Abstract
Ca2+/calmodulin-dependent protein kinase II (αCaMKII) is thought to exert its role in memory formation by autonomous Ca2+-independent persistent activity conferred by Thr286 autophosphorylation, allowing the enzyme to remain active even when intracellular [Ca2+] has returned to resting levels. Ca2+ sequestration-induced inhibition, caused by a burst of Thr305/306 autophosphorylation via calmodulin (CaM) dissociation from the Thr305/306 sites, is in conflict with this view. The processes of CaM binding, autophosphorylation, and inactivation are dissected to resolve this conflict. Upon Ca2+ withdrawal, CaM sequential domain dissociation is observed, starting with the rapid release of the first (presumed N-terminal) CaM lobe, thought to be bound at the Thr305/306 sites. The time courses of Thr305/306 autophosphorylation and inactivation, however, correlate with the slow dissociation of the second (presumed C-terminal) CaM lobe. Exposure of the Thr305/306 sites is thus not sufficient for their autophosphorylation. Moreover, Thr305/306 autophosphorylation and autoinactivation are shown to occur in the continuous presence of Ca2+ and bound Ca2+/CaM by time courses similar to those seen following Ca2+ sequestration. Our investigation of the activity and mechanisms of phospho-Thr286-αCaMKII thus shows time-dependent autoinactivation, irrespective of the continued presence of Ca2+ and CaM, allowing a very short, if any, time window for Ca2+/CaM-free phospho-Thr286-αCaMKII activity. Physiologically, the time-dependent autoinactivation mechanisms of phospho-Thr286-αCaMKII (t½ of ∼50 s at 37 °C) suggest a transient kinase activity of ∼1 min duration in the induction of long term potentiation and thus memory formation.
Ca2+/calmodulin-dependent protein kinase II (αCaMKII)2 is essential in hippocampal learning and N-methyl-d-aspartate receptor-dependent synaptic plasticity, causing long term potentiation (1, 2). The exact mechanisms of αCaMKII in memory functions have not yet been identified.
αCaMKII is a broad specificity Ser/Thr protein kinase, which catalyzes the phosphorylation of over 100 protein and peptide substrates in vitro (3). Uniquely, the CaMKII family possesses two distinct kinase mechanisms. The first mechanism is a “canonical” intrasubunit phosphorylation, commonly found in monomeric kinases, in which the phosphorylatable residue of the substrate bound to the helical subdomain of the catalytic domain at the active site is lined up with the terminal phosphate of ATP (4). Although there is a large number of potential “canonical” substrates for αCaMKII at the synapse (5), so far AMPA receptors have been shown to be possible physiological substrates of αCaMKII (6). For the purpose of this study, syntide 2, a commonly used peptide substrate derived from phosphorylation site 2 of glycogen synthase (7), was chosen.
The second mechanism, intersubunit autophosphorylation, takes advantage of the oligomeric organization of CaMKII (8). The most important autophosphorylation site in the α isoform is Thr286, which resides in the vicinity of the autoinhibitory domain (9). Peptide substrates with homologous sequences to this region have been reported to be phosphorylated by αCaMKII. This, however, occurs with a low Vmax, and these substrates show properties of a non-competitive inhibitor with respect to phosphorylation of “canonical” substrates (10) and of Thr286 autophosphorylation itself (11). Examples of such substrates include autocamtide, a peptide substrate derived from the autoinhibitory region (12) and the NR2B subunit of the N-methyl-d-aspartate receptor, which has been identified as a potential physiological target of phospho-Thr286-αCaMKII at the postsynaptic membrane (13). The possible physiological significance of NR2B phosphorylation is not yet known. There is evidence to suggest that Thr286 autophosphorylation is required to achieve full activity of the enzyme, since the unphosphorylatable T286A mutant enzyme has much diminished activity compared with wild type enzyme (14, 15).
Thr286 autophosphorylation causes CaM “trapping,” a >104-fold increase in the affinity of αCaMKII for Ca2+/CaM (16–18). At the same time, Thr286 autophosphorylation is also attributed to confer Ca2+- and CaM-independent persistent “autonomous” kinase activity to αCaMKII. However, due to the extremely high affinity of phospho-Thr286-αCaMKII for Ca2+/CaM, [Ca2+] of <10 nm is required to achieve full dissociation of Ca2+/CaM, since CaM trapping occurs by virtue of Ca2+ trapping (19). Partial activity measured upon partial Ca2+ withdrawal therefore may not always reflect Ca2+/CaM-free enzyme (9). Furthermore, the physiological resting [Ca2+] range is 50–100 nm; therefore, phospho-Thr286-αCaMKII is likely always to have residual Ca2+/CaM bound. This may be partially Ca2+-saturated CaM (19).
Persistent autonomous activity conferred by Thr286 autophosphorylation is thought to enable αCaMKII to function as a memory molecule (20, 21). In contrast, however, following the development of chemical long term potentiation, rapid inactivation has also been reported (22). The extent of an autonomous activity is further obscured by the finding that Ca2+ sequestration induces a burst of autophosphorylation at residues Thr305/306, followed by a loss of activity (23). Moreover, when examined across a broad range of [Ca2+], the Ca2+/CaM dependence of phospho-Thr286-αCaMKII activity is apparent (19). It is thus vital to establish the mechanisms of activation and inactivation of αCaMKII at the molecular level in order to understand how it may function physiologically in learning and memory. To this end, it is necessary to dissect the mechanisms of Ca2+/CaM dissociation, Thr305/306 autophosphorylation, and inactivation of phospho-Thr286-αCaMKII and to establish the time window for autonomous Ca2+/CaM-independent activity.
MATERIALS AND METHODS
Proteins
Mouse αCaMKII (cDNA kindly provided by Dr. D. Brickey and Prof. T. R. Soderling, Vollum Institute, Oregon Health and Science University, Portland, OR) was overexpressed in baculovirus-transfected Sf9 insect cells and purified by CaM-Sepharose and Mono Q fast protein liquid chromatography (17). Rat CaMKIV (cDNA kindly provided by Dr. K. A. Anderson and Prof. A. R. Means, Department of Pharmacology and Cancer Biology, Duke University Medical Center, Durham, NC) was purified by the same method. Human liver recombinant calmodulin, Lys75-FL-calmodulin (FL-CaM), and AEDANS/DDP-T34C/T110C-CaM (DA-CaM) were generated as described previously (17, 24). Mouse monoclonal anti-phospho-Thr286- and rabbit polyclonal anti-phospho-Thr305-αCaMKII antibodies were purchased from Millpore Chemicon. Western blotting was carried out as previously described, and antibodies were used at a 1:1000 dilution (19).
Protein Concentration Measurements
Protein concentrations were determined spectrophotometrically using molar extinction coefficients (ϵo) calculated from the amino acid composition: αCaMKII (subunits), ϵo = 64,805 m−1 cm−1 (280 nm); CaM, ϵo = 3,300 m−1 cm−1 (278 nm); CaMKIV, ϵo = 47,330 m−1 cm−1 (280 nm) (17). The concentration of FL-CaM was determined using ϵo = 70,000 m−1 cm−1 (492 nm) at pH 9.0 (Molecular Probes).
Time Course of Thr286 and Thr305/306 Autophosphorylation of αCaMKII
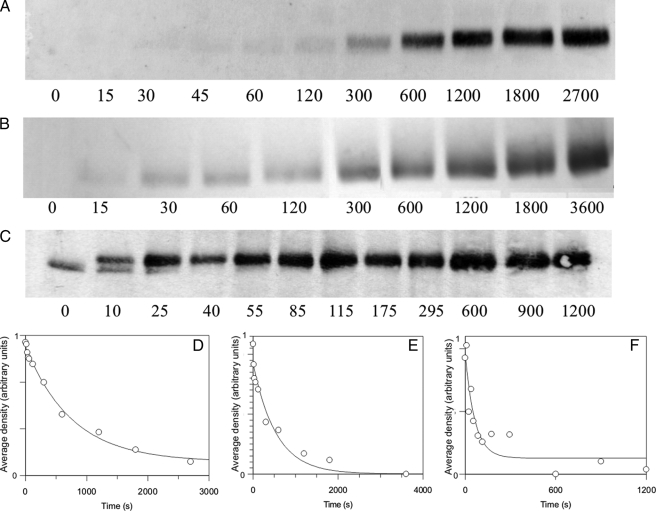
6 μm αCaMKII, 8 μm calmodulin, and 1 mm ATP were incubated in 50 mm K+-PIPES, pH 7.0, 100 mm KCl, 2 mm MgCl2, 5 mm 1,4-dithiothreitol, and 0.05 mm CaCl2 at 21, 30, and 37 °C for various times from 0 to 3600 s. The reaction was either allowed to proceed in the presence of Ca2+, or 4.2 mm EGTA was added at 15 s, and the reaction was terminated using 4× SDS sample buffer at predefined time points. The phospho-Thr286-αCaMKII was detected by Western blotting using a specific mouse monoclonal anti-phospho-Thr286-αCaMKII and anti-phospho-Thr305/306-αCaMKII antibodies as described in Ref. 19. The time course was assessed by densitometry of Western blots using a Fuji Film Luminescent Image Analyzer with LAS4000IR software. Densitometry was carried out using ImageJ software from the National Institutes of Health as follows. The average density reading for each band on the Western blot and a reading for the background was taken. The following formula was used to calculate relative density on an inverted scale: (Dmax − Dt)/(Dmax − DB), where Dmax represents the average density of the darkest band, Dt is the average density at a particular time point, and DB is the average density of the background. The scale was thus inverted by subtracting each value from the highest density value. Relative density was calculated with reference to the highest density, and the data were fitted with an exponential function using the GraFit software program, version 4.0.
Steady-state Assay of αCaMKII Enzyme Activity
A continuous enzyme-linked spectrofluorometric assay was used to determine ADP production by monitoring the decrease in NADH fluorescence due to its oxidation to NAD+. Fluorescence excitation was set to 340 nm, and emission was detected at 460 nm. The experiments were carried out at the specified temperatures. Typically, 5 mm 1,4-dithiothreitol, 4.5 units of lactate dehydrogenase, 2 units of pyruvate kinase, 2 mm phosphoenolpyruvate, 22 μm NADH, 6 μm calmodulin, 50 μm syntide-2, and 1 mm ATP were present in the assay mix. 0.1 μm αCaMKII or phospho-Thr286-αCaMKII was added last to a final volume of 0.05 ml of assay solution (19).
Free Ca2+ concentrations of Ca2+/EGTA mixtures were calculated using a Kd value of 4.35 × 10−7 m, which was determined in buffer conditions and ionic strength similar to those used in this study (25). A Fortran program was written to solve the quadratic equation, [Ca2+] = (b ± √(b2 − 4x [Ca2+]o × [EGTA]o))/2, where b = Kd + [Ca2+]o + [EGTA]o from total Ca2+ [Ca2+]o, total EGTA [EGTA]o, and the Kd. To verify the calculated [Ca2+] concentrations, the fluorescent Ca2+ indicator fluo3 was titrated in Ca2+/EGTA mixtures. Best fit to the titration curve gave a Kd value of 433 ± 55 nm for fluo3, in good agreement with the Kd value of 390 nm previously described for fluo3 (Molecular Probes), thus verifying our calculated [Ca2+] values (19).
Fluorescence Spectroscopy
Stopped-flow kinetic measurement of DA-CaM dissociation was carried out using a Hi-Tech Scientific SF-61DX2 stopped-flow system as follows. Fluorescence excitation was set to 365 nm with a 1-nm slit width, and fluorescence emission from AEDANS was collected using a 400 nm cut-off filter. The assay solution contained 50 mm K+-PIPES, pH 7.0, 100 mm KCl, 2 mm MgCl2. Equilibrium fluorescence measurements of DA-CaM and FL-CaM polarization were carried out using a SLM Model 8000 spectrofluorimeter by ISS, Inc. (champaign, IL).
Software
Data were analyzed using the GraFit software program, version 4.0. Stopped-flow kinetic data were fitted using the KinetAsyst software program (Hi-Tech Scientific).
RESULTS
Thr286 Autophosphorylation in the Presence of an Exogenous “S” Type Peptide Substrate
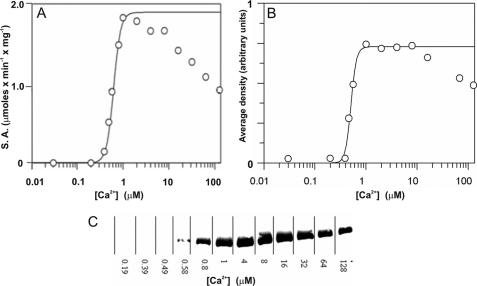
Thr286 autophosphorylation is a rapid reaction that occurs immediately upon Ca2+/CaM and ATP activation of αCaMKII and changes the enzyme properties (19). It was therefore important to know whether αCaMKII in our steady-state assays was Thr286-phosphorylated. To determine the state of αCaMKII with regard to Thr286 autophosphorylation, first the Ca2+ dependence of phosphorylation of exogenous peptide substrate syntide 2 was compared with that of Thr286 autophosphorylation. The Ca2+ dependence of phosphorylation of peptide substrate syntide 2, shown in Fig. 1A, revealed a Km of 612 ± 26 nm for Ca2+, a Vmax of 2.0 ± 0.2 μmol × min−1 × mg of enzyme, and a Hill coefficient (n) of 5.6 ± 0.5 at 25 °C. The Km and n values were similar to those previously determined for the Ca2+ dependence of αCaMKII activation of phosphorylation of protein substrate smooth muscle myosin light chain kinase, whereas the Vmax for syntide 2 was higher than that for smooth muscle myosin light chain kinase (19).
FIGURE 1.
Ca2+ dependence of peptide substrate and Thr286 autophosphorylation by αCaMKII. A, steady-state activity of 50 nm enzyme in the presence of 50 μm syntide 2 as peptide substrate was measured at 25 °C at the indicated Ca2+ concentrations. B, Thr286 autophosphorylation was quantified by densitometry of Western blots as shown in C. A mouse monoclonal anti-phospho-αCaMKII (Thr286) antibody was used (Millipore). C, autophosphorylation mixtures containing 6 μm αCaMKII, 8 μm CaM, 50 μm syntide 2, and 1 mm ATP at the specified [Ca2+] were incubated for 15 s at 25 °C, and the reaction was quenched with SDS sample buffer. Western blot of samples is shown.
Thr286 autophosphorylation of αCaMKII in the presence of saturating peptide substrate syntide 2 was analyzed by Western blotting. As shown in Fig. 1, B and C, a Km of 500 ± 14 nm for Ca2+ and an n value of 8.2 ± 1.7 for Thr286 autophosphorylation were measured. These data showed that exogenous substrate phosphorylation and Thr286 autophosphorylation by αCaMKII were activated in the same [Ca2+] concentration range and that saturating exogenous peptide substrate did not effectively compete with Thr286 autophosphorylation. These data support the existence of distinct phosphorylation mechanisms at the “canonical” and the Thr286 site with a low level of competition presented by “canonical” phosphorylation to Thr286 autophosphorylation.
Ca2+ Sequestration-induced Inactivation of Ca2+/CaM·Phospho-Thr286-αCaMKII
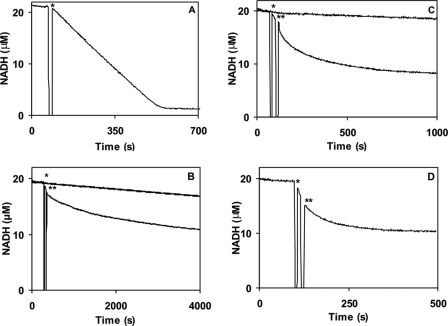
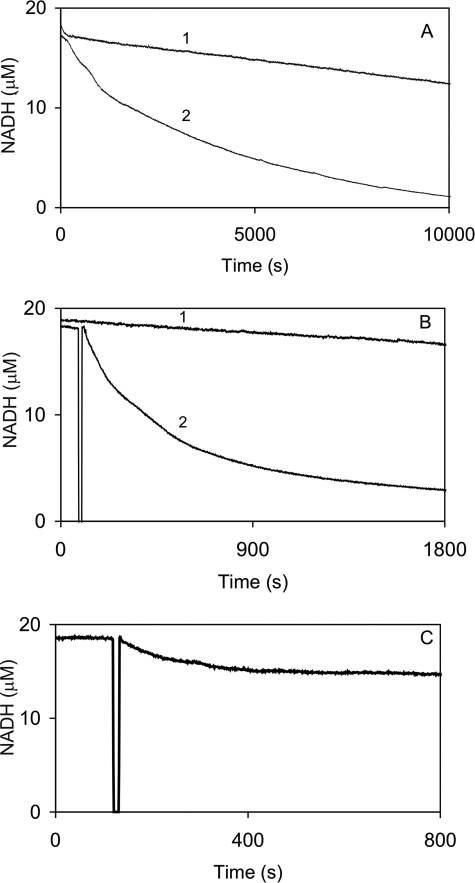
Followingactivation and thus Thr286 autophosphorylation, EGTA is thought to expose “autonomous” activity of αCaMKII (9), but the enzyme is also reported to undergo a burst of inhibitory Thr305/306 autophosphorylation (7). In order to dissect this process, first the time course of kinase activity during syntide 2 phosphorylation activity was monitored. As shown in Fig. 2, A–D, steady-state activity was initiated by the addition of αCaMKII into the assay as denoted by the asterisks. At the points indicated by double asterisks, 4.2 mm EGTA was added, and this induced an exponential decay of activity. The rate constants of EGTA-induced inactivation were 0.0017 s−1, 0.0053 s−1, and 0.0125 s−1 at 21, 30, and 37 °C, respectively. In order to exclude the possibility that inactivation occurred due to unfolding, Trp fluorescence, an indicator of the integrity of the tertiary structure of proteins, of αCaMKII in the assay conditions was monitored. EGTA caused a rapid quenching of Trp fluorescence; there were, however, no further changes in the slow time course of inactivation (data not shown). The EGTA-induced autoinactivation time courses were next compared with those of Thr305/306 autophosphorylation, the dissociation of Ca2+, and the subsequent release of CaM from the Ca2+/CaM·phospho-Thr286-αCaMKII complex.
FIGURE 2.
Temperature-dependent αCaMKII activity and Ca2+ sequestration-induced inactivation of Ca2+/CaM·phospho-Thr286-αCaMKII. A, steady-state activity measured by an NADH-coupled assay as described under “Materials and Methods.” 50 nm αCaMKII was added (indicated by the asterisk) to start the reaction in the presence of 50 μm syntide 2 as peptide substrate at 10 μm [Ca2+] concentration at 21 °C. Temperature-dependent steady-state activity and inactivation induced by the addition of 4.2 mm EGTA at 21 °C (B), 30 °C (C), and 37 °C (D) were measured. The reaction was started by the addition of the enzyme, marked by an asterisk. A double asterisk denotes where 4.2 mm EGTA was added. As a base-line control, NADH fluorescence was monitored prior to starting the reaction by the addition of the enzyme (the section before that marked with an asterisk). In B and C, which had the longest time courses, additionally independent photobleaching controls were also run for the same duration as the experiment (top lines). The fluorescence decay rates due to enzyme activity or inactivation by EGTA were corrected for the appropriate base line.
Time Course of Thr305/306 Autophosphorylation Induced by Ca2+ Sequestration
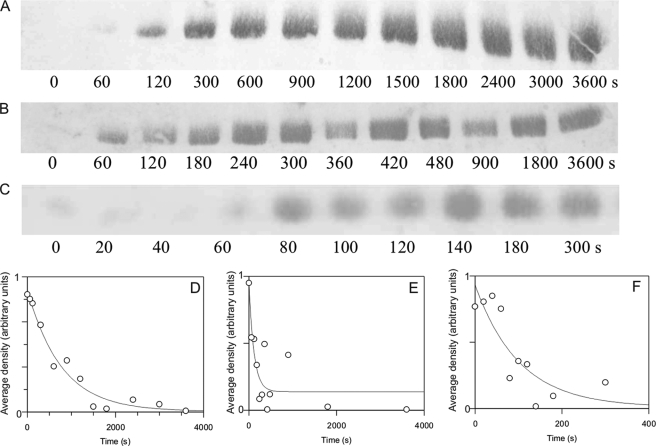
The rate of Thr305/306 autophosphorylation of Ca2+/CaM·phospho-Thr286-αCaMKII induced by EGTA was estimated from the semiquantitative densitometric analysis of Western blots (Fig. 3, A–C). Rate constants of 0.0013 s−1 at 21 °C, 0.0086 s−1 at 30 °C, and 0.0098 s−1 at 37 °C were obtained (see Table 1). The EGTA-induced Thr305/306 autophosphorylation and autoinactivation rates (shown above; Fig. 2) were thus closely related.
FIGURE 3.
Thr305/306 autophosphorylation time course estimated by Western blotting. 1 μm αCaMKII was allowed to autophosphorylate for 15 s, as described under “Materials and Methods.” At that point, 4.2 mm EGTA was added, and aliquots were taken at the indicated time points; the reaction was quenched by pipetting the aliquots taken into SDS sample buffer. A rabbit polyclonal anti-phospho-αCaMKII (Thr305) (Millipore) was used. Shown are Western blots and respective densitometric analyses of representative time courses at 21 (A and D), 30 (B and E), and 37 °C (C and F). Densitometric analysis of Western blots was carried out as described under “Materials and Methods.”
TABLE 1.
Inactivation and dissociation rates of the Ca2+/αCaM·phospho-Thr286-CaMKII complex
The autoinactivation processes of the Ca2+/CaM·phospho-Thr286-αCaMKII complex were dissected by measurements of Ca2+ and CaM dissociation, inactivation, and Thr305 autophosphorylation rates.
| Temperature |
k |
||||||
|---|---|---|---|---|---|---|---|
| Ca2+ offa | CaM “stretching” | CaM dissociation (polarization) (slow phase) | Inhibition (EGTA) | Thr305 autophosphorylation (EGTA) | Inhibition (Ca2+) | Thr305 autophosphorylation (Ca2+) | |
| s−1 | |||||||
| 21 °C | 8 ± 1.4 | 0.00105 ± 0.00047 (n = 4)c | 0.0013 ± 0.0002 | 0.000450 ± 0.000002d(n = 2)c | 0.0013 ± 0.0002 | ||
| 0.31 ± 0.03 | 0.82 ± 0.02(0.70)b | ||||||
| 0.023 ± 0.016 | 0.088 ± 0.002(0.30)b | ||||||
| 30 °C | 0.00485 ± 0.00064(n = 2)c | 0.0086 ± 0.0002 | 0.0043 ± 0.0028(n = 3)c | 0.0060 ± 0.0051(n = 2)c | |||
| 37 °C | 0.0200 ± 0.0075e(n = 5)c | 0.01255 ± 0.00007(n = 2)c | 0.0098 ± 0.0064 | 0.01335 ± 0.00092(n = 2)c | 0.0157 ± 0.0050 | ||
a Data from Tzortzopoulos et al. (19).
b Relative amplitude.
c n specifies the number of independent experiments; the mean and the S.D. are shown. Where n = 1, the fitted value and the S.E. of the fit is given.
d The temperature in this experiment was 22 °C.
e A p value of 0.244 obtained using an unpaired two-tailed t test showed this value not to be significantly different from the rate constant of 0.01255 ± 0.000071 (S.D.) s−1 (n = 2) obtained for inhibition induced by EGTA.
Mechanism of CaM Dissociation in Ca2+ Sequestration-induced Autoinactivation of Phospho-Thr286-αCaMKII
The kinetics of Ca2+ dissociation from Ca2+/CaM·phospho-Thr286-αCaMKII had previously been examined. Ca2+ dissociation rate constants were 8 s−1 for one site, 0.31 s−1 for two of the sites, and 0.023 s−1 for the one remaining site at 21 °C (19).
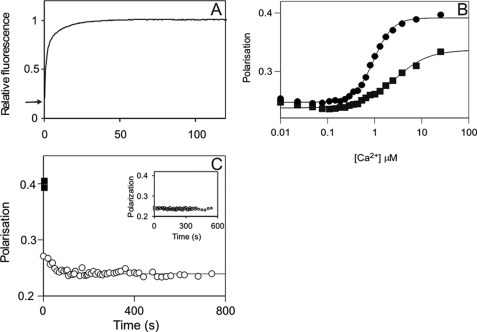
To study CaM dissociation kinetics, DA-CaM, a probe of CaM conformation with the fluorescent donor quenching reporting distance changes between the N and C lobes of CaM (17), was used. Conformational changes of CaM were monitored during the time course of inactivation of phospho-Thr286-αCaMKII upon the addition of EGTA. Stopped-flow kinetic experiments in which αCaMKII, DA-CaM, and ATP premixed in the presence of Ca2+ were rapidly reacted with 25 mm EGTA showed a biphasic process; DA-CaM became extended with two rate constants: 0.82 s−1 (amplitude 0.70) and 0.088 s−1 (amplitude 0.30) at 21 °C (Fig. 4A and Table 1).
FIGURE 4.
Mechanism of CaM dissociation from its complex with phospho-Thr286-αCaMKII upon Ca2+ sequestration. A, calmodulin stretching shown by DA-CaM unquenching. The mixture of 1 μm αCaMKII, 1 μm DA-CaM, and 0.5 mm ATP in 50 mm K-PIPES, pH 7.0, 100 mm KCl, 2 mm MgCl2, 1 mm 1,4-dithiothreitol, and 0.1 mm CaCl2 was rapidly mixed with 50 mm EGTA adjusted to pH 7.0 with KOH at 21 °C. DA-CaM fluorescence (donor AEDANS was excited at 365 nm, and emission was detected using a 455 nm cut-off filter) was quenched in complex with phospho-Thr286-αCaMKII. The arrow indicates relative fluorescence 0.2. Upon EGTA addition, DA-CaM fluorescence was unquenched in a biphasic process. B, polarization of FL-CaM upon Ca2+-dependent binding to αCaMKII (squares) and CaMKIV (diamonds). The mixture of 120 nm FL-CaM and 1.4 μm CaMKIV or 2.0 μm αCaMKII in the same conditions as above except for the presence of 2 mm EGTA was titrated with CaCl2. [Ca2+] was determined as described under “Materials and Methods” (19). C, FL-CaM dissociation from phospho-Thr286-αCaMKII was monitored by polarization. ■, the starting values of polarization of the Ca2+/FL-CaM·phospho-Thr286-αCaMKII; the time course of polarization decay (○) upon the addition of 4.2 mm EGTA is shown. Inset, controls that contained no ATP but in which 2 mm EGTA was present from the start (n = 4); these show no time-dependent change. In the main panel, a mean rate constant of 0.0200 ± 0.0075 s−1 (±S.D.) was obtained for the polarization data on CaM dissociation (n = 5).
These data showed that both Ca2+ dissociation and CaM “stretching” were more rapid than Thr305/306 autophosphorylation and inactivation (Table 1). However, DA-CaM unquenching may not have signified its complete dissociation, and instead it may have indicated that DA-CaM adopted an extended conformation as a result of the dissociation of one of its lobes only (17); thus, fluorescence polarization was used to further probe the state of CaM binding to phospho-Thr286-αCaMKII.
Ca2+ Sequestration-induced FL-CaM Dissociation Monitored by Polarization
Steady-state polarization measurements showed that FL-CaM (24) was a useful probe of CaM binding to protein kinases αCaMKII and CaMKIV. As seen in Fig. 4B, FL-CaM polarization increased from 0.24 to 0.4 and 0.33 upon Ca2+-dependent association with CaMKIV and αCaMKII, respectively, in the absence of ATP or protein substrates in the [Ca2+] range applied. The high polarization of Ca2+/CaM in complex with CaMKIV (P, 0.39) was consistent with compact Ca2+/CaM binding to CaMKIV indicating that both CaM lobes were engaged3; similarly, the lower polarization of Ca2+/CaM in complex with unphosphorylated αCaMKII in the absence of ATP and protein substrates (P, 0.34) was consistent with Ca2+/CaM binding in an extended conformation, mainly with one lobe only (17). The differences in affinities governed by the binding conformations were also in accord with a greater effect on the Ca2+ binding affinities in the Ca2+/CaM·CaMKIV complex (Kd for Ca2+, 0.96 ± 0.03 μm) compared with that in the Ca2+/CaM· αCaMKII complex (Kd for Ca2+, 2.70 ± 0.37 μm). The lack of involvement of both lobes of CaM in binding to αCaMKII was also reflected in a low Hill coefficient, n = 1.22 ± 0.14 in contrast with n = 2.13 ± 0.10 for CaMKIV.
In order to probe the dissociation process of CaM by polarization, Ca2+/CaM·phospho-Thr286-αCaMKII was generated in a mixture of αCaMKII with Ca2+/FL-CaM and ATP; this resulted in an increase of polarization from 0.23 to 0.4 consistent with high affinity compact binding of Ca2+/CaM (17). Upon the addition of EGTA, most of the polarization was rapidly reversed, reaching 0.27 after 15 s, indicating that CaM became substantially more flexible than it was in the presence of Ca2+; this was most likely due to the dissociation of one of its lobes. The rapid phase of depolarization was, however, followed by a slow phase with a rate constant of 0.0200 s−1 at 37 °C (Fig. 4C). The EGTA-induced inactivation rate constant was 0.0125 s−1 at the same temperature. There was no significant difference between the two values (Fig. 4C and Table 1).
Temperature Dependence of Autoinactivation of Phospho-Thr286-αCaMKII in the Presence of Ca2+
A burst of autoinhibition triggered by the addition of EGTA has been well documented (7). We have, however, previously also observed a loss of activity upon incubating αCaMKII in the presence of Ca2+, CaM, ATP at 30 °C for 2 min4 and have decided to further investigate the phenomenon. From the data shown in Fig. 1, it is evident that the enzyme complex that undergoes this inactivation is Ca2+/CaM·phospho-Thr286-αCaMKII. First, autoinactivation of Ca2+/CaM·phospho-Thr286-αCaMKII was measured in the presence of peptide substrate syntide 2. Fig. 5, A–C, shows the time courses of activity in the presence of syntide 2 and an ATP-regenerating system at 22, 30, and 37 °C. It can be seen that αCaMKII activity was lost in an exponential process, and the rate constants of Ca2+-dependent autoinactivation were 0.0004 s−1 at 22 °C, 0.0043 s−1 at 30 °C, and 0.0127 s−1 at 37 °C, similar to those obtained by inactivation in EGTA (Table 1).
FIGURE 5.
Autoinactivation of phospho-Thr286-αCaMKII in the presence of Ca2+. Temperature-dependent αCaMKII activity and time-dependent inactivation of Ca2+/CaM·phospho-Thr286-αCaMKII was monitored in the NADH-coupled assay. Activity of 50 nm enzyme in the presence of 50 μm syntide 2 as peptide substrate at 10 μm [Ca2+] concentration was measured at 22 (A), 30 (B), and 37 °C (C). Exponential decays of activity (traces 2 at 22 and 30 °C) were corrected for photobleaching (traces 1 at 22 and 30 °C) and were fitted with rate constants.
Thr305/306 Autophosphorylation in the Presence of Ca2+
We then asked if Thr305/306 autophosphorylation occurred in the presence of Ca2+. As seen in Fig. 6, A–C, Thr305/306 autophosphorylation occurred in these conditions. At 50 μm Ca2+, the rate constants for Thr305/306 autophosphorylation were estimated from densitometric analysis of Western blots as 0.0013 s−1 at 21 °C, 0.0024 s−1 at 30 °C, and 0.0157 s−1 at 37 °C (Fig. 6, D–F). These values were not significantly different from those seen when Thr305/306 autophosphorylation was thought to be induced by Ca2+ removal by the addition of 4.2 mm EGTA. These data also highlight that autoinactivation of Ca2+/CaM·phospho-Thr286-αCaMKII in the presence and, as described above, in the absence of Ca2+ has a physiologically relevant time course, with a half-life of ∼50 s at 37 °C.
FIGURE 6.
Thr305/306 autophosphorylation in the presence of Ca2+. Thr305/306 autophosphorylation time course estimated by Western blotting. 1 μm αCaMKII was allowed to autophosphorylate for the indicated lengths of time as described under “Materials and Methods.” Aliquots were taken at the indicated time points; the reaction was quenched by pipetting the aliquots taken into SDS sample buffer. Shown are Western blots and respective densitometric analyses of representative time courses at 21 (A and D), 30 (B and E), and 37 °C (C and F). Care was taken that the intensity of the bands was in the quasilinear range of densities (for details, see Fig. 3). Densitometric analysis of Western blots was carried out as described under “Materials and Methods.”
CaM Conformation during Autoinactivation of αCaMKII in Ca2+
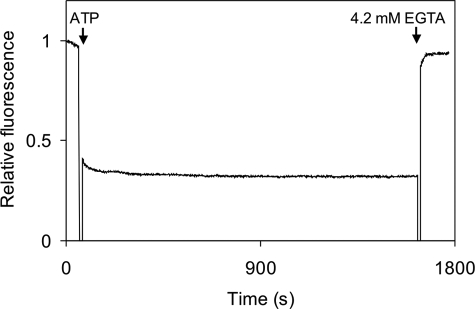
We have shown above that αCaMKII becomes inactivated upon continued incubation with Ca2+/CaM and ATP following its activation. Thr305/306 autophosphorylation was also detected during the period of inactivation. Since the Thr305/306 sites are located in the CaM binding domain, it was expected that Ca2+/CaM may be displaced by Thr305/306 autophosphorylation. We thus checked if Ca2+/CaM remained bound or dissociated during inactivation, using DA-CaM (see above). The fluorescence of DA-CaM is strongly quenched when bound to a target in a compact conformation, due to the donor and acceptor labels on the N and C lobes of CaM being in a closer proximity than the critical distance between them for resonance energy transfer. Unbound CaM is more extended, and less energy transfer is shown by the greater, unquenched fluorescence. A complex of Ca2+/DA-CaM·αCaMKII also has high fluorescence (relative fluorescence 1), since CaM is bound in an extended conformation (17). Fig. 7 shows that upon the addition of ATP, which results in immediate Thr286 autophosphorylation and Ca2+/CaM trapping, the fluorescence of DA-CaM becomes quenched, indicating that Ca2+/DA-CaM is bound in a compact conformation. It was expected that at least one CaM lobe, assumed to be the N lobe covering the Thr305/306 sites, dissociates, allowing CaM to adopt the extended conformation during Thr305/306 autophosphorylation. It is clearly seen however, that DA-CaM fluorescence remained quenched in the autoinhibited and presumed Thr286/305/306-phosphoenzyme complex, as monitored for 4000 s at 21 °C (not shown) and 1600 s at 37 °C (Fig. 7). In the latter conditions, as shown above, autoinactivation occurred with a t½ of 50 s. Ca2+/DA-CaM supports both Thr286 and Thr305/306 autophosphorylation (data not shown). These data show that Thr305/306 autophosphorylation of phospho-Thr286-αCaMKII proceeds in the presence of bound Ca2+/CaM, contrary to previous reports suggesting that CaM dissociation is a prerequisite for it to occur.
FIGURE 7.
Ca2+/DA-CaM trapping in phospho-Thr286-αCaMKII during Thr305/306-autophosphorylation. 0.5 μm DA-CaM and 1 μm αCaMKII were preincubated at 37 °C; DA-CaM fluorescence intensity was defined as 1. At the point indicated by an arrow, 1 mm ATP was added, resulting in substantial quenching of DA-CaM fluorescence, indicating its adopting a compact conformation bound to phospho-Thr286-αCaMKII. As indicated by the second arrow, at 1600 s, 4.2 mm EGTA was added, resulting in unquenching of DA-CaM fluorescence, indicating the stretching of DA-CaM to an extended conformation.
Arrhenius Plots of Temperature Dependences of Activation and Autoinactivation Processes of αCaMKII
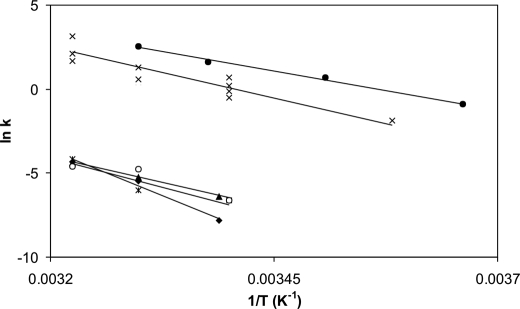
Fig. 8 shows the Arrhenius plots of the measured reactions. Data set 1 represents the temperature dependence of the turnover rate of αCaMKII-phosphorylating peptide substrate syntide-2. This reaction had a high ln A value and an Ea of 101 kJ/mol; these values were consistent with a favorable reaction characterized by a transition state that was more highly disordered than the ground state, an average activation energy, and a fast rate constant (14.6 s−1 at 37 °C). The values for data set 2 were estimated using published data for the Thr286 autophosphorylation reaction (26). These values show a reaction similarly favorable and rapid as substrate phosphorylation, consistent with the observation that Thr286 autophosphorylation occurs in preference to substrate phosphorylation (Fig. 1) (27).
FIGURE 8.
Arrhenius plots of activation and inactivation processes of αCaMKII. Turnover rates were calculated from steady-state activity measurements (data set 1; ●); Thr286 autophosphorylation rates were estimated using data from a previous publication (26) where autonomous activity was used as a measure of Thr286 autophosphorylation (data set 2, ×); inactivation rates were obtained in EGTA (data set 3; ▴); and Ca2+ (data set 4; ♦) and Thr305/306 autophosphorylation rates were measured in EGTA (data set 5; *) and Ca2+ (data set 6; ○), as described above (also see Table 1).
The autoinactivation reactions were represented by a distinctly different group of Arrhenius plots compared with those of activation. Data set 3 illustrates the inactivation induced by EGTA addition, and data set 4 corresponds to autoinactivation that occurs in the presence of Ca2+, following activation and Thr286 autophosphorylation. The Ea value for EGTA-induced inactivation (data set 3) was 106 kJ/mol; the ln A value was, however, much lower than that for the activation processes. Similar values were obtained for Thr305/306 autophosphorylation (data set 5 and Table 2), indicating an unfavorable reaction in which the transition state may be more ordered than the ground state. A low rate of reaction may thus be due to a reduction in the degrees of freedom during acquisition of the transition state. The rates and temperature dependence of autoinactivation in the presence of Ca2+ were similar to those in EGTA in the 30–37 °C range (data set 6). Although the inactivation rates in Ca2+ when fitted gave an Ea value of 172 kJ/mol, this value was not significantly different from the others for inactivation and Thr305/306 autophosphorylation.
TABLE 2.
Arrhenius parameters of activity, autophosphorylation, and inactivation of the Ca2+/CaM·phospho-Thr286-αCaMKII complex
Arrhenius plots were constructed from temperature-dependent activation and inactivation data sets obtained in the specified conditions. Each set of data was fitted by linear regression.
| Activity |
Thr286 autophosphorylation |
Inactivation (EGTA) |
Thr305 autophosphorylation (EGTA) |
Inactivation (Ca2+) |
Thr305 autophosphorylation (Ca2+) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E | ln A | E | ln A | E | ln A | E | ln A | E | ln A | E | ln A |
| kJ/mol | kJ/mol | kJ/mol | kJ/mol | kJ/mol | kJ/mol | ||||||
| 101 | 41.4 | 77 | 33.10 | 106 | 36.7 | 99 | 34.1 | 172 | 62.5 | 114 | 39.9 |
The temperature dependence of the reaction rates shows that distinct, less favorable structural changes underlie the autoinactivation processes compared with those for activation. Correspondingly, there is a difference of approximately 3 orders of magnitude between the activation and inactivation rates of αCaMKII. At physiological temperature (37 °C), however, the autoinactivation processes occur with a t½ of ∼1 min, which is in the range of relevant rates for signal transduction and may explain the observed inactivation in chemically induced long term potentiation (22).
DISCUSSION
First, the conditions of phospho-Thr286-αCaMKII formation were studied. In our observation, Thr286 autophosphorylation and phosphorylation of an exogenous peptide substrate have similar Ca2+ dependences with Km values of ∼500 nm for Ca2+. This value is typical for the [Ca2+] responsible for activation. It is, however, at variance with the previously reported Km or EC50 values for Ca2+ for Thr286 autophosphorylation of 1.6 μm (26) and 2 μm (28), obtained by the measurement of “autonomous” activity and Thr286 autophosphorylation by Western blotting, respectively. An n value of ∼8 for Ca2+ activation was measured, suggesting that the Ca2+ sites from two CaM molecules are simultaneously involved, supporting the previously determined intersubunit mechanism for Thr286 autophosphorylation (29). Such a high n value has previously been reported for the measurement of “autonomous” activity in the presence of phosphatase only (26). These discrepancies are probably related to “autonomous” activity measurements being greatly dependent on experimental conditions. Our data furthermore clearly show that Thr286 autophosphorylation occurs immediately upon activation and that it proceeds regardless of the presence of saturating exogenous peptide substrate, demonstrating the highly preferential nature of Thr286 autophosphorylation over an exogenous substrate (27).
Autoinactivation of phospho-Thr286-αCaMKII was investigated in two different conditions. Ca2+ sequestration and Ca2+-induced autoinactivation had common and distinct features; both were characterized by Thr305/306 autophosphorylation, but the fate of CaM was distinct. Upon Ca2+ sequestration, Ca2+/CaM sequential lobe dissociation occurred, whereas in the presence of Ca2+, Ca2+/CaM remained trapped in phospho-Thr286-αCaMKII during Thr305/306 autophosphorylation and autoinactivation.
In the mechanism of inactivation upon Ca2+ sequestration following initial activation by Ca2+/CaM, one CaM lobe was shown to dissociate rapidly, but the second lobe remained attached, probably by virtue of a high affinity Ca2+ binding site in trapped CaM (19). The structures of the Ca2+/CaM·peptide complexes of peptides derived from the CaM binding domain of αCaMKII show anti-parallel binding (30), suggesting that the N lobe of CaM is attached to the C-terminal region of the CaM binding domain in the proximity of or over the Thr305/306 sites. Thus, according to previous models, dissociation of the N lobe, which is likely to correspond to the rapid phase of CaM dissociation, should allow Thr305/306 autophosphorylation. If N lobe dissociation controlled Thr305/306 autophosphorylation, it would thus be rapid (31). However, this was not seen. Thr305/306 autophosphorylation correlated with the slow phase of dissociation, assumed to represent the C lobe (Scheme 1). Thus, our data show that exposure of the Thr305/306 residues is not sufficient to allow their autophosphorylation.
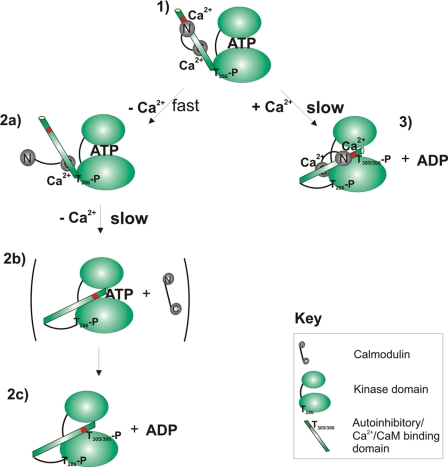
SCHEME 1.
Mechanisms of auto-inactivation of Ca2+/CaM·phospho-Thr286-αCaMKII. Two alternative pathways of autoinactivation leading to Thr305/306 autophosphorylation are illustrated from the perspective of a single kinase domain. The top and bottom ellipses represent the β-sheeted propeller domain and the α-helical substrate binding domain connected by a hinge and with the ATP binding site in between (4). The pathway in the left column includes initial rapid dissociation of the CaM N lobe followed by a slow conformational change accompanying the dissociation of the CaM C lobe, allowing a conformational change permissive for Thr305/306 autophosphorylation to occur. This path results in an inactivated enzyme complex free of CaM. 1, active Ca2+/CaM·phospho-Thr286-αCaMKII; the autoinhibitory domain is detached from the kinase domain and is Ca2+/CaM-bound. 2, upon Ca2+ chelation by EGTA, rapid dissociation of the N lobe of CaM is seen. 2b, slow dissociation of CaM C lobe; slow conformational change positions Thr305/306 as a “canonical” substrate, adjacent to the ATP binding pocket. 2c, Thr305/306 autophosphorylation of Ca2+/CaM-free kinase. In the right-hand column, Thr305/306 autophosphorylation of the Ca2+/CaM-bound phospho-Thr286-αCaMKII is shown. 3, slow conformational change leads to the conformation permissible for Thr305/306-autophosphorylation.
Is complete dissociation of CaM required for Thr305/306 autophosphorylation? Thr305/306 autophosphorylation is shown here to proceed in the continued presence of Ca2+ with rates similar to those in its absence. Thr305/306 autophosphorylation observed in the presence of Ca2+ was expected to occur with the concomitant dissociation of at least the CaM lobe that binds at or near residues Thr305/306. Our DA-CaM experiments, however, showed no detectable change in the compact structure of Ca2+/CaM, indicating that Ca2+/CaM remained bound throughout the experiment, ∼30 min at 37 °C. Despite predictions, Ca2+/CaM dissociation was not required for Thr305/306 autophosphorylation to occur. Interestingly, the rate of Thr305/306 autophosphorylation resembled that of Ca2+/CaM dissociation in Ca2+ (17), suggesting that CaM binding dynamics may play a role.
Since autoinactivation is contemporaneous with the slow dissociation of the second CaM lobe, it is further evident that rapid dissociation of the presumed N lobe of CaM does not cause inhibition of phospho-Thr286-αCaMKII. It is important to consider whether the state in which phospho-Thr286-αCaMKII has CaM attached by one lobe only (presumed to be the C lobe) has any special properties in terms of activity and if it limits the rate of Thr305/306 autophosphorylation. Although the existence of stable, partially Ca2+-saturated CaM-bound phospho-Thr286-αCaMKII has been demonstrated at Ca2+ concentrations that correspond to resting intracellular [Ca2+] (19), further work is required to establish their functional significance.
How does autonomous activity fit into the observed processes? Interestingly, whether autonomy is considered during Ca2+ sequestration or in the continuous presence of Ca2+, it is difficult to find a time window for such a process. The enzyme is not inactive until both CaM lobes have dissociated; thus, C lobe-attached partially Ca2+-saturated CaM may be the active species. Upon Ca2+ dissociation from the high affinity Ca2+ site and the subsequent dissociation of the second CaM lobe, inactivation occurs, and thus there appears to be no time window for autonomous activity.
The activity of Ca2+/CaM-bound phospho-Thr286-αCaMKII also declines in time. It is unclear at present by what mechanism phospho-Thr286-αCaMKII becomes inactivated upon the continued presence of Ca2+/CaM. Inactivation in the presence of Ca2+ has been shown to occur by self-association (32). Autoinactivation in Ca2+ observed here, however, does not appear to be the same process as self-association, which was seen at 10 μm ATP and pH 6.3 (32). Our experimental conditions of 1 mm ATP and pH 7.0 would not be expected to give rise to self-association.
As seen in the Arrhenius plots, the rates of autoinactivation correlated well with those of Thr305/306 autophosphorylation. This suggests that similar intermediates and mechanisms may lead up to Thr305/306 autophosphorylation and autoinactivation irrespective of the presence or absence of bound Ca2+/CaM. It is worth noting that the existence of phospho-Thr286/305/306-αCaMKII itself has not been directly confirmed, since detection of autophosphorylation by immunotechniques does not allow definitive determination of whether the same subunit of αCaMKII becomes autophosphorylated in all three sites. If phospho-Thr286/305/306-αCaMKII can be reactivated by selective dephosphorylation at Thr305/306, the Ca2+/CaM-bound and free forms may have different susceptibilities to phosphatases (33, 34).
The NR2B subunit of the NMDA receptor has been reported to fix αCaMKII in an active state (35). It has, however, also been shown that the resulting activity is very low compared with that with other substrates (11), that NR2B in fact is an effective non-competitive inhibitor of αCaMKII activity (10), and that it does not prevent Thr305/306 autophosphorylation (10) and is thus unlikely to prevent the above described autoinhibition.
It is concluded that Ca2+/CaM binding does not determine the rate of Thr305/306 autophosphorylation but that slow conformational changes in the phospho-Thr286-αCaMKII enzyme result in a conformation permissible to Thr305/306-autophosphorylation with or without Ca2+ and CaM present. Thr305/306 autophosphorylation may be the common process that is responsible for autoinhibition both in the presence and absence of Ca2+ (Scheme 1). Further questions do, however, still remain as to by what mechanism Thr305/306 autophosphorylation may cause inactivation.
In conclusion, phospho-Thr286-αCaMKII, universally the functionally essential form of αCaMKII, appears to be programmed for autoinactivation, having a brief time window of ∼1 min to exert its activity. After this, new enzyme is required to respond to new Ca2+ stimuli. These observations suggest a transient activity by αCaMKII at the synapse and may explain why new protein synthesis is required from local mRNA in synaptic plasticity (36). Coincidentally, transient activation of αCaMKII in single dendritic spines lasting for ∼1 min has been reported during long term potentiation; however, the mechanism of inactivation has yet to be determined in vivo (34).
This work was supported by Wellcome Trust Project Grant 075931 (to K. T.).
K. Török, unpublished data.
C. Fraser and K. Török, unpublished observation.
- αCaMKII
- Ca2+/calmodulin-dependent protein kinase II
- AEDANS
- 5-(2-acetylamino)-ethylamino-naphtalene-1-sulfonic acid
- AMPA
- α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- CaM
- calmodulin
- CaMKIV
- Ca2+/calmodulin-dependent protein kinase IV
- DA-CaM
- AEDANS/DDP-T34C/T110C-calmodulin
- DDP
- N-(4-dimethylamino-3,5-dinitrophenyl)
- Ea
- activation energy
- FL
- 5-(4,6-dichlorotriazinyl)aminofluorescein
- NR2B
- subunit 2B of N-methyl-d-aspartate receptor
- PIPES
- piperazine-N,N′-bis-(2-ethanesulfonic acid).
REFERENCES
- 1.Fukunaga K., Miyamoto E. (1999) Jpn. J. Pharmacol. 79, 7–15 [DOI] [PubMed] [Google Scholar]
- 2.Nicoll R. A., Malenka R. C. (1999) Ann. N.Y. Acad. Sci. 868, 515–525 [DOI] [PubMed] [Google Scholar]
- 3.Schulman H., Lou L. L. (1989) Trends Biochem. Sci. 14, 62–66 [DOI] [PubMed] [Google Scholar]
- 4.Johnson L. N. (2001) Ernst Schering Res. Found. Workshop 34, 47–69 [DOI] [PubMed] [Google Scholar]
- 5.Wang Z. W. (2008) Mol. Neurobiol. 38, 153–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barria A., Muller D., Derkach V., Griffith L. C., Soderling T. R. (1997) Science 276, 2042–2045 [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto Y., Soderling T. R. (1987) Arch. Biochem. Biophys. 252, 418–425 [DOI] [PubMed] [Google Scholar]
- 8.Morris E. P., Török K. (2001) J. Mol. Biol. 308, 1–8 [DOI] [PubMed] [Google Scholar]
- 9.Miller S. G., Kennedy M. B. (1986) Cell 44, 861–870 [DOI] [PubMed] [Google Scholar]
- 10.Robison A. J., Bartlett R. K., Bass M. A., Colbran R. J. (2005) J. Biol. Chem. 280, 39316–39323 [DOI] [PubMed] [Google Scholar]
- 11.Pradeep K. K., Cheriyan J., Suma Priya S. D., Rajeevkumar R., Mayadevi M., Praseeda M., Omkumar R. V. (2009) Biochem. J. 419, 123–132 [DOI] [PubMed] [Google Scholar]
- 12.Ishida A., Fujisawa H. (1995) J. Biol. Chem. 270, 2163–2170 [DOI] [PubMed] [Google Scholar]
- 13.Strack S., Colbran R. J. (1998) J. Biol. Chem. 273, 20689–20692 [DOI] [PubMed] [Google Scholar]
- 14.Ishida A., Kitani T., Fujisawa H. (1996) Biochim. Biophys. Acta 1311, 211–217 [DOI] [PubMed] [Google Scholar]
- 15.Tzortzopoulos A., Török K. (2004) Biochemistry 43, 6404–6414 [DOI] [PubMed] [Google Scholar]
- 16.Meyer T., Hanson P. I., Stryer L., Schulman H. (1992) Science 256, 1199–1202 [DOI] [PubMed] [Google Scholar]
- 17.Török K., Tzortzopoulos A., Grabarek Z., Best S. L., Thorogate R. (2001) Biochemistry 40, 14878–14890 [DOI] [PubMed] [Google Scholar]
- 18.Tse J. K., Giannetti A. M., Bradshaw J. M. (2007) Biochemistry 46, 4017–4027 [DOI] [PubMed] [Google Scholar]
- 19.Tzortzopoulos A., Best S. L., Kalamida D., Török K. (2004) Biochemistry 43, 6270–6280 [DOI] [PubMed] [Google Scholar]
- 20.Lisman J., Schulman H., Cline H. (2002) Nat. Rev. Neurosci. 3, 175–190 [DOI] [PubMed] [Google Scholar]
- 21.Wang H., Feng R., Phillip Wang L., Li F., Cao X., Tsien J. Z. (2008) Curr. Biol. 18, 1546–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lengyel I., Voss K., Cammarota M., Bradshaw K., Brent V., Murphy K. P., Giese K. P., Rostas J. A., Bliss T. V. (2004) Eur. J. Neurosci. 20, 3063–3072 [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto Y., Schworer C. M., Colbran R. J., Soderling T. R. (1987) J. Biol. Chem. 262, 8051–8055 [PubMed] [Google Scholar]
- 24.Török K., Wilding M., Groigno L., Patel R., Whitaker M. (1998) Curr. Biol. 8, 692–699 [DOI] [PubMed] [Google Scholar]
- 25.Smith G. L., Miller D. J. (1985) Biochim. Biophys. Acta 839, 287–299 [DOI] [PubMed] [Google Scholar]
- 26.Bradshaw J. M., Kubota Y., Meyer T., Schulman H. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 10512–10517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant P. A., Best S. L., Sanmugalingam N., Alessio R., Jama A. M., Török K. (2008) Cell Calcium 44, 465–478 [DOI] [PubMed] [Google Scholar]
- 28.Shifman J. M., Choi M. H., Mihalas S., Mayo S. L., Kennedy M. B. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 13968–13973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanson P. I., Meyer T., Stryer L., Schulman H. (1994) Neuron 12, 943–956 [DOI] [PubMed] [Google Scholar]
- 30.Meador W. E., Means A. R., Quiocho F. A. (1992) Science 257, 1251–1255 [DOI] [PubMed] [Google Scholar]
- 31.Mukherji S., Soderling T. R. (1995) J. Biol. Chem. 270, 14062–14067 [DOI] [PubMed] [Google Scholar]
- 32.Hudmon A., Aronowski J., Kolb S. J., Waxham M. N. (1996) J. Biol. Chem. 271, 8800–8808 [DOI] [PubMed] [Google Scholar]
- 33.Strack S., Barban M. A., Wadzinski B. E., Colbran R. J. (1997) J. Neurochem. 68, 2119–2128 [DOI] [PubMed] [Google Scholar]
- 34.Lee S. J., Escobedo-Lozoya Y., Szatmari E. M., Yasuda R. (2009) Nature 458, 299–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bayer K. U., De Koninck P., Leonard A. S., Hell J. W., Schulman H. (2001) Nature 411, 801–805 [DOI] [PubMed] [Google Scholar]
- 36.Roberts L. A., Large C. H., Higgins M. J., Stone T. W., O'Shaughnessy C. T., Morris B. J. (1998) Brain Res. Mol. Brain Res. 56, 38–44 [DOI] [PubMed] [Google Scholar]