Abstract
A growing body of research supports the development of recombinant adeno-associated viral (rAAV) vectors for delivery of gene expression cassettes to striated musculature as a method of treating severe neuromuscular conditions. However, it is unclear whether delivery protocols that achieve extensive gene transfer in mice can be adapted to produce similarly extensive gene transfer in larger mammals and ultimately patients. Consequently, we sought to investigate methodological modifications that would facilitate rAAV-mediated gene transfer to the striated musculature of canines. A simple procedure incorporating acute (i) occlusion of limb blood flow, (ii) exsanguination via compression bandage, and (iii) vector “dwell” time of <20 minutes, markedly enhanced the transduction of limb muscles, compared with a simple bolus limb infusion of vector. A complementary method whereby vector was infused into the jugular vein led to efficient transduction of cardiomyocytes and to a lesser degree the diaphragm. Together these methods can be used to achieve transgene expression in heart, diaphragm, and limb muscles of juvenile dogs using rAAV6 vectors. These results establish that rAAV-mediated gene delivery is a viable approach to achieving systemic transduction of striated musculature in mammals approaching the dimensions of newborn humans.
Introduction
Individuals who experience significant loss of striated (skeletal and cardiac) muscle mass and contractile function associated with serious neuromuscular disorders are placed at risk of premature death. As an example, Duchenne muscular dystrophy (DMD) is presently a lethal condition without cure, characterized by progressive and debilitating degeneration of the striated musculature, culminating in death by respiratory or cardiac failure. Because the disease features of DMD are a consequence of a monogenic defect in the dystrophin gene,1,2 it has long been proposed that patients with this condition may benefit from therapies that repair or compensate for the genetic lesion.3
In the absence of sufficiently mature technologies that specifically and efficiently repair the genetic defect in DMD patients' cells, considerable effort has been invested into therapeutic strategies founded on “gene replacement.” By taking advantage of specific recombinant adeno-associated viral (rAAV) vector serotypes/pseudotypes that exhibit marked propensity to enter striated muscle cells in vivo, a number of studies have demonstrated encouraging evidence of vector-based interventions achieving functional improvement via therapeutic transgene expression in striated muscles of dystrophic mice.4,5,6,7,8,9
To develop viable new interventions for severe neuromuscular disorders, promising methods demonstrated in mice must be adapted to perform with similar or better efficacy and safety in mammalian models closer in size and immunological function to patients. As a step toward developing clinical gene delivery interventions, we evaluated the potential for achieving practical levels of gene transfer to axial and appendicular striated musculature in a canine model. Not only are dogs considerably larger than mice, but canine breeding lines have been established with mutations in the dystrophin gene, producing animals that exhibit pathological features comparable to those of DMD patients.10,11,12,13 Establishing successful protocols for gene delivery in wild-type dogs would be an important first step toward undertaking subsequent evaluations of the therapeutic potential of dystrophin-based vectors in dystrophic dogs.
Although intravascular vector delivery methods have been consistently demonstrated in mice, comparatively little is known concerning the adaptation of these techniques for larger animals. The principal aim of this investigation was to test the hypothesis that rAAV6 vectors, which can efficiently deliver genes body-wide to murine striated musculature via intravascular administration, can achieve significant transduction of the axial and appendicular musculature of a juvenile canine model following intravascular administration. Toward this goal, we conducted experiments utilizing reporter gene vectors administered to nondystrophic animals, expecting that well-developed protocols could subsequently be screened in therapeutic studies using dystrophic animals that are presently available in limited supply and which are very expensive to maintain and breed.
We achieved rAAV6 transduction in heart, diaphragm, and limb musculature at an efficiency similar to that previously obtained in mice. Given the size of dogs, and the advantages of localizing vector dissemination to the target tissues, our results suggest that intravascular infusion of vector at multiple sites could lead to efficient whole-body gene transfer to the musculature of large mammals and eventually patients.
Results
Effect of immunosuppression
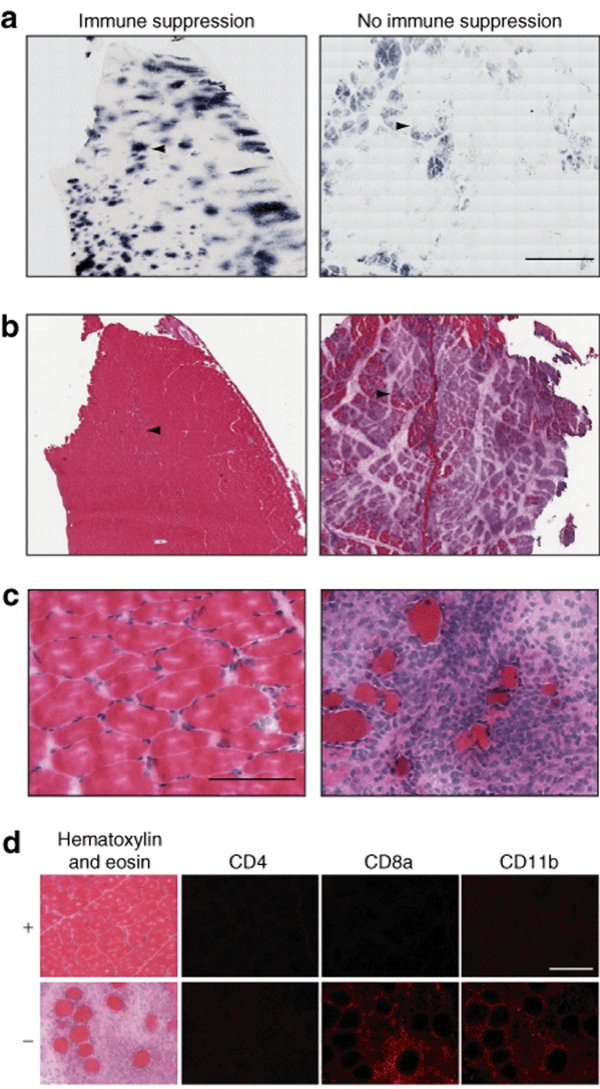
Experiments described by Wang et al. reported a cellular immune response against AAV capsid proteins following intramuscular injection of vector into both wild-type and dystrophic dogs, which was completely blocked by a short course of immune suppression.14,15 To determine whether immune suppression would be required to prevent cellular immunity against rAAV6-human placental alkaline phosphatase (hPLAP) vectors delivered by an intravascular route, we compared transgene expression, muscle necrosis, and inflammation in two dogs, 355 and 371. While each animal received the same infusion protocol (described below), Dog 355 received a combined administration of oral cyclosporine and mycophenolate mofetil (see Materials and Methods). Dog 371 was not immune suppressed. Both animals exhibited transgene expression in lower hindlimb muscles following limb artery infusion (Figure 1a) with similarly unremarkable blood toxicology profiles at intervals post-treatment. However, morphological analysis of muscle cryosections from these animals revealed a marked myofiber necrosis in the nonimmune suppressed animal, while little-to-no tissue breakdown or mononuclear cell accumulation was evident in the sections from the dog receiving immune suppression (Figure 1b,c). CD8+ T-lymphocytes and CD11b+ cells were the predominant cell-types detected by immunofluorescence, while CD4+ T-lymphocytes were scarce (Figure 1d). We conclude that transient immune suppression can enhance transgene expression from intravascularly delivered rAAV vectors, similar to results obtained with intramuscular administration.15
Figure 1.
Inflammation and tissue clearance in the absence of immune suppression. Two dogs were administered 2 × 1013 vg rAAV6-CMV-hPLAP via the femoral artery, with the same infusion protocol. At week 6 after infusion, the animals were euthanized and tissues examined for transgene expression and inflammation. (a,b) Mosaic images of serial gastrocnemius sections for each animal. (a) Sections stained for the presence of hPLAP. (b) Sections stained by hematoxylin and eosin. (c) Higher magnification of the section in b, located at the arrowhead in b. The arrowheads in a demark the same region of each respective section. (d) Serial gastrocnemius sections with hematoxylin and eosin staining and immunofluorescent detection of CD4+, CD8a+, and CD11b+ cells in the immune suppressed (+) and nonimmune suppressed (−) animals. Bar = (a,b) 2 mm, (c,d) 100 µm.
Transduction of hindlimb muscles
We sought to develop a method for widespread transduction of canine limb muscles using a minimally invasive, low-pressure infusion of rAAV6. To enable facile detection of transduced cells, we used hPLAP as a reporter gene, driven by the cytomegalovirus promoter. Due to the relative ease of access to the major femoral vessels in the femoral triangle of the thigh, we chose the femoral artery as the site of infusion. This approach incorporates a principal advantage in that it constrains the region of potential gene delivery to the limb, thereby minimizing the potential for first-pass uptake of vector by nonmuscle organs. Based on results from mouse experiments, in which strong whole-body transduction was obtained at ~3 × 1014 vector genomes/kg (vg/kg),9 we chose a dose of ~5 × 1012 vg/kg to infuse into the hindlimbs of beagle pups (2–4 months, 2.0–6.3 kg). Although this dose was significantly lower by total body weight than was previously used in mice, we hypothesized that this approach would enable an initial and economically feasible assessment of the potential for regional vascular gene delivery in large mammals. Except where noted, these animals received the immune suppression regimen mentioned above, intended to attenuate cellular responses to either the vector or the transgene product, as our goal was to monitor gene delivery efficiency, not immunogenicity.
We compared the effects of a simple infusion into the femoral artery to combinations of either exsanguinating the limb before infusion, and/or allowing the vector to dwell in the hindlimb vasculature after infusion. The vessel occlusion and compression wrap protocol was based on commonly used surgical and regional anesthetic clinical procedures, in which blood flow is obstructed to limb vasculature for up to 2 hours (ref. 16). Upon histological examination at the conclusion of experiments, no ischemic fibers were apparent in any muscles. In our procedure, each animal was placed under general anesthesia, and a confined surgical cut-down was applied to the femoral triangle to expose the femoral artery and vein. Dog 0022 had its femoral vessels occluded with vascular clamps, and received 2 × 1013 vg (~5 × 1012 vg/kg) delivered by an angiocatheter into the femoral artery, distal to the occlusion site. Both vessels remained occluded for 15 minutes to allow the vector to dwell in the hindlimb, after which blood flow was restored and the surgical site was repaired. Dog 9997 underwent a similar procedure; however, before incision, a compression wrap was applied to the length of the leg beginning distal to the surgical site and concluding past the ankle, in order to temporarily evacuate blood from the limb vasculature. The compression wrap was removed once the femoral triangle was exposed and the principal vessels were occluded. Vector was delivered and allowed to dwell under local circulation stasis for 15 minutes. Dogs 355 and 371 underwent a similar procedure, but the compression wrap was applied and remained in place during vector infusion and dwell time. Dog 0006 experienced a two-step infusion process. First, it received a femoral artery infusion as described, with neither a compression wrap nor dwell time. Only the femoral artery was occluded, and blood flow was restored as soon as the artery was repaired following infusion (~10 minutes). After Dog 0006 regained consciousness following anesthesia and was confirmed to be stable (~30 minutes after anesthesia removal), the second infusion began, consisting of a slow infusion into the saphenous vein of the un-injected leg of 8 × 1013 vg (~1 × 1013 vg/kg) in 20 ml, over 2 hours.
Except where noted, the muscles and organs of the dogs were collected 3 weeks after vector infusion and stained for the presence of hPLAP. Each limb muscle was sectioned and stained at three different locations: a proximal, middle, and distal section. The muscles of animals that had not received application of a compression wrap demonstrated little detectable hPLAP expression in any muscle tissue, regardless of whether the vector had been allowed to dwell in the limb vasculature before restoring blood flow (Figure 2). Dog 0022 (15-minute dwell) exhibited transgene expression in a few fibers in the proximal and middle tibialis cranialis (TC), and in occasional liver cells (data not shown). Dog 0006 (no dwell time + slow saphenous infusion), despite receiving a total of 1.6 × 1013 vg/kg, had almost no detectable positive fibers in any muscle, including heart and diaphragm, except for rare fibers in TC and flexor hallucis longus muscles (Figure 2 and data not shown). However, the liver samples of Dog 0006 contained more positive cells than did the liver samples of Dog 0022 (data not shown). Neither of these animals exhibited appreciable hPLAP expression in other organs including lung, kidney, or spleen (data not shown).
Figure 2.
Effect of compression wrap and dwell time on rAAV6-CMV-hPLAP transduction. Four dogs were each administered 2 × 1013 vg rAAV6-CMV-hPLAP into the femoral artery at the femoral triangle. In animals with a vector dwell time, blood flow into and out of the leg was occluded before infusion and restored after the noted time. Depending on the animal, an external compression wrap was applied to the leg, and either removed before infusion, or maintained during vector dwell time. Tissues were harvested and stained for the presence of hPLAP. Bar = 200 µm. TC, tibialis cranialis; FHL, flexor hallucis longus.
The animals whose limbs were temporarily exsanguinated via compression wrap all exhibited substantial transduction in muscles distal to the infusion site (Figure 2). Tissues from Dogs 355 and 371 were harvested 6 weeks after vector infusion. In the regions of surviving muscle tissue in Dog 371 (not immune suppressed), the proximal sections of TC and gastrocnemius had near-confluent staining of the remaining fibers, with diminished staining in the middle and distal TC sections and moderately reduced expression in the middle and distal gastrocnemius sections (data not shown). The flexor hallucis longus muscle of the treated limb presented with groups of positive fibers in regions of the proximal section, but sparse evidence of positive fibers in the mid-belly and distal sections (data not shown). The only other muscle with positive staining was the triceps of the thoracic limb, ipsilateral to the injected leg, which comprised some scattered groups of positive fibers in one head of the muscle (data not shown). The contralateral triceps muscle was not harvested. No positive staining was observed in heart, diaphragm, lung, or liver (data not shown). Dog 355 had strong staining in fiber groups throughout the proximal and middle sections of the TC and gastrocnemius (Figure 2 and data not shown). Staining in the distal gastrocnemius and throughout the flexor hallucis longus was considerable, but less dense than the other sections (data not shown). As expected of limb infusion–based delivery, a scattering of positive cells was found in sections of liver, but no staining was detected in other limb muscles, or in the heart, diaphragm, or lung (data not shown). In contrast to the positive fibers found in discrete fiber groups in the muscles of Dog 355, the positive fibers in Dog 9997 were more evenly dispersed across each muscle, as exemplified in Figure 3. The proximal portion of the TC muscle of the treated limb of Dog 9997 exhibited an ~50% population of muscle fibers with marked transgene activity, with a lessening percentage of positive fibers observable in the middle and distal sections (Figures 3 and 4). The proximal sections of the flexor hallucis longus showed patches of positive cells in small areas of each muscle, with diminishing positive fiber numbers in the middle and distal sections (Figure 4). A similar pattern was observed in the gastrocnemius muscle (data not shown). The proximal quadriceps region presented a low percentage of positive fibers, as did the heart (data not shown). An occasional positive cell was seen in liver, but not in kidney, lung, or spleen (data not shown).
Figure 3.
Effect of removing compression wrap before vector infusion of hindlimb. Animals underwent application of an external compression wrap followed by occlusion of the major femoral vessels. With the wrap either in place or removed, and the blood flow still occluded, rAAV6-CMV-hPLAP was injected into the femoral artery at a dose of 2 × 1013 vg. Tibialis cranialis and extensor digitorum longus cross sections were placed on slides together and stained for the presence of hPLAP. Bar = 5 mm.
Figure 4.
rAAV6-CMV-hPLAP expression at varying levels of hindlimb muscles. Dog 9997 was injected with 2 × 1013 vg rAAV6-CMV-hPLAP via the femoral artery, with a preinfusion compression wrap and a 15-minute vector dwell time. Three weeks after infusion, the animal was euthanized and tissues harvested. Each muscle was sectioned at 25% (proximal), 50% (middle), and 75% (distal) of its length, and stained for the presence of hPLAP. Bar = 200 µm. TC, tibialis cranialis; FHL, flexor hallucis longus.
Transduction of heart and diaphragm
In addition to addressing the peripheral musculature, treatment of the heart and diaphragm will be a necessary part of therapy for DMD. Unfortunately, none of the limb perfusion studies described above resulted in appreciable transduction of the diaphragm or heart muscles (although it was not intended that limb-targeted infusion approaches would simultaneously achieve widespread axial musculature transduction). We therefore attempted to deliver vector to the respiratory and cardiac muscles by infusion directly into the external jugular vein of a 2-month-old beagle pup. In this case, rAAV6-RSV-hPLAP was injected at a dose of 2.5 × 1013 vg (1 × 1013 vg/kg) in a volume of 20 ml. This animal and an un-injected control received an immunosuppression regimen of cyclosporine and azathioprine for the duration of the experiment. Twenty-one days after injection, the animals were euthanized and tissues were harvested. In stained sections, hPLAP expression was present in large groups of fibers throughout the heart, especially surrounding blood vessels (Figure 5). The diaphragm also exhibited scattered groups of positive fibers, but at reduced levels of expression (Figure 5). No expression was observed in intercostal muscles or in limb muscles, nor were any positively staining fibers found in sections from the un-injected animal (Figure 5 and data not shown).
Figure 5.
rAAV6 transduction of heart and diaphragm via jugular vein infusion. A dog was infused with 2 × 1013 vg rAAV-RSV-hPLAP via the jugular vein. An un-injected dog served as a negative control. Three weeks after vector administration, both animals were euthanized, tissues were harvested and stained for the presence of hPLAP. (a) Three sections each of heart and diaphragm from the injected animal are shown, alongside a counterpart section from the un-injected animal (Neg). (b) Sections stained by hematoxylin and eosin and for the presence of hPLAP. Bar = (a) 200 µm, (b) 100 µm. H&E, hematoxylin and eosin.
Discussion
Although rAAV-mediated body-wide transduction in striated musculature has proven to be a relatively simple venture in mice, an effective therapy for DMD patients will require an understanding of how to scale up gene delivery in a safe and economical manner, followed by validation of therapeutic constructs in larger dystrophic animals. We therefore set out to determine the efficacy and efficiency of muscle transduction in a juvenile canine via intravascular rAAV6 delivery. Despite utilizing suboptimal vector doses (as compared with studies in mice), we demonstrated gene delivery to canine hindlimb, diaphragmatic, and cardiac muscles, without chemical modulators or high-pressure infusion. The most important findings of the studies described are that intravascular administration of rAAV6 vectors can achieve significant transduction of striated muscle in a mammalian model approaching the physical dimensions of a newborn human.
A number of recent studies have described targeting canine musculature with various serotypes of rAAV.17,18,19,20,21 In particular, rAAV1, 8, and 9 have been shown to successfully transduce canine muscle with vascular delivery methods. In this study, we have achieved transduction with rAAV serotype 6, a serotype that is known to also efficiently transduce murine striated muscle.22,23 It is not yet clear whether any one of these serotypes is a better choice than another for transduction of canine skeletal musculature. However, serotypes 8 and 9 have thus far been surprisingly inefficient in canine heart, considering their tropism for murine cardiac muscle.19,21 Of those tested in a study of percutaneous transendocardial delivery, involving 40 injections directly into the endomyocardium, AAV6 appears to be the most efficient serotype for canine cardiac transduction, by a factor of 10 (ref. 19). In this report, we present data showing rAAV transduction in canine heart for the first time by systemic administration. It is premature to determine how serotype tropism in mouse and dog tissue will reflect the behavior of various serotypes in humans, but gene delivery to cardiac muscle will be crucially important for treating muscle diseases with cardiac involvement, such as DMD.24
In addition to transduction of central musculature, gene delivery to appendicular skeletal musculature becomes a challenge as the size of the organism increases. The incorporation of simple methodological modifications such as the application of a compression bandage before vector infusion, and the permission of vector to “dwell” in the vasculature of the target region via temporary occlusion of the arterial and venous vessels proximal to the infusion site, markedly increased the degree of transduction subsequently observed in the region of interest. Due to the extensive muscle breakdown of the nonimmune suppressed animal, we base our evaluations on the relative hPLAP expression in animals whose muscles remained intact and therefore are more fairly interpretable. Our results suggest that removing the compression wrap before vector infusion allows more uniform vector dissemination than allowing the wrap to remain in place during infusion and dwell time. Fortuitously, removing the wrap should also comparatively minimize the intravascular pressure during infusion. As this technique is performed routinely in clinical surgical and emergent situations, and because our occlusion lasts for a shorter time period than does the typical clinical practice of the procedure,16 we feel this is a safe, effective method of vector delivery that will be pursued in future studies. Our findings support the hypothesis that rAAV6 vectors, which have demonstrable capacity to deliver genes body-wide to the striated musculature of mice via intravascular administration, can achieve significant transduction of the appendicular skeletal musculature of a nondystrophic juvenile canine model when administered via the circulatory vasculature. Eliminating the blood from the limb before infusion may aid transduction in a number of ways, including (i) creating potential space in the vasculature for the vector to occupy before extravasation, thus aiding localized delivery, and (ii) reducing vector-neutralizing agents in the blood, such as antiviral antibodies.
The animals we utilized had received initial rounds of canine parvovirus vaccinations, before the start of our study. Although immunized animals may not be optimal for experimental purposes of delivering rAAV (a parvovirus family member with potentially similar peptide epitopes, as featured in the attenuated immunogens comprising the vaccine), our experiments may serve as a model for treating human patients, who are likely to have been exposed to variants of AAV during childhood and early adolescence, and/or who would require multiple treatments of rAAV vectors. Conceivably, certain serotypes may prove to be more immunogenic than others on the basis of prevalence in the human population, or because of the presentation of particular capsid protein epitopes.25,26 As support, CD8+ T-cells have been shown to cross-react with different serotypes,27 likely via common epitope motifs. Furthermore, canine dendritic cells have been shown to be activated by AAV2 and by AAV8 capsids.18 Although our primary goal was not to perform an immunological study, we determined that achieving widespread, sustained expression of a human reporter gene in the musculature of canine subjects was facilitated with a regimen of immune suppression. Interestingly, the nonimmune suppressed animal continued to express the transgene in relatively widespread regions of muscle tissue; however, extensive inflammation and muscle fiber breakdown was observed in the vicinity of the surviving islands of transduced myofibers. This observation is consistent with those of Wang et al., in which some transduced fibers remain intact without immune suppression, but in reduced numbers compared to animals treated under immune suppressive conditions, even after local inflammation has subsided.14,15 Our findings may also be consistent with reports from two recent studies of rAAV8 vascular infusion into canine limbs, in which transgene expression levels declined over time, although no inflammation was observed.18,20 In addition, neonatal injections of rAAV9 vectors to dog pups appear to avoid immune reactions while achieving widespread vector dissemination;21 however, it will not be possible to treat every human patient as a newborn, and thus, methods to overcome immune responses to AAV will likely be required, especially in sporadic cases of the disorder.
The results in our isolated limb transduction experiments are highly encouraging, considering that the doses we used for hindlimb infusion were less than those used in our mouse studies and in other recent studies of canine hindlimb transduction (5 × 1012 vg/kg versus 1 × 1013 to 1 × 1014 vg/kg) (refs. 9,18,20). Although we did not expect to observe complete transduction in each limb muscle at this vector dose, the results represent substantial progress toward optimizing vascular delivery in a readily executable, low injectate–pressure configuration. We are also encouraged by our demonstration of cardiac and diaphragmatic muscle transduction. Body-wide transduction in large mammals may ultimately benefit from selective infusion of vectors via multiple routes of administration. Future studies are therefore required to determine the optimal doses required for each infusion intended for a given downstream mass of targeted tissue, the minimal degree of transient immune suppression needed to support long-term expression following vector infusion, and the most favorable combination of infusion sites needed for maximal transduction of the critical muscles required for maintaining quality of life, respiration, and for preventing heart failure.
Materials and Methods
Vector production. Recombinant AAV6 vector was produced and prepared as previously described.22 Briefly, genome-containing vectors were produced by CaPO4 transfection of HEK293 cells with plasmids containing rAAV-CMV-hPLAP vector genomes and pDGM6 packaging/helper plasmids.28 Cells were harvested, processed through a microfluidizer (Microfluidics, Newton, MA), and vector particles were purified on a HiTrap heparin column (GE Healthcare, Chalfont St Giles, UK) before ultracentrifugation for additional concentration. Vector genome titer was determined by Southern blot with a DNA standard of known quantity.
Animal use. Beagle pups (8 weeks old) were acquired from Marshall Farm (North Rose, NY) at which time they had commenced, though not completed their course of routine canine vaccinations: Intra-Trac 3 (Schering Plough, Kenilworth, NJ), Bronchicine (Biocor Animal Health, Omaha, NE), and Galaxy Pv Parvo (Schering Plough, Kenilworth, NJ). Upon arrival, animals were provided with an acclimation period of >1 week before commencing immune suppression (if assigned). During the course of the studies, blood was drawn from enrolled animals at least twice per week, for blood analysis (complete blood count, morphology), chemistry screen (Phoenix Labs, Bothell, WA), and cyclosporine levels. All procedures using animals were approved by the University of Washington Institutional Animal Care and Use Committee.
Hindlimb vector delivery. Animals subjected to general anesthesia inhaled isofluorane in oxygen during procedures, which was applied initially via facemask, and subsequently via tracheal intubation. In preparation for vector administration, the inguinal area proximal to the hindlimb was shaved and sterilized with betadine. A surgical incision was made to expose the femoral artery and vein in the femoral triangle. In animals for which blood was exsanguinated from the limb, an external compression bandage was applied between the paw and the surgical site. For animals undergoing dwell time of vector, either a tourniquet was placed around the limb proximal to the exposed vessels, or vascular clamps were placed on the vessels proximal to the vector delivery site. A 22 gauge angiocatheter was placed into the femoral artery, and vector was infused at a rate of 1 ml/minute. When vector administration was complete, the angiocatheter was removed and the artery repaired with suture to ensure vessel patency. Depending on the animal, blood flow would be restored to the limb either immediately after vessel repair, or after a 15-minute dwell time. Once blood flow was restored to the limb, the overlying surgical incision was repaired, and the animal brought out of anesthesia. The slow saphenous vein infusion in Dog 0006 was controlled by a syringe pump (KD Scientific, Holliston, MA) running at 0.15 ml/minute, connected to an in-dwelling catheter in the saphenous vein. Vector in the line was flushed with isotonic saline.
Jugular vein delivery. Animals were placed under isofluorane anesthesia on O2. A catheter was inserted into the external jugular vein. Vector was infused at a rate of 4 ml/minute in a volume of 20 ml.
Immunosuppression. Animals receiving immune suppression were administered oral cyclosporine (Neoral Oral Solution; Novartis, Basel, Switzerland) twice daily to achieve a whole blood trough level concentration of 400–600 ng/ml. Concurrently, animals were administered oral mycophenolate mofetil (CellCept Oral Suspension; Roche Laboratories, Nutley, NJ) twice daily at 20 mg/kg. Where noted, animals received azathioprine instead of mycophenolate mofetil, every 2 days at 5 mg/kg. The experiment utilizing azathioprine was conducted before development of a regimen using mycophenolate mofetil;15 the regimens in our subsequent experiments were thus adjusted.
Tissue processing. Animals were humanely euthanized (gas anesthesia followed by lethal sodium pentobarbital overdose), and tissues of interest were collected via precise excision. Tissues for sectioning were embedded in a cryocassette filled with cryoprotectant O.C.T. Compound (Sakura Finetek USA, Torrance, CA), and frozen in isopentane cooled by liquid nitrogen. Frozen tissues were cut into 10-µm thick sections and transferred to glass slides.
Histochemical staining. For hPLAP staining, sections were fixed with ice cold 4% paraformaldehyde, washed three times in cold phosphate-buffered saline, placed in 65 °C phosphate-buffered saline for 90 minutes, rinsed in room temperature phosphate-buffered saline, and washed in alkaline phosphatase buffer (0.1 mol/l Tris–HCl pH 9.5, 0.1 mol/l NaCl, 0.01 mol/l MgCl2) for 10 minutes. Excess liquid was removed from the sections, and Sigma FAST BCIP/NBT substrate solution (Sigma, St Louis, MO) was applied to each section for 30 minutes at room temperature in the dark. Slides were rinsed three times in room temperature phosphate-buffered saline, dehydrated in 70% EtOH for 5 minutes, 2× (95% EtOH for 2 minutes), 2× (100% EtOH for 2 minutes), 2× (xylene for 3 minutes), and coverslipped with Permount mounting media (Fisher Scientific, Fair Lawn, NJ). For hematoxylin and eosin staining, muscle sections were first fixed for 5 minutes in absolute methanol, stained according to a standard hematoxylin and eosin protocol, and dehydrated and mounted as described above. Immunohistochemical detection of immune cells was performed with mouse monoclonal canine-specific anti-CD4 (CA13.1E4), anti-CD8a (CA9.JD3), and anti-CD11b (CA16.3E10) antibodies (AbD Serotec, Oxford, UK), each at 1:10 dilution. Alexa Fluor 594 goat anti-mouse IgG antibodies (Invitrogen, Carlsbad, CA) were used as secondary antibodies (1:1,200 dilution). Sections were mounted with VECTASHIELD HardSet with DAPI (Vector Labs, Burlingame, CA). Tissue sections were visualized under white light or epifluorescent microscopy with a Nikon Eclipse E1000 microscope (Nikon Instruments, Melville, NY) and images captured with QCapture software (Qimaging, Pleasanton, CA). Mosaic images were captured with Syncroscan software (Syncroscopy, Frederick, MD). Uniform contrast adjustment was made to the immunofluorescent images as an entire panel.
Acknowledgments
We acknowledge the excellent assistance provided by the veterinarians and the veterinary technicians in the UW Department of Comparative Medicine. We thank Miki Haraguchi for performing cryosectioning and histochemistry. This work was supported by grants (to J.S.C.) from the National Institutes of Health (NIH R37 AR040864) and the Muscular Dystrophy Association (USA). P.G. was supported in part by a development grant from the MDA, and B.R.S. was supported in part by the University of Washington Medical Scientist Training Program and by an ARCS Foundation Fellowship. This work was performed in Seattle, Washington, USA.
REFERENCES
- Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C., and , Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH., and , Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Chamberlain J., and , Caskey C.Duchenne Muscular Dystrophy Current Neurology 1990Yearbook Medical Publishers: Chicago; 65–103.In: Appel, S (ed) [Google Scholar]
- Wang B, Li J, Fu FH., and , Xiao X. Systemic human minidystrophin gene transfer improves functions and life span of dystrophin and dystrophin/utrophin-deficient mice. J Orthop Res. 2009;27:421–426. doi: 10.1002/jor.20781. [DOI] [PubMed] [Google Scholar]
- Pacak CA, Walter GA, Gaidosh G, Bryant N, Lewis MA, Germain S, et al. Long-term skeletal muscle protection after gene transfer in a mouse model of LGMD-2D. Mol Ther. 2007;15:1775–1781. doi: 10.1038/sj.mt.6300246. [DOI] [PubMed] [Google Scholar]
- Townsend D, Blankinship MJ, Allen JM, Gregorevic P, Chamberlain JS., and , Metzger JM. Systemic administration of micro-dystrophin restores cardiac geometry and prevents dobutamine-induced cardiac pump failure. Mol Ther. 2007;15:1086–1092. doi: 10.1038/sj.mt.6300144. [DOI] [PubMed] [Google Scholar]
- Bostick B, Yue Y, Lai Y, Long C, Li D., and , Duan D. Adeno-associated virus serotype-9 microdystrophin gene therapy ameliorates electrocardiographic abnormalities in mdx mice. Hum Gene Ther. 2008;19:851–856. doi: 10.1089/hum.2008.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom GL, Gregorevic P, Allen JM, Finn E., and , Chamberlain JS. Microutrophin delivery through rAAV6 increases lifespan and improves muscle function in dystrophic dystrophin/utrophin-deficient mice. Mol Ther. 2008;16:1539–1545. doi: 10.1038/mt.2008.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorevic P, Allen JM, Minami E, Blankinship MJ, Haraguchi M, Meuse L, et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med. 2006;12:787–789. doi: 10.1038/nm1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BJ, Winand NJ, Stedman H, Valentine BA, Hoffman EP, Kunkel LM, et al. The homologue of the Duchenne locus is defective in X-linked muscular dystrophy of dogs. Nature. 1988;334:154–156. doi: 10.1038/334154a0. [DOI] [PubMed] [Google Scholar]
- Valentine BA, Cooper BJ, de Lahunta A, O'Quinn R., and , Blue JT. Canine X-linked muscular dystrophy. An animal model of Duchenne muscular dystrophy: clinical studies. J Neurol Sci. 1988;88:69–81. doi: 10.1016/0022-510x(88)90206-7. [DOI] [PubMed] [Google Scholar]
- Shimatsu Y, Katagiri K, Furuta T, Nakura M, Tanioka Y, Yuasa K, et al. Canine X-linked muscular dystrophy in Japan (CXMDJ) Exp Anim. 2003;52:93–97. doi: 10.1538/expanim.52.93. [DOI] [PubMed] [Google Scholar]
- Shimatsu Y, Yoshimura M, Yuasa K, Urasawa N, Tomohiro M, Nakura M, et al. Major clinical and histopathological characteristics of canine X-linked muscular dystrophy in Japan, CXMDJ. Acta Myol. 2005;24:145–154. [PubMed] [Google Scholar]
- Wang Z, Allen JM, Riddell SR, Gregorevic P, Storb R, Tapscott SJ, et al. Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum Gene Ther. 2007;18:18–26. doi: 10.1089/hum.2006.093. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kuhr CS, Allen JM, Blankinship M, Gregorevic P, Chamberlain JS, et al. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol Ther. 2007;15:1160–1166. doi: 10.1038/sj.mt.6300161. [DOI] [PubMed] [Google Scholar]
- Roberts JR.Intravenous regional anesthesia Clinical Procedures in Emergency Medicine 20044th edn., Saunders: Philadelphia; 591–595.In: Roberts, JR, Hedges, JR, Chanmugam, AS, Chudnofsky, CR, Custalow, CB and Dronen, SC (eds) [Google Scholar]
- Su LT, Gopal K, Wang Z, Yin X, Nelson A, Kozyak BW, et al. Uniform scale-independent gene transfer to striated muscle after transvenular extravasation of vector. Circulation. 2005;112:1780–1788. doi: 10.1161/CIRCULATIONAHA.105.534008. [DOI] [PubMed] [Google Scholar]
- Ohshima S, Shin JH, Yuasa K, Nishiyama A, Kira J, Okada T, et al. Transduction efficiency and immune response associated with the administration of AAV8 vector into dog skeletal muscle. Mol Ther. 2009;17:73–80. doi: 10.1038/mt.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bish LT, Sleeper MM, Brainard B, Cole S, Russell N, Withnall E, et al. Percutaneous transendocardial delivery of self-complementary adeno-associated virus 6 achieves global cardiac gene transfer in canines. Mol Ther. 2008;16:1953–1959. doi: 10.1038/mt.2008.202. [DOI] [PubMed] [Google Scholar]
- Qiao C, Li J, Zheng H, Bogan J, Li J, Yuan Z, et al. Hydrodynamic limb vein injection of AAV8 canine myostatin propeptide gene in normal dogs enhances muscle growth Hum Gene Ther 2008. epub ahead of print [DOI] [PMC free article] [PubMed]
- Yue Y, Ghosh A, Long C, Bostick B, Smith BF, Kornegay JN, et al. A single intravenous injection of adeno-associated virus serotype-9 leads to whole body skeletal muscle transduction in dogs. Mol Ther. 2008;16:1944–1952. doi: 10.1038/mt.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankinship M, Gregorevic P, Allen J, Harper S, Harper H, Halbert C, et al. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol Ther. 2004;10:671–678. doi: 10.1016/j.ymthe.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Zincarelli C, Soltys S, Rengo G., and , Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- Townsend D, Yasuda S, Li S, Chamberlain JS., and , Metzger JM. Emergent dilated cardiomyopathy caused by targeted repair of dystrophic skeletal muscle. Mol Ther. 2008;16:832–835. doi: 10.1038/mt.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe L, Wang L, Somanathan S, Zhi Y, Figueredo J, Calcedo R, et al. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat Med. 2006;12:967–971. doi: 10.1038/nm1445. [DOI] [PubMed] [Google Scholar]
- Mingozzi F., and , High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2007;7:316–324. doi: 10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Blankinship M, Allen J, Crawford R, Meuse L, Miller D, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]