Abstract
The extracellular sulfatases Sulf1 and Sulf2 remodel the 6O-sulfation state of heparan sulfate proteoglycans on the cell surface, thereby modulating growth factor signaling. Different from all other sulfatases, the Sulfs contain a unique, positively charged hydrophilic domain (HD) of about 320 amino acid residues. Using various HD deletion mutants and glutathione S-transferase (GST)-HD fusion proteins, this study demonstrates that the HD is required for enzymatic activity and acts as a high affinity heparin/heparan sulfate interaction domain. Association of the HD with the cell surface is sensitive to heparinase treatment, underlining specificity toward heparan sulfate chains. Correspondingly, isolated GST-HD binds strongly to both heparin and heparan sulfate in vitro and also to living cells. Surface plasmon resonance studies indicate nanomolar affinity of GST-HD toward immobilized heparin. The comparison of different mutants reveals that especially the outer regions of the HD mediate heparan sulfate binding, probably involving “tandem” interactions. Interestingly, binding to heparan sulfate depends on the presence of 6O-sulfate substrate groups, suggesting that substrate turnover facilitates release of the enzyme from its substrate. Deletion of the inner, less conserved region of the HD drastically increases Sulf1 secretion without affecting enzymatic activity or substrate specificity, thus providing a tool for the in vitro modulation of HS-dependent signaling as demonstrated here for the signal transduction of fibroblast growth factor 2. Taken together, the present study shows that specific regions of the HD influence different aspects of HS binding, cellular localization, and enzyme function.
The human sulfatases represent a family of 17 enzymes responsible for the turnover and remodeling of sulfate esters and sulfamates. Their reaction mechanism relies on a special amino acid residue, Cα-formylglycine, which is generated post-translationally via oxidation of a conserved cysteine residue in the active site (1–3). Besides the lysosomal sulfatases involved in the cellular degradation of various sulfated substrates (4), two extracellular sulfatases, Sulf1 and Sulf2 (the Sulfs), have been described (5, 6). The Sulfs are endosulfatases with restricted substrate specificity toward 6O-sulfate groups of heparan sulfate (HS),2 an information-rich glycosaminoglycan (GAG) polymer attached to proteoglycans at the cell surface and in the extracellular matrix (6–8). HS proteoglycans (HSPGs) act as co-receptors in cell signaling pathways and provide binding sites for growth factors and morphogens via specific sulfation patterns on their HS chains. By enzymatically removing 6O-sulfate groups from HSPGs on the cell surface, Sulf1 and Sulf2 differentially regulate the activity of FGF, vascular endothelial growth factor, Wnt, and other HS ligands, thereby modulating important processes such as development, cell growth, and differentiation (9–12). Misregulation of the Sulfs has been linked with both tumor progression and suppression, depending on either activating or inhibitory effects upon cell signaling (13–16).
To investigate the physiological role of Sulf1 and Sulf2, single and double knock-out mice were generated (17–21). Both Sulf1 and Sulf2 knock-out mice are characterized by increased embryonic lethality, impaired neurite outgrowth, and other neurological abnormalities in the developing and adult nervous system (22). The corresponding double knock-out mice display an obvious reduction in body weight and developmental malformations, including skeletal and renal defects (18, 19, 23). Together with biochemical analyses on the impact of Sulf loss on HS sulfation, the phenotypic observations suggest a functional cooperativity between Sulf1 and Sulf2 in modulating the 6O-sulfation of UA(2S)-GlcNS(6S) disaccharide units within the S-domains of HS chains (17, 24). Moreover, analyses of heparan sulfate disaccharide compositions from Sulf1 and Sulf2 knock-out mice cell lines have indicated dynamic influences of Sulf loss also on non-substrate N-, 2O-, and 6O-sulfate groups via modulation of sulfotransferase expression, which may contribute to the developmental defects associated with the Sulf knock-out mice (24).
From the biochemical perspective, it is an important question how the Sulfs are able to recognize their HSPG substrates and how cell surface localization is achieved, despite a lack of transmembrane domains or lipid anchors. Classical GAG-binding proteins, such as antithrombin III (25) or FGF1 (26), interact with their negatively charged GAG partners via small clusters of positively charged amino acid residues. Although some consensus sequences for heparin binding have been identified (XBBXBX, XBBBXXBX, and XBBXXBBBXXBBX, where B is a basic residue and X a hydropathic) (27–29), they are neither required nor sufficient. Unlike these classical GAG-binding proteins, Sulf1 and Sulf2 contain a large hydrophilic domain (HD), located between the N-terminal catalytic domain and the C-terminal domain. The HD is a unique feature of the extracellular sulfatases that is neither found in other sulfatases nor shows any homology with other known protein domains. According to sequence alignments, the HD of human Sulf1 has a size of ∼320 amino acid residues, 27% of which are basic and 14% acidic, resulting in a strong positive charge at neutral pH and a high theoretical pI of 9.8. Remarkably, the C-terminal end of the HD is composed of a cluster of 12 basic amino acid residues. Whereas the outer regions of the HD are highly conserved between Sulf1 and Sulf2 as well as between human, murine, and avian orthologs, the inner region, encoded by exons 13 and 14 in the case of human Sulf1 (6), is significantly less conserved.
The role of the HD has previously been investigated for the avian ortholog QSulf2 (30). Results from this study indicated that the HD binds to negatively charged ligands and might serve to anchor the enzyme on the cell surface. Sulfate release assays indicated the necessity of the avian HD for enzymatic activity. Moreover, a very recent analysis of the HD of human Sulf1/Sulf2 revealed the presence of two furin-type proteinase cleavage sites within the inner region, explaining their partial processing into disulfide-linked subunits of 75 and 50 kDa (31). Sulf1/2 mutants, in which these sites were deleted, retained enzymatic activity but failed to potentiate Wnt signaling when overexpressed in human embryonic kidney 293 cells.
Due to the observed differences in enzyme secretion and detergent solubility between the human and avian orthologs (24, 30) and the likely importance of this domain for mammalian Sulf localization and activity, we analyzed the function of the HD of human Sulf1 in mediating enzyme activity, cell surface targeting, secretion, and substrate recognition. Using different Sulf1 deletion mutants and glutathione S-transferase (GST)-HD fusion proteins, this study demonstrates that specific regions of the HD, especially at the conserved N and C termini, are responsible for heparin/HS binding, cell surface localization, and enzymatic activity of human Sulf1. Interaction analyses show that binding of the HD to heparin is significantly stronger compared with other typical heparin-binding proteins, suggesting a new mode of GAG binding. The deletion of the inner region of the HD leads to significantly increased secretion of the enzyme, allowing the purification of an active variant that is able to modulate FGF signaling in cell culture experiments.
EXPERIMENTAL PROCEDURES
Construction of Expression Plasmids
The human full-length Sulf1 cDNA sequence was cloned into the pCI-neo expression vector (Promega) and equipped with a C-terminal RGS-His6 tag as described (24). The deletion mutants Sulf1ΔHD (ΔK417-K735), Sulf1ΔHDB (ΔK417-N720), and Sulf1ΔHDC (ΔQ459-H616) were generated by site-directed mutagenesis according to the QuikChange protocol (Stratagene, for primer sequences see supplemental Table S1). The preparation of the enzymatically inactive Sulf1 C87A/C88A mutant (Sulf1 CA) and cloning of human full-length Sulf2 cDNA have previously been described (24). For heterologous expression of the isolated HD of Sulf1 in Escherichia coli, cDNA fragments encoding Sulf1HD (residues Lys417–Lys735), Sulf1HDB (residues Lys417–Asn720), and Sulf1HDC (residues Lys417–Lys735, ΔQ459-H616) were amplified by PCR using Sulf1 or Sulf1ΔHDC as a template and cloned via NheI/XhoI restriction sites into pET21b+ (Novagen). To the coding sequences, an N-terminal TEV-cleavable RGS-His6 tag and a C-terminal Strep tag II were added (for primer sequences see supplemental Table S2). Due to the formation of inclusion bodies upon expression in E. coli, the constructs were subcloned into pKM263 (32) via XhoI/BamHI sites, thereby obtaining the corresponding N-terminal GST fusion constructs, GST-HD, GST-HDB, and GST-HDC (for primer sequences see supplemental Table S2). For surface plasmon resonance experiments with streptavidin biosensor chips, corresponding constructs without the C-terminal Strep tag II were prepared (supplemental Table S2). All plasmid constructs were sequenced in the coding region to preclude any PCR-derived errors.
Cell Culture and Transfection
HT1080 human fibrosarcoma cells were grown and transfected as previously described (24). Stable clones were screened for protein expression by Western blot analysis of cell lysates and cell culture supernatants, using a mouse monoclonal antibody directed against the RGS-His6 epitope (Qiagen) as described (24, 33).
Protein Purification
Cell culture supernatants from HT1080 cells stably overexpressing Sulf1ΔHDC were collected and enriched by Ni2+ affinity chromatography as previously described for human arylsulfatase G (33). Sulf1ΔHDC eluted at a concentration of ∼150–200 mm imidazole. Peak fractions, as analyzed by Western blotting and 4-methylumbelliferyl sulfate (4-MUS) assays were pooled. Protein concentrations were determined by Western blotting using purified, RGS-His6-tagged formylglycine-generating enzyme as a standard (34).
For purification of GST fusion proteins, expression plasmids were transformed into E. coli BL21(DE3) cells. Cells were grown in LB medium at 37 °C to an A600 of 0.5 and induced with isopropyl 1-thio-β-d-galactopyranoside at a final concentration of 0.1 mm. Proteins were expressed at 30 °C for 2 h. The cells were harvested by centrifugation and lysed in lysis buffer (1 mm EDTA, 1% Triton X-100 in PBS, pH 7.3) using a French press. GST fusion proteins were purified from the soluble fraction by affinity chromatography on a GSTrap HP 1-ml column (GE Healthcare). After washing with PBS, proteins were eluted with elution buffer (50 mm Tris, 10 mm reduced glutathione, pH 8.0). Peak fractions, as determined by SDS-PAGE/Coomassie staining and Western blotting with a polyclonal goat anti-GST antibody (GE Healthcare), were pooled and loaded onto a HiTrap heparin HP 1-ml column (GE Healthcare). After washing with binding buffer (20 mm Tris, pH 7.4), proteins were gradually eluted with elution buffer (20 mm Tris, 1.5 m NaCl, pH 7.4). Peak fractions were subsequently desalted on a HiTrap Desalt 5-ml column (GE Healthcare) using PBS as running buffer. Protein concentrations were determined using Coomassie Plus Bradford reagent (Pierce). Average yields of soluble proteins were about 50 or 25 μg/liter LB medium for GST-HD or GST-HDB and GST-HDC, respectively.
In Vitro Sulf Activity Assays
Activities of Sulf1 and Sulf1 mutants toward the fluorogenic pseudosubstrate 4-MUS were determined by incubating cell lysates with 2 mm 4-MUS in 10 mm HEPES (pH 7.4) for 1 h at 37 °C. Reactions were analyzed as previously described (33). Endosulfatase activities toward HS were investigated using [3H]glucosamine-labeled HS isolated from Sulf1/Sulf2 double knock-out mouse embryonic fibroblasts as a substrate (24). Assays were performed with either cellular or secreted Sulfs, purified from cell lysates on nickel-Sepharose beads (GE Healthcare) or enriched from conditioned medium, respectively (24). In the case of purified Sulf1ΔHDC, unlabeled HS or heparin from porcine intestinal mucosa (from Celsus Laboratories or Sigma, respectively) was used as substrates. Therefore, 1 mg of HS or heparin was incubated with Sulf1ΔHDC at a final substrate concentration of 1 mg/ml for 1 h at 37 °C and pH 7.4. Samples were digested to disaccharides and resolved by strong anion exchange HPLC as described (24). Disaccharide peaks were identified from UV absorbance of the Δ4,5 bond at 232 nm. Experiments with chondroitin sulfate B (Sigma) were performed accordingly, using chondroitinase ABC (Sigma) for disaccharide composition analysis.
Preparation of HS Affinity Columns
HS from porcine intestinal mucosa (Celsus) or HS material 6O-desulfated by Sulf1ΔHDC (see above) were desalted on PD10 columns, freeze dried, and reductively aminated at the reducing terminus based on a previously published protocol (35). Briefly, 50 mg of GAG material in 2 ml of 2 m NH4Cl was incubated with 100 mg of NaCNBH3 for 2 days at 60 °C. A further 50 mg of NaCNBH3 was added and incubated for an additional 3 days at 60 °C. After desalting, GAGs were coupled to HiTrap NHS 1-ml columns (GE Healthcare) at a concentration of 10 mg/ml in coupling buffer (0.2 m NaHCO3, pH 8.3) overnight at 4 °C. Unreacted NHS was quenched with 0.5 m ethanolamine (pH 8.3). As a control, a reference column without GAGs but inactivated with ethanolamine was generated. GST-HD was applied to the columns and gradually eluted with NaCl as described above.
BaSO4 Assay
Release of inorganic sulfate from HS or heparin was quantified via a BaSO4 precipitation assay adapted from Lundquist et al. (36). In brief, 200 μl of each sample were mixed with 100 μl of Ba2+ reagent (40 mm BaCl2, 7.5% (w/v) polyethylene glycol 6000, 60 μm K2SO4). After a 5-min incubation at room temperature, turbidimetry was measured at 600 nm using a TECAN infinite M200 microplate reader (TECAN, Crailsheim, Germany) and compared with a standard curve with K2SO4 from 5 to 250 μm.
Immunofluorescence Microscopy
The cell surface localization of Sulf1 and the Sulf1 deletion mutants, overexpressed in HT1080 cells, was analyzed by immunofluorescence microscopy as previously described (24). Binding of GST-HD to the cell surface of untransfected HT1080 cells was assayed by incubating the cells with purified protein at a final concentration of 20 ng/μl for 1 h at 37 °C. Purified GST was used as a control for unspecific binding. The cells were washed with PBS and labeled with polyclonal goat anti-GST antibodies (GE Healthcare) for 1 h at 4 °C. After fixation and blocking, cells were incubated with Alexa 546-conjugated donkey anti-goat IgG secondary antibodies and processed as above.
FACS Experiments
To assay the binding of GST-HD to the cell surface, untransfected HT1080 cells were grown to subconfluence, harvested with a cell scraper, and incubated with purified GST-HD, GST-HDB, or GST-HDC fusion proteins for 1 h at 4 °C at a final concentration of 1.5 μm (100 ng/μl) in PBS. Purified GST (1.5 μm, 40 ng/μl) and PBS were used as negative controls. The cells were washed three times with PBS and incubated with polyclonal goat anti-GST antibodies (GE Healthcare) for 1 h at 4 °C in Dulbecco's modified Eagle's medium. After washing three times with PBS, primary antibodies were detected with Alexa 546-conjugated donkey anti-goat IgG secondary antibodies in Dulbecco's modified Eagle's medium, 1% fetal calf serum. Again, the cells were washed, resuspended in HEPES buffer (10 mm HEPES, 150 mm NaCl, pH 7.4), and analyzed using a FACScalibur system (BD Biosciences). To determine binding specificity, cells were treated with either trypsin or heparinases II and III from Flavobacterium heparinum prior to incubation with GST fusion proteins. Therefore, the cells were incubated with 2 ml of 0.05% trypsin/EDTA (Invitrogen) for 10 min at 37 °C or with 2 mIU of each heparinases II and III (IBEX) in Dulbecco's modified Eagle's medium for 1 h at 37 °C. In both cases, the cells were extensively washed with PBS, incubated with GST-HD as described above, and exposed to primary and secondary antibodies for subsequent FACS analysis.
Heparan Sulfate Binding Assay
30 μmol of GST-HD, GST-HDB, or GST-HDC fusion proteins in PBS were immobilized on 100 μl of glutathione-agarose beads (Sigma). The beads were incubated with 50,000 cpm of 3H-labeled HS from Sulf1/2 double-deficient mouse embryonic fibroblasts (24) in 500 μl of PBS for 1 h at room temperature and washed three times with 500 μl of PBS to remove unspecifically bound HS. Specifically bound HS was eluted with 500 μl of 2 m NaCl after incubation for 10 min at room temperature with gentle agitation. The amount of [3H]HS in the wash and elution fractions was quantified via liquid scintillation counting.
FGF Signaling Assay
Growth factor signaling experiments were performed as previously described using wild type and Sulf1/Sulf2 double knock-out mouse embryonic fibroblasts (24). Prior to addition of FGF2, the cells were treated for 1 h at 37 °C with purified Sulf1ΔHDC in serum-free medium at a final concentration of 1.5 μg/ml. As a negative control, binding buffer with 270 mm imidazole was used instead of Sulf1ΔHDC. Subsequently, cells were washed twice with PBS and incubated with 1 ng/ml FGF2 for 10 min at 25 °C. Equal protein amounts of cell lysates were analyzed for diphospho and non-phosphorylated ERK1/2 signal intensities on Western blots and quantified as previously described (37).
Surface Plasmon Resonance
Biotinylated heparin (from porcine intestinal mucosa, average molecular mass 15 kDa, Merck) was immobilized (∼100 response units) on the surface of a Biacore sensor chip SA (GE Healthcare) according to the manufacturer's instructions using a Biacore 3000 system. Flow cell 1 served as a reference without immobilized heparin. PBS (pH 7.3) was used as running buffer for all experiments at a flow rate of 30 μl/min. Different concentrations of GST-HD without the C-terminal Strep tag II (125 to 1000 nm in PBS) were injected onto the chip surface and proteins were allowed to associate for 3 min, followed by a dissociation phase of 15 min. The sensor surface was regenerated by two short pulses (20 s) of 0.05% SDS with subsequent stabilization for 30 min.
RESULTS
Generation and Characterization of Sulf1 Deletion Mutants
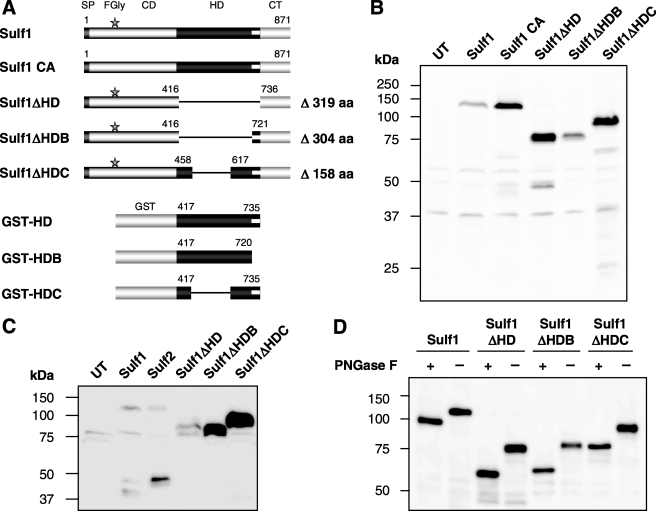
To investigate the functional importance of the HD of human Sulf1, we generated HT1080 cell lines stably overexpressing Sulf1 mutants in which the HD was either partially or completely deleted (Fig. 1A). In the first mutant, Sulf1ΔHD, the entire HD (ΔK417-K735) was removed. In contrast, the basic cluster at the C-terminal end of the HD was not deleted in the second mutant, referred to as Sulf1ΔHDB (ΔK417-N720). A third mutant, Sulf1ΔHDC, was prepared, containing only the evolutionary conserved outer regions of the HD but lacking the divergent inner region, encoded by exons 13 and 14 (ΔQ459-H616).
FIGURE 1.
Expression, secretion, and glycosylation of Sulf1 and Sulf1 mutants. Full-length Sulf1 is composed of a signal peptide (SP), the catalytic domain (CD), the hydrophilic domain (HD), and a C-terminal domain (CT). The C-terminal end of the HD contains a highly basic cluster, represented by a white bar. Sulf1 CA (lacking the active site Cα-formylglycine (FGly) residue) and the three deletion mutants of Sulf1 were generated as described in the text. As a complementary approach, GST fusion proteins containing different regions of the HD were prepared for production in E. coli (A). The expression of Sulf1 and Sulf1 mutants in cell lysates of stably transfected HT1080 cell lines was analyzed by Western blotting using a mouse anti-RGS-His6 antibody. As a negative control, untransfected cells (UT) were used (B). Accordingly, secretion into the conditioned medium was analyzed in 100-fold concentrated medium samples and compared with Sulf2 (C). Furthermore, cell lysate samples from the HT1080 cell lines were subjected to deglycosylation with peptide-N-glycosidase F (PNGase F) and analyzed by Western blotting (D).
Based on its amino acid sequence, a molecular mass of 100 kDa was expected for the RGS-His6-tagged full-length Sulf1 protein after signal peptide cleavage (residues 1–22). Sizes of 62, 64, and 82 kDa were anticipated for Sulf1ΔHD, -ΔHDB, and -ΔHDC, respectively. Analysis of cell lysates (Fig. 1B) revealed the presence of ∼120-kDa bands for the full-length Sulf1 protein and the previously described inactive Sulf1 mutant C87A/C88A (Sulf1 CA) (24). Sulf1ΔHD, -ΔHDB, and -ΔHDC migrated at apparent sizes of ∼75, 80, and 95 kDa, respectively, and were expressed significantly better than full-length Sulf1. As described below, the observed differences in size as compared with theoretical masses are due to protein N-glycosylation.
Full-length Sulf1 is released into the medium of transfected cells at very low levels. As previously described (6, 24, 31), processed forms of full-length Sulf1 and Sulf2 were detected in the conditioned medium, indicated by C-terminal fragments of ∼40 and 45 kDa. In the case of deletion mutants Sulf1ΔHD, Sulf1ΔHDB, and Sulf1ΔHDC, less processing was observed, corresponding to recent findings that furin-type proteinase processing occurs at two cleavage sites within the inner region of the HD (31). Interestingly, deletion of the HD or parts thereof significantly promotes secretion of the enzyme as reflected by increased amounts of the mutants in the conditioned medium (Fig. 1C). The most dramatic increase in secretion was observed for deletion mutant Sulf1ΔHDC which, compared with the full-length Sulf1, revealed about 30–50-fold higher abundance in the conditioned medium. These findings correspond in part to the increased cellular expression levels (Fig. 1B). On the other hand, the strong increase in secretion also suggested a role of the HD in interactions with the cell surface.
Deletion of the HD Does Not Alter Protein Glycosylation
The sequence of human Sulf1 contains 10 potential N-glycosylation sites (positions 64, 111, 131, 148, 170, 197, 240, 623, 773, and 783) but no predicted positions for O-glycosylation or sialylation. One of the potential N-glycosylation sites is located within the HD (position 623). In the case of the quail ortholog, QSulf1, two N-glycosylation sites are predicted in the HD (positions 527 and 620). A systematic analysis of the N-glycosylation of QSulf1 has revealed that neither of these two sites are utilized in vivo (38). Furthermore, protein N-glycosylation was shown to be essential for enzymatic activity and membrane targeting of QSulf1. To exclude that deletion of the HD of human Sulf1 affects N-glycosylation of the mutants, lysates from Sulf1 and Sulf1 mutant expressing cells were subjected to treatment with peptide-N-glycosidase F to release all N-linked glycan structures (Fig. 1D). Removal of N-glycans resulted in protein bands close to the calculated sizes without signal peptide (104, 61, 65, and 81 kDa for Sulf1, Sulf1ΔHD, -ΔHDB, and -ΔHDC, respectively). In accordance with full-length Sulf1, the observed shift in electrophoretic mobility was ∼15 kDa for all proteins, suggesting that deletion of the HD does not lead to major differences in protein glycosylation and confirming that the HD itself is not glycosylated.
In Vitro Activity of HD Deletion Mutants
To compare the enzymatic activities and specificities of full-length Sulf1 and the three HD deletion mutants toward their physiological substrate, we used an in vitro activity assay based on [3H]glucosamine-labeled heparan sulfate as a substrate, isolated from Sulf1/2 double-deficient mouse embryonic fibroblasts (24). Because this HS material has not been exposed to endogenous Sulf activity, maximum sensitivity is provided by a high degree of physiological 6O-sulfation.
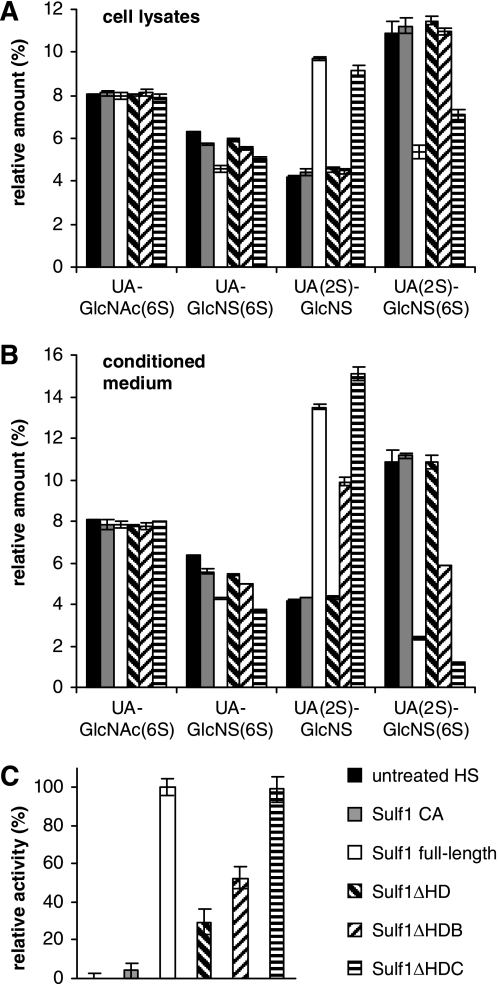
Lysates of transfected cells, expressing full-length Sulf1, inactive Sulf1 CA, Sulf1ΔHD, -ΔHDB, or -ΔHDC mutants were partially purified on nickel-Sepharose beads and normalized protein amounts were incubated with 3H-labeled HS. Following depolymerization, the corresponding disaccharides were resolved by strong anion exchange HPLC (Fig. 2A). Furthermore, the activities of the secreted Sulfs were analyzed from conditioned medium samples (Fig. 2B).
FIGURE 2.
Activity of Sulf1 and Sulf1 mutants toward heparan sulfate and 4MUS. [3H]Glucosamine-labeled HS material, isolated from Sulf1/2 double knock-out mouse embryonic fibroblasts, was used as a substrate for in vitro activity assays from either cell lysates (A) or conditioned medium (B). The HS substrate was incubated with Sulf1 CA (C87A/C88A), full-length Sulf1, Sulf1ΔHD, -ΔHDB, or -ΔHDC, digested to disaccharides, resolved by strong anion exchange HPLC, and quantified as described under “Experimental Procedures.” Alternatively, activities toward the pseudosubstrate 4-MUS were analyzed from cell lysate samples (C). Due to differences in expression levels (compare Fig. 1B), data were normalized according to band intensities on Western blots or, in the case of untransfected cells, to total protein amounts. Activity of full-length Sulf1 was set to 100%. The error bar at the very left represents the background from endogenous sulfatases in untransfected HT1080 cells. All error bars indicate S.D. from three independent experiments.
Importantly, deletion of the HD or parts thereof led to a differential loss of enzymatic activity. In the case of proteins purified from cell lysates, incubation of HS chains with full-length Sulf1 resulted in a ∼50% reduction of the trisulfated UA(2S)-GlcNS(6S) disaccharide and a concomitant increase of the corresponding disulfated UA(2S)-GlcNS unit (Fig. 2A). The amount of disulfated UA-GlcNS(6S) was reduced by ∼20%. No activity toward the transition zone-associated disaccharide unit UA-GlcNAc(6S) was detected. Negative controls with cell lysates from untransfected cells and inactive Sulf1 CA expressing cells indicated no background activity compared with untreated HS. Among the three deletion mutants, only Sulf1ΔHDC had significant activity, resulting in a ∼35% reduction of UA(2S)-GlcNS(6S). Disulfated UA-GlcNS(6S) was reduced by only 15%. Sulf1ΔHD and Sulf1ΔHDB were inactive toward all detectable disaccharide units within the HS chains.
Compared with intracellular Sulf activities, assays with secreted Sulfs from the conditioned medium showed endosulfatase activities not only for Sulf1 and Sulf1ΔHDC but also for Sulf1ΔHDB (Fig. 2B). In accordance with cell lysate results, no activity could be detected for Sulf1ΔHD. In the case of full-length Sulf1, the amount of trisulfated UA(2S)-GlcNS(6S) was reduced by ∼80%. Sulf1ΔHDC had even higher activity with ∼90% reduction, probably also due to better secretion of the enzyme as described above. It should be noted that due to the low secretion of full-length Sulf1, a reliable normalization of protein concentrations from the conditioned medium was not possible. Treatment of HS with secreted Sulf1ΔHDB resulted in a ∼50% reduction of the trisulfated UA(2S)-GlcNS(6S) disaccharide. The inactivity of Sulf1ΔHDB in the cell lysate samples might be explained by a high degree of misfolding of the intracellular protein. Activity trends toward the disulfated UA-GlcNS(6S) unit were similar to those observed toward the trisulfated UA(2S)-GlcNS(6S) species with 25, 35, 10, or 0% reduction upon incubation with full-length Sulf1, Sulf1ΔHDC, Sulf1ΔHDB, or Sulf1ΔHD, respectively. As observed for full-length Sulf1, none of the mutants were active toward monosulfated UA-GlcNAc(6S) disaccharide units (Fig. 2, A and B). Furthermore, no activity against N- or 2O-sulfate moieties or toward chondroitin sulfate/dermatan sulfate was observed for any Sulf variant (data not shown). Taken together, these findings confirm that deletion of parts of the HD in the case of Sulf1ΔHDC and ΔHDB does not alter the substrate specificity of Sulf1 within HS chains. A deletion of the entire HD leads to a complete loss of enzymatic activity toward the physiological substrate.
To determine whether this loss of activity is a result of protein misfolding or reduced substrate affinity, the small fluorogenic pseudosubstrate 4-MUS was used as an alternative substrate in enzyme assays based on normalized cell lysates (Fig. 2C). No differences in activities were detected between full-length Sulf1 and Sulf1ΔHDC, whereas ΔHDB had only about 50% activity. Interestingly, Sulf1ΔHD, which is inactive toward HS, had about 30% activity against 4-MUS, suggesting a role of the HD and especially the basic cluster at its C-terminal end in directing substrate specificity.
Biochemical Characterization of Sulf1ΔHDC
Biochemical analysis of the Sulfs has been complicated by the poor secretion of the enzymes in eukaryotic expression systems, reported by us and other groups (6, 24, 30), and fragmentation upon expression in E. coli (data not shown). Due to the high activity in vitro and significantly enhanced secretion compared with full-length Sulf1, the Sulf1ΔHDC mutant opened up the possibility for production and purification at a larger scale, allowing a detailed enzymatic characterization. Therefore, conditioned medium from stably transfected HT1080 cells was partially purified by Ni2+ affinity chromatography. This approach enabled acquisition of ∼10 μg of Sulf1ΔHDC per liter of cell culture medium, as determined by Western blot analysis. To find optimal conditions for 4-MUS hydrolysis, we analyzed the pH and temperature dependence of the reaction and found a broad pH optimum between pH 5.5 and 7.5 with a peak at 6.6 (Fig. 3A). At pH 8, Sulf1ΔHDC has only 25% residual activity, whereas full-length Sulf1, according to previous reports, has comparable activities at pH 7 and 8 (6). During incubation for 1 h, Sulf1ΔHDC cleaved 4-MUS most efficiently at ∼35 °C and rapidly lost activity at higher temperatures (Fig. 3B). At 37 °C, the turnover of 4-MUS is stable for about 2 h, whereas no further activity was observed after 8 h (Fig. 3C). According to substrate dose-response curves (Fig. 3D), Sulf1ΔHDC cleaves 4-MUS with a specific activity Vmax of 2400 nmol/(min/mg). This result is similar to the activity that was previously estimated for full-length Sulf1 (1000–2000 nmol/(min/mg)) (6). The affinity of Sulf1ΔHDC toward the artificial substrate 4-MUS is rather low, as indicated by an estimated Km value of 15 mm. In addition to 4-MUS, para-nitrocatechol sulfate was also found to be cleaved by Sulf1ΔHDC, albeit with very low activity of ∼0.05 nmol/(min/mg).
FIGURE 3.
Enzyme kinetics of Sulf1ΔHDC with 4-MUS. Secreted Sulf1ΔHDC was enriched from conditioned medium of stably transfected HT1080 cells and analyzed with 4-MUS as a substrate. The activities at different pH values (pH 5–9 at 37 °C) (A) or temperatures (4–65 °C at pH 7.0) (B) were investigated at a substrate concentration of 2 mm 4-MUS using 60 ng of Sulf1ΔHDC and a 1-h incubation time. The enzymatic stability of Sulf1ΔHDC was investigated at pH 7.0 and 37 °C under conditions not limited by substrate concentrations (C). Furthermore, the substrate dependence of the 4-MUS turnover was monitored in the range between 0 and 50 mm 4-MUS at 37 °C and pH 7.0 using 40 ng of Sulf1ΔHDC per reaction. Turnover rates were determined from a calibration curve with 4-MUS. At high concentrations, substrate inhibition was observed (D). The effects of inorganic phosphate (E) and sulfate (F) on the activity of Sulf1ΔHDC were analyzed at a 4-MUS concentration of 10 mm.
Similar to many other sulfatases such as arylsulfatase G (33), the activity of Sulf1ΔHDC is inhibited by inorganic phosphate (Fig. 3E). The calculated IC50 value is about 1 mm. At concentrations <3 mm, inorganic sulfate also has an increasingly inhibitory effect on 4-MUS cleavage (Fig. 3F). Surprisingly, concentrations above ∼3 mm result in a strong activation of the enzyme. At a concentration of 30 mm sulfate, the reaction was accelerated almost 10-fold compared with a control without sulfate addition. In experiments where HS was used as a substrate, the addition of sulfate did not result in increased activity but in weak inhibition (data not shown), suggesting that the activating effect is restricted to the turnover of the pseudosubstrate.
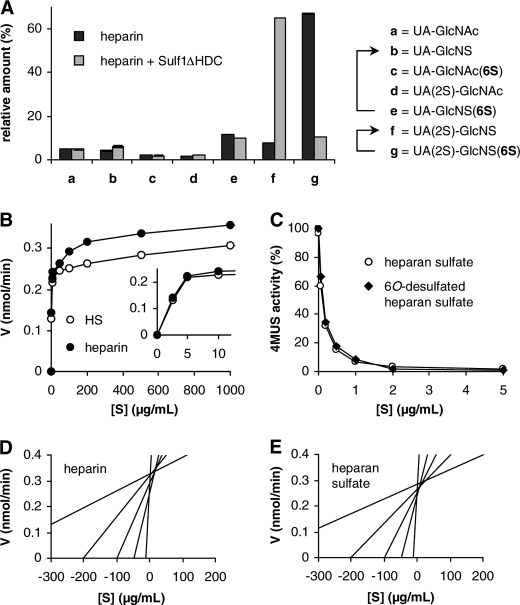
Although the experiments with endogenous HS material as a substrate enabled a comparison of the Sulf1 deletion mutants (Fig. 2, A and B), the unknown chemical amounts of substrate used in this radioactive assay precluded further quantitative or kinetic analyses. Therefore, partially purified Sulf1ΔHDC was incubated with defined amounts of unlabeled heparin. Heparin was tested as a substrate due to its structural similarity to the highly sulfated S-domains found in endogenous HS. As shown in Fig. 4A, the trisulfated UA(2S)-GlcNS(6S), representing the major disaccharide repeat unit of heparin, was reduced by 85% upon incubation of 1 mg of heparin with 0.7 μg of Sulf1ΔHDC. The disulfated UA-GlcNS(6S) unit was reduced by 20%, whereas no activity was observed toward other disaccharide units within the heparin chains. Using repeated enzyme treatments, preparative amounts of 20 mg of HS were also specifically 6O-desulfated by Sulf1ΔHDC to the same extent, thereby providing an important tool for further functional studies.
FIGURE 4.
Enzyme kinetics of Sulf1ΔHDC with heparin and heparan sulfate. To assay the activity of Sulf1ΔHDC toward heparin as a substrate, 1 mg of heparin was incubated with 0.7 μg of Sulf1ΔHDC for 24 h at 37 °C, digested to disaccharides, and resolved by strong anion exchange HPLC. Disaccharides were detected by UV absorbance of the unsaturated uronic acid residues at 232 nm. As a negative control, heparin was incubated with buffer instead of Sulf1ΔHDC. The 6O-sulfated substrate units and the corresponding products are indicated by arrows (A). To compare the activity of Sulf1ΔHDC toward heparin and HS, different concentrations of GAGs were incubated with Sulf1ΔHDC as above and the amount of released sulfate was determined by BaSO4 turbidimetry (B). The inset shows the curve trends at low substrate concentrations. Furthermore, the effect of heparan sulfate and 6O-desulfated heparan sulfate on the hydrolysis of 4-MUS was analyzed at different GAG concentrations and 10 mm 4-MUS. 4-MUS hydrolysis rates in the absence of competitor were set to 100% (C). To determine the kinetic parameters, the data from panel B, obtained at a concentration range of 10–500 μg/ml, were transformed into direct-linear Eisenthal-Cornish-Bowden plots (D and E).
To directly compare the kinetics of the desulfation of heparin or heparan sulfate by Sulf1ΔHDC, the amount of released sulfate at different substrate concentrations was analyzed by precipitation with Ba2+ and turbidimetric quantification. This assay allowed, for the first time, characterization of Sulf1 enzyme kinetics toward its endogenous substrates. As shown in Fig. 4B, increasing concentrations of substrate resulted in a hyperbolic curve shape with substrate saturation at concentrations of ∼1 mg/ml for both heparin and heparan sulfate. The highest activities were observed for heparin, probably due to the increased amount of trisulfated UA(2S)-GlcNS(6S) substrate units. Indeed, because heparin lacks the varied domain structure of endogenous heparan sulfate, it is also conceivable that the turnover of heparin by Sulf1ΔHDC is characterized by a higher processivity without dissociation of the enzyme from the GAG chain. The kinetic parameters Vmax and Km were determined from the corresponding direct-linear plots (39) (Fig. 4, D and E). In the case of heparin, a Vmax of 6200 nmol/(min/mg) and a Km of ∼13 μg/ml were estimated from the intersection of the lines. HS was desulfated with Vmax = 5100 nmol/(min/mg) and Km ∼5 μg/ml. Both activities are significantly higher as compared with the artificial substrate 4-MUS (Vmax = 2400 nmol/(min/mg), see above). Furthermore, the difference in Km values suggests that Sulf1ΔHDC has an increased affinity toward HS, the physiologically relevant substrate, and might have a preference toward the domain structure of HS.
To further investigate the substrate preference of Sulf1ΔHDC, assays with the artificial substrate 4-MUS were performed in the presence of different concentrations of heparan sulfate as a competitor (Fig. 4C). Already at low concentrations, HS represents the preferred substrate of Sulf1ΔHDC. A concentration of 230 ng/ml of HS (∼5 nm assuming an average HS chain length of 100 disaccharides) is sufficient to reduce the hydrolysis of 10 mm 4-MUS in the substrate mixture by 50%. Similar competition efficiencies were observed in the case of 6O-desulfated HS as a competitor. This finding might suggest that binding of the HS substrate blocks the entry of 4-MUS to the active site.
Sulf1ΔHDC Modulates FGF Signaling ex Vivo
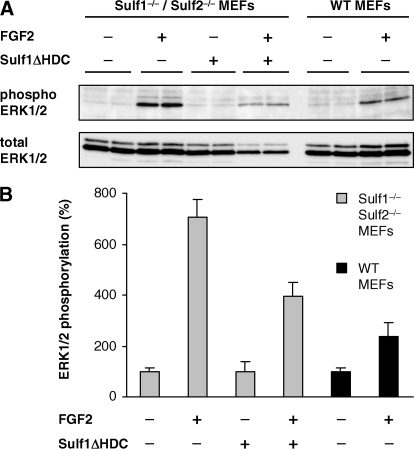
To investigate whether partially purified Sulf1ΔHDC is also active toward HSPGs on the cell surface, the effect of Sulf1ΔHDC on FGF signaling was analyzed in an ex vivo experiment. As previously reported (24), mouse embryonic fibroblasts (MEFs) isolated from Sulf1/Sulf2 double-deficient mice were characterized by a strong increase in HSPG 6O-sulfation. The presence of 6O-sulfate groups leads to an increased signaling response upon stimulation with the heparan sulfate-dependent growth factor FGF2 as compared with wild type MEFs. Preincubation of these cells with Sulf1ΔHDC significantly reduced the FGF signaling response, as measured by the ratio of phosphorylated ERK relative to total ERK, to a level slightly above the corresponding wild type MEFs (Fig. 5). This finding suggests that Sulf1ΔHDC is able to desulfate HSPGs at the cell surface, thereby influencing growth factor signaling in the same way as endogenous full-length Sulf1.
FIGURE 5.
Sulf1ΔHDC modulates FGF signaling ex vivo. MEFs, isolated from wild type and Sulf1/Sulf2 double knock-out mice, were induced with 1 ng/ml FGF2 for 10 min with or without prior treatment with Sulf1ΔHDC for 1 h at 37 °C. The FGF signaling response was quantified via Western blotting by an increase in the intensities of phosphorylated ERK relative to the amount of total ERK (A). Non-induced controls were set to 100% and compared with the FGF2-induced cells. Treatment of the cells with Sulf1ΔHDC reduces the FGF signaling response to a level slightly above the wild type controls (B). Error bars represent S.D. from three independent experiments.
The Isolated HD of Sulf1 Interacts with Heparin/HS Depending on 6O-Sulfate Substrate Groups
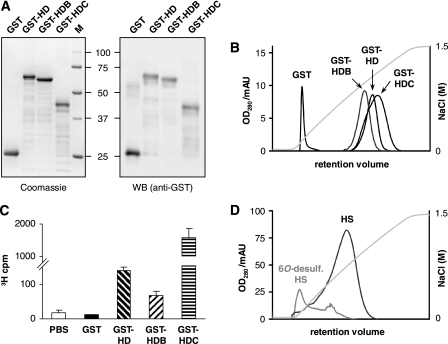
In addition to the Sulf1 deletion mutants, the isolated HD of Sulf1 was analyzed. Based on glycosylation analysis of human Sulf1 (see above) and previous results from the quail ortholog QSulf1 (38), the absence of glycan structures within the HD enables heterologous expression of the isolated HD in E. coli. Therefore, N-terminal GST fusion constructs GST-HD, GST-HDB, and GST-HDC were generated, containing either the entire HD of human Sulf1 or parts thereof, corresponding to the above described Sulf1 deletion mutants (Fig. 1A). The proteins showed poor solubility upon expression in E. coli, but could be purified via glutathione affinity chromatography with modest yields from the soluble fraction of bacterial lysates. In a second chromatography step, the proteins were bound onto a heparin matrix at pH 7.4 and eluted with a linear NaCl gradient. After heparin chromatography and subsequent gel filtration, GST fusion proteins GST-HD, GST-HDB, and GST-HDC were obtained with good purity and the expected molecular masses of 66, 64, and 48 kDa according to SDS-PAGE (Fig. 6A). This approach allowed for the first time investigation of the isolated HD of Sulf1 and its binding properties. Indeed, previous attempts to characterize the HD of QSulf2 are difficult to interpret due to a lack of purification and significant protein fragmentation upon expression (30). Compared with the GST control, GST-HD, GST-HDB, and GST-HDC all showed strong interactions with immobilized heparin and were displaced from the heparin column at unphysiologically high NaCl concentrations. GST-HD, GST-HDB, and GST-HDC eluted at 1050, 950, and 1100 mm, respectively, whereas GST alone eluted at 130 mm NaCl (Fig. 6B). The same system has previously been used to classify heparin interactions of other typical heparin-binding proteins (40) (see “Discussion”).
FIGURE 6.
Purification and heparin/HS binding of the isolated HD of Sulf1. The fusion proteins GST-HD, GST-HDB, GST-HDC, and GST alone were overexpressed in E. coli, purified as described under “Experimental Procedures,” and analyzed by SDS-PAGE (stained with Coomassie) or by Western blot using anti-GST antibodies (A). The affinity of GST fusion proteins to heparin was determined via heparin affinity chromatography. Proteins were resolved with a linear NaCl gradient and compared with GST as a control (B). To investigate the affinity of the HD constructs toward HS, normalized amounts of GST fusion proteins were immobilized on glutathione-agarose and incubated with 3H-labeled HS from Sulf1/2 double knock-out mouse embryonic fibroblasts. Specifically bound HS was eluted with 2 m NaCl and quantified (C). To address the role of 6O-sulfate groups, HS was specifically 6O-desulfated with Sulf1ΔHDC and immobilized on a column. For comparison, a column with equal amounts of untreated HS was prepared. GST-HD affinity was assayed using a NaCl gradient as described above (D). Error bars represent S.D. from three independent experiments.
To investigate the interaction with the physiological substrate HS, binding experiments were carried out using 3H-labeled HS isolated from Sulf1/Sulf2 double knock-out MEFs (24). To quantify the degree of binding, normalized amounts of GST fusion proteins were immobilized on glutathione-agarose and incubated with HS. After stringent washing, the specifically bound radiolabeled HS was eluted from the bead-bound GST fusion proteins with 2 m NaCl and quantified via scintillation counting (Fig. 6C). A significant amount of radioactivity was found in the elution fractions of GST-HD, GST-HDB, and GST-HDC but not in the eluates of beads loaded with GST alone or beads without any protein, both of which served as negative controls. Interestingly, whereas GST-HD and GST-HDB showed comparable binding of HS, about 8-fold increased retention was found in the case of GST-HDC, reflecting the elution order also observed in heparin affinity chromatography. Furthermore, we investigated the specific role of 6O-sulfate substrate groups in HS binding. To this end, commercial HS was treated with Sulf1ΔHDC as described above, yielding about 75% reduction in 6O-sulfation. The material was reductively aminated at the reducing end and immobilized on NHS-activated Sepharose beads to obtain an affinity column with specifically 6O-desulfated HS ligands. Whereas GST-HD eluted at a concentration of about 850 mm NaCl from control columns conjugated with the same amount of untreated HS, almost no specific interaction was detected on columns with HS that had been treated with Sulf1ΔHDC (Fig. 6D). Indeed, about 95% of GST-HD loaded onto this column was found in the flow-through fraction (data not shown), suggesting a pivotal role of 6O-sulfate groups in HD binding and release.
The HD Is Required for Targeting of Sulf1 to the Cell Surface
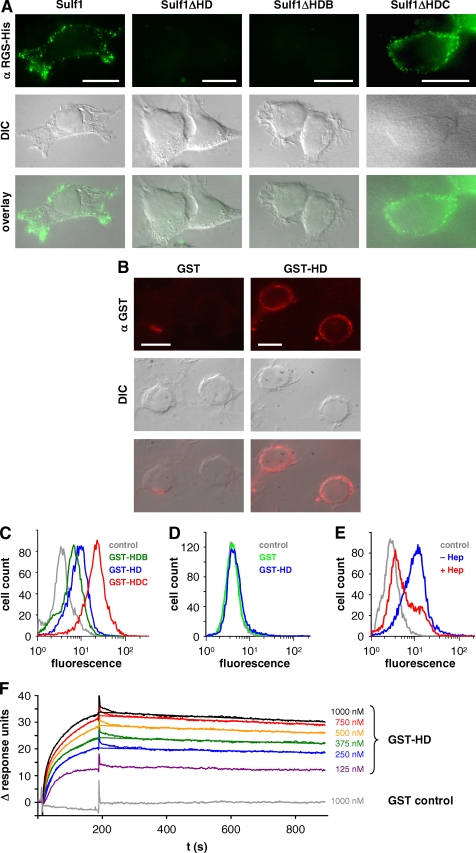
To determine whether heparin/heparan sulfate binding of the HD plays a role in targeting Sulf1 to the cell surface, we analyzed the effect of HD deletion on membrane targeting. Imaging of cells transfected with the Sulf1 deletion mutants in immunofluorescence microscopy revealed a clear cell surface localization of full-length Sulf1 and Sulf1ΔHDC, but not in the case of ΔHD or ΔHDB (Fig. 7A), indicating that the HD is responsible for cell surface localization of the enzyme. The basic cluster at the C-terminal end of the HD, which is present in the case of Sulf1ΔHDB but not in Sulf1ΔHD, is not sufficient to localize the enzyme on the cell surface.
FIGURE 7.
Cell surface targeting of Sulf1 and binding of the HD to heparan sulfate and heparin. Non-permeabilized cells from HT1080 cell lines expressing full-length Sulf1, Sulf1ΔHD, -ΔHDB, or -ΔHDC were analyzed by indirect immunofluorescence microscopy with an antibody directed against the C-terminal RGS-His6 tag (A). Bars represent 10 μm. The overlays of the respective fluorescence images with differential interference contrast (DIC) images reveal a cell surface localization of Sulf1 and Sulf1ΔHDC. As a complementary approach to the deletion mutants, purified GST-HD was added to untransfected HT1080 cells and detected on the cell surface using anti-GST antibodies (B). GST was used as a negative control. The binding of the different fusion proteins GST-HD, GST-HDB, and GST-HDC was compared by FACS analysis (C). In the case of panel D, the HT1080 cells were pre-treated with trypsin to remove all cell surface proteins and extensively washed before GST-HD was applied. The experiment was repeated with cells that had instead been incubated with heparinases to remove HS chains of cell surface HSPGs (E). Binding of GST-HD to immobilized heparin was further analyzed by surface plasmon resonance studies as described in the text and compared with GST as a control (F). The data from 250 to 1000 nm GST-Sufl1HD were used for curve fitting, based on the heterogeneous ligand model as described in the text (χ2 = 0.046).
The HD Preferentially Binds to Heparan Sulfate at the Cell Surface
As a complementary approach to the cell surface binding experiments using Sulf1 deletion mutants, the ability of the HD alone to interact with the cell surface was investigated. To this end, purified GST-HD was added to untransfected fibroblasts and detected using GST antibodies. Compared with the GST control, a clear cell surface association was seen for GST-HD (Fig. 7B). This binding could also be detected and quantified in FACS experiments, again using an antibody directed against the GST tag (Fig. 7C). At identical protein concentrations, the largest shift in fluorescence intensities was observed for GST-HDC, whereas GST-HDB, lacking the basic cluster, showed lower intensities compared with GST-HD, corroborating the results from the heparin affinity chromatography and the HS binding assays as described above.
To answer the question whether the HD selectively binds to the cell surface specifically via HS chains of HSPGs or, as proposed for the QSulfs, this binding involves the association with other interaction partners such as chondroitin sulfate or membrane phospholipids (30), FACS assays were performed with cells that had been treated either with trypsin to remove all cell surface proteins or heparinases II/III to selectively remove the HS chains of HSPGs. Binding of GST-HD was completely abolished on cells treated with trypsin, thereby demonstrating that phospholipids are unlikely to contribute to cell surface binding (Fig. 7D). Incubation with heparinases II/III (Fig. 7E) resulted in a major loss of cell surface binding, suggesting that the HS chains of HSPGs are the major interaction partners of the HD.
The HD Binds to HS with Nanomolar Affinity
The binding properties of GST-HD were further investigated by surface plasmon resonance experiments. For this purpose, biotinylated heparin was immobilized on the surface of a streptavidin biosensor chip. As mentioned above, heparin resembles the highly sulfated S-domain structures found in endogenous HS. For this experiment, purified GST-HD was applied onto the heparin chip surface. As shown in Fig. 7F, stable complexes were formed as indicated by the fast association and very slow dissociation. GST alone did not interact with the chip surface. Using the BIAevaluation software, the binding curves of GST-HD were fitted to determine dissociation constants and kinetic parameters. The best fit was obtained using the heterogeneous ligand model, corresponding to the heterogeneity of the heparin chains (χ2 = 0.046, i.e. <0.2% of the maximum response signal Rmax). This model is based on the assumption of two independent binding sites, a high affinity and a low affinity site. Although binding of the HD might be even more complex due to the structural variability of heparin and especially HS, the nanomolar dissociation constants KD1 = 0.6 nm (kon1 = 1.4 × 105 m−1 s−1, koff1 = 8.4 × 10−5 s−1) and KD2 = 17 nm (kon2 = 1.2 × 104 m−1 s−1, koff2 = 2.1 × 10−4 s−1) underline that the HD mediates high affinity binding toward heparin and the S-domains of HSPGs.
DISCUSSION
Editing of 6O-sulfation of cell surface HSPGs represents a novel mechanism by which the signal transduction pathways of various growth factors and morphogens can be modulated. How the two endosulfatases Sulf1 and Sulf2 recognize their HS substrates and how membrane targeting is achieved are critical questions for understanding their function in cell signaling processes. The present study demonstrates that the HD and distinct regions thereof have important functions in mediating high affinity GAG binding and enzyme activity.
The HD Is a Novel Heparin/HS-binding Domain That Interacts in a 6O-Sulfate-dependent Manner
Deletion of the HD of Sulf1 strongly reduces the cell surface localization of Sulf1 and leads to increased secretion of the enzyme into the medium. Taken together, the results from the deletion mutants underline that the HD and especially the outer regions are essential for cell surface targeting. For direct or indirect attachment to the cell membrane, several potential interaction partners have to be considered, including different classes of proteoglycans, other proteins, and also negatively charged phospholipids. As shown by FACS experiments with heparinase-treated fibroblasts, the HD of human Sulf1 preferentially binds to heparan sulfate chains, indicating that the HD mediates interactions with the HS chains of HSPGs, the substrate of Sulf1 and Sulf2. These findings contradict previous reports on QSulf1, which had either excluded HS as an interaction partner (5) or assumed non-selective binding of the HD to negatively charged GAGs (30).
To compare the heparin/HS binding properties of the HD with other GAG-binding proteins, heparin affinity chromatography was employed, using immobilized heparin as a matrix and gradient elution with increasing concentrations of NaCl. In previous studies, typical heparin-binding proteins, such as thrombin, antithrombin III, and heparin cofactor II, exhibited elution peaks at 470, 760, or 220 mm NaCl, respectively (40). In contrast, binding of GST-HD was significantly stronger with elution peaks at >1000 mm NaCl, similar to the affinity of FGF1 (41). Combined with the data from surface plasmon resonance experiments, indicating stable complex formation between the HD and heparin with nanomolar affinity, these results imply that the HD represents a novel high affinity heparin/HS-binding domain that localizes the enzyme close to the 6O-sulfate substrate groups.
Yet, the Sulfs also need to be released from their substrate to fulfill their function at the cell surface. We could show that the binding of GST-HD to HS was almost completely abrogated after treatment of HS with enzymatically active Sulf1ΔHDC. This result indicates that the binding of GST-HD specifically depends on the 6O-sulfated substrate disaccharides, i.e. UA(2S)-GlcNS(6S) and UA-GlcNS(6S) (24), and that dissociation and mobility of Sulf1 are promoted by substrate turnover. This would allow the enzyme to reach other proteoglycan substrates at the cell surface and also in the extracellular matrix.
Due to the large size of the HD, it seems likely that multiple contacts to adjacent or more distant sites are required for a stable interaction with heparin/HS. Based on the analysis of the different deletion mutants, the inner region of the HD, encoded by the less conserved exons 13 and 14, appears not to be involved in GAG binding. As seen for Sulf1ΔHDC, the loss of the inner region does not affect the cell surface localization. Consistent with these data, the GST-HDC fusion protein exhibited high affinity toward heparan sulfate, heparin, and mammalian cell surfaces. Compared with GST-HD, the increased affinity of GST-HDC toward heparin and especially HS might be explained by a hypothetical model in which the N- and C-terminal regions of the HD contain at least two independent GAG-binding sites, which come into close contact upon deletion of the HD inner region. This proximity could allow binding of the protein within the same S-domain of HS, whereas the presence of the inner region in GST-HD might result in orientation of the binding sites where only one site can interact with one S-domain (Fig. 8). This model would also explain why the observed differences in binding efficiencies were less prominent with homogeneously sulfated heparin as the binding partner. In the case of full-length Sulf1, the inner region might have structural importance to regulate HS affinity or to enable “tandem” interactions of the HD outer regions with two different GAG chains simultaneously.
FIGURE 8.
Hypothetical model of the interaction of the HD with HS chains. Due to the large size of the HD, several binding sites could be present within the HD sequence. In this model, only the conserved outer regions contribute to HS binding. In the HDC mutant, two HS binding sites come into close contact, allowing high affinity binding of the shortened HD within one S-domain, whereas in the full-length HD, both binding sites cannot bind within the same S-domain. Thus, tight binding of HD requires “tandem” contacts with two S-domains.
Biochemical Properties of Sulf1 with and without HD
Using different Sulf1 deletion mutants, the role of the HD in enzymatic activity was investigated. Enzyme assays with the physiological substrate, HS, as well as with the pseudosubstrate 4-MUS demonstrate that the HD of Sulf1 is required for enzyme function, corresponding to previous reports on QSulf2 (30). A deletion of the inner region of the HD does not affect activity or substrate specificity toward HS or 4-MUS. In contrast, the loss of the entire HD leads to a reduced activity toward 4-MUS, whereas activity is completely lost against HS, supporting recent results also from Sulf2 (31). Taken together, these findings support a model in which the HS chain is first bound by the HD to neutralize its negative charge and then presented to the active site for efficient catalysis (30). The small pseudosubstrate 4-MUS might simply diffuse into the active site and thus does not require the presence of the HD. In this context, the sulfate-induced activation of Sulf1ΔHDC toward 4-MUS, but not toward HS as a substrate, might indicate that the sulfate ions are able to occupy binding sites within the HD, mimicking the HS chain and leading to neutralization of the positive charge and subsequent conformational changes. Also the basic cluster at the C-terminal end of the HD appears to be essential for Sulf1 activity, as deletion of this cluster represents the only difference between Sulf1ΔHD (inactive) and Sulf1ΔHDB (active). Finally, it was shown for the first time that deletion of large parts of the HD does not alter HS substrate specificity of Sulf1. In both active mutants Sulf1ΔHDB and Sulf1ΔHDC, the trisulfated UA(2S)-GlcNS(6S) unit was the preferred substrate group within HS chains, confirming that the selection of specific positions is mediated by the active site rather then by the HD.
Perspectives
Based on its high activity in vitro and significantly increased secretion, the Sulf1ΔHDC variant allowed the biochemical and kinetic characterization of heparin and heparan sulfate desulfation. Furthermore, this mutant opens up new possibilities to obtain larger amounts of functional enzyme, which will be useful for further applications such as cell culture-based assays, also in comparison to Sulf2 (12). The general applicability of Sulf1ΔHDC has been demonstrated by the reduction of the FGF signaling response after incubation of Sulf1/Sulf2 double-deficient mouse embryonic fibroblasts with Sulf1ΔHDC. This assay suggests that the purified protein will be helpful to study also other growth factor signaling pathways dependent on heparan sulfate 6O-sulfate groups. Interestingly, it has recently been shown that deletion of the furin cleavage sites within the HD of Sulf1 and Sulf2 renders both enzymes unable to activate Wnt signaling (31). This finding was attributed to the reduced localization of the uncleavable Sulf mutants into lipid rafts. In contrast to Wnt signaling, we show here that Sulf1ΔHDC, a mutant lacking both furin cleavage sites due to the deletion of the inner region of the HD, is able to modulate FGF2 signal transduction. This difference is likely due to the highly soluble nature of exogenous Sulf1ΔHDC, which might allow the enzyme to freely access raft and non-raft located proteoglycans on the cell surface. In addition to its ability to modulate cell signaling, Sulf1ΔHDC will serve as an important tool for further research investigating the role of 6O-sulfate groups. 6O-Desulfated HS material, generated by Sulf1ΔHDC treatment, can be used for example, in surface plasmon resonance studies to explicitly investigate the role of substrate sulfates in binding of the HD. Protein crystallography of the isolated HD in complex with defined HS oligosaccharides will help to further understand the role of the HD with regard to substrate recognition and enzyme mechanism. Moreover, structural analysis of the HD will also contribute to a more detailed understanding of how the analog code of HS sulfation is recognized and deciphered by proteins at the molecular level.
Supplementary Material
Acknowledgments
We thank Katrin Wollschläger for help with surface plasmon resonance experiments, valuable discussions, and critically reading the manuscript. We thank Norbert Sewald for provision of the Biacore 3000 system.
This work was supported by the Deutsche Forschungsgemeinschaft, the Fonds der Chemischen Industrie, and Shire Human Genetic Therapies, Inc. (Cambridge, MA).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2.
- HS
- heparan sulfate
- FGF
- fibroblast growth factor
- GlcNAc
- N-acetylglucosamine
- GlcNS
- N-sulfoglucosamine
- GST
- glutathione S-transferase
- HPLC
- high performance liquid chromatography
- HSPG
- heparan sulfate proteoglycan
- NS
- N-sulfate
- 2S
- 2O-sulfate
- 6S
- 6O-sulfate
- UA
- hexuronic acid
- GAG
- glycosaminoglycan
- MEF
- mouse embryonic fibroblasts
- 4-MUS
- 4-methylumbelliferyl sulfate
- HD
- hydrophilic domain
- PBS
- phosphate-buffered saline
- FACS
- fluorescence-activated cell sorter
- ERK
- extracellular signal-regulated kinase.
REFERENCES
- 1.Dierks T., Schmidt B., Borissenko L. V., Peng J., Preusser A., Mariappan M., von Figura K. (2003) Cell 113, 435–444 [DOI] [PubMed] [Google Scholar]
- 2.Cosma M. P., Pepe S., Annunziata I., Newbold R. F., Grompe M., Parenti G., Ballabio A. (2003) Cell 113, 445–456 [DOI] [PubMed] [Google Scholar]
- 3.Dierks T., Schlotawa L., Frese M. A., Radhakrishnan K., von Figura K., Schmidt B. (2009) Biochim. Biophys. Acta Mol. Cell Res. 1793, 710–725 [DOI] [PubMed] [Google Scholar]
- 4.Diez-Roux G., Ballabio A. (2005) Annu. Rev. Genomics Hum. Genet. 6, 355–379 [DOI] [PubMed] [Google Scholar]
- 5.Dhoot G. K., Gustafsson M. K., Ai X., Sun W., Standiford D. M., Emerson C. P., Jr. (2001) Science 293, 1663–1666 [DOI] [PubMed] [Google Scholar]
- 6.Morimoto-Tomita M., Uchimura K., Werb Z., Hemmerich S., Rosen S. D. (2002) J. Biol. Chem. 277, 49175–49185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernfield M., Götte M., Park P. W., Reizes O., Fitzgerald M. L., Lincecum J., Zako M. (1999) Annu. Rev. Biochem. 68, 729–777 [DOI] [PubMed] [Google Scholar]
- 8.Sasisekharan R., Raman R., Prabhakar V. (2006) Annu. Rev. Biomed. Eng. 8, 181–231 [DOI] [PubMed] [Google Scholar]
- 9.Ai X., Do A. T., Lozynska O., Kusche-Gullberg M., Lindahl U., Emerson C. P., Jr. (2003) J. Cell Biol. 162, 341–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viviano B. L., Paine-Saunders S., Gasiunas N., Gallagher J., Saunders S. (2004) J. Biol. Chem. 279, 5604–5611 [DOI] [PubMed] [Google Scholar]
- 11.Wang S., Ai X., Freeman S. D., Pownall M. E., Lu Q., Kessler D. S., Emerson C. P., Jr. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 4833–4838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchimura K., Morimoto-Tomita M., Bistrup A., Li J., Lyon M., Gallagher J., Werb Z., Rosen S. D. (2006) BMC Biochem. 7, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nawroth R., van Zante A., Cervantes S., McManus M., Hebrok M., Rosen S. D. (2007) PLoS ONE 2, e392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morimoto-Tomita M., Uchimura K., Bistrup A., Lum D. H., Egeblad M., Boudreau N., Werb Z., Rosen S. D. (2005) Neoplasia 7, 1001–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narita K., Staub J., Chien J., Meyer K., Bauer M., Friedl A., Ramakrishnan S., Shridhar V. (2006) Cancer Res. 66, 6025–6032 [DOI] [PubMed] [Google Scholar]
- 16.Lai J., Chien J., Staub J., Avula R., Greene E. L., Matthews T. A., Smith D. I., Kaufmann S. H., Roberts L. R., Shridhar V. (2003) J. Biol. Chem. 278, 23107–23117 [DOI] [PubMed] [Google Scholar]
- 17.Lamanna W. C., Baldwin R. J., Padva M., Kalus I., Ten Dam G., van Kuppevelt T. H., Gallagher J. T., von Figura K., Dierks T., Merry C. L. (2006) Biochem. J. 400, 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ai X., Kitazawa T., Do A. T., Kusche-Gullberg M., Labosky P. A., Emerson C. P., Jr. (2007) Development 134, 3327–3338 [DOI] [PubMed] [Google Scholar]
- 19.Holst C. R., Bou-Reslan H., Gore B. B., Wong K., Grant D., Chalasani S., Carano R. A., Frantz G. D., Tessier-Lavigne M., Bolon B., French D. M., Ashkenazi A. (2007) PLoS ONE 2, e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lum D. H., Tan J., Rosen S. D., Werb Z. (2007) Mol. Cell. Biol. 27, 678–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamanna W. C., Kalus I., Padva M., Baldwin R. J., Merry C. L., Dierks T. (2007) J. Biotechnol. 129, 290–307 [DOI] [PubMed] [Google Scholar]
- 22.Kalus I., Salmen B., Viebahn C., von Figura K., Schmitz D., D'Hooge R., Dierks T. (2009) J. Cell. Mol. Med. in press, DOI 10.1111/j.1582-4934.2008.00558.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ratzka A., Kalus I., Moser M., Dierks T., Mundlos S., Vortkamp A. (2008) Dev. Dyn. 237, 339–353 [DOI] [PubMed] [Google Scholar]
- 24.Lamanna W. C., Frese M. A., Balleininger M., Dierks T. (2008) J. Biol. Chem. 283, 27724–27735 [DOI] [PubMed] [Google Scholar]
- 25.Li W., Johnson D. J., Esmon C. T., Huntington J. A. (2004) Nat. Struct. Mol. Biol. 11, 857–862 [DOI] [PubMed] [Google Scholar]
- 26.Pellegrini L., Burke D. F., von Delft F., Mulloy B., Blundell T. L. (2000) Nature 407, 1029–1034 [DOI] [PubMed] [Google Scholar]
- 27.Cardin A. D., Weintraub H. J. (1989) Arteriosclerosis 9, 21–32 [DOI] [PubMed] [Google Scholar]
- 28.Sobel M., Soler D. F., Kermode J. C., Harris R. B. (1992) J. Biol. Chem. 267, 8857–8862 [PubMed] [Google Scholar]
- 29.Hileman R. E., Fromm J. R., Weiler J. M., Linhardt R. J. (1998) BioEssays 20, 156–167 [DOI] [PubMed] [Google Scholar]
- 30.Ai X., Do A. T., Kusche-Gullberg M., Lindahl U., Lu K., Emerson C. P., Jr. (2006) J. Biol. Chem. 281, 4969–4976 [DOI] [PubMed] [Google Scholar]
- 31.Tang R., Rosen S. D. (2009) J. Biol. Chem. 284, 21505–21514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melcher K. (2000) Anal. Biochem. 277, 109–120 [DOI] [PubMed] [Google Scholar]
- 33.Frese M. A., Schulz S., Dierks T. (2008) J. Biol. Chem. 283, 11388–11395 [DOI] [PubMed] [Google Scholar]
- 34.Preusser-Kunze A., Mariappan M., Schmidt B., Gande S. L., Mutenda K, Wenzel D., von Figura K., Dierks T. (2005) J. Biol. Chem. 280, 14900–14910 [DOI] [PubMed] [Google Scholar]
- 35.Osmond R. I., Kett W. C., Skett S. E., Coombe D. R. (2002) Anal. Biochem. 310, 199–207 [DOI] [PubMed] [Google Scholar]
- 36.Lundquist P., Mårtensson J., Sörbo B., Ohman S. (1980) Clin. Chem. 26, 1178–1181 [PubMed] [Google Scholar]
- 37.Merry C. L., Bullock S. L., Swan D. C., Backen A. C., Lyon M., Beddington R. S., Wilson V. A., Gallagher J. T. (2001) J. Biol. Chem. 276, 35429–35434 [DOI] [PubMed] [Google Scholar]
- 38.Ambasta R. K., Ai X., Emerson C. P., Jr. (2007) J. Biol. Chem. 282, 34492–34499 [DOI] [PubMed] [Google Scholar]
- 39.Eisenthal R., Cornish-Bowden A. (1974) Biochem. J. 139, 715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eckert R., Ragg H. (2003) FEBS Lett. 541, 121–125 [DOI] [PubMed] [Google Scholar]
- 41.Klagsbrun M. (1990) Curr. Opin. Cell Biol. 2, 857–863 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.