Abstract
Abscisic acid (ABA) is a plant hormone regulating fundamental physiological functions in plants, such as response to abiotic stress. Recently, ABA was shown to be produced and released by human granulocytes, by insulin-producing rat insulinoma cells, and by human and murine pancreatic β cells. ABA autocrinally stimulates the functional activities specific for each cell type through a receptor-operated signal transduction pathway, sequentially involving a pertussis toxin-sensitive receptor/G-protein complex, cAMP, CD38-produced cADP-ribose and intracellular calcium. Here we show that the lanthionine synthetase C-like protein LANCL2 is required for ABA binding on the membrane of human granulocytes and that LANCL2 is necessary for transduction of the ABA signal into the cell-specific functional responses in granulocytes and in rat insulinoma cells. Co-expression of LANCL2 and CD38 in the human HeLa cell line reproduces the ABA-signaling pathway. Results obtained with granulocytes and CD38+/LANCL2+ HeLa transfected with a chimeric G-protein (Gαq/i) suggest that the pertussis toxin-sensitive G-protein coupled to LANCL2 is a Gi. Identification of LANCL2 as a critical component of the ABA-sensing protein complex will enable the screening of synthetic ABA antagonists as prospective new anti-inflammatory and anti-diabetic agents.
The plant hormone abscisic acid (ABA)4 plays a fundamental role in the regulation of plant response to environmental conditions, as well as in plant tissue development (1). Although the ABA biosynthetic pathway in plants and in fungi has been largely detailed, identification of the components of the ABA signaling pathway, particularly of the ABA receptor(s), has remained elusive. Two ABA-binding proteins have been identified in different plant tissues: the chloroplast Mg-chelatase subunit H (2) and, most recently, the G-protein-coupled receptor GCR2, which appears to mediate ABA-controlled stomatal closure and seed dormancy in Arabidopsis (3), although the role of GCR2 in the control of seed germination is still controversial (4–6) and its coupling to a G-protein has been refuted on the basis of sequence analyses (7–8). The Mg-chelatase subunit H was proposed as an intracellular ABA receptor, whereas GCR2 is a plasmamembrane protein, which interacts with the only Gα subunit (GPA 1) present in Arabidopsis (3). Although the Mg-chelatase subunit H does not show any significant homology with mammalian proteins, GCR2 shares a high amino acid identity with the mammalian peptide-modifying lanthionine synthetase C-like protein (LANCL) family (7). The animal LANCL protein family in turn shows structural similarities with the prokaryotic lanthionine synthetase component C proteins (9) involved in the synthesis of lanthionine-containing antimicrobial peptides known as lantibiotics (10).
The fact that lantibiotics are not produced in animals suggests that LANCL proteins have a different function than prokaryotic lanthionine synthetase component C proteins. The human genome contains three LANCL genes, LANCL1, LANCL2, and LANCL3, located on chromosomes 2 and 7 and the X chromosome, respectively (11, 12). LANCL1 was the first member of the family to be isolated from human erythrocyte membranes (13). The LANCL2 mRNA was identified in a screening procedure for genes whose down-regulation resulted in anticancer drug resistance; thus, LANCL2 was also called testis-specific Adriamicin sensitivity protein (14). The structural assignment for the human LANCL proteins remains controversial. Based on the presence of seven putative transmembrane domains, LANCL1 and -2 were originally described as new G-protein-coupled receptors (GPCR69A and GPR69B, respectively); however, subsequent studies performed on human epithelial cells overexpressing LANCL1 or LANCL2 fused to the green fluorescent protein (LANCL1-GFP and LANCL2-GFP) showed that LANCL1-GFP is mainly found in the cytosol and in the nucleus, whereas LANCL2-GFP is associated with the plasmamembrane through N-terminal myristoylation (15). Similarly, the debate over the structurally related GCR2 is still open (3–6, 8).
ABA has recently been demonstrated to be an endogenous pro-inflammatory hormone in human granulocytes, stimulating several cell functions (phagocytosis, reactive oxygen species and nitric oxide production, chemotaxis, and chemokinesis) through a pathway involving a pertussis toxin (PTX)-sensitive G-protein/receptor complex located on the plasmamembrane, cAMP overproduction, protein kinase A-dependent phosphorylation of the human ADP-ribosyl cyclase CD38, and consequent cADP-ribose (cADPR) generation, leading to an increase of the intracellular Ca2+ concentration (16; see also Ref. 17). This signaling pathway is similar to that triggered by ABA in plants (18). Fluorescence microscopy confirmed binding of biotinylated ABA to the granulocyte plasmamembrane. Scatchard plot analysis of [3H]ABA binding demonstrated presence of both high and low affinity ABA binding sites (Kd 11 nm and 500 μm, respectively) on human granulocytes (16). Most recently, nanomolar ABA has been shown to stimulate insulin secretion by human and murine pancreatic β cells and by rat insulinoma cell lines through a signaling pathway similar to the one described in human granulocytes (19). The autocrine release of ABA from glucose-stimulated human and rodent insulin-releasing cells, together with the fact that ABA is also produced by activated inflammatory cells, granulocytes (16), and monocytes (20), suggests that this hormone may contribute to the network of cytokine signals exchanged between inflammatory cells and pancreatic β cells, which is increasingly recognized as a fundamental mechanism in the development of the metabolic syndrome and type II diabetes (21–24).
Based on (i) the sequence homology between the putative Arabidopsis ABA-receptor protein GCR2 and the human LANCL protein family, and (ii) the reported association of LANCL2 with the plasmamembrane, we investigated whether LANCL2 might be involved in ABA sensing in mammalian ABA-responsive cells. The results obtained indicate that LANCL2 is indeed, (i) required for ABA binding to the plasmamembrane of human granulocytes and (ii) necessary for the activation of the ABA signaling pathway, leading to the stimulation of the functional responses induced by ABA in human granulocytes and in rat insulinoma cells.
EXPERIMENTAL PROCEDURES
Materials
Fura2/AM was purchased from Calbiochem (Milan, Italy). The [3H]cAMP assay system, Ficoll-Paque Plus and [3H](±)-ABA (40 Ci/mmol) were from Amersham Biosciences. Dulbecco's modified Eagle's medium and RPMI cell culture medium were purchased from Lonza (Milano, Italy). All other chemicals were obtained from Sigma.
Isolation of Human Granulocytes
Buffy coats, prepared from blood freshly drawn from healthy human volunteers after informed consent, were provided by Galliera Hospital (Genova, Italy). Granulocytes were isolated as described previously (16).
Cell Culture
The rat insulinoma RIN-m cell line was obtained from ATCC and cultured as described previously (19). The rat insulinoma cell line INS-1 was kindly provided by Prof. F. Beguinot (Federico II University of Napoli, Italy) and cultured in the same medium as RIN-m cells, supplemented with 50 μm 2-mercaptoethanol. HeLa stably transfected with sense (CD38+) and antisense (CD38−) CD38 cDNA (25) were cultured in Dulbecco's modified Eagle's medium supplemented with fetal calf serum (10%), penicillin (50 units/ml), and streptomycin (50 μg/ml). Cells were maintained in a humidified 5% CO2 atmosphere at 37 °C.
siRNA Transfection
Transient transfection of human granulocytes, INS-1, and RIN-m cells was performed using the Nucleofector System (Amaxa GmbH, Cologne, Germany). Freshly isolated granulocytes (20 × 106 cells) were transfected using the Nucleofector program X-005, without (control), with 2 μm LANCL2-targeting StealthTM duplex short interference RNA (siRNA#1, #2 or #3) or with 2 μm StealthTM Negative Control (negative control), as described (26). After transfection, granulocytes were resuspended in 2.5 ml of RPMI supplemented with fetal calf serum (10%), penicillin (50 units/ml), and streptomycin (50 μg/ml) and incubated in a 5% CO2 at 37 °C. All experiments were performed 24 h after transfection.
RIN-m and INS-1 cells were grown to 50–75% confluence. Cells were trypsinized and 5 × 106 cells were electroporated with 1 μm LANCL2-targeting StealthTM duplex siRNA, using the Amaxa Nucleofector, program T20, solution T or V for INS-1 or RIN-m cells, respectively. To exclude nonspecific knockdown, cells were also transfected with StealthTM Negative Control (negative control). All experiments were performed 48 h after transfection.
The LANCL2-targeting StealthTM siRNAs for human LANCL2 (siRNA#1, LANCL2-HSS125242; siRNA#2, LANCL2- HSS125243; and siRNA#3, LANCL2-HSS125244) and for rat LANCL2 (siRNA, NM_001014187_stealth_208) were obtained from Invitrogen (Milan, Italy); the negative control siRNA for transfection of human (cat. no. 12935-300), or of rat cells (NM_001014187_stealth_control_208), were also from Invitrogen.
Fluorimetric Measurement of the [Ca2+]i
Twenty-four hours after transfection, [Ca2+]i measurements were performed on Fura2/AM-loaded granulocytes or rat insulinoma cells (control, negative control siRNA, and LANCL2 siRNA) seeded on 20-mm glass coverslips, as described in a previous study (16).
After transfection, CD38+ and CD38− HeLa transfected with the control or with the LANCL2 plasmid were seeded on 20-mm glass coverslips, and [Ca2+]i determinations were performed after 24 h, as described in a previous study (25).
Determination of Intracellular cAMP and IP3 Levels
Twenty- four hours after transfection, granulocytes (control, negative control, and siRNA) were resuspended in Hanks' balanced salt solution (HBSS, 25 × 106/ml), preincubated for 5 min at 25 °C in the presence of 10 μm 4-(3-butoxy-4-methoxybenzyl)imidazolidin-2-one (Ro 20-1724), a cAMP phosphodiesterase inhibitor, and then challenged with 10 μm ABA. At 0 and 60 s, a 300-μl aliquot of the suspension was withdrawn, and incubations were stopped by adding perchloric acid (PCA) to a final concentration of 0.6 m, at 4 °C. The PCA was removed as described in a previous study (27). Intracellular cAMP levels were determined by radioimmunoassay (Amersham Biosciences), according to the manufacturer's protocol.
For measurement of the [cAMP]i in CD38+/LANCL2+ HeLa, cells (1.5 × 106/determination in a total volume of 0.7 ml of HBSS) were preincubated for 10 min at 25 °C with 10 μm Ro 20-1724, the supernatant was removed, and 0.7 ml of HBSS with or without 100 nm ABA was added; after 0.5 and 1 min the incubation was stopped with PCA (0.6 m final concentration). The cell extracts were recovered and processed as described above. For measurement of the [IP3]i, CD38+ and CD38+/LANCL2+ HeLa or granulocytes were transfected with the pcDNAI/Gαq/i plasmid. Twenty-four hours after transfection, the HeLa cell supernatant was removed and 0.7 ml of HBSS with or without ABA was added to the adherent cells (1 × 106/determination); granulocytes were centrifuged, resuspended in HBSS, and stimulated with 10 μm ABA (6 × 106/determination); and then incubations were stopped with PCA (0.6 m final concentration) after 10, 30, and 60 s. The cell extracts were recovered, PCA was removed, and intracellular IP3 levels were determined by radioimmunoassay (Amersham Biosciences), according to the manufacturer's protocol. The protein content of each sample was measured (28) on the protein precipitate solubilized with 1 n NaOH.
Vector Construction and Transfection
The full-length LANCL2 cDNA was amplified by PCR using cDNA obtained with reverse transcription of total RNA from human granulocytes (purified as described before) and using the following primers: 5′-CACCATGGGCGAGACCATGTCAAAG-AG-3′ (foward) and 5′-TTAATCCCTCTTCGAAGAGTCAAGT-3′ (reverse).
The PCR was performed in 25 μl containing 1× reaction buffer, 200 μm dNTP, 5 pmol of primers, and using 1.25 units of Herculase HotStart DNA polymerase. The PCR reaction profile was 1 cycle at 94 °C for 2 min, 35 cycles at 94 °C for 15 s, 62 °C for 30 s, and 72 °C for 1 min with a final extension for 5 min at 72 °C. The PCR product was purified with a Nucleospin® Extract Kit (Macherey-Nagel) and cloned into pcDNA6.2/V5/ GW/D-TOPO©. This vector allows the synthesis of the recombinant protein as a C-terminal fusion to the V5 epitope. The LANCL2 plasmid was purified using PureLinkTM HiPure Plasmid Filter Kit (Invitrogen) and sequenced by TibMolbiol (Genova, Italy). The pCS2+/α-transducin plasmid (Addgene plasmide 16681) was purchased from Addgene Inc. (Cambridge, MA). The plasmid pcDNAI/Gαq/i and the corresponding empty vector were kindly provided by Dr. R. A. Nicholas (Chapel Hill, NC).
Human granulocytes or CD38+ and CD38− HeLa, were transfected in parallel with pcDNA6.2/V5/GW/D-TOPO© (empty, control plasmid) and with LANCL2-pcDNA6.2/V5/ GW/D-TOPO© (LANCL2 plasmid). Moreover, granulocytes, CD38+, and CD38+/LANCL2+ HeLa cells were also transfected with pCS2+/α-transducin or with pcDNAI/Gαq/i plasmids. Human granulocytes were transfected using the Nucleofector System as described above; HeLa were transfected with Nucleofector System (Cell Line Nucleofector Kit R, program I-013).
Western Blot
LANCL2 protein expression was determined by Western blot analysis using a monoclonal antibody against the V5 epitope (Invitrogen). Twenty-four hours after transfection with the plasmid, granulocytes and HeLa were washed twice with ice-cold phosphate-buffered saline (PBS) and lysed in RIPA buffer (150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mm Tris-HCl, pH 8) in the presence of a protease inhibitor mixture for mammalian cells (Sigma). Homogenates were incubated for 30 min at 4 °C with an orbital rotator and then centrifuged at 10,000 × g for 10 min at 4 °C. The protein concentration of the supernatants was determined by a DC Protein Assay (Bio-Rad). After SDS-PAGE, performed according to the standard method on 10% gels, the proteins were transferred to a polyvinylidene difluoride membrane (Bio-Rad). The membrane was blocked for 1 h with PBS-0.1% Tween 20 (PBST) containing 5% nonfat dry milk and incubated for 1 h at room temperature with Anti-V5 mouse monoclonal antibody (Invitrogen). After washing with PBST, the membrane was incubated with an anti-mouse IgG antibody conjugated with horseradish peroxidase (HRP) (Santa Cruz Biotechnology, Santa Cruz, CA) and developed with ImmobilonTM Western Chemiluminescent HRP Substrate (Millipore), following the manufacturer's instructions. Blots were also probed with a primary goat anti-actin antibody (Santa Cruz Biotechnology) and a secondary anti-goat IgG-HRP antibody.
Real-time PCR
Total RNA was extracted from human granulocytes, HeLa, INS-1, or RIN-m using the RNeasy micro kit (Qiagen) according to the manufacturer's instructions and reverse transcribed into cDNA using the iScriptTM cDNA Synthesis Kit (Bio-Rad). The cDNA was used as template for real-time PCR analysis: reactions were performed in an iQ5 real-time PCR detection system (Bio-Rad). The human- and rat-specific primers were designed by using Beacon Designer 2.0 software (Bio-Rad), and their sequences were as shown in Table 1.
TABLE 1.
Human- and rat-specific primers used in real-time PCR
| Sequence, 5′–3′ | |
|---|---|
| Human genes | |
| LANCL1 | |
| Forward | TCTCACAACGCTTGACCAATAAG |
| Reverse | GCCCAGCCAGTGTAACCG |
| LANCL2 | |
| Forward | AGGCGTACAAGGTCTTTAAGGAG |
| Reverse | GCTCGGTAGAGGTACTTCTTATCC |
| LANCL3 | |
| Forward | GCTCTGTGTGCCGTCTGC |
| Reverse | CTCCTGGGCGAGTTTCTGC |
| GAPDH | |
| Forward | CCTGTTCGACAGTCAGCCG |
| Reverse | CGACCAAATCCGTTGACTCC |
| HPRT-1 | |
| Forward | GGTCAGGCAGTATAATCCAAAG |
| Reverse | TTCATTATAGTCAAGGGCATATCC |
| Rat genes | |
| LANCL2 | |
| Forward | GGTGCCACGGTGCTCCAG |
| Reverse | CCTCGCTGCCAAATCACATCAC |
| GAPDH | |
| Forward | ATGATTCTACCCACGGCAAG |
| Reverse | CTGGAAGATGGTGATGGGTT |
| β-Actin | |
| Forward | GGGAAATCGTGCGTGACATT |
| Reverse | GCGGCAGTGGCCATCTC |
Each sample was assayed in triplicate in a 25-μl amplification reaction, containing 4 ng of cDNA, primers mixture (0.4 μm each of sense and antisense primers), and 12.5 μl of 2× iQ SYBR Green Supermix Sample (Bio-Rad). The amplification program included 40 cycles of two steps, each comprising heating to 95 °C and to 62 °C, respectively. Fluorescence products were detected at the last step of each cycle. To verify the purity of the products, a melting curve was produced after each run. Values for human genes were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and hypoxanthine-guanine phosphoribosyltransferase-1 mRNA expression, and values for rat genes to GAPDH and β-actin. Statistical analysis of the quantitative real-time PCR was obtained using the iQ5 Optical System Software version 1.0 (Bio-Rad) based on the 2−ΔΔCt method, which calculated relative changes in gene expression of the target normalized to GAPDH and hypoxanthine-guanine phosphoribosyltransferase-1. The quantitative PCR efficiencies were determined by series of 5-fold dilutions for each experiment and each gene, and were always between 90 and 95%. The relative expression of two genes in the same sample was estimated (29).
Absolute quantification of cDNA copy number was achieved using a standard curve constructed from known amounts of DNA amplicons of the relative target, after purification with QIAquick® gel extraction kit (Qiagen) and absorbance determination (30).
Insulin Release
Following siRNA transfection, RIN-m and INS-1 cells were seeded in a 24 well-plate (105 cells/well). After 48 h, the culture medium was replaced, and cells were incubated for 4 h in the low glucose buffer (LG-KRH: 129 mm NaCl, 5 mm NaHCO3, 4.8 mm KCl, 1.2 mm KH2PO4, 1 mm CaCl2, 1.2 mm MgSO4, 10 mm Hepes, 0.5% bovine serum albumin, 2 mm glucose). The buffer was then removed, and cells were incubated for 30 min at 37 °C with fresh LG or high glucose buffer (HG-KRH: same as LG-KRH, mixed with glucose to 20 mm final concentration), in the presence or absence of ABA. The buffer was recovered, centrifuged at 5000 × g for 1 min, and insulin concentration was determined by EIA (Rat Insulin Enzyme Immunoassay Kit, SPI-BIO, Montigny Le Bretonneux, France), following the manufacturer's instruction.
Assays of GDP-ribosyl Cyclase in CD38+ HeLa
CD38+ HeLa were transfected in parallel with the empty plasmid or with the LANCL2 plasmid, as described above. After 24 h, cells were resuspended in HBSS (5 × 106/ml). Ectocellular GDP-ribosyl cyclase activity was measured on nicotinamide guanine dinucleotide, an NAD+ analogue used to determine cyclase activity, because cGDPR is not a substrate of the hydrolase (31). Nicotinamide guanine dinucleotide was added at a final concentration of 0.4 mm with or without 100 nm ABA. Enzymatic activities were carried out at 37 °C: at various times from the addition of the substrate (0, 5, 15, and 30 min), 100-μl aliquots were withdrawn and centrifuged at 5000 × g for 15 s (supernatants were recovered and deproteinized with 2.5% (v/v) trichloroacetic acid). High-performance liquid chromatography analyses of nucleotides to measure the production of cGDPR were performed as described (32). Protein determination (28) was performed on aliquots of each incubation saved prior to trichloroacetic acid addition.
Chemotaxis and Chemokinesis
Granulocytes were resuspended at 107/ml in chemotaxis buffer (HBSS, PBS, and 0.5% albumin, 39:16:1). Chemotaxis and chemokinesis assays were performed using 96-well ChemoTx system microplates (Neuro Probe, Gaithersburg, MD) with a 3-μm pore size polycarbonate filter. For chemotaxis assays, ABA was diluted to 10 μm in chemotaxis buffer and added to the bottom wells. For chemokinesis assays, cells were preincubated for 15 min in the presence or absence of 10 μm ABA; chemotaxis buffer was added in the bottom wells. Cell suspensions (25 μl) were placed directly on top of the filter, and the plates were incubated for 60 min at 37 °C. The transmigrated cells were collected following ChemoTx system instructions, transferred into a 96-well plate, and quantified by adding 60 μl of a solution composed of 0.2% Nonidet P-40 and 1 mm SYTOX Green. After 20 min of incubation at 37 °C, fluorescence was recorded (excitation, 485 nm; emission, 520 nm). A standard curve was obtained by placing a serial dilution of the cell suspension in the bottom wells. The results were expressed as chemotaxis index (chemotaxis index = number of cells migrated toward chemoattractant/number of cells migrated toward medium) and chemokinesis index (chemokinesis index = number of ABA-treated cells migrated toward medium/number of untreated cells migrated toward medium).
Phagocytosis and ROS Production
Twenty-four hours after transfection, phagocytosis and reactive oxygen species (ROS) production were determined on granulocytes (untreated, negative control- and siRNA-transfected) as described before (16).
[3H]ABA Binding
CD38+ HeLa transfected with the control, empty plasmid, or with the LANCL2 plasmid (2 × 105/determination) were incubated in duplicate at 20 °C for 60 min in 100 μl of HBSS with or without excess unlabeled ABA (to determine nonspecific binding), in the presence of increasing concentrations of [3H]ABA. At the end of the incubation, cells were centrifuged (30 s at 5000 × g), the supernatant was discarded, cell pellets were washed once in excess HBSS by centrifugation, and the radioactivity of the cells was determined on a Packard β-counter. The specific [3H]ABA binding was calculated as the difference between total binding and nonspecific binding.
Staining of CD38+ HeLa with Biotinylated ABA and with Anti-V5 mAb
CD38+ HeLa transfected with the empty plasmid or with the LANCL2 plasmid were seeded on eight-wells chambered coverglasses (Lab-Tek, Nalge Nunc International, Naperville, IL) at a cell density of 3 × 104/well. Cells were incubated in 100 μl of HBSS with 10 μm biotinylated ABA (bio-ABA), in the presence or absence of excess unlabeled ABA (1 mm) for 5 min on ice. Cells were then washed with HBSS and incubated for further 5 min on ice with 5 μg/ml Alexa 633-conjugated streptavidin (Alexa-strp, Molecular Probes and Invitrogen). As a control, cells were incubated with Alexa-strp alone.
The anti-V5 peptide monoclonal antibody (mAb) was used for LANCL2 protein staining on transfected HeLa. Briefly, 24 h after transfection cells, previously seeded on eight-well chambered coverglasses at a cell density of 3 × 104/well, were incubated with or without 50 μm cycloheximide to prevent new protein synthesis and then washed twice with PBS and fixed for 10 min with 3.7% paraformaldehyde. After fixation, cells were either permeabilized or not with 0.1% Triton X-100 for 8 min, washed four times, and then incubated with 5 μg/ml anti-V5 mAb in 5% bovine serum albumin-PBS for 30 min. Cells were again washed four times and incubated for 30 min with 1 μg/ml Alexa 488-secondary Ab, then, 5 min before the end of incubation, 1 μg/ml propidium iodide was added. Finally cells were washed four times with PBS and observed at the confocal microscope. As a control, cells were incubated with the Alexa 488-secondary Ab alone.
Images were obtained using a Leica TCS SL confocal microscope equipped with argon/He-Ne laser sources and an HCX PL APO CS 63.0 × 1.40 oil objective. During image acquisition of bio-ABA, the 633 laser was set at 20% energy, the emission range was between 650 and 700 nm, and the photomultiplier voltage gain was set to eliminate cell autofluorescence. During image acquisition of LANCL2-V5, the 488 laser was set at 20% energy, and emission ranges were 500–550 and 600–700 nm for V5-Alexa 488 and propidium iodide, respectively. Single plane images were taken at the center of cell thickness.
RESULTS
Abrogation of the ABA-triggered Signaling Pathway in Human Granulocytes by Silencing of LANCL2
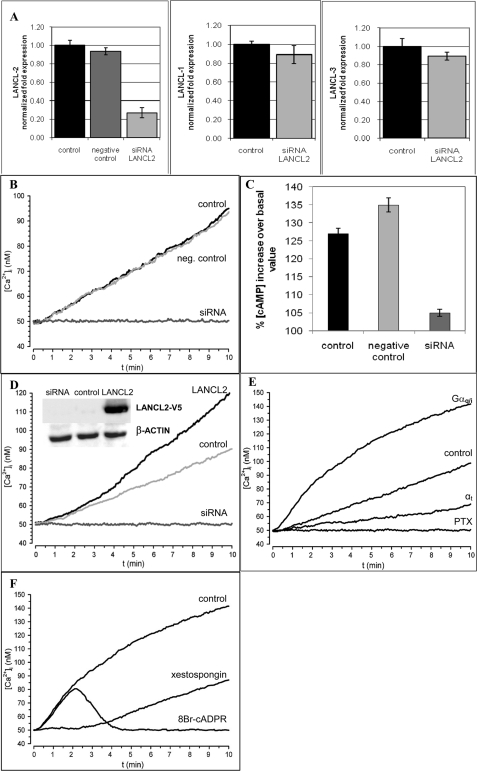
Real-time PCR performed on the cDNA obtained from freshly isolated human granulocytes showed that LANCL2 mRNA is present, in these cells, along with the LANCL1 transcript (1.4 ± 0.2-fold relative to LANCL2, n = 3) and, to a far lesser extent, the LANCL3 transcript (0.17 ± 0.03-fold relative to LANCL2, n = 3). Granulocytes were transfected with either one of three specific small interfering RNAs (siRNAs#1–3) for LANCL2 and in parallel with a negative control siRNA. Similar results were obtained with the three different LANCL2-specific siRNAs: thus, only results obtained with siRNA#1 are shown in Fig. 1. After 24 h, LANCL2 mRNA levels were decreased to ∼25% compared with control cells (granulocytes electroporated in the absence of siRNA), as revealed by real-time PCR analysis (Fig. 1A). Conversely, neither LANCL1 nor LANCL3 transcription were significantly affected by LANCL2 silencing (Fig. 1A). The presence of a negative control siRNA did not induce any significant modification of LANCL2 mRNA levels (Fig. 1A). The ABA-induced Ca2+ response in Fura2/AM-loaded granulocytes was measured 24 h after transfection. As the Ca2+ response of granulocytes to ABA is concentration-dependent (16), cells were stimulated with 10 μm ABA to obtain a maximal [Ca2+]i increase. As shown in Fig. 1B, silencing of LANCL2 was accompanied by abrogation of the ABA-triggered Ca2+ response, whereas the [Ca2+]i rise induced by ABA in cells transfected with the negative control siRNA was comparable to that of control cells. LANCL2 silencing also abrogated the ABA-triggered increase of the [cAMP]i in human granulocytes, whereas the [cAMP]i increase was comparable in control and in negative control-transfected cells (Fig. 1C).
FIGURE 1.
LANCL2-expression modifications alter the ABA-induced increase of the [Ca2+]i and of the [cAMP]i in human granulocytes. After 24 h from transfection, control (electroporated in the absence of siRNA), negative control siRNA-transfected, or LANCL2 siRNA-transfected cells were treated as follows: A, subjected to total RNA extraction and real-time PCR were performed (n = 3); B, loaded with Fura2/AM and exposed to 10 μm ABA (to obtain maximal response (16)) at 20 °C. The [Ca2+]i was measured as described in Ref. 16. Each microscopic field contained ≥30 cells. Representative traces from three experiments, yielding closely comparable results, are shown; C, challenged with 10 μm ABA and processed for measurement of [cAMP]i levels (at 60 s). Results are expressed as percentage increase over basal values, measured at time = 0 (n = 3). D, 24 h after transfection, control granulocytes (electroporated in the presence of the empty vector pcDNA6.2), LANCL2-transfected granulocytes, and granulocytes transfected simultaneously with the empty plasmid and with the siRNA for LANCL2 (siRNA) were processed for [Ca2+]i measurement and challenged with 10 μm ABA (added at time = 0). Representative traces from three experiments, yielding closely comparable results, are shown. Inset: Western blot analysis of cell lysates from control, LANCL2-transfected granulocytes or empty plasmid/siRNA-transfected granulocytes stained with a monoclonal antibody against the V5 epitope (see “Experimental Procedures”). E, 24 h after transfection, granulocytes transfected with Gαq/i, control granulocytes (electroporated in the presence of the empty vector), granulocytes transfected with αt, and granulocytes transfected with the empty vector and preincubated with PTX, 2 μg/ml, were processed for [Ca2+]i measurement and challenged with 10 μm ABA (added at time = 0). Representative traces from three experiments, yielding closely comparable results, are shown. F, 24 h after transfection, control granulocytes (electroporated in the presence of the empty vector) and granulocytes transfected with Gαq/i preincubated with 1 μm xestospongin or with 100 μm 8-Br-cADPR, were processed for [Ca2+]i measurement and challenged with 10 μm ABA (added at time = 0). Representative traces from three experiments, yielding closely comparable results, are shown.
Amplification of the ABA-induced [Ca2+]i Rise by Overexpression of LANCL2 in Human Granulocytes
Human granulocytes were transfected with the plasmid vector pcDNA6.2/V5/GW/ D-TOPO© containing the full-length LANCL2 cDNA. In parallel, granulocytes were also transfected with the empty plasmid (control) or with the same plasmid in the presence of the siRNA#1 for LANCL2. After 24 h, Western blot analysis of total cell lysates stained with an antibody against the tag epitope V5 at the C-terminal of LANCL2 demonstrated the efficient expression of recombinant LANCL2 (inset to Fig. 1D). The ABA-induced Ca2+ response in granulocytes expressing recombinant LANCL2 was significantly increased over the [Ca2+]i rise observed in granulocytes transfected with the empty plasmid (Fig. 1D). The [Ca2+]i values measured after 10 min from the addition of 10 μm ABA were 122 ± 11 nm and 90 ± 4 nm for LANCL2- and for empty plasmid-transfected cells, respectively (n = 3, p < 0.01 by t test). Cells transfected with the empty vector and silenced for LANCL2 did not respond to ABA (Fig. 1D), confirming that silencing of LANCL2 abrogates the ABA-induced Ca2+ response in human granulocytes. The steady [Ca2+]i increase triggered by ABA on LANCL2 overexpressing granulocytes reached a plateau after 30–40 min (not shown), similarly to what observed in untransfected granulocytes (16).
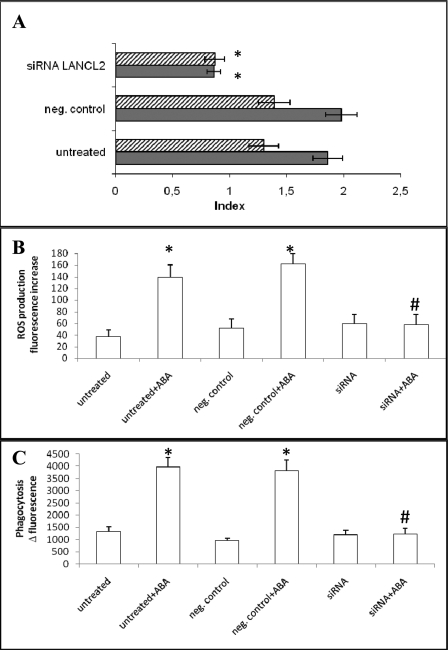
Abrogation of ABA-induced Functional Effects in Granulocytes by LANCL2 Silencing
The chemotactic response of granulocytes to ABA is concentration-dependent (16). Thus, to induce maximal cell migration, 10 μm ABA was placed in the bottom well of a chemotaxis migration chamber. LANCL2 silencing abrogated the ABA-induced chemotaxis (Fig. 2A).
FIGURE 2.
LANCL2 silencing abrogates ABA-induced functional effects in human granulocytes. After 24 h from transfection, untreated, negative control siRNA-transfected, or LANCL2 siRNA-transfected granulocytes were subjected to the following functional assays, following stimulation with 10 μm ABA: A, chemotaxis (gray bars) and chemokinesis (striped bars) assays; *, p < 0.05 by Tukey test, compared with negative control and untreated cells; B, measurement of ROS production; C, phagocytosis of fluorescent latex beads. *, p < 0.01 and #, p > 0.5 by t test, compared with the respective unstimulated cell type (n = 3).
To assess the effect of ABA on untargeted cell movement (chemokinesis), granulocytes were preincubated with 10 μm ABA and then placed on top of the filter of a chemotaxis chamber containing buffer in the bottom wells: cell migration through the filter was again abolished in LANCL2-silenced cells compared with untreated controls and negative control-transfected granulocytes (Fig. 2A). Finally, LANCL2 silencing also abrogated the ABA-induced stimulation of ROS production (Fig. 2B) and of particle phagocytosis (Fig. 2C) by granulocytes.
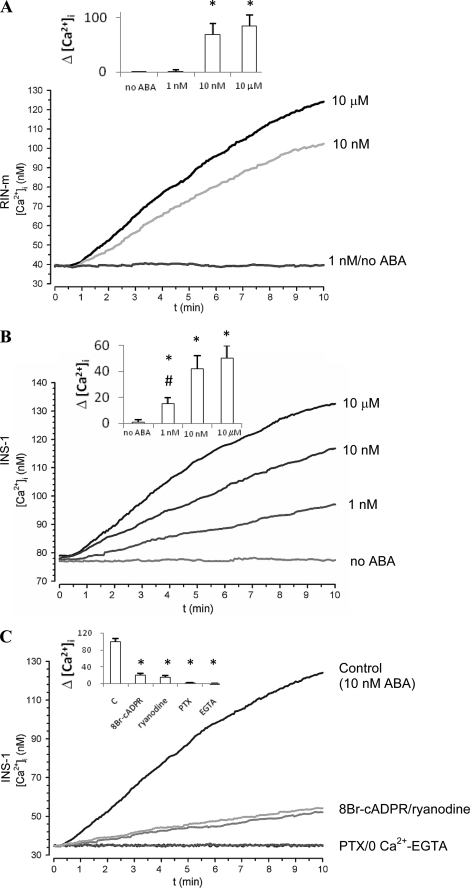
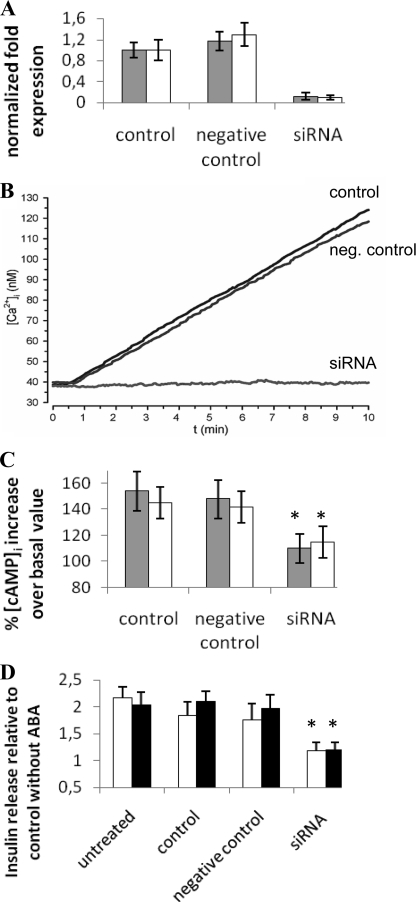
Silencing of LANCL2 in the Rat Insulinoma Cell Lines RIN-m and INS-1 Abrogates the ABA-triggered Signaling Pathway
Nanomolar ABA (1–10 nm) stimulates insulin release from human and murine pancreatic β cells and from RIN-m and INS-1 cells, and this effect is abrogated by PTX, by a protein kinase A-specific inhibitor preventing CD38 phosphorylation, by the cADPR antagonists 8-Br-cADPR and ryanodine and by the intracellular Ca2+ chelator EGTA- AM (19). ABA triggers a steady, concentration-dependent increase of the [Ca2+]i in RIN-m and INS-1 cells (Fig. 3, A and B), which is abrogated in the presence of PTX and severely reduced (by ≥80%) by the cADPR antagonists 8-Br-cADPR and ryanodine and by EGTA (Fig. 3C). Thus, in insulin-secreting cells the ABA-triggered [Ca2+]i rise is primarily due to a cADPR-dependent extracellular Ca2+ influx, similarly to what observed in human granulocytes (16). The rat insulinoma cells RIN-m and INS-1, as well as human and murine pancreatic β cells, proved to express LANCL2, as demonstrated by real-time PCR analysis (not shown). RIN-m and INS-1 cells were transfected with a specific siRNA for rat LANCL2 and in parallel with a negative control siRNA. Closely comparable results were obtained on the two insulinoma cell lines (Fig. 4). After 48 h, LANCL2 mRNA levels were decreased by ∼90% compared with control cells (electroporated in the absence of siRNA), as revealed by real-time PCR analysis (Fig. 4A). The presence of a negative control siRNA did not induce any significant modification of LANCL2 mRNA levels (Fig. 4A). The Ca2+ response to 10 μm ABA (i.e. the maximal response, Fig. 4B) was measured in Fura2/AM-loaded cells 48 h after transfection: silencing of LANCL2 was accompanied by abrogation of the ABA-triggered Ca2+ rise, whereas the [Ca2+]i increase induced by ABA in cells transfected with the negative control siRNA was comparable to that of control cells (Fig. 4B). The effect of LANCL2 silencing on the ABA-triggered increase of the [cAMP]i was also explored. The ABA-induced [cAMP]i increase was comparable in control cells (electroporated in the absence of siRNA) and in negative control-transfected cells (Fig. 4C), whereas it was abrogated in LANCL2 siRNA-transfected cells. Finally, LANCL2 silencing abrogated the ABA-triggered insulin release in both high and low glucose, while transfection with the negative control siRNA was without effect (Fig. 4D).
FIGURE 3.
ABA-induced [Ca2+]i increase in RIN-m and INS-1 cells. Fura2/AM-loaded RIN-m (A) and INS-1 (B) were treated with the indicated concentrations of ABA in HBSS (with Ca2+); insets to panels: difference between the [Ca2+]i values recorded at 10 min and at time = 0 (Δ[Ca2+]i) for each experimental condition. *, p < 0.05 by Tukey test, compared with the Δ[Ca2+]i without ABA; #, p < 0.05 by Tukey test, compared with the Δ[Ca2+]i with 10 nm and 10 μm ABA. C, Fura2/AM-loaded INS-1 were treated with 10 nm ABA in Ca2+ buffer (HBSS, control) or in Ca2+-free HBSS, containing 0.05 mm EGTA (0 Ca2+-EGTA), or preincubated for 30 min with 50 μm 8-Br-cADPR, 50 μm ryanodine, or 2 μg/ml PTX prior to ABA addition. Inset to C: Δ[Ca2+]i for each condition. *, p < 0.05 by Dunnett's method compared with Δ[Ca2+]i in the control. [Ca2+]i changes were measured at 20 °C, on microscopic fields containing ∼25 cells. A similar kinetics of the Ca2+ increase was observed on single cells (not shown). Representative traces from individual experiments are shown (n = 4). To verify trace stability, fluorescence signals were monitored for at least 5 min before ABA was added (at time = 10 s in all traces shown).
FIGURE 4.
LANCL2 silencing impairs the ABA-induced increase of the [Ca2+]i, of the [cAMP]i, and of insulin release in the rat insulinoma cell lines INS-1 and RIN-m. After 48 h from transfection, control (electroporated in the absence of siRNA), negative control siRNA-transfected, or LANCL2 siRNA-transfected cells were treated as follows: A, RIN-m (white bars) or INS-1 cells (gray bars) were subjected to total RNA extraction and real-time PCR analysis of LANCL2. Expression of LANCL2 is normalized to that of the housekeeping genes GAPDH and β-actin (n = 3); B, INS-1 cells were loaded with Fura2/AM and exposed to 10 μm ABA at 20 °C. The [Ca2+]i was measured as described before (25). Each microscopic field contained ≥25 cells. Representative traces from three experiments, yielding closely comparable results, are shown. Similar traces were obtained with RIN-m cells (not shown). C, RIN-m (white bars) or INS-1 cells (gray bars) cells were challenged with 10 μm ABA and extracted after 60 s for measurement of [cAMP]i levels. Results are expressed as percentage increase over basal values, measured at time = 0 (n = 3). D, INS-1 cells were incubated for 30 min at 37 °C in LG- (white bars) or HG-KRH (black bars), in the absence or in the presence of 100 nm ABA. Results are expressed as insulin secretion over unstimulated samples and are the mean ± S.D. of three different experiments. Untreated cells were not electroporated. Similar results were obtained with RIN-m cells (not shown). Basal insulin secretion was 4.0 ± 0.8 and 33.3 ± 7.5 ng/106 cells/30 min in RIN-m and INS-1 cells, respectively. *, p < 0.01 by t test, compared with the respective negative control (transfected with negative control siRNA).
Reproduction of the Granulocyte ABA-signaling Pathway by Expression of LANCL2 in CD38-transfected HeLa Cells
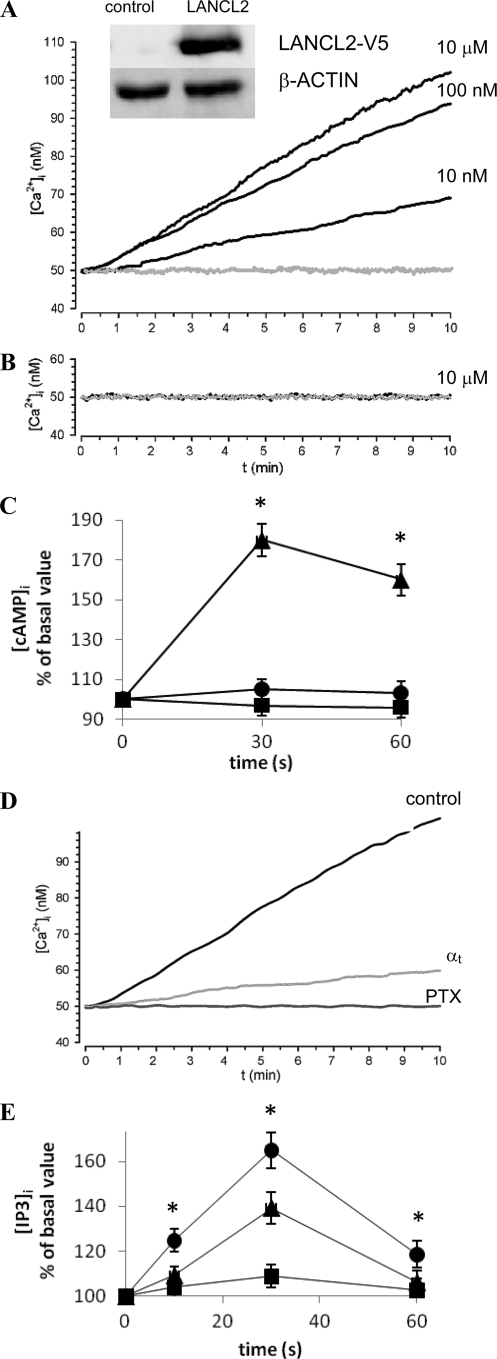
We tried to reproduce the ABA-signaling pathway of human granulocytes and of the rat insulinoma cell lines in an ABA-unresponsive cell line. The human ovarian carcinoma cell line HeLa does not express detectable ADPRC activity (25), and HeLa transfected with the ADPRC of human granulocytes, CD38, express basal levels of LANCL2 detectable by real-time PCR (Supplemental data, Fig. 1), which proved however functionally insufficient to transduce the ABA signal into a detectable increase of the [Ca2+]i (not shown). Thus, HeLa transfected with CD38 sense (CD38+) or antisense (CD38−) (25), were further transfected with LANCL2. After 24 h from transfection with the empty plasmid or with LANCL2 plasmid, Western blot analysis on cell lysates confirmed expression of recombinant LANCL2 in CD38+ (inset to Fig. 5A) and CD38− (not shown) HeLa. Addition of 10 μm ABA to CD38− HeLa transfected with either the empty vector or with the LANCL2 plasmid did not affect the [Ca2+]i (Fig. 5B). Conversely, ABA induced a concentration-dependent, sustained [Ca2+]i increase in CD38+ HeLa co-expressing LANCL2 (Fig. 5A, black traces). Finally, the [Ca2+]i of CD38+ HeLa electroporated in the presence of the empty plasmid was not affected by ABA (Fig. 5A, gray trace). Thus, only the concomitant presence of LANCL2 and of CD38 allows generation of the ABA-triggered [Ca2+]i increase.
FIGURE 5.
ABA induces an increase of the [Ca2+]i and of the [cAMP]i in HeLa co-transfected with CD38 and LANCL2 through a Gi-dependent mechanism. 24 h after transfection with the empty vector (gray traces) or with LANCL2 plasmid (black traces), CD38+ (A) or CD38− (B) HeLa were loaded with Fura2/AM and exposed to the indicated ABA concentrations at 20 °C. The [Ca2+]i was measured as described before (16). Each microscopic field contained ≥20 cells. Representative traces from three experiments, yielding closely comparable results, are shown. Inset: Western blot analysis of cell lysates from control (CD38+) and CD38+/LANCL2+ HeLa stained with a monoclonal antibody against the V5 epitope. C, CD38+ HeLa transfected with LANCL2 (CD38+/LANCL2+) were preincubated without (triangles), or with (squares) 2 μg/ml PTX for 1 h, then challenged with 100 nm ABA and processed for measurement of [cAMP]i levels. Alternatively, CD38+/LANCL2+ HeLa were further transfected with αt (circles) and then challenged with 100 nm ABA. No increase of the [cAMP]i was observed upon addition of ABA in the following controls: CD38+ HeLa transfected with the empty vector for LANCL2; CD38+ HeLa transfected with αt alone (in the absence of LANCL2) (not shown). *, p < 0.01 by t test, compared with values measured in cells transfected with the empty vector, or with αt, or compared with values measured in PTX-preincubated cells. D, CD38+ HeLa were transfected with LANCL2-pcDNA6.2/V5/GW/D-TOPO© and without, control or with αt (pCS2+/α-transducin). After 24 h, cells were loaded with Fura2/AM, preincubated for 1 h at 37 °C without or with 2 μg/ml PTX, and then exposed to 100 nm ABA at 25 °C. The [Ca2+]i was measured as described before (19). Each microscopic field contained ∼20 cells. Representative traces from three experiments, yielding closely comparable results, are shown. E, CD38+/LANCL2+ HeLa were transfected, or not (triangles), with Gαq/i (circles) and then challenged with 100 nm ABA and processed for measurement of [IP3]i levels. No significant increase of the [IP3]i was observed upon addition of ABA to CD38+ HeLa transfected with Gαq/i alone (in the absence of LANCL2, squares). *, p < 0.05 by t test, compared with values measured in cells not transfected with Gαq/i. Results in C and D are expressed as percentage increase over basal values, measured at time = 0 for each cell type (n = 4).
ADPRC activity of CD38+/LANCL2+ HeLa was activated following addition of 100 nm ABA from a basal value of 15.5 ± 0.4 to 20.1 ± 0.5 nmol of cGDPR/min/mg of protein after 5 min (n = 3; p < 0.01 by paired t test), confirming that ADPRC stimulation follows ABA binding, similarly to what observed in human granulocytes (16).
Finally, the [cAMP]i increased rapidly in CD38+/LANCL2+ HeLa upon addition of 100 nm ABA, the highest increase over basal values (180%) being measured after 30 s, and the [cAMP]i remained elevated (160% of basal values) at 1 min (Fig. 5C). Conversely, the [cAMP]i was not significantly modified upon addition of ABA to CD38+ HeLa transfected with the empty vector (not shown).
LANCL2 Coupling to Gi in Granulocytes and LANCL2+/CD38+ HeLa
Pre-treatment with PTX abrogated the [cAMP]i increase triggered by ABA in LANCL2+/CD38+ HeLa (Fig. 5C), similarly to what already observed in human granulocytes (16) and in human and rat insulin-releasing cells (19). Sensitivity of the [cAMP]i increase to PTX points to Gi as the G-protein coupled to LANCL2: activation of adenylic cyclase (AC) by Gi has been reported to occur via a synergistic mechanism involving (i) interaction of AC with the βγ subunits released from activated Gi and (ii) AC phosphorylation by protein kinase C (33). Indeed, a protein kinase C-specific inhibitor down-regulates the ABA-triggered increase of the [cAMP]i in human granulocytes (16).
To verify the involvement of βγ subunits in the activation of AC induced by ABA in CD38+/LANCL2+ HeLa, cells were transfected with the pCS2+ plasmid encoding α-transducin (αt), which is known to function as a scavenger of free βγ (33, 34). Indeed, the [cAMP]i increase and the [Ca2+]i rise triggered by ABA in CD38+/LANCL2+ HeLa were abrogated or strongly reduced, respectively, following transfection of the cells with αt (Fig. 5, C and D). Transfection of granulocytes with αt also significantly reduced (by ∼60%) the ABA-triggered [Ca2+]i increase compared with controls, transfected with the empty vector (Fig. 1E).
To obtain further evidence that LANCL2 is coupled to Gi, granulocytes and CD38+/LANCL2+ HeLa were also transfected with the pcDNAI encoding for a chimeric G-protein, resulting from the fusion of Gαq with the last 5 amino acids of Gαi (Gαq/i). This G-protein allows the coupling to PLC of receptors normally activating Gαi (35). Indeed, CD38+/LANCL2+ HeLa transfected with Gαq/i responded to ABA with a significantly higher increase of the [IP3]i compared with controls (Fig. 5E). Similarly to what observed in CD38+/LANCL2+/Gαq/i+ HeLa, the [IP3]i increased 76 ± 5% and 37 ± 4% over time zero values at 10 and 30 s, respectively, in Gαq/i-transfected granulocytes upon addition of ABA, while at the same time points the [IP3]i increase was significantly lower in granulocytes transfected with the empty vector and stimulated with ABA (28 ± 2% and 3 ± 1%, respectively, n = 3). Activation of PLC by Gi (34, 36), is mediated by the βγ subunits released from activated Gi (34). Activation of AC and of PLC by βγ subunits is less efficient than activation by Gαs and Gαq, respectively (33): indeed, the [cAMP]i and the [IP3]i increase ∼1.7-fold in CD38+/LANCL2+ HeLa stimulated with ABA (Fig. 5, C and E). In granulocytes transfected with Gαq/i, addition of ABA triggered a significantly higher sustained increase of the [Ca2+]i compared with cells transfected with the empty vector (Fig. 1E). Xestospongin, an inhibitor of IP3-mediated Ca2+ release, inhibited the ABA-triggered [Ca2+]i increase in Gαq/i-transfected granulocytes by ∼70% (Fig. 1F), i.e. to a greater extent compared with controls transfected with the empty vector (15 ± 4%) (n = 4). The apparent lag preceding the slow [Ca2+]i increase in xestospongin-pretreated cells suggested a major contribution by IP3 to the initial Ca2+ rise in Gαq/i-transfected granulocytes. Indeed, preincubation of Gαq/i-transfected granulocytes with 8-Br-cADPR, an antagonist of cADPR, revealed a fast and transient [Ca2+]i rise immediately upon addition of ABA, which was not followed by a sustained Ca2+ increase (Fig. 1F). These results suggest that two mechanisms underlie the ABA-triggered Ca2+ increase in Gαq/i-transfected granulocytes: a transient, IP3-dependent, xestospongin-sensitive Ca2+ increase, followed by a sustained, cADPR-mediated Ca2+ rise.
Altogether, results obtained on Gαq/i-transfected granulocytes and HeLa suggest that LANCL2 is coupled to Gi, enabling the ABA-induced activation of both PLC and AC. The formylmethionylleucylphenylalanine receptor in granulocytes is also mainly coupled to Gi, and ligand binding induces an increase of both the [IP3]i and the [cAMP]i (37, 38).
ABA Binding to CD38+/LANCL2+ HeLa
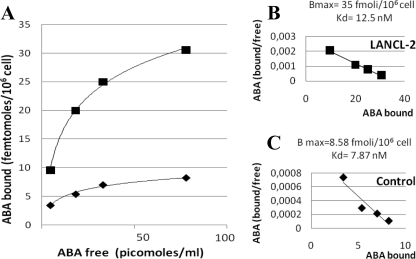
The presence of ABA-binding sites in CD38+/LANCL2+ HeLa was investigated with radioactive ABA. Intact cells were incubated with [3H]ABA at increasing concentrations, in the presence or absence of excess unlabeled ABA. The specific binding of ABA to CD38+/LANCL2+ HeLa was saturated with increasing concentrations of ABA; the saturation curve was much higher compared with that of control CD38+ HeLa, transfected with the empty plasmid (Fig. 6A). Scatchard plot analysis of the results confirmed presence of ABA-binding sites in LANCL2+ HeLa (Fig. 6B). The Kd of the binding site was 12.5 nm, similar to that described in granulocytes (16), and the number of ABA-binding sites per cell, as calculated from the Bmax value, was 2.1 × 104, significantly higher than that of CD38+ HeLa transfected with the empty vector (5.1 × 103, Fig. 6C). The Kd in cells transfected with the empty vector (∼8 nm) was similar to that in LANCL2+ cells, in line with presence of endogenous LANCL2 expression (supplemental Fig. S1). Both ABA enantiomers, (+)- and (−)-ABA, were equally effective in displacing [3H]ABA (not shown), in line with the previous observations that they induced a similar [Ca2+]i response in human granulocytes (16) and in human pancreatic islets (19).
FIGURE 6.
Binding of [3H]ABA to CD38+/LANCL2+ HeLa. 24 h after transfection of CD38+ HeLa with the empty vector pcDNA6.2/V5/GW/D-TOPO© (control, rhombus) or with LANCL2-pcDNA6.2/V5/GW/D-TOPO© (LANCL2, squares), cells were incubated at 20 °C for 60 min in the presence of increasing concentrations of [3H]ABA, with or without excess unlabeled ABA. Radioactivity of washed cell pellets was determined on a Packard β-counter. A, ABA binding (n = 5); B and C, Scatchard plot analysis of ABA-binding sites.
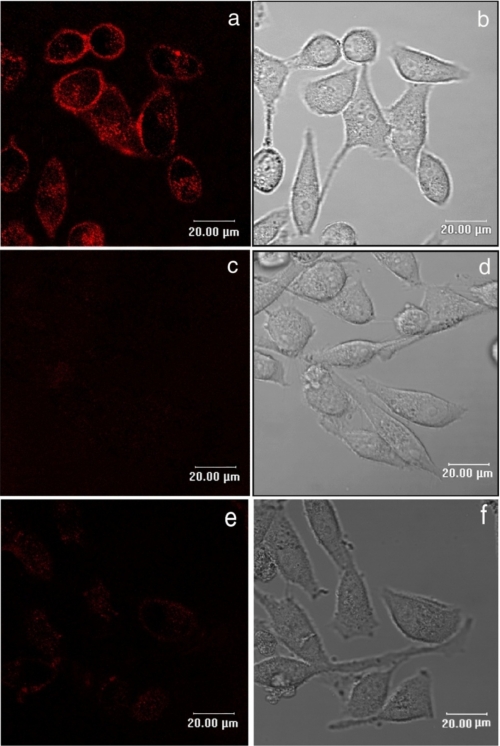
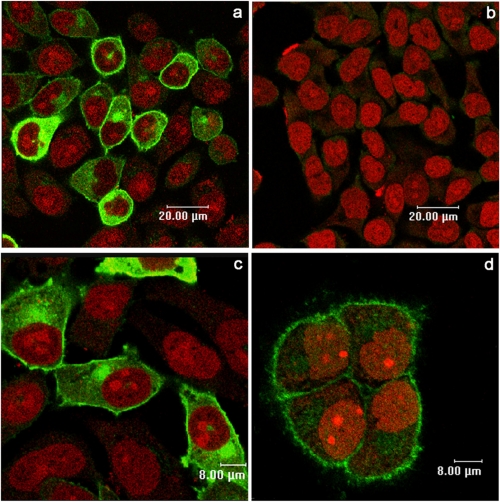
Finally, the presence of plasmamembrane ABA-binding sites on CD38+/LANCL2+ HeLa was investigated by fluorescence microscopy with bio-ABA. CD38+/LANCL2+ HeLa were incubated with bio-ABA and subsequently with Alexa-strp: surface fluorescence was indeed detected in LANCL2+ cells (Fig. 7a), and excess unconjugated ABA prevented cell staining (Fig. 7c). CD38+ HeLa transfected with the empty vector did not show detectable surface fluorescence with bio-ABA (Fig. 7e), confirming the presence of low levels of endogenous ABA-binding sites, in line with the absence of a [Ca2+]i increase in response to ABA (Fig. 5A, gray trace). As Alexa-strp (molecular mass 60 kDa) is unlikely to cross the plasmamembrane, these results suggest that the ABA-binding sites on LANCL2+ HeLa are exposed on the extracellular side of the plasmamembrane. Conversely, intracellular staining of LANCL2+ HeLa was observed after incubation of permeabilized (Fig. 8a), but not of intact cells (Fig. 8b), with the anti-V5 mAb. The diffuse cytoplasmic fluorescence observed in permeabilized LANCL2+ cells was attributable to accumulation of overexpressed LANCL2 in the cytoplasm: when protein synthesis was inhibited with cycloheximide for 6 h prior to cell staining, cytoplasmic fluorescence was reduced and membrane fluorescence became more evident (Fig. 8d). Altogether, these results indicate that the C-terminal of LANCL2 is intracellular, whereas the ABA-binding sites on LANCL2+ HeLa are extracellular: if ABA binding occurs directly on LANCL2, this protein should then span the plasmamembrane. Alternatively, LANCL2 is necessary for the activity of an ABA-binding protein complex, where the ABA-binding domain is extracellular and LANCL2 lies on the intracellular side of the plasmamembrane.
FIGURE 7.
Binding of bio-ABA to CD38+/LANCL2+ HeLa cells. 24 h after transfection with the empty vector (e and f), or with LANCL2 plasmid (a–d), cells were incubated with 10 μm bio-ABA, in the absence (a and e) or in the presence (c and d) of excess unlabeled ABA (1 mm) for 5 min at 0 °C. Cells were then washed and incubated with Alexa 633-strp for a further 5 min. Images are from one representative experiment out of four, giving closely comparable results. No fluorescence was detected on cells incubated with Alexa-strp alone (not shown). b, d, and f are phase contrast images of a, c, and e, respectively. Images are from one representative experiment out of three performed, yielding closely comparable results.
FIGURE 8.
Staining of intact and permeabilized CD38+/LANCL2+ HeLa with anti-V5 mAb. 24 h after transfection with LANCL2 plasmid, cells were incubated for 6 h in the absence (a, b, and c), or in the presence (d) of 100 μm cycloheximide, to prevent protein synthesis. Subsequently, cells were fixed and either permeabilized (a, c, and d) or not (b) with 0.1% Triton X-100, and then stained with the anti-V5 peptide mAb, followed by Alexa 488 secondary antibody (green), while nuclei were highlighted in red with propidium iodide. Images are from one representative experiment out of three performed, yielding closely comparable results.
DISCUSSION
The following lines of evidence support the conclusion that LANCL2 is necessary for ABA binding and signaling in mammalian cells: (i) silencing of LANCL2 abrogates the ABA-induced increase of the [Ca2+]i and of the [cAMP]i in human granulocytes (Fig. 1) and also prevents the ABA-triggered stimulation of cell migration, ROS production, and phagocytosis (Fig. 2); (ii) LANCL2 overexpression conversely potentiates the [Ca2+]i increase induced by ABA in granulocytes (Fig. 1D) and confers ABA responsiveness (i.e. [Ca2+]i and [cAMP]i increase, Fig. 5) to CD38+ HeLa; and (iii), finally, LANCL2 silencing abrogates the ABA-induced biochemical (increase of the [Ca2+]i and of the [cAMP]i) and functional (insulin release) responses also in two rat insulinoma cell lines (Fig. 4). Thus, LANCL2 mediates the effects of ABA in four different cell types (granulocytes, HeLa cells, and RIN-m and INS-1 cells) from two mammalian species. Admittedly, in the absence of a direct evidence of ABA binding to LANCL2, we cannot rule out the possibility that LANCL2 is required for the binding of ABA to a multicomponent ABA receptor complex, but is not directly responsible for ABA binding.
Regarding the plasmamembrane localization of LANCL2, the C-terminal of LANCL2 lies intracellularly, because it is stained by the specific mAb in permeabilized cells only (Fig. 8). Whether LANCL2 spans the plasmamembrane cannot be inferred from these results. If LANCL2 directly binds ABA, then it would be a transmembrane protein, because binding of biotinylated ABA occurs on the extracellular side of the plasmamembrane of LANCL2+ HeLa (Fig. 7). The reported N-myristoylation of LANCL2 (15) would not contrast with a transmembrane localization of the protein, because lipid modifications of G-protein-coupled receptors have been already observed (39, 40). The fact that the procaryotic homolog of LANCL2, NisC, is a soluble protein does not allow to draw a similar conclusion regarding LANCL2: a 30% sequence homology, as observed between LANCL2 and NisC, is also present between the Aplysia californica ADPRC and the mammalian ADPRC CD38: nonetheless, the Aplysia cyclase is a soluble protein, whereas the mammalian homolog is a transmembrane protein (41).
LANCL2 appears to be coupled to Gi. This conclusion is sustained by the following results: (i) abrogation by PTX of the ABA-triggered [cAMP]i rise in human granulocytes (16), in rat insulinoma cells (Fig. 3C) and in CD38+/LANCL2+ HeLa (Fig. 5C); (ii) inhibition of the ABA-induced [Ca2+]i rise in granulocytes (Fig. 1E) and in CD38+/LANCL2+ HeLa (Fig. 5D) transfected with αt, a scavenger of βγ subunits; (iii) stimulation of the [IP3]i rise induced by ABA in granulocytes and in CD38+/LANCL2+ HeLa transfected with Gαq/i (“Results” and Fig. 5E). Admittedly, none of these results is a direct evidence that LANCL2 and Gi physically interact. Recently, Pandey et al. (42) identified two Arabidopsis proteins endowed with ABA binding and with endogenous GTPase activity, apparently functioning as a new class of membrane-bound receptor. The fact that transfection of granulocytes with Gαq/i increased the ABA-triggered IP3 production could not be explained if LANCL2 per se did activate PLC. Thus, it appears that several different models of receptor-G-protein complex have evolved in Metaphyta and Metazoa to transduce the ABA signal. LANCL2 coupling to Gi suggests that ABA sensing in mammals occurs via a receptor-G-protein complex. The ABA receptor GCR2 was shown to be coupled to a G-protein in Arabidopsis (3). An ABA-binding protein endowed with endogenous GTP binding and GTPase activity was also described in Arabidopsis (42). On the one hand, presence of multiple ABA-sensing protein complexes may help explain the fact that loss-of-function mutants of a single receptor may not show a drastic reduction of ABA-sensing capacity (4, 5). On the other hand, the ancient evolutionary origin of ABA as a stress signal may justify this apparent redundancy of ABA receptor types. A primitive ABA-binding protein, also functioning as a G-protein-like signal transducer, may have evolved into G-protein-coupled receptor(s). Presence of multiple ABA-binding proteins in mammals cannot be ruled out: indeed, both high and low affinity ABA-binding sites have been observed in human granulocytes (16). The Kd of ABA binding to LANCL2 in granulocytes and in LANCL2+ HeLa is ∼10 nm: this high affinity is in agreement with the fact that nanomolar concentrations of the hormone elicit an almost maximal Ca2+ response and functional effects in several cell types (pancreatic islets, insulinoma cells, and microglia (19, 43)).
LANCL2 expression on ABA-responsive human-, murine-, and rat insulin-releasing cells, as well as on granulocytes, is particularly significant. Because ABA has been shown to be released by activated human granulocytes (16) and monocytes (20), paracrine generation of ABA by these inflammatory cells may result in potentiation of insulin secretion under conditions of chronic inflammation, such as those occurring in the adipose tissue and believed to be pathogenetic of the metabolic syndrome and type II diabetes. Prolonged stimulation of β cells by ABA released from inflammatory cells may result in an increased, glucose-independent insulin secretion, eventually leading to insulin resistance (21–24). The functional stimulation by ABA of cells involved in the innate immune response and in the production of the arguably most important mammalian hormone-regulating energy metabolism highlights the conserved role of ABA as a stress hormone from plants to mammals. Identification of LANCL2 as a critical component of the ABA-sensing receptor-G-protein complex in granulocytes and in insulin-releasing cells will enable the screening of compounds, both natural and synthetic, active as ABA antagonists, with potential anti-inflammatory and anti-diabetic applications.
Supplementary Material
Acknowledgments
We thank Dr. Randall Moon for the pCS2+/α-transducin plasmid and Dr. R. A. Nicholas for the pcDNAI/Gαq/i.
This work was supported by grants from Regione Liguria, Ministero dell'Istruzione, dell'Università e della Ricerca, the University of Genova and Fondazione Cassa di Risparmio di Genova e Imperia.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- ABA
- abscisic acid
- [Ca2+]i
- intracellular calcium concentration
- cADPR
- cADP-ribose
- 8-Br-cADPR
- 8-bromo-cADPR
- ADPRC
- ADP-ribosyl cyclase
- ROS
- reactive oxygen species
- PTX
- pertussis toxin
- GDPRC
- GDP-ribosyl cyclase
- αt
- α-transducin
- bio-ABA
- biotinylated ABA
- mAb
- monoclonal antibody
- LANCL
- lanthionine synthetase C-like protein
- GFP
- green fluorescent protein
- siRNA
- small interference RNA
- HBSS
- Hanks' balanced salt solution
- PCA
- perchloric acid
- IP3
- inositol 1,4,5-trisphosphate
- PBS
- phosphate-buffered saline
- HRP
- horseradish peroxidase
- AC
- adenylic cyclase
- strp
- streptomycin
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase.
REFERENCES
- 1.Nambara E., Marion-Poll A. (2005) Annu. Rev. Plant Biol. 56, 165–185 [DOI] [PubMed] [Google Scholar]
- 2.Shen Y. Y., Wang X. F., Wu F. Q., Du S. Y., Cao Z., Shang Y., Wang X. L., Peng C. C., Yu X. C., Zhu S. Y., Fan R. C., Xu Y. H., Zhang D. P. (2006) Nature 443, 823–826 [DOI] [PubMed] [Google Scholar]
- 3.Liu X., Yue Y., Li B., Nie Y., Li W., Wu W. H., Ma L. (2007) Science 315, 1712–1716 [DOI] [PubMed] [Google Scholar]
- 4.Gao Y., Zeng Q., Guo J., Cheng J., Ellis B. E., Chen J. G. (2007) Plant. J. 52, 1001–1013 [DOI] [PubMed] [Google Scholar]
- 5.Guo J., Zeng Q., Emami M., Ellis B. E., Chen J. G. (2008) PLoS ONE 3, e2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCourt P., Creelman R. (2008) Curr. Opin. Plant Biol. 11, 474–478 [DOI] [PubMed] [Google Scholar]
- 7.Johnston C. A., Temple B. R., Chen J. G., Gao Y., Moriyama E. N., Jones A. M., Siderovski D. P., Willard F. S. (2007) Science 318, 914. [DOI] [PubMed] [Google Scholar]
- 8.Illingworth C. J., Parkes K. E., Snell C. R., Mullineaux P. M., Reynolds C. A. (2008) Biophys. Chem. 133, 28–35 [DOI] [PubMed] [Google Scholar]
- 9.Bauer H., Mayer H., Marchler-Bauer A., Salzer U., Prohaska R. (2000) Biochem. Biophys. Res. Commun. 275, 69–74 [DOI] [PubMed] [Google Scholar]
- 10.Sahl H. G., Bierbaum G. (1998) Annu. Rev. Microbiol. 52, 41–79 [DOI] [PubMed] [Google Scholar]
- 11.Mayer H., Bauer H., Prohaska R. (2001) Cytogenet. Cell Genet. 93, 100–104 [DOI] [PubMed] [Google Scholar]
- 12.Katoh M., Katoh M. (2003) Int. J. Mol. Med. 12, 399–404 [PubMed] [Google Scholar]
- 13.Mayer H., Salzer U., Breuss J., Ziegler S., Marchler-Bauer A., Prohaska R. (1998) Biochim. Biophys. Acta 1395, 301–308 [DOI] [PubMed] [Google Scholar]
- 14.Park S., James C. D. (2003) Cancer Res. 63, 723–727 [PubMed] [Google Scholar]
- 15.Landlinger C., Salzer U., Prohaska R. (2006) Biochim. Biophys. Acta 1758, 1759–1767 [DOI] [PubMed] [Google Scholar]
- 16.Bruzzone S., Moreschi I., Usai C., Guida L., Damonte G., Salis A., Scarfì S., Millo E., De Flora A., Zocchi E. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 5759–5764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeBrasseur N. (2007) J. Cell Biol. 177, 187 [Google Scholar]
- 18.Wu Y., Kuzma J., Maréchal E., Graeff R., Lee H. C., Foster R., Chua N. H. (1997) Science 278, 2126–2130 [DOI] [PubMed] [Google Scholar]
- 19.Bruzzone S., Bodrato N., Usai C., Guida L., Moreschi I., Nano R., Antonioli B., Fruscione F., Magnone M., Scarfì S., De Flora A., Zocchi E. (2008) J. Biol. Chem. 283, 32188–32197 [DOI] [PubMed] [Google Scholar]
- 20.Magnone M., Bruzzone S., Guida L., Damonte G., Millo E., Scarfì S., Usai C., Sturla L., Palombo D., De Flora A., Zocchi E. (2009) J. Biol. Chem. 284, 17808–17818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dandona P., Aljada A., Bandyopadhyay A. (2004) Trends Immunol. 25, 4–7 [DOI] [PubMed] [Google Scholar]
- 22.Shoelson S. E., Herrero L., Naaz A. (2007) Gastroenterology 132, 2169–2180 [DOI] [PubMed] [Google Scholar]
- 23.de Luca C., Olefsky J. M. (2008) FEBS Lett. 582, 97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muoio D. M., Newgard C. B. (2008) Nat. Rev. Mol. Cell Biol. 9, 193–205 [DOI] [PubMed] [Google Scholar]
- 25.Zocchi E., Daga A., Usai C., Franco L., Guida L., Bruzzone S., Costa A., Marchetti C., De Flora A. (1998) J. Biol. Chem. 273, 8017–8024 [DOI] [PubMed] [Google Scholar]
- 26.Moreschi I., Bruzzone S., Nicholas R. A., Fruscione F., Sturla L., Benvenuto F., Usai C., Meis S., Kassack M. U., Zocchi E., De Flora A. (2006) J. Biol. Chem. 281, 31419–31429 [DOI] [PubMed] [Google Scholar]
- 27.Bruzzone S., De Flora A., Usai C., Graeff R., Lee H. C. (2003) Biochem. J. 375, 395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradford M. M. (1976) Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 29.Ståhlberg A., Zoric N., Aman P., Kubista M. (2005) Expert. Rev. Mol. Diagn. 5, 221–230 [DOI] [PubMed] [Google Scholar]
- 30.Rutledge R. G., Côté C. (2003) Nucleic Acids Res., 31, e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graeff R. M., Walseth T. F., Fryxell K., Branton W. D., Lee H. C. (1994) J. Biol. Chem. 269, 30260–30267 [PubMed] [Google Scholar]
- 32.Guida L., Franco L., Zocchi E., De Flora A. (1995) FEBS Lett. 368, 481–484 [DOI] [PubMed] [Google Scholar]
- 33.Tsu R. C., Wong Y. H. (1996) J. Neurosci. 16, 1317–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dorn G. W., 2nd, Oswald K. J., McCluskey T. S., Kuhel D. G., Liggett S. B. (1997) Biochemistry 36, 6415–6423 [DOI] [PubMed] [Google Scholar]
- 35.Coward P., Chan S. D., Wada H. G., Humphries G. M., Conklin B. R. (1999) Anal. Biochem. 270, 242–248 [DOI] [PubMed] [Google Scholar]
- 36.Kim K., Kim H. L., Lee Y. K., Han M., Sacket S. J., Jo J. Y., Kim Y. L., Im D. S. (2008) Arch. Pharm. Res. 31, 310–317 [DOI] [PubMed] [Google Scholar]
- 37.Gierschik P., Sidiropoulos D., Jakobs K. H. (1989) J. Biol. Chem. 264, 21470–21473 [PubMed] [Google Scholar]
- 38.Simchowitz L., Fischbein L. C., Spilberg I., Atkinson J. P. (1980) J. Immunol. 124, 1482–1491 [PubMed] [Google Scholar]
- 39.Escribá P. V., Wedegaertner P. B., Goñi F. M., Vögler O. (2007) Biochim. Biophys. Acta 1768, 836–852 [DOI] [PubMed] [Google Scholar]
- 40.Utsumi T., Ohta H., Kayano Y., Sakurai N., Ozoe Y. (2005) FEBS J. 272, 472–481 [DOI] [PubMed] [Google Scholar]
- 41.Lee H. C. (ed) (2002) Cyclic ADP-ribose and NAADP; Structure, Metabolism and Functions, Kluver Academic Publisher, Norwell, MA, pp. 23–43 [Google Scholar]
- 42.Pandey S., Nelson D. C., Assmann S. M. (2009) Cell 136, 136–148 [DOI] [PubMed] [Google Scholar]
- 43.Bodrato N., Franco L., Fresia C., Guida L., Usai C., Salis A., Moreschi I., Ferraris C., Verderio C., Basile G., Bruzzone S., Scarfì S., De Flora A., Zocchi E. (2009) J. Biol. Chem. 284, 14777–14787 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.