Abstract
Background
Colorectal carcinoma is the most common type of tumor in Western countries. The risk of developing colorectal carcinoma depends both on genetic factors (familial predisposition) and on lifestyle-related factors such as body-mass index, level of physical activity, and nutritional behavior. Regular physical activity is important in primary prevention, and there is also evidence that the prognosis after treatment of a colorectal carcinoma can be improved by exercise.
Methods
The PubMed database was searched for relevant articles that appeared in the last 10 years, and selected articles were evaluated.
Results
Cross-sectional studies have shown that regular physical activity (ca. 7 hours of brisk walking per week) lowers the risk of colon carcinoma by 40%. Physical activity also improves the outcome of patients already diagnosed with colorectal carcinoma: for example, patients with advanced disease (UICC stage II or III) have been found to survive significantly longer if they perform 4 hours of brisk walking per week, or the equivalent degree of physical exercise.
Conclusions
Cross-sectional studies show that physically active persons are less likely to develop colorectal carcinoma than physically inactive persons, and that they have better outcomes in the event that they do develop the disease. The positive findings with respect to secondary prevention still need to be confirmed in interventional trials, but in primary prevention, at least, physical activity should be actively promoted, along with other beneficial lifestyle habits and screening measures.
Keywords: colorectal carcinoma, physical activity, cancer prophylaxis, cancer therapy, prevention
Colorectal carcinoma is the most common type of tumor in Germany, with an incidence of ca. 70 000 new cases annually. Each year approximately 29 000 patients die of the disease; overall, men are slightly more often affected. The incidence of colorectal carcinoma varies widely across the world, ranging from 1 to 5 diagnoses per 100 000 inhabitants in the developing countries to 20 to 60 per 100 000 in the Western industrialized nations (e1). Genetic disposition is one of the most important risk factors for colorectal carcinoma (e2, e3). A sedentary lifestyle, overweight, immoderate consumption of alcohol, and smoking are recognized as modifiable factors that favor the development of colorectal carcinoma (1, 2, e4– e8). Studies of low-fiber, high-fat nutrition have reached no clear conclusions. Meta-analyses have shown that neither a high-fiber diet nor antioxidant micronutrients such as beta-carotene, vitamin C, or vitamin E can be definitely stated to have a protective effect (e9, e10).
Physical activity in primary prevention
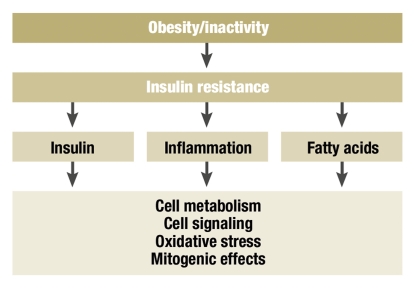
Epidemiological studies have unequivocally demonstrated that a sedentary lifestyle and obesity strongly influence the development of various tumor diseases (2, 5, 6, e4, e11– e14). The connection is significant for colon carcinoma (relative risk reduction of up to 40%; for incidence, see above), but not for rectal carcinoma. The important role of excess weight in carcinogenesis is explained by obesity-induced insulin resistance, which leads to elevated plasma levels of insulin, glucose, and fatty acids. Insulin appears to have a mitogenic effect on the colonic mucosa (figure 1). Furthermore, a significant part seems to be played by a chronic inflammatory reaction associated with insulin resistance (3, 4).
Figure 1.
Possible relationships between overweight, inactivity, insulin resistance, and tumorigenesis
Sport in primary prevention of colon carcinoma
Several epidemiological investigations and prospective cohort studies have agreed in showing a reduced risk of developing colon carcinoma among men and women who participate regularly in physical activity (2, 5, 6, e4, e11– e14). One epidemiological study investigated the link between physical activity and the risk of colon carcinoma in more than 150 000 people (70 403 men and 80 771 women; mean age 63 years) over a period of 6 years. Risk reduction was examined, not overall survival. In this study, 14 933 men and 13 329 women were regularly physically active for more than 7 hours per week. The authors showed that the risk of colon carcinoma was reduced by 40% in persons who exercised for over 7 hours each week (7).
The effect of physical activity on the prognosis was not decisively influenced by the subjects’ previous level of sporting activity, e.g., 10 years or more before the observation period (7). This indicates that physical activity exerts a direct protective action against the development of colon carcinoma and thus reduces the risk of colon carcinoma (e15). The recurrence of colonic polyps seems not to be prevented, however, suggesting that the protective effect of exercise and sport is exerted only after the development of adenoma in the adenoma-carcinoma sequence (8). In two prospective studies in which the participants were asked only about their habitual exercise, no connection could be established between physical activity and the incidence of colorectal carcinoma (9, 10). Further prospective studies are certainly required to validate the influence of exercise on the development of colon carcinoma.
Notwithstanding, the possible effect of physical activity on the development of colon carcinoma is essentially unknown. Coups et al. established that only 15% of those surveyed were aware of the postulated preventive effect of sport and leisure time exercise on the risk of colon carcinoma. Especially persons over 50 years of age with a low level of education were poorly informed (11). Doctors should consistently provide this information to their patients.
The data for rectal carcinoma are less unequivocal. While individual studies have described risk reduction similar to that for colon carcinoma (12, e16, e17), other investigations have found no benefit of physical activity in this regard (13, e18– e20). Such studies are complicated by the need to take account of other factors, because nutrition, weight, and other aspects of lifestyle differ between physically active and inactive persons. Those who perform sporting activity display, for example:
A more favorable diet
Lower consumption of stimulants (alcohol, tobacco)
A good energy balance with avoidance of overweight.
Physiological mechanisms
To date few investigations have concerned themselves with the (patho-)physiological links between physical exercise and the development of cancer. It is clear that we are not dealing with isolated factors; rather, risk reduction is based on complex multifactorial interrelationships. Primarily the influence of insulin and insulin-like growth factor (IGF) on carcinogenesis is discussed, not least on the basis of epidemiological studies in which an increased risk of colon carcinoma was observed in obesity and insulin resistance (3). Changes in the constellation of inflammation and in immunity have also been proposed to be responsible for carcinogenesis (14, e13, e21). Furthermore, those who perform sport exhibit faster intestinal transit and thus a shorter contact time of potentially carcinogenic substances with the intestinal mucosa.
Role of exercise in tumor treatment
The diagnosis of a tumor, followed by stays in the hospital and treatment measures such as surgery, radiotherapy, and chemotherapy, leads to reduced activity on the part of the patients with a resulting decrease in performance. The tumor disease itself, together with the adverse effects of treatment such as anemia and chemotherapy-induced cardiomyopathy or neuropathy, can further impair physical capacity. The result is a tumor-associated fatigue syndrome (cancer-related fatigue, CRF) with breathlessness, tachycardia, marked fatigability, pronounced weakness, and depressive mood. This pattern of symptoms is more severe in tumor cachexia (15, e22). Physical training can counteract these symptoms. Exercise is therefore an essential component of treatment for CRF. Regular physical activity can also improve the subjective quality of life (16, e23, e24).
Physical training in colon carcinoma
Only a small proportion of patients modify their lifestyle. Merely 23% of patients with colorectal carcinoma follow the recommendations with regard to physical activity, 12% continue to smoke after diagnosis, and 16% carry on drinking moderate or large amounts of alcohol (17). This assumes even greater importance in light of the fact that three prospective studies on the effect of lifestyle modifications on disease outcome have shown that physical activity improves the prognosis of patients diagnosed with colon carcinoma, even at an advanced stage (18– 21). In an Australian population (Melbourne Collaborative Cohort Study, MCSS), 41 528 study participants, recruited between 1990 and 1994, were questioned about their physical activity in the previous 6 months:
“How many times per week did you perform vigorous exercise for at least 20 minutes?”
“How many times per week did you perform less intensive activities, such as walking, gardening, etc.?
On the basis of their answers the patients were divided into two groups: “ no activity” and “activity once or more per week”. The study participants were observed for 10 to 14 years, and the findings were evaluated in 2004. Overall 526 persons developed colon carcinoma during the study period, 229 from the “active” and 297 from the “non-active” cohort. Both groups of patients received the same standardized treatment: surgery, adjuvant chemotherapy (30% in each group), and radiotherapy (comparably frequently: 8% vs. 11%). The median duration of follow-up of the colon carcinoma patients was 5.5 years from the time of diagnosis. Within the observation period the likelihood of survival was distinctly higher for those in the active group. Their overall mortality (hazard ratio 0.77, 95% confidence interval [95% CI] 0.58 to 1.03) was considerably lower (19) than that in the non-active group. This was limited to UICC (Union International Contre le Cancer) stages II and III, in which physical activity lowered overall mortality by 39% and disease-specific mortality by 51%. In very early disease (UICC stage I) and in the metastatic stage (UICC IV), no significant differences were observed (19, 20). A limitation of the MCSS is that no account was taken of social factors such as family background, education level, occupation, or previous illnesses—except for the measurement of central obesity.
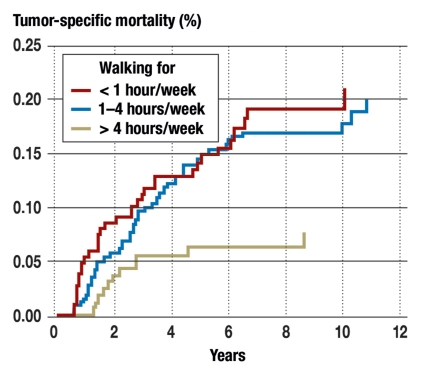
Similarly, data from a prospective study that formed part of the Cancer and Leukemia Group B Study (CALGB) showed that regular exercise for at least three times 45 minutes every week was associated with a 45% relative reduction in mortality among colon carcinoma patients (18) (figure 2). The threshold above which this positive effect was observed corresponds to weekly activity of 18 MET × hours (18). MET (metabolic equivalent task) describes the intensity of exercise, which is multiplied by duration. An hour of brisk walking, for example, corresponds to 4 to 5 MET × hour (e25). It can be concluded from the findings of this study that brisk walking for at least 4 hours per week is associated with these positive effects.
Figure 2.
Influence of physical activity in MET × hours/week on disease-specific mortality in patients with colorectal carcinoma (MET, metabolic equivalent task). From (18): Meyerhardt JA, et al.: Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol 2006; 24: 3527–34; reproduced by kind permission of the American Society of Clinical Oncology
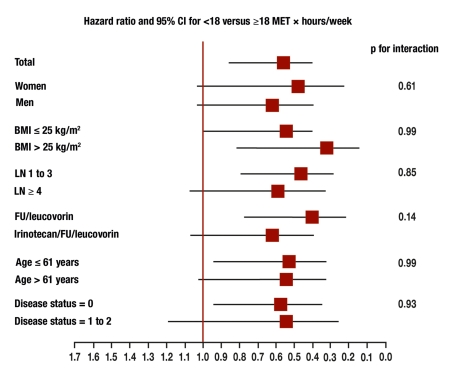
Interestingly, this result corresponds exactly with the findings of studies on the treatment of type 2 diabetes mellitus. In diabetes patients, too, regular exercise in the form of at least 4 hours of brisk walking per week reduces the metabolic-cardiovascular risk factors and thus mortality (e26). The importance in both diseases (colon carcinoma and diabetes mellitus) of improvement in insulin resistance and reduction of growth factors such as IGF-1 has not yet been investigated in any detail (figure 1). Nonetheless, in the studies cited physical activity went together with an improvement in disease-specific overall mortality, with identical tumor treatment in both groups (Figures 3 and 4) (18, 21).
Figure 3.
Hazard ratio of increased physical activity by sex, body-mass index (BMI), lymph node involvement (LN), type of chemotherapy, age, and disease severity. From (21): Meyerhardt JA, Heseltine D, Niedzwiecki D, et al.: Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol 2006; 24: 3535–41; reproduced by kind permission of the American Society of Clinical Oncology
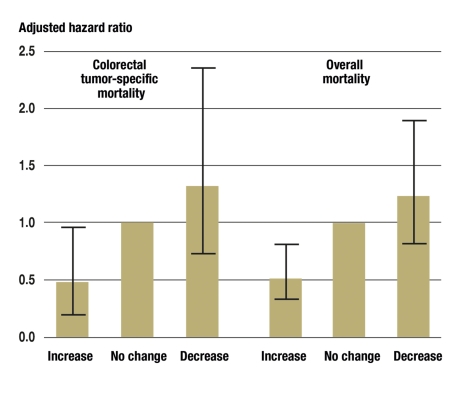
Figure 4.
Association of change in physical activity (increase, no change, decrease) before and after diagnosis of colon carcinoma with disease-specific mortality and overall mortality. In comparison with women who did not change their exercise behavior (n = 203), patients who increased their sporting activity (n = 144) had an adjusted hazard ratio (HR) of 0.48 (95% confidence interval [95% CI] 0.24 to 0.97) for colorectal carcinoma-specific mortality and an adjusted HR of 0.51 (95% CI 0.30 to 0.85) for overall mortality. In contrast, women whose sporting activity decreased (n = 176) showed a moderate, albeit non-significant, increase in tumor-specific mortality (HR 1.32, 95% CI 0.74 to 2.34) and overall mortality (HR 1.23, 95% CI 0.79 to 1.91). From (18): Meyerhardt JA, et al.: Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol 2006; 24: 3527–34; reproduced by kind permission of the American Society of Clinical Oncology
Interestingly, again, the positive effect of exercise does not depend on weight or body-mass index (BMI). In a prospective study, Meyerhardt et al. established that in contrast to primary prevention, in secondary prevention overweight and/or weight increase have no influence on the risk of recurrence and mortality following the diagnosis of colorectal carcinoma (22).
Exercise therapy in secondary prevention
Patients who have just had a tumor diagnosed are very receptive to suggestions regarding lifestyle modification. Such recommendations are implemented long-term particularly by young, educated women (23). In future, primary care physicians and oncologists should provide information on this subject and give appropriate advice. Young women, however, are rarely affected by colon carcinoma. The greatest concentration of patients is found in the fifth to seventh decades of life, so physicians will need to focus strongly on increasing awareness among those in this age group.
Our own practice shows that in order to motivate people to increase physical activity or perform sporting activity, expert advice must be accompanied by a sense of achievement on the part of the patient, the earlier the better. This is particularly important in patients with no previous experience of sports or exercise and those whose health was already impaired before diagnosis of their tumor. A deliberately gradual introduction to the program, with individual adjustment of exercise intensity and careful augmentation, is essential for success.
Training recommendations for colon carcinoma patients
The first step should always be a clinical examination with recording of the findings, resting ECG, and a standardized exercise test. Particularly in patients with previous cardiac disease, clinical cardiac insufficiency, or cardiotoxic chemotherapy, echocardiography should be performed. In previously heavy smokers and in suspicion of impaired pulmonary function, lung function testing with blood gas analysis should be carried out before and after ergometry.
There are still no scientifically founded recommendations for exercise and exercise therapy during and after treatment of colon carcinoma, because it depends on the individual patient’s capacity, perceptions, motivation, and desires. We have found that a combination of endurance training and light strength training is beneficial, because it improves patient motivation and compliance.
The primary goal of every individualized training program is improvement in aerobic capacity. Strength training aims more at “activation of the musculature” than at a measurable increase in strength. Initially the training should be structured so that the exercise and regeneration phases alternate in a defined pattern. Particularly at the beginning, the exercise intensity should not be too high (start low—go slow) and the regeneration time should be 48 hours, to avoid subjective exhaustion. Every unit of training must be adapted to the accompanying treatment (e.g., chemo- or radiotherapy), the current blood count, and the patient’s subjective perception of illness. Thus, exercise intensity and duration should be gradually increased or – in the case of subjective over-exhaustion – reduced. The aim is to achieve 3 to 5 units of training per week, each lasting for 30 minutes and then, eventually, 60 minutes. Endurance training can theoretically be performed daily, but units of strength training should be spaced 1 to 2 days apart. The type of exercises should be selected according to the patient’s requirements and preferences.
Contraindications and complications
There are no specific studies on the contraindications and complications of exercise during and after treatment for colon carcinoma. The only scientifically founded recommendations relate to breast cancer but can, with reservations, be applied to patients with colorectal carcinoma (24).
In any event exercise training during treatment for colorectal carcinoma necessitates close cooperation among all medical disciplines involved, e.g., primary care physician, gastroenterologist, oncologist, sports medicine and exercise specialist, and psychologist. Following surgery the wound-healing process must be taken into consideration: premature and unplanned exercise favor complications and scar instability. Particularly in stoma patients, parastomal hernias can lead to problems. Swimming should be postponed at least until the patient is confident in dealing with the stoma.
Tumor treatment frequently causes a reduction in hematopoiesis. If the thrombocyte count goes below 20 000/µL, vigorous activity is prohibited because of the risk of bleeding. Before strength training or intensive exercise that provokes an increase in blood pressure, a thrombocyte count over 50 000/µL must be documented. Light endurance training is permitted with thrombocyte counts between 20 000 and 50 000/µL. Anemia with hemoglobin levels below 8 mg/dL may lead to ischemic complications owing to the restricted oxygen supply. If the hemoglobin concentrations are only slightly decreased (9 to 12 mg/dL), correspondingly adapted endurance training is possible. Neutropenia—a frequent adverse effect of chemotherapy with irinotecan or 5-fluorouracil—does not constitute a contraindication to sporting activity. Nevertheless, attention must be paid to hygiene precautions such as hand disinfection, face masks, and avoidance of physical contact with other athletes.
Although there is no scientific evidence in this regard, patients should avoid vigorous activity for 24 hours after administration of chemotherapy because of the potential for cardio- and nephrotoxicity. The same holds for toxic diarrhea following irinotecan-based chemotherapy. During treatment with oxaliplatin it is important to avoid exposure to cold owing to the increased risk of transient peripheral neuropathy. If bony metastases are suspected, skeletal stability must be investigated before the commencement of training.
Key Messages.
Primary prevention: Regular exercise is associated with a reduction in colon carcinoma risk; this effect is less pronounced in rectal carcinoma. According to Chao et al., 35 MET × hours/week, corresponding to 7 hours’ brisk walking per week, are necessary to achieve this effect.
Before tumor patients start exercise, they should undergo clinical exercise testing. On the basis of the results, the composition of the individual training program can be decided.
Physical training of 18 MET × hours/week (4 hours’ brisk walking per week) seems effective in improving the prognosis of tumor patients.
The subjectively perceived training intensity should be 12 to 13 on the Borg scale (25).
In some states of Germany sports clubs offer “Sport in Cancer Care” (Sport in der Krebsnachsorge). Further information is available in German from the German Olympic Sports Federation (Deutscher Olympischer Sportbund) in Frankfurt/Main.
Acknowledgments
The authors thank Karin Mair and Dr. Jan D’Haese of the Red Cross Hospital, Munich for their critical comments.
This article was prepared with the support of German Cancer Aid (Deutsche Krebshilfe).
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Heitkamp Ch, Bott M. Kolorektalkarzinome und körperliche Aktivität. Dtsch Arztebl. 2001;98(10) [Google Scholar]
- 2.Martinez ME, Giovannucci E, Spiegelman D, Hunter DJ, Willett WC, Colditz GA. Leisure-time physical activity, body size, and colon -cancer in women. Nurses’ Health Study Research Group. J Natl Cancer Inst. 1997;89:948–955. doi: 10.1093/jnci/89.13.948. [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:s836–s842. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 5.Mai PL, Sullivan-Halley J, Ursin G, et al. Physical activity and colon cancer risk among women in the California Teachers Study. Cancer Epidemiol Biomarkers Prev. 2007;16:517–525. doi: 10.1158/1055-9965.EPI-06-0747. [DOI] [PubMed] [Google Scholar]
- 6.Inoue M, Yamamoto S, Kurahashi N, Iwasaki M, Sasazuki S, Tsugane S. Daily total physical activity level and total cancer risk in men and women: results from a large-scale population-based -cohort study in Japan. Am J Epidemiol. 2008;168:391–403. doi: 10.1093/aje/kwn146. [DOI] [PubMed] [Google Scholar]
- 7.Chao A, Connell CJ, Jacobs EJ, et al. Amount, type, and timing of recreational physical activity in relation to colon and rectal cancer in older adults: the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2004;13:2187–2195. [PubMed] [Google Scholar]
- 8.Colbert LH, Lanza E, Ballard-Barbash R, et al. Adenomatous polyp recurrence and physical activity in the Polyp Prevention Trial (United States) Cancer Causes Control. 2002;13:445–453. doi: 10.1023/a:1015736524447. [DOI] [PubMed] [Google Scholar]
- 9.Schnohr P, Gronbaek M, Petersen L, Hein HO, Sorensen T. Physical activity in leisure-time and risk of cancer: 14-year follow-up of 28,000 Danish men and women. Scand J Public Health. 2005;33:244–249. doi: 10.1080/14034940510005752. [DOI] [PubMed] [Google Scholar]
- 10.Calton BA, Lacey JV, Jr, Schatzkin A, et al. Physical activity and the risk of colon cancer among women: a prospective cohort study (United States) Int J Cancer. 2006;119:385–391. doi: 10.1002/ijc.21840. [DOI] [PubMed] [Google Scholar]
- 11.Coups EJ, Hay J, Ford JS. Awareness of the role of physical activity in colon cancer prevention. Patient Educ Couns. 2008 doi: 10.1016/j.pec.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slattery ML, Edwards S, Curtin K, et al. Physical activity and -colorectal cancer. Am J Epidemiol. 2003;158:214–224. doi: 10.1093/aje/kwg134. [DOI] [PubMed] [Google Scholar]
- 13.Colbert LH, Hartman TJ, Malila N, et al. Physical activity in relation to cancer of the colon and rectum in a cohort of male smokers. Cancer Epidemiol Biomarkers Prev. 2001;10:265–268. [PubMed] [Google Scholar]
- 14.Demarzo MM, Martins LV, Fernandes CR, et al. Exercise reduces inflammation and cell proliferation in rat colon carcinogenesis. Med Sci Sports Exerc. 2008;40:618–621. doi: 10.1249/MSS.0b013e318163274d. [DOI] [PubMed] [Google Scholar]
- 15.Dimeo FC, Stieglitz RD, Novelli-Fischer U, Fetscher S, Keul J. Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer. 1999;85:2273–2277. [PubMed] [Google Scholar]
- 16.Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Fairey AS. A randomized trial of exercise and quality of life in -colorectal cancer survivors. Eur J Cancer Care (Engl) 2003;12:347–357. doi: 10.1046/j.1365-2354.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 17.Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control -intervention. J Clin Oncol. 2005;23:8884–8893. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 18.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 19.Haydon AM, Macinnis RJ, English DR, Morris H, Giles GG. Physical activity, insulin-like growth factor 1, insulin-like growth factor -binding protein 3, and survival from colorectal cancer. Gut. 2006;55:689–694. doi: 10.1136/gut.2005.081547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haydon AM, Macinnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55:62–67. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 22.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol. 2008;26:4109–4115. doi: 10.1200/JCO.2007.15.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salminen E, Heikkila S, Poussa T, Lagstrom H, Saario R, Salminen S. Female patients tend to alter their diet following the diagnosis of rheumatoid arthritis and breast cancer. Prev Med. 2002;34:529–535. doi: 10.1006/pmed.2002.1015. [DOI] [PubMed] [Google Scholar]
- 24.Kirshbaum MN. A review of the benefits of whole body exercise -dur-ing and after treatment for breast cancer. J Clin Nursing. 2007;16:104–121. doi: 10.1111/j.1365-2702.2006.01638.x. [DOI] [PubMed] [Google Scholar]
- 25.Borg G. Anstrengungsempfinden und körperliche Aktivität. Dtsch Arztebl. 2004;101(15) [Google Scholar]
- e1.Washington MK. Colorectal carcinoma: selected issues in pathologic examination and staging and determination of prognostic factors. Arch Pathol Lab Med. 2008;132:1600–1607. doi: 10.5858/2008-132-1600-CCSIIP. [DOI] [PubMed] [Google Scholar]
- e2.Park JG, Park YJ, Wijnen JT, Vasen HF. Gene-environment interaction in hereditary nonpolyposis colorectal cancer with implications for diagnosis and genetic testing. Int J Cancer. 1999;82:516–519. doi: 10.1002/(sici)1097-0215(19990812)82:4<516::aid-ijc8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- e3.Rahner N, Steinke V. Hereditary Cancer Syndromes [Erbliche Krebserkrankungen] Dtsch Arztebl Int. 2008;105(41):706–714. doi: 10.3238/arztebl.2008.0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e4.Nilsen TI, Romundstad PR, Petersen H, Gunnell D, Vatten LJ. Recreational physical activity and cancer risk in subsites of the colon (the Nord-Trondelag Health Study) Cancer Epidemiol Biomarkers Prev. 2008;17:183–188. doi: 10.1158/1055-9965.EPI-07-0746. [DOI] [PubMed] [Google Scholar]
- e5.Trojian TH, Mody K, Chain P. Exercise and colon cancer: primary and secondary prevention. Curr Sports Med Rep. 2007;6:120–124. doi: 10.1007/BF02941153. [DOI] [PubMed] [Google Scholar]
- e6.Cronin KA, Krebs-Smith SM, Feuer EJ, Troiano RP, Ballard-Barb-ash R. Evaluating the impact of population changes in diet, physical activity, and weight status on population risk for colon cancer (United States) Cancer Causes Control. 2001;12:305–316. doi: 10.1023/a:1011244700531. [DOI] [PubMed] [Google Scholar]
- e7.MacLennan R, Jensen OM, Mosbech J, Vuori H. Diet, transit time, stool weight, and colon cancer in two Scandinavian populations. Am J Clin Nutr. 1978;31:239–242. doi: 10.1093/ajcn/31.10.S239. [DOI] [PubMed] [Google Scholar]
- e8.Calle EE, Teras LR, Thun MJ. Obesity and mortality. N Engl J Med. 2005;353:2197–2199. doi: 10.1056/NEJM200511173532020. [DOI] [PubMed] [Google Scholar]
- e9.Park Y, Hunter DJ, Spiegelman D, et al. Dietary fiber intake and risk of colorectal cancer: a pooled analysis of prospective cohort studies. JAMA. 2005;294:2849–2857. doi: 10.1001/jama.294.22.2849. [DOI] [PubMed] [Google Scholar]
- e10.Koushik A, Hunter DJ, Spiegelman D, et al. Fruits, vegetables, and colon cancer risk in a pooled analysis of 14 cohort studies. J Natl Cancer Inst. 2007;99:1471–1483. doi: 10.1093/jnci/djm155. [DOI] [PubMed] [Google Scholar]
- e11.Johnsen NF, Christensen J, Thomsen BL, et al. Physical activity and risk of colon cancer in a cohort of Danish middle-aged men and women. Eur J Epidemiol. 2006;21:877–884. doi: 10.1007/s10654-006-9076-z. [DOI] [PubMed] [Google Scholar]
- e12.Zhang Y, Cantor KP, Dosemeci M, Lynch CF, Zhu Y, Zheng T. Occupational and leisure-time physical activity and risk of colon cancer by subsite. J Occup Environ Med. 2006;48:236–243. doi: 10.1097/01.jom.0000199521.72764.26. [DOI] [PubMed] [Google Scholar]
- e13.Harriss DJ, Cable NT, George K, Reilly T, Renehan AG, Haboubi N. Physical activity before and after diagnosis of colorectal cancer: disease risk, clinical outcomes, response pathways and biomarkers. Sports Med. 2007;37:947–960. doi: 10.2165/00007256-200737110-00003. [DOI] [PubMed] [Google Scholar]
- e14.Lee KJ, Inoue M, Otani T, Iwasaki M, Sasazuki S, Tsugane S. Physical activity and risk of colorectal cancer in Japanese men and women: the Japan Public Health Center-based prospective study. Cancer Causes Control. 2007;18:199–209. doi: 10.1007/s10552-006-0098-3. [DOI] [PubMed] [Google Scholar]
- e15.Slattery ML, Potter JD. Physical activity and colon cancer: confounding or interaction? Med Sci Sports Exerc. 2002;34:913–919. doi: 10.1097/00005768-200206000-00002. [DOI] [PubMed] [Google Scholar]
- e16.Severson RK, Nomura AM, Grove JS, Stemmermann GN. A prospective analysis of physical activity and cancer. Am J Epidemiol. 1989;130:522–529. doi: 10.1093/oxfordjournals.aje.a115366. [DOI] [PubMed] [Google Scholar]
- e17.Le ML, Wilkens LR, Kolonel LN, Hankin JH, Lyu LC. Associations of sedentary lifestyle, obesity, smoking, alcohol use, and diabetes with the risk of colorectal cancer. Cancer Res. 1997;57:4787–4794. [PubMed] [Google Scholar]
- e18.Gerhardsson dV, Steineck G, Hagman U, Rieger A, Norell SE. Physical activity and colon cancer: a case-referent study in Stockholm. Int J Cancer. 1990;46:985–989. doi: 10.1002/ijc.2910460606. [DOI] [PubMed] [Google Scholar]
- e19.Longnecker MP, Gerhardsson IV, Frumkin H, Carpenter C. A case-control study of physical activity in relation to risk of cancer of the right colon and rectum in men. Int J Epidemiol. 1995;24:42–50. doi: 10.1093/ije/24.1.42. [DOI] [PubMed] [Google Scholar]
- e20.Fraser G, Pearce N. Occupational physical activity and risk of cancer of the colon and rectum in New Zealand males. Cancer Causes Control. 1993;4:45–50. doi: 10.1007/BF00051713. [DOI] [PubMed] [Google Scholar]
- e21.Buehlmeyer K, Doering F, Daniel H, Kindermann B, Schulz T, Michna H. Alteration of gene expression in rat colon mucosa after exercise. Ann Anat. 2008;190:71–80. doi: 10.1016/j.aanat.2007.04.002. [DOI] [PubMed] [Google Scholar]
- e22.Mock V, Pickett M, Ropka ME, et al. Fatigue and quality of life outcomes of exercise during cancer treatment. Cancer Pract. 2001;9:119–127. doi: 10.1046/j.1523-5394.2001.009003119.x. [DOI] [PubMed] [Google Scholar]
- e23.Galvao DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol. 2005;23:899–909. doi: 10.1200/JCO.2005.06.085. [DOI] [PubMed] [Google Scholar]
- e24.Adamsen L, Midtgaard J, Rorth M, et al. Feasibility, physical capacity, and health benefits of a multidimensional exercise program for cancer patients undergoing chemotherapy. Support Care Cancer. 2003;11:707–716. doi: 10.1007/s00520-003-0504-2. [DOI] [PubMed] [Google Scholar]
- e25.Löllgen H. Primärprävention kardialer Erkrankungen: Stellenwert der körperlichen Aktivität. Dtsch Arztebl. 2003;100:A987–A996. [Google Scholar]
- e26.Ribisl PM, Lang W, Jaramillo SA, et al. Exercise capacity and cardiovascular/metabolic characteristics of overweight and obese individuals with type 2 diabetes: the Look AHEAD clinical trial. Diabetes Care. 2007;30:2679–2684. doi: 10.2337/dc06-2487. [DOI] [PubMed] [Google Scholar]