Abstract
The role of hepatitis C virus (HCV) protein non-structural (NS) 5A in HCV-associated pathogenesis is still enigmatic. To investigate the in vivo role of NS5A for viral persistence and virus-associated pathogenesis a transgenic (Tg) mouse model was established. Mice with liver-targeted NS5A transgene expression were generated using the albumin promoter. Alterations in the hepatic immune response were determined by Western blot, infection by lymphocytic choriomeningitis virus (LCMV), and using transient NS3/4A Tg mice generated by hydrodynamic injection. Cytotoxic T lymphocyte (CTL) activity was investigated by the Cr-release assay. The stable NS5A Tg mice did not reveal signs of spontaneous liver disease. The intrahepatic immunity was disrupted in the NS5A Tg mice as determined by clearance of LCMV infection or transiently NS3/4A Tg hepatocytes in vivo. This impaired immunity was explained by a reduced induction of interferon β, 2′,5′-OAS, and PKR after LCMV infection and an impairment of the CTL-mediated elimination of NS3-expressing hepatocytes. In conclusion, these data indicate that in the present transgenic mouse model, NS5A does not cause spontaneous liver disease. However, we discovered that NS5A could impair both the innate and the adaptive immune response to promote chronic HCV infection.
Chronic hepatitis C virus (HCV)4 infection is associated with an increased risk of liver cirrhosis and hepatocellular carcinoma (HCC). The HCV genome is a single-stranded positive-sense RNA molecule of ∼9600 bp (1). The viral RNA codes for one large polyprotein of ∼3100 amino acids that is post-translationally processed by cellular and viral proteases, leading to the structural proteins core, E1 and E2, the p7 protein, and the non-structural proteins NS2, NS3, NS4A, NS4B, NS5A, and NS5B (2). The mature NS5A protein is generated by the action of the NS3/NS4A serine protease. NS5A is a phosphoprotein that exists in a basal or in a hyperphosphorylated state (p56 and p58) (3). Through an amphipathic α-helix, NS5A is associated with the cytoplasmic face of the ER (4) and is an integral part of the replication complex (5). Mutations in NS5A affect the rate of HCV replication suggesting a role of NS5A in modulating viral expression and replication (6). Moreover, NS5A is able to interfere with a variety of cellular proteins. Some of these interaction partners, such as Grb2, PI3K, p53, or Raf-1 are important key players in host cell signal transduction, enabling NS5A to deregulate important cellular check points (7–10). Recent reports even suggest that NS5A may deregulate cell cycle progression by modulating the expression of cell cycle regulatory genes (11). In light of these observations and that it has been suggested to transform murine fibroblasts (12), it is speculated that NS5A could represent an important factor for the development of HCV-associated HCC (13).
Infection of transgenic mice expressing the complete HCV polyprotein with lymphocytic choriomeningitis virus (LCMV) showed a reduced IFN response and a delayed viral elimination (14). Cell culture-based experiments have shown that NS5A interacts directly with the interferon-dependent induced protein kinase R (PKR), a key player in the cellular antiviral response and that this interaction results in an inhibition of PKR function (15). Therefore, a role of NS5A for the establishment of a chronic HCV infection by inhibiting the innate immunity is conceivable.
To enable in vivo studies of NS5A-specific effects transgenic mice were generated with a liver-specific expression of NS5A. We used these mice to show that NS5A affects both the innate and the adaptive hepatic immunity.
MATERIALS AND METHODS
Generation and Characterization of Transgenic Mice
The NS5A gene derived from a genotype 1b isolate (GenBankTM accession number D16435) was kindly provided by Dr. Kunitada Shimotohno (Kyoto University, Japan). The coding sequence for an N-terminal His6 tag and a C-terminal V5 epitope was fused to the NS5A coding sequence. The expression was performed under control of the mouse albumin promoter/enhancer (16–19). The β-globin intron was included to increase the expression level (16, 20). The poly(A) site was taken from SV40 (18, 21). The NS5A expression cassette was obtained by NotI/XhoI digestion of the vector palbNS5A and purified by preparative agarose gel electrophoresis. The fragment was microinjected into FVB/N mouse embryos that were implanted into pseudopregnant FVB/N mice. Cell culture experiments comparing the untagged with the tagged construct (data not shown) and previous work (17, 18) demonstrated that the functionality of NS5A was not affected by fusion of the tags. Chromosomal DNA was isolated as described and analyzed by PCR using a β-globin intron-specific forward primer (5′-cgtgctggttattgtgctgtctc-3′) and a NS5A-specific backward primer (5′-attggttgagcccgacctggaatg-3′). A tubulin-specific primer pair was used as control. Glutamyl pyruvic transaminase (GPT) serum levels were determined using the reflotron assay system according to the instructions of the manufacturer (Roche).
Infection of Mice and Virus Titration
The plaque-purified WE strain of LCMV was propagated in NCTC clone 929 L cells. Mice were infected with 105 plaque-forming units (PFU); after 6 and 12 days the livers and spleens of the animals were homogenized, and infectious virus was titrated as PFU in liter cell monolayer cultures (22).
Histochemistry and Immunohistochemistry
Sections and staining of the paraffin or cryosections was performed as described recently (18, 23, 24). For detection of NS5A, a rabbit-derived V5-epitope-specific serum (Invitrogen) was used. NS3 was detected using a mouse-derived serum (24) and CD3 by a commercial rabbit-derived serum (Dako).
In the case of lectin staining, paraffin sections were treated as described earlier (25). The following lectins were used: UEA I, Con A, GNA, ML I, HPA, CMA, WGA, MAA, SNA I, and PHA-L (25).
Immunoblotting
The following antisera were used: anti-phospho Raf-1-Ser-338 (Upstate), anti-P-MAPK (Promega), anti-P-MEK Ser-217/221 (Promega), anti-Raf-1 (Signal Transduction Laboratories) anti-MEK (Santa Cruz Biotechnology), anti-MAP kinase (Promega, Santa Cruz Biotechnology), anti-actin (Santa Cruz Biotechnology), anti-Erk2 (Santa Cruz Biotechnology), anti-PCNA (Santa Cruz Biotechnology) anti-2′,5′-OAS (AbGent), anti-PKR (Cell Signaling Technologies). For detection of NS5A rabbit of NS3 mouse-derived sera were used (9, 24). Bound antibodies were visualized by a peroxidase-conjugated secondary antibody. Immunostaining was quantified using the Fujifilm Las 3000 system.
Immunoprecipitation
Immunoprecipitation and immunocomplex assays were performed as described (9, 26, 27).
Immunization and Hydrodynamic Injection
Immunization and hydrodynamic injection of mice using a codon optimized (co) NS3/4A gene (genotype 1.a) has been described recently (26, 27). In brief, mice were immunized with 2 μg of coNS3/4A-pVAX1-labeled gold beads using the gene gun. After 13 days, 1.8 ml of Ringer solution containing 100 μg of coNS3/4A-pVAX1 were injected intravenously in the tail vein within ∼5–10 s. 72-h posthydrodynamic injection, mice were sacrificed, and liver tissue was taken for Western blot and immunohistochemistry experiments.
51Cr Release Assay
Chromium release assay was performed as described (26, 27) (for details see additional supplemental information).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 4 software. The S.E. was used for deviation and for significance analysis Student's test was used (p ≤ 0.05, *; p ≤ 0.01, **; p ≤ 0.001, ***). To calculate the significance of LCMV titer at day 12 p.i. the values for wild-type animals were set as 102, the border of detection limit.
Isolation of Mouse Liver RNA Microarrays
RNA was isolated from snap-frozen liver samples derived from three NS5A transgenic 3-month-old mice and three corresponding sex- and age-matched wild-type littermates as described (28). Each sample or reference RNA was transcribed into cDNA in the presence of Cy3- or Cy5-labeled dUTP, respectively. Hybridizations were performed in the presence of an equal amount of reference RNA (Stratagene, La Jolla, CA) as described by Boldrick et al. (29). The microarray results are based on three independent experiments.
RT-PCR Analysis
For RT-PCR analysis RNA was isolated from snap-frozen tissue using TRIzol (Invitrogen) according to the instructions of the manufacturer. The INFβ-, PKR-, and 2′,5′-OAS-specific RT-PCR was performed as described (30). Serial dilutions of the corresponding cDNA served in every PCR as a standard. In addition, induction of 2′,5′-OAS and of IFNβ was analyzed by Lightcycler PCR.
RESULTS
Generation of NS5A Transgenic Mice
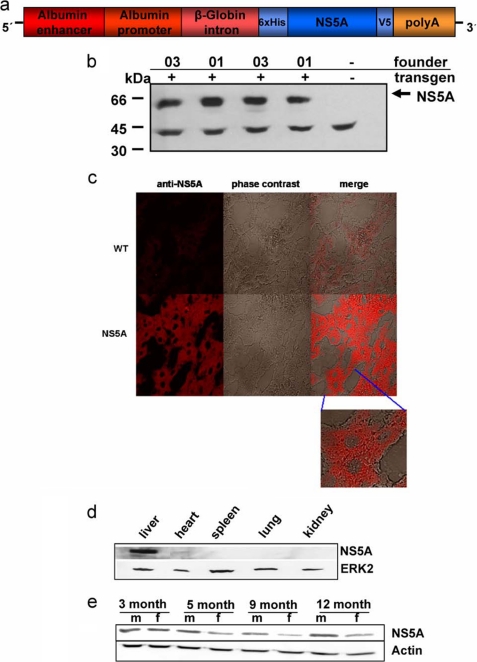
To study the function of NS5A in vivo, transgenic mice were established that express the NS5A protein in the liver. The schematic structure of the transgene is shown in Fig. 1a. Based on this construct, two independent transgenic lines (01 and 03) were established that had comparable expression levels of NS5A of the expected size (Fig. 1b).
FIGURE 1.
Liver-specific expression of the transgene encoding NS5A. a, schematic structure of the transgene encoding HCV NS5A genotype 1b. To direct the expression of the transgene to the liver, the albumin promoter was instrumental. A sufficient expression level was ensured by including the β-globin intron and for stabilization of the mRNA a poly(A) site was introduced at the 3′-end. For an efficient enrichment from tissue lysate and simple detection, NS5A was produced as a fusion protein with an N-terminal His6 tag and a C-terminal V5 epitope. b, Western blot analysis of liver-derived lysates derived from two different male mice per the two founder lineages 01 and 03. A V5-specific antiserum served for detection of NS5A. c, immunofluorescence microscopy of liver sections derived from 3-month-old male NS5A-transgenic mice. Sections derived from sex- and age-matched wild-type littermates served as control. A V5-specific antiserum was used for detection. The staining was performed using a Cy3-conjugated secondary antibody. The weak staining in the negative control is due to autofluorescence and background staining of the secondary antibody. Merge was obtained by overlay of the immunofluorescence and of the phase contrast. The micrographs were taken at 400× magnification, the inlet at 630× magnification. d, Western blot analysis of lysates derived from liver, heart, spleen, lung, and kidney of a NS5A-transgenic mouse. A V5-specific antiserum served for detection of NS5A, an Erk2-specific serum was used to control equal loading. e, Western blot analysis of lysates derived from liver of 3-, 5-, 9-, or 12-month-old male or female mice. A V5-specific antiserum served for detection of NS5A, an actin-specific serum was used to control equal loading.
The expression of the transgene was analyzed by immunofluorescence microscopy of liver sections derived from transgenic animals and the respective wild-type littermates using a V5 tag-specific antiserum. As expected for an ER-attached protein, the immunofluorescence microscopy for NS5A showed an inhomogeneous cytoplasmic staining with a concentration in the perinuclear region, while the nucleus remains unstained (Fig. 1c).
To verify the liver-specific expression, lysates derived from liver and various other tissues (heart, kidney, spleen, and lung) were analyzed by Western blotting using V5 tag-specific antisera. The Western blot shows that NS5A was solely detectable in the liver-derived lysates, confirming a liver-specific transgene expression (Fig. 1d).
To study whether the expression of the transgene was dependent on sex or age, liver-derived lysates from 3–12-month-aged mice were analyzed by Western blotting. The blot shows (i) that the transgene is constitutively expressed and (ii) that the NS5A expression is slightly increased in male mice compared with the female animals (Fig. 1e).
To investigate if the transgenic NS5A is functional, we investigated the ability of NS5A to interact with Raf-1 and to mediate increase Raf-1 phosphorylation, similar to reports from cell culture (9). We could confirm an interaction of NS5A and Raf-1 with increased phosphorylation/activation of Raf-1 in the transgenic mice. Moreover, consistent with the cell culture experiments, the increased Raf-1 phosphorylation does not result in an activation of MEK/MAP2 kinase (see additional online information).
Gene Expression Profile in the Liver of NS5A-expressing Transgenic Mice
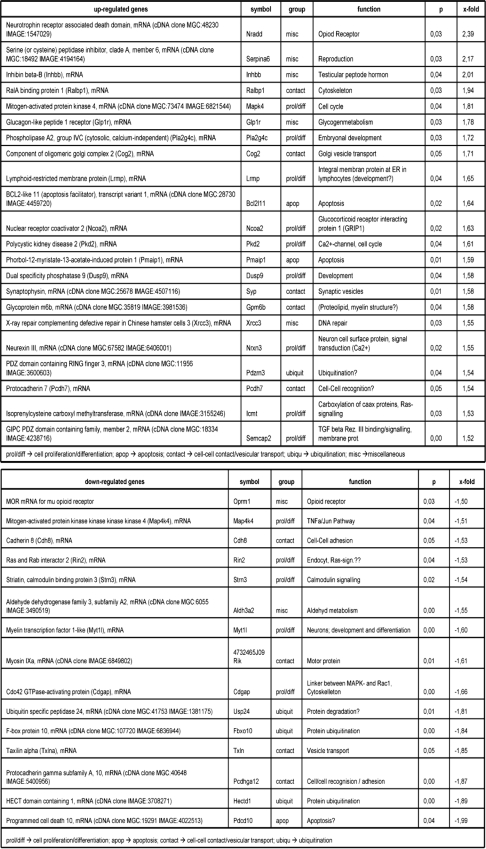
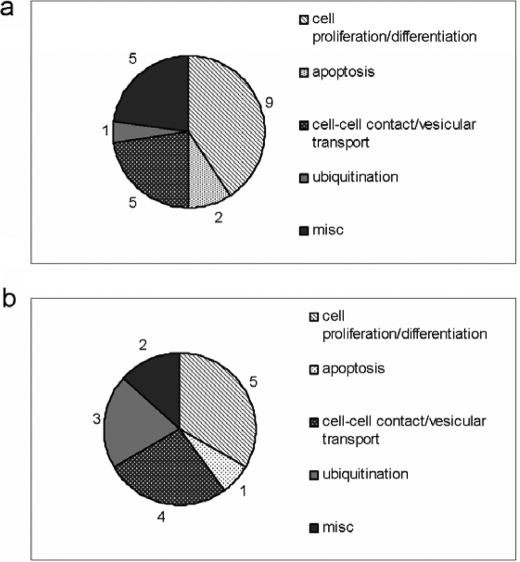
To investigate the effect of NS5A on the gene expression profile, oligonucleotide arrays complementary to 15,000 genes were performed, comparing in independent experiments three transgenic mice to the corresponding three wild-type littermates. Genes that differed in expression levels by 1.5-fold or greater in each comparison, and in all experiments with a p value of less than 0.05 were considered. Based on these parameters, 37 genes could be identified as deregulated by the presence of NS5A. These are presented in Table 1, top and bottom and summarized in Fig. 2, a and b. The observed induction of Serpina 6, Inhibb, Ralbp1, and the repression of TXln, Pcdhga12, and Pdcd10 were confirmed by RT-PCR (data not shown). Thus, in these Tg mice, NS5A seems to affect the expression of several genes related to cell proliferation, differentiation, cell-cell contact, apoptosis, and protein degradation.
TABLE 1.
Deregulated gene expression in NS5A transgenic mice determined by microarray analysis
In the top section, genes more than 1.5-fold up-regulated in NS5A transgenic mice are shown. In the bottom section, genes more than 1.5-fold down-regulated in NS5A transgenic mice are shown.
FIGURE 2.
Summary representation of genes affected by NS5A. According to their functions, genes that were affected by NS5A are summarized in different gene groups. The numbers reflect their quantity, respectively. a summarizes NS5A-dependent induced genes; b, the repressed genes.
NS5A Does Not Display a Transforming Potential in Vivo
NS5A is considered as a factor possibly involved in the process of HCV-associated pathogenesis (13). To investigate whether the expression of NS5A is able to induce liver damage such as cirrhosis or HCC, the livers from 31 transgenic mice were analyzed histologically in comparison to their wild-type littermates. Neither in the liver nor in any other organ, could tumors be detected even in the case of mice that were kept up to an age of 17 months. Besides the absence of tumors, there were no other pathological changes detectable such as neoplastic or preneoplastic changes as investigated by histological analysis based on HE and PAS staining (data not shown). Additionally there was no difference in markers for cell proliferation, such as PCNA, and ongoing cell transformation such as altered glycosylation pattern of the cell surface (investigated by lectin staining of paraffin sections) between NS5A transgenic mice and corresponding wild-type littermates. Consistent with previous data (31), these data indicate that at least on the genetic background of FVB/N mice, NS5A per se is not sufficient to induce any pathological changes in liver in vivo.
NS5A Impairs Antiviral Response
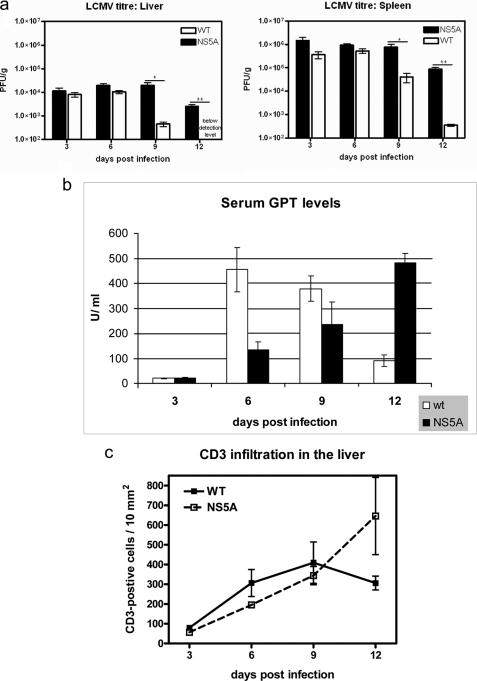
To investigate the potential role of NS5A in the establishment of a persistent viral infections in vivo, 12 wild-type and 12 NS5A transgenic mice were infected with 105 PFU LCMV. Six Tg and wild-type animals were sacrificed at days 3, 6 (peak of LCMV infection) 9, or 12 days postinfection, and the LCMV titers in the liver and in the spleen were determined. At days 3 and 6, there was a slightly elevated viral titer in the liver and in the spleen of the transgenic animals. However, at days 9 and 12 a significantly higher viral titer was found in the Tg mice in both the liver and in the spleen compared with the wild-type mice (Fig. 3a). The minor difference in viral titers seen at days 3 and 6 and the major differences seen at days 9 and 12 indicate that virus elimination is impaired by NS5A, but not the susceptibility to infection.
FIGURE 3.
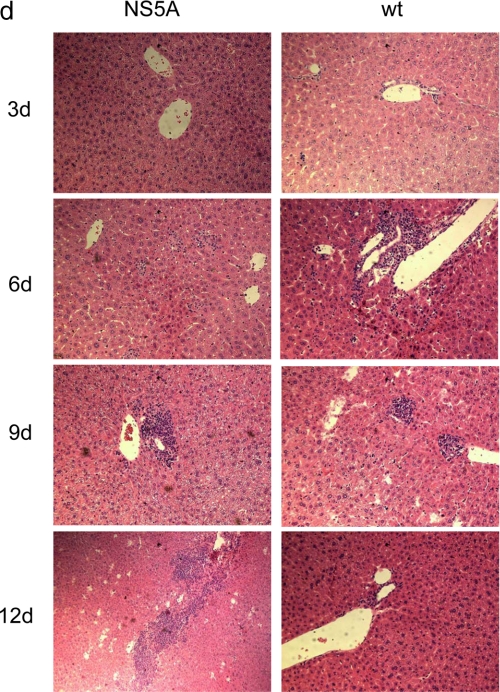
Impaired virus elimination in NS5A transgenic mice. a, NS5A-transgenic mice and the corresponding wild-type littermates were infected with LCMV (WE strain). The virus titer in the liver and spleen was determined 3, 6, 9, and 12 days after infection (22). The data are mean values from 6 animals per group. (**, p ≤ 0.01; ***, p ≤ 0.001). b, GPT activity in 6 NS5A and 6 wild-type mice at days 3, 6, 9, and 12 after LCMV infection. The values are given in units/liter (**, p ≤ 0.01). c, number of T cells was determined by immunohistochemistry of liver tissue using a CD3-specific serum. d, HE staining of paraffin sections derived from wild-type or NS5A transgenic mice sacrificed 3, 6, 9, and 12 days after LCMV infection (200× magnification).
LCMV infection triggers a potent CTL response causing rapid viral clearance around day 10 postinfection (32, 33) and that ultimately leads to severe liver damage. Therefore, the prolonged infection in the transgenics should be associated with ongoing release of liver-specific enzymes. Serum levels of GPT levels were determined in infected animals at days 3, 6, 9, and 12. Fully consistent with the impaired elimination of LCMV in the transgenic animals, in the sera derived from the NS5A transgenic mice, significantly lower GPT levels were found for days 6 and 9 and significantly higher GPT levels were found for day 12 (Fig. 3b). This is in accordance to the persistent elevated amount of T cells in the liver of the NS5A transgenics after 12 days. In wt mice, a reduction in the amount of T cells can be observed after 12 days. T cells were visualized by a CD3-specific antiserum (Fig. 3c). In addition, HE staining of liver tissues from the different time points revealed in the case of the transgenics 12 days after infection, a persistant increased infiltration indicating an ongoing immune response in the case of the NS5A transgenics compared with the wild-type mice (Fig. 3d). Taken together, these data indicate that clearance of LCMV is impaired in NS5A transgenic animals.
NS5A Impairs Interferon Immune Response
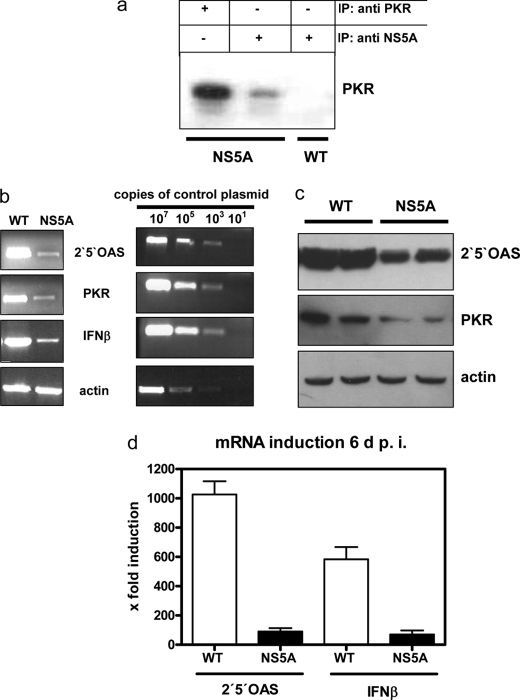
To test whether an inhibited innate immune response contributes to the delayed virus elimination in the NS5A-Tg mice we analyzed the NS5A-PKR interaction. In cell culture experiments, it has been shown that NS5A (HCV genotype 1b) directly binds to PKR, thereby inhibiting PKR activity (15). This is important since PKR is involved in the double-stranded RNA-dependent induction of IFNβ, PKR, and 2′,5′-OAS are induced subsequently by interferon α/β (34). Co-immunoprecipitation experiments of liver lysates demonstrated that PKR indeed bound NS5A also in vivo (Fig. 4a). To study the effect of NS5A on the IFN response in greater detail, we analyzed the induction of IFNβ, PKR, and 2′,5′-OAS by RT-PCR and Western blotting at day 6 after infection. RT-PCR analysis showed a strong induction of IFNβ, 2′,5′-OAS and PKR in wild-type mice, and these were all inhibited in the NS5A-Tg mice (Fig. 4b). For IFNβ and 2′,5′-OAS, this was confirmed by Lightcycler PCR (Fig. 4d). Also, Western blotting using 2′,5′-OAS- or PKR-specific antisera showed that the induction of proteins was impaired in NS5A-Tg (Fig. 4c). Taken together, these results suggest that NS5A has a strong inhibitory effect on the innate immune response that contributes to impaired virus elimination.
FIGURE 4.
NS5A impairs interferon immune response. a, co-immunoprecipitation of liver lysates derived from wild-type and NS5A transgenic mice using rabbit-derived NS5A- and PKR-specific antisera. For detection a PKR-specific monoclonal was used. b, RT-PCR for quantification of IFNβ-, 2′,5′-OAS-, and PKR-specific transcripts. RT-PCR using actin-specific primers served as control. Serial dilutions of the corresponding cDNA served in every PCR as a standard. Three wild-type and three transgenic mice were analyzed. One representative experiment is shown. c, Western blot analysis of liver-derived lysates of LCMV-infected NS5A-transgenic or wild-type mice using PKR- or 2′,5′-OAS-specific antisera. An actin-specific serum was used to control equal loading. Three wild-type and three transgenic mice were analyzed in duplicate. One representative experiment is shown. d, Lightcycler PCR for quantification of the 2′,5′-OAS- and the IFNβ-specific transcripts. Lightcycler RT-PCR was performed as described (43). The data are given as fold inductions and are mean values from three animals per group.
NS5A Interferes with CTL Response
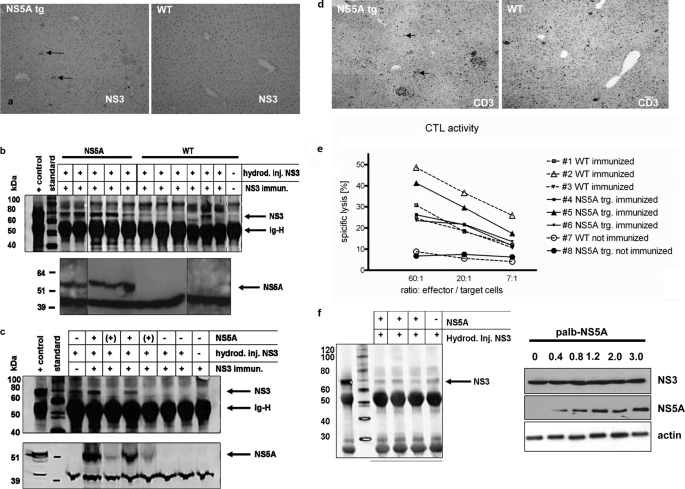
The difference in the viral titers between infected NS5A-Tg and wild-type mice was most pronounced at day 12. Taken into consideration that T-cell response peaks after the first week postinfection (33), an effect of NS5A on the adaptive immune response may be postulated. To test this possible interference of NS5A with the CTL response, we made the NS5A-Tg mice transiently transgenic also for NS33/4A proteins by hydrodynamic injection. In this model, NS3/4A-expressing liver cells are effectively eliminated by vaccine-primed CTLs (24), whereby an impaired clearance indicates an effect restricted to CTL function. Thus, NS5A-Tg and wild-type mice were vaccinated with coNS3/4A-DNA, and 13 days later they were all given a hydrodynamic injection of the same coNS3/4A plasmid (26). Three days later, the mice were sacrificed, and the presence of NS3-expressing cells was determined by immunohistochemistry and Western blot (Fig. 5a). This revealed that most NS3/4A-expressing cells were eliminated in the wild-type mice, whereas NS5A-Tg mice had a significantly higher number of NS3-positive hepatocytes. This was confirmed by Western blot analysis of liver lysates, where we also noted that mice with a high level expression of NS5A had the highest levels of NS3 (Fig. 5, b and c). This suggests that a certain amount of NS5A is needed to protect NS3/4A-positive cells from CTL-mediated elimination (Fig. 5c).
FIGURE 5.
NS5A interferes with the CTL response. Wild-type and NS5A-transgenic mice were immunized with coNS3/4A-pVAX1 or empty pVAX1 vector, indicated as not immunized, 2 weeks prior to hydrodynamic injection of coNS3/4A-pVAX1 into the tail vein. Control mice without injection were used as a negative controls as indicated. Three days after the hydrodynamic injection, mice were sacrificed and analyzed for NS3 expression in the liver. a, by immunohistochemistry of paraffin sections using a NS3-specific serum. b and c, by IP/Western blot of liver lysates. d, presence of T cells was visualized by immunohistochemistry staining of liver tissue using a CD3-specific antiserum. e, CTL activity was analyzed by 51chromium release assay using spleenic CTLs. Mice no. 7 and 8 have not been immunized. f, Western blot analysis using a NS3-specific serum of (left panel) liver lysate derived from wt and NS5A transgenic mice after hydrodynamic injection of pVaxNS3/4A or (right panel) lysate derived from HuH7.5 cells that were cotransfected with a constant amount of pVaxNS3/4A and variable amounts of palbNS5A.
To exclude that NS5A affects the expression of NS3/4A, pVaxNS3/4a was cotransfected with palbNS5A or with the empty control vector (palb) into HuH7.5 cells. The Western blot analysis revealed that the presence or absence of NS5A does not affect the amount of NS3 (Fig. 5f). Comparable results were obtained after hydrodynamic injection of pVaxNS3/4a in wt and NS5A transgenic mice and subsequent Western blot analysis using an NS3-specific antiserum confirming that NS5A does not affect the amount of NS3.
There are two ways by which NS5A could protect NS3/4A-expressing cells from elimination: either to prevent entry of CTLs to the liver or by interfering with one or more of the effector molecule pathways. To investigate whether the entry of CTLs into the liver was affected by NS5A, we monitored hepatic CD3+ cells at the time of elimination by immunohistochemistry. This revealed that comparable amounts of CD3-positive cells were present in wild-type and NS5A-Tg livers. This suggested that NS5A expression did not affect the entry of CTLs into the liver (Fig. 5d). To control the possibility that a reduced effector role of CTLs may cause the impaired elimination of NS3/4A-positive hepatocytes, the lytic activity was determined by a 51Cr-release assay. This revealed a comparable CTL activity in spleens from wild-type and NS5A-Tg mice (Fig. 5e). Taken together, these data indicate that NS5A does not affect entry of T cells into the liver or the activity of CTLs. Thus, this suggests NS5A directly protects the NS3/4A-expressing hepatocytes from elimination by the activated CTLs.
DISCUSSION
The role of NS5A on the molecular level has been well characterized, but much less is known about the relevance of NS5A for HCV-associated pathogenesis or its possible role in viral persistence. To gain more insight regarding these issues, we generated a novel transgenic mouse model that displayed a liver-specific expression of NS5A. There is a variety of reports describing the capacity of NS5A to interact with different cellular proteins and thereby modulate intracellular signal transduction cascades (reviewed in Ref. 35). We therefore investigated whether this was reflected by changes in the hepatic gene expression pattern in NS5A-Tg mice. The microarray analysis revealed significant but moderate alterations in 37 genes. At first glance, none of the genes deregulated by NS5A are known to have an obvious association with pathogenic processes. Moreover, promoter analysis of the deregulated genes does not reveal a common pattern of promoter elements shared by NS5A-dependent deregulated genes. Earlier reports on cells stably or transiently producing NS5A of genotype 1b have revealed a variety of genes that are strongly deregulated by NS5A (36). However, comparing these to the list of genes that were significantly deregulated in NS5A-Tg mice, no overlap was identified. This might be due to the difference of the experimental systems and external factors that seem to affect significantly the expression pattern of NS5A-dependent regulated genes. With respect to the moderate deregulation of gene expression in the NS5A-Tg mice, reflecting an equilibrium between transgene-dependent deregulation and compensatory mechanisms, this did not result in a clear phenotype of the NS5A gt 1a Tg mice (31). However, if this equilibrium between transgene-dependent deregulation and compensatory mechanisms is disturbed. i.e. by a viral infection, the NS5A-transgenics display a clear phenotype. This was demonstrated by impaired clearance of LCMV and reduced induction of 2′,5′-OAS or PKR in LCMV-infected NS5A-Tg mice. Apart from its direct role as an essential part of the replication complex (6, 37), NS5A can be considered as an important factor that facilitates the establishment or persistence of the HCV infection. The impaired virus elimination in LCMV-infected mice and the delayed elimination of NS3/4A-positive cells after hydrodynamic injection corroborate this. This suggests that two, possibly independent, mechanisms by which NS5A is able to promote the viral infection are described in this study: impairment of the antiviral interferon response (innate immunity) and the interference with the CTL response (adaptive immunity).
The binding of NS5A genotype 1b to PKR (15) in vivo inhibits PKR that is an essential player for induction and transduction of the interferon-dependent antiviral response (38). In accord with this, the LCMV-infected NS5A-Tg mice had a decreased induction of IFNβ, 2′,5′-OAS and PKR, reflecting the inhibitory interference of NS5A with the antiviral interferon response.
Many viruses have the potential to interfere with different steps in the antiviral interferon response to escape the host defense system (39). The HCV NS3/4A complex targets MAVS/IPS-1/VISA/Cardif for cleavage as an immune evasion strategy (40). The NS5A-dependent inactivation of PKR could provide an additional escape mechanism from the host antiviral response and thereby favors viral replication and presumably also persistence. The results from the LCMV infection experiments with the NS5A-Tgs identify NS5A as at least one factor that confers reduced viral clearance in LCMV-infected Tg mice expressing the complete HCV genome (14).
Apart from the effect of NS5A on the innate immune response, the observed NS5A-dependent interference with the CTL response might be an additional factor that confers impaired LCMV elimination in the NS5A-Tgs. The most pronounced differences in LCMV titers were observed after day 6, a time point when T-cell response has been primed. Evidence for the interference of NS5A with the acquired immune response was obtained from the inhibited elimination of NS3/4A-expressing hepatocytes generated by a hydrodynamic injection model. The clearance of NS3-positive cells in this model is exclusively CTL-mediated (24). Because neither CTL activity nor entry of T cells into the liver was altered in the NS5A-Tg mice, NS5A could exert a protective effect on the liver cell. Since the CTL-dependent clearance is in part mediated by TNFα, the inhibitory effect of NS5A on TNFα-dependent apoptosis (41) could be relevant. Moreover, one could speculate that NS5A is the molecule that interferes with Fas-mediated apoptosis through Bid as reported from mice expressing the full HCV polyprotein (42). However incubation of primary hepatocytes derived from wt or NS5A transgenic mice with TNFα demonstrated no difference in their sensitivity to TNFα-mediated apoptosis (supplemental Fig. S3).
In conclusion, NS5A expression in vivo may help HCV to establish and/or maintain the chronic infection by inhibiting both the innate interferon response and the adaptive CTL response. Considering these new aspects of the relevance of NS5A for the HCV infection could help to find new approaches to prevent the establishment of a chronic HCV infection.
Supplementary Material
Acknowledgments
We thank Prof. Hans Will, HPI Hamburg, for the generous support of this study and Prof. U. Schumacher, UKE Hamburg, for helping with the histological analysis.
This work was supported in part by grants from the Swedish Society of Medical Research, the Swedish Society of Medicine, the Royal Swedish Academy of Sciences, Goljes Memorial Fund, the Ruth and Richard Juhlin Foundation, and the Karolinska Institutet (to L. F.). This work was also supported by a grant from the DFG-excellence cluster (to E. H.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental data and Figs. S1–S3.
- HCV
- hepatitis C virus
- NS
- non-structural
- Tg
- transgenic
- ER
- endoplasmic reticulum
- wt
- wild type
- GPT
- glutamyl pyruvic transaminase
- CTL
- cytotoxic T lymphocyte
- LCMV
- lymphocytic choriomeningitis virus
- PFU
- plaque-forming units
- PKR
- protein kinase R
- IFN
- interferon
- OAS
- oligodenylate synthetase
- HE
- hematoxylin and eosin.
REFERENCES
- 1.Hoofnagle J. H. (2002) Hepatology 36, S21–29 [DOI] [PubMed] [Google Scholar]
- 2.Hijikata M., Mizushima H., Tanji Y., Komoda Y., Hirowatari Y., Akagi T., Kato N., Kimura K., Shimotohno K. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 10773–10777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaneko T., Tanji Y., Satoh S., Hijikata M., Asabe S., Kimura K., Shimotohno K. (1994) Biochem. Biophys. Res. Commun. 205, 320–326 [DOI] [PubMed] [Google Scholar]
- 4.Penin F., Dubuisson J., Rey F. A., Moradpour D., Pawlotsky J. M. (2004) Hepatology 39, 5–19 [DOI] [PubMed] [Google Scholar]
- 5.Pietschmann T., Lohmann V., Rutter G., Kurpanek K., Bartenschlager R. (2001) J. Virol. 75, 1252–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appel N., Pietschmann T., Bartenschlager R. (2005) J. Virol. 79, 3187–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qadri I., Iwahashi M., Simon F. (2002) Biochim. Biophys. Acta 1592, 193–204 [DOI] [PubMed] [Google Scholar]
- 8.Tan S. L., Nakao H., He Y., Vijaysri S., Neddermann P., Jacobs B. L., Mayer B. J., Katze M. G. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 5533–5538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bürckstümmer T., Kriegs M., Lupberger J., Pauli E. K., Schmittel S., Hildt E. (2006) FEBS Lett. 580, 575–580 [DOI] [PubMed] [Google Scholar]
- 10.Street A., Macdonald A., Crowder K., Harris M. (2004) J. Biol. Chem. 279, 12232–12241 [DOI] [PubMed] [Google Scholar]
- 11.Ghosh A. K., Steele R., Meyer K., Ray R., Ray R. B. (1999) J. Gen. Virol. 80, 1179–1183 [DOI] [PubMed] [Google Scholar]
- 12.Gale M., Jr., Kwieciszewski B., Dossett M., Nakao H., Katze M. G. (1999) J. Virol. 73, 6506–6516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levrero M. (2006) Oncogene 25, 3834–3847 [DOI] [PubMed] [Google Scholar]
- 14.Blindenbacher A., Duong F. H., Hunziker L., Stutvoet S. T., Wang X., Terracciano L., Moradpour D., Blum H. E., Alonzi T., Tripodi M., La Monica N., Heim M. H. (2003) Gastroenterology 124, 1465–1475 [DOI] [PubMed] [Google Scholar]
- 15.Gale M. J., Jr., Korth M. J., Tang N. M., Tan S. L., Hopkins D. A., Dever T. E., Polyak S. J., Gretch D. R., Katze M. G. (1997) Virology 230, 217–227 [DOI] [PubMed] [Google Scholar]
- 16.Brinster R. L., Allen J. M., Behringer R. R., Gelinas R. E., Palmiter R. D. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 836–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinkert C. A., Ornitz D. M., Brinster R. L., Palmiter R. D. (1987) Genes Dev. 1, 268–276 [DOI] [PubMed] [Google Scholar]
- 18.Hildt E., Munz B., Saher G., Reifenberg K., Hofschneider P. H. (2002) EMBO J. 21, 525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chisari F. V. (1989) Mol. Biol. Med. 6, 143–149 [PubMed] [Google Scholar]
- 20.Werner S., Weinberg W., Liao X., Peters K. G., Blessing M., Yuspa S. H., Weiner R. L., Williams L. T. (1993) EMBO J. 12, 2635–2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauer U., Weiss L., Hofschneider P. H., Kekulé A. S. (1992) J. Virol. 66, 5284–5289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruns M., Gessner A., Lother H., Lehmann-Grube F. (1988) Virology 166, 133–139 [DOI] [PubMed] [Google Scholar]
- 23.Saher G., Hildt E. (1999) J. Biol. Chem. 274, 27651–27657 [DOI] [PubMed] [Google Scholar]
- 24.Ahlen G., Nystrom J., Pult I., Frelin L., Hultgren C., Sallberg M. (2005) J. Infect. Dis. 192, 2112–2116 [DOI] [PubMed] [Google Scholar]
- 25.Jörns J., Mangold U., Neumann U., Van , Damme E. J., Peumans W. J., Pfüller U., Schumacher U. (2003) Anat. Embryol. 207, 85–94 [DOI] [PubMed] [Google Scholar]
- 26.Frelin L., Ahlén G., Alheim M., Weiland O., Barnfield C., Liljeström P., Sällberg M. (2004) Gene Ther. 11, 522–533 [DOI] [PubMed] [Google Scholar]
- 27.Frelin L., Alheim M., Chen A., Söderholm J., Rozell B., Barnfield C., Liljeström P., Sällberg M. (2003) Gene Ther. 10, 686–699 [DOI] [PubMed] [Google Scholar]
- 28.Donauer J., Rumberger B., Klein M., Faller D., Wilpert J., Sparna T., Schieren G., Rohrbach R., Dern P., Timmer J., Pisarski P., Kirste G., Walz G. (2003) Transplantation 76, 539–547 [DOI] [PubMed] [Google Scholar]
- 29.Boldrick J. C., Alizadeh A. A., Diehn M., Dudoit S., Liu C. L., Belcher C. E., Botstein D., Staudt L. M., Brown P. O., Relman D. A. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 972–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malmgaard L., Salazar-Mather T. P., Lewis C. A., Biron C. A. (2002) J. Virol. 76, 4520–4525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majumder M., Steele R., Ghosh A. K., Zhou X. Y., Thornburg L., Ray R., Phillips N. J., Ray R. B. (2003) FEBS Lett. 555, 528–532 [DOI] [PubMed] [Google Scholar]
- 32.Assmann-Wischer U., Simon M. M., Lehmann-Grube F. (1985) Med. Microbiol. Immunol. 174, 249–256 [DOI] [PubMed] [Google Scholar]
- 33.Lehmann-Grube F., Assmann U., Löliger C., Moskophidis D., Löhler J. (1985) J. Immunol. 134, 608–615 [PubMed] [Google Scholar]
- 34.Haller O., Kochs G., Weber F. (2006) Virology 344, 119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macdonald A., Harris M. (2004) J. Gen. Virol. 85, 2485–2502 [DOI] [PubMed] [Google Scholar]
- 36.Girard S., Vossman E., Misek D. E., Podevin P., Hanash S., Bréchot C., Beretta L. (2004) Hepatology 40, 708–718 [DOI] [PubMed] [Google Scholar]
- 37.Penin F., Brass V., Appel N., Ramboarina S., Montserret R., Ficheux D., Blum H. E., Bartenschlager R., Moradpour D. (2004) J. Biol. Chem. 279, 40835–40843 [DOI] [PubMed] [Google Scholar]
- 38.Samuel C. E. (2001) Clin. Microbiol. Rev. 14, 778–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hengel H., Koszinowski U. H., Conzelmann K. K. (2005) Trends Immunol. 26, 396–401 [DOI] [PubMed] [Google Scholar]
- 40.Cheng G., Zhong J., Chisari F. V. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 8499–8504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majumder M., Ghosh A. K., Steele R., Zhou X. Y., Phillips N. J., Ray R., Ray R. B. (2002) Virology 294, 94–105 [DOI] [PubMed] [Google Scholar]
- 42.Disson O., Haouzi D., Desagher S., Loesch K., Hahne M., Kremer E. J., Jacquet C., Lemon S. M., Hibner U., Lerat H. (2004) Gastroenterology 126, 859–872 [DOI] [PubMed] [Google Scholar]
- 43.Stobart M. J., Parchaliuk D., Simon S. L., Lemaistre J., Lazar J., Rubenstein R., Knox J. D. (2007) Mol. Neurodegener. 2, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.