Abstract
Impaired epithelial barrier function plays a crucial role in the pathogenesis of inflammatory bowel disease. Elevated levels of the pro-inflammatory cytokine, interferon-γ (IFNγ), are believed to be prominently involved in the pathogenesis of Crohn disease. Treatment of T84 intestinal epithelial cells with IFNγ severely impairs their barrier properties measured as transepithelial electrical resistance (TER) or permeability and reduces the expression of tight junction proteins such as occludin and zonula occludens-1 (ZO-1). However, little is known about the signaling events that are involved. The cellular energy sensor, AMP-activated protein kinase (AMPK), is activated in response to cellular stress, as occurs during inflammation. The aim of this study was to investigate a possible role for AMPK in mediating IFNγ-induced effects on the intestinal epithelial barrier. We found that IFNγ activates AMPK by phosphorylation, independent of intracellular energy levels. Inhibition of AMPK prevents, at least in part, the IFNγ-induced decrease in TER. Furthermore, AMPK knockdown prevented the increased epithelial permeability, the decreased TER, and the decrease in occludin and ZO-1 caused by IFNγ treatment of T84 cells. However, AMPK activity alone was not sufficient to cause alterations in epithelial barrier function. These data show a novel role for AMPK, in concert with other signals induced by IFNγ, in mediating reduced epithelial barrier function in a cell model of chronic intestinal inflammation. These findings may implicate AMPK in the pathogenesis of chronic intestinal inflammatory conditions, such as inflammatory bowel disease.
Inflammatory bowel disease (IBD)2 consists of two major subgroups, ulcerative colitis and Crohn disease (CD). A complex cascade of genetic, immunological, and bacterial factors contributes to IBD pathogenesis (1). In the healthy intestine, the epithelial barrier separates the luminal bacterial microbiota and other aspects of the external environment from cells of the mucosal immune system. In CD in particular, an impaired epithelial barrier (2, 3) leads to increased exposure of the immune system to commensal bacteria. Along with possible genetic defects in bacterial sensing, this might contribute to a dysregulated immune response leading to further epithelial damage and active episodes of IBD (4). Epithelial barrier dysfunction in CD is characterized by alterations in intercellular tight junctions (5), as well as by an excessive loss of water and salt into the lumen. An important immunological marker in CD is the existence of excessively high levels of the pro-inflammatory cytokine, interferon gamma (IFNγ) (6).
IFNγ treatment of intestinal epithelial cell monolayers severely compromises their barrier integrity. Most importantly from a functional perspective, IFNγ causes a decrease in transepithelial electrical resistance (TER) and increases epithelial permeability (7, 8). These defects closely resemble observations in CD, where there is a disruption of intercellular tight junctional complexes. This effect is due to disruption of the apical actin cytoskeleton in conjunction with decreased expression, as well as increased internalization, of important tight junction proteins such as occludin and zonula occludens-1 (ZO-1) (8–11). Conversely, induction of epithelial apoptosis by IFNγ is believed to contribute little to barrier dysfunction (12). IFNγ also induces further alterations in epithelial function that include reduced expression of various ion transporters and associated decreases in epithelial ion transport (13, 14). Despite the influence of IFNγ on a number of epithelial functions, relatively little is known about intracellular signaling mechanisms mediating its effects following receptor activation. Recent studies demonstrated the involvement of phosphatidylinositol 3′-kinase (PI3K) in mediating IFNγ-induced effects on epithelial barrier function (11, 15). However, this is unlikely to be the only regulatory pathway involved. Indeed, increased expression of receptors for tumor necrosis factor core family members, such as the tumor necrosis factor receptor and LIGHT (homologous to lymphotoxin, shows inducible expression and competes with herpes simplex virus glycoprotein D for herpes virus entry mediator (HVEM), a receptor expressed by T lymphocytes), can also occur in response to IFNγ and lead to changes in intestinal barrier function (16–18).
The effects of IFNγ in intestinal epithelial cells resemble, at least in part, those of the cellular energy sensor, AMP-activated protein kinase (AMPK). Upon activation, AMPK restores intracellular ATP levels by stimulating energy-producing pathways, such as glucose uptake (19) and glycolysis, while inhibiting energy-consuming pathways, such as the synthesis of fatty acids or triglycerides (20, 21). In the intestine, energy-consuming processes include epithelial ion transport, and, indeed, AMPK has been shown to decrease intestinal ATP-consuming ion transport as well as the synthesis of various proteins (22, 23). Moreover, it has previously been demonstrated that ion transport processes are suppressed in intestinal biopsies from IBD patients (24–26).
AMPK is usually activated in response to cellular stress that depletes intracellular ATP and elevates the AMP:ATP ratio (27, 28). AMPK-activating conditions include oxidative stress (29), hypoxia (30), and hypoglycemia (31). Binding of AMP to AMPK causes an increase in activity of 5-fold or less (32). Further, binding of AMP to AMPK makes AMPK a better substrate for upstream kinase activation, resulting in phosphorylation of the catalytic α-subunit of AMPK on the Thr172 residue and subsequently in a 50- to 100-fold activation of the enzyme (32). A number of upstream kinases for AMPK have been identified, with LKB1 (33, 34) or calmodulin kinase II (35–37) being the most important and well studied. However, recent studies also indicate that PI3K can activate AMPK (38, 39).
The goal of this study was to determine whether AMPK mediates IFNγ-induced alterations in intestinal epithelial barrier function. We found that IFNγ activates AMPK in intestinal epithelial cells and AMPK inhibition prevents, at least in part, IFNγ-induced barrier dysfunction. Our data indicate a novel role for the cellular energy sensor, AMPK, in the regulation of intestinal epithelial barrier properties in a cell model of chronic inflammation. These findings may have implications for barrier function in the setting of chronic inflammatory processes, such as IBD.
EXPERIMENTAL PROCEDURES
Material
Human IFNγ (Roche Diagnostics, Mannheim, Germany), Compound C (Calbiochem), LY294002 (Calbiochem), carbachol (Sigma), hydrogen peroxide (H2O2, Sigma), rabbit anti-lamin A/C-antibody (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-phospho-AMPKα (Thr172) antibody, rabbit anti-AMPK-α antibody, rabbit anti-phospho-Akt (Ser473) antibody, and rabbit anti-Akt antibody (Cell Signaling Technologies, Danvers, MA) were obtained from the sources noted. Mouse anti-claudin-2 antibody, mouse anti-claudin-4 antibody, rabbit-anti-occludin antibody, and mouse-anti-ZO-1 antibody were obtained from Zymed Laboratories Inc. (Carlsbad, CA). All other reagents were of analytical grade and acquired commercially.
Cell Culture
Human colonic T84 epithelial cells were cultured in a humidified atmosphere with 5% CO2 as described previously (40) in Dulbecco's modified Eagle's/F-12 medium (Mediatech, Inc., Herndon, VA) supplemented with 5% newborn calf serum. Cells were separated by trypsinization, and 1 × 106 cells were seeded onto either 12- or 30-mm Millicell-HA semipermeable filter supports (Millipore, Bedford, MA). When seeded on filters, T84 cells develop monolayers with the polarized phenotype of native intestinal epithelial cells (41). Before treatment, cells were cultured for 8–14 days. According to its receptor localization, IFNγ (1000 units/ml) was added basolaterally while Compound C (50 μm) and LY294002 (20 μm) were added bilaterally.
Preparation of Whole Cell Lysates
After stimulation, T84 cells were washed three times with ice-cold Ringer's solution (140 mm Na+, 5.2 mm K+, 1.2 mm Ca2+, 0.8 mm Mg2+, 120 mm Cl−, 25 mm HCO3−, 2.4 mm H2PO4−, 0.4 mm HPO42−, 10 mm glucose) and lysed in ice-cold lysis buffer (1% Triton X-100, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 μg/ml antipain, 100 μg/ml phenylmethylsulfonyl fluoride, 1 mm sodium vanadate, 1 mm sodium fluoride, and 1 mm EDTA in PBS) for 45 min. T84 cells were scraped from the filters, transferred to a microcentrifuge tube, and centrifuged for 10 min at 12,000 rpm. Cell lysate supernatants were assayed for protein content using a Bio-Rad protein assay kit (Bio-Rad).
Western Blotting
An aliquot of each lysate was mixed with an equal amount of 2× gel loading buffer (50 mm Tris, pH 6.8, 2% SDS, 200 mm dithiothreitol, 40% glycerol, 0.2% bromphenol blue) and boiled for 4 min. Proteins were separated by SDS-PAGE and transferred onto polyvinylidene fluoride membranes (Millipore). Membranes were blocked with 1% blocking solution, and an appropriate concentration of primary antibody was added in 1% blocking buffer over night. Membranes were washed with Tris-buffered saline containing 1% Tween 20 (1% TBST) for 1 h, horseradish peroxidase-labeled secondary anti-mouse- or anti-rabbit-IgG-antibody (BD Biosciences) in 1% blocking solution (1:2500) was added for 30 min, and membranes were washed for 1 h with 1% TBST. Finally, immunoreactive proteins were detected using an enhanced chemiluminescence detection kit (Amersham Biosciences). Densitometric analysis of Western blots was performed by using Image software (National Institutes of Health).
ATP Assay
T84 cells were seeded on 0.6-cm2 semi-permeable filter supports for 8–10 days. After treatment, cells were washed twice with warm Ringer's solution. Cellular ATP levels were assessed using the CellTiter-Glo Luminescence Cell Viability Assay (Promega) according to the manufacturer's instructions. Briefly, equal volumes of Ringer's and CellTiter-Glo reagent were added apically to lyse the cells. After 10-min shaking, cells were scraped off the filters, and luminescence was read with open range settings on a SpectraMax Fluorescence Microplate reader using the SoftMax Pro v5 software (both from Molecular Devices, Sunnyvale, CA). To normalize cellular ATP levels, the cellular double strand DNA content was analyzed using the Quant-itTM Pico Green dsDNA Assay Kit (Molecular Probes, Eugene, OR) according to the manufacturer's instructions.
TER
T84 cells were seeded onto either 12- or 30-mm semi-permeable filter supports (pore size, 0.45 μm) and cultured for ∼10 days until the TER was at least 900 Ohm.cm2. The TER of each monolayer was then assessed before and after treatment using a voltohmeter (WPI, Sarasota, FL) and companion electrodes (Millipore). To normalize the variation in the absolute TER values of each monolayer, the data are expressed as the percentage of pre-treatment TER values (15, 42).
Electrophysiological Studies
After treatment, T84 cell monolayers were mounted in Ussing chambers with a window area of 0.6 cm2 and bathed in oxygenated (95% O2, 5% CO2) Ringer's solution at 37 °C. Using short-circuit current (Isc) the monolayers were continuously voltage-clamped to zero potential difference. Cells were allowed to equilibrate for 10 min before baseline Isc and conductance were assessed. Changes in Isc (ΔIsc) under these conditions are exclusively due to changes in electrogenic chloride secretion (43) and were measured for a period of 20 min after stimulation with carbachol (100 μm, basolaterally). Conductance was calculated according to Ohm's law as mS/cm2.
Small Interfering RNA Transfection
2 × 106 T84 cells were seeded 3 days before transfection and grown to 50–70% confluency in T75 flasks. Three different annealed Silencer pre-designed siRNA oligonucleotides targeting AMPKα1 were obtained from Applied Biosystems (Foster City, CA). For transfection reactions, 100 pmol of each of the three gene-specific siRNA oligonucleotides were transfected into T84 cells using the Amaxa nucleofector system (Amaxa Inc., Gaithersburg, MD) according to the manufacturer's instructions. After transfection, T84 cells were cultured on filter membranes for 48 h before further treatment. A nonspecific control siRNA SMARTpool (100 pmol, Upstate Biotechnology/Dharmacon, Chicago, IL) was used as a negative control.
Transepithelial Permeability
Transepithelial permeability was assessed by measuring the flux of fluorescein isothiocyanate-dextran (FITC-dextran, 10 kDa, Sigma) across T84 cell monolayers. Following treatment, T84 cells were washed (3×) with Ringer's solution and incubated in Ringer's solution for 30 min at 37 °C to equilibrate. FITC-dextran (1 mg/ml) was added to the apical side of the monolayer. 1 h later, 100 μl of the basolateral solution was removed, and fluorescence of FITC-dextran in this compartment was detected using excitation at 490 nm and emission at 520 nm.
Confocal Microscopy
T84 cells were seeded onto 12-mm Millicell-HA filters for 2 days after siRNA transfection and before stimulation. After treatment, cells were washed twice with PBS, fixed with 10% Formalin in PBS for 10 min at room temperature, washed (3×) with PBS, and permeabilized with 0.3% Triton X-100 in PBS for 10 min. After three washes with PBS, cells were blocked with 20% donkey serum (Sigma) in PBS for 1 h and washed with PBS once. Mouse anti-ZO-1 antibody (1 μg/ml) in 20 mg/ml bovine serum albumin in PBS was applied overnight. Cells were washed (3×) with PBS and secondary Alexa-488-conjugated donkey anti-mouse antibody (excitation/emission maxima at 495/519 nm, Molecular Probes) was added in a 1:500 dilution in 20 mg/ml bovine serum albumin in PBS for 30 min at room temperature. Cells were washed (3×) with PBS, incubated with Hoechst 33258 (excitation/emission maxima at 352/461 nm, Molecular Probes) in PBS (1:500) for 20 min at room temperature, and washed four times with PBS. Finally, the cells on the filter membrane were transferred onto glass slides and mounted with ProLong Gold Antifade Reagent (Molecular Probes). Confocal microscopy was performed using a Zeiss LSM 510 Laser Scanning Confocal system on a Zeiss Axioscope 2 Upright Microscope (Zeiss, Jena, Germany). Data were analyzed using the Zeiss LSM 5 Image Examiner software.
Statistical Analysis
Data are presented as means ± S.E. for a series of n experiments. Data are expressed as raw data, arbitrary units or as a percentage of the respective control. Statistical analysis was performed by analysis of variance followed by Student-Newman-Keuls post hoc test unless otherwise noted. p values of <0.05 were considered significant.
RESULTS
IFNγ Activates AMPK in Human T84 Intestinal Epithelial Cells
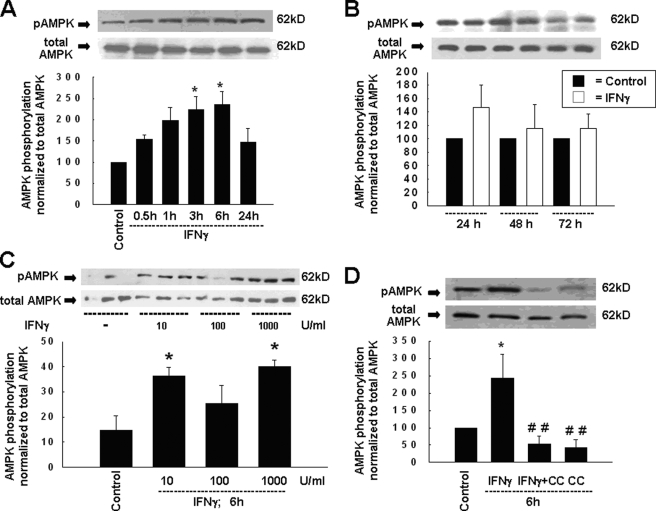
To study whether the cellular energy sensor, AMPK, participates in IFNγ-induced effects on epithelial barrier function, we initially investigated whether IFNγ activates AMPK. Polarized monolayers of T84 cells were treated with IFNγ at a concentration of 1000 units/ml for 0.5–72 h. At this concentration, IFNγ has been shown to exert significant effects on intestinal epithelial cell barrier properties (7, 44, 45). Furthermore, in a previous study by Sasaki et al. (46), cultured biopsies from patients with active CD were shown to release IFNγ at levels as high as ∼1000 units/ml/mg of tissue, although the mean level was 75 ± 215 units/ml/mg of tissue. So, although the majority of our data were generated using 1000 units/ml (100 ng/ml) of IFNγ (in contrast to a number of other studies that used 10 or 20 ng/ml IFNγ), the experimental use of this concentration is not unreasonable. IFNγ treatment for 6 h caused maximal activation of AMPK in human T84 intestinal epithelial cells as assessed by phosphorylation of Thr172 of the AMPK catalytic α1-subunit, which is an established marker of AMPK activation (47). Representative Western blots showed that IFNγ stimulated AMPK activation as early as 30 min after treatment; thereafter phosphorylation increased in a time-dependent manner reaching a peak at 6 h (Fig. 1A). Thereafter AMPK phosphorylation declined in a time-dependent manner almost to control levels by 48 and 72 h of IFNγ treatment (Fig. 1B). To assess concentration-dependent effects of IFNγ on the phosphorylation of AMPK, we administered 10, 100, and 1000 units/ml IFNγ for 6 h, the time point showing a maximal IFNγ effect on AMPK phosphorylation. As shown in Fig. 1C, a concentration of 1000 units/ml IFNγ caused the strongest increase in AMPK phosphorylation in our cell model, whereas reduced effects were seen with of 10 and 100 units/ml IFNγ. Furthermore, the pharmacological AMPK inhibitor, Compound C (CC, 50 μm), which competes with intracellular ATP for binding to AMPK (48), prevented the IFNγ-induced activation of AMPK (Fig. 1D). In a separate and as yet unpublished study, we generated a concentration curve for the inhibitory effect of CC on AMPK activation and observed peak inhibition with a dose of 50 μm CC (data not shown). Therefore, we used this inhibitor concentration for subsequent experiments. Of note, 50 μm CC is at the lower end of the standard range for AMPK inhibition in epithelial cells: Walker et al. (23) used 75 μm CC in their T84 cell studies and Woollhead et al. (49) used 80 μm CC in their studies in lung epithelial cells, although lower doses may be required for other cell types such as endothelial cells (50).
FIGURE 1.
IFNγ-induced activation of AMPK is sensitive to CC in T84 cells. A, representative Western blots of phosphorylated and total AMPK-α1 after IFNγ treatment for 0–24 h and densitometric analysis (n = 3–7). B, representative Western blots of phosphorylated and total AMPK-α1 after IFNγ treatment for 24–72 h, and densitometric analysis (n = 4). C, representative Western blots of phosphorylated and total AMPK-α1 after treatment for 6 h with different concentrations of IFNγ (10, 100, and 1000 units/ml), and densitometric analysis (n = 3). D, representative Western blots show phosphorylated and total AMPK after treatment with IFNγ and/or CC, added bilaterally (50 μm), and densitometric analysis of five similar experiments. Data are presented as percentage of control. Asterisks indicate significant difference versus the respective control, *, p < 0.05; ##, p < 0.01 versus 6 h IFNγ treatment of T84 cells.
IFNγ-induced AMPK Activation Is Independent of Changes in Intracellular ATP
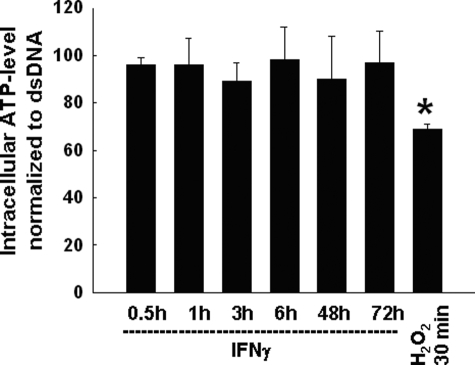
Because AMPK is usually activated by declining levels of intracellular ATP, we next investigated if AMPK activation in response to IFNγ was dependent on the intracellular ATP level. T84 cells were treated with IFNγ for 0.5–72 h. Treatment with H2O2 (100 μm; 30 min, bilaterally) was used as a positive control to deplete ATP (51). Interestingly, IFNγ treatment had no significant effect on intracellular ATP at any time point. Although the cellular ATP level declined somewhat within 3 h of IFNγ treatment, this effect was not significant and therefore unlikely to explain IFNγ-induced AMPK activation, which occurred as early as 30 min after addition of IFNγ. Additionally, long term treatment with IFNγ for up to 72 h did not significantly alter the cellular ATP level, correlating with a lack of AMPK phosphorylation at these time points. In contrast, H2O2 significantly reduced ATP levels within 30 min (Fig. 2). These data indicate that IFNγ activates AMPK independent of changes in intracellular ATP.
FIGURE 2.
IFNγ-induced activation of AMPK is independent of changes in intracellular ATP. T84 cells were treated with IFNγ (1000 units/ml) for the indicated times (n = 4–8). H2O2 (100 μm) treatment for 0.5 h was used as a positive control. In contrast to H2O2 treatment, IFNγ treatment did not cause a significant decrease in cellular ATP levels. Data are shown as a percentage of control ATP normalized to double strand DNA. Asterisks indicate significant difference versus control; *, p < 0.05.
CC Ameliorates the IFNγ-induced Decrease in TER across T84 Monolayers
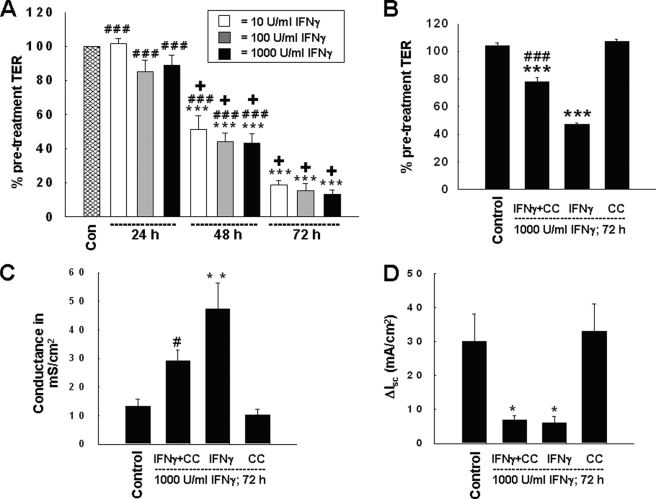
Long term treatment with IFNγ decreases TER across T84 cell monolayers. Because AMPK is activated by IFNγ, our next goal was to investigate whether AMPK inhibition influences IFNγ-induced effects on epithelial barrier function. First, we determined the time and concentration dependence of the IFNγ-induced alterations in TER across T84 monolayers. Therefore we administered 10, 100, or 1000 units/ml IFNγ for 24, 48, or 72 h. In keeping with previous studies (7), IFNγ did not affect TER within 24 h of treatment, but decreased TER significantly by 48 h and even more so by 72 h (Fig. 3A). Interestingly, whereas the IFNγ effect was shown to be clearly time-dependent, different doses of IFNγ had similar effects on TER (Fig. 3A). Based on these observations, we chose the 72-h time point and a concentration of 1000 units/ml IFNγ for further analysis, and treated T84 monolayers with IFNγ in the presence or absence of the AMPK inhibitor, CC (50 μm, bilaterally) for 72 h. TER was assessed by a voltohmeter, and the findings were confirmed by measuring the conductance across monolayers mounted in Ussing chambers. IFNγ treatment for 72 h caused a significant drop in TER, whereas CC had no effect on TER by itself (Fig. 3B). However, CC partially, and significantly, reversed the IFNγ-induced decrease in TER (Fig. 3B) and increase in conductance (Fig. 3C). However, AMPK inhibition did not affect the IFNγ-induced suppression of another parameter of colonic epithelial cell function, Ca2+-dependent chloride secretion, as stimulated by the muscarinic receptor agonist, carbachol (100 μm, basolaterally) (Fig. 3D). These data indicate that AMPK activity is, at least in part, specifically required for the effects of IFNγ on epithelial barrier function in T84 cells.
FIGURE 3.
AMPK inhibition partially prevents the IFNγ-induced decrease in intestinal epithelial barrier function in T84 cells. A, IFNγ treatment caused a significant decrease in TER across T84 monolayers dependent on the duration of the respective treatment but independent of the IFNγ dose used (n = 5). B, AMPK inhibition by Compound C partially prevented the IFNγ-induced decrease in TER following co-treatment for 72 h (n = 5). Data are expressed as a percentage of the control value. C, the IFNγ-induced increase in conductance across T84 monolayers was partially diminished by Compound C (n = 4). D, AMPK inhibition by Compound C was unable to restore IFNγ-induced inhibition of chloride secretion in response to the muscarinic receptor agonist, carbachol (100 μm; n = 4). Asterisks indicate significant differences versus the respective control (*, p < 0.05; **, p < 0.01; and ***, p < 0.001). #, indicates p < 0.05; ###, indicates p < 0.001 versus 72 h IFNγ treatment of T84 cells. +, indicates p < 0.001 versus 24-h IFNγ treatment of T84 cells.
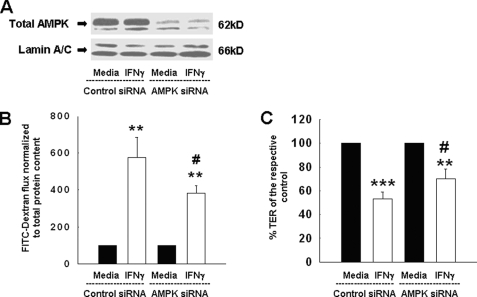
AMPK Knockdown Diminishes the IFNγ-induced Increase in Epithelial Permeability
Mindful of the possible limitations of pharmacological inhibition of AMPK with respect to specificity, we also sought a molecular approach to confirm a role for AMPK in the ability of IFNγ to reduce epithelial barrier function. T84 cells were therefore transfected with either siRNA oligonucleotides targeting AMPK or with nonspecific siRNA sequences as a control. 48 h later, cells were treated for a further 72 h with IFNγ. As shown in Fig. 4A, AMPK-specific siRNA clearly decreased the expression of AMPK protein. Additionally, no nonspecific effects on cellular protein expression could be observed, as shown by equivalent levels of the nuclear envelope protein, lamin A/C, which was used as a loading control. To examine the functional correlate of these results, we first assessed the flux of FITC-labeled dextran (10 kDa) across T84 monolayers. In control siRNA-transfected cells, IFNγ significantly increased permeability to FITC-dextran, an observation that is in good accordance with previous findings by Watson et al. (8). However, knockdown of AMPK ameliorated this effect, at least in part. Although tracer flux was still increased in IFNγ-treated, AMPK-deficient cells compared with cells not treated with IFNγ, it was significantly lower than the flux across IFNγ-treated cells transfected with the nonspecific control siRNA (Fig. 4B). Furthermore, we also assessed if AMPK knockdown affected the IFNγ-induced decrease in TER across T84 monolayers. Although IFNγ treatment caused a significant decrease in TER in cells transfected with control siRNA, this effect was partially prevented in AMPK-deficient cells. Although IFNγ still caused a detectable reduction in the TER across AMPK-deficient cells, this effect was significantly lower than in cells transfected with nonspecific siRNA (Fig. 4C). Therefore, these data confirm a role for AMPK in IFNγ-induced alterations in the barrier properties of the intestinal epithelium, and extend this not only to TER, but also to macromolecular permeability.
FIGURE 4.
siRNA knockdown of AMPK ameliorates the IFNγ-induced increase in epithelial permeability and decrease in TER. A, representative Western blots of three similar experiments showing the expression of total AMPK-α1 and the loading control Lamin A/C, in T84 cells transfected with either control siRNA, or AMPK-specific siRNA. AMPK siRNA caused a clear decrease in AMPK protein expression, whereas no nonspecific effects on Lamin A/C protein expression were observed. B, AMPK knockdown partially reversed the IFNγ-induced increase in epithelial permeability (n = 3). C, AMPK knockdown partially prevented the IFNγ-induced decrease in TER across T84 monolayers (n = 3). Data are expressed as percentage of control. Asterisks indicate significant difference versus the respective control (**, p < 0.01; ***, p < 0.001). #, p < 0.05 versus 72-h IFNγ treatment of T84 cells transfected with control siRNA.
AMPK Mediates IFNγ-induced Down-regulation of the Tight Junction Proteins Occludin and ZO-1
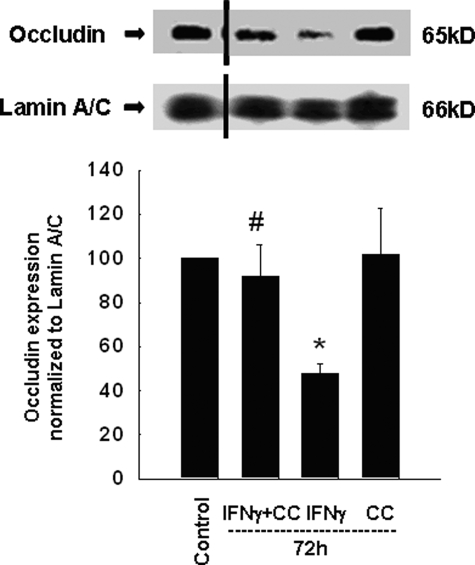
A well-described effect of IFNγ that correlates with reduced epithelial barrier integrity is the down-regulation of the tight junction protein, occludin (8, 9, 11). Having shown that AMPK inhibition reverses the effects of IFNγ on epithelial barrier function, we investigated a possible effect on tight junction proteins that contributes to the epithelial barrier. To assess the involvement of AMPK, we incubated T84 cells with IFNγ (1000 units/ml) and/or Compound C (50 μm) for 72 h. In IFNγ-treated cells, occludin expression was significantly reduced, whereas Compound C treatment alone had no significant effect (Fig. 5). However, co-treatment with Compound C prevented the ability of IFNγ to reduce occludin levels (Fig. 5).
FIGURE 5.
AMPK inhibition by Compound C inhibits the IFNγ-induced down-regulation of the tight junction protein, occludin. T84 cells were treated with IFNγ ± Compound C for 72 h. Representative Western blots of cell lysates demonstrate reduced expression of occludin in IFNγ treated cells that was prevented by Compound C co-treatment. A blot of the loading control, Lamin A/C, is also shown. The black dash indicates that the gel has been cropped at this position to remove irrelevant lanes. The histogram shows the densitometric analysis of four similar experiments. Data are expressed as percentage of control. Asterisks indicate significant difference versus control (*, p < 0.05). #, indicates p < 0.05 versus 72-h IFNγ treatment of T84 cells.
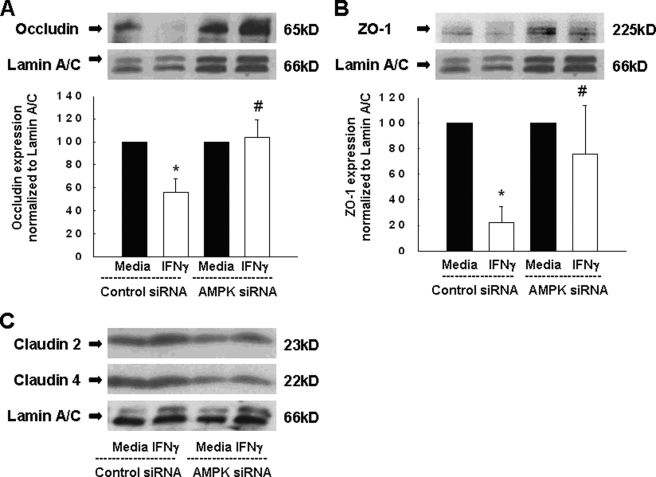
To confirm and extend these findings, we assessed the expression of various tight junction proteins in IFNγ-treated cells that were also rendered AMPK-deficient. Occludin, ZO-1, and claudin-4 play important roles in maintaining epithelial barrier function, whereas increased levels of the pore-forming claudin-2 are associated with increased epithelial permeability (52). As already shown in Fig. 4A, AMPK-specific siRNA reduced AMPK protein expression, whereas the levels of lamin A/C were unaffected in either control siRNA or AMPK siRNA-transfected T84 cells. Treatment with IFNγ for 72 h caused a significant reduction in the protein expression of occludin and ZO-1 in cells transfected with nonspecific siRNA. However, knockdown of AMPK largely abrogated the effect of IFNγ on occludin (Fig. 6A) or ZO-1 (Fig. 6B) expression. In contrast, neither the pore-forming protein, claudin-2, nor claudin-4 was affected by either IFNγ or the knockdown of AMPK or the combination of these treatments (Fig. 6C). These data indicate that AMPK activity is, at least in part, required to mediate the effect of IFNγ on the expression of occludin and ZO-1 in intestinal epithelial cells, but not of the two claudins examined.
FIGURE 6.
AMPK knockdown prevents the IFNγ-induced decrease in expression of the tight junction proteins, occludin and ZO-1. A, representative Western blots show expression of occludin and the loading control Lamin A/C in either control siRNA, or AMPK siRNA, transfected cells. Densitometric analysis of three similar experiments is also shown. B, protein expression of ZO-1 and Lamin A/C demonstrated by representative Western blots. The densitometric analysis of three similar experiments is shown in the histogram below. C, the expression of claudin-2 and claudin-4 as well as of the loading control, Lamin A/C, is shown by representative Western blots (n = 3). Data are presented as a percentage of control. Asterisks indicate significant differences versus the respective control (p < 0.05). #, indicates p < 0.05 versus 72-h IFNγ treatment of T84 cells transfected with control siRNA.
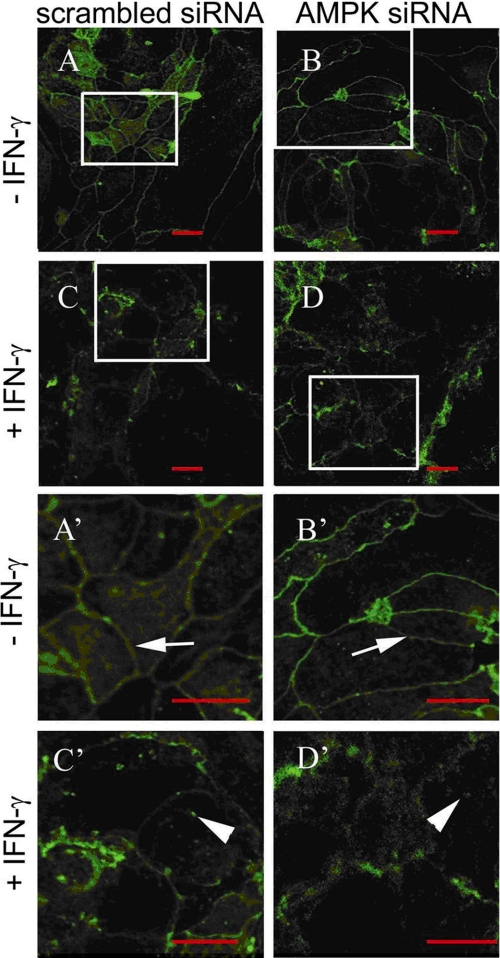
Loss of AMPK Does Not Affect IFNγ-induced Internalization of the Tight Junction Protein ZO-1
IFNγ has been demonstrated to cause internalization of various tight junction proteins (9, 12). We therefore set out to investigate the involvement of AMPK in the regulation of IFNγ-induced internalization of ZO-1. We transfected T84 cells with either nonspecific control or AMPK-specific siRNA and stimulated these cells for 72 h with 1000 units/ml IFNγ. As shown in Fig. 7, ZO-1 appeared in unstimulated control siRNA (Fig. 7, A and A′) as well as in AMPK siRNA-transfected cells (Fig. 7, B plus B′) to be mainly localized within the cellular membrane. In contrast, and in good agreement with our protein data (cf. Fig. 6), in IFNγ-treated control-siRNA cells, ZO-1 was less prominent. Additionally, ZO-1 was more prominent within the intracellular compartment of these cells than in the cell membrane, even showing aggregated, intracellular protein complexes and a heavily disturbed pattern (Fig. 7, C and C′). Similarly, in IFNγ-treated AMPK-deficient cells, ZO-1 also appeared to be more prominent within the cytoplasmic cell compartment and removed from the cell membrane (Fig. 7, D and D′). Nevertheless, correlating with our protein data, the abundance of ZO-1 in IFNγ-treated AMPK knockdown cells was apparently greater than in IFNγ-treated control siRNA-transfected cells (Fig. 7, C, C′, D, and D′). The impression that the overall shape of the cells subjected to AMPK knockdown seems to be altered in comparison to AMPK-competent cells might be due to altered tight junction assembly in AMPK-deficient cells (50, 53, 54). These data indicate disruption of the cell membrane and ZO-1 localization with possible internalization, in addition to decreased overall expression of ZO-1 as indicated by Western blotting (cf. Fig. 6) in response to IFNγ. Furthermore, they suggest that AMPK is likely not involved in regulating the internalization of ZO-1, at least in our T84 cell model.
FIGURE 7.
AMPK knockdown does not affect IFNγ-induced relocalization of the tight junction protein, ZO-1. T84 cells were transfected with either nonspecific control siRNA or AMPK-specific siRNA and subsequently treated with IFNγ (1000 units/ml) for 72 h (n = 3). ZO-1 staining appears in green. The size bar represents 10 μm in each figure. Arrows indicate intact cell membranes. Arrowheads demonstrate possible internalization of ZO-1. A′, B′, C′, and D′ show zoomed sections of the respective original parts A, B, C, and D. A and A′, in untreated control (scrambled) siRNA cells, ZO-1 was mainly localized within the cytoplasmic membrane, and the white arrow indicates intact ZO-1 pattern. B and B′, similarly, in untreated T84 cells transfected with AMPK-specific siRNA, ZO-1 was mainly localized at the cytoplasmic membrane indicating intact cellular membrane pattern (white arrow). C and C′, treatment with IFNγ caused a decrease in ZO-1 protein, disruption of the epithelial monolayer and of ZO-1 localization, as well as aggregation and possible internalization of ZO-1 in control siRNA-transfected cells (white arrowhead). D and D′, similarly, IFNγ treatment also caused monolayer disruption and aggregation as well as possible ZO-1 internalization in cells transfected with AMPK siRNA (white arrowhead).
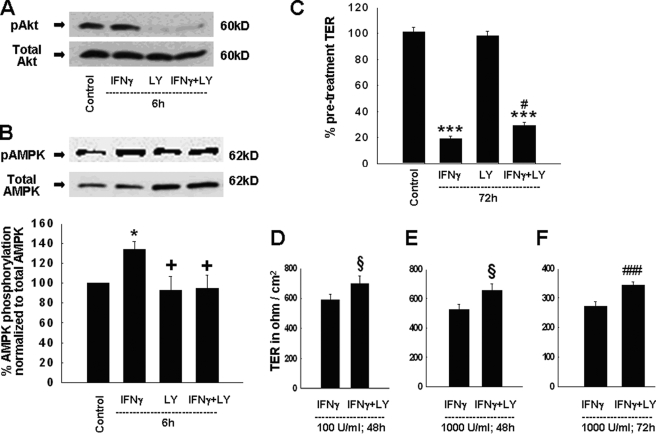
PI3K Inhibition Partially Prevents IFNγ-induced Activation of AMPK
Having shown that IFNγ exerts its effects on intestinal epithelial barrier function, at least in part, via AMPK, we next sought to explain the connection between IFNγ and AMPK. Recent studies showed that the PI3K inhibitor, LY294002, diminished some aspects of IFNγ-induced effects on the epithelial barrier (11, 15). Therefore, we investigated if PI3K inhibition could affect IFNγ-induced activation of AMPK. We first confirmed that LY294002 inhibits the activity of PI3K by assessing the phosphorylation of Akt, a well described marker for PI3K activity (55, 56). IFNγ alone did not significantly affect Akt phosphorylation, thus mirroring previous findings of constitutive Akt phosphorylation in T84 cells (15). However, incubation of T84 cells with LY294002, as well as the combination of IFNγ and LY294002, resulted in a dramatic decrease in Akt phosphorylation (Fig. 8A). We next investigated whether PI3K inhibition affected IFNγ-induced increases in AMPK phosphorylation. Treatment with LY294002 had no significant effect on AMPK phosphorylation by itself, but completely prevented the IFNγ-induced increase in AMPK phosphorylation (Fig. 8B). Interestingly, and in contrast to AMPK inhibition by Compound C, PI3K inhibition did not reduce AMPK phosphorylation below control levels (compare Figs. 1B and 8B). These data indicate that IFNγ likely requires activation of PI3K signaling to facilitate AMPK activation.
FIGURE 8.
PI3K inhibition diminishes IFNγ-induced effects on AMPK phosphorylation and epithelial barrier function. A, Ser473 phosphorylation of Akt was assessed as a marker of PI3K activity. Treatment of T84 cells with LY294002 (20 μm, bilaterally) for 6 h significantly inhibited PI3K activity as shown by representative Western blots of phosphorylated and total Akt (n = 3). B, PI3K inhibition by LY294002 diminished IFNγ-induced Thr172 phosphorylation of AMPK-α1 in T84 cells following a 6-h co-treatment. Representative Western blots show phosphorylation of AMPK as well as the expression of AMPK-α1 protein. The histogram shows the densitometric analysis of nine similar experiments. C, PI3K inhibition by LY294002 partially ameliorated the IFNγ-induced decrease in TER following co-treatment for 72 h. Data are expressed as percentage of control (n = 9). D, co-treatment with the PI3K inhibitor, LY294002, restricted the IFNγ-induced (100 units/ml; 48 h) decrease in TER (n = 6). Data are presented in Ohm/cm2. E, LY294002 diminished the IFNγ-induced (1000 units/ml) decrease in TER after co-treatment for 48 h (n = 6). Data are presented in Ohm/cm2. F, PI3K inhibition by LY294002 ameliorated the IFNγ-induced (1000 units/ml) decrease in TER by co-treatment for 72 h (n = 6). Data are presented in Ohm/cm2. Statistical analysis in D–F was performed by Student t test. Asterisks indicate significant differences versus the respective control (*, p < 0.05; ***, p < 0.001). +, p < 0.05 versus 6 h IFNγ treatment of T84 cells. #, p < 0.05; ###, p < 0.001 versus treatment of T84 cells with IFNγ for 72 h. §, p < 0.05 versus treatment with IFNγ for 48 h.
PI3K Inhibition Ameliorates the IFNγ-induced Decrease in TER across T84 Monolayers
Having shown that PI3K inhibition prevented the IFNγ-induced rise in AMPK phosphorylation, we sought to correlate this finding with IFNγ-induced effects on intestinal epithelial barrier function. McKay et al. studied the effects of pharmacological PI3K inhibition on the response to 20 ng/ml IFNγ for a 48-h treatment period (15), whereas Boivin et al. tested the role of PI3K activity in the effects of a lower concentration of IFNγ (10 ng/ml) also with a 48-h treatment (11). For purposes of comparison with our previous experiments, we investigated if PI3K inhibition could also modulate effects of IFNγ produced by a concentration of 1000 units/ml (100 ng/ml) for 72 h. As expected, IFNγ alone significantly decreased TER across T84 monolayers, whereas treatment with LY294002 (20 μm) alone had no effect. PI3K inhibition by LY294002 did, however, significantly attenuate the IFNγ-induced decrease in TER (Fig. 8C). However, this effect was, in keeping with data on AMPK phosphorylation, clearly less pronounced than the effect of Compound C on AMPK activity and was also lower than the effect of LY294002 on IFNγ-induced TER alterations observed by Boivin et al. (11) and McKay et al. (15). Nevertheless, these findings confirm our biochemical data, indicating that AMPK activation by IFNγ in T84 cells occurs in the context of PI3K activation. To further define the ability of LY294002 to ameliorate the IFNγ-induced decrease in TER, we also performed time- and dose-response experiments using T84 cells treated with either IFNγ, or LY294002, or a combination of both agents. Because our previous data have demonstrated that the IFNγ-induced effect on the TER is independent of the actual concentration of the cytokine, but critically dependent on the duration of cytokine treatment (cf. Fig. 3A), we treated T84 cells for 48 or 72 h with 100 or 1000 units/ml (10 or 100 ng/ml) IFNγ and/or LY294002. As shown in Fig. 8 (D and E), and similar to Fig. 8 (C and F), PI3K inhibition had a small, but consistently significant effect in ameliorating the IFNγ-induced decrease in TER across T84 monolayers by 48-h treatment independent of the IFNγ dose used (100 or 1000 units/ml), as well as by 72-h treatment (100 units/ml, data not shown, or 1000 units/ml). These data demonstrate that inhibition of PI3K is able to partially, but significantly, diminish the IFNγ-induced decrease in TER across T84 cell monolayers, independent of the concentration or treatment duration of IFNγ. Furthermore, they suggest that additional factors, besides PI3K, are likely required for IFNγ-induced activation of AMPK, as well as altered barrier function, under these experimental conditions.
DISCUSSION
It is well described that the pro-inflammatory cytokine, IFNγ, compromises the intestinal epithelial barrier by causing internalization and reduced expression of tight junction proteins, resulting in increased transepithelial permeability and decreased TER. IFNγ also produces inhibition of epithelial ion transport (7–11, 13). However, the intracellular signaling pathways that mediate the various effects of IFNγ are poorly understood. Recent studies have shown an involvement of PI3K in mediating the effects of IFNγ on epithelial barrier properties (11, 15). In contrast, a major role for the most established mediator of IFNγ signaling, the signal transducer and activator of transcription 1, in epithelial barrier defects has not been confirmed (15, 39, 57).
Here, we show that IFNγ activates the cellular energy sensor, AMPK, in intestinal epithelial cells and that inhibition of AMPK diminishes the detrimental effect of this inflammatory cytokine on epithelial barrier function. Previously, AMPK was thought to be activated predominantly in response to decreased levels of intracellular ATP (24). For example, the production of reactive oxygen species, such as hydrogen peroxide, during intestinal inflammation can lead to oxidative stress, reduced ATP levels, and consequent cellular dysfunction or damage (58). H2O2 can also promote activation of AMPK (26). However, IFNγ alone, which plays a crucial role in the pathogenesis of IBD, had not previously been reported to activate AMPK in vitro or in vivo. Additionally, our data suggest that IFNγ activates AMPK independent of changes in the levels of intracellular ATP.
Nevertheless, a recent study from Riboulet-Chavey et al. using pancreatic β-cells demonstrated that AMPK phosphorylation in glucose-pretreated cells was increased 48 h after administration of a cytokine mix consisting of tumor necrosis factor, interleukin-1β, and IFNγ (59). Furthermore, administration of the cytokine mix for 48 h caused a significant decrease in the cellular ATP level in glucose-pretreated cells. However, this study did not investigate the effects of IFNγ alone on either the phosphorylation of AMPK or the cellular ATP level. Because we detected neither an increase in AMPK phosphorylation with 48- or 72-h IFNγ treatment nor significant alterations in the cellular ATP level at these respective time points, the discrepancy between these observations could be due either to the different cell types used in the respective studies (intestinal epithelial cells versus pancreatic β-cells), to the glucose pretreatment, or to the additional cytokines used (tumor necrosis factor and/or interleukin-1β).
Our studies suggest that AMPK-mediated alterations in barrier function and composition are dependent on the signaling context in which AMPK is activated, with IFNγ altering the cytosolic milieu to conditions that reveal a role for AMPK in altering barrier function. Because the intestinal epithelium is subjected to small changes in intracellular ATP very frequently, we can speculate that it is an appropriate adaptation that AMPK activity alone would be insufficient to impair intestinal epithelial barrier function. From a physiological perspective, this makes sense as it would be extremely undesirable for every stimulus of AMPK, i.e. any event that causes a decrease in ATP levels, to result in barrier dysfunction in a tissue that is so frequently subjected to hypoxia. Interestingly, AMPK inhibition could not restore impaired carbachol-induced chloride secretion seen in cells treated with IFNγ, indicating that the cytokine exerts this latter effect independent of AMPK.
These findings suggest a novel role for AMPK as not only a cellular energy sensor, but also an important signal transducer for pro-inflammatory cytokines such as IFNγ. This finding may also have implications for chronic inflammatory conditions, such as IBD, which exhibit high levels of inflammatory cytokines. The exact molecular mechanism responsible for AMPK activation by IFNγ will be the subject of future studies.
We demonstrated that inhibition of AMPK attenuated IFNγ-induced barrier dysfunction. AMPK inhibition prevented both the decline in TER and the increase in conductance across T84 monolayers by ∼50%. Using an AMPK knockdown approach, we also uncovered a role for AMPK in the regulation of IFNγ-induced epithelial permeability to macromolecules, such as 10-kDa FITC-dextran. The latter finding is of particular interest, because increased permeability to macromolecules, specifically bacterial components or products, is believed to contribute significantly to the pathogenesis of CD. We also investigated one or more possible mechanisms by which AMPK mediates IFNγ-induced changes in epithelial barrier function. A well established effect of chronic IFNγ treatment is to alter the expression of tight junction proteins, such as occludin and ZO-1 (9–11, 60). AMPK inhibition completely prevented IFNγ-induced down-regulation of occludin and partially restricted the reduction in ZO-1 expression. Thus we have identified a new signaling component mediating the effect of IFNγ on the expression of tight junction proteins. The almost complete restoration of ZO-1 and occludin by AMPK knockdown was sufficient to partially ameliorate the IFNγ-induced decrease in TER and the increases in conductance and permeability. Our observed effects of amelioration of IFNγ-induced effects on occludin and permeability to 10-kDa FITC-dextran by AMPK knockdown, correlate with studies by Watson et al. (8) who demonstrated that IFNγ increased epithelial permeability via increased frequency of a large macromolecule (10 kDa) pore, and this was associated with changes in occludin expression and phosphorylation. Interestingly, IFNγ did not affect the expression of the pore-forming claudin-2 or of claudin-4, and, subsequently, loss of AMPK had no effect on their expression, either. Because AMPK inhibition or knockdown were only partially able to mitigate IFNγ-induced changes in epithelial barrier function, we speculate that there may also be additional AMPK-independent modifications of tight junction proteins, either through post-translational events or other transcriptional effects of IFNγ. Additionally, the effect of pharmacological AMPK inhibition by CC was greater than the effect of AMPK knockdown as regards amelioration of the IFNγ-induced decrease in TER. This finding correlates with the greater inhibition of AMPK phosphorylation, even below control levels, in IFNγ and CC co-treated cells. Although these data support our evidence of a pre-existing “basal” level of AMPK phosphorylation in these cells, one might hypothesize, that CC, which has already been shown to have off-target effects in non-epithelial cell lines (61–63), or possibly to even cause AMPK knockdown itself, may also have AMPK-independent effects, that could contribute to an enhanced protective effect on epithelial barrier function versus AMPK knockdown. However, as stated above, the concentration of CC chosen for this study was based on its functional efficacy and was actually lower than used in similar studies of AMPK function in epithelial cell lines, including T84 cells.
Interestingly, AMPK knockdown did not affect IFNγ-induced internalization of the tight junction protein ZO-1. A possible candidate for IFNγ-induced regulation of tight junction protein localization is myosin light chain kinase, which can also be activated in response to IFNγ treatment and plays an important role in tight junction protein internalization as established by Turner and colleagues (64, 65). However, a previous report using smooth muscle cells indicates that myosin light chain kinase is a substrate of, and is inactivated by, AMPK (66). Whether AMPK regulates myosin light chain kinase function in epithelial cells in response to IFNγ or other stimuli has not been identified, but this avenue of investigation will likely yield findings of general interest in the field of tight junction regulation.
Peak AMPK activation in response to IFNγ occurred at 6 h. This is in keeping with the observations of McKay et al. and Boivin et al. regarding the role of PI3K in IFNγ-induced barrier defects (11, 15). In the McKay study, PI3K inhibition could only prevent IFNγ-induced effects on TER when LY294002 was administered within the first 6 h after IFNγ treatment, whereas the Boivin study showed that IFNγ-induced PI3K activation occurs within 10 min after IFNγ exposure. In contrast, a detectable effect of IFNγ on epithelial barrier function did not occur until 48 h after treatment in both studies. Our data confirmed the involvement of PI3K in the signaling events that underlie IFNγ-induced barrier dysfunction. Interestingly, Akt, a common downstream target of PI3K signaling, is likely not involved based on the lack of an effect of IFNγ on Akt phosphorylation (cf. Fig. 8A) (15). However, even though PI3K inhibition reduced IFNγ-stimulated AMPK phosphorylation to control levels, LY294002 only partially diminished the IFNγ-induced decrease in TER, and to a far lesser extent than specific AMPK inhibition by Compound C, or to levels observed in the studies of McKay et al. (15) and Boivin et al. (11). To investigate the mechanism for the different findings in our study compared with previous work (11, 15), we also administered IFNγ for 48 h, as the previous data were generated using this time point. However, in our cell model we did not detect an increased effect of PI3K inhibition on the IFNγ-induced effects on the TER at the different time points (48 versus 72 h) or with different concentrations of IFNγ (100 versus 1000 units/ml). Therefore, our data suggest that additional mediators, supplementary to PI3K, may be necessary to display the full effect of IFNγ on AMPK activity, and subsequently to diminish intestinal epithelial barrier function. The identification of these additional factors was beyond the scope of the present study. Nevertheless, we have identified a central role for AMPK as a mediator of IFNγ-induced signaling and downstream pathophysiological events.
In conclusion, our data indicate an important role for the cellular energy sensor, AMPK, in the regulation of IFNγ-induced changes in intestinal epithelial barrier function, independent of cellular energy status. In addition, besides its well established regulatory effects in energy homeostasis and cell survival, AMPK participates in regulating the integrity of the epithelial barrier. In a pathophysiological setting, these findings suggest a novel role for AMPK in mediating the consequences of chronic intestinal inflammation, and thus may have implications for chronic inflammatory processes in the intestine, such as CD.
Acknowledgment
We are grateful to Dr. Ronald R. Marchelletta (University of California at San Diego) for technical assistance with the confocal microscopy studies.
This work was supported, in whole or in part, by National Institutes of Health Grant DK080506. This work was also supported by a Career Development Award and a Senior Research Award from the Crohn's and Colitis Foundation of America (to D. F. M.), scholarships from the German Research Foundation (Deutsche Forschungsgemeinschaft) (to M. S. and G. P.), and by the UCSD Digestive Diseases Research Development Center.
- IBD
- inflammatory bowel disease
- CD
- Crohn disease
- PBS
- phosphate-buffered saline
- CC
- Compound C
- AMPK
- AMP-activated protein kinase
- Akt
- protein kinase B
- FITC
- fluorescein isothiocyanate
- IFNγ
- interferon γ
- LY294002
- 2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride
- PI3K
- phosphatidylinositol 3′-kinase
- siRNA
- small interfering RNA
- TER
- transepithelial electrical resistance
- ZO-1
- zonula occludens-1.
REFERENCES
- 1.Podolsky D. K. (2002) N. Engl. J. Med. 347, 417–429 [DOI] [PubMed] [Google Scholar]
- 2.Marin M. L., Greenstein A. J., Geller S. A., Gordon R. E., Aufses A. H., Jr. (1983) Am. J. Gastroenterol. 78, 537–547 [PubMed] [Google Scholar]
- 3.Marin M. L., Geller S. A., Greenstein A. J., Marin R. H., Gordon R. E., Aufses A. H., Jr. (1983) Am. J. Gastroenterol. 78, 355–364 [PubMed] [Google Scholar]
- 4.Wehkamp J., Schmid M., Fellermann K., Stange E. F. (2005) J. Leukoc. Biol 77, 460–465 [DOI] [PubMed] [Google Scholar]
- 5.Zeissig S., Bürgel N., Günzel D., Richter J., Mankertz J., Wahnschaffe U., Kroesen A. J., Zeitz M., Fromm M., Schulzke J. D. (2007) Gut 56, 61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuss I. J., Neurath M., Boirivant M., Klein J. S., de la Motte C., Strong S. A., Fiocchi C., Strober W. (1996) J. Immunol. 157, 1261–1270 [PubMed] [Google Scholar]
- 7.Madara J. L., Stafford J. (1989) J. Clin. Invest. 83, 724–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson C. J., Hoare C. J., Garrod D. R., Carlson G. L., Warhurst G. (2005) J. Cell Sci. 118, 5221–5230 [DOI] [PubMed] [Google Scholar]
- 9.Youakim A., Ahdieh M. (1999) Am. J. Physiol. 276, G1279–G1288 [DOI] [PubMed] [Google Scholar]
- 10.Utech M., Ivanov A. I., Samarin S. N., Bruewer M., Turner J. R., Mrsny R. J., Parkos C. A., Nusrat A. (2005) Mol. Biol. Cell 16, 5040–5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boivin M. A., Roy P. K., Bradley A., Kennedy J. C., Rihani T., Ma T. Y. (2009) J. Interferon Cytokine Res. 29, 45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruewer M., Luegering A., Kucharzik T., Parkos C. A., Madara J. L., Hopkins A. M., Nusrat A. (2003) J. Immunol. 171, 6164–6172 [DOI] [PubMed] [Google Scholar]
- 13.Bertelsen L. S., Eckmann L., Barrett K. E. (2004) Am. J. Physiol. Gastrointest. Liver Physiol. 286, G157–G165 [DOI] [PubMed] [Google Scholar]
- 14.Sugi K., Musch M. W., Field M., Chang E. B. (2001) Gastroenterology 120, 1393–1403 [DOI] [PubMed] [Google Scholar]
- 15.McKay D. M., Watson J. L., Wang A., Caldwell J., Prescott D., Ceponis P. M., Di Leo. V., Lu J. (2007) J. Pharmacol. Exp. Ther. 320, 1013–1022 [DOI] [PubMed] [Google Scholar]
- 16.Taylor C. T., Dzus A. L., Colgan S. P. (1998) Gastroenterology 114, 657–668 [DOI] [PubMed] [Google Scholar]
- 17.Wang F., Schwarz B. T., Graham W. V., Wang Y., Su L., Clayburgh D. R., Abraham C., Turner J. R. (2006) Gastroenterology 131, 1153–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarz B. T., Wang F., Shen L., Clayburgh D. R., Su L., Wang Y., Fu Y. X., Turner J. R. (2007) Gastroenterology 132, 2383–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fryer L. G., Hajduch E., Rencurel F., Salt I. P., Hundal H. S., Hardie D. G., Carling D. (2000) Diabetes 49, 1978–1985 [DOI] [PubMed] [Google Scholar]
- 20.Marsin A. S., Bouzin C., Bertrand L., Hue L. (2002) J. Biol. Chem. 277, 30778–30783 [DOI] [PubMed] [Google Scholar]
- 21.Hardie D. G., Pan D. A. (2002) Biochem. Soc. Trans. 30, 1064–1070 [DOI] [PubMed] [Google Scholar]
- 22.Hallows K. R., Kobinger G. P., Wilson J. M., Witters L. A., Foskett J. K. (2003) Am. J. Physiol. Cell Physiol. 284, C1297–C1308 [DOI] [PubMed] [Google Scholar]
- 23.Walker J., Jijon H. B., Churchill T., Kulka M., Madsen K. L. (2003) Am. J. Physiol. Gastrointest. Liver Physiol. 285, G850–G860 [DOI] [PubMed] [Google Scholar]
- 24.Greig E. R., Boot-Handford R. P., Mani V., Sandle G. I. (2004) J. Pathol. 204, 84–92 [DOI] [PubMed] [Google Scholar]
- 25.Amasheh S., Barmeyer C., Koch C. S., Tavalali S., Mankertz J., Epple H. J., Gehring M. M., Florian P., Kroesen A. J., Zeitz M., Fromm M., Schulzke J. D. (2004) Gastroenterology 126, 1711–1720 [DOI] [PubMed] [Google Scholar]
- 26.Schmitz H., Barmeyer C., Gitter A. H., Wullstein F., Bentzel C. J., Fromm M., Riecken E. O., Schulzke J. D. (2000) Ann. N.Y. Acad. Sci. 915, 312–326 [DOI] [PubMed] [Google Scholar]
- 27.Hardie D. G., Salt I. P., Hawley S. A., Davies S. P. (1999) Biochem. J. 338, 717–722 [PMC free article] [PubMed] [Google Scholar]
- 28.Hardie D. G., Carling D. (1997) Eur. J. Biochem. 246, 259–273 [DOI] [PubMed] [Google Scholar]
- 29.Choi S. L., Kim S. J., Lee K. T., Kim J., Mu J., Birnbaum M. J., Soo Kim S., Ha J. (2001) Biochem. Biophys. Res. Commun. 287, 92–97 [DOI] [PubMed] [Google Scholar]
- 30.Kemp B. E., Mitchelhill K. I., Stapleton D., Michell B. J., Chen Z. P., Witters L. A. (1999) Trends Biochem. Sci. 24, 22–25 [DOI] [PubMed] [Google Scholar]
- 31.Salt I. P., Johnson G., Ashcroft S. J., Hardie D. G. (1998) Biochem. J. 335, 533–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corton J. M., Gillespie J. G., Hardie D. G. (1994) Curr. Biol. 4, 315–324 [DOI] [PubMed] [Google Scholar]
- 33.Woods A., Johnstone S. R., Dickerson K., Leiper F. C., Fryer L. G., Neumann D., Schlattner U., Wallimann T., Carlson M., Carling D. (2003) Curr. Biol. 13, 2004–2008 [DOI] [PubMed] [Google Scholar]
- 34.Shaw R. J., Kosmatka M., Bardeesy N., Hurley R. L., Witters L. A., DePinho R. A., Cantley L. C. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 3329–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawley S. A., Pan D. A., Mustard K. J., Ross L., Bain J., Edelman A. M., Frenguelli B. G., Hardie D. G. (2005) Cell Metab. 2, 9–19 [DOI] [PubMed] [Google Scholar]
- 36.Hurley R. L., Anderson K. A., Franzone J. M., Kemp B. E., Means A. R., Witters L. A. (2005) J. Biol. Chem. 280, 29060–29066 [DOI] [PubMed] [Google Scholar]
- 37.Woods A., Dickerson K., Heath R., Hong S. P., Momcilovic M., Johnstone S. R., Carlson M., Carling D. (2005) Cell Metab. 2, 21–33 [DOI] [PubMed] [Google Scholar]
- 38.Huang N. L., Chiang S. H., Hsueh C. H., Liang Y. J., Chen Y. J., Lai L. P. (2009) Int. J. Cardiol. 134, 169–175 [DOI] [PubMed] [Google Scholar]
- 39.Cheng P. Y., Lee Y. M., Law K. K., Lin C. W., Yen M. H. (2007) Biochem. Pharmacol. 74, 1758–1765 [DOI] [PubMed] [Google Scholar]
- 40.Uribe J. M., Keely S. J., Traynor-Kaplan A. E., Barrett K. E. (1996) J. Biol. Chem. 271, 26588–26595 [DOI] [PubMed] [Google Scholar]
- 41.Madara J. L., Dharmsathaphorn K. (1985) J. Cell Biol. 101, 2124–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson J. L., Ansari S., Cameron H., Wang A., Akhtar M., McKay D. M. (2004) Am. J. Physiol. Gastrointest. Liver Physiol. 287, G954–G961 [DOI] [PubMed] [Google Scholar]
- 43.Cartwright C. A., McRoberts J. A., Mandel K. G., Dharmsathaphorn K. (1985) J. Clin. Invest. 76, 1837–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fish S. M., Proujansky R., Reenstra W. W. (1999) Gut 45, 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uribe J. M., McCole D. F., Barrett K. E. (2002) Am. J. Physiol. Gastrointest. Liver Physiol. 283, G923–G931 [DOI] [PubMed] [Google Scholar]
- 46.Sasaki T., Hiwatashi N., Yamazaki H., Noguchi M., Toyota T. (1992) Gastroenterol. Jpn. 27, 29–36 [PubMed] [Google Scholar]
- 47.Hawley S. A., Davison M., Woods A., Davies S. P., Beri R. K., Carling D., Hardie D. G. (1996) J. Biol. Chem. 271, 27879–27887 [DOI] [PubMed] [Google Scholar]
- 48.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., Musi N., Hirshman M. F., Goodyear L. J., Moller D. E. (2001) J. Clin. Invest. 108, 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woollhead A. M., Baines D. L. (2006) J. Biol. Chem. 281, 5158–5168 [DOI] [PubMed] [Google Scholar]
- 50.Blume C., Benz P. M., Walter U., Ha J., Kemp B. E., Renné T. (2007) J. Biol. Chem. 282, 4601–461250 [DOI] [PubMed] [Google Scholar]
- 51.DuVall M. D., Guo Y., Matalon S. (1998) Am. J. Physiol. 275, C1313–C1322 [DOI] [PubMed] [Google Scholar]
- 52.Van Itallie C. M., Holmes J., Bridges A., Gookin J. L., Coccaro M. R., Proctor W., Colegio O. R., Anderson J. M. (2008) J. Cell Sci. 121, 298–305 [DOI] [PubMed] [Google Scholar]
- 53.Zhang L., Li J., Young L. H., Caplan M. J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 17272–17277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng B., Cantley L. C. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 819–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franke T. F., Yang S. I., Chan T. O., Datta K., Kazlauskas A., Morrison D. K., Kaplan D. R., Tsichlis P. N. (1995) Cell 81, 727–736 [DOI] [PubMed] [Google Scholar]
- 56.Datta K., Bellacosa A., Chan T. O., Tsichlis P. N. (1996) J. Biol. Chem. 271, 30835–30839 [DOI] [PubMed] [Google Scholar]
- 57.Beaurepaire C., Smyth D., McKay D. M. (2009) J. Interferon Cytokine Res. 29, 133–144 [DOI] [PubMed] [Google Scholar]
- 58.Tüzün A., Erdil A., Inal V., Aydin A., Bağci S., Yeşilova Z., Sayal A., Karaeren N., Dağalp K. (2002) Clin. Biochem. 35, 569–572 [DOI] [PubMed] [Google Scholar]
- 59.Riboulet-Chavey A., Diraison F., Siew L. K., Wong F. S., Rutter G. A. (2008) Diabetes 57, 415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prasad S., Mingrino R., Kaukinen K., Hayes K. L., Powell R. M., MacDonald T. T., Collins J. E. (2005) Lab. Invest. 85, 1139–1162 [DOI] [PubMed] [Google Scholar]
- 61.Emerling B. M., Viollet B., Tormos K. V., Chandel N. S. (2007) FEBS Lett. 581, 5727–5731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nam M., Lee W. H., Bae E. J., Kim S. G. (2008) Arch. Biochem. Biophys. 479, 74–81 [DOI] [PubMed] [Google Scholar]
- 63.Vucicevic L., Misirkic M., Janjetovic K., Harhaji-Trajkovic L., Prica M., Stevanovic D., Isenovic E., Sudar E., Sumarac-Dumanovic M., Micic D., Trajkovic V. (2009) Biochem. Pharmacol. 77, 1684–1693 [DOI] [PubMed] [Google Scholar]
- 64.Clayburgh D. R., Rosen S., Witkowski E. D., Wang F., Blair S., Dudek S., Garcia J. G., Alverdy J. C., Turner J. R. (2004) J. Biol. Chem. 279, 55506–55513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang F., Graham W. V., Wang Y., Witkowski E. D., Schwarz B. T., Turner J. R. (2005) Am. J. Pathol. 166, 409–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Horman S., Morel N., Vertommen D., Hussain N., Neumann D., Beauloye C., El Najjar N., Forcet C., Viollet B., Walsh M. P., Hue L., Rider M. H. (2008) J. Biol. Chem. 283, 18505–18512 [DOI] [PubMed] [Google Scholar]