Abstract
Carotenoids have been demonstrated to possess antioxidative and anti-inflammatory effects. However, there is no report that the effects of carotenoids on degranulation of mast cell is critical for type I allergy. In this study, we focused on the effect of carotenoids on antigen-induced degranulation of mast cells. Fucoxanthin, astaxanthin, zeaxanthin, and β-carotene significantly inhibited the antigen-induced release of β-hexosaminidase in rat basophilic leukemia 2H3 cells and mouse bone marrow-derived mast cells. Those carotenoids also inhibited antigen-induced aggregation of the high affinity IgE receptor (FcϵRI), which is the most upstream of the degranulating signals of mast cells. Furthermore, carotenoids inhibited FcϵRI-mediated intracellular signaling, such as phosphorylation of Lyn kinase and Fyn kinase. It suggests that the inhibitory effect of carotenoids on the degranulation of mast cells were mainly due to suppressing the aggregation of FcϵRI followed by intracellular signaling. In addition, those carotenoids inhibited antigen-induced translocation of FcϵRI to lipid rafts, which are known as platforms of the aggregation of FcϵRI. We assume that carotenoids may modulate the function of lipid rafts and inhibit the translocation of FcϵRI to lipid rafts. This is the first report that focused on the aggregation of FcϵRI to investigate the mechanism of the inhibitory effects on the degranulation of mast cells and evaluated the functional activity of carotenoids associated with lipid rafts.
Mast cells play pivotal roles in inflammation and immediate-type allergic reactions by secreting biologically active substances including histamine, eicosanoids, proteolytic enzymes, cytokines, and chemokines. The antigen-induced aggregation of the high affinity IgE receptor (FcϵRI)2 expressed on the cell surface triggers the degranulation of mast cells. FcϵRI has a tetrameric structure comprised of an IgE binding α-chain, a β-chain, and a disulfide-linked γ-chain dimer (1). The aggregation of FcϵRI by means of multivalent antigen-IgE complexes activates cytosolic Src protein-tyrosine kinases, such as Fyn and Lyn, which then regulate the activation of mast cells (2). Fyn kinase plays a key role in mast cell degranulation and in cytokine production by regulating Gab2 and phosphatidylinositol 3-kinase (3). Phosphorylated Lyn activates immunoreceptor tyrosine-based activation motifs of the β- and γ-chains, and the phosphorylated immunoreceptor tyrosine-based activation motifs of the γ-chain phosphorylate Syk kinase. Thereafter, a number of other signaling and adaptor molecules, such as phospholipase Cγ and protein kinase C (PKC), are phosphorylated (4). Phospholipase Cγ catalyzes the generation both of inositol 1,4,5-trisphosphate and diacylglycerol. Inositol 1,4,5-trisphosphate is an inducer of intracellular Ca2+ mobilization, which is critical for degranulation, and diacylglycerol is an activator of PKC (5). Activated PKC is translocated from the cytosol to the plasma membrane fraction. PKC regulates many functions of mast cells, including leukotriene generation, cytokine synthesis, and degranulation (6, 7).
Many studies have provided evidence that lipid rafts are involved in the activation of intracellular signaling molecules mediated by FcϵRI, the T cell receptor, the B cell receptor, and other cell surface receptors (8, 9). Lipid rafts are originally defined as microdomains in terms of their resistance to solubilization by non-ionic detergents such as Triton X-100, and are enriched in sphingolipids and cholesterol (10). Because numerous cell surface receptors and palmitoyl-anchored signaling molecules, including Src family tyrosine kinases, are associated with lipid rafts, it has been suggested that lipid rafts function as platforms regulating the induction of signaling pathways. Aggregated, but not non-aggregated, FcϵRIs are localized in lipid rafts fractionated by sucrose density gradient ultracentrifugation of detergent-treated cells (11, 12). The translocation of FcϵRI to lipid rafts is the key event that initiates the degranulation.
Carotenoids are a class of widespread natural pigments that have multiple functions (13). Dietary carotenoids have been associated with a decreased risk for certain types of immune diseases, such as asthma and atopic dermatitis. Consumption of β-carotene suppresses the production of specific IgE and IgG1 and decreases antigen-induced anaphylactic responses due to an improvement of the Th1-Th2 balance (14). Furthermore, β-carotene blocks nuclear translocation of the NF-κB p65 subunit, which correlates with the prevention of IκBα phosphorylation and degradation (15). It has been reported that fucoxanthin, a major carotenoid of edible brown algae, shows an anti-inflammatory effect on endotoxin-induced uveitis by decreasing the production of prostaglandin E2 and tumor necrosis factor-α (16). Astaxanthin, found in the red pigment of crustacean shells and salmon, also has anti-inflammatory effects due to its suppression of NF-κB activation (17, 18). It has been assumed that these anti-inflammatory activities of carotenoids are due to their antioxidant activity. However, there is no information to date about the direct effect of carotenoids on the degranulation of mast cells.
In the present study, we investigated the effects of carotenoids on antigen-induced degranulation of RBL-2H3 cells and mouse bone marrow-derived mast cells. In addition, to elucidate the mechanism of the modulation of degranulation by carotenoids, we focused on FcϵRI-mediated signaling in mast cells.
EXPERIMENTAL PROCEDURES
Materials
Fucoxanthin was extracted and refined from brown alga (Undaria pinnatifida) as reported previously (19). Astaxanthin and β-carotene were purchased from Cayman Chemical (Ann Arbor, MI) and Wako Pure Chemical Industries (Osaka, Japan), respectively. Zeaxanthin was obtained from Extrasynthese (Genay, France). Geraniol and β-citronellol were purchased from Tokyo Chemical Industry Co. (Tokyo, Japan). Fucoxanthin, astaxanthin, and zeaxanthin were solubilized in dimethyl sulfoxide, although β-carotene was solubilized in tetrahydrofuran.
Cell Culture
RBL-2H3 cells (Health Science Resources Bank, Osaka, Japan) were cultured in RPMI 1640 medium containing 10% fetal bovine serum and antibiotics (100 unit/ml penicillin and 100 μg/ml streptomycin) at 37 °C in a humidified atmosphere in the presence of 5% CO2.
BALB/c mice at the age of 10 weeks (Japan SLC Inc., Shizuoka, Japan) were used as donors of bone marrow-derived mast cells (BMMCs). The mice were bred in accordance with the guidelines of Kyoto University for the use and care of laboratory animals. Mouse bone marrow cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 10 ng/ml interleukin-3, 100 unit/ml penicillin, and 100 μg/ml streptomycin at 37 °C in a humidified atmosphere in the presence of 5% CO2. After 4 weeks, >98% of the non-adherent cells were defined as mast cells by metachromatic staining with toluidine blue (20, 21).
β-Hexosaminidase Release Assay
The degree of degranulation of mast cells stimulated by IgE-antigen was determined by the β-hexosaminidase release assay. RBL-2H3 cells or BMMCs were seeded in 96-well plates (3 × 104 cells/well) with 0.45 μg/ml anti-dinitrophenyl (DNP) IgE (Sigma). After overnight incubation, IgE-sensitized cells were treated with the indicated concentrations of carotenoids or monoterpenes dissolved in serum-free RPMI 1640 medium for 4 h (0.1% dimethyl sulfoxide or 0.2% tetrahydrofuran). After washing twice with Tyrode buffer (pH 7.7), the cells were incubated with 120 μl/well of Tyrode buffer containing 10 μg/ml DNP-BSA (Molecular Probes, Eugene, OR) for 30 min. The released β-hexosaminidase was measured (22).
To investigate whether carotenoids inhibited the binding of IgE and antigen directly, IgE-sensitized RBL-2H3 cells were incubated with DNP-BSA and each carotenoid simultaneously for 30 min, and the released β-hexosaminidase was measured. In the case of stimulation with another agent, IgE-sensitized RBL-2H3 cells were treated with 10 μg/ml anti-FcϵRI α-chain antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for 30 min to induce degranulation and the released β-hexosaminidase was measured.
Immunofluorescence Microscopy (23–25)
RBL-2H3 cells or BMMCs were cultured with 0.45 μg/ml of anti-DNP IgE overnight in 8-well chamber slides (Nunc, Naperville, IL) at 4.0 × 103 cells/well and treated with 10 μm of each carotenoid or 5 μm monoterpene as described above. The cells were then stimulated by 1 μg/ml of DNP-BSA or 10 μg/ml of anti-FcϵRI antibody in Tyrode buffer for 20 min. After washing with ice-cold PBS immediately, the cells were fixed with 3.7% formaldehyde in PBS for 20 min and blocked with 1% BSA in PBS. The IgE/α-chain of FcϵRI complexes in cells were detected using an anti-mouse IgE antiserum (Bethyl Laboratories, Inc., Montgomery, TE), and an appropriate fluorescein isothiocyanate-labeled secondary antibody (Chemicon International, Temecula, CA). Fluorescence images were observed with a FLUOVIEW FV 1000 (Olympus, Tokyo, Japan). The data were quantified by counting the aggregation number of FcϵRI positive cells and presented as aggregation positive cells/total cells. Two hundred cells were counted in six independent micrographs.
Membrane Translocation of PKC (26, 27)
DNP-IgE-sensitized RBL-2H3 cells (1.0 × 106 cells/dish) were treated with 10 μm carotenoids for 4 h. The cells were stimulated with 1 μg/ml DNP-BSA for 20 min and then sonicated in lysis buffer (25 mm Tris-buffered saline, 50 mm sodium fluoride, 1 mm Na3VO4, and protease inhibitor mixture (Roche)). The resultant homogenate was centrifuged at 20,400 × g at 4 °C for 1 h and the supernatant was considered as the cytosolic fraction. The pellet was resuspended in lysis buffer containing 1% Triton X-100 as the membrane fraction. Equal proteins of each fraction (20 μg) were separated by SDS-PAGE and protein was then transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA). The membranes were blocked with Blocking One-P (Nacarai Tesque, Inc., Kyoto, Japan), and probed with the anti-PKC-β antibody (Sigma) at 1:1000 dilution for 2 h. The secondary antibody used was horseradish peroxidase anti-rabbit antibody (Nacalai) at 1:2000 dilutions for 1 h. Detection was performed using Chemi-Lumi One L (Nacalai) and image analyzer LAS-3000 (FUJIFILM, Tokyo, Japan).
Immunoprecipitation (28, 29)
DNP-IgE-sensitized cells (1.0 × 106 cells in 6-cm dishes) were treated with 10 μm of each carotenoid for 4 h and then the cells were stimulated with 1 μg/ml DNP-BSA for 5 min. Cells were lysed by incubation for 30 min on ice with lysis buffer containing 1% Triton X-100. For immunoprecipitation, the equal amount of protein (20 μg) of the cell lysates was incubated with Lyn- or Fyn-specific antibodies coupled to protein G-Sepharose (Sigma) with slow rotation overnight at 4 °C. Each precipitated protein was subjected to Western blot analysis using anti-phospho-Lyn (Santa Cruz) or anti-phosphotyrosine antibodies (Sigma).
Measurement of Cytosolic Ca2+ Concentrations
Cytosolic Ca2+ concentrations were measured using the fluorescent indicator Fluo-4/AM (Dojindo Laboratories, Kumamoto, Japan) (30). IgE-sensitized cells (1.5 × 104 cells/well) were treated with 10 μm of each carotenoid in RPMI 1640 medium for 4 h, and then cells were loaded with 4 μm Fluo-4/AM in Tyrode buffer at 37 °C for 1 h. The cells were stimulated with 10 μg/ml DNP-BSA for 90 s. Intracellular calcium mobilization was detected at a 485-nm excitation wavelength and a 535-nm emission wavelength with a Wallac 1420 ARVOSX-FL spectrophotometer (Wallac, Waltham, MA).
Sucrose Density Gradient Ultracentrifugation (11, 31)
IgE-sensitized cells (5.0 × 107 cells) were treated with 10 μm carotenoid for 4 h, and then cells were stimulated with 1 μg/ml DNP-BSA for 20 min. Cells were harvested in 500 μl of lysis buffer containing 1% Triton X-100 and incubated at 4 °C for 30 min. Each cell lysate (500 μl) were added to an equal volume of 80% sucrose in Tris-buffered saline. The solubilized cells (in 40% sucrose) were overlaid successively with 3.0 ml of 30% sucrose and 2.0 ml of 5% sucrose. The gradients were centrifuged at 200,000 × g (4 °C) in a Beckman 90 Ti rotor for 19 h, and 0.5 ml of each fraction was collected from the top of the gradient. For detection of flotillin and FcϵRI, each fraction was immunoprecipitated using anti-flotillin antibody (Santa Cruz) and anti-FcϵRI α-chain antibody before Western blot analysis.
Localization of GM1
RBL-2H3 cells (4.0 × 103 cells/well) were cultured overnight in 8-well chamber slides with 0.45 mg/ml DNP-IgE and treated with 10 μm of each carotenoid for 4 h. The cells were then stimulated by 1 μg/ml DNP-BSA in Tyrode buffer for 20 min. After washing three times in ice-cold PBS, the cells were incubated with cholera toxin B subunit (CTxB) Alexa 594 (Molecular Probes) in 1% BSA in PBS for 1 h at 4 °C. Then, cells were fixed with 3.7% formaldehyde in PBS for 20 min and blocked with 1% BSA in PBS for 30 min. The IgE/α-chain of FcϵRI complexes in cells were detected as described above. Fluorescence images were observed with BIOREVO BZ-9000 (Keyence, Osaka, Japan).
Statistical Analysis
Data are reported as mean ± S.D. Statistical analyses were performed by one-way analysis of variance with a Dunnett test to identify levels of significance between the groups.
RESULTS
Effect of Carotenoids on Antigen-induced Degranulation of Mast Cells
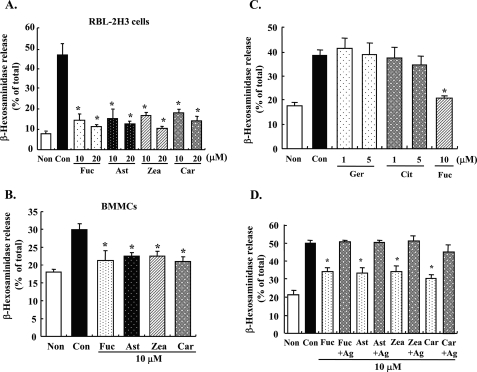
To examine the effects of carotenoids on FcϵRI-mediated degranulation, antigen-induced release of β-hexosaminidase from mast cells was determined. Pre-treatment with each carotenoid (β-carotene, zeaxanthin, astaxanthin, or fucoxanthin) for 4 h significantly suppressed the release of β-hexosaminidase from RBL-2H3 cells and BMMCs stimulated with DNP-BSA (Fig. 1, A and B). The release of β-hexosaminidase in carotenoid-treated cells was decreased to ∼30–40% the level of cells treated with antigen alone. There was no difference in the inhibitory effect among the carotenoids examined. These carotenoids did not directly inhibit the enzyme activity of β-hexosaminidase (data not shown). Monoterpenes did not inhibit the degranulation of RBL-2H3 cells (Fig. 1C). Geraniol and β-citronellol showed cytotoxicity at 10 μm concentration. Because carotenoids did not affect the degranulation of mast cells when treated with 10 μm of each carotenoid and 10 μg/ml DNP-BSA simultaneously (Fig. 1D), the binding of antigen and IgE could not be directly inhibited by carotenoids.
FIGURE 1.
Effect of carotenoids and monoterpenes on degranulation of mast cells. A and B, DNP-IgE-sensitized RBL-2H3 cells (A) or BMMCs (B) were incubated with the indicated concentrations of carotenoids for 4 h and stimulated with DNP-BSA for 30 min. Released β-hexosaminidase was measured. C, DNP-IgE-sensitized RBL-2H3 cells were treated with the indicated concentrations of monoterpenes for 4 h and then stimulated by DNP-BSA for 30 min. Released β-hexosaminidase was measured. D, DNP-IgE-sensitized cells were stimulated after incubation with each carotenoid for 4 h or co-incubated with DNP-BSA and carotenoids simultaneously for 30 min. Released β-hexosaminidase was measured. Values are mean ± S.D., error bars represent the S.D., n = 4. *, significantly different from control, p < 0.05. Non, nonstimulation; Con, stimulation; Fuc, fucoxanthin: Ast, astaxanthin; Zea, zeaxanthin; Car, β-carotene; Ger, geraniol; Cit, β-citronellol; +Ag, incubated with DNP-BSA and each carotenoid simultaneously for 30 min.
Effect of Carotenoids on Antigen-induced Aggregation of FcϵRI
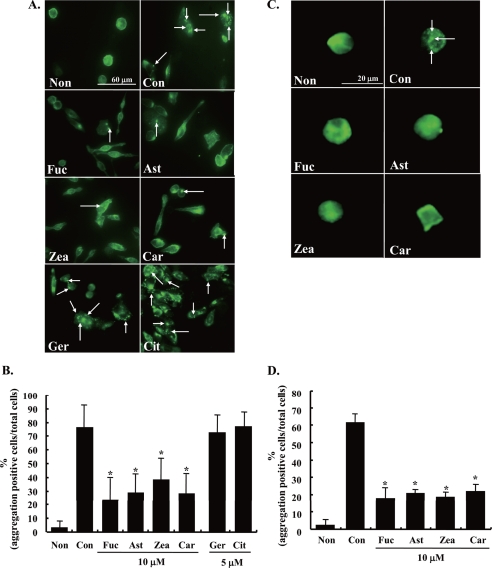
The aggregation of FcϵRI is the initiation factor of the degranulating signal (1). Thus, the effect of carotenoids on FcϵRI aggregation was examined by immunofluorescence staining using an antibody for rat IgE and a secondary fluorescein isothiocyanate-conjugated rabbit IgG antibody. The aggregation of FcϵRI was observed as condensed fluorescence signals in RBL-2H3 cells stimulated with DNP-BSA for 20 min (Fig. 2A). The aggregation was quantified by counting the number of the cells that were positive for aggregation of FcϵRI (Fig. 2B). Compared with control cells, the aggregation of FcϵRI in RBL-2H3 cells stimulated for 20 min was apparently inhibited by pre-treatment with 10 μm of each carotenoid for 4 h. Similar results were obtained as in the case of BMMCs. On the other hand, geraniol and β-citronellol showed no inhibitory effect on the aggregation of FcϵRI in RBL-2H3 cells (Fig. 2, A and B). These results suggested that the inhibitory effect of carotenoids on the degranulation of mast cells is due to suppressing the aggregation of FcϵRI.
FIGURE 2.
Effect of carotenoids on antigen-induced aggregation of FcϵRI. A and C, sensitized RBL-2H3 cells (A) or BMMCs (C) were incubated with each carotenoid for 4 h and then stimulated with DNP-BSA for 20 min. Cells were fixed by 3.7% paraformaldehyde, and the IgE/α-chain of FcϵRI complexes were detected using anti-rat IgE antibody and fluorescein isothiocyanate anti-rabbit IgG antibody. The arrowhead shows the aggregation of FcϵRI. B and D, the quantified data of the aggregation by counting number of the RBL-2H3 cells (B) or BMMCs (D). Data are represented by the number of aggregation positive cells/total cell number. Means were represented independent of six micrographs (total number was more than a 100 cells). Values are mean ± S.D., error bars represent the S.D. *, significantly different from control, p < 0.05. The abbreviations are the same as those given in the legend to Fig. 1.
Inhibition of the Downstream of FcϵRI-mediated Degranulating Signals by Carotenoids
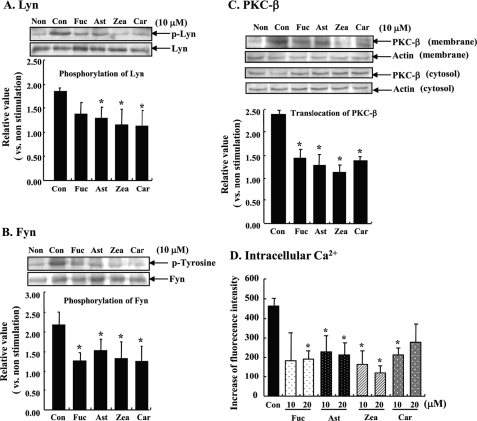
To confirm that carotenoids inhibit FcϵRI-mediated degranulating signals following FcϵRI aggregation, we examined tyrosine phosphorylation of Src family tyrosine kinases, Lyn and Fyn. Levels of both phosphorylated Lyn and Fyn were increased by stimulation with DNP-BSA for 5 min. In carotenoid-treated cells, FcϵRI-mediated tyrosine phosphorylations of Lyn and Fyn were significantly lower than in control cells (Fig. 3, A and B). Furthermore, we investigated the effect on the activation of PKC-β, which is downstream of the phosphorylation of Lyn. PKC is known to be translocated from the cytosolic fraction to the plasma membrane fraction after activation. In the plasma membrane fraction of cells stimulated by antigen for 20 min, the amount of PKC-β was higher than in non-stimulated cells. At the same time point, activation of PKC-β in cells pre-treated with 10 μm carotenoid for 4 h was significantly inhibited compared with control cells (Fig. 3C). Because it is well known that elevating the intracellular Ca2+ concentration triggers the degranulation of mast cells, the effects of carotenoids on antigen-induced intracellular Ca2+ influx were evaluated. RBL-2H3 cells stimulated with DNP-BSA increased intracellular Ca2+ levels within a few minutes. Pre-treatment with a carotenoid for 4 h, on the contrary, significantly suppressed the antigen-induced elevation of intracellular Ca2+ at 90 s compared with cells treated with antigen alone (Fig. 3D). These results demonstrate that carotenoids inhibit the degranulation of mast cells due to suppressing the aggregation of FcϵRI followed by intracellular degranulating signals.
FIGURE 3.
Effect of carotenoids on the downstream of FcϵRI signal transduction. A and B, sensitized RBL-2H3 cells were incubated with each carotenoid for 4 h and stimulated with DNP-BSA for 5 min. Cell lysate was immunoprecipitated with Lyn- or Fyn-specific antibodies, and then phosphorylation of Lyn or Fyn was detected by Western blot analysis using phospho-Lyn (A) or phosphotyrosine antibody (B). Western blotting was performed independent three experiments. Data were analyzed by the density of bands and represented the intensity of the bands of phosphorylated Lyn/normal Lyn (A) and phosphorylated Fyn/normal Fyn (B), as a relative value for that of the nonstimulated cells. Means are represented by three different experiments. Values are mean ± S.D. C, cells were treated with each carotenoid for 4 h and stimulated with DNP-BSA for 20 min. Cell lysate was fractionated by centrifugation for 4 h at 20,400 × g (4 °C). Membrane and cytosolic PKC-β were detected by Western blotting using PKC-β-specific antibodies. Data were analyzed by the density of bands and represent the intensity of the bands of the membrane fraction/cytosol fraction as a relative value for that of the nonstimulated cells. Each fraction was corrected by β-actin before calculation. Means are represented by four different experiments. Values are mean ± S.D. D, IgE-sensitized cells were loaded with 4 μm Ca2+ indicator Fluo-4/AM after treatment with each carotenoid for 4 h and then the cells were stimulated with DNP-BSA for 90 s, and fluorescence was measured. Values are mean ± S.D., error bars represent the S.D., n = 6. *, significantly different from control, p < 0.05. The abbreviations are the same as those given in the legend to Fig. 1.
Inhibition of FcϵRI Translocation to Lipid Rafts by Carotenoids
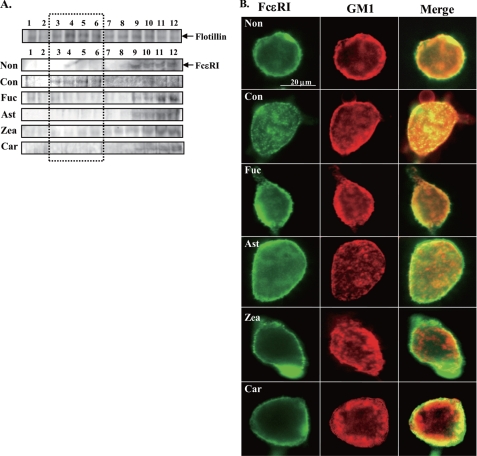
Because FcϵRI is translocated to lipid rafts when stimulated by multivalent antigens and then it leads to the aggregation of FcϵRI, we examined the effects of carotenoids on the association of FcϵRI with the lipid raft. As shown in Fig. 4A, flotillin, which is a marker protein of lipid rafts, was detected in fractions 3–6 (low density) prepared by sucrose density gradient ultracentrifugation. FcϵRI α-chain was translocated from fractions 9–12 (high density) to fractions 3–6 (low density) by stimulation with antigen for 20 min (Con, Fig. 4A). Each carotenoid suppressed the antigen-induced translocation of FcϵRI to lipid rafts.
FIGURE 4.
Effect of carotenoids on the translocation of FcϵRI to lipid rafts and localization of GM1. A, RBL-2H3 cells treated with each carotenoid for 4 h were lysed by 1% Triton X-100 lysis buffer. Cell lysate was fractionated by sucrose density gradient centrifugation (4 °C, 200,000 × g, 19 h). Each fraction was immunoprecipitated with flotillin or FcϵRI. Immunoprecipitated protein was applied to SDS-PAGE and Western blot using anti-flotillin antibody or anti-FcϵRIα antibody. A replicate experiment verified a similar trend. B, sensitized RBL-2H3 cells were treated with each carotenoid for 4 h and stained with cholera toxin B subunit (CTxB) Alexa 594 in Tyrode buffer containing 1% BSA for 1 h at 4 °C and then immunostained by anti-rat IgE antibody and fluorescein isothiocyanate anti-rabbit IgG antibody. The abbreviations are the same as those given in the legend to Fig. 1.
The aggregated FcϵRI and GM1 were co-localized in positive control cells but not in carotenoid-treated cells (Fig. 4B). These findings supported the suppressive effect of carotenoids on the translocation of FcϵRI to lipid rafts. Moreover, each carotenoid did not affect the localization of GM1 defined as a marker of lipid rafts. Thus, carotenoids may modulate the function of lipid rafts without disrupting lipid rafts.
Effect of Carotenoids on Degranulation and Aggregation of FcϵRI Stimulated by Anti-FcϵRI Antibody
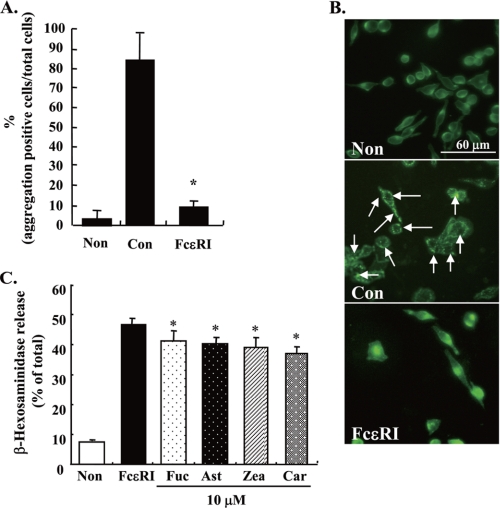
We investigated whether carotenoids inhibit degranulation induced by another stimulating agent, anti-FcϵRI antibody. IgE-sensitized cells, which were stimulated by 10 μg/ml of anti-FcϵRI antibody for 20 min, were not induced by the aggregation of FcϵRI, whereas 1 μg/ml of DNP-BSA for 20 min markedly induced aggregation of FcϵRI (Fig. 5, A and B). In this condition, the suppressive effects of carotenoids were apparently less than those by antigen (Fig. 5C). These results also supported that suppressing the aggregation of FcϵRI is important for the inhibitory effect of carotenoids on the degranulation of mast cells.
FIGURE 5.
Effect of carotenoids on degranulation and aggregation of FcϵRI in RBL-2H3 cells stimulated by anti-FcϵRI antibody. A and B, IgE-sensitized cells were stimulated by DNP-BSA or anti-FcϵRI antibody for 20 min after incubation with serum-free RPMI 1640 for 4 h. Aggregation of FcϵRI was detected and aggregation-positive cells were counted. The arrowhead shows the aggregation of FcϵRI. C, IgE-sensitized cells were pretreated with each carotenoid for 4 h and then stimulated by 10 μg/ml of anti-FcϵRI antibody for 30 min. Released β-hexosaminidase was measured. Values are mean ± S.D., error bars represent the S.D. *, significantly different from control, p < 0.05. The abbreviations are the same as those given in the legend to Fig. 1. FcϵRI, stimulated by anti-FcϵRI antibody.
DISCUSSION
In the present study, we demonstrated the inhibitory effect of certain carotenoids but not monoterpenes on the release of β-hexosaminidase from mast cells, which is an index of mast cell degranulation. β-Citronellol and geraniol belong to monoterpene and carotenoids are tetraterpene. Thus, it is assumed that the different activities of monoterpenes and carotenoids depend on their chain length. Carotenoids also inhibited the antigen-induced activation of PKC-β, the phosphorylation of Lyn and Fyn, and influx of intracellular Ca2+ via the suppression of FcϵRI aggregation. The inhibitory effect of carotenoids on the degranulation of mast cells would be due to suppressing the translocation of FcϵRI to lipid rafts that are the putative platform of the FcϵRI aggregation.
In this study, we examined the effect of carotenoids on the antigen-induced aggregation of FcϵRI because aggregation is the first step in activation of mast cells and induces intracellular signal transduction (2). Many studies have screened anti-inflammatory materials that could inhibit the FcϵRI-mediated signal transduction, yet there are no reports about the inhibitory mechanism of mast cell degranulation focused on FcϵRI aggregation. We evaluated the effect of carotenoids on antigen-induced aggregation of FcϵRI using immunofluorescence staining. Each carotenoid markedly suppressed the aggregation of FcϵRI followed by activation of intracellular signaling (Figs. 2 and 3). These results support that the suppressive effect of carotenoids on the FcϵRI-mediated degranulation of mast cells is due to inhibition of antigen-induced aggregation of FcϵRI followed by the degranulating signals. It seems most likely that evaluation of the visualized FcϵRI aggregation is useful as a screening method for anti-inflammatory function.
Carotenoids did not affect the degranulation stimulated with each carotenoid and DNP-BSA simultaneously (Fig. 1C). Therefore, the inhibitory effects of carotenoids were not due to inhibiting the binding of antigen-IgE. We evaluated whether carotenoids inhibit degranulation induced by the anti-FcϵRI antibody, another stimulating agent. It was reported that anti-FcϵRI antibody also induces the release of histamine from basophilis (32). Anti-FcϵRI antibody may evoke the phosphorylation of the immunoreceptor tyrosine-based activation motif of FcϵRI β-chain followed by activation of Syk kinase. In the case of stimulation by anti-FcϵRI antibody, anti-FcϵRI antibody did not induce the aggregation of FcϵRI (Fig. 5, A and B). In this case, the inhibitory effects of carotenoids on the release of β-hexosaminidase were markedly lower compared with the case of stimulation by antigen (Fig. 5C). Thus, carotenoids could not inhibit the activation of Syk kinase or some other signaling molecules directly. These results support that the inhibitory effect of carotenoids on the degranulation of mast cells could be mainly due to the suppression of the aggregation of FcϵRI.
The aggregation of FcϵRI induced an association with lipid rafts and subsequent activation events (11). Our results strongly suggested that the effect of carotenoids on aggregation of FcϵRI was due to inhibition of the translocation of FcϵRI to lipid rafts. It has been reported that the length of the dihydroxycarotenoid molecules (the distance between hydroxyl groups is 30.2 Å) is matched to the thickness of the hydrocarbon interior of the phosphatidylcholine bilayer, and it is assumed that lutein and zeaxanthin adopt an orientation mainly perpendicular to the membrane surface (33). Van de Ven et al. (34) indicated that the orientation of β-carotene is parallel to the membrane surface in the dioleoylphosphatidylcholine bilayer, perpendicular to the membrane surface in a soybean-phosphatidylcholine bilayer, and both parallel and perpendicular in dimyristoylphosphatidylcholine membranes (34). It was also reported that the localization of macular xanthophylls, such as zeaxanthin and lutein, in detergent-soluble membranes was about six times more abundant than in the detergent-resistant membranes considered as lipid rafts (35). These reports indicate that carotenoids can localize not only to the cell membrane but also lipid rafts. In our results, treatment of RBL-2H3 cells with carotenoids for 4 h did not cause visually a disruption of lipid rafts (Fig. 4B). Thus, it is assumed that carotenoids may modify the functions of lipid rafts by locating in the cell membrane and inhibit the translocation of FcϵRI to lipid rafts. It is important to verify whether carotenoids affect the other signaling involved in lipid rafts for understanding the biological relationship between carotenoids and lipid rafts.
Our results indicate for the first time that carotenoids suppress the degranulation of mast cells by inhibiting aggregation of FcϵRI and the subsequent intracellular signal transduction. Carotenoids presumably have an immunoregulatory action to modulate immunocompetent cells. On the other hand, many studies have indicated that carotenoids have various physiological functions such as an antioxidative activity, antiangiogenic effects, and an anti-tumor efficacy (36–39). It is interesting to verify the relationship between the multiple bioactive effects of carotenoids and modulation of lipid rafts function.
Acknowledgments
We thank Dr. Y. Sako and Dr. I. Yoshinaga (Graduate School of Agriculture, Kyoto University) for excellent technical advice on the fluorescence microscope.
This work was supported in part by the Mishima Kaiun Memorial Foundation and the Iijima Memorial Foundation for the Promotion of Food Science and Technology.
- FcϵRI
- high affinity IgE receptor
- RBL-2H3
- rat basophilic leukemia cells
- PKC
- protein kinase C
- BMMC
- bone marrow-derived mast cell
- DNP
- dinitrophenyl
- BSA
- bovine serum albumin
- PBS
- phosphate-buffered saline.
REFERENCES
- 1.Gilfillan A. M., Tkaczyk C. (2006) Nat. Rev. Immunol. 6, 218–230 [DOI] [PubMed] [Google Scholar]
- 2.Gomez G., Gonzalez-Espinosa C., Odom S., Baez G., Cid M. E., Ryan J. J., Rivera J. (2005) J. Immunol. 175, 7602–7610 [DOI] [PubMed] [Google Scholar]
- 3.Parravicini V., Gadina M., Kovarova M., Odom S., Gonzalez-Espinosa C., Furumoto Y., Saitoh S., Samelson L. E., O'Shea J. J., Rivera J. (2002) Nat. Immunol. 3, 741–748 [DOI] [PubMed] [Google Scholar]
- 4.Leitges M., Gimborn K., Elis W., Kalesnikoff J., Hughes M. R., Krystal G., Huber M. (2002) Mol. Cell. Biol. 22, 3970–3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho S. H., Woo C. H., Yoon S. B., Kim J. H. (2004) J. Allergy Clin. Immunol. 114, 1085–1092 [DOI] [PubMed] [Google Scholar]
- 6.Ozawa K., Yamada K., Kazanietz M. G., Blumberg P. M., Beaven M. A. (1993) J. Biol. Chem. 268, 2280–2283 [PubMed] [Google Scholar]
- 7.Nechushtan H., Leitges M., Cohen C., Kay G., Razin E. (2000) Blood 95, 1752–1757 [PubMed] [Google Scholar]
- 8.Holowka D., Gosse J. A., Hammond A. T., Han X., Sengupta P., Smith N. L., Wagenknecht-Wiesner A., Wu M., Young R. M., Baird B. (2005) Biochim. Biophys. Acta 1746, 252–259 [DOI] [PubMed] [Google Scholar]
- 9.Dráber P., Dráberová L. (2002) Mol. Immunol. 38, 1247–1252 [DOI] [PubMed] [Google Scholar]
- 10.Simons K., Ikonen E. (1997) Nature 387, 569–572 [DOI] [PubMed] [Google Scholar]
- 11.Yamashita T., Yamaguchi T., Murakami K., Nagasawa S. (2001) J. Biochem. 129, 861–868 [DOI] [PubMed] [Google Scholar]
- 12.Field K. A., Holowka D., Baird B. (1997) J. Biol. Chem. 272, 4276–4280 [DOI] [PubMed] [Google Scholar]
- 13.Socaciu C., Jessel R., Diehl H. A. (2000) Spectrochim. Acta A Mol. Biomol. Spectrosc. 56, 2799–2809 [DOI] [PubMed] [Google Scholar]
- 14.Sato Y., Akiyama H., Suganuma H., Watanabe T., Nagaoka M. H., Inakuma T., Goda Y., Maitani T. (2004) Biol. Pharm. Bull. 27, 978–984 [DOI] [PubMed] [Google Scholar]
- 15.Bai S. K., Lee S. J., Na H. J., Ha K. S., Han J. A., Lee H., Kwon Y. G., Chung C. K., Kim Y. M. (2005) Exp. Mol. Med. 37, 323–334 [DOI] [PubMed] [Google Scholar]
- 16.Shiratori K., Ohgami K., Ilieva I., Jin X. H., Koyama Y., Miyashita K., Yoshida K., Kase S., Ohno S. (2005) Exp. Eye Res. 81, 422–428 [DOI] [PubMed] [Google Scholar]
- 17.Lee S. J., Bai S. K., Lee K. S., Namkoong S., Na H. J., Ha K. S., Han J. A., Yim S. V., Chang K., Kwon Y. G., Lee S. K., Kim Y. M. (2003) Mol. Cells 16, 97–105 [PubMed] [Google Scholar]
- 18.Ohgami K., Shiratori K., Kotake S., Nishida T., Mizuki N., Yazawa K., Ohno S. (2003) Invest. Ophthalmol. Vis. Sci. 44, 2694–2701 [DOI] [PubMed] [Google Scholar]
- 19.Sugawara T., Kushiro M., Zhang H., Nara E., Ono H., Nagao A. (2001) J. Nutr. 131, 2921–2927 [DOI] [PubMed] [Google Scholar]
- 20.Hültner L., Moeller J., Schmitt E., Jäger G., Reisbach G., Ring J., Dörmer P. (1989) J. Immunol. 142, 3440–3446 [PubMed] [Google Scholar]
- 21.Raizman M. B., Austen K. F., Katz H. R. (1990) J. Immunol. 145, 1463–1468 [PubMed] [Google Scholar]
- 22.Gehlhar K., Peters M., Brockmann K., van Schijndel H., Bufe A. (2005) Int. Arch. Allergy Immunol. 136, 311–319 [DOI] [PubMed] [Google Scholar]
- 23.Xu K., Williams R. M., Holowka D., Baird B. (1998) J. Cell Sci. 111, 2385–2396 [DOI] [PubMed] [Google Scholar]
- 24.Okabe T., Teshima R., Furuno T., Torigoe C., Sawada J., Nakanishi M. (1996) Biochem. Biophys. Res. Commun. 223, 245–249 [DOI] [PubMed] [Google Scholar]
- 25.Asai K., Fujimoto K., Harazaki M., Kusunoki T., Korematsu S., Ide C., Ra C., Hosoi S. (2000) J. Histochem. Cytochem. 48, 1705–1716 [DOI] [PubMed] [Google Scholar]
- 26.Kurkinen K., Busto R., Goldsteins G., Koistinaho J., Pérez-Pinzón M. A. (2001) Neurochem. Res. 26, 1139–1144 [DOI] [PubMed] [Google Scholar]
- 27.Maeda-Yamamoto M., Inagaki N., Kitaura J., Chikumoto T., Kawahara H., Kawakami Y., Sano M., Miyase T., Tachibana H., Nagai H., Kawakami T. (2004) J. Immunol. 172, 4486–4492 [DOI] [PubMed] [Google Scholar]
- 28.Iwaki S., Tkaczyk C., Satterthwaite A. B., Halcomb K., Beaven M. A., Metcalfe D. D., Gilfillan A. M. (2005) J. Biol. Chem. 280, 40261–40270 [DOI] [PubMed] [Google Scholar]
- 29.Tkaczyk C., Horejsi V., Iwaki S., Draber P., Samelson L. E., Satterthwaite A. B., Nahm D. H., Metcalfe D. D., Gilfillan A. M. (2004) Blood 104, 207–214 [DOI] [PubMed] [Google Scholar]
- 30.Takahashi A., Camacho P., Lechleiter J. D., Herman B. (1999) Physiol. Rev. 79, 1089–1125 [DOI] [PubMed] [Google Scholar]
- 31.Kovárová M., Tolar P., Arudchandran R., Dráberová L., Rivera J., Dráber P. (2001) Mol. Cell Biol. 21, 8318–8328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzukawa M., Hirai K., Iikura M., Nagase H., Komiya A., Yoshimura-Uchiyama C., Yamada H., Ra C., Ohta K., Yamamoto K., Yamaguchi M. (2005) Int. Immunol. 17, 1249–1255 [DOI] [PubMed] [Google Scholar]
- 33.Wisniewska A., Widomska J., Subczynski W. K. (2006) Acta Biochim. Pol. 53, 475–484 [PubMed] [Google Scholar]
- 34.van de Ven M., Kattenberg M., van Ginkel G., Levine Y. K. (1984) Biophys. J. 45, 1203–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wisniewska A., Subczynski W. K. (2006) Free Radic. Biol. Med. 40, 1820–1826 [DOI] [PubMed] [Google Scholar]
- 36.Kotake-Nara E., Kushiro M., Zhang H., Sugawara T., Miyashita K., Nagao A. (2001) J. Nutr. 131, 3303–3306 [DOI] [PubMed] [Google Scholar]
- 37.Sugawara T., Yamashita K., Sakai S., Asai A., Nagao A., Shiraishi T., Imai I., Hirata T. (2007) Biosci. Biotechnol. Biochem. 71, 1069–1072 [DOI] [PubMed] [Google Scholar]
- 38.Sugawara T., Matsubara K., Akagi R., Mori M., Hirata T. (2006) J. Agric. Food Chem. 54, 9805–9810 [DOI] [PubMed] [Google Scholar]
- 39.Sugawara T., Baskaran V., Tsuzuki W., Nagao A. (2002) J. Nutr. 132, 946–951 [DOI] [PubMed] [Google Scholar]