Abstract
The E3 ubiquitin ligase c-Cbl ubiquitinates the G protein-coupled receptor protease-activated receptor 2 (PAR2), which is required for postendocytic sorting of activated receptors to lysosomes, where degradation terminates signaling. The mechanisms of PAR2 deubiquitination and its importance in trafficking and signaling of endocytosed PAR2 are unknown. We report that receptor deubiquitination occurs between early endosomes and lysosomes and involves the endosomal deubiquitinating proteases AMSH and UBPY. Expression of the catalytically inactive mutants, AMSH(D348A) and UBPY(C786S), caused an increase in PAR2 ubiquitination and trapped the receptor in early endosomes, thereby preventing lysosomal trafficking and degradation. Small interfering RNA knockdown of AMSH or UBPY also impaired deubiquitination, lysosomal trafficking, and degradation of PAR2. Trapping PAR2 in endosomes through expression of AMSH(D348A) or UBPY(C786S) did not prolong the association of PAR2 with β-arrestin2 or the duration of PAR2-induced ERK2 activation. Thus, AMSH and UBPY are essential for trafficking and down-regulation of PAR2 but not for regulating PAR2 dissociation from β-arrestin2 or PAR2-mediated ERK2 activation.
Ubiquitination of certain G protein-coupled receptors (GPCRs)3 is an essential signal for their postendocytic trafficking to lysosomes, which prevents uncontrolled signaling during chronic stimulation. Agonists stimulate ubiquitination of the β2-adrenergic receptor (β2AR), chemokine (CXC motif) receptor 4, and protease-activated receptor 2 (PAR2), and the E3 ubiquitin ligases that mediate ubiquitination of these GPCRs and associated proteins, such as β-arrestins, have been identified (1–3). Although ubiquitination of these receptors is not required for endocytosis, ubiquitin-resistant mutant receptors show diminished postendocytic sorting to lysosomes and impaired down-regulation. However, despite of the reversible nature of this post-translational modification, little is known about the role of deubiquitinating proteases (DUBs) in the postendocytic trafficking and signaling of GPCRs.
Our understanding of the role of DUBs in postendocytic receptor trafficking mostly derives from studies of receptor tyrosine kinases, such as epidermal growth factor receptor (EGFR). Two endosomal DUBs, AMSH (associated molecule with the Src homology 3 domain of STAM (signal-transducing adapter molecule)) and UBPY (ubiquitin-specific protease Y) (also known as USP8), regulate deubiquitination and postendocytic trafficking of EGFR (4). AMSH belongs to the JAMM (JAB1/MPN/Mov34) family of metalloproteases and shows specificity for Lys63- over Lys48-linked ubiquitin chains (5, 6). UBPY is a cysteine protease of the ubiquitin-specific protease (USP) family and does not discriminate between Lys48- and Lys63-linked ubiquitin (7, 8). Activated EGFR recruits the E3 ligase c-Cbl, which ubiquitinates the receptor (9). Ubiquitinated EGFR then interacts with the Hrs (hepatocyte growth factor-regulated tyrosine kinase substrate)-STAM complex in early endosomes (10). Hrs-STAM forms part of the ESCRT (endosomal sorting complex required for transport)-I, -II, -III complex that sorts ubiquitinated receptors in multivesicular bodies (MVBs) to intralumenal vesicles that eventually fuse with lysosomes, where degradation occurs (11). Before receptors are incorporated into the intralumenal vesicles, they are deubiquitinated, which serves to maintain levels of free ubiquitin (11). AMSH and UBPY interact directly with STAM through a common binding site within its Src homology 3 domain (12–14). The balance of EGFR ubiquitination by c-Cbl and deubiquitination by AMSH and UBPY controls the postendocytic trafficking and down-regulation of the EGFR. c-Cbl promotes lysosomal degradation of the EGFR (9), AMSH opposes c-Cbl action and promotes EGFR recycling (5), and UBPY is required for lysosomal sorting and degradation of EGFR (8, 15–17). The role of AMSH and UBPY in regulating deubiquitination, trafficking, and signaling of GPCRs in endosomes is largely unknown. A recent study has shown, however, that AMSH and UBPY regulate the down-regulation of the δ-opioid receptor (DOR), a GPCR that is ubiquitinated and degraded following activation (18). Expression of catalytically inactive mutants of AMSH or UBPY or knockdown of AMSH or UBPY levels using siRNA inhibits down-regulation of DOR. Interestingly, the roles of AMSH and UBPY in DOR down-regulation appear to be nonredundant, since depletion of either DUB produced comparable effects, and simultaneous depletion of both DUBs did not have additional consequences (18). Different DUBs, USP20 and -33, have been recently shown to reverse agonist-induced ubiquitination of the β2AR (19).
We examined the roles of AMSH and UBPY in the ubiquitination, postendocytic trafficking, and lysosomal degradation of PAR2. We also determined whether AMSH and UBPY regulate PAR2 association with β-arrestins in endosomes and control β-arrestin-mediated extracellular signal-regulated kinase (ERK) activation. PAR2 is a receptor for multiple serine proteases that are generated during injury and inflammation (20). Activated PAR2 promotes inflammation and pain, and PAR2 contributes to inflammatory diseases of the airway, joints, and intestine. PAR2 levels are elevated during inflammation, due to increased mRNA expression or perhaps decreased receptor degradation, which amplifies the proinflammatory actions of proteases (21). Given the irreversible nature of proteolytic activation, and since the internalized receptor probably signals by the β-arrestin-dependent recruitment of mitogen-activated protein kinase (MAPK) to endosomes (22), termination of PAR2 signaling requires receptor degradation in lysosomes, which in turn is ubiquitination-dependent (3, 23). It is therefore important to understand mechanisms of PAR2 ubiquitination and lysosomal targeting and also how these processes can be reversed. We have reported that activated PAR2 is monoubiquitinated at multiple sites by the E3 ligase c-Cbl and targeted to lysosomes by an Hrs-dependent pathway (3, 24). Nothing is known about the mechanism and function of PAR2 deubiquitination. Herein, we examined the role of AMSH and UBPY in regulating the deubiquitination, lysosomal trafficking, and degradation of PAR2, the interaction of PAR2 with β-arrestin2, and β-arrestin-mediated ERK2 activation. We demonstrate that endosomal DUBs are key regulators of GPCR down-regulation.
EXPERIMENTAL PROCEDURES
Reagents
Antibodies were from the following sources: rabbit anti-FLAG, rabbit anti-HA11, mouse anti-β-actin, and rabbit anti-USP8/UBPY (Sigma); mouse anti-human transferrin receptor (TfR; Invitrogen); mouse anti-EEA1 (early endosomal antigen 1; BD Transduction Laboratories); mouse anti- human LAMP1 (lysosome-associated glycomembrane protein-1; Developmental Studies Hybridoma Bank, Iowa City, IA); rat high affinity anti-HA11 (Roche Applied Science); mouse anti-ubiquitin (P4D1), mouse anti-pERK1/2 (E-4), rabbit anti-ERK2 (C-14), goat anti-PAR2 (C-17), rabbit anti-Rab5a (S-19), mouse nonspecific IgG1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); rabbit anti-AMSH (ProteinTech Group, Chicago, IL); goat anti-HSV (Abcam, Cambridge, MA); goat anti-mouse IgG, goat anti-rabbit IgG, donkey anti-rabbit IgG, or donkey anti-goat IgG, coupled to fluorescein isothiocyanate, rhodamine red-X, or Cy5 (Jackson ImmunoResearch, West Grove, PA); goat anti-mouse or rabbit IgG coupled to AlexaFluor®680 (Invitrogen) and coupled to IRDye™800 (Rockland Immunochemicals, Gilbertsville, PA). NeutrAvidin-agarose and EZ-Link™-Sulfo-NHS-Biotin were from Pierce. Human EGF was from Invitrogen.
cDNAs
cDNA for PAR2 has been described (3, 24). GFP-AMSH, GFP-UBPY, GFP-AMSH(D348A), and GFP-UBPY(C786S) were from Dr. S. Urbe (University of Liverpool, Liverpool, UK). Myc-Hrs and Rab5aQ79L-GFP were from Dr. M. von Zastrow (University of California, San Francisco, CA), and a CFP tag was added to Rab5aQ79L by subcloning. Human β-arrestin2 was cloned from human embryonic kidney 293 (HEK) cells by reverse transcription-PCR, and an HSV tag was added to the C terminus by PCR. pcDNA5/FRT was from Invitrogen.
Transfected Cells and Cell Lines
HEK cells were grown in Dulbecco's modified Eagle's medium containing 10% heat-inactivated FBS (95% air, 5% CO2, 37 °C). The generation and maintenance of HEK-FLP cells (Invitrogen) stably expressing PAR2 (HEK-PAR2 cells) with an N-terminal FLAG and C-terminal HA11 or T7 epitopes have been described (3, 24). HEK cells were transiently transfected using Lipofectamine™ 2000 (Invitrogen) according to the manufacturer's guidelines. Cells were plated 48 h prior to experiments and incubated in Dulbecco's modified Eagle's medium, 0.1% bovine serum albumin for treatments. For analysis of ERK2 activation, cells were deprived of serum (Dulbecco's modified Eagle's medium, 0.1% bovine serum albumin) overnight before experiments.
Activation of PAR2 and Drug Treatments
Cells were stimulated with PAR2 activating peptide (AP; tethered ligand of mouse PAR2, SLIGRL-NH2, 100 μm; CPC Scientific, San Jose, CA), bovine pancreatic trypsin (10 nm; Worthington), or human neutrophil elastase (0.5 μm; Calbiochem). To inhibit lysosomal proteases, cells were treated with ZPAD, E64d (200 and 20 μm, respectively; Bachem), and pepstatin A (10 μm; Roche Applied Science). To inhibit clathrin-mediated endocytosis, cells were treated with hypertonic sucrose (0.45 m). To inhibit EGFR tyrosine kinase activity, cells were treated with AG1478 (1 μm; Calbiochem). Inhibitors were preincubated with cells 30–60 min prior to stimulation with AP and were present throughout experiments. To inhibit N-linked glycosylation, cells were treated with 10 μg/ml tunicamycin (Sigma) for 24 h.
Immunofluorescence and Confocal Microscopy
Cells were plated at ∼3 × 105 per 35-mm dish onto coverslips coated with poly-d-lysine (100 μg/ml). Cells were washed in 100 mm PBS, pH 7.4, and fixed in PBS containing 4% paraformaldehyde, pH 7.4 (20 min, 4 °C). Cells were washed with PBS containing 0.1% saponin and 1% normal goat serum or 2% normal donkey serum for 30 min. Proteins were localized using the primary antibodies HA11 (rabbit, 1:200), EEA1 (1:500), LAMP1 (1:1,000), and HSV (1:1000) (overnight, 4 °C). Cells were washed and incubated with secondary antibodies coupled to fluorescein isothiocyanate, rhodamine red-X, or Cy5 (1:200, 2 h, room temperature). To examine trafficking of PAR2 from the plasma membrane, cell surface PAR2 was labeled by incubating cells with antibody to an extracellular epitope tag (FLAG; 1:100, 1 h, 37 °C). Cells were washed with PBS, stimulated with AP, fixed and incubated with secondary antibodies. Antibody-tagged PAR2 traffics similarly to non-tagged receptor (3). Cells were observed with a Zeiss laser-scanning confocal microscope (LSM Meta 510) using a Fluar Plan Apochromat ×63 oil immersion objective (numerical aperture 1.4) or a Plan Apochromat ×100 oil immersion objective (numerical aperture 1.4). Images were collected at a zoom of 2–3 and an iris of <3 μm, and eight optical sections were taken at intervals of 0.5 μm. Single sections are shown. Images were processed (colored and merged) with the Zeiss (LSM 510) software. Colocalization of proteins in organelles was analyzed by drawing regions of interest around the outside of a cell in the merged image and measuring the overlap coefficient, with a coefficient of 0 indicating no colocalization and of 1 indicating complete colocalization. >20 cells were analyzed for each experiment.
SDS-PAGE and Western Blotting
Cells were lysed in 50 mm Tris/HCl, pH 7.4, 1% SDS. For analysis of ERK2 activation, cells were lysed in 50 mm Tris/HCl, pH 7.4, 150 mm NaCl, 5 mm MgCl2, 1 mm EGTA, 10 mm NaF, 10 mm Na4P2O7, 10 mm Na3VO4, 1% Nonidet P-40. Lysates were separated by SDS-PAGE (8, 10, or 12%). Proteins were transferred to polyvinylidene difluoride membranes (Immobilon-FL, Millipore, Billerica, MA) and blocked for 1 h at room temperature (Odyssey Blocking Buffer, LiCOR, Lincoln, NE). Membranes were incubated with antibodies to HA11 (rabbit, 1:5000), PAR2 C-17 (1:1000), TfR (1:1000), ubiquitin P4D1 (1:1000), β-actin (1:20,000), pERK1/2 (1:1000), ERK2 (1:5000), AMSH (1:1000), or UBPY (1:1000) (overnight, 4 °C). Membranes were washed (1× PBS, 0.1% Tween 20, 30 min) and incubated with secondary antibodies coupled to AlexaFluor®680 or IRDye™800 (1:20,000, 1 h, room temperature), and blots were analyzed with the Odyssey Infrared Imaging System (LiCOR). PAR2 is detected as several major forms by Western blotting due to differential glycosylation of the receptor (3, 25). To quantify PAR2 levels, we included all forms of PAR2 receptor detected. Quantified signals are indicated in the figures by a bracket beside the Western blots. To quantify PAR2 degradation, PAR2 signals were compared with TfR signals; to quantify PAR2 ubiquitination, PAR2-ubiquitin signals were compared with total PAR2 signals; and to quantify ERK2 activation, pERK2 signals were compared with total ERK2 signals.
Cell Surface Biotinylation
Cells were plated at ∼1 × 106 cells/35-mm dish coated with poly-d-lysine. After 48 h, cells were washed in 100 mm PBS, pH 7.4, and incubated with 0.3 mg/ml EZ-Link™-Sulfo-NHS-Biotin in PBS for 30 min at 4 °C to biotinylate cell surface proteins. Cells were washed in PBS, stimulated with AP, lysed in RIPA buffer (50 mm Tris/HCl, pH 7.4, 150 mm NaCl, 5 mm MgCl2, 1 mm EGTA, 10 mm NaF, 10 mm Na4P2O7, 0.1 mm Na3VO4, 0.5% Nonidet P-40), and centrifuged. Biotinylated proteins were recovered by incubation with 40 μl of NeutrAvidin-agarose (overnight, 4 °C), pelleted, washed with RIPA buffer, boiled in Laemmli buffer, and analyzed by Western blotting.
Immunoprecipitation
For denaturing immunoprecipitation, cells were lysed in 50 mm Tris/HCl, pH 7.4, 1% SDS; sonicated; mixed with 9 volumes of RIPA buffer; and centrifuged. Supernatants were rotated with immunoprecipitating antibody (rat HA11, 500 ng) for 1 h at 4 °C. Protein A/G PLUS (Santa Cruz Biotechnology) was added (30 μl), and samples were rotated for 1 h at 4 °C. Immunoprecipitates were pelleted, washed with RIPA buffer, boiled in Laemmli buffer, and analyzed by SDS-PAGE and Western blotting.
siRNA
siRNA reagents were from Dharmacon (Chicago, IL). ON-TARGETplus SMARTpool L-005203-00 and L-012202-00 each consisted of four distinct siRNA duplexes targeted to knockdown of human UBPY mRNA and of human AMSH mRNA, respectively. siCONTROL nontargeting siRNApool (D-001206) consisted of four off-target siRNA duplexes. HEK cells (0.3 × 106 cells/well of a 6-well plate in antibiotic-free medium) were transfected with 200 μmol of siRNA and 5 μl of DharmaFECT1 according to the manufacturer's instructions. Cells were incubated in the transfection medium for 72 h and then used for experiments.
Endosome Isolation
Cells were washed with PBS and suspended in 10 mm HEPES, pH 7.2, 100 mm KCl, 1 mm EDTA, 25 mm sucrose. The cell suspension was passed through a 22-gauge syringe needle 10 times and centrifuged at 3000 × g for 10 min at 4 °C. Supernatants were rotated with mouse anti-EEA1 antibody (2.5 μg, 1 h, 4 °C). Immune complexes were captured with rat anti-mouse IgG1 magnetic microbeads (1 h, 4 °C) and purified using MACS MS separation columns (Miltenyi Biotec, Auburn, CA) (26).
Statistics
Results are expressed as mean ± S.E. of n ≥ 3 experiments and were compared by Student's t test, with p < 0.05 (asterisk) considered to be significant. Immunofluorescence images and blots represent n ≥ 3 experiments.
RESULTS
Detection of PAR2 by Western Blotting and Immunoprecipitation
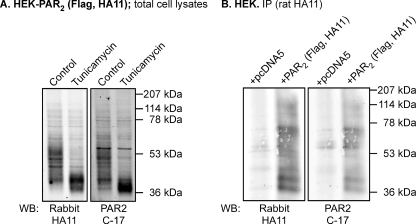
We evaluated the expression of PAR2 in HEK cells by Western blotting using antibodies to a C-terminal HA11 epitope (rabbit anti-HA11) and to the C terminus of human PAR2 (C-17). Both antibodies detected several forms of PAR2 in HEK-PAR2 cells (Fig. 1A). We treated cells with tunicamycin, which inhibits N-linked glycosylation, to determine whether the multiple forms of PAR2 represent variably glycosylated receptors. Treatment of HEK-PAR2 cells with tunicamycin for 24 h reduced the molecular mass of PAR2 to ∼40–45 kDa, similar to the predicted molecular mass of PAR2 (44 kDa) (Fig. 1A). Both antibodies detected the same deglycosylated protein. Thus, PAR2 is variably glycosylated in HEK cells, as we have previously reported (3, 25). To confirm specific detection of PAR2 in immunoprecipitates, we analyzed HEK cells transfected with PAR2 (N-terminal FLAG and C-terminal HA11 epitopes) or empty vector (pcDNA5). PAR2 was immunoprecipitated using an antibody to the HA11 epitope (rat anti-HA11), and Western blots were probed using antibodies to the HA11 epitope (rabbit anti-HA11) or the C terminus of human PAR2 (C-17). Antibodies to the HA11 epitope and the C terminus of human PAR2 detected the same proteins in cells expressing PAR2, and there were no signals in cells expressing empty vector, confirming specific detection of PAR2 (Fig. 1B).
FIGURE 1.
Expression and glycosylation of PAR2 in HEK cells. A, HEK-PAR2 cells were treated with tunicamycin (10 μg/ml) for 24 h to inhibit N-linked glycosylation. Total cell lysates were analyzed by Western blotting (WB) for HA11 (rabbit) or PAR2 (C-17). Treatment with tunicamycin decreased the molecular mass of PAR2 to ∼40–45 kDa. B, HEK cells were transiently transfected with pcDNA5 (control) or PAR2. PAR2 was immunoprecipitated (IP; rat HA11) and analyzed by Western blotting for HA11 (rabbit) or PAR2 (C-17). Both antibodies detected multiple PAR2 protein species in PAR2-transfected cells.
PAR2 Activating Peptide and Trypsin but Not Elastase Induce Ubiquitination and Degradation of PAR2
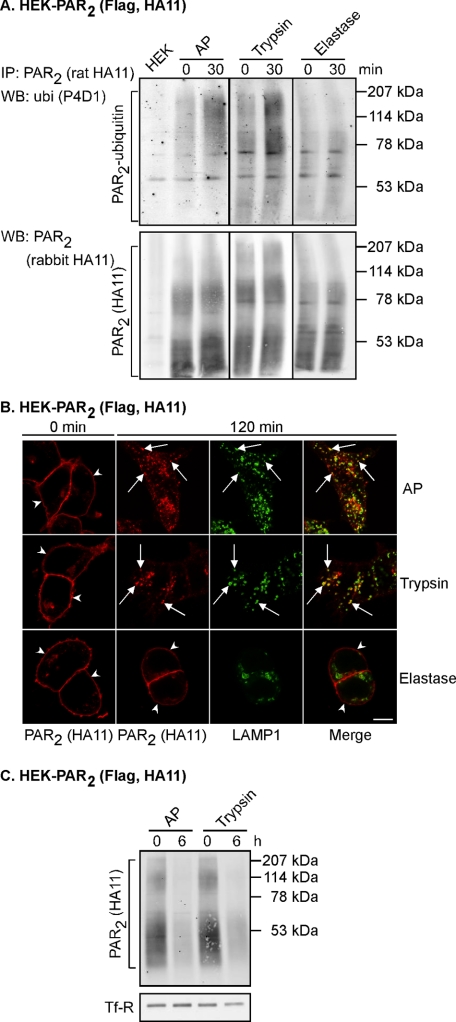
To confirm agonist-induced ubiquitination of PAR2, as we have reported previously (3), HEK-PAR2 cells were treated for 30 min with PAR2 AP or the physiological agonist trypsin. As a control, HEK-PAR2 cells were incubated for 30 min with elastase, which cleaves but does not activate PAR2 (27). PAR2 ubiquitination was examined by immunoprecipitation and Western blotting under denaturing conditions. As expected, AP and trypsin both induced ubiquitination of PAR2 (Fig. 2A). In contrast, there was no detectable ubiquitination of PAR2 following treatment of cells with the non-activating protease elastase (Fig. 2A). The HA antibody detected multiple forms of PAR2 (Fig. 2A). We similarly detected multiple forms of variably glycosylated PAR2 by Western blotting of total cell lysates and immunoprecipitated PAR2 from HEK cells transfected with PAR2, using both epitope tag (HA) and PAR2 (C-17) antibodies (Fig. 1, A and B).
FIGURE 2.
AP and trypsin, but not elastase, induce PAR2 ubiquitination, trafficking to lysosomes, and degradation. A, HEK-PAR2 cells were stimulated for 0 or 30 min with AP (100 μm), trypsin (10 nm), or elastase (0.5 μm). PAR2 was immunoprecipitated (IP), and membranes were analyzed by Western blotting (WB) for ubiquitin (ubi) and PAR2. Only AP and trypsin induced ubiquitination of PAR2. B, HEK-PAR2 cells were challenged for 0 or 120 min with AP (100 μm), trypsin (10 nm), or elastase (0.5 μm). Cells were fixed, and proteins were localized by indirect immunofluorescence with antibodies to HA11 (PAR2) and LAMP1. In unstimulated cells, PAR2 was located at the plasma membrane (arrowheads) and in a perinuclear pool. Stimulation with AP or trypsin induced PAR2 translocation to LAMP1-containing vesicles (arrows). In contrast, PAR2 remained at the plasma membrane following treatment with elastase (arrowheads). Scale bar, 10 μm. C, cell surface proteins on HEK-PAR2 cells were biotinylated prior to stimulation with AP (100 μm) or trypsin (10 nm) for 0 or 6 h. Biotinylated proteins were precipitated using avidin beads, and PAR2 and TfR were detected by Western blotting. Stimulation with AP or trypsin both induced degradation of PAR2. Results shown representative of n = 3 experiments.
To confirm that activation of PAR2 induces trafficking of the receptor to lysosomes as we have reported previously (3, 23), HEK-PAR2 cells were stimulated with AP, trypsin, or elastase for 0 or 120 min prior to fixation and localization of PAR2 and the lysosome marker LAMP1 by immunofluorescence microscopy. In unstimulated cells, PAR2 was detected at the plasma membrane (Fig. 2B). We also detected a small amount of PAR2 at an intracellular site in unstimulated cells, which we have previously identified as the Golgi apparatus (23, 28). Treatment of cells with AP or trypsin for 120 min induced trafficking of PAR2 to LAMP-1-containing vesicles (Fig. 2B). In contrast, PAR2 remained at the plasma membrane following incubation of cells with the non-activating protease elastase for 120 min (Fig. 2B). Thus, activation of PAR2 induces translocation of the receptor from the plasma membrane to lysosomes.
To determine whether activated PAR2 that traffics to lysosomes is degraded, we biotinylated cell surface proteins of HEK-PAR2 cells prior to stimulation with AP or trypsin for 0 or 6 h. The levels of biotinylated PAR2 remaining after treatments were determined by pull-down with avidin beads and Western blotting. Both AP and trypsin induced degradation of PAR2 (Fig. 2C). Thus, activation of PAR2 by AP or the physiological agonist trypsin has the same outcome of PAR2 ubiquitination, trafficking to lysosomes, and degradation.
PAR2 Is Ubiquitinated at the Plasma Membrane and in Early Endosomes and Deubiquitinated during Trafficking from Early Endosomes to Lysosomes
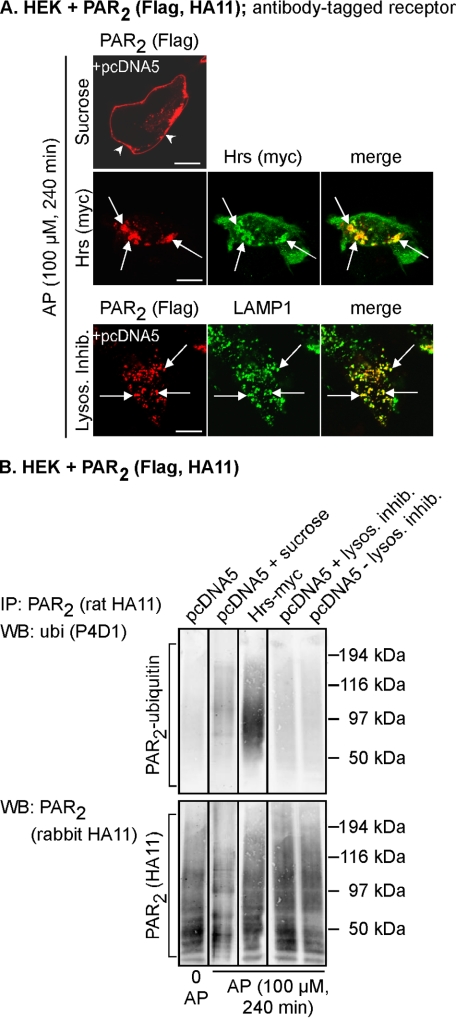
c-Cbl mediates agonist-induced ubiquitination, degradation, and down-regulation of PAR2 (3). Upon activation of PAR2, c-Cbl traffics to the plasma membrane to colocalize with PAR2, and then PAR2 and c-Cbl redistribute to the same early endosomes. Thus, PAR2 is ubiquitinated at an early stage of the endosomal pathway. To identify the subcellular compartments where PAR2 is ubiquitinated and deubiquitinated, we blocked trafficking of PAR2 at various stages of the endosomal pathway by using hypertonic sucrose (which prevents receptor internalization (29)), Hrs overexpression (which traps PAR2 in early endosomes (24)), and lysosomal protease inhibitors (which allows accumulation of PAR2 in lysosomes (3, 23)). Cell surface PAR2 was labeled with an antibody to an extracellular epitope, which allowed examination of trafficking from the plasma membrane by immunofluorescence and confocal microscopy. PAR2 ubiquitination was examined by immunoprecipitation and Western blotting under denaturing conditions. HEK-PAR2 cells were stimulated with PAR2 AP (240 min), which induces trafficking of PAR2 to lysosomes (23). In cells treated with hypertonic sucrose, PAR2 failed to internalize and was present at the plasma membrane (Fig. 3A), where it was clearly ubiquitinated (Fig. 3B). In cells overexpressing Hrs, PAR2 accumulated in enlarged Hrs-containing early endosomes (Fig. 3A) as described (24) and was highly ubiquitinated (Fig. 3B). Thus, PAR2 ubiquitination occurs at the plasma membrane and in early endosomes, which is consistent with colocalization of PAR2 and c-Cbl at these sites (3). In cells treated with lysosomal protease inhibitors, PAR2 accumulated in LAMP1-positive lysosomes (Fig. 3A), but PAR2 was not ubiquitinated in lysosomes regardless of the presence of lysosomal inhibitors (Fig. 3B). Thus, activated PAR2 is ubiquitinated at the plasma membrane and in early endosomes and is deubiquitinated en route from early endosomes to lysosomes.
FIGURE 3.
PAR2 is ubiquitinated at the plasma membrane and early endosomes and deubiquitinated before transfer to lysosomes. HEK cells were transiently transfected with PAR2 and pcDNA5 (control) or Hrs-Myc. A, cell surface PAR2 was labeled with antibody to extracellular FLAG epitope. Cells were stimulated with AP (100 μm, 240 min), and PAR2, Hrs, and LAMP1 were localized. In cells treated with hypertonic sucrose, PAR2 remained at the plasma membrane (arrowheads). Expression of Hrs prevented trafficking of PAR2 from early endosomes. In cells treated with lysosomal protease inhibitors, PAR2 accumulated in LAMP1-positive lysosomes (arrows). Scale bar, 10 μm. B, cells were treated as in A. PAR2 was immunoprecipitated (IP), and membranes analyzed by Western blotting (WB) for ubiquitin (ubi) and PAR2. In unstimulated cells, there was no detectable PAR2 ubiquitination. AP-induced PAR2 ubiquitination was detected when trafficking of PAR2 was blocked at the plasma membrane with sucrose or when PAR2 was trapped in early endosomes by overexpression of Hrs. PAR2 ubiquitination was not detected when PAR2 had accumulated in lysosomes in the presence or absence of lysosomal inhibitors. Results shown are representative of n = 3 experiments.
Activated PAR2 Colocalizes with AMSH and UBPY in Endosomes
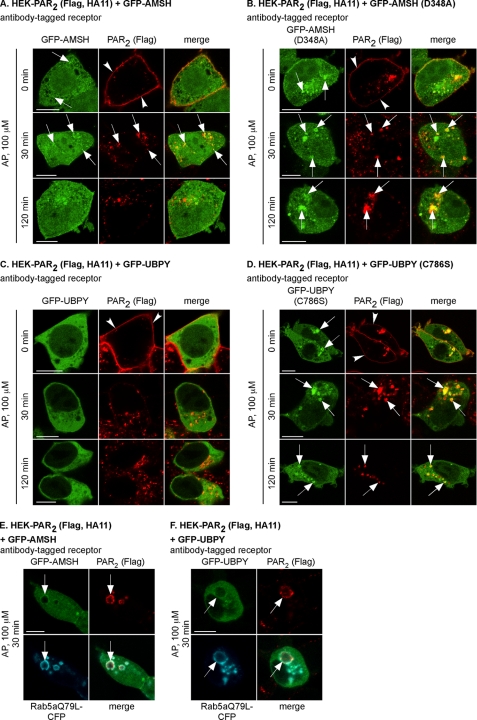
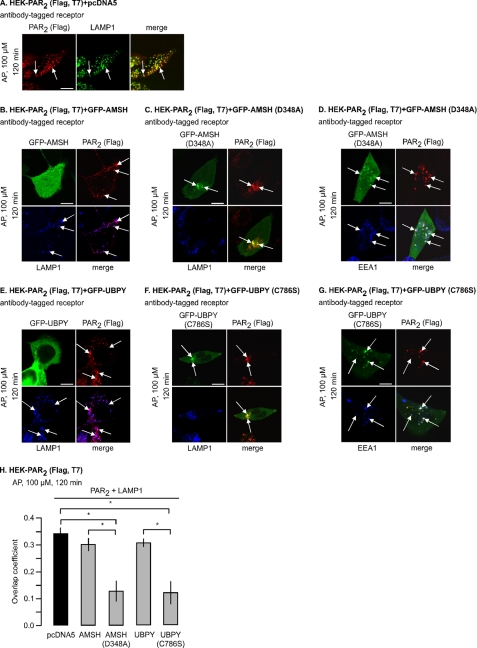
Since deubiquitination of PAR2 occurs during trafficking between early endosomes and lysosomes, we investigated the role of the endosomal DUBs AMSH and UBPY, which associate with the Hrs-STAM complex that also interacts with PAR2 during its postendocytic trafficking (24). To examine the effect of PAR2 stimulation on the subcellular distribution of AMSH and UBPY, we localized PAR2 with GFP-tagged wild-type AMSH and UBPY or catalytically inactive mutants AMSH(D348A) and UBPY(C786S) in HEK cells. Cell surface PAR2 was labeled with an antibody to an extracellular epitope, cells were stimulated with AP (0–120 min), and proteins were localized by confocal microscopy. In unstimulated cells (0 min), PAR2 was at the plasma membrane, AMSH was diffusely distributed in the cytoplasm and nucleus and sometimes concentrated in vesicles (Fig. 4A), and UBPY was diffusely distributed in the cytoplasm (Fig. 4C). AP (30 min) stimulated trafficking of PAR2 to AMSH-containing endosomes (Fig. 4A). After 120 min, PAR2 trafficked to LAMP1-positive lysosomes (Fig. 5A) and was no longer present in the same vesicles as AMSH (Fig. 4A). There was no obvious presence of wild-type UBPY in endosomes containing PAR2 (Fig. 4C). In unstimulated cells, AMSH(D348A) and UBPY(C786S) exhibited a different subcellular distribution from the wild-type proteins, being localized in enlarged vesicles and the cytosol. Activated PAR2 trafficked to AMSH(D348A)- or UBPY(C786S)-containing vesicles and remained there even after 120 min (Fig. 4, B and D). Activated PAR2, AMSH, and UBPY were also prominently detected in enlarged endosomes in cells expressing Rab5aQ79L, a constitutively active mutant that causes endosomal fusion (Fig. 4, E and F). Thus, following activation of PAR2, AMSH, UBPY, and PAR2 are transiently recruited to the same endosomes, whereas AMSH(D348A) and UBPY(C786S) show sustained colocalization with PAR2 in enlarged endosomes.
FIGURE 4.
PAR2 colocalizes with AMSH and UBPY in endosomes. HEK-PAR2 cells were transiently transfected with GFP-AMSH (A), GFP-AMSH(D348A) (B), GFP-UBPY (C), GFP-UBPY(C786S) (D), GFP-AMSH and Rab5aQ79L-CFP (E), or GFP-UBPY and Rab5aQ79L-CFP (F). Cell surface PAR2 was labeled with FLAG antibody. Cells were challenged with AP (100 μm; 0, 30, 120 min), and PAR2 was localized. In unstimulated cells, PAR2 was at the plasma membrane (arrowheads), GFP-AMSH was cytosolic and present in endosomes (arrows), GFP-UBPY was cytosolic, and GFP-AMSH(D348A) and GFP-UBPY(C786S) were in enlarged cytosolic vesicles (arrows). AP (30 min) induced trafficking of PAR2 from the plasma membrane to colocalize with GFP-AMSH, GFP-AMSH(D348A), and GFP-UBPY(C786S) in vesicles (presumably endosomes; arrows, yellow in merged images). At 120 min, PAR2 was still present in vesicles (presumably lysosomes) and did not colocalize with GFP-AMSH or GFP-UBPY. In GFP-AMSH(D348A)- and GFP-UBPY(C786S)-expressing cells, GFP-AMSH(D348A) and GFP-UBPY(C786S) remained colocalized with PAR2. E and F, AP (30 min) induced trafficking of PAR2 to enlarged endosomes containing Rab5aQ79L-CFP (arrows). GFP-AMSH and GFP-UBPY were also present in these enlarged endosomes (arrows). Scale bars, 10 μm.
FIGURE 5.
Catalytically inactive AMSH and UBPY accumulate with PAR2 in early endosomes and prevent lysosomal targeting. HEK-PAR2 cells were transiently transfected with pcDNA5 (control) (A), GFP-AMSH (B), GFP-AMSH(D348A) (C and D), GFP-UBPY (E), or GFP-UBPY(C786S) (F and G). Cell surface PAR2 was labeled with FLAG antibody. Cells were challenged with AP (100 μm, 120 min). PAR2, LAMP1, and EEA1 were localized. In control cells, PAR2 colocalized with LAMP1. Similar results were obtained in GFP-AMSH- and GFP-UBPY-expressing cells (B and E, colocalization of PAR2 and LAMP1; pink in merged image). In cells expressing GFP-AMSH(D348A) or GFP-UBPY(C786S), PAR2 did not colocalize with LAMP1 (C and F, colocalization of PAR2 and GFP; yellow in merged image) but was retained in enlarged EEA1-positive endosomes (D and G, colocalization of PAR2, GFP, and EEA1; white in merged image). Scale bars, 10 μm. H, colocalization of PAR2 and LAMP1 in cells expressing wild-type and catalytically inactive mutants of AMSH and UBPY after 120 min (0, no overlap; 1, complete overlap). n = 23 cells each. *, p < 0.05.
Expression of Catalytically Inactive AMSH or UBPY Prevents Lysosomal Targeting and Degradation of PAR2
To determine the role of AMSH and UBPY in lysosomal targeting of PAR2, HEK cells were transfected with PAR2 and control vector (pcDNA5), AMSH, UBPY, AMSH(D348A), or UBPY(C786S). We localized activated PAR2 with markers of early endosomes (EEA1) and lysosomes (LAMP1). In control cells, PAR2 trafficked to LAMP1-containing lysosomes after 120 min of stimulation (Fig. 5A). Trafficking of PAR2 to lysosomes was unaffected by overexpression of wild-type AMSH (Fig. 5B) or UBPY (Fig. 5E). In cells expressing AMSH(D348A) or UBPY(C786S), PAR2 showed markedly diminished co-localization with LAMP1 after 120 min (Fig. 5, C and F) and instead co-localized with EEA1 and AMSH(D348A) or UBPY(C786S) in early endosomes (Fig. 5, D and G). Quantitative analysis revealed that the colocalization of PAR2 and LAMP1 was significantly diminished in cells expressing AMSH(D348A) or UBPY(C786S) compared with cells expressing control vector, AMSH, or UBPY (Fig. 5H). Thus, the catalytic activities of AMSH or UBPY are required for trafficking of PAR2 from early endosomes to lysosomes.
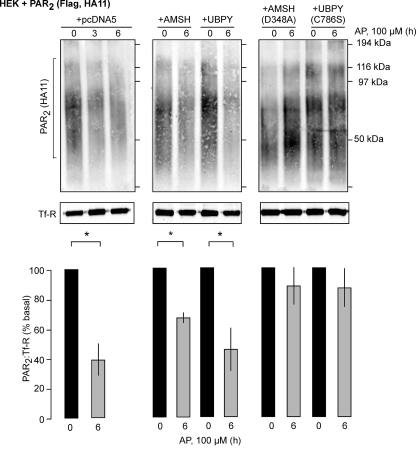
To determine the role of AMSH or UBPY in degradation of PAR2, we biotinylated cell surface proteins, stimulated cells with AP (0–6 h), and determined the levels of biotinylated PAR2 by Western blotting. In cells expressing control vector, PAR2 was markedly degraded (39 ± 10% of basal, 100%, at 6 h) (Fig. 6). In cells expressing AMSH, degradation of PAR2 was modestly reduced (65 ± 2%, 6 h), whereas expression of UBPY did not affect degradation (46 ± 11%, 6 h). In cells expressing AMSH(D348A) or UBPY(C786S), PAR2 degradation was markedly reduced (AMSH(D348A), 87 ± 9.8%, 6 h; UBPY(C786S), 87 ± 10%, 6 h) (Fig. 6). Thus, AMSH or UBPY is required for lysosomal degradation of PAR2.
FIGURE 6.
Catalytically inactive AMSH or UBPY prevent lysosomal degradation of PAR2. HEK cells were transiently transfected with PAR2 and pcDNA5 (control), GFP-AMSH, GFP-AMSH(D348A), GFP-UBPY, or GFP-UBPY(C786S). Cell surface proteins were biotinylated, cells were stimulated with AP (0–6 h), and biotinylated proteins were precipitated using avidin beads. PAR2 and TfR were determined by Western blotting. In cells expressing control vector, GFP-AMSH, or GFP-UBPY, similar levels of PAR2 degradation occurred. Expression of GFP-AMSH(D348A) or GFP-UBPY(C786S) blocked degradation. n = 3. *, p < 0.05.
Catalytic Activity of AMSH or UBPY Is Required for Deubiquitination of PAR2
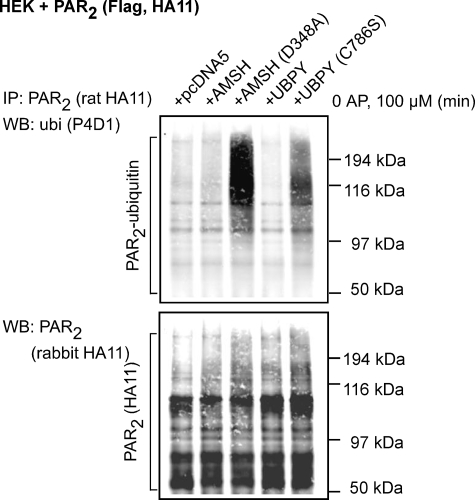
To examine the role of AMSH and UBPY in regulating PAR2 ubiquitination, we examined the effects of expressing catalytically inactive AMSH or UBPY on PAR2 ubiquitination. PAR2 ubiquitination was assessed by immunoprecipitating PAR2 and blotting for ubiquitin and PAR2. In unstimulated cells, there was a low level of PAR2 ubiquitination that was unaffected by expression of AMSH or UBPY (Fig. 7). Expression of AMSH(D348A) or UBPY(C786S) caused a marked increase in the basal levels of PAR2 ubiquitination (Fig. 7). Thus, expression of catalytically inactive AMSH or UBPY prevents PAR2 deubiquitination under basal conditions. These results suggest that AMSH and UBPY are necessary for maintaining a low level of PAR2 ubiquitination in unstimulated cells.
FIGURE 7.
Expression of catalytically inactive AMSH or UBPY mutants enhances PAR2 ubiquitination under basal conditions. HEK cells were transiently transfected with PAR2 and pcDNA5 (control), GFP-AMSH, GFP-AMSH(D348A), GFP-UBPY, or GFP-UBPY(C786S). Cells were unstimulated. PAR2 was immunoprecipitated (IP), and membranes were analyzed by Western blotting (WB) for ubiquitin (ubi) and PAR2. There was a low base-line level of PAR2 ubiquitination in cells expressing empty vector (pcDNA5) that was unaffected by overexpression of AMSH or UBPY. Overexpression of AMSH(D348A) or UBPY(C786S) markedly increased basal levels of PAR2 ubiquitination. Shown is a representative blot of n = 4 experiments.
AMSH or UBPY Knockdown Prevents Lysosomal Trafficking, Degradation, and Deubiquitination of PAR2
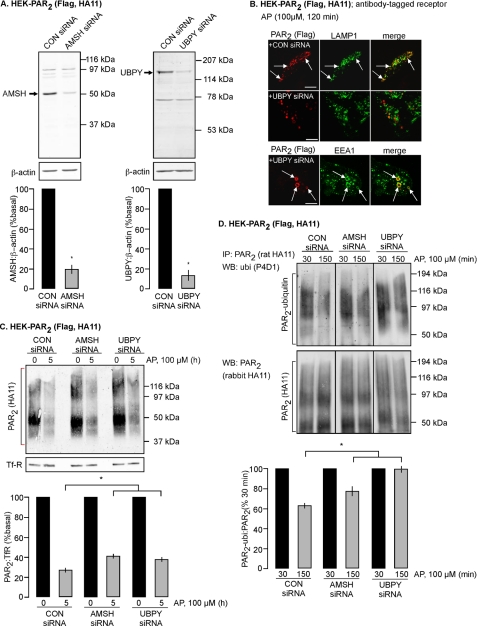
To confirm the roles of AMSH and UBPY in the postendocytic trafficking, degradation, and ubiquitination of PAR2, we depleted endogenous AMSH or UBPY using siRNA, since the effects of the catalytically inactive mutants could reflect an indirect consequence of their overexpression. HEK cells were transfected with control or AMSH- or UBPY-specific siRNA. siRNA suppressed levels of AMSH by 80.1 ± 4.3% and UBPY by 87.9 ± 4.9% after 72 h, compared with control siRNA (100%) (Fig. 8A). UBPY knockdown caused formation of enlarged early endosomes containing EEA1 (Fig. 8B), an expected consequence (8, 15–17). No morphological changes were observed in cells treated with control siRNA, and PAR2 activation (AP; 120 min) resulted in strong co-localization of PAR2 with LAMP1 in lysosomes (Fig. 8B). In cells treated with UBPY siRNA, there was markedly diminished colocalization of PAR2 and LAMP1, and PAR2 was instead retained in enlarged EEA1-positive early endosomes. Unlike UBPY siRNA, AMSH siRNA did not cause an apparent morphological change, and we were therefore unable to identify AMSH knockdown cells and examine the effects on lysosomal trafficking of PAR2. Thus, UBPY is necessary for efficient trafficking of PAR2 to lysosomes.
FIGURE 8.
AMSH or UBPY knockdown prevent lysosomal trafficking, degradation, and deubiquitination of PAR2. HEK-PAR2 cells were treated with control (CON), AMSH, or UBPY siRNA and used for experiments after 72 h. A, Western blots show efficient knockdown of endogenous AMSH or UBPY. The lower panels show densitometric analyses of AMSH, UBPY, and β-actin. n = 3. *, p < 0.05. B, cell surface PAR2 was labeled with FLAG antibody. Cells were challenged with AP (100 μm, 120 min), and PAR2, LAMP1, and EEA1 were localized. In control siRNA-treated cells, PAR2 colocalized with LAMP1. UBPY siRNA treatment diminished PAR2 colocalization with LAMP1 and caused retention of PAR2 in enlarged endosomes containing EEA1. Scale bars, 10 μm. C, cell surface proteins were biotinylated, cells were stimulated with AP (0 or 5 h), and biotinylated proteins were precipitated using avidin beads. PAR2 and TfR were determined by Western blotting. PAR2 degradation was significantly reduced in cells treated with AMSH or UBPY siRNA compared with control siRNA. n = 3. *, p < 0.05. D, cells were stimulated with AP (100 μm; 30 or 150 min), PAR2 was immunoprecipitated (IP) and membranes were analyzed by Western blotting (WB) for ubiquitin (ubi) and PAR2. In cells treated with control siRNA, PAR2 was ubiquitinated at 30 min and deubiquitinated at 150 min. In cells treated with AMSH or UBPY-siRNA, PAR2 remained highly ubiquitinated at 150 min. n = 3. *, p < 0.05. IP, immunoprecipitation; WB, Western blot.
We examined the effects of AMSH or UBPY knockdown on degradation of biotinylated PAR2. Cells were stimulated with AP (0 or 5 h), and PAR2 levels were determined by Western blotting. In cells treated with control siRNA, PAR2 was substantially degraded (26.9 ± 2.2% of basal (100%) at 5 h) (Fig. 8C). In cells treated with AMSH or UBPY siRNA, PAR2 degradation was significantly reduced (AMSH siRNA, 40.9 ± 2.2%; UBPY siRNA, 37.6 ± 2.3%) (Fig. 8C). Thus, AMSH and UBPY are necessary for efficient degradation of PAR2 in lysosomes.
We examined the effects of AMSH or UBPY knockdown on PAR2 deubiquitination. Cells were stimulated with AP (30 or 150 min), and PAR2-ubiquitination was determined by immunoprecipitation and Western blotting. In cells treated with control siRNA, PAR2 was strongly ubiquitinated at 30 min, and ubiquitination was markedly diminished after 150 min (37.7 ± 3.2% of levels at 30 min), indicating that deubiquitination had occurred (Fig. 8D). In cells treated with AMSH or UBPY-siRNA, deubiquitination of PAR2 at 150 min was significantly reduced (AMSH, 22.7 ± 6.8%; UBPY, 0.6 ± 4.7% of levels at 30 min) (Fig. 8D). Thus, AMSH and UBPY are necessary for the efficient deubiquitination of PAR2. In summary, depletion of endogenous AMSH or UBPY resulted in impaired deubiquitination of PAR2 and prevented lysosomal trafficking and degradation of PAR2.
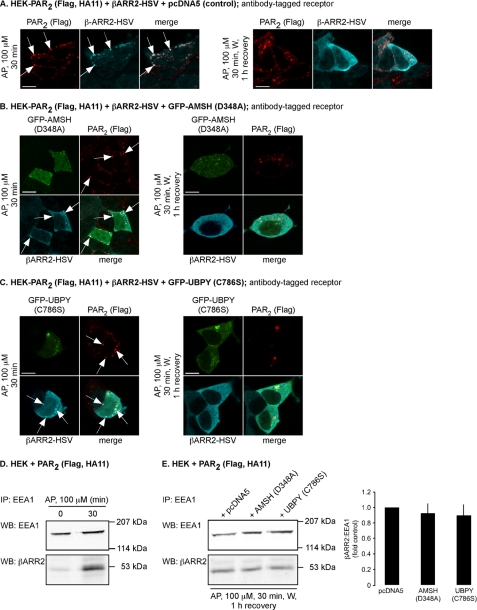
Expression of Catalytically Inactive AMSH or UBPY Does Not Prolong PAR2 Association with β-Arrestin2 in Endosomes or the Duration of AP-induced ERK Activation
Following activation, GPCRs are phosphorylated by G protein receptor kinases, which promotes receptor interaction with β-arrestins. These cytosolic proteins translocate to the receptor at the plasma membrane and serve to uncouple the receptor from heterotrimeric G proteins, which mediates desensitization, and to couple the receptor to clathrin and AP2, which mediate endocytosis (30–32). β-Arrestins also recruit components of the MAPK signaling cascade, such as Raf-1, MEKK, and ERK, allowing receptors to signal by G protein-independent mechanisms (22, 33, 34). PAR2 activates ERK1/2 by β-arrestin-dependent mechanisms (22), and β-arrestins redistribute from PAR2-containing endosomes to the cytosol prior to PAR2 degradation in lysosomes (28). Since expression of catalytically inactive AMSH or UBPY traps PAR2 in early endosomes, thereby preventing PAR2 transit to lysosomes, we hypothesized that this arrest of PAR2 trafficking would prolong the association of PAR2 with β-arrestin2 in endosomes. In cells transfected with PAR2, β-arrestin2-HSV, and control vector (pcDNA5), AP stimulation (30 min) caused internalization of PAR2 and β-arrestin2 to the same endosomes (Fig. 9A). After 1 h of recovery in AP-free medium, β-arrestin2 had returned to the cytosol, and PAR2 was still present in vesicles (Fig. 9A). Expression of AMSH(D348A) or UBPY(C786S) did not affect AP-induced trafficking of PAR2 and β-arrestin2 to the same endosomes after 30 min or subsequent redistribution of β-arrestin2 to the cytosol after 1 h of recovery (Fig. 9, B and C). To further examine whether expression of catalytically inactive AMSH or UBPY prolongs β-arrestin2 association with endosomes, we purified endosomes by immunoprecipitation with antibodies to EEA1 (26). Isolated endosomes were analyzed by Western blotting for EEA1 and Rab5a (endosome markers) and β-actin (cytosolic marker). This analysis confirmed that the endosomal fractions were highly enriched due to the presence of EEA1 and Rab5a (supplemental Fig. S1). The absence of β-actin indicated that the endosomal fractions were free of cytosolic proteins (supplemental Fig. S1). Thus, β-arrestin2 present in endosomal fractions represents endosome-associated β-arrestin2 only. In HEK cells expressing PAR2, levels of β-arrestin2 were low in endosomes isolated from unstimulated cells (Fig. 9D). AP (30 min) induced a substantial increase of β-arrestin2 in endosomes (Fig. 9D). To examine whether expression of catalytically inactive AMSH or UBPY prolongs β-arrestin2 association with endosomes, we challenged HEK cells transfected with PAR2 and pcDNA5 (control), AMSH(D348A), or UBPY(C786S) with AP for 30 min, followed by a recovery in AP-free medium for 1 h. Endosomes were isolated and analyzed for β-arrestin2 and normalized to EEA1 (Fig. 9E). The levels of β-arrestin2 in endosomes were similar in cells expressing pcDNA5 (control), AMSH(D348A), or UBPY(C786S) (Fig. 9E). Together, these results indicate that expression of catalytically inactive AMSH or UBPY does not prolong the association of PAR2 and β-arrestin2 in endosomes.
FIGURE 9.
Expression of catalytically inactive AMSH or UBPY does not prolong the interaction between β-arrestin2 and PAR2 in endosomes. HEK-PAR2 cells were transiently transfected with β-arrestin2-HSV and pcDNA5 (control) (A), GFP-AMSH(D348A) (B), or GFP-UBPY(C786S) (C). Cell surface PAR2 was labeled with FLAG antibody. Cells were challenged with AP (100 μm, 30 min) or with AP (100 μm, 30 min), followed by washing and recovery in AP-free medium for 1 h. In control cells, AP (30 min) induced trafficking of PAR2 and β-arrestin2 to the same vesicles (presumably endosomes; arrows). After a 1-h recovery, PAR2 was still present in vesicles, and β-arrestin2 had returned to the cytosol. Similar results were obtained in cells expressing GFP-AMSH(D348A) or GFP-UBPY(C786S). Scale bars, 10 μm. D, HEK cells transiently transfected with PAR2 were stimulated with AP (100 μm) for 0 or 30 min. Endosomes were isolated and analyzed for β-arrestin2 and EEA1 by Western blotting. AP (30 min) increased the levels of β-arrestin2 in endosomes. E, HEK cells transiently transfected with PAR2 and pcDNA5 (control), GFP-AMSH(D348A), or GFP-UBPY(C786S) were challenged with AP (100 μm, 30 min), followed by washing and recovery in AP-free medium for 1 h. Endosomes were isolated and analyzed for β-arrestin2 and EEA1 by Western blotting and quantified using densitometry. Expression of GFP-AMSH(D348A) or GFP-UBPY(C786S) did not cause retention of β-arrestin2 in endosomes. n = 3. IP, immunoprecipitation; WB, Western blot.
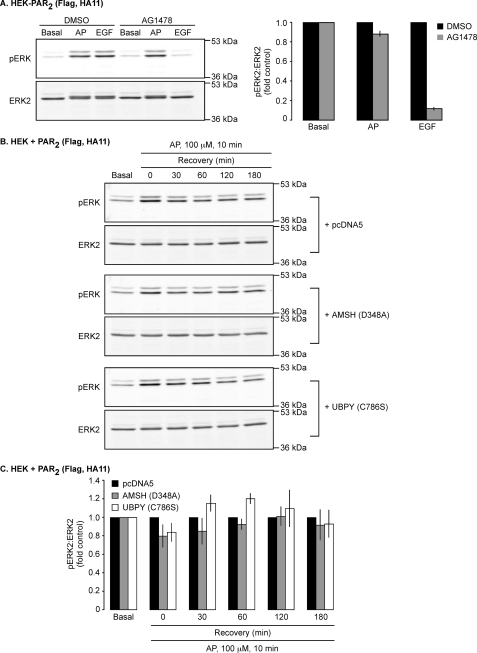
GPCRs can activate ERKs by mechanisms that include transactivation of the EGFR and the formation of β-arrestin-based MAPK signaling complexes (36, 37). Initially, to determine the contribution of the EGFR transactivation to PAR2-dependent ERK activation in HEK cells, we examined the effect of the EGFR tyrosine kinase inhibitor AG1478 on AP-induced ERK2 phosphorylation. Stimulation of HEK cells expressing PAR2 with either AP or EGF (5 min) resulted in ERK2 phosphorylation (Fig. 10A). AG1478 inhibited EGF-induced ERK2 phosphorylation by ∼90%, indicating that AG1478 inhibits EGFR tyrosine kinase activity (Fig. 10A). In contrast, AG1478 inhibited AP-induced ERK2 phosphorylation by ∼10% (Fig. 10A). Thus, EGFR transactivation does not have a major role in PAR2-induced ERK activation in HEK cells.
FIGURE 10.
PAR2-mediated ERK2 activation is not regulated by AMSH or UBPY catalytic activities. A, HEK-PAR2 cells were incubated with vehicle (DMSO; 1:10,000) or 1 μm AG1478 for 1 h prior to stimulation with AP (100 μm) or EGF (100 ng/ml) for 5 min. Cell lysates were analyzed by Western blotting for pERK and ERK2. Densitometry indicated that AG1478 abolished EGF-dependent ERK2 activation but had little effect on AP-dependent ERK2 activation. n = 3. HEK cells were transiently transfected with PAR2 and pcDNA5 (control), GFP-AMSH(D348A), or GFP-UBPY(C786S). Serum-starved cells were challenged with AP (100 μm, 0–10 min), washed, and incubated in AP-free medium (0–180 min). pERK and ERK2 levels were analyzed by Western blotting (B) and quantified using densitometry (C). The levels of ERK2 activation in cells expressing GFP-AMSH(D348A) or GFP-UBPY(C786S) were similar to control cells. n = 3.
PAR2 predominantly activates the MAPK cascade by forming a complex with β-arrestins, Raf-1, and activated ERK in endosomes (22). Since AMSH and UBPY regulate PAR2 trafficking from endosomes to lysosomes, which is required for PAR2 degradation and signal termination, we examined the role of AMSH and UBPY in regulating PAR2-induced ERK signaling. In cells transfected with PAR2 and control vector (pcDNA5), AP (10 min) stimulated ERK2 phosphorylation, which declined after recovery in AP-free medium (0–180 min) (Fig. 10B). Expression of AMSH(D348A) or UBPY(C786S) did not affect the magnitude or duration of AP-induced ERK2 activation. Quantitative analysis revealed that at both early and later time points following AP stimulation, the levels of phosphorylated ERK2 in cells expressing AMSH(D348A) or UBPY(C786S) were similar to those in control cells (Fig. 10C). Thus, PAR2-induced ERK signaling is not regulated by AMSH or UBPY.
DISCUSSION
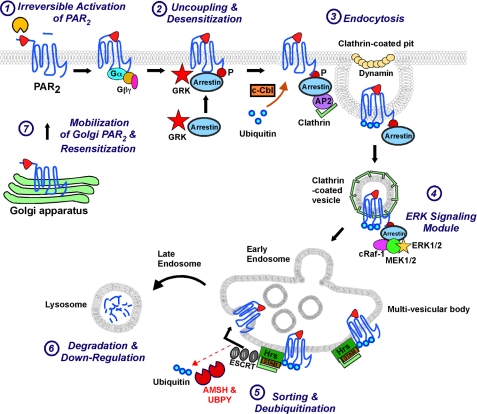
Ubiquitination of certain GPCRs is a targeting signal for postendocytic sorting to lysosomes (Fig. 11). Since ubiquitination is highly dynamic, a clear understanding of ubiquitin-dependent GPCR trafficking requires knowledge of the role of DUBs, which act downstream in ubiquitination pathways and have the potential to determine the ultimate fate of receptors. Here we identified two endosomal DUBs, AMSH and UBPY, as key regulators of PAR2 ubiquitination and down-regulation.
FIGURE 11.
Proposed model for AMSH and UBPY function in the pathway of agonist-induced trafficking of PAR2. 1, proteases cleave PAR2 to expose a tethered ligand that binds to and irreversibly activates the receptor. 2, G protein receptor kinases (GRK) phosphorylate PAR2, which induces membrane translocation of β-arrestins. β-Arrestins interact with phosphorylated PAR2 to uncouple the receptor from heterotrimeric G proteins, resulting in desensitization. The E3 ubiquitin ligase c-Cbl translocates to the plasma membrane and endosomes to mediate multi-monoubiquitination of activated PAR2. 3, β-arrestins serve as adaptors for clathrin- and AP2-mediated endocytosis of PAR2. 4, β-arrestins co-internalize with the receptor and recruit a MAPK complex that mediates PAR2 signaling through the ERK1/2 pathway. 5, PAR2 associates with the Hrs-STAM complex in clathrin-coated microdomains of the early endosome. Hrs/STAM recruit further components of the ESCRT machinery, leading to receptor sorting into intralumenal vesicles. These vesicles give rise to MVBs and late endosomes that ultimately fuse with lysosomes and release their internal vesicles within the lysosomal lumen. AMSH and UBPY deubiquitinate PAR2, allowing the deubiquitinated receptor to invaginate into the lumen of the MVB vesicles. 6, PAR2 is degraded in lysosomes, resulting in permanent down-regulation of PAR2 signaling. 7, resensitization of protease signaling requires mobilization of PAR2 from prominent stores in the Golgi apparatus and eventually synthesis of new receptors.
By disrupting various stages of the endocytic pathway, we determined the subcellular sites of PAR2 ubiquitination and deubiquitination (Fig. 11). PAR2 ubiquitination was unaffected by disruption of endocytosis with hypertonic sucrose or trapping PAR2 in early endosomes by overexpression of Hrs (24). Thus, PAR2 is ubiquitinated at the plasma membrane and in early endosomes, which is consistent with colocalization of PAR2 and c-Cbl (3). PAR2 is subsequently deubiquitinated between early endosomes and lysosomes, since the receptor was deubiquitinated when it reached lysosomes in the presence of lysosomal inhibitors. Our observations are supported by previous studies showing that ubiquitin molecules need to be released from receptors prior to their delivery to lysosomes for degradation in order to maintain levels of free ubiquitin (11). Thus, although PAR2 is ultimately in a deubiquitinated state when it reaches lysosomes, it does require ubiquitination at the earlier stages of the pathway for correct lysosomal sorting (3). Ubiquitination has a quite different regulatory effect on another member of the PAR family, PAR1, where ubiquitination retains the receptor at the cell surface and is not required for agonist-induced lysosomal sorting and degradation (38).
AMSH and UBPY are strong candidates for deubiquitinating PAR2. Activated PAR2 prominently colocalized with AMSH in endosomes, and expression of catalytically inactive AMSH or UBPY enhanced colocalization of PAR2 and DUBs in early endosomes. EGF causes similar trafficking of EGFR to endosomes containing AMSH or UBPY in HeLa cells (5, 8). AMSH and UBPY have a common binding site within the Src homology 3 domain of STAM, which constitutively interacts with Hrs through coiled-coil regions (12–14). It remains to be resolved whether the association of PAR2 with AMSH and UBPY in endosomes is a result of a direct interaction or an indirect interaction via the Hrs-STAM complex, since PAR2 engages with Hrs in early endosomes (24). The UBPY-EGFR interaction is also mediated by the Hrs-STAM complex (39).
Overexpression of wild-type AMSH or UBPY did not affect PAR2 ubiquitination, trafficking, or degradation, suggesting that adequate levels of endogenous AMSH and UBPY are already present in HEK cells. This is consistent with reports that the overexpression of AMSH and UBPY does not affect EGFR ubiquitination (40), although overexpression of UBPY has also been shown to reduce EGFR ubiquitination (39). In contrast, overexpression of catalytically inactive AMSH or UBPY markedly increased base-line levels of PAR2 ubiquitination and, in agonist-stimulated cells, caused retention of ubiquitinated PAR2 in enlarged early endosomes, with which AMSH(D348A) and UBPY(C786S) are constitutively associated, suggesting that their catalytic activity is necessary for dissociation from endosomal membranes. AMSH(D348A) and UBPY(C786S) cause endosomal accumulation of ubiquitinated proteins, including cargo, such as EGFR or PAR2, and components of the endosomal sorting machinery, such as STAM (5, 8, 40). However, the accumulation of ubiquitinated STAM is promoted by AMSH(D348A) but not UBPY(C786S). AMSH(D348A) or UBPY(C786S) also inhibited lysosomal trafficking and degradation of PAR2, which could be explained by diminished deubiquitination of PAR2 in endosomes and reduced receptor incorporation into intralumenal vesicles of MVBs. Overall, our results suggest that the catalytic activity of AMSH and UBPY is required for PAR2 deubiquitination and subsequent lysosomal trafficking and degradation.
Since catalytically inactive DUBs are constitutively associated with endosomal membranes, they may displace either endogenous AMSH or UBPY. To further evaluate the role of endogenous AMSH or UBPY in PAR2 regulation, we used specific siRNAs. Knockdown of AMSH or UBPY inhibited PAR2 deubiquitination and degradation, which could be explained in several ways. First, by suppressing PAR2 deubiquitination, AMSH or UBPY depletion may interfere with the incorporation of the receptor into intralumenal vesicles of MVBs, thereby disrupting lysosomal delivery and degradation, as reported for the EGFR after UBPY knockdown (8). Impaired deubiquitination could cause endosomal accumulation of ubiquitinated PAR2 or EGFR, which may overwhelm the sorting machinery and disrupt receptor sorting. Second, DUB knockdown may suppress PAR2 trafficking from endosomes to lysosomes due to changes in endosomal morphology. UBPY knockdown caused PAR2 retention in abnormally enlarged early endosomes. UBPY knockdown results in aberrantly enlarged early endosomes by increasing the number and size of multivesicular endosomal structures (8, 15–17). UBPY knockdown inhibits lysosomal trafficking and degradation of the EGFR, which is directly due to loss of UBPY, since overexpression of siRNA-resistant UBPY rescues this phenotype (8, 15). The inhibitory effect of AMSH knockdown on PAR2 degradation is unlikely to be due to changes in endosomal morphology, since endosomes had normal morphology. Third, AMSH or UBPY knockdown may influence the ubiquitination of key endosomal sorting proteins, such as Hrs and STAM. Thus, the effects on PAR2 could be due to changes in the stability of sorting proteins in endosomes. UBPY knockdown depletes STAM, suggesting that UBPY is necessary to reverse STAM ubiquitination, which targets STAM for proteasomal degradation (8, 17). Although STAM directly stimulates AMSH activity in vitro (6), overexpression of STAM in UBPY knockdown cells cannot rescue the observed defect in EGFR degradation (8). Unlike UBPY knockdown, AMSH knockdown does not affect the stability of components of the endosomal sorting machinery, such as STAM (5, 8). Thus, the effect of AMSH knockdown on PAR2 degradation is unlikely to be due to changes in the stability of the endosomal sorting machinery. However, AMSH is diminished by UBPY knockdown (5, 8), which could partially explain the effects of UBPY depletion on PAR2. Finally, AMSH or UBPY knockdown may reduce levels of free ubiquitin and consequently affect the efficient ubiquitination and lysosomal sorting of PAR2. Depletion of the yeast protein Doa4, which is homologous to mammalian UBPY, reduces free ubiquitin levels and impairs ubiquitin-dependent endocytosis and vacuolar degradation of the yeast GPCR Ste3 (41). Doa4 activity at the prevacuolar compartment is required to recover ubiquitin from ubiquitinated receptors en route to the vacuole (the yeast lysosome) (42). However, UBPY knockdown in mammalian cells does not deplete levels of free ubiquitin (8, 17), suggesting that defects in the maintenance of free ubiquitin levels are unlikely to account for reduced lysosomal degradation of PAR2 after UBPY knockdown. We propose that AMSH or UBPY knockdown directly suppresses PAR2 deubiquitination and thereby impairs incorporation of the receptor into intralumenal vesicles of MVBs and delivery to lysosomes for degradation.
Our results support the conclusion that both AMSH and UBPY deubiquitinate PAR2 and thereby promote lysosomal targeting and degradation of the receptor (Fig. 11). Since disruption of either AMSH or UBPY function produced similar effects, these DUBs appear to have non-redundant roles in PAR2 trafficking to lysosomes. PAR2 deubiquitination probably occurs downstream of Hrs and after the lysosomal sorting decision has been made. A different model has been proposed for the EGFR, where AMSH and UBPY may act in opposition (43). UBPY knockdown suppresses EGFR degradation (8, 15), whereas AMSH knockdown enhances EGFR degradation (5), suggesting that UBPY promotes and AMSH impedes EGFR degradation. AMSH may thus act at an early stage of the sorting process to reverse EGFR ubiquitination prior to commitment to the lysosomal pathway and may thereby divert the receptor to a recycling route (5, 43). If AMSH deubiquitinated PAR2 at an earlier stage, it could interfere with lysosomal sorting and thereby prevent PAR2 degradation, which does not appear to be the case. However, PAR2 that has been deubiquitinated by AMSH at an early stage could be ubiquitinated again by c-Cbl and deubiquitinated by UBPY prior to sequestration into intralumenal vesicles. Thus, the cycle of ubiquitination and deubiquitination may determine the rate at which degradation occurs. AMSH or UBPY may also deubiquitinate other proteins that regulate PAR2 trafficking to lysosomes. β-Arrestins mediate desensitization of GPCRs, couple GPCRs to clathrin and AP2 to mediate endocytosis, and act as scaffolds for recruitment of MAPK cascade components (22, 30–34). β-Arrestin itself is ubiquitinated in response to GPCR activation, and expression of a β-arrestin-ubiquitin chimera promotes GPCR internalization (1, 44). β-Arrestin is deubiquitinated by USP33, which is thought to enhance the dissociation of β-arrestin from activated GPCRs and promote the termination of β-arrestin-dependent ERK activation (45). Thus, other cell machinery that regulates the trafficking and function of activated PAR2 may similarly be subject to ubiquitination and deubiquitination. AMSH and UBPY also have non-redundant roles in the down-regulation of DOR, a GPCR that is ubiquitinated and degraded following activation (18). Further studies are necessary to understand the precise mechanisms by which AMSH and UBPY regulate PAR2 and DOR down-regulation to reconcile the seemingly non-redundant roles of these DUBs.
Other DUBs also regulate the ubiquitination state of GPCRs, with important functional consequences. In the case of the β2AR, β-arrestins recruit the E3 ligase Nedd4 to the β2AR to mediate receptor ubiquitination, which is necessary for lysosomal degradation and down-regulation after chronic stimulation (46). USP33 and USP20 reverse this ubiquitination and thereby suppress down-regulation and promote recycling and resensitization of β2ARs (19). Thus, whereas AMSH and UBPY deubiquitinate PAR2 and DOR to promote receptor degradation, USP33 and USP20 deubiquitinate β2AR to allow receptors to escape degradation and recycle.
PAR2 activation causes a redistribution of β-arrestin from the cytosol to the plasma membrane and internalization of PAR2 and β-arrestin to the same endosomes (28). PAR2 can activate MAPKs by both β-arrestin-dependent and β-arrestin-independent mechanisms (22). PAR2 activation induces the formation of a signaling complex comprising PAR2, β-arrestin, Raf-1, and activated ERK (22). Whether internalization of PAR2 is necessary for β-arrestin-dependent activation of ERK is currently unknown. Since PAR2 and β-arrestin colocalize in endosomes following PAR2 activation and β-arrestin forms part of an ERK signaling complex, we hypothesized that trapping PAR2 in early endosomes by expression of catalytically inactive AMSH and UBPY would prolong both PAR2 association with β-arrestin and PAR2-mediated ERK activation. However, expression of catalytically inactive AMSH or UBPY did not cause sustained colocalization of PAR2 and β-arrestin2 in endosomes, sustained association of β-arrestin2 with endosomes, or sustained PAR2-induced ERK signaling. Thus, AMSH and UBPY do not appear to regulate these processes. Other proteases can regulate trafficking and signaling of GPCRs and β-arrestins in endosomes. Endothelin-converting enzyme-1 degrades substance P and calcitonin gene-related peptide in acidified endosomes to disrupt the ligand-GPCR-β-arrestin complex, which promotes β-arrestin return to the cytosol and receptor recycling to the plasma membrane (47, 48). This mechanism also attenuates substance P-induced ERK activation (35). Although the ligand for PAR2 is tethered to the receptor, other endosomal proteases may degrade the ligand to disrupt the PAR2-β-arrestin-MAPK complex and thereby attenuate ERK signaling.
The functional implications of AMSH- and UBPY-induced PAR2 degradation remain to be determined. Drugs that activate DUBs may promote PAR2 degradation and thereby prevent the uncontrolled inflammation that would be expected to result from sustained PAR2 activation (20). It also remains to be resolved whether the requirement of AMSH and UBPY in receptor down-regulation applies to GPCRs other than PAR2 and DOR (18).
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants DK43207, DK57480, and DK39957.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- GPCR
- G protein-coupled receptor
- β2AR
- β2-adrenergic receptor
- DOR
- δ-opioid receptor
- DUB
- deubiquitinating enzyme
- EGF
- epidermal growth factor
- EGFR
- epidermal growth factor receptor
- ERK
- extracellular signal-regulated kinase
- MAPK
- mitogen activated protein kinase
- MVB
- multivesicular body
- PAR2
- protease-activated receptor 2
- pERK
- phosphorylated extracellular signal-regulated kinase
- TfR
- transferrin receptor
- USP
- ubiquitin-specific protease
- E3
- ubiquitin-protein isopeptide ligase
- siRNA
- small interfering RNA
- CFP
- cyan fluorescent protein
- HEK
- human embryonic kidney 293
- PBS
- phosphate-buffered saline
- AP
- activating peptide
- RIPA
- radioimmune precipitation
- HA
- hemagglutinin.
REFERENCES
- 1.Shenoy S. K., McDonald P. H., Kohout T. A., Lefkowitz R. J. (2001) Science 294, 1307–1313 [DOI] [PubMed] [Google Scholar]
- 2.Marchese A., Raiborg C., Santini F., Keen J. H., Stenmark H., Benovic J. L. (2003) Dev. Cell 5, 709–722 [DOI] [PubMed] [Google Scholar]
- 3.Jacob C., Cottrell G. S., Gehringer D., Schmidlin F., Grady E. F., Bunnett N. W. (2005) J. Biol. Chem. 280, 16076–16087 [DOI] [PubMed] [Google Scholar]
- 4.Clague M. J., Urbé S. (2006) Trends Cell Biol. 16, 551–559 [DOI] [PubMed] [Google Scholar]
- 5.McCullough J., Clague M. J., Urbé S. (2004) J. Cell Biol. 166, 487–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCullough J., Row P. E., Lorenzo O., Doherty M., Beynon R., Clague M. J., Urbé S. (2006) Curr. Biol. 16, 160–165 [DOI] [PubMed] [Google Scholar]
- 7.Naviglio S., Mattecucci C., Matoskova B., Nagase T., Nomura N., Di Fiore P. P., Draetta G. F. (1998) EMBO J. 17, 3241–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Row P. E., Prior I. A., McCullough J., Clague M. J., Urbé S. (2006) J. Biol. Chem. 281, 12618–12624 [DOI] [PubMed] [Google Scholar]
- 9.Levkowitz G., Waterman H., Zamir E., Kam Z., Oved S., Langdon W. Y., Beguinot L., Geiger B., Yarden Y. (1998) Genes Dev. 12, 3663–3674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urbé S., Sachse M., Row P. E., Preisinger C., Barr F. A., Strous G., Klumperman J., Clague M. J. (2003) J. Cell Sci. 116, 4169–4179 [DOI] [PubMed] [Google Scholar]
- 11.Williams R. L., Urbé S. (2007) Nat. Rev. Mol. Cell Biol. 8, 355–368 [DOI] [PubMed] [Google Scholar]
- 12.Asao H., Sasaki Y., Arita T., Tanaka N., Endo K., Kasai H., Takeshita T., Endo Y., Fujita T., Sugamura K. (1997) J. Biol. Chem. 272, 32785–32791 [DOI] [PubMed] [Google Scholar]
- 13.Tanaka N., Kaneko K., Asao H., Kasai H., Endo Y., Fujita T., Takeshita T., Sugamura K. (1999) J. Biol. Chem. 274, 19129–19135 [DOI] [PubMed] [Google Scholar]
- 14.Kato M., Miyazawa K., Kitamura N. (2000) J. Biol. Chem. 275, 37481–37487 [DOI] [PubMed] [Google Scholar]
- 15.Bowers K., Piper S. C., Edeling M. A., Gray S. R., Owen D. J., Lehner P. J., Luzio J. P. (2006) J. Biol. Chem. 281, 5094–5105 [DOI] [PubMed] [Google Scholar]
- 16.Mizuno E., Kobayashi K., Yamamoto A., Kitamura N., Komada M. (2006) Traffic 7, 1017–1031 [DOI] [PubMed] [Google Scholar]
- 17.Niendorf S., Oksche A., Kisser A., Löhler J., Prinz M., Schorle H., Feller S., Lewitzky M., Horak I., Knobeloch K. P. (2007) Mol. Cell Biol. 27, 5029–5039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hislop J. N., Henry A. G., Marchese A., von Zastrow M. (2009) J. Biol. Chem. 284, 19361–19370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berthouze M., Venkataramanan V., Li Y., Shenoy S. K. (2009) EMBO J. 28, 1684–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ossovskaya V. S., Bunnett N. W. (2004) Physiol. Rev. 84, 579–621 [DOI] [PubMed] [Google Scholar]
- 21.Nystedt S., Ramakrishnan V., Sundelin J. (1996) J. Biol. Chem. 271, 14910–14915 [DOI] [PubMed] [Google Scholar]
- 22.DeFea K. A., Zalevsky J., Thoma M. S., Déry O., Mullins R. D., Bunnett N. W. (2000) J. Cell Biol. 148, 1267–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Böhm S. K., Khitin L. M., Grady E. F., Aponte G., Payan D. G., Bunnett N. W. (1996) J. Biol. Chem. 271, 22003–22016 [DOI] [PubMed] [Google Scholar]
- 24.Hasdemir B., Bunnett N. W., Cottrell G. S. (2007) J. Biol. Chem. 282, 29646–29657 [DOI] [PubMed] [Google Scholar]
- 25.Compton S. J., Sandhu S., Wijesuriya S. J., Hollenberg M. D. (2002) Biochem. J. 368, 495–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H. S., Stolz D. B., Romero G. (2005) Traffic 6, 324–334 [DOI] [PubMed] [Google Scholar]
- 27.Dulon S., Candé C., Bunnett N. W., Hollenberg M. D., Chignard M., Pidard D. (2003) Am. J. Respir. Cell Mol. Biol. 28, 339–346 [DOI] [PubMed] [Google Scholar]
- 28.Déry O., Thoma M. S., Wong H., Grady E. F., Bunnett N. W. (1999) J. Biol. Chem. 274, 18524–18535 [DOI] [PubMed] [Google Scholar]
- 29.Garland A. M., Grady E. F., Payan D. G., Vigna S. R., Bunnett N. W. (1994) Biochem. J. 303, 177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lohse M. J., Benovic J. L., Codina J., Caron M. G., Lefkowitz R. J. (1990) Science 248, 1547–1550 [DOI] [PubMed] [Google Scholar]
- 31.Goodman O. B., Jr., Krupnick J. G., Santini F., Gurevich V. V., Penn R. B., Gagnon A. W., Keen J. H., Benovic J. L. (1996) Nature 383, 447–450 [DOI] [PubMed] [Google Scholar]
- 32.Ferguson S. S., Downey W. E., 3rd, Colapietro A. M., Barak L. S., Ménard L., Caron M. G. (1996) Science 271, 363–366 [DOI] [PubMed] [Google Scholar]
- 33.DeFea K. A., Vaughn Z. D., O'Bryan E. M., Nishijima D., Déry O., Bunnett N. W. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 11086–11091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luttrell L. M., Ferguson S. S., Daaka Y., Miller W. E., Maudsley S., Della Rocca G. J., Lin F., Kawakatsu H., Owada K., Luttrell D. K., Caron M. G., Lefkowitz R. J. (1999) Science 283, 655–661 [DOI] [PubMed] [Google Scholar]
- 35.Cottrell G. S., Padilla B. E., Amadesi S., Poole D. P., Murphy J. E., Hardt M., Roosterman D., Steinhoff M., Bunnett N. W. (2009) J. Biol. Chem. 284, 22411–22425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daub H., Weiss F. U., Wallasch C., Ullrich A. (1996) Nature 379, 557–560 [DOI] [PubMed] [Google Scholar]
- 37.Luttrell L. M. (2003) J. Mol. Endocrinol. 30, 117–126 [DOI] [PubMed] [Google Scholar]
- 38.Wolfe B. L., Marchese A., Trejo J. (2007) J. Cell Biol. 177, 905–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizuno E., Iura T., Mukai A., Yoshimori T., Kitamura N., Komada M. (2005) Mol. Biol. Cell 16, 5163–5174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alwan H. A., van Leeuwen J. E. (2007) J. Biol. Chem. 282, 1658–1669 [DOI] [PubMed] [Google Scholar]
- 41.Chen L., Davis N. G. (2002) Traffic 3, 110–123 [DOI] [PubMed] [Google Scholar]
- 42.Amerik A. Y., Nowak J., Swaminathan S., Hochstrasser M. (2000) Mol. Biol. Cell 11, 3365–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urbé S., McCullough J., Row P., Prior I. A., Welchman R., Clague M. J. (2006) Biochem. Soc. Trans. 34, 754–756 [DOI] [PubMed] [Google Scholar]
- 44.Shenoy S. K., Lefkowitz R. J. (2003) J. Biol. Chem. 278, 14498–14506 [DOI] [PubMed] [Google Scholar]
- 45.Shenoy S. K., Modi A. S., Shukla A. K., Xiao K., Berthouze M., Ahn S., Wilkinson K. D., Miller W. E., Lefkowitz R. J. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 6650–6655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shenoy S. K., Xiao K., Venkataramanan V., Snyder P. M., Freedman N. J., Weissman A. M. (2008) J. Biol. Chem. 283, 22166–22176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Padilla B. E., Cottrell G. S., Roosterman D., Pikios S., Muller L., Steinhoff M., Bunnett N. W. (2007) J. Cell Biol. 179, 981–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roosterman D., Cottrell G. S., Padilla B. E., Muller L., Eckman C. B., Bunnett N. W., Steinhoff M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 11838–11843 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.