89 S19, sc-293, rabbit, 1:100), XPA (XPA FL-273, sc-853, rabbit, 1:100), and cyclin A (cyclin A H-432, sc-751, rabbit, 1:200) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies against MSH3 (catalogue number 611390, mouse, 1:100) and NPAT (catalogue number 611344, mouse, 1:200) were obtained from BD Transduction Laboratories. Antibody against XPF (ms-1385-P1ABX, mouse, 1:100) was obtained from Neomarkers, whereas XPC antibody (GTX-70309, mouse, 1:500) was obtained from GeneTex Inc. Antibodies against FLAG (F3165, mouse, 1:200) and digoxigenin (catalogue number 11333062910, mouse, 1:100) were obtained from Sigma and Roche Applied Science, respectively. Stained cells were visualized and imaged using a Hamamatsu EM-CCD digital camera attached to the Nikon Eclipse TE2000 confocal microscope. 20–25 cells were examined for each experimental point. In each experiment, images of cells at various time points were acquired using the same exposure, gain, sensitivity, and contrast settings. Digoxigenin signal removal measurements were obtained by measuring mean GFP intensity of the signal normalized against background from 20 cells/time point.
RESULTS
Psoralen Adduct Repair in Repair-proficient G1 Phase Cell
We first asked if repair-proficient SW480 cells were able to remove the dig-tagged adducts in the absence of replication (i.e. in G1 phase). Some experiments were carried out in cells synchronized in G1 phase (see “Experimental Procedures”). Alternatively, in experiments with random cultures, cells were immunostained for NPAT, a cell cycle marker, to distinguish G1 phase cells (two spots) from S/G2 phase cells (four spots) (56). The cells were incubated with either dig-ang or dig-pso (Fig. 1B). The compounds were photoactivated by laser exposure in defined subnuclear regions (see “Experimental Procedures”) at various times prior to fixing and staining with an antibody against the dig tag. Our previous confocal analysis of the stained cells indicated that adducts were restricted to a limited region within the nucleus (54). We estimate that photoactivation of the region of interest (∼0.5 × 3 μm) results in adduct occupancy of 1–2% of the nuclear volume (see supplemental Fig. S1).
The dig-ang signal was removed between 15 and 30 min after laser treatment, whereas the dig-pso signal was removed between 45 and 60 min after. The same results were obtained in G1 phase synchronized cells (not shown) or in cells identified as in G1 phase by the NPAT pattern (Fig. 1C). The cells remained viable and capable of division, as revealed by inspection after 24 h. In order to ask if transcription was a factor in the removal of the dig-pso adduct, we repeated the experiments in G1 phase cells pretreated with actinomycin D, an RNA synthesis inhibitor. The persistence time of the dig-pso and dig-ang was identical to that in cells without the inhibitor (data not shown). These results demonstrated a difference in the time of repair of adducts formed by the two compounds and indicated that transcription was not essential for the process.
Recruitment of NER Proteins
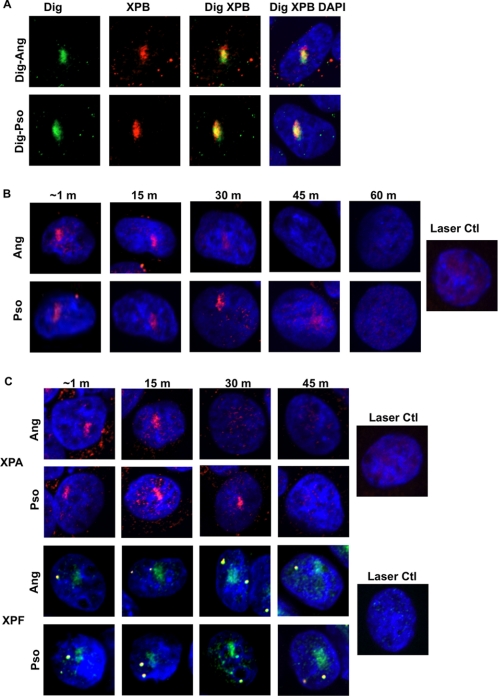
We were interested in monitoring the recruitment of repair proteins to the photoadducts. Cells were treated with the dig-pso or dig-ang, laser-photoactivated, incubated for 15 min, and then stained with antibodies to both the dig tag and to the XPB protein, which is involved in NER as a helicase subunit of TFIIH. XPB was recruited to and colocalized with dig-pso and dig-ang adducts (Fig. 2A; for a confocal analysis, see supplemental Fig. S1).
FIGURE 2.
Recruitment of repair proteins to sites of psoralen and angelicin adducts. A, repair-proficient SW580 cells were incubated with dig-pso or dig-ang and followed by laser treatment. Cells were fixed after 15 min and stained with anti-dig (green) and anti-XPB (red). B, SW480 cells synchronized in G1 phase were incubated with psoralen or angelicin prior to laser treatment. The cells were immunostained for XPB (in red). C, cells synchronized in G1 phase were stained for XPA (in red). In a separate experiment, cells in random culture were treated and immunostained for XPF (green) and NPAT (orange). The results with the cells 15 min after treatment with the laser alone are shown for each protein.
The psoralen and angelicin derivatives were linked via a side chain to the dig tag. It was important to ask if these elements of the compounds played a role in the recruitment of repair proteins. To address this issue, we incubated cells synchronized in G1 phase with unconjugated psoralen or angelicin and laser-photoactivated a defined region as before. We then monitored the accumulation and persistence of XPB protein (Fig. 2B). There was rapid accumulation of XPB to both adducts and a longer persistence at sites of psoralen photoadducts. In companion experiments with the tagged compounds, we observed the same results (not shown). Consequently, we concluded that the dig tag did not influence the recruitment of repair proteins. The staining pattern in the 45 min psoralen sample illustrates a feature of several of our experiments. The signal tended to broaden and become more diffuse over time. This may reflect a spreading of the chromatin during repair as has been reported by others (58).
We also studied the accumulation of two other NER pathway proteins, XPA and XPF. Both XPA and XPF proteins were rapidly recruited to psoralen and angelicin adducts (Fig. 2C). The intensity of the XPA signal declined more rapidly at the angelicin adducts than the psoralen adducts. The XPF signal was equally persistent with both compounds.
In control experiments, the cells were exposed to the laser in the absence of either compound. We observed no recruitment of the NER proteins to the region of interest. These results demonstrated the engagement of NER proteins by adducts formed by both compounds. The longer persistence of the XPB and XPA proteins in the region of psoralen adducts, relative to the angelicin adducts, was consistent with the presence of cross-links in the psoralen adduct population, which would take longer than monoadducts to repair. However, it was clearly possible that monoadducts were also in that population.
Role of Early Recognition Proteins
In light of these results and our focus on repair in the absence of replication, it was of interest to examine the role of NER proteins involved in the early response to DNA damage. Accordingly, we determined the sensitivity to psoralen/UVA of repair-proficient and XPC-, XPE-, and XPA-deficient, cells in a conventional survival assay. XPE-deficient cells were as resistant as wild type cells. XPC- and XPA-deficient cells showed increased sensitivity as compared with wild type cells. The sensitivity of the XPC-deficient cells was partially reversed by complementation by expression of wild type XPC protein (Fig. 3A). Unhooking, as measured by comet tail recovery, by XPE-deficient cells synchronized in G1 phase was similar to wild type cells. Unhooking did not occur in G1 phase XPC-deficient cells (Fig. 3, B and C).
FIGURE 3.
Sensitivity of repair deficient cells to psoralen. A, SW480 (empty circle); cells deficient in XPC (filled square), XPA (filled circle), and XPE (cross); and XPC-deficient cells complemented by expression of wild type XPC (triangle) were exposed to psoralen/UVA, and survival was measured in a WST-1 conversion assay. B, alkaline comet tail recovery assay of cells synchronized in G1 phase treated with 8-methoxypsoralen/UVA and fixed immediately (gray columns) or 6 h later (black columns). Control cells were not exposed to psoralen (white columns). C, quantitation of the comet tail analysis. D, dig-ang and pso repair in XPE-, XPC-, and MSH2/MSH3-deficient cells. The secondary antibody for the NPAT stain was Alexa 633 (red, XPE and XPC cells) or Alexa 568 (orange, LoVo cells). E, dig signal intensity was measured and plotted versus time after photoactivation. dig-psoralen intensity was measured immediately (white columns) or 1 h after photoactivation (black columns). The dig-angelicin signal was measured immediately (white columns), 1 h after (gray columns), or 2 h after photoactivation (darker gray columns).
Psoralen and Angelicin Adduct Removal in Repair-deficient G1 Phase Cells
We then examined the persistence of the dig-pso and dig-ang in XPC or XPE cells. The assay was also performed in cells deficient in the MSH2 protein, a component of the MutSβ complex, which has been implicated in ICL recognition (44). In XPE cells, the dig-pso signal declined with kinetics similar to that in wild type cells. However, the dig-ang signal was only slightly diminished after 45 min (Fig. 3, D and E). The XPE cells were able to completely remove the dig-ang signal after 2 h. In similar experiments, XPC cells were unable to remove the dig-pso and dig-ang adducts (Fig. 3D), even after a 4-h incubation (not shown). Complementation of these cells by expression of wild type XPC protein restored the ability to remove both adducts (not shown). MSH2/MSH3-deficient LoVo cells exhibited wild type activity in the removal of the dig-ang and dig-pso adducts.
The delayed removal of the dig-ang signal in XPE cells was consistent with a contribution by, but not an absolute requirement for, the DDB1-DDB2 complex to NER, as previously described (59–61). Accordingly, we asked if recruitment of XPB protein to sites of angelicin adducts was affected by the DDB2 deficiency. Accumulation of XPB protein was retarded in the XPE cells, and it persisted for a longer time than in wild type cells (compare Fig. 2B with Fig. 4). In contrast, there was no change from wild type in recruitment and persistence dynamics of XPB with the psoralen adducts.
FIGURE 4.
Recruitment of XPB to psoralen and angelicin adducts in G1 phase XPE deficient cells. Cells were treated with angelicin or psoralen, laser-photoactivated at various times, and then immunostained for XPB protein. The time after photoactivation is indicated.
DDB2, XPC, and MSH3 Recruitment in WT G1 Phase Cells
The preceding results were in accord with an XPC-dependent repair of psoralen adducts and a DDB2 involvement with angelicin, but not psoralen, adduct repair. Repair of adducts formed by both compounds appeared to be independent of MSH3/MSH2. In light of these conclusions, it was of interest to examine the recruitment of XPC, DDB2, and MSH3 proteins to adducts of the two compounds. The experiments were performed in wild type cells synchronized in G1 phase.
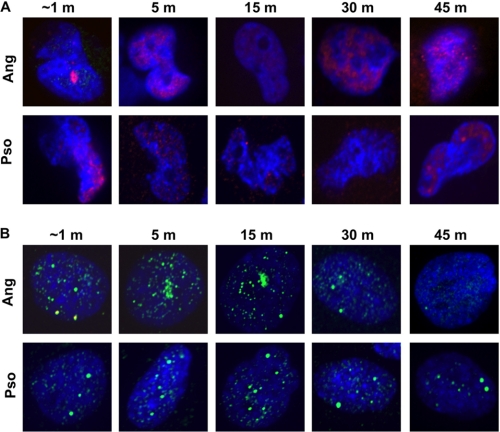
XPC protein appeared at both angelicin and psoralen sites as early as the cells could be fixed (<1 min). The levels declined to base line in angelicin-treated cells by 30 min but were more persistent in the psoralen-treated cells (Fig. 5A). Thus, both psoralen and angelicin adducts were rapidly recognized by XPC.
FIGURE 5.
Recruitment of “early” response candidate repair proteins to psoralen and angelicin adducts in G1 phase SW480 cells. Shown is recruitment of XPC (A), MSH3 (B), and DDB2-FLAG (C). The 15 min laser alone controls (Ctl) are shown.
MSH3 did appear at sites of angelicin adducts; however, accumulation did not begin immediately. Instead, it was clearly apparent at 5 min after laser activation (Fig. 5B). The appearance was slower in the cells treated with psoralen, with a weak signal at 5 min, but quite strong by 15 min. These results suggested that MSH3 recruitment was a secondary event in the repair response to adducts of both compounds, particularly to psoralen.
In order to monitor DDB2 recruitment, we transfected the cells with a plasmid encoding FLAG-tagged DDB2 (62). DDB2-FLAG recruitment was rapid at sites of laser-activated angelicin (Fig. 5C). However, residence of the protein was relatively brief, with a return to base line within 15 min. The results in the experiment with psoralen were quite different. There was no early recruitment of DDB2-FLAG. Instead the protein appeared at about 10 min and declined to base line in 20–30 min.
In control experiments, the cells were treated with the laser in the absence of either compound. There was no accumulation of XPC, MSH3, or DDB2-FLAG in the region of interest. The results of these experiments demonstrated a difference in the repair protein response to adducts formed by the two compounds. The DDB2-FLAG recruitment dynamics clearly distinguished the two adduct populations.
Repair Protein Recruitment in XPC-deficient Cells
We interpreted the results of the preceding experiments as a demonstration of psoralen adduct repair by an NER pathway, with XPC performing an early function. Consequently, it was of interest to ask if the accumulation of DDB2-FLAG and MSH3 was dependent on XPC. We treated XPC-deficient cells with the two compounds and laser-photoactivated as before. DDB2-FLAG was recruited to angelicin adducts as rapidly as in wild type cells and then declined to base-line levels (Fig. 6). In contrast to wild type cells, there was no recruitment of DDB2-FLAG to psoralen adducts over the 45 min of the experiment. Similarly, we observed accumulation of MSH3 to angelicin adducts but saw no appearance in the region of the psoralen adducts. In other experiments, XPB and XPA proteins were not recruited to the sites of angelicin or psoralen adducts (not shown). Complementation of the XPC-deficient cells by expression of wild type XPC protein restored NER protein recruitment (not shown). These data demonstrated that recruitment of DDB2-FLAG and also MSH3 to psoralen adducts was dependent on functional XPC protein. These results further supported the conclusion that recruitment of DDB2 and MSH3 was secondary to a primary, XPC-dependent, event.
FIGURE 6.
Recruitment of DDB2-FLAG and MSH3 to psoralen and angelicin adducts in G1 phase XPC deficient cells. A, DDB2-FLAG; B, MSH3.
Influence of Repair Synthesis Inhibition on Protein Recruitment
The dual incisions on either side of monoadducts can occur without a requirement for repair synthesis that fills the gap formed during NER (63, 64). During cross-link repair, a monoadduct in the context of a duplex would appear only after synthesis to fill the gap formed during the first repair cycle. Thus, inhibition of repair synthesis would not block loss of monoadducts of dig-ang and dig-pso but would preclude removal of dig-pso engaged in ICLs. We reasoned that this would be another test of the distribution of monoadducts and ICLs in the region treated with the laser/dig-pso. Repair-proficient SW480 cells were pretreated with Ara-C, a nucleoside analogue that blocks DNA synthesis for 1 h prior to incubation with the dig compounds (65, 66). The persistence of the dig signal was monitored as before over a 1-h period. There was a complete decline in the dig-ang signal in the Ara-C-treated cells (Fig. 7A). However, there was no loss of the dig-pso signal, as shown in Fig. 7A and confirmed by quantitation of the intensity of the dig-pso signal at 0 and 60 min (not shown). We then asked how the Ara-C treatment would affect the recruitment of DDB2-FLAG (Fig. 7B). The rapid appearance and decline of this protein in the region containing angelicin adducts was the same in treated and control cells. In contrast, there was no accumulation of DDB2-FLAG to psoralen adducts in the cells treated with Ara-C. These results demonstrated that the recruitment of DDB2-FLAG to the psoralen adducts was dependent on repair synthesis. We also found that Ara-C blocked the appearance MSH3 at sites of psoralen adducts. As a control, we monitored XPA protein, whose recruitment to psoralen adducts was unaffected by the Ara-C treatment (not shown). This indicated that protein accumulation at psoralen adducts was not generally disabled by Ara-C.
FIGURE 7.
Repair of psoralen and angelicin adducts and recruitment of DDB2 in cells in the presence of a repair synthesis inhibitor. A, SW480 cells were incubated with dig-pso or dig-ang and laser-photoactivated in defined regions of interest. The cells were either untreated or pretreated with Ara-C for 1 h. The cells were fixed and immunostained for the dig tag. B, SW480 cells were transfected with a plasmid expressing DDB2-FLAG. After 24 h, they were treated with Ara-C for 1 h, and localized angelicin or psoralen adducts were introduced. After fixation, they were immunostained for the FLAG tag.
DISCUSSION
Despite investigation by many groups, a detailed understanding of the repair of ICLs in mammalian cells remains to be developed. Here we have employed antigen-tagged psoralens (54) to address some basic questions about this process. Although our results provide initial answers to some of these, they also address key technical issues, whose resolution is essential for the interpretation of the data presented here.
Technical Issues
Laser localization of DNA damage has become an important and popular tool in DNA repair studies. However, as has been emphasized in recent reports, multiple forms of DNA damage can be introduced by the lasers, as a function of beam intensity, with a corresponding induction of multiple repair pathways (53, 67, 68). During the development of the technology employed in our experiments, we established conditions under which activation of the repair response to the psoralen photoadducts was dependent on both laser and psoralen. No response was seen in cells exposed to the laser alone. Thus, repair proteins were recruited to sites of angelicin or psoralen photoproducts rather than some undefined DNA lesion(s) introduced by the laser.
The conjugated psoralens were attached to the antigenic digoxigenin tag. At the outset of our experiments, we were concerned that the dig tag, linked to the DNA via the psoralen, could be the necessary signal for repair protein recruitment. However, the results in Fig. 2 argued against this possibility, since repair protein recruitment, with identical kinetics, was observed in cells in which unconjugated psoralen or angelicin was activated by exposure to the laser. Consequently, we concluded that the presence or absence of the dig tag did not influence the response of the repair proteins.
Perhaps the most critical concern is the relative frequency of monoadducts and ICLs following laser activation of psoralen. DNA-damaging agents rarely generate a single adduct; instead, multiple chemical structures are formed. For example, mitomycin C, commonly used as a cross-linking agent, is enzymatically reduced in the cell, yielding oxygen radicals that are DNA reactive. In addition, activated mitomycin C reacts with DNA to form six major adducts, with a 10-fold greater frequency of monoadducts than ICLs (69, 70). Psoralen reacts with DNA in a two-step, two-photon process. Absorption of the first photon by an intercalated psoralen produces a monoadduct. After a brief period (1 μs), during which conformational changes are thought to occur, absorption of an additional photon produces an ICL (71). The process is not absolutely efficient, and exposure of cellular DNA to psoralen and conventional laboratory UVA lamps typically results in a mixture of monoadducts and ICLs (72).
Given this feature of psoralen photochemistry, proteins recruited to the site of laser-activated psoralen adducts could be responding to monoadducts rather than ICLs. This would be of particular concern with proteins involved in the repair of both kinds of adducts, such as the NER factors. To address this issue, we performed experiments in parallel with psoralen and angelicin. We reasoned that events that were unique to the psoralen would reflect the presence of cross-links. The most striking example was seen with the recruitment dynamics of DDB2. In contrast to the situation with angelicin, the response to sites of psoralen adducts required XPC and repair synthesis. In effect, the repair pathway dependent on these functions generated a structure that, like the angelicin adduct, was recognized by DDB1-DDB2. This is consistent with our current models of cross-link repair and difficult to rationalize if the laser/psoralen combination formed only monoadducts.
It should be noted that there is an important distinction between the use of a high powered laser and a conventional laboratory UVA lamp to photoactivate psoralen. We estimate that the laser beam as it emerges from the back aperture of the objective is ∼1000 times more powerful than a standard laboratory lamp. Additionally, our protocol involved two successive cycles of laser pulses to the region of interest. Multiple laser pulses have been shown to drive monoadducts to ICLs (57). In the absence of an option for direct measurement, we suggest that the recruitment dynamics of the proteins in the DDB1-DDB2 complex, in wild type and XPC-deficient G1 phase cells, can be a diagnostic test of the presence of cross-links following laser activation of psoralen.
Repair of Cross-links in the Absence of a Replication Fork
Considerable recent attention has been given to cross-link repair in the context of a stalled replication fork (23, 26). On the other hand, studies in yeast establish an NER-dependent pathway for ICL repair in the G1 phase of the cell cycle (37, 38). Unhooking of ICLs in G1 phase has been demonstrated in mammalian cells (39), and we found that this was dependent on NER activities (40). The experiments reported here confirm repair of ICL in G1 phase mammalian cells and also address the role of candidate early response proteins.
XPC-HR23B Is Involved in Early Events in Cross-link Repair
Our results demonstrate that removal of the dig-pso signal in G1 phase is dependent on the XPC complex. The failure of a transcription inhibitor to suppress removal of the psoralen adducts is in support of a requirement for the global NER pathway for G1 phase repair. As noted above, the digoxigenin persistence assay cannot distinguish blocks to ICL repair in the first or the second cycle. However, the results of the comet assay indicate that XPC-deficient G1 phase cells, like other NER-deficient cells (40), are defective in the unhooking step. The XPC-deficient cells were also sensitive to psoralen in a survival assay. These data are consistent with repair of cross-links by an NER pathway in G1 phase mammalian cells, as observed in yeast (37).
The XPC-HR23B complex has been shown to recognize DNA containing helical distortions. The complex binds both double-stranded DNA and the single strand DNA induced on the strand opposite a DNA lesion (36, 73–75). Crystallographic structural studies on the yeast XPC orthologue Rad4 bound to DNA containing a cyclopyrimidine dimer demonstrate that both the adducted bases and the opposing undamaged nucleotides on the other strand are flipped out of the helix (75). The emphasis on helical distortion and base unstacking, rather than on the structure of individual adducts, explains the well known versatility of adduct recognition by NER and provides a unifying theme for damage recognition (76).
The rapid recruitment of XPC protein to regions containing psoralen ICLs raises the possibility that the XPC-HR23B complex recognizes these lesions directly. NMR spectroscopy of psoralen-cross-linked DNA indicated that there is significant local destabilization adjacent to the cross-link (71). The helix structure returns to normal within 3 base pairs. Analysis of crystal structures of psoralen-cross-linked DNA showed considerable distortion of the thymine base linked to the pyrone ring of psoralen (77). If the XPC complex directly binds psoralen-cross-linked DNA, it would most likely be via recognition of the unstacked region adjacent to the ICL.
DDB1-DDB2 Is Not Required for Cross-link Recognition or Repair
We found that cells deficient in the DDB2 (XPE) were not more sensitive to psoralen than wild type cells (Fig. 3). Additionally, the decline in the dig-pso signal was independent of this protein, as was unhooking in G1 phase cells. These results are consistent with much earlier reports of normal levels of incision of psoralen/UVA adducts in cells from an XPE patient (78). It has been suggested that the discrimination between damaged and undamaged DNA reflects sensing of adduct-induced helical distortion by DDB1-DDB2 complex (79). Recently, the crystallographic structure of DDB1-DDB2, bound to a 6-4 photodimer was determined. DDB2 has a three-amino acid hairpin that inserts into the DNA from the minor groove, displacing the lesion into a binding site located in the major groove. DDB2 interacts with the phosphodiester backbone of the lesion as well as the damaged base in the flipped out configuration (80, 81). Interstrand cross-linked bases cannot flip out of the helix, which may explain the non-involvement of the DDB1-DDB2 complex in cross-link recognition. Psoralen monoadducts, as well as cross-links, are poor substrates for binding by DDB1-DDB2 (82). As mentioned above, entry into the repair pathway generated a structure that was recognized by DDB1-DDB2. The requirement for binding, by DDB2, of DNA damage in the context of a duplex would explain the Ara-C sensitivity of DDB2 recruitment. Repair synthesis, following unhooking, would restore the duplex, thus completing the first repair cycle. This would produce a monoadduct consisting of a thymine cross-linked minimally to another thymine, maximally to the oligonucleotide product of the unhooking incisions (38, 83).
MutSβ Is Not Required for Psoralen Cross-link Repair in G1 Phase Cells
A role for mismatch repair functions in cross-link repair in yeast and mammalian cells is supported by reports from several laboratories (12, 25, 44, 84, 85). It has been proposed that ICLs are recognized by MutSβ, stimulated by interaction with proliferating cell nuclear antigen. This would be followed by recruitment of several proteins, including the Werner syndrome helicase, replication protein A, the Pso4 pre-mRNA splicing complex, and ERCC1-XPF (45).
MutSβ also plays a role in the repair of other forms of DNA damage. For example, the involvement in processing of chromium-DNA-protein adducts has been described recently (86). Proliferating cell nuclear antigen-dependent recruitment of MutSβ has been observed at sites of UV photoproducts (87). The appearance of the MutSβ proteins was additionally dependent on functional NER. The authors of this study presented a model in which, following UV-induced DNA damage, proliferating cell nuclear antigen localizes to an NER dependent intermediate and recruits MutSβ. They speculated that the MutSβ complex could be involved in mismatch repair during repair synthesis.
We found that the decline of the dig-psoralen signal was unaffected by deficiency in MutSβ, suggesting that repair of the psoralen adducts was not dependent on this activity in G1 phase cells. Furthermore, recruitment of MSH3 to psoralen sites was clearly slower than the appearance of XPC protein. Recruitment was dependent on functional XPC and blocked by inhibition of repair synthesis. Consequently, we conclude that, like DDB2, MutSβ is not necessary for psoralen recognition and repair in G1 phase cells, although it can be recruited in a post-recognition stage in the repair process. The involvement of this complex in very early steps of cross-link repair, as proposed by other groups, may reflect a role in processes operative in S phase, when homologous recombination and post-replication repair pathways are available (12, 45).
Cross-link Repair in G1 Phase
We interpret our results as indicating that psoralen ICLs are repaired in G1 phase cells in an efficient pathway that involves NER functions. Recognition is dependent on the XPC-HR23B complex, and the ensuing repair pathway engages and is dependent on classical NER activities, such as XPA and XPF. Although there is no requirement for DDB1-DDB2, this complex does recognize the appearance of the monoadduct produced by unhooking and the first round of repair synthesis. Thus, DDB1-DDB2 recruitment acts as a marker of the completion of the first repair cycle and the start of the second. Similarly, the timing of MutSβ recruitment and the dependence on XPC and repair synthesis are consistent with entry after the initial recognition step (87).
Although the DDB2 deficiency of the XPE cells clearly retarded angelicin removal, there was no apparent influence on psoralen repair, which includes a monoadduct repair cycle in the second step. The DDB1-DDB2 activity has been shown to be important for chromatin remodeling as a prelude for XPC recruitment and monoadduct repair (59, 60, 62, 88). Presumably, entry of the ICL into an XPC-dependent NER pathway obviates the requirement for the contribution of the DDB1-DDB2 complex during the second repair cycle (64). Consequently, we suggest that the recruitment of DDB1-DDB2 to the monoadduct at the end of the first repair cycle can be seen as gratuitous. It happens because the complex recognizes the lesion, but since the site is already the locus of repair, that interaction is not essential for completion of the second cycle.
Finally, it should be noted that, although we have confined the studies described here to events in G1 phase, we have observed similar repair and protein recruitment dynamics in S phase cells. This is an indication of an XPC-dependent repair pathway operative throughout the cell cycle, in agreement with the earlier conclusions based on experiments in yeast (37). It will be important to account for this pathway in experiments intended to address ICL repair in S phase, in the context of a replication fork.
Supplementary Material
Acknowledgments
We thank Dr. Vesna Rapič-Otrin for the DDB2-FLAG plasmid and Sam Broder, John Hearst, James McNally, Larry Phillips, and Scott Randall for helpful discussions and advice.
This work was supported, in whole or in part, by the National Institutes of Health, NIA, Intramural Research Program. This work was also supported by the Fanconi Anemia Research Fund.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- ICL
- interstrand cross-link
- ang
- angelicin
- Ara-C
- cytosine arabinoside
- dig
- digoxigenin
- NER
- nucleotide excision repair
- pso
- psoralen
- PBS
- phosphate-buffered saline.
REFERENCES
- 1.Niedernhofer L. J., Daniels J. S., Rouzer C. A., Greene R. E., Marnett L. J. (2003) J. Biol. Chem. 278, 31426–31433 [DOI] [PubMed] [Google Scholar]
- 2.Mitchell J. R., Hoeijmakers J. H., Niedernhofer L. J. (2003) Curr. Opin. Cell Biol. 15, 232–240 [DOI] [PubMed] [Google Scholar]
- 3.Dronkert M. L., Kanaar R. (2001) Mutat. Res. 486, 217–247 [DOI] [PubMed] [Google Scholar]
- 4.McHugh P. J., Spanswick V. J., Hartley J. A. (2001) Lancet Oncol. 2, 483–490 [DOI] [PubMed] [Google Scholar]
- 5.Weeda G., Donker I., de Wit J., Morreau H., Janssens R., Vissers C. J., Nigg A., van Steeg H., Bootsma D., Hoeijmakers J. H. (1997) Curr. Biol. 7, 427–439 [DOI] [PubMed] [Google Scholar]
- 6.Houtsmuller A. B., Rademakers S., Nigg A. L., Hoogstraten D., Hoeijmakers J. H., Vermeulen W. (1999) Science 284, 958–961 [DOI] [PubMed] [Google Scholar]
- 7.Tian M., Shinkura R., Shinkura N., Alt F. W. (2004) Mol. Cell. Biol. 24, 1200–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaspers N. G., Raams A., Silengo M. C., Wijgers N., Niedernhofer L. J., Robinson A. R., Giglia-Mari G., Hoogstraten D., Kleijer W. J., Hoeijmakers J. H., Vermeulen W. (2007) Am. J. Hum. Genet. 80, 457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grompe M., D'Andrea A. (2001) Hum. Mol. Genet. 10, 2253–2259 [DOI] [PubMed] [Google Scholar]
- 10.Grossmann K. F., Ward A. M., Matkovic M. E., Folias A. E., Moses R. E. (2001) Mutat. Res. 487, 73–83 [DOI] [PubMed] [Google Scholar]
- 11.Saffran W. A., Ahmed S., Bellevue S., Pereira G., Patrick T., Sanchez W., Thomas S., Alberti M., Hearst J. E. (2004) J. Biol. Chem. 279, 36462–36469 [DOI] [PubMed] [Google Scholar]
- 12.Barber L. J., Ward T. A., Hartley J. A., McHugh P. J. (2005) Mol. Cell. Biol. 25, 2297–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noronha A. M., Noll D. M., Wilds C. J., Miller P. S. (2002) Biochemistry 41, 760–771 [DOI] [PubMed] [Google Scholar]
- 14.Cole R. S. (1973) Proc. Natl. Acad. Sci. U.S.A. 70, 1064–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole R. S., Levitan D., Sinden R. R. (1976) J. Mol. Biol. 103, 39–59 [DOI] [PubMed] [Google Scholar]
- 16.Sladek F. M., Munn M. M., Rupp W. D., Howard-Flanders P. (1989) J. Biol. Chem. 264, 6755–6765 [PubMed] [Google Scholar]
- 17.Sladek F. M., Melian A., Howard-Flanders P. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 3982–3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berardini M., Foster P. L., Loechler E. L. (1999) J. Bacteriol. 181, 2878–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuraoka I., Kobertz W. R., Ariza R. R., Biggerstaff M., Essigmann J. M., Wood R. D. (2000) J. Biol. Chem. 275, 26632–26636 [DOI] [PubMed] [Google Scholar]
- 20.Cromie G. A., Connelly J. C., Leach D. R. (2001) Mol. Cell 8, 1163–1174 [DOI] [PubMed] [Google Scholar]
- 21.De Silva I. U., McHugh P. J., Clingen P. H., Hartley J. A. (2000) Mol. Cell. Biol. 20, 7980–7990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akkari Y. M., Bateman R. L., Reifsteck C. A., Olson S. B., Grompe M. (2000) Mol. Cell. Biol. 20, 8283–8289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niedernhofer L. J., Odijk H., Budzowska M., van Drunen E., Maas A., Theil A. F., de Wit J., Jaspers N. G., Beverloo H. B., Hoeijmakers J. H., Kanaar R. (2004) Mol. Cell. Biol. 24, 5776–5787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanada K., Budzowska M., Modesti M., Maas A., Wyman C., Essers J., Kanaar R. (2006) EMBO J. 25, 4921–4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang N., Liu X., Li L., Legerski R. (2007) DNA Repair 6, 1670–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Räschle M., Knipscheer P., Enoiu M., Angelov T., Sun J., Griffith J. D., Ellenberger T. E., Schärer O. D., Walter J. C. (2008) Cell 134, 969–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergstralh D. T., Sekelsky J. (2008) Trends Genet. 24, 70–76 [DOI] [PubMed] [Google Scholar]
- 28.Fousteri M., Mullenders L. H. (2008) Cell Res. 18, 73–84 [DOI] [PubMed] [Google Scholar]
- 29.Mocquet V., Kropachev K., Kolbanovskiy M., Kolbanovskiy A., Tapias A., Cai Y., Broyde S., Geacintov N. E., Egly J. M. (2007) EMBO J. 26, 2923–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maillard O., Camenisch U., Clement F. C., Blagoev K. B., Naegeli H. (2007) Trends Biochem. Sci. 32, 494–499 [DOI] [PubMed] [Google Scholar]
- 31.Maillard O., Camenisch U., Blagoev K. B., Naegeli H. (2008) Mutat. Res. 658, 271–286 [DOI] [PubMed] [Google Scholar]
- 32.Wittschieben B. Ø., Iwai S., Wood R. D. (2005) J. Biol. Chem. 280, 39982–39989 [DOI] [PubMed] [Google Scholar]
- 33.Fujiwara Y., Masutani C., Mizukoshi T., Kondo J., Hanaoka F., Iwai S. (1999) J. Biol. Chem. 274, 20027–20033 [DOI] [PubMed] [Google Scholar]
- 34.Moser J., Volker M., Kool H., Alekseev S., Vrieling H., Yasui A., van Zeeland A. A., Mullenders L. H. (2005) DNA Repair 4, 571–582 [DOI] [PubMed] [Google Scholar]
- 35.Kusumoto R., Masutani C., Sugasawa K., Iwai S., Araki M., Uchida A., Mizukoshi T., Hanaoka F. (2001) Mutat. Res. 485, 219–227 [DOI] [PubMed] [Google Scholar]
- 36.Maillard O., Solyom S., Naegeli H. (2007) PLoS Biol. 5, e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarkar S., Davies A. A., Ulrich H. D., McHugh P. J. (2006) EMBO J. 25, 1285–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McHugh P. J., Sarkar S. (2006) Cell Cycle 5, 1044–1047 [DOI] [PubMed] [Google Scholar]
- 39.Rothfuss A., Grompe M. (2004) Mol. Cell. Biol. 24, 123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richards S., Liu S. T., Majumdar A., Liu J. L., Nairn R. S., Bernier M., Maher V., Seidman M. M. (2005) Nucleic Acids Res. 33, 5382–5393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X., Peterson C. A., Zheng H., Nairn R. S., Legerski R. J., Li L. (2001) Mol. Cell. Biol. 21, 713–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mu D., Bessho T., Nechev L. V., Chen D. J., Harris T. M., Hearst J. E., Sancar A. (2000) Mol. Cell. Biol. 20, 2446–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smeaton M. B., Hlavin E. M., McGregor Mason T., Noronha A. M., Wilds C. J., Miller P. S. (2008) Biochemistry 47, 9920–9930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang N., Lu X., Zhang X., Peterson C. A., Legerski R. J. (2002) Mol. Cell. Biol. 22, 2388–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang N., Kaur R., Lu X., Shen X., Li L., Legerski R. J. (2005) J. Biol. Chem. 280, 40559–40567 [DOI] [PubMed] [Google Scholar]
- 46.Derheimer F. A., Hicks J. K., Paulsen M. T., Canman C. E., Ljungman M. (2009) Mol. Pharmacol. 75, 599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Islas A. L., Baker F. J., Hanawalt P. C. (1994) Biochemistry 33, 10794–10799 [DOI] [PubMed] [Google Scholar]
- 48.Zheng H., Wang X., Warren A. J., Legerski R. J., Nairn R. S., Hamilton J. W., Li L. (2003) Mol. Cell. Biol. 23, 754–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelms B. E., Maser R. S., MacKay J. F., Lagally M. G., Petrini J. H. (1998) Science 280, 590–592 [DOI] [PubMed] [Google Scholar]
- 50.Volker M., Moné M. J., Karmakar P., van Hoffen A., Schul W., Vermeulen W., Hoeijmakers J. H., van Driel R., van Zeeland A. A., Mullenders L. H. (2001) Mol. Cell 8, 213–224 [DOI] [PubMed] [Google Scholar]
- 51.Rogakou E. P., Boon C., Redon C., Bonner W. M. (1999) J. Cell Biol. 146, 905–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fousteri M., Vermeulen W., van Zeeland A. A., Mullenders L. H. (2006) Mol. Cell 23, 471–482 [DOI] [PubMed] [Google Scholar]
- 53.Lan L., Nakajima S., Oohata Y., Takao M., Okano S., Masutani M., Wilson S. H., Yasui A. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 13738–13743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thazhathveetil A. K., Liu S. T., Indig F. E., Seidman M. M. (2007) Bioconjug. Chem. 18, 431–437 [DOI] [PubMed] [Google Scholar]
- 55.Emmert S., Kobayashi N., Khan S. G., Kraemer K. H. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 2151–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao J., Kennedy B. K., Lawrence B. D., Barbie D. A., Matera A. G., Fletcher J. A., Harlow E. (2000) Genes Dev. 14, 2283–2297 [PMC free article] [PubMed] [Google Scholar]
- 57.Johnston B. H., Johnson M. A., Moore C. B., Hearst J. E. (1977) Science 197, 906–908 [DOI] [PubMed] [Google Scholar]
- 58.Kruhlak M. J., Celeste A., Nussenzweig A. (2006) Cell Cycle 5, 1910–1912 [DOI] [PubMed] [Google Scholar]
- 59.Fitch M. E., Nakajima S., Yasui A., Ford J. M. (2003) J. Biol. Chem. 278, 46906–46910 [DOI] [PubMed] [Google Scholar]
- 60.El-Mahdy M. A., Zhu Q., Wang Q. E., Wani G., Praetorius-Ibba M., Wani A. A. (2006) J. Biol. Chem. 281, 13404–13411 [DOI] [PubMed] [Google Scholar]
- 61.Nishi R., Alekseev S., Dinant C., Hoogstraten D., Houtsmuller A. B., Hoeijmakers J. H., Vermeulen W., Hanaoka F., Sugasawa K. (2009) DNA Repair 8, 767–776 [DOI] [PubMed] [Google Scholar]
- 62.Kapetanaki M. G., Guerrero-Santoro J., Bisi D. C., Hsieh C. L., Rapiæ-Otrin V., Levine A. S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 2588–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moggs J. G., Yarema K. J., Essigmann J. M., Wood R. D. (1996) J. Biol. Chem. 271, 7177–7186 [DOI] [PubMed] [Google Scholar]
- 64.Staresincic L., Fagbemi A. F., Enzlin J. H., Gourdin A. M., Wijgers N., Dunand-Sauthier I., Giglia-Mari G., Clarkson S. G., Vermeulen W., Schärer O. D. (2009) EMBO J. 28, 1111–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsumoto M., Yaginuma K., Igarashi A., Imura M., Hasegawa M., Iwabuchi K., Date T., Mori T., Ishizaki K., Yamashita K., Inobe M., Matsunaga T. (2007) J. Cell Sci. 120, 1104–1112 [DOI] [PubMed] [Google Scholar]
- 66.Hanasoge S., Ljungman M. (2007) Carcinogenesis 28, 2298–2304 [DOI] [PubMed] [Google Scholar]
- 67.Kong X., Mohanty S. K., Stephens J., Heale J. T., Gomez-Godinez V., Shi L. Z., Kim J. S., Yokomori K., Berns M. W. (2009) Nucleic Acids Res. 37, e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dinant C., de Jager M., Essers J., van Cappellen W. A., Kanaar R., Houtsmuller A. B., Vermeulen W. (2007) J. Cell Sci. 120, 2731–2740 [DOI] [PubMed] [Google Scholar]
- 69.Sartorelli A. C., Hodnick W. F., Belcourt M. F., Tomasz M., Haffty B., Fischer J. J., Rockwell S. (1994) Oncol. Res. 6, 501–508 [PubMed] [Google Scholar]
- 70.Palom Y., Suresh Kumar G., Tang L. Q., Paz M. M., Musser S. M., Rockwell S., Tomasz M. (2002) Chem Res. Toxicol. 15, 1398–1406 [DOI] [PubMed] [Google Scholar]
- 71.Spielmann H. P., Dwyer T. J., Sastry S. S., Hearst J. E., Wemmer D. E. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 2345–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lai C., Cao H., Hearst J. E., Corash L., Luo H., Wang Y. (2008) Anal. Chem. 80, 8790–8798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dip R., Camenisch U., Naegeli H. (2004) DNA Repair 3, 1409–1423 [DOI] [PubMed] [Google Scholar]
- 74.Sugasawa K., Shimizu Y., Iwai S., Hanaoka F. (2002) DNA Repair 1, 95–107 [DOI] [PubMed] [Google Scholar]
- 75.Min J. H., Pavletich N. P. (2007) Nature 449, 570–575 [DOI] [PubMed] [Google Scholar]
- 76.Yang W. (2008) Cell Res. 18, 184–197 [DOI] [PubMed] [Google Scholar]
- 77.Eichman B. F., Mooers B. H., Alberti M., Hearst J. E., Ho P. S. (2001) J. Mol. Biol. 308, 15–26 [DOI] [PubMed] [Google Scholar]
- 78.Bredberg A., Söderhäll S. (1985) Biochim. Biophys. Acta. 824, 268–271 [DOI] [PubMed] [Google Scholar]
- 79.Tang J., Chu G. (2002) DNA Repair 1, 601–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scrima A., Konícková R., Czyzewski B. K., Kawasaki Y., Jeffrey P. D., Groisman R., Nakatani Y., Iwai S., Pavletich N. P., Thomä N. H. (2008) Cell 135, 1213–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schärer O. D., Campbell A. J. (2009) Nat. Struct. Mol. Biol. 16, 102–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Payne A., Chu G. (1994) Mutat. Res. 310, 89–102 [DOI] [PubMed] [Google Scholar]
- 83.Minko I. G., Harbut M. B., Kozekov I. D., Kozekova A., Jakobs P. M., Olson S. B., Moses R. E., Harris T. M., Rizzo C. J., Lloyd R. S. (2008) J. Biol. Chem. 283, 17075–17082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lan L., Hayashi T., Rabeya R. M., Nakajima S., Kanno S., Takao M., Matsunaga T., Yoshino M., Ichikawa M., Riele H., Tsuchiya S., Tanaka K., Yasui A. (2004) DNA Repair 3, 135–143 [DOI] [PubMed] [Google Scholar]
- 85.Wu Q., Christensen L. A., Legerski R. J., Vasquez K. M. (2005) EMBO Rep. 6, 551–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reynolds M. F., Peterson-Roth E. C., Bespalov I. A., Johnston T., Gurel V. M., Menard H. L., Zhitkovich A. (2009) Cancer Res. 69, 1071–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hong Z., Jiang J., Hashiguchi K., Hoshi M., Lan L., Yasui A. (2008) J. Cell Sci. 121, 3146–3154 [DOI] [PubMed] [Google Scholar]
- 88.Luijsterburg M. S., Goedhart J., Moser J., Kool H., Geverts B., Houtsmuller A. B., Mullenders L. H., Vermeulen W., van Driel R. (2007) J. Cell Sci. 120, 2706–2716 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.