Abstract
Background
Stroke is the third most common cause of death in industrialized countries, accounting for more than 10% of deaths over age 65. Most strokes are due to arteriosclerosis. Regular physical activity lowers arterial blood pressure and body weight and improves glucose and lipid metabolism, thereby slowing the development of arteriosclerosis and its cardiovascular complications, particularly myocardial infarction.
This review focuses on the question whether physical activity might also have a preventive effect on cerebral infarction and hemorrhage.
Methods
This analysis is based on 33 prospective cohort studies and 10 case-control studies that addressed the potential effect of physical activity on stroke-related morbidity and mortality.
Results
Our meta-analysis shows that physical activity reduces the risk of all types of stroke (infarction, hemorrhage, and stroke of unspecified type). The relative risk (RR) of fatal or non-fatal cerebral infarction is 0.75, while the corresponding figures for cerebral hemorrhage and stroke of unspecified type are 0.67 and 0.71, respectively. The reduction of risk is only statistically significant for men. The case-control studies show an RR of 0.32 for men and women combined.
Conclusions
When a multivariate analysis is performed that takes other vascular risk factors into account, physical activity is found to have an independent protective effect against cerebrovascular events.
The effect is statistically significant only for men, not for women.
Keywords: stroke, cerebral hemorrhage, risk of stroke, physical activity, health-related behavior
Stroke is the third most common cause of death in industrialized countries (1). About 30% of all deaths before age 65 and 50% of all deaths thereafter are due to cardiovascular diseases, and one-fifth of these are due to stroke (2). Every third stroke has a fatal outcome (3). Stroke is also the most important cause of disability: 15% to 30% of stroke patients have persistent deficits, which often lead them to require nursing home care (4). Despite advances in stroke treatment, including systemic clot-lysis therapy, the opportunities for treatment remain limited (1).
The risk of stroke doubles every ten years after age 55; the risk of stroke is 50% higher in men than in women from age 55 to 75, but the risk in the two sexes equalizes thereafter (4). The main treatable causes of stroke are the following (4):
Cardiovascular diseases
Arterial hypertension
Smoking
Diabetes mellitus
Carotid stenosis
Atrial fibrillation
Dyslipidemia
Overweight
Excessive alcohol consumption
Hypercoagulable states
The consumption of oral contraceptive drugs.
Regular exercise lowers arterial blood pressure, reduces weight, and improves glucose and lipid metabolism, endothelial function, and the rheological properties of the blood (2, 3, 5). Accordingly, regular exercise also lowers the risk of myocardial infarction (2).
This review article concerns the question whether regular exercise can also lower the risk of suffering or dying from a stroke, and, if so, whether ischemic or hemorrhagic strokes, or both, can be prevented by exercise.
Methods
The PubMed database was searched for original articles dealing with the frequency of ischemic or hemorrhagic strokes in physically active and physically inactive persons. The search terms employed were “stroke” AND “physical activity” OR “sport” AND “prevention” (for all listed years; last update December 2008). Many of the 535 publications retrieved were experimental studies, review articles, or studies on therapeutic aspects and the like. Thus, only a few publications were relevant to the question we sought to answer: this was the case for 33 prospective cohort studies and 10 case-control studies. In addition to these publications, all of the studies included in previously published meta-analyses (3, 6) were included in the present meta-analysis as well.
In all of the studies included in the present analysis, the level of physical activity of each person included in the study was determined from statements made by the participants themselves. The quantitative assessment of physical activity over long periods of time is impractical because of the immense amount of work it would require, and it has thus not been carried out in any study to date. Data from men and women in the cohort studies were analyzed separately as far as possible; in the case-control studies, this distinction could not be drawn, because of the relatively low number of study participants. The present study does not differentiate between occupational and leisure-time physical activity, as its goal is to determine the effect of exercise as such on stroke.
The risk of stroke in physically active persons was calculated in relation to the risk seen in the least physically active group of participants in each of the studies reviewed for the present article. Among the physically active persons, the groups that had the lowest risk of stroke in comparison to physically inactive persons were included in the calculation. The studies listed were subjected to a meta-analysis. All calculations were performed with the aid of the “meta” packet in the “R” statistics software, version 2.8.0 (7). The cohort studies were meta-analyzed separately from the case-control studies.
More specifically, within the class of cohort studies, separate meta-analyses were performed for three subgroups—ischemic, hemorrhagic, and undifferentiated stroke—and for men and women. The “undifferentiated stroke” subgroup consisted of publications in which no diagnostic imaging was carried out, as well as those that grouped the patients with ischemic stroke together with those with intracerebral (sometimes also subarachnoid) hemorrhage. Subarachnoid hemorrhage was not considered in a separate category, as it is rare compared to other types of stroke. The studies in which subarachnoid hemorrhage was listed as an entity in its own right were not eliminated, because a small number of patients with subarachnoid hemorrhage might also be concealed in the statistics of the other studies in which no imaging was performed. The meta-analysis included only those studies whose results were available as an estimate of the strength of the effect accompanied by a confidence interval. The results from the individual studies were combined on a logarithmic scale. Because of heterogeneity among studies, a random-effects model was used consistently. In the Figures, the relative risk (RR) is always shown on a logarithmic scale. The meta-analytic estimators were retransformed back onto the original RR scale.
Results
In a meta-analysis of 18 cohort and 5 case-control studies, Lee et al. (3) concluded that the RR of stroke, or of fatal stroke, is 27% lower in very physically active persons than in those whose level of physical activity is low (RR = 0.73, 95% confidence interval [95% CI]: 0.67–0.79, p<0.001). Even persons who were only moderately physically active had a significantly reduced risk, with RR = 0.80. The risk of both ischemic stroke and intracerebral hemorrhage was reduced. In the cohort studies, the risk reduction for a fatal or non-fatal stroke in very active, as compared to relatively inactive, persons was 21% for ischemic events (RR = 0.79, 95% CI: 0.69–0.91, p<0.001) and 34% for hemorrhagic events (RR = 0.66, 95% CI: 0.48–0.91, p<0.001). The risk reduction remained significant, though of lesser magnitude, for only moderately physically active persons.
In a further meta-analysis, Wendel-Vos et al. (7) distinguished occupational physical activity from physical activity during free time. They reviewed 24 cohort and 7 case-control studies and calculated that a high level of physical activity during free time, as compared with a low one, reduced the risk of all types of stroke by 22% (RR = 0.78, 95% CI: 0.71–0.85, p<0.001), the risk of ischemic stroke by 21% (RR = 0.79, 95% CI: 0.69–0.91, p<0.001), and the risk of intracerebral hemorrhage by 26% (RR = 0.74, 95% CI: 0.57–0.96, p < 0.001). In contrast, when they studied occupational physical activity, they found that moderate physical activity on the job lowered the risk of stroke to a greater extent than a high level of physical activity on the job (36% versus 23%), although they did not state whether this difference was statistically significant. Alevizos et al. (8) also concluded in their review that exercise reduces the risk of stroke. There is as yet no definitive answer to the question whether the extent and intensity of physical activity are significantly correlated with its preventive effect (5). The literature does not even contain a uniform definition of different levels of physical activity, let alone a quantitative measure.
The authors’ findings
eTables e1 and e2 show the relative risk that a physically active person will suffer a stroke or die from one, as compared to the least physically active group of study participants in each study. The eTables also contain further details about the level of activity in each of the groups being compared, and about the duration of observation in each study. The studies listed often give the risk of stroke in various subpopulations; in the present analysis, the two groups presented for comparison are always the group with the lowest level of physical activity as a control group, and the group with the highest level of physical activity or the one with the greatest risk reduction.
eTable 1. The relative risk of (fatal or non-fatal) ischemic and hemorrhagic stroke depending as a function of physical exercise in prospective cohort studies.
| Source | Patient collective (sex, [median] age), median follow-up interval | Cofactors considered | Exercise group | Comparison group | n | RR | p |
| Ischemic stroke | |||||||
| Abbott et al. 1994 (15) | M, 45–68 yr | age, arterial hypertension, smoking, alcohol consumption, glucose, cholesterol, uric acid, hematocrit, LVH | exercise | no exercise | 7530 | nonsmokers: 0.6 | nonsmokers: p < 0.05 smokers: ns |
| 22 yr | (0.3–0.9)*1 | ||||||
| smokers: 0.8 | |||||||
| (0.6–1.3) *1 | |||||||
| Agnarsson et al. 1999 (16) | M, 45–80 yr | age, smoking, glucose, arterial hypertension, pulmonary function | leisure activities from age 40 onward | no sports | 4484 | 0.62 (0.40–0.97) | p = 0.03 |
| 10.6 ± 3.6 yr | |||||||
| Chiuve et al. 2008 (11) | M, 40–75 yr | Heart attack in a parent before age 60, acetylsalicylic acid, vitamin E supplementation, hormone therapy (women), arterial hypertension, hypercholesterolemia, diabetes mellitus | 6 h sports/wk | no sports | 43 685 M, | M: 0.57 (0.43–0.75) | p < 0.05 |
| 2 yr | 71 243 F | F: 0.60 (0.45–0.79) | |||||
| F, 30–55 yr | |||||||
| 6 yr | |||||||
| Evenson et al. 1999 (17) | F, 45–64 yr | sex, age, ethnic group, education, smoking, arterial hypertension,fibrinogen, BMI, diabetes mellitus | among 4 groups, the one that was most active in sports | among 4 groups, the one that was least active in sports | 8296 | 1.29 (0.89–1.87) | NS |
| 7.2 yr | |||||||
| Evenson et al. 1999 (17) | M, 45–64 yr | sex, age, ethnic group, education, smoking, arterial hypertension, fibrinogen, BMI, diabetes mellitus | among 4 groups, the one that was most active in sports | among 4 groups, the one that was least active in sports | 6279 | 0.74 (0.50–1.10) | NS |
| 7.2 yr | |||||||
| Gillum et al. 1996 (18) | M & F, 45–74 yr | age, smoking, diabetes mellitus, coronary heart disease, education, systolic blood pressure, cholesterol, BMI, hemoglobin concentration | moderate to large amount of exercise in free time | little exercise in free time | 5852 | women 45–64 yr | NS |
| 11.6 yr | 0.35 (0.10–1.15) | ||||||
| women 65–74yr | |||||||
| 0.68 (0.41–1.14) | |||||||
| men 45–64 yr | |||||||
| 0.91 (0.45–1.85) | |||||||
| men 65–74 yr | |||||||
| 0.75 (0.50–1.11) | |||||||
| Harmsen et al. 1990 (19) | M, 47–55 yr | blood pressure, BMI, cholesterol, smoking, emotional stress, atrial fibrillation, myocardial infarction, stroke, history of TIA | among 4 groups, the 3 groups that were more active in sports | among 4 groups, the one that was least active in sports | 7495 | 0.83 (0.7–1.2) | NS |
| 28 yr | |||||||
| Hu et al. 2000 (20) | F, 40–65 yr | age, smoking, alcohol consumption, BMI, menopausal status, aspirin consumption, family history of myocardial infarction under age 60, diabetes mellitus, hypercholesterolemia, high blood pressure | ≥ 21.7 | ≤ 2.0 | 72 488 | 0.52 (0.33–0.80) | p = 0.003 |
| 8 yr | MET × h/week | MET × h/week | |||||
| Hu et al. 2005 (9) | M & F, 25–64 yr | age, sex, BMI, systolic blood pressure, cholesterol, education, smoking, alcohol consumption, diabetes mellitus | Intense exercise in free time (>3 h/wk: running, swimming, intense gardening, martial arts) | Little or no exercise | 474 721 | 0.80 (0.68–0.93) | p < 0.001 |
| 19.0 yr | |||||||
| Lee et al. 1999 (21) | M, 40–84 yr | age, smoking, alcohol consumption, angina pectoris, arterial hypertension, diabetes mellitus, hypercholesterolemia, myocardial infarction in a parent | intense exercise (leading to sweating) at least once a week | intense exercise (leading to sweating) less than once a week | 21 823 | 0.90 (0.66–1.22) | NS |
| 11.1 yr | |||||||
| Mora et al. 2007 (13) | F > 45 yr | age, high blood pressure, diabetes mellitus, lipids, laboratory evidence of inflammation and coagulopathy, BMI, homocysteine | energy consumption 200–599 kcal/wk | energy consumption ≥1 500 kcal/wk | 27 055 | 0.76 (0.58–0.99) | p < 0.05 |
| 10.9 ± 1.6 yr | |||||||
| Nakayama et al. 1997 (22) | F > 40 yr | age | intense exercise | mild exercise | 1469 | 2.10 (0.89–4.97) | NS |
| 15.5 yr | |||||||
| Nakayama et al. 1997 (22) | M > 40 yr | age | intense exercise | mild exercise | 1182 | 1.11 (0.52–2.38) | NS |
| 15.5 yr | |||||||
| Noda et al. 2005 (12) | F, 40–79 yr | age, BMI, arterial hypertension, diabetes mellitus, smoking, alcohol consumption, sleep, occupation, emotional stress, frequency of fish consumption | ≥ 5 h sports/wk | 1–2 h sports/wk | 64 327 | 0.73 (0.31–1.70) | NS |
| 9.7 yr | |||||||
| Noda et al. 2005 (12) | M, 40–79 yr | age, BMI, arterial hypertension, diabetes mellitus, smoking, alcohol consumption, sleep, occupation, emotional stress, frequency of fish consumption | ≥ 5 h sports/wk | 1–2 h sports/wk | 31 023 | 0.84 (0.45–1.57) | NS |
| 9.7 yr | |||||||
| Okada et al. 1976 (23) | M & F, 40–79 yr | age, sex | regular exercise on the job | No exercise on the job | 3649 | 0.83 | NS |
| 6 yr | |||||||
| Paganini-Hill & Perez Barreto 2001 (24) | F, 44–101 yr | arterial hypertension and its treatment, diabetes mellitus, coronary heart disease, digitalis, smoking, acetylsalicylic acid, gynecological history | exercise > 1 h/d | exercise | 8532 | 0.81 | NS |
| (median: 74 yr) | <0.5 h/d | ||||||
| 17 yr | |||||||
| Paganini-Hill & Perez Barreto 2001 (24) | M, 44–101 yr | arterial hypertension and its treatment, diabetes mellitus, coronary heart disease, digitalis, smoking, acetylsalicylic acid, gynecological history | exercise > 1 h/d | exercise | 4722 | 0.96 | NS |
| (median: 74 yr) | <0.5 h/d | ||||||
| 17 yr | |||||||
| Hemorrhagic stroke | |||||||
| Abbott et al. 1994 (15) | M, 45–68 yr | age, systolic blood pressure, smoking, alcohol consumption, glucose, uric acid, hematocrit, LVH | exercise | no exercise | 7530 | 45–54 yr: 0.5 | 45–54yr: |
| 22 yr | (0.2–1.3)*1 | NS | |||||
| 55–68 yr: 0.3 | 55–68 yr: | ||||||
| (0.1–0.8) *1 | p < 0.05 | ||||||
| Chiuve et al. 2008 (11) | M, 40–75 yr | myocardial infarction in a parent under age 60, acetylsalicylic acid, vitamin E supplementation, hormone therapy (women), arterial hypertension, hypercholesterolemia, diabetes mellitus | ≥ 6 h sports/wk | no sports | 43 685 M, | M: 0.65 (0.39–1.09) | NS |
| 2 yr | 71 243 F | F: 0.79 (0.50–1.26) | |||||
| F, 30–55 yr | |||||||
| 6 yr | |||||||
| Harmsen et al. 1990 (19) | M, 47–55 yr | blood pressure, BMI, cholesterol, smoking, emotional stress, atrial fibrillation, myocardial infarction, stroke, history of TIA | the 3 more active of 4 groups | the least active of 4 groups | 7495 | 0.91 (0.27–2.50) | NS |
| 28 yr | |||||||
| Hu et al. 2000 (20) | F, 40–65 yr | age, smoking, alcohol consumption, BMI, menopausal status, aspirin consumption, family history of myocardial infarction under age 60, diabetes mellitus, hypercholesterolemia, high blood pressure | 4.7–10.4 | ≤ 2.0 | 72 488 | 0.72 (0.25–2.08) | NS |
| 8 yr | MET × h/week | MET × h/week | |||||
| Hu et al. 2005 (9) | M & F, 25–64 yr | age, sex, BMI, systolic blood pressure, cholesterol, education, smoking, alcohol consumption, diabetes mellitus | intense leisure activities (>3 h/wk running, swimming, intense gardening, competitive sports) | little or no exercise | 47 721 | 0.63 (0.42–0.95) | p < 0.001 |
| 19.0 yr | |||||||
| Lee et al. 1999 (21) | M, 40–84 yr | age, smoking, alcohol consumption, angina pectoris, arterial hypertension, diabetes mellitus, hypercholesterolemia, myocardial infarction in a parent | intense exercise leading to sweating at least once a week | intense exercise leading to sweating less than once a week | 21 823 | 0.54 (0.25–1.13) | NS |
| 11.1 yr | |||||||
| Nakayama et al. 1997 (22) | M > 40 yr | age | mild exercise | no exercise | 1182 | 1.38 (0.27–7.16) | NS |
| 15.5 yr | |||||||
| Nakayama et al. 1997 (22) | F, > 40 yr | age, BMI, high blood pressure, smoking, alcohol consumption, albumin- and glycosuria, cholesterol, hematocrit, fundus abnormalities, ECG | mild exercise | no exercise | 1469 | 4.13 (1.09–15.69) | p < 0.05 |
| 15.5 yr | |||||||
| Noda et al. 2005 (12) | F, 40–79 yr | age, BMI, arterial hypertension, diabetes mellitus, smoking, alcohol consumption, sleep, occupation, emotional stress, frequency of fish consumption | ≥ 5 h sports/wk | 1–2 h sports/wk | 42 242 | 0.40 (0.11–1.41) | NS |
| 9.7 yr | |||||||
| Noda et al. 2005 (12) | M, 40–79 yr | age, BMI, arterial hypertension, diabetes mellitus, smoking, alcohol consumption, sleep, occupation, emotional stress, frequency of fish consumption | ≥ 5 h sports/wk | 1–2 h sports/wk | 31 023 | 0.67 (0.27–1.64) | NS |
| 9.7 yr | |||||||
| Okada et al. 1976 (23) | M & F, 40–79 yr | age, sex | regular exercise on the job | no exercise on the job | 3649 | 0.43 | NS |
| 6 yr | |||||||
| Paganini-Hill & Perez Barreto 2001 (24) | F, 44–101 yr | arterial hypertension and its treatment, diabetes mellitus, coronary heart disease, digitalis, smoking, acetylsalicylic acid, gynecological history | exercise > 1 h/d | exercise | 8532 | 1.00 | NS |
| (median: 74 yr) | <0.5 h/d | ||||||
| 17 yr | |||||||
| Paganini-Hill & Perez Barreto 2001 (24) | M, 44–101 yr | arterial hypertension and its treatment, diabetes mellitus, coronary heart disease, digitalis, smoking, acetylsalicylic acid, gynecological history | exercise > 1 h/d | exercise | 4722 | 0.69 | NS |
| (median: 74 yr) | <0.5 h/d | ||||||
| 17 yr | |||||||
| Undifferentiated stroke*2 | |||||||
| Agnarsson et al. 1999 (16) | M, 45–80 yr | age, smoking, glucose, arterial hypertension, pulmonary function | leisure activities from age 40 onward | no sports | 4484 | 0.69 (0.47–1.01) | NS |
| 10.6 ± 3.6 yr | |||||||
| Bijnen et al. 1998 (25) | M, 64–84 yr | age, smoking, alcohol consumption, chronic illnesses | exercise | inactive lifestyle | 802 | 0.95 (0.52–1.74) | NS |
| 10 yr | |||||||
| Chiuve et al. 2008 (11) | F, 30–55 yr | myocardial infarction in a parent under age 60, acetylsalicylic acid, vitamin E supplementation, hormone therapy (women), arterial hypertension, hypercholesterolemia, diabetes mellitus | ≥ 6 h sports/wk | no sports | 71 243 | 0.58 (0.47–0.71) | p < 0.05 |
| 6 yr | |||||||
| Chiuve et al. 2008 (11) | M, 40–75 yr | myocardial infarction in a parent under age 60, acetylsalicylic acid, vitamin E supplementation, hormone therapy (women), arterial hypertension, hypercholesterolemia, diabetes mellitus | ≥ 6 h sports/wk | no sports | 43 685 | 0.60 (0.49–0.74) | p < 0.05 |
| 2 yr | |||||||
| Ellekjær et al. 2000 (e1) | F, 50–101 yr | age, BMI, systolic blood pressure, antihypertensive drugs, diabetes mellitus, coronary heart disease, smoking | intense exercise | little exercise | 14 101 | 0.52 (0.38–0.72) | p < 0.001 |
| 9.0 yr | |||||||
| Folsom et al. 1990 (e2) | F, 55–69 yr | age, arterial hypertension, diabetes mellitus | the most active among 3 groups | the least active among 3 groups | 41 837 | 0.8 (0.5–1.1) | NS |
| 2 yr | |||||||
| Gillum et al. 1996 (18) | M & F, 45–74 yr | age, smoking, diabetes mellitus, coronary heart disease, education, systolic blood pressure, cholesterol, BMI, hemoglobin concentration | large amount of exercise in free time | small amount of exercise in free time | 5852 | women 45–64 yr | NS |
| 11.6 yr | 0.31 (0.10–1.05) | ||||||
| women 65–74 yr | |||||||
| 0.65 (0.40–1.05) | |||||||
| men 45–64 yr | |||||||
| 0.81 (0.41–1.59) | |||||||
| men 65–74 yr | |||||||
| 0.78 (0.53–1.14) | |||||||
| Håheim et al. 1993 (e3) | M, 40–49 yr | none | moderate to intense exercise in spare time | no exercise in spare time | 14 403 | 0.36 (0.15–0.80) | p < 0.05 |
| 12 yr | |||||||
| Harmsen et al. 1990 (19) (SAB) | M, 47–55 yr | blood pressure, BMI, cholesterol, smoking, emotional stress, atrial fibrillation, myocardial infarction, stroke, TIA in the history | the 3 more active of 4 groups | the least active of 4 groups | 7495 | 0.83 (0.56–1.25) | NS |
| 28 yr | |||||||
| Harmsen et al. 2006 (e4) | M, 47 – 55 yr | age, systolic blood pressure, antihypertensive drugs, TIA, atrial fibrillation, stroke or coronary event in a parent, diabetes mellitus, angina pectoris, smoking, emotional stress, BMI, cholesterol, social status | moderate to intense exercise | little exercise | 7457 | 0.90 (0.74–1.11)*2 | NS |
| 28 yr | |||||||
| Hu et al. 2000 (20) (SAB) | F, 40–65 yr | age, smoking, alcohol consumption, BMI, menopausal status, aspirin consumption, family history of myocardial infarction under age 60, diabetes mellitus, hypercholesterolemia, high blood pressure | 4.7–10.4 | ≤ 2.0 | 72 488 | 0.66 (0.47–0.91) | p < 0.05 |
| 8 yr | MET × h/week | MET × h/week | |||||
| Hu et al. 2005 (9) (SAB) | F, 25–64 yr | age, sex, BMI, systolic blood pressure, cholesterol, education, smoking, alcohol consumption, diabetes mellitus | intense exercise in free time (>3 h/wk running, swimming, intense gardening, competitive sports) | little or no exercise | 24 880 | 0.77 (0.62–0.97) | p < 0.001 |
| 19.0 yr | |||||||
| Hu et al. 2005 (9) (SAB) | M, 25–64 yr | age, sex, BMI, systolic blood pressure, cholesterol, education, smoking, alcohol consumption, diabetes mellitus | intense exercise in free time (>3 h/wk running, swimming, intense gardening, competitive sports) | little or no exercise | 22 841 | 0.72 (0.60–0.87) | p < 0.001 |
| 19.0 yr | |||||||
| Kiely et al. 1994 (e5) | F, 49.7 and | age, systolic blood pressure, cholesterol, smoking, glucose intolerance, vital capacity, BMI, LVH, atrial fibrillation, cardiac valvular defects | the most physically active of 3 groups | the least physically active of 3 groups | 2873 | 1.21 (0.75–1.96) | NS |
| 49.9 yr | |||||||
| up to 32 yr | |||||||
| Kiely et al. 1994 (e5) | M, 49.7 and | age, systolic blood pressure, cholesterol, smoking, glucose intolerance, vital capacity, BMI, LVH, atrial fibrillation, cardiac valvular defects | the most physically active of 3 groups | the least physically active of 3 groups | 2336 | 0.53 (0.34–0.84) | p < 0.05 |
| 49.9 yr | |||||||
| up to 32 yr | |||||||
| Lapidus & Bengtsson 1986 (e6) | F, 38–60 yr | age, BMI, waist-to-hip ratio, systolic blood pressure, triglycerides, cholesterol, smoking, socioeconomic status | At least a half hour walking or bicycling per day, regular exercise or competitive sports | no exercise | 1462 | 0.1 (0.1–0.3) | p < 0.001 |
| 12 yr | |||||||
| Lee & Blair 2002 (e7) | M, 40–87 yr | age, smoking, alcohol consumption, BMI, arterial hypertension, diabetes mellitus, coronary heart disease in a parent | the 40% of the group that had the highest cardiovascular fitness (treadmill test) | the 20% of the group that had the lowest cardiovascular fitness | 16 878 | 0.32 (0.12–0.82) | p < 0.05 |
| 10 yr | |||||||
| Lee & Paffenbarger Jr. 1998 (e8) | M, 58 yr | age, smoking, alcohol consumption, early death in a parent | weekly energy consumption by exercise <1000 kcal | weekly energy consumption by exercise 2000 to 2999 kcal | 11 130 | 0.54 (0.38–0.76) | p < 0.05 |
| (43–88 yr) | |||||||
| 11 yr | |||||||
| Lindenstrøm et al. 1993 (e9) | F, >35 yr | age, BMI, smoking, alcohol consumption, tranquilizers and hypnotics, oral contraception, hormone replacement therapy | at least 2 h of intense or 4 h of mild exercise/wk | less than 2 h of intense or 4 h of mild exercise/wk | 7060 | 0.69 (0.48–1.00)*1 | p < 0.05 |
| 12 yr | |||||||
| Lindsted et al. 1991 (e10) | M, 49.9–54.7 ± | ethnic group, smoking, education, BMI, family status, eating habits | moderate exercise | little exercise | 9484 | 0.78 (0.61–1.00) | NS |
| 12.0–16.5 yr | |||||||
| 26 yr | |||||||
| Menotti & Seccareccia 1985 (e11) | M, 40–59 yr | age | calorie consumption >3000/d | calorie consumption <2400/d | 99 029 | almost 1.0 | NS |
| up to 5 yr | |||||||
| Myint et al. 2006 (10) | F, 40–76 yr | age, sex, smoking, systolic blood pressure, BMI, cholesterol, diabetes mellitus | physically active | physically inactive | 12 523 | 0.71 (0.41–1.26) | NS |
| 8.5 yr | |||||||
| Myint et al. 2006 (10) | M, 40–76 yr | age, sex, smoking, systolic blood pressure, BMI, cholesterol, diabetes mellitus | physically active | physically inactive | 10 079 | 0.67 (0.43–1.05) | NS |
| 8.5 yr | |||||||
| Nakayama et al. 1997 (22) (SAB) | F > 40 yr | age, BMI, high blood pressure, smoking, alcohol consumption, albumin- and glycosuria, cholesterol, hematocrit, fundus abnormalities, ECG | mild exercise | no exercise | 1469 | 1.95 (1.03–3.68) | p < 0.05 |
| 15.5 yr | |||||||
| Nakayama et al. 1997 (22) (SAB) | M > 40 yr | age | mild exercise | no exercise | 1182 | 1.38 (0.77–2.48) | NS |
| 15.5 yr | |||||||
| Noda et al. 2005 (12) (SAB) | F, 40–79 yr | age, BMI, arterial hypertension, diabetes mellitus, smoking, alcohol consumption, sleep, occupation, emotional stress, frequency of fish consumption | ≥ 5 h sports/wk | 1–2 h sports/wk | 42 242 | 1.09 (0.90–1.32) | NS |
| 9.7 yr | |||||||
| Noda et al. 2005 (12) (SAB) | M, 40–79 yr | age, BMI, arterial hypertension, diabetes mellitus, smoking, alcohol consumption, sleep, occupation, emotional stress, frequency of fish consumption | ≥ 5 h sports/wk | 1–2 h sports/wk | 31 023 | 0.87 (0.59–1.27) | NS |
| 9.7 yr | |||||||
| Paffenbarger et al. 1984 (e12) | M, 49.7 ± 5.7 yr | age, arterial hypertension, smoking | energy consumption/wk >2000 kcal | energy consumption/wk <500 kcal | 16 936 | 0.37 | p < 0.001 |
| 10 yr | |||||||
| Paganini-Hill & Perez Barreto 2001 (24) | F, 44–101 yr | arterial hypertension and its treatment, diabetes mellitus, coronary heart disease, digitalis, smoking, acetylsalicylic acid, gynecological history | exercise >1 h/d | exercise | 8532 | 0.83 | p < 0.01 |
| (median: 74 yr) | <0.5 h/d | ||||||
| 17 yr | |||||||
| Paganini-Hill & Perez Barreto 2001 (24) | M, 44–101 yr | arterial hypertension and its treatment, diabetes mellitus, coronary heart disease, digitalis, smoking, acetylsalicylic acid, gynecological history | exercise >1 h/d | exercise | 4722 | 0.85 | NS |
| (median: 74 yr) | <0.5 h/d | ||||||
| 17 yr | |||||||
| Panagiotakos et al. 2003 (e13) | M, 49.7 ± 5.7 yr | age, high blood pressure, cholesterol, smoking, BMI | physically active | physically inactive | 529 | 0.59 (0.32–0.92) | p < 0.05 |
| 40 yr | |||||||
| Salonen et al. 1982 (e14) | F, 30–59 yr | age, cholesterol, diastolic blood pressure, BMI, smoking | large amount of exercise in free time | small amount of exercise in free time | 3688 | 0.8 (0.5–1.3)*1 | NS |
| 7 yr | |||||||
| Salonen et al. 1982 (e14) | M, 30–59 yr | age, cholesterol, diastolic blood pressure, BMI, smoking | large amount of exercise in free time | small amount of exercise in free time | 3978 | 1.0 (0.7–1.4)*1 | NS |
| 7 yr | |||||||
| Simonsick et al. 1993 (e15) | M and F, | age, sex, education, occupation, smoking, depression, respiratory symptoms, angina pectoris, myocardial infarction, stroke or diabetes mellitus in the history | the most physically active 16% to 26% | the most physically inactive 26% to 32% | East Boston: | East Boston: | p < 0.05 only in iowa, not in east boston or new haven |
| 65–74 yr | 1728 | 0.58 (0.17–1.95) | |||||
| 6 yr | |||||||
| New Haven: | New Haven: | ||||||
| 1387 | 1.06 (0.38–2.95) | ||||||
| Iowa: | Iowa: | ||||||
| 1725 | 0.22 (0.08–0.61) | ||||||
| Wannamethee & Shaper 1992 (e16)*3 | M, 40–59 yr | age, social status, smoking, alcohol consumption, BMI | intense exercise | little or no exercise | 7 735 | 0.3 | p < 0.01 |
| 9.5 yr | |||||||
| Wannamethee et al. 1998 (e17) | M, 40–59 yr | BMI, smoking, alcohol consumption | exercise | no exercise | 7 142 | 0.54 (0.38–0.77) | p < 0.05 |
| 15 yr | |||||||
BMI, body-mass index; d, day; F, female; LVH, left ventricular hypertrophy; M, male; MET, multiple of energy consumption at rest when exercising; n, number of study participants; NA, some items of (non-significant) data not mentioned because of the multiplicity of subgroups and limited space; p, level of statistical significance; PAOD, peripheral arterial occlusive disease; RR, the relative risk of having, or dying of, a stroke; SAB, including subarachnoid hemorrhage; TIA, transient ischemic attack; wk, week; yr, year;
*1 the original data give the relative risk of the inactive persons; the data for both sexes are given together, whenever available in the original publication
*2 ischemic and hemorrhagic strokes were not differentiated by imaging studies;
*3 not considered in the meta-analysis, because the results are incorporated in the published continuation of the same study (e17)
eTable 2. The relative risk of (fatal or non-fatal) ischemic and hemorrhagic stroke depending as a function of physical exercise in case-control studies.
| Source | Patient collective | Cofactors considered | Exercise group | Comparison group | RR | p |
| Ischemic stroke | ||||||
| Choi-Kwon & Kim 1998 (e18) | 135 F, 66 F in the comparison group, median age 65–66 yr ± 6–10 yr | age, arterial hypertension, diabetes mellitus, smoking, alcohol consumption, eating habits, BMI, overall body fat content, subcutaneous fat | recent exercise | no recent exercise | 0.2 (0.1–0.5) | p < 0.01 |
| Ellekjær et al. 1992 (e19) | 95 M, 68 F, median age 69 yr (62–75 yr) 652 in the comparison group | none | walking, skiing, bicycling or the like at least once a week | walking, skiing, bicycling or the like less than once a week | 0.88 (0.58–1.39) | NS |
| Krarup et al. 2007 (14) | M & F, 127 in exercise group, median age 71.8 yr ± 11.7 yr 301 in the comparison group, median age 71.5 yr ± 11.6 yr | systolic blood pressure, stroke or myocardial infarction in the history, atrial fibrillation, diabetes mellitus, alcohol consumption, education, BMI, smoking | activity index for the preceding week in the highest category | activity index for the preceding week in the lowest category | 0.09 (0.03–0.25) | p < 0.05 |
| Sacco et al. 1998 (e20) | 57% F, 43% M, 369 in exercise group, 678 in comparison group, 69.9 ± 12.0 yr | age, sex, ethnic group, coronary heart disease, PAOD, arterial hypertension, diabetes mellitus, smoking, alcohol consumption, obesity, season | intense exercise (hiking, tennis, swimming, bicycling, jogging, aerobics, handball and the like) | no exercise | 0.23 (0.10–0.54) | p < 0.05 |
| Shinton & Sagar 1993 (e21) | 125 M & F in exercise group, 198 M & F in control group; 35–74 yr | age, sex, cigarette smoking | walking: rapid walking, at least 1 mile/mo.; intense sports: running, swimming, bicycling and the like | less exercise than this | walking: | walking: p < 0.05 |
| 0.3 (0.1–0.7) | ||||||
| intense sports: | intense sports: NS | |||||
| 0.61 (0.2–1.5) | ||||||
| You et al. 1995 (e22) | 203 M, 203 F, | sex, age, arterial hypertension, diabetes mellitus, alcohol consumption, smoking, oral contraceptives | exercise leading to sweating and shortness of breath at least three times a week | rarely, or never, exercise leading to sweating and shortness of breath | 0.3 (0.1–0.7) | p < 0.05 |
| 15–85 yr | ||||||
| You et al. 1997 (e23) | 201 M, 201 F, | sex, age, arterial hypertension, diabetes mellitus, alcohol consumption, smoking, coronary heart disease, hypercholesterolemia, oral contraceptives | exercise leading to sweating and shortness of breath at least three times a week | rarely, or never, exercise leading to sweating and shortness of breath | 0.6 (0.3–1.3) | NS |
| 15–45 yr (median age 45.5 yr) | ||||||
| Hemorrhagic stroke | ||||||
| Choi-Kwon & Kim 1998 (e18) | 135 F, 66 F in the comparison group, median age | age, arterial hypertension, diabetes mellitus, smoking, alcohol consumption, eating habits, BMI, overall body fat content, subcutaneous fat | recent exercise | no recent exercise | 0.2 (0.1–1.0) | p = 0.05 |
| 65–66 yr ± 6–10 yr | ||||||
| Thrift et al. 2002 (e24) | 331 M & F in the exercise group and 331 M & F in the control group, | age, sex, high blood pressure, cholesterol, coronary heart disease, diabetes mellitus, BMI, smoking, hormone replacement therapy, educational level | exercise leading to sweating at least once per month (up to the event) | no exercise leading to sweating (up to the event) | 0.66 (0.39–1.11) | NS |
| 63.4 ± 1.4 yr | ||||||
| Undifferentiated stroke * | ||||||
| Herman et al. 1982 (e25) | M & F, 40–74 yr, 126 stroke patients, 212 control subjects | age | regular, intense exercise | little exercise | 0.4 (0.2–0.9) | p < 0.01 |
BMI, body-mass index; d, day; F, female; LVH, left ventricular hypertrophy; M, male; MET, multiple of energy consumption at rest when exercising; n, number of study participants; NA, some items of (non-significant) data not mentioned because of the multiplicity of subgroups and limited space; p, level of statistical significance; PAOD, peripheral arterial occlusive disease; RR, the relative risk of having, or dying of, a stroke; SAB, including subarachnoid hemorrhage; TIA, transient ischemic attack; wk, week; yr, year; * ischemic and hemorrhagic strokes were not differentiated with imaging studies
Cohort studies
28 of the 33 cohort studies were included in the meta-analysis because both effect estimators and confidence intervals were reported on.
Ischemic stroke
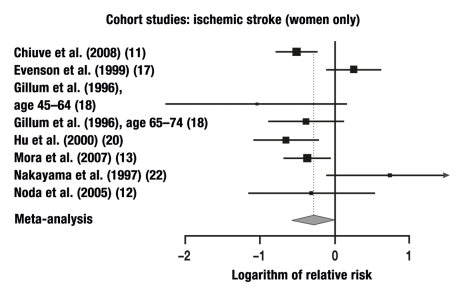
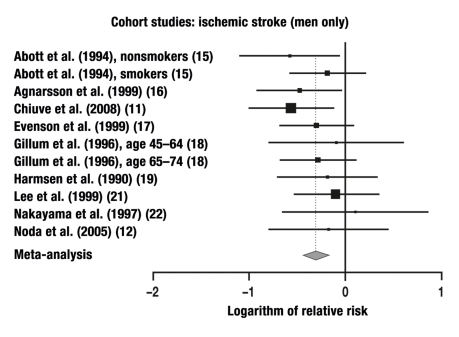
12 of the 14 studies in which ischemic strokes were studied were combined in a meta-analysis. These studies yielded a total of 20 individual comparisons for meta-analysis: RR = 0.75 (95% CI: 0.67–0.84, p < 0.0001; Q = 30.13, p = 0.05, I2 = 36.9%).
Cerebral hemorrhage
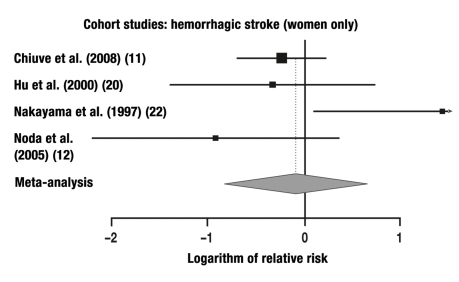
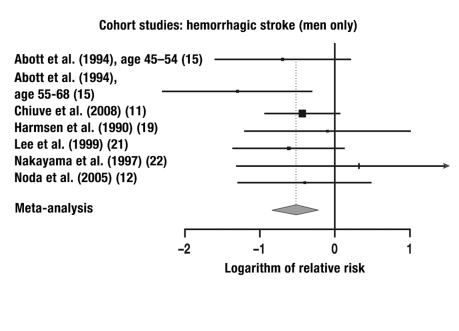
7 of the 9 studies in which the risk of cerebral hemorrhage was studied were combined in a meta-analysis. These 7 studies yielded a total of 12 individual comparisons for meta-analysis: RR = 0.67 (95% CI: 0.52–0.86, p = 0.0013; Q = 13.20, p = 0.28, I2 = 16.7%).
Stroke of undifferentiated type
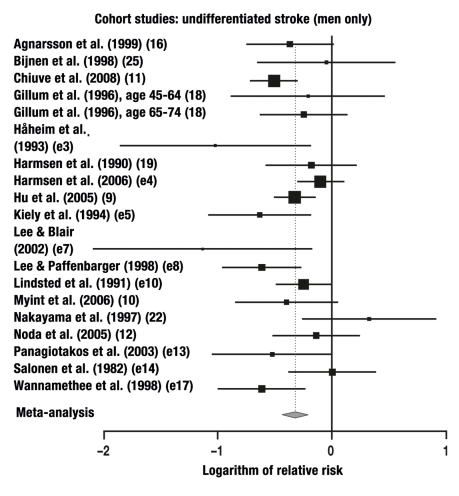
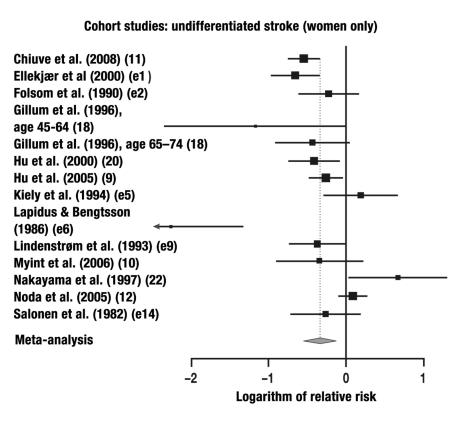
23 of the 27 studies in which the risk of stroke was studied without any differentiation between ischemic and hemorrhagic events were combined in a meta-analysis. These studies yielded a total of 23 individual comparisons for meta-analysis: RR = 0.71 (95% CI: 0.64–0.80, p<0.0001; Q = 97.1, p<0.0001, I2 = 64%).
Case-control studies
Results for ischemic strokes were reported in 7 of the 10 case-control studies. Meta-analysis yielded the following results: RR = 0.32 (95% CI: 0.17–0.59, p = 0.0003; Q = 26.1, p = 0.0002, I2 = 77%). Two studies gave results for cerebral hemorrhages: one result was barely significant, the other insignificant. The results for strokes of undifferentiated type from two studies were significant.
The relative risks that were calculated separately for men and women are given in the Table. The results of our meta-analysis are given in Figures 1–6 and are, in general, consistent with the findings of Lee et al. (3) and Wendel-Vos et al. (7).
Table. The relative risk of a cerebrovascular event as a function of study design.
| Study design | Number of studies | Number of comparisons | Sex | Type of stroke | RR | Remarks |
| Cohort study | 7 | 8 | F | Ischemic | 0.76 | 95% CI: 0.56–1.02, |
| p=0.065; Q=20.54, | ||||||
| p=0.005, I2=65.9% | ||||||
| Cohort study | 9 | 11 | M | Ischemic | 0.73 | 95% CI: 0.65–0.83, |
| p<0.001; q=8.71, | ||||||
| p=0.56, I2=0% | ||||||
| Cohort study | 4 | 4 | F | Hemorrhagic | 0.92 | 95% CI: 0.44–1.93, |
| p=0.8236; Q=6.95, | ||||||
| p=0.073, I2=56.9% | ||||||
| Cohort study | 6 | 7 | M | Hemorrhagic | 0.60 | 95% CI: 0.43–0.83, |
| p=0.001; Q=4.26, | ||||||
| p=0.64, I2=0% | ||||||
| Cohort study | 13 | 14 | F | Undifferentiated | 0.71 | 95% CI: 0.58–0.88, |
| p=0.002; Q=59.4, | ||||||
| p<0.001, i2=78.1% | ||||||
| Cohort study | 18 | 19 | M | Undifferentiated | 0.72 | 95% CI: 0.64–0.80, |
| p<0.001; q=31.2, | ||||||
| p=0.027, I2=42.3% | ||||||
| Case-control study | 7 | 7 | M + F | Ischemic | 0.32 | 95% CI: 0.17–0.59, |
| p<0.001; q=26.1, | ||||||
| p<0.001, i2=77% |
M=male; F=female; CI=confidence interval; p=p-value; Q=Cochrane’s homogeneity statistic; RR=relative risk; I2=Higgins’ I2 (a measure of the percentage of the differences between studies that is not due to chance)
Figure 1.
Figure 6.
16 studies in which physical activity was graded on a scale with three or more levels showed that the risk of stroke declined with increasing physical activity (9, 11, 12, 15, 18, 20, 25, e1, e2, e3, e5, e7, e10, e12, e16, e17). Eight studies revealed a U-shaped dependence, i.e., the risk of stroke was less with intermediate degrees of physical activity than with lower or higher physical activity (10, 11, 15, 18, 20, e3, e8, e11). On the other hand, five studies revealed an inverted U-shaped dependence, in which the risk of stroke was highest for an intermediate degree of activity (9, 15, 18, e5, e15). In the four studies in which physical activity was graded on a scale with at least four levels, the risk of stroke was found to depend on the level of physical activity in an unsystematic way (10, 11, 12, 21). A number of studies appear in our tables more than once, because many studies involved several different populations or types of activity (e.g., on the job or during free time). Nonetheless, the differences in risk between the different levels of activity were mostly insignificant.
Discussion
The studies available to date show that regular exercise can lower the risk of a fatal or non-fatal stroke (infarct, cerebral hemorrhage, or—rarely—subarachnoid hemorrhage) by about 20% to 30%. There is a trend suggesting that a higher level of physical activity is more effective than a lower one for the prevention of ischemic stroke. Not all of the relevant studies yielded significant results, as is usually the case in epidemiology. Inadequate cohort size may explain this in some cases.
In the present meta-analysis, which is based on 33 cohort studies, the risk of a fatal or non-fatal ischemic stroke was lowered by 24% in women and by 27% in men, while that of a cerebral hemorrhage was lowered by 8% in women and by 40% in men. For strokes that were not radiologically classified as being either ischemic or hemorrhagic, the risk was 29% lower and 28% lower (in women and men, respectively) than in the physically inactive persons constituting the control group, but only the risk reduction in men was statistically significant. It remains an open question whether the insignificant results in women were due to the smaller number of studies including women. In the case-control studies, the risk reduction for men and women combined reached the very high figure of 68%.
As can be seen from Figures 1–6, there was one study for each type of stroke that actually showed the risk of stroke in physically active men to be higher than in physically inactive men. In women, a higher risk associated with physical activity was seen in two studies on ischemic stroke, one on cerebral hemorrhage, and three on undifferentiated strokes. All other studies showed that physically active persons were less likely to have a stroke. No case-control study indicated a higher risk for physically active persons.
The statistically significant preventive effect of physical activity on stroke in all of the calculations undertaken here (in agreement with two earlier meta-analyses [3, 7]) does not preclude the possibility that such an effect might be absent in certain subgroups. Thus, voluntary exercise during one’s free time might conceivably have a different effect from compulsory exercise on the job. The effect might also vary from one ethnic group to another. It is known, for example, that black US-Americans suffer strokes at least twice as commonly as their white compatriots, and tend to have them at different sites and with different pathophysiological mechanisms (e28).
A clear dependence of the magnitude of risk reduction on the extent of physical activity (i.e., a dose-response effect) has not yet been demonstrated. Most studies indicate, however, that the risk is less with higher levels of activity. There may be a saturation effect, however, or even an increased risk when physical activity is very frequent or very intense.
Most of the original articles that we evaluated here to calculate the risk of a fatal or non-fatal stroke took a number of the known risk factors for vascular disease into account, e.g., high blood pressure, diabetes mellitus, lipid metabolic disorders, obesity, smoking, and alcohol consumption. Nonetheless, some publications left some risk factors out of consideration (e.g., dietary habits) and did not assess others precisely or in detail (e.g., only overall cholesterol concentrations were given, rather than subfractions), as to do otherwise would presumably have been too expensive and impractical in cohort studies involving up to 70 000 study participants. Thus, it remains conceivable that a beneficial effect of exercise on the vascular risk factors that have already been recognized accounts for at least some of the observed risk reduction. Postulated mechanisms of risk reduction that are currently under discussion include an antihypertensive effect of exercise and a beneficial effect on lipid metabolism, e.g., a reduction of elevated concentrations of HDL-cholesterol. It has also been proposed that the effect may be mediated by an improvement in endothelial function—for example, increased activity of endothelial nitric oxide synthase (eNOS) (5) and of extracellular superoxide dismutase (ecSOD) expression: nitric oxide, a potent vasodilator, inhibits platelet aggregation and adhesion (1). Other mechanisms that may play a role include a lowering of blood viscosity, a tendency toward platelet aggregation, and increased fibrinolysis (8). Further factors include a reduction of the plasma fibrinogen concentration, increased activity of plasma tissue plasminogen activator, and a raised concentration of HDL-cholesterol (8, 17).
In view of the fact that the multivariate analyses of most of the studies reviewed here took account of the more important known cerebrovascular risk factors that can be positively influenced by exercise (obesity, glucose metabolism, arterial blood pressure, platelet aggregation tendency [e27]), one may suspect that the “gross” preventive effect of exercise is actually higher than the “net” effect on the order of 8% to 40% that is cited above. As already mentioned, the lowest risk of stroke was not always seen in the group of subjects who were most physically active; in some studies, a group with intermediate physical activity had the lowest risk. Yet, when we performed our meta-analysis, we used the data from the group whose risk was lowest in each study. It follows, therefore, that if we had compared only the (nearly) identical levels of physical activity across studies, we would have arrived at a somewhat lower figure for the reduction of stroke risk by physical activity.
The risk reduction for cerebrovascular events is of the same order of magnitude as that for coronary heart disease. The risk of coronary heart disease in men who exercise regularly in their free time is 24% lower than that of men who are physically inactive in their free time. In women, there is an analogous risk reduction of 23%. The effect is less pronounced at intermediate levels of physical activity (e29). Traveling to work on foot or by bicycle has been shown to lower the risk of cardiovascular events significantly only in women: RR = 0.87 (95% CI: 0.77–0.98, p = 0.02; for men, RR = 0.91, 95% CI: 0.80–1.04) (e30).
Although the description of physical activity was not quite uniform across studies, the type of activity reported in most of them corresponded to aerobic exercise. Even the types of highly intense physical activity that were named in most studies were usually leisure sports such as jogging, swimming, cycling, or the like. Walking is considered to be a mildly to moderately intense form of physical activity (3). The term “intense physical activity” in the epidemiological studies cited here thus has little to do with what an athlete would consider intense activity, i.e., an anaerobic or competitive stress. No data on this type of activity are available. Thus, regular cardiovascular exercise with moderately intense physical activity for at least 30 minutes per day is recommended (4) for persons who do not get adequate exercise on the job. Moderately intense physical activity can be provided by, for example, rapid walking, cycling, moderately fast swimming, or slow canoeing.
Figure 2.
Figure 3.
Figure 4.
Figure 5.
Key Messages.
Physical activity, generally consisting of aerobic endurance tasks, has been found in earlier meta-analyses and in the present one to lower the risk of cerebrovascular events and the associated mortality, in a manner that is probably independent of the known risk factors for such events, at least in men.
In men, regular physical activity reduces the risk of suffering an ischemic stroke or dying from one by 27% and the risk of a cerebral hemorrhage by 40%. In women, no significant preventive effects are seen.
For persons whose occupations do not provide them with adequate physical activity, regular exercise for about 30 minutes per day is recommended, both to prevent cerebrovascular events and to obtain other health benefits as well.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
The authors declare that they have no conflict of interest as defined by the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Endres M, Gertz K, Lindauer U, et al. Mechanisms of stroke protection by physical activity. Ann Neurol. 2003;54:582–590. doi: 10.1002/ana.10722. [DOI] [PubMed] [Google Scholar]
- 2.Vinereanu D. Risk factors for atherosclerotic disease: present and future. Herz. 2006;(31 Suppl. III):5–24. [PubMed] [Google Scholar]
- 3.Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk. A meta-analysis. Stroke. 2003;34:2475–2482. doi: 10.1161/01.STR.0000091843.02517.9D. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke. A guideline from the American Heart Associa-tion/American Stroke Association Council: Cosponsored by the Artherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Car-di–ol-ogy Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Inter-disciplinary Working Group. Stroke. 2006;37:1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 5.Chrysohoou C, Pitsavos C, Kokkinos P, Panagiotakos DB, Singh SN, Stefanadis C. The role of physical activity in the prevention of stroke. Cent Eur J Publ Health. 2005;13:132–136. [PubMed] [Google Scholar]
- 6.R Development Core Team. R Foundation for Statistical Computing. Vienna, Austria: 2008. R: A language and environment for statistical computing. ISBN 3-900051-07-0, www.R-project.org. [Google Scholar]
- 7.Wendel-Vos GCW, Schuit AJ, Feskens EJM, Boshuizen HC, Verschuren WMM, Saris WHM, Kromhut D. Physical activity and stroke. A meta-analysis of observational data. Int J Epidemiol. 2004;33:787–798. doi: 10.1093/ije/dyh168. [DOI] [PubMed] [Google Scholar]
- 8.Alevizos A, Lentzas J, Kokkoris S, Mariolis A, Korantzopoulos P. Physical activity and stroke risk. Int J Clin Pract. 2005;59:922–930. doi: 10.1111/j.1742-1241.2005.00536.x. [DOI] [PubMed] [Google Scholar]
- 9.Hu G, Sarti C, Jousilahti P, Silventoinen K, Barengo NC, Tuomilehto J. Leisure time, occupational, and commuting physical a-ctivity and the risk of stroke. Stroke. 2005;36:1994–1999. doi: 10.1161/01.STR.0000177868.89946.0c. [DOI] [PubMed] [Google Scholar]
- 10.Myint PK, Luben RN, Wareham NJ, Welch AA, Bingham SA, Day NE, Khaw K-T. Combined work and leisure physical activity and risk of stroke in men and women in the European Prospective Investigation into Cancer-Norfolk Prospective Population Study. Neuroepidemiol. 2006;27:122–129. doi: 10.1159/000095551. [DOI] [PubMed] [Google Scholar]
- 11.Chiuve SE, Rexrode KM, Spiegelman D, Logroscino G, Manson JE, Rimm EB. Primary prevention of stroke by healthy lifestyle. Circulation. 2008;118:947–954. doi: 10.1161/CIRCULATIONAHA.108.781062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noda H, Iso H, Tokoshima H, et al. JACC Study Group. Walking and sports participation and mortality from coronary heart disease and stroke. J Am Coll Cardiol. 2005;46:1761–1767. doi: 10.1016/j.jacc.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 13.Mora S, Cook N, Buring JE, Ridker PM, Lee I-M. Physical activity and reduced risk of cardiovascular events. Potential mediating mechanisms. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krarup L-H, Truelsen T, Pedersen A, Lerke H, Lindahl M, Hansen L, Schnohr P, Boysen G. Level of physical activity in the week preceding an ischemic stroke. Cerebrovasc Dis. 2007;24:296–300. doi: 10.1159/000105683. [DOI] [PubMed] [Google Scholar]
- 15.Abbott RD, Rodriguez BL, Buchfiel CM, Curb JD. Physical activity in older middle-aged men and reduced risk of stroke: The Honolulu Heart Program. Am J Epidemiol. 1994;139:881–893. doi: 10.1093/oxfordjournals.aje.a117094. [DOI] [PubMed] [Google Scholar]
- 16.Agnarsson U, Thorgeirsson G, Sigvaldason H, Sigfusson N. Effects of leisure-time physical and ventilator function on risk for stroke in men: The Reykjavík Study. Ann Intern Med. 1999;130:987–990. doi: 10.7326/0003-4819-130-12-199906150-00006. [DOI] [PubMed] [Google Scholar]
- 17.Evenson KR, Rosamond WD, Cai J, Toole JF, Hutchinson RG, Shahar E, Folsom AR. Physical activity and ischemic stroke risk. The arteriosclerosis risk in communities study. Stroke. 1999;30:1333–1339. doi: 10.1161/01.str.30.7.1333. [DOI] [PubMed] [Google Scholar]
- 18.Gillum RF, Mussolino ME, Ingram DD. Physical activity and stroke incidence in women and men. The NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1996;143:860–869. doi: 10.1093/oxfordjournals.aje.a008829. [DOI] [PubMed] [Google Scholar]
- 19.Harmsen P, Rosengren A, Tsipogianni A, Wilhelmsen L. Risk factors for stroke in middle-aged men in Göteborg, Sweden. Stroke. 1990;21:223–229. doi: 10.1161/01.str.21.2.223. [DOI] [PubMed] [Google Scholar]
- 20.Hu FB, Stampfer MJ, Colditz GA, Ascherio A, Rexrode KM, Willett WC, Manson JE. Physical activity and risk of stroke in women. JAMA. 2000;283:2961–2967. doi: 10.1001/jama.283.22.2961. [DOI] [PubMed] [Google Scholar]
- 21.Lee I-M, Henneckens CH, Berger K, Buring JE, Manson JE. Exercise and risk of stroke in male physicians. Stroke. 1999;30:1–6. doi: 10.1161/01.str.30.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama T, Date C, Yokoyama T, Yoshiike N, Yamaguchi M, Tanaka H. A 155-year follow-up study of stroke in a Japanese provincial city. The Shibata Study. Stroke. 1997;28:45–52. doi: 10.1161/01.str.28.1.45. [DOI] [PubMed] [Google Scholar]
- 23.Okada H, Horibe H, Yoshiyuki O, Hayakawa N, Aoki N. A prospective study of cerebrovascular disease in Japanese rural communities, Akabane and Asahi. Part 1: evaluation of risk factors in the occurrence of cerebral hemorrhage and thrombosis. Stroke. 1976;7:599–607. doi: 10.1161/01.str.7.6.599. [DOI] [PubMed] [Google Scholar]
- 24.Paganini-Hill A, Barreto Perez M. Stroke risk in older men and women: aspirin, estrogen, exercise, vitamins, and other factors. J Gend Specif Med. 2001;4:18–28. [PubMed] [Google Scholar]
- 25.Bijnen FCH, Caspersen CJ, Feskens EJM, Saris WHM, Mosterd WL, Kromhout D. Physical activity and 10-year mortality from cardiovascular diseases and all causes. Arch Intern Med. 1998;158:1499–1505. doi: 10.1001/archinte.158.14.1499. [DOI] [PubMed] [Google Scholar]
- e1.Ellekjær EF, Holemn J, Ellekjær E, Vatten L. Physical activity and stroke mortality in women. Ten-year follow-up of the Nord–Trøndelag Healthy Survey, 1984-1986. Stroke. 2000;31:14–18. doi: 10.1161/01.str.31.1.14. [DOI] [PubMed] [Google Scholar]
- e2.Folsom AR, Prineas RJ, Kaye SA, Munger RG. Incidence of hypertension and stroke in relation to body fat distribution and other risk factors in older women. Stroke. 1990;21:701–706. doi: 10.1161/01.str.21.5.701. [DOI] [PubMed] [Google Scholar]
- e3.Håheim LL, Holme I, Hjermann I, Leren P. Risk factors of stroke incidence and mortality. A 12-year follow-up of the Oslo Study. Stroke. 1993;24:1484–1489. doi: 10.1161/01.str.24.10.1484. [DOI] [PubMed] [Google Scholar]
- e4.Harmsen P, Lappas G, Rosengren A, Wilhelmsen L. Long-term risk factors for stroke. Twenty-eight years of follow-up of 7 457 middle-aged men in Göteborg, Sweden. Stroke. 2006;37:1663–1667. doi: 10.1161/01.STR.0000226604.10877.fc. [DOI] [PubMed] [Google Scholar]
- e5.Kiely DK, Wolf PA, Cupples LA, Beiser AS, Kannel WB. Physical activity and stroke risk: The Framingham Study. Am J Epidemiol. 1994;140:608–620. doi: 10.1093/oxfordjournals.aje.a117298. [DOI] [PubMed] [Google Scholar]
- e6.Lapidus L, Bengtsson C. Socioeconomic factors and physical activ-ity in relation to cardiovascular disease and death. A 12 year follow up of participation in a population study of women in Gothenburg, Sweden. Br Heart J. 1986;55:295–301. doi: 10.1136/hrt.55.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e7.Lee CD, Blair SN. Cardiorespiratory fitness and stroke mortality in men. Med Sci Sports Exerc. 2002;34:592–595. doi: 10.1097/00005768-200204000-00005. [DOI] [PubMed] [Google Scholar]
- e8.Lee I-M, Paffenbarger RS., Jr Physical activity and stroke incidence. The Havard Alumni Healthy Study. Stroke. 1998;29:2049–2054. doi: 10.1161/01.str.29.10.2049. [DOI] [PubMed] [Google Scholar]
- e9.Lindenstrøm E, Boysen G, Nyboe J. Lifestyle factors and risk of cerebrovascular disease in women. The Copenhagen City Heart Study. Stroke. 1993;24:1468–1472. doi: 10.1161/01.str.24.10.1468. [DOI] [PubMed] [Google Scholar]
- e10.Lindsted KD, Tonstad S, Kuzma JW. Self-report of physical activity and patterns of mortality in Seventh-Day Adventist men. J Clin Epidemiol. 1991;44:355–364. doi: 10.1016/0895-4356(91)90074-j. [DOI] [PubMed] [Google Scholar]
- e11.Menotti A, Seccareccia F. Physical activity at work and job -responsibility as risk factors for fatal coronary heart disease and other causes of death. J Epidemiol Community Health. 1985;39:325–329. doi: 10.1136/jech.39.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Paffenbarger RS, Hyde DT, Wing AL, Steinmetz CH. A natural -history of athleticism and cardiovascular health. JAMA. 1984;252:491–495. [PubMed] [Google Scholar]
- e13.Panagiotakos DB, Chrysohoou C, Pitsavos C, et al. Risk factors of stroke mortality: A 40-year follow-up of the Corfu Cohort from the Seven-Country Study. Neuroepidemiology. 2003;22:332–338. doi: 10.1159/000072922. [DOI] [PubMed] [Google Scholar]
- e14.Salonen JT, Puska P, Tuomilehto J. Physical activity and risk of myocardial infarction, cerebral stroke and death. Am J Epidemiol. 1982;115:526–537. doi: 10.1093/oxfordjournals.aje.a113334. [DOI] [PubMed] [Google Scholar]
- e15.Simonsick EM, Lafferty ME, Phillips CL, Mendes de Leon CF, Kasl SV, Seeman TE, Fillenbaum G, Hebert P, Lemke JH. Risk due to -inactivity in physically capable older adults. Am J Public Health. 1993;83:1443–1450. doi: 10.2105/ajph.83.10.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e16.Wannamethee G, Shaper AG. Physical activity and stroke in British middle aged men. BMJ. 1992;304:597–601. doi: 10.1136/bmj.304.6827.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e17.Wannamethee SG, Shaper AG, Walker M, Ebrahim S. Lifestyle and 15-year survival free of heart attack, stroke, and diabetes in middle-aged British men. Arch Intern Med. 1998;158:2433–2440. doi: 10.1001/archinte.158.22.2433. [DOI] [PubMed] [Google Scholar]
- e18.Choi-Kwon S, Kim JS. Lifestyle factors and risk of stroke in Seoul, South Korea. J Stroke Cerebrovasc Dis. 1998;7:414–420. doi: 10.1016/s1052-3057(98)80125-0. [DOI] [PubMed] [Google Scholar]
- e19.Ellekjær EF, Wyller TB, Sverre JM, Holmen J. Lifestyle factors and risk of cerebral infarction. Stroke. 1992;23:829–834. doi: 10.1161/01.str.23.6.829. [DOI] [PubMed] [Google Scholar]
- e20.Sacco RL, Gan R, Boden-Albala B, et al. Leisure-time physical activity and ischemic stroke risk. The Northern Manhattan Stroke Study. Stroke. 1998;29:380–387. doi: 10.1161/01.str.29.2.380. [DOI] [PubMed] [Google Scholar]
- e21.Shinton R, Sagar G. Lifelong exercise and stroke. BMJ. 1993;307:231–234. doi: 10.1136/bmj.307.6898.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e22.You R, McNeil JJ, O’Malley HM, Davis SM, Donnan GA. Risk -factors for lacunar infarction syndromes. Neurology. 1995;45:1483–1487. doi: 10.1212/wnl.45.8.1483. [DOI] [PubMed] [Google Scholar]
- e23.You R, McNeil JJ, O’Malley HM, Davis SM, Thrift AG, Donnan GA. Risk factors for stroke due to cerebral infarction in young adults. Stroke. 1997;28:1913–1918. doi: 10.1161/01.str.28.10.1913. [DOI] [PubMed] [Google Scholar]
- e24.Thrift AG, Donnan GA, McNeil JJ. Reduced risk of intracerebral hemorrhage with dynamic recreational exercise but not with heavy work activity. Stroke. 2002;33:559–564. doi: 10.1161/hs0202.102878. [DOI] [PubMed] [Google Scholar]
- e25.Herman B, Leyten ACM, van Lujik JH, Frenken CWGM, Op de Coul AAW, Schulte BPM. An evaluation of risk factors for stroke in a Dutch community. Stroke. 1982;13:334–339. doi: 10.1161/01.str.13.3.334. [DOI] [PubMed] [Google Scholar]
- e26.Herman B, Schmitz PIM, Leyten ACM, van Lujik JH, Frenken CWGM, Op de Coul AAW, Schulte BPM. Multivariate logistic anal-ysis of risk factors for stroke in Tilburg, The Netherlands. Am J Epidemiol. 1983;118:514–525. doi: 10.1093/oxfordjournals.aje.a113657. [DOI] [PubMed] [Google Scholar]
- e27.Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e28.Wolf PA, Kannel WB. Preventing stroke: Does race/ethnicity matter? Circulation. 2007;116:2099–2100. doi: 10.1161/CIRCULATIONAHA.107.736942. [DOI] [PubMed] [Google Scholar]
- e29.Sofi F, Capalbo A, Cesari F, Abbate R, Gensini GF. Physical activity during leisure time and primary prevention of coronary heart -disease: an update meta-analysis of cohort studies. Eur J Cardiovasc Prev Rehabil. 2008;15:247–257. doi: 10.1097/HJR.0b013e3282f232ac. [DOI] [PubMed] [Google Scholar]
- e30.Hamer M, Chida Y. Active commuting and cardiovascular risk: A meta-analytic review. Prev Med. 2008;46:9–13. doi: 10.1016/j.ypmed.2007.03.006. [DOI] [PubMed] [Google Scholar]