Abstract
Skeletal muscles are frequently analyzed for composition of phenotypically distinct myofibers, as a functional determinant. We describe an improved myofiber phenotyping procedure, involving cryosection co-incubation with fluorophore-labeled myosin heavy-chain isoform-specific antibodies. This technique identifies multiple fiber “types” on a single section, thereby reducing reagents and processing, and offers side-by-side comparison of samples from multiple species including mice. These advances are valuable for studying the physiological attributes of skeletal muscle in health and disease.
Keywords: fiber type, fluorophore, muscle, myosin-specific antibody
Introduction
As mammalian skeletal muscle fibers can contain functionally distinct isoforms of myosin heavy chain (MyHC)1, muscles are frequently characterized by MyHC isoform composition. Histological fiber “type” determination has been improved by the development of specific monoclonal antibodies raised against individual MyHC isoforms5,6. However, as the antibodies originate from mouse hybridomas, analyzing a muscle for fiber types requires multiple sections incubated with separate antibody stocks to definitively prevent cross-labeling by secondary antibodies, followed by time consuming, and often spatially imperfect image overlay. Furthermore, additional processing is necessary to minimize the non-specific interaction of “anti-mouse” secondary labeling products when using mouse-derived primary antibodies on mouse muscle samples (an increasingly common necessity given the expanding range of transgenic mice for muscle research)2. Here, we demonstrate that the process of characterizing myofiber types can be considerably improved by co-incubating sections with MyHC isoform-specific antibodies directly labeled with distinct fluorophores.
Materials and Methods
Hybridoma lines BA-D5, SC-71 and BF-F3 (which detect types I, IIa and IIb MyHC isoforms, respectively6, American Type Culture Collection, Manassas, VA, banked by Dr S Schiaffino) were cultured (initially ∼3 × 105 cells/ml and passaged every 3−4 days) in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (Hyclone, Logan, UT), 1% L-glutamine and penicillin-streptomycin (Invitrogen). Plated cells approaching 1 × 106 cells/ml were switched to serum-free media 4 days prior to antibody harvest, after which the media was filtered (0.22 μm Stericup; Millipore, Billerica, MA) and the antibodies concentrated over IgG (BA-D5, SC-71) or IgM (BF-F3) columns (Amersham HiTrap; GE Healthcare, Piscataway, NJ). Retained fractions were supplemented to 0.02% sodium azide concentration and stored at −80°C. For antibody visualization, BA-D5, SC-71 and BF-F3 aliquots adjusted for concentration (1 mg/ml) and pH (7.5 − 8.5) were processed for 12 hours at 4°C with Alexa Fluor 350, 594, or 488 protein labeling kits (Molecular Probes; Invitrogen), and separated from unincorporated dye on >40 kDa-size exclusion columns with 2 mM sodium azide-supplemented elution buffer. Protein concentration and labeling efficiency was determined spectrophotometrically according to protocol guidelines (Molecular Probes; Invitrogen), and aliquots were stored at −20°C in buffered saline containing 10 mg/ml bovine serum albumin (BSA; Sigma, St Louis, MO). Antibodies stored in this fashion remain usable for over 12 months. For optimizing methods and eventual application of the labeled antibodies, rehydrated 10-μm cryosections of mouse (12-wk C57BL/10), rat (12-wk Sprague-Dawley), dog (12-wk Beagle pup) and non-human primate (6-yr M. Fasicularis) muscle samples (obtained from humanely euthanized animals according to approved institution protocols) were blocked with 1% Tween 20 (USB, Cleveland, OH) and 0.03% BSA in 0.2% gelatin-supplemented phosphate-buffered saline (PBS-G, 15 min), briefly washed (PBS-G, 5min), incubated with individual or cocktail mixtures of the primary antibodies (90 min), washed again (PBS-G, 3 × 5 min), coverslipped (ProLong Gold; Invitrogen) and shielded from direct light. Processed sections were examined on an upright microscope (Eclipse E1000. Nikon, Kanagawa, Japan) selecting for blue, red and green channels separately. Digital images of each channel were recorded and merged (Retiga QICam with QCapture; QImaging, Burnaby, BC, Canada) to produce multicolor images as presented here.
Results
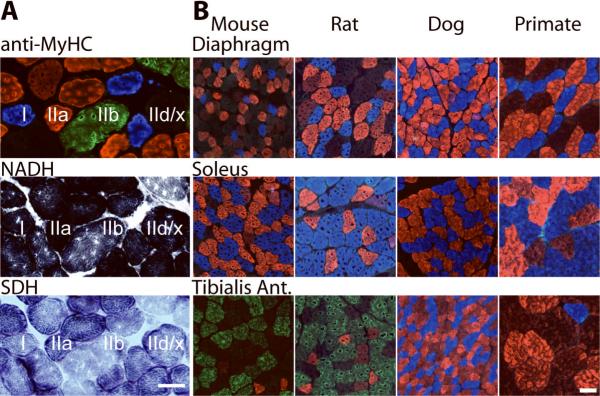
Figure 1 illustrates that co-incubation of skeletal muscle cryosections with directly-labeled antibodies as described enables quick identification of multiple myofiber types on a single section, and simplifies side-by-side classification of myofiber type distribution between muscles and across species (including mice) without additional mouse-specific blocking steps. This antibody combination positively labels three (I, blue; IIa, red; IIb, green.) of the four MyHC isoforms that routinely comprise the majority of mammalian trunk and limb skeletal-muscle fibers, and identifies by elimination the fourth (IId/x), which is unlabeled in this instance (Fig. 1A). Incorporation of this method in parallel with other rapid histological procedures for comparing the properties of muscle fibers [such as a reduced nicotinamide adenine dinucleotide (NADH) or succinate dehydrogenase (SDH) -based protocol], provides a convenient and consistent battery of assays with which to quickly compare muscle fibers (Fig. 1A). Our results highlight that the diaphragm of the dog contains few, if any, IId/x-dominant myofibers compared with the diaphragm of the mouse, rat, and non-human primate (Fig. 1B). None of the species displayed a significant number of IIb fibers in this muscle. The rat soleus contained more type I than IIa fibers, whereas the other three species’ soleus muscles exhibited a more equivalent composition of type I and IIa fibers. Samples of rodent TA muscles contained a high proportion of IIb and IId/x fibers, whereas dog muscles were composed of type I and IIa fibers only, and primate TA muscles contained mostly IId/x fibers, some IIa fibers, and a minor type I population.
Figure 1.
Fluorophore-labeled antibodies simplify classification of mammalian skeletal muscle-fiber types according to myosin heavy chain isoform composition. A) A muscle rat quadriceps cryosection incubated with directly-labeled antibodies raised against different isoforms of myosin heavy chain identifies several different myofiber “types” (here, I, blue; IIa, red; IIb, green; IId/x, unlabeled) and considerably aids discrimination between individual myofibers, compared with examination of only oxidative activity via histochemical methods [where darkest represents most oxidative using a tetrazolium reductase nicotinamide adenine dinucleotide (TR NADH) or succinate dehydrogenase (SDH) – based method]. B) Transverse cryosections of diaphragm, tibialis anterior and soleus muscle biopsies obtained from a mouse, rat, dog and non-human primate are readily amenable to simultaneous characterization using the described procedure, and can demonstrate variability in fiber type composition between functionally distinct muscles, and species. Scale bars, 100 μm.
Discussion
This methodological advance achieves a major reduction in the tissue and reagent quantities required to identify successfully the major MyHC isoforms of mammalian skeletal muscle with antibodies, and requires considerably less time than previous methods to process successfully any type of mammalian muscle biopsy, let alone mouse muscles that require additional processing to minimize (often incompletely) non-specific labeling. For instance, section processing and image acquisition for three muscles from four species (as presented in Fig. 1) could conceivably be accomplished within 3 hours using only one cryosection from each tissue. Rapid comparison of samples from different muscles and from different species (especially mice) by this manner is especially useful. Merged multicolor images readily identify the MyHC composition of the majority of muscle fibers, and single color images obtained prior to merging, or by switching off selected color channels in the merged digital images can easily identify muscle fibers that contain more than one MyHC isoform.
Using this method we demonstrate that the fiber type composition of mouse muscles does not always reproduce the fiber composition of equivalent muscles in other species, an important consideration when selecting samples for experiments intended to model the musculature of another organism. Antibodies developed against specific myosin heavy chain isoforms can distinguish between muscle fibers of differing phenotype in correlation with the traditional means of muscle-fiber typing via myosin ATPase activity with acid pre-incubation6. However, a major advantage of antibody-based myofiber identification is the opportunity to incorporate additional reagents to detect other proteins of interest on the same tissue section. In this study, we were constrained to the use of three detection channels by our hardware, but the methodology described can incorporate additional antibodies labeled with other fluorophores if the necessary detection optics are available, as labeling products offer a variety of additional emission wavelengths. For instance, it would be desirable to utilize a total of six emission/detection channels in order to use not only the three antibodies employed here, but also an antibody to positively identify fibers comprising the IId/x isoform3, and antibodies specific to embryonic and neonatal MyHC isoforms5 observed during muscle fiber formation/regeneration4. Commercial products employing alternate labeling chemistries also exist and provide a means of adapting this approach to employ particular antibodies that might otherwise demonstrate reduced binding affinity when labeled using the described reagents. We suggest that the approach described herein is a valuable means of considerably simplifying and accelerating the analysis of mammalian skeletal muscle sections by MyHC isoform prevalence and will prove useful for simultaneously identifying other proteins of interest.
Acknowledgements
Supported by a Development Grant from the Muscular Dystrophy Association (to PG), and by grants from the Muscular Dystrophy Association and the National Institutes of Health (to JSC).
Abbreviations
- ATPase
adenosine triphosphatase
- BSA
bovine serum albumin
- MyHC
myosin heavy chain
- NADH
reduced nicotinamide adenine dinucleotide
- PBS-G
gelatin-supplemented phosphate-buffered saline
- SDH
succinate dehydrogenase
- TA
tibialis anterior hindlimb muscle
References
- 1.Bottinelli R, Schiaffino S, Reggiani C. Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol (Lond.) 1991;437:655–672. doi: 10.1113/jphysiol.1991.sp018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu QL, Partridge TA. A new blocking method for application of murine monoclonal antibody to mouse tissue sections. J Histochem Cytochem. 1998;46:977–984. doi: 10.1177/002215549804600813. [DOI] [PubMed] [Google Scholar]
- 3.Lucas CA, Kang LH, Hoh JF. Monospecific antibodies against the three mammalian fast limb myosin heavy chains. Biochem Biophys Res Commun. 2000;272:303–308. doi: 10.1006/bbrc.2000.2768. [DOI] [PubMed] [Google Scholar]
- 4.Marini JF, Pons F, Leger J, Loffreda N, Anoal M, Chevallay M, et al. Expression of myosin heavy chain isoforms in Duchenne muscular dystrophy patients and carriers. Neuromuscul Disord. 1991;1:397–409. doi: 10.1016/0960-8966(91)90003-b. [DOI] [PubMed] [Google Scholar]
- 5.Schiaffino S, Gorza L, Pitton G, Saggin L, Ausoni S, Sartore S, et al. Embryonic and neonatal myosin heavy chain in denervated and paralyzed rat skeletal muscle. Dev Biol. 1988;127:1–11. doi: 10.1016/0012-1606(88)90183-2. [DOI] [PubMed] [Google Scholar]
- 6.Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, et al. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]