Abstract
Soy phytoestrogens have been proposed as an alternative to estrogen replacement therapy and have demonstrated potential neuroprotective effects in the brain. We have shown that a high soy diet significantly reduces infarct size following permanent middle cerebral artery occlusion (MCAO). Here, we tested the hypothesis that a high soy diet would attenuate programmed cell death after stroke. Adult female Sprague-Dawley rats were ovariectomized and fed either an isoflavone-reduced diet (IFP) or a high soy diet (SP) for 2 weeks before undergoing 90 minutes of transient MCAO (tMCAO) followed by 22.5 hr reperfusion. Infarct size, as assessed by TTC staining, was significantly reduced by a high soy diet (p< 0.05). Apoptosis in the ischemic cortex, measured by TUNEL staining, was significantly reduced by the high soy diet. The number of active caspase-3 positive cells and caspase-mediated α-spectrin cleavage was also significantly decreased in the ischemic cortex of SP rats. Furthermore, nuclear translocation of apoptosis-inducing factor (AIF) was significantly reduced in the ischemic cortex of SP rats. Soy significantly increased bcl-xL mRNA and protein expression in the ischemic cortex compared to IFP rats. Immunohistochemistry revealed increased neuronal expression of bcl-2 and bcl-xL in the ischemic cortex of both IFP and SP rats following tMCAO. These results suggest that a high soy diet decreases both caspase-dependent and caspase-independent programmed cell death following tMCAO. Further, a high soy diet enhances expression of the cell survival factor bcl-xL following tMCAO, contributing to the neuroprotective effects of soy in the ischemic cortex.
Keywords: apoptosis, bcl-2, neuroprotection, caspase-3
Recent studies suggest that dietary soy is neuroprotective in rat models of cerebral ischemia. We have shown that a high soy diet decreases infarct size after permanent middle cerebral artery occlusion (MCAO) in ovariectomized female rats (Schreihofer et al., 2005). Dietary soy isoflavones also improve stroke outcome and decrease stroke size in male rats following transient MCAO (tMCAO) (Burguete et al., 2006). The isoflavones contained in soy have been postulated to account for their neuroprotective actions. Genistein and daidzein, as well as their metabolites, are phytoestrogens, natural compounds that can bind to estrogen receptors (ERs) and mimic some of estrogen’s effects (Vaya and Tamir, 2004). Indeed, the soy isoflavone genistein is neuroprotective in a mouse model of ischemic stroke (Trieu and Uckun, 1999). However, the mechanism of soy neuroprotection in the brain remains to be determined.
Estrogen is well established as a neuroprotective agent in many models of brain injury, including stroke (Wise et al., 2001, McCullough and Hurn, 2003, Alonso de Lecinana and Egido, 2006). Pretreatment with a physiological dose of estradiol protects the ischemic cortex against delayed cell death induced by MCAO, reducing both caspase activity and DNA fragmentation in the ischemic penumbra following permanent MCAO (Rau et al., 2003). One potential mechanism for estradiol-induced neuroprotection is that it modulates expression of genes involved in control of cell death and apoptosis, including anti-apoptotic bcl-2 family proteins. In a permanent MCAO model, estradiol prevents the injury-induced down-regulation of bcl-2 mRNA (Dubal et al., 1999, Won et al., 2006). Following tMCAO, bcl-2 mRNA and protein are induced in the ischemic penumbra of both intact females and ovariectomized females treated with estrogen (Alkayed et al., 2001). Transgenic overexpression of bcl-2 in neurons has also been shown to decrease infarct size in male mice (Alkayed et al., 2001). Furthermore, overexpression of bcl-2 in adult rat brain enhances neurogenesis and survival of newborn neurons (Zhang et al., 2006b). The induction and/or maintainance of bcl-2 following MCAO may represent a survival mechanism for neurons after stroke and may account for at least some of the neuroprotective effects of estrogen.
Following recent clinical studies suggesting possible negative health consequences of hormone therapy (Rossouw et al., 2002, Brass, 2004), the use of soy as a natural alternative to estrogen replacement after menopause has increased (Newton et al., 2002, Kurzer, 2003). Whether soy is acting like estrogen in the brain to provide neuroprotection is unclear. The purpose of this study was to elucidate potential mechanisms by which soy decreases infarct size. Multiple modes of cell death have been described in the ischemic penumbra following MCAO, including caspase-3 dependent apoptosis due to mitochondrial metabolic compromise, and caspase-independent apoptosis induced by apoptosis-inducing factor, or AIF (Ferrer and Planas, 2003). It is unclear whether a high soy diet reduces apoptotic cell death following MCAO and which mechanisms may be involved. Because estrogen has been shown to modulate caspase-3 dependent apoptosis and expression of the anti-apoptotic gene bcl-2 in ischemic injury, we tested the hypothesis that a high soy diet would similarly inhibit caspase-3 dependent apoptosis and induce bcl-2 family gene expression in the ischemic penumbra following focal cerebral ischemia.
Experimental Procedures
Animals and Treatments
The Medical College of Georgia Institutional Animal Care and Use Committee approved all animal protocols in accordance with the guidelines in the NIH Guide for the Care and Use of Laboratory Animals. Female Sprague-Dawley rats (200–225 g) were purchased from Harlan (Indianapolis, IN) and maintained in our animal facility in a temperature-controlled room (22–25 °C) with 12hr dark-light cycles. All rats had free access to standard laboratory chow and water. After acclimating for one week, animals were randomly assigned to one of three groups: IFP (isoflavone-reduced, placebo), IFE (isoflavone-reduced, estrogen), or SP (high soy, placebo). The isoflavone-reduced diet was custom made by Ziegler Brothers Inc. (Gardners, PA) and is designed to match macro- and micro-nutrient content of the high soy diet, Teklad 8604 (Harlan Teklad, Madison, WI). The Teklad diet has been shown to contain 600 μg/g soy isoflavones and results in an average of 6 μM circulating total isoflavones (Schreihofer et al., 2005), an amount equal to or greater than a typical Asian diet which is high in soy (Setchell, 2001). One week after initiation of diets, animals were bilaterally ovariectomized and subcutaneously implanted with either a placebo pellet or a 0.05 mg, 21-day release estrogen pellet (Innovative Research of America, Sarasota, FL). Animals were kept on assigned diet for two weeks after ovariectomy before further surgery. Because the effect of estradiol on infarct size and DNA fragmentation has been reported previously (Rau et al., 2003), an estrogen treated group (IFE) was not included in all studies presented here to reduce animal use. The IFE group was included in gene expression results since soy had different effects than estradiol in those studies.
Transient Middle Cerebral Artery Occlusion
Two weeks after ovariectomy, rats underwent middle cerebral artery occlusion according to a modified version of the Longa method (Longa et al., 1989). Rats were anesthetized with 5% halothane in 100% oxygen and maintained on 1.5–2.0% halothane during the procedure. Body temperature was maintained at 37 +/− 0.5°C with a heating pad controlled by a rectal probe (CWE Instruments, Wood Dale, IL). A laser-Doppler flow (LDF) probe (Perimed, North Royalton, OH) was attached to the left side of the dorsal surface of the skull 2 mm caudal and 6 mm lateral to bregma. Rectal temperature and LDF were monitored continuously through an analog-to-digital converter and collected on a computer with Spike 2 software (Cambridge Electronic Design, Cambridge, England) for subsequent analysis. A sterile, silicone-coated 4-0 monofilament nylon suture was introduced retrogradely into the left external carotid artery and advanced cranially into the internal carotid artery until resistance was felt (~19 mm from the bifurcation of the common carotid artery). MCAO was verified by a rapid drop in blood flow to the left cerebral hemisphere. LDF was observed for 90 min before the suture was withdrawn and reperfusion monitored by LDF. 32% of animals did not demonstrate reperfusion and were excluded from the study. Failure to reperfuse was mainly due to hemorrhage following suture withdrawal. Animals were allowed to recover for 24hr from the time of stroke initiation with free access to soft food and water.
Measurement of infarct size
Twenty-four hours after initiation of MCAO, rats were deeply anesthetized with urethane (1.7 g/kg ip), transcardially perfused with cold sterile saline, and decapitated with a guillotine. The brain was rapidly removed, placed in ice-cold sterile saline for 2 minutes and cut into 2 mm coronal sections in a brain matrix (Braintree Scientific) starting at the frontal pole. Sections were stained with triphenyltetrazolium chloride (TTC) to assess infarct size (Hatfield et al., 1991). After fixation in 4% paraformaldehyde, each section was electronically scanned. Inclusion criteria included a drop in LDF between 50–75% for 90 min followed by a reperfusion and a visible infarct. The lesion size was determined using NIH Image and the method of Swanson et al (Swanson et al., 1990), which accounts for regional edema on the infarcted side. Ischemic volumes are thus expressed as percentage of the contralateral side.
DNA Fragmentation
A separate group of rats was used to measure DNA fragmentation 22.5hr following tMCAO. Brains were removed, sliced in 2 mm sections starting 4 mm from the frontal pole, and frozen in Tissue-Tek OCT compound (Sakura Finetechnical Co, Tokyo, Japan) at −20°C. Consecutive coronal sections (10 μm) were cut on a cryostat from 3 coronal levels corresponding to approximately 0, −2.0, and −4.0 mm relative to bregma (three sections per slide) and frozen until use. TUNEL staining was performed using the TdT-FragEL DNA fragmentation Detection Kit according to the manufacturer’s instructions (Calbiochem, La Jolla, CA). Detection was performed with DAB solution and sections were counterstained with methyl green. The sections were observed under a light microscope using the 40X objective and TUNEL-positive cells in the entire cortex were mapped using NeuroLucida (MicroBrightField, Inc., Colchester, VT). The total number of TUNEL-positive cells in the ischemic cortex of a comparable section from each animal was then compared (n=4 /group).
Active Caspase-3 and AIF Immunohistochemistry and Cell Counts
Cryosections were fixed in 4% formaldehyde and incubated with either active caspase-3 (Promega, G748; 1:250) or AIF (Santa Cruz, sc-9416; 1:50) antibodies overnight at 4°C followed by biotinylated anti-rabbit (for active caspase-3) or anti-goat (for AIF) immunoglobulin (Jackson Immunoresearch, West Grove, PA) and strepavidin-Cy3 for fluorescent detection. AIF slides were then counterstained with DAPI (Pierce, Rockford, IL) to detect nuclei. Each slide contained three sections. In each section, three images were taken in identical regions of the ischemic cortex with a 20X objective. Active caspase-3 positive cells were counted in the three 20X microscope fields, each 2.4 mm2, using NIH Image. Cell counts were averaged for each animal and expressed as cells/mm2. For AIF, both cytoplasmic and nuclear staining was counted using the 40X objective in the same regions of the cortex as described above. In each of three sections per animal, three fields of 2.4 mm2 each were counted in the ischemic cortex using the MeanderScan function of NeuroLucida (MicroBrightField, Inc., Williston, VT). Percent nuclear staining was then calculated for each animal.
RNA collection and real time RT-PCR
For RNA collection, a separate set of animals was sacrificed as described above. Brain slices corresponding to AP +2 to 0 and 0 to −2 mm relative to bregma were frozen on glass slides on dry ice. The remaining sections were stained with TTC to estimate the infarct boundaries and ensure that tissue punches were made in the peri-infarct zone. Total RNA was isolated from the dorsal cortex using a commercial kit with a DNAse treatment step to remove any DNA contamination (RNEasy, Qiagen, Valencia, CA). RNA concentration was determined in triplicate using RiboGreen RNA-binding dye (Molecular Probes, Invitrogen, Carlsbad, CA) and RNA was stored at −80° C until used. Total RNA (500 ng) was reverse transcribed with oligo-dT using a commercial kit (OmniScript, Qiagen, Valencia, CA) and real-time RT-PCR was performed on 25 ng equivalents in triplicate on an Applied Biosystems (AB) 7500 Sequence Detection System using AB TaqMan Gene Expression Assays for Bcl-xL (Rn00580568_g1), Bcl-2 (Mm00477631_m1), and primer limited GAPDH as an endogenous control gene. No significant differences in GAPDH expression were detected between groups. Threshold amplification cycle number (Ct) data from multiple plates was combined using Applied Biosystems Relative Quantitation software (SDS1.2) and the ΔΔCt method with GAPDH as the endogenous control. All data are expressed as mean fold change ± SE.
Protein collection and immunoblotting
For protein collection, a separate group of animals were sacrificed as described above. Coronal sections were made in a brain matrix and the sections corresponding to AP +2 to −3 relative to bregma were placed on ice-cold glass slides. The cortex was carefully peeled away from the underlying tissue and frozen at −80°C. Protein was extracted using T-PER reagent (Pierce, Rockford, IL) supplemented with HALT Protease Inhibitor Cocktail (Pierce, Rockford, IL). Concentrations were determined using a BCA protein assay kit (Pierce, Rockford, IL), and samples were aliquoted to avoid multiple freeze-thaw cycles. Total protein (15–50 μg) from each sample was separated on precast 4–20% polyacrylamide gels (Pierce Precise) under reducing conditions. Samples were transferred to nitrocellulose and blocked for one hour at room temperature using Odyssey Blocking Buffer (LiCor, Lincoln, NE). Blots were subjected to immunoblotting in primary antibody overnight at 4°C. Antibodies used were anti-bclxL (Santa Cruz sc-8392; Santa Cruz, CA), anti-bcl-2 (Santa Cruz sc-492), anti-spectrin (Chemicon MAB1622; Temecula, CA), and anti-AIF (Santa Cruz sc-9416). Secondary detection was accomplished with corresponding IRDye Conjugated antibodies (LiCor, Lincoln, NE). Blots were visualized using a LiCor Odyssey Infrared Scanner and quantified using Odyssey software. Blots were subsequently stripped using Pierce Stripping Buffer, and reprobed for β-actin (Sigma, A5441; St. Louis, MO) to normalize for loading.
Bcl-2 and Bcl-xL Immunohistochemistry
Cryosections were prepared as described above. For immunostaining, sections were incubated with Bcl-xL (Cell Signaling #2762, 1:50) or bcl-2 (Santa Cruz, sc-492; 1:50) overnight at 4°C followed by biotinylated anti-rabbit immunoglobulin (Jackson Immunoresearch, West Grove, PA) and strepavidin-Cy3 (Jackson Immunoresearch) for fluorescent detection. To indicate whether bcl-2 family proteins co-localized to neurons or other cell populations after ischemia, double-label immunoflourescent staining was performed with either the neuronal marker NeuN (Chemicon, MAB377; 1:500) or the glial marker glial fibrillary acidic protein (GFAP) (Sigma; 1:500) detected with an AlexaFlour 488 anti-mouse secondary (Molecular Probes, Eugene, OR).
Statistical Analysis
Differences among groups were assessed by t-test or analysis of variance with post-hoc comparisons between groups performed with Tukey-Kramer. A p< 0.05 was considered significant.
Results
High soy diet decreases infarct size after transient MCAO
Twenty-four hours after initiation of a 90 min ischemic period (22.5hr post-MCAO), rats fed an isoflavone-reduced diet (IFP) had a mean infarct of 31.1 +/− 5.2%. Rats fed a high soy diet (SP) had significantly smaller strokes, averaging 16.0 +/− 2.2% (n=4/ group, p< 0.05).
High soy diet decreases DNA fragmentation after transient MCAO
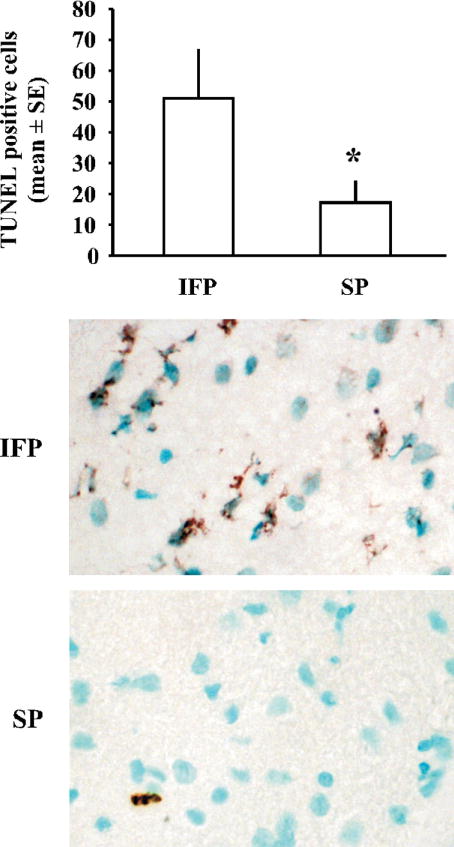
We analyzed DNA fragmentation 22.5hr following tMCAO by counting the number of TUNEL-positive cells in the ischemic cortex. Rats fed a high soy diet had significantly less TUNEL-positive staining in the ischemic cortex following tMCAO compared to IFP animals, suggesting decreased apoptosis (Fig 1).
Figure 1.
TUNEL staining demonstrates decreased DNA fragmentation 22.5hr following tMCAO in rats fed a high soy diet (SP) compared to control ovariectomized female rats (IFP). Bars represent mean +/− SE of TUNEL-positive cells in the ischemic cortex (n=4/ group, *p< 0.05). Shown are representative images of TUNEL staining in the ischemic cortex of IFP and SP rats. TUNEL-positive cells are stained brown, while other cells are counterstained with methyl green.
High soy diet decreases active caspase-3
We determined the number of active caspase-3 positive cells in the ischemic cortex of IFP and SP rats 22.5hr after tMCAO using IHC. While IFP rats had an average of 21.3 +/− 2.0 cells/mm2 in the ischemic cortex, this was significantly reduced to 13.0 +/− 1.3 cell/mm2 in SP rats (Fig 2). To assess active caspase-3 activity, we measured relative amounts of intact α-spectrin (240 kDa) and spectrin breakdown products (120 kDa and 150 kDa) in the ischemic cortex of IFP (n=4) and SP rats (n=8) by western blot analysis. The caspase-mediated spectrin breakdown product (120/240 kDa ratio) was significantly decreased by high soy diet compared to IFP (Fig 3). In contrast, the calpain-mediated spectrin breakdown product (150/240 kDa ratio) was significantly increased by a high soy diet compared to IFP (Fig 3).
Figure 2.
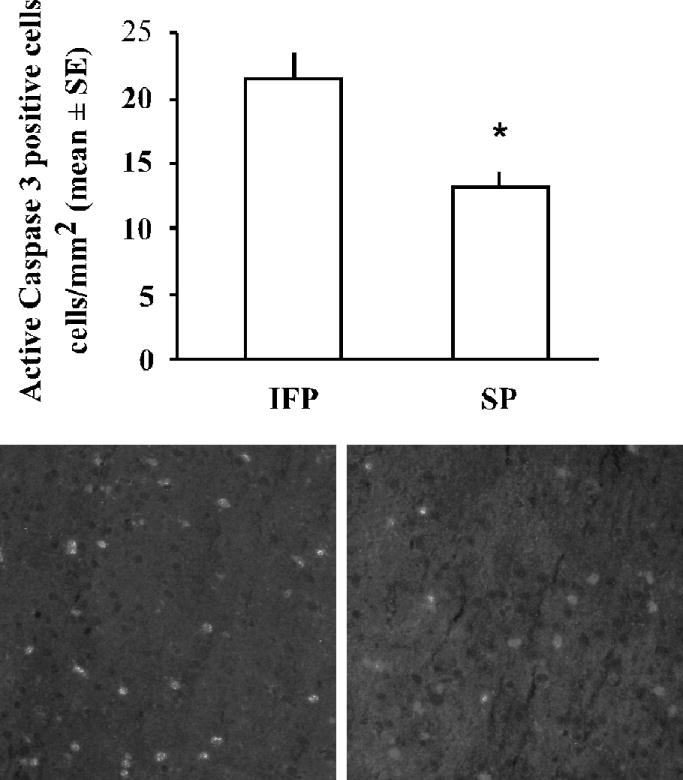
Active caspase-3 immunoreactivity in the ischemic cortex 22.5hr following tMCAO. Bars represent mean +/− SE of active caspase-3 positive cell/mm2 in three regions of the ischemic cortex from three sections per animal (n=5/group, *p< 0.05). Shown are representative images of active caspase-3 immunostaining in the ischemic cortex of IFP and SP rats.
Figure 3.
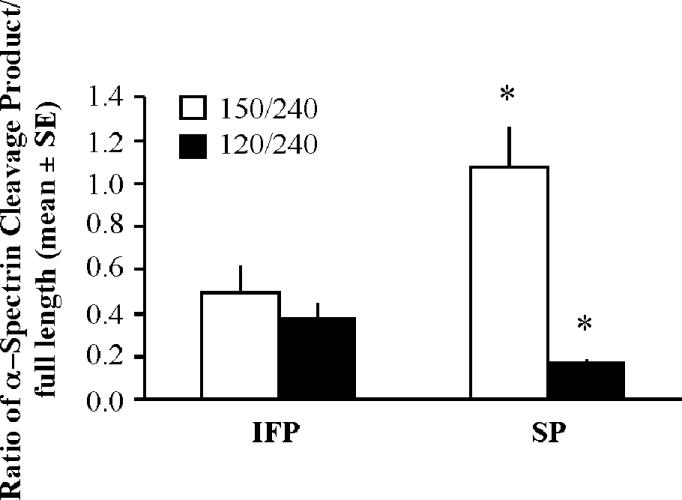
Spectrin cleavage in the ischemic cortex 22.5hr following tMCAO. Proteins were analyzed by western blotting to detect intact spectrin (240 kDa) and 120 kDa and 150 kDa spectrin breakdown products. Bars represent mean +/− SE of the ratio of spectrin-breakdown products to full-length spectrin normalized to β-actin from the same sample (n= 4–8/ group, *p< 0.05).
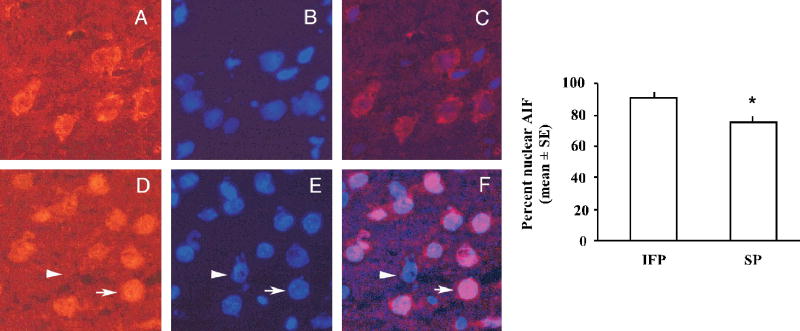
High soy diet decreases the percentage of nuclear apoptosis inducing factor (AIF)
AIF is a caspase-independent apoptosis pathway recently shown to be involved in cell death following ischemia (Ferrer et al., 2003). AIF protein was measured by western blot and IHC. Total AIF protein in the ischemic cortex was not different among treatment groups as measured by western blot (data not shown). However, because it is the nuclear translocation of AIF following ischemia that initiates DNA fragmentation, we counted both nuclear and cytoplasmic AIF staining post-ischemia using IHC in three regions of the ischemic cortex in three sections from each animal. The percent of nuclear AIF expression was significantly decreased in the SP group compared to the IFP group (Fig 4).
Figure 4.
Nuclear translocation of AIF in the ischemic cortex 22.5hr following tMCAO. Bars represent mean +/− SE of percent nuclear AIF localization in the ischemic cortex (n= 4/ group, *p< 0.05). Also shown are representative pictures of AIF immunostaining demonstrating both cytoplasmic (A) and nuclear (D) expression of AIF co-labeled with DAPI (B, E) for nuclear detection. Both cytoplasmic (C) and nuclear (F) AIF are visible in the overlayed images. The arrowhead shows a non-AIF expressing cell, while the arrow shows nuclear translocation of AIF.
High soy diet increases bclxL mRNA and protein expression after transient MCAO
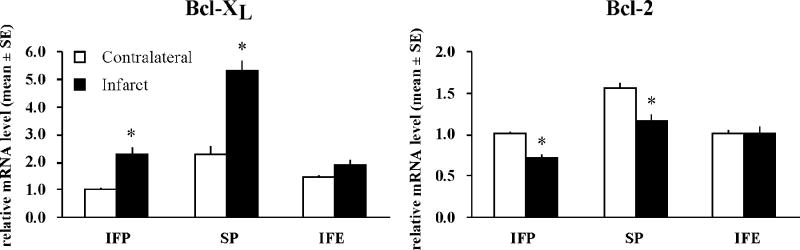
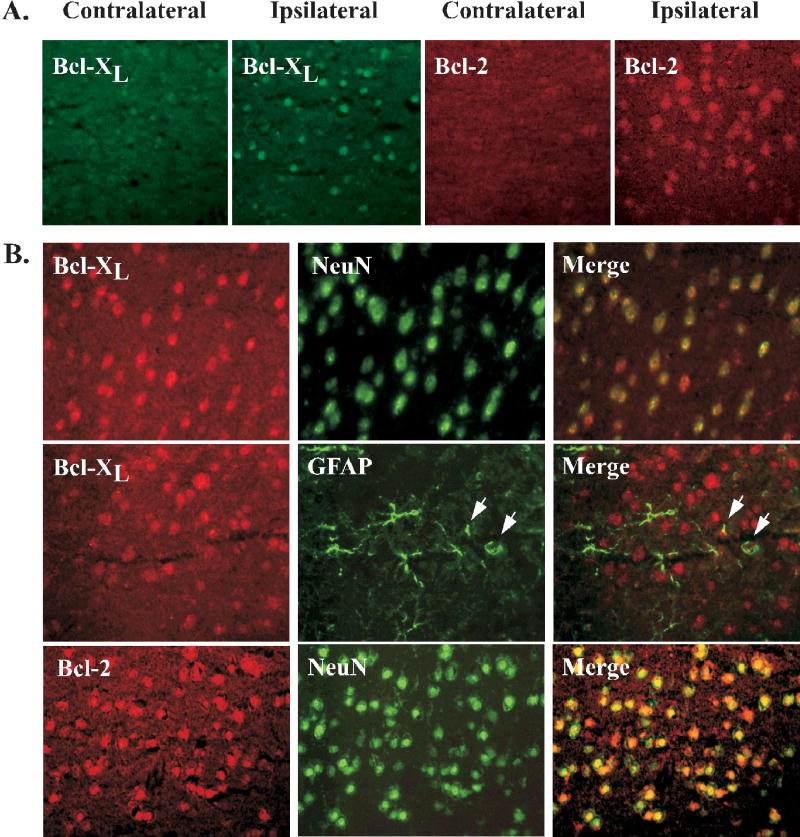
To explore the mechanism for the protective effect of a high soy diet, we analyzed mRNA expression of the anti-apoptotic bcl-2 family members bcl-2 and bcl-xL using real-time RT-PCR. Because estradiol has been shown to alter expression of bcl-2, we compared the effects of soy to estradiol. Tissue punches were collected from the dorsal cortex of both the non-stroked (contralateral) and stroked (ipsilateral) sides of the brain 22.5hr after tMCAO. Bcl-xL mRNA was significantly increased after stroke in both IFP and SP groups, but not in the IFE group (Fig 5). Bcl-2 mRNA expression was significantly decreased after stroke in both IFP and SP groups, but estrogen prevented this decrease in bcl-2 (Fig 5). Next, we analyzed the protein expression of both bcl-2 and bcl-xL using IHC and western blot. While bcl-2 and bcl-xL were weakly expressed in the contralateral hemisphere, protein expression was increased in the ischemic hemisphere in all groups (Fig 6a). Further, double-label IHC revealed that both bcl-2 and bcl-xL were found almost exclusively in neurons (Fig 6b). Protein expression in the ischemic cortex was compared among groups using western blot analysis. Bcl-xL protein in the ischemic cortex was significantly increased in rats fed a high soy diet (SP) following MCAO compared to IFP. This effect was not seen in the IFE group (Fig. 7). Bcl-2 protein expression in the ischemic cortex was not significantly different among the groups 22.5hr following MCAO as measured by western blot (data not shown).
Figure 5.
Relative mRNA expression of bcl-xL and bcl-2 22.5hr following tMCAO. RNA was extracted from the dorsal cerebral cortex adjacent to the infarct (black bars) and from the corresponding contralateral side (white bars). Real time RT-PCR gene expression was normalized to GAPDH as an endogenous control and expressed relative to the contralateral cortex from the IFP group. Data are represented as mean +/− SE (n= 6/ group, *p< 0.05).
Figure 6.
IHC analysis of bcl-xL and bcl-2 22.5hr following tMCAO. (A) Representative pictures showing differential expression of bcl-xL and bcl-2 protein in the cerebral cortex of IFP rats after cerebral ischemia. In response to injury, both bcl-2 and bcl-xL expression was upregulated in the ipsilateral (ischemic) cortex of all groups compared to the contralateral (non-stroked) side. (B) Localization of bcl-xL and bcl-2 to neurons is shown in representative pictures in which bcl was double-labeled with the neuronal marker NeuN in the ischemic cortex 22.5hr following tMCAO. Overlayed images demonstrate predominant neuronal expression of both bcl-xL and bcl-2. A panel showing bcl-xL double-labeled with the astrocyte marker GFAP is included to demonstrate that bcl-xL is not found in these cells. However, arrows show close association of bcl-xL and GFAP-expressing cells.
Figure 7.
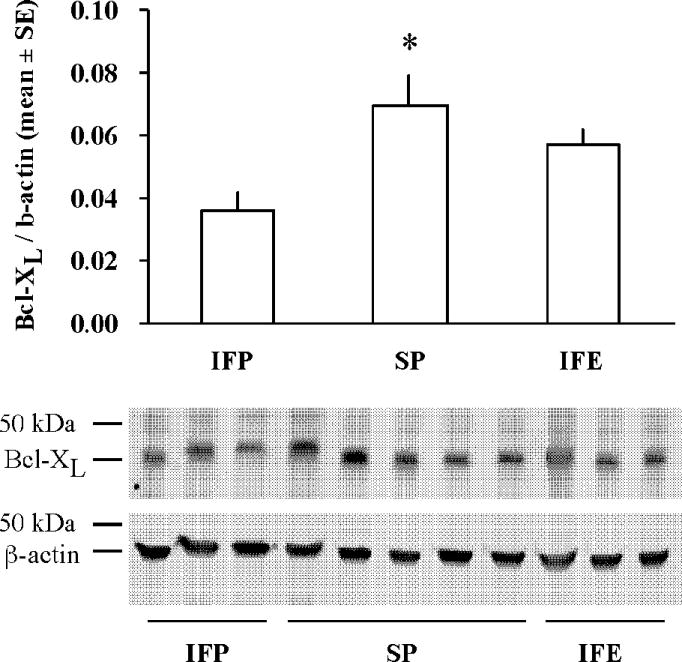
High soy diet increases Bcl-xL protein expression 22.5hr following tMCAO. Proteins extracted from the ischemic cortex were analyzed by western blotting to detect bcl-xL. Bars represent mean +/− SE of bcl-xL normalized to β-actin (n= 4–8/ group, *p< 0.05). Shown is a representative immunoblot of bcl-xL and β-actin.
Discussion
The present study demonstrates that a high soy diet reduces infarct size after tMCAO in ovariectomized female rats, similar to effects previously shown with the permanent MCAO model (Schreihofer et al., 2005) and in male rats following tMCAO (Burguete et al., 2006). This is the first study to show attenuation of programmed cell death by a high soy diet, similar to effects previously shown with estradiol (Rau et al., 2003). Rats fed a high soy diet for two weeks prior to tMCAO had decreased DNA fragmentation 22.5hr after tMCAO. Further, rats fed a high soy diet had reduced active caspase-3 protein expression and reduced caspase-mediated α-spectrin cleavage 22.5hr after tMCAO. A high soy diet also decreased nuclear AIF translocation after tMCAO. These results suggest that soy decreases both caspase-dependent and caspase-independent cell death in the ischemic cortex following tMCAO, leading to a reduced infarct size. Finally, this is the first study to show that a high soy diet increases both mRNA and protein expression of the anti-apoptotic gene bclxL in the ischemic cortex following tMCAO, suggesting a possible mechanism for soy-mediated neuroprotection.
Focal cerebral ischemia and reperfusion result in massive cell death in the core of the infarct. Neurons in the core of the infarct die by necrosis, while cells in the ischemic penumbra undergo programmed cell death, or apoptosis (Ferrer and Planas, 2003). Cells in the penumbra have reduced blood flow and may be exposed to deleterious factors produced by neighboring cells. In the hours and days following a stroke, the infarct area will expand as cells in the penumbra die by apoptosis. However, cells in the penumbra may be “rescued” by decreasing the amount of programmed cell death after ischemia, leading to a reduced infarct size (Rau et al., 2003, Wise et al., 2005). Estradiol attenuates stroke-related injury in animal models of ischemia, and several potential mechanisms have been proposed to account for estrogen’s neuroprotective effects (Alonso de Lecinana and Egido, 2006). Rao et al. concluded that estradiol protects the brain against ischemic injury by delaying and decreasing the extent of apoptosis over the course of 24hr following ischemia (Rau et al., 2003). Estrogen decreases TUNEL staining in the cortex after tMCAO (Fan et al., 2003), indicating decreased DNA fragmentation and apoptosis. Here, we demonstrate that a high soy diet also decreases DNA fragmentation after tMCAO, leading to a reduction in infarct size.
During apoptosis, intracellular activation of caspases in a cascade leads to degradation of cellular constituents and ultimately, cell death. Caspase-3 is believed to be the main executioner protease of apoptotic caspase (Rami, 2003). Caspase-3 exerts is effects by cleaving DNA and disabling DNA repair processes. There are some conflicting reports on whether caspase-3 is activated following ischemia in some rodent models (Zhao et al., 2003). However, in our tMCAO model, we observed active caspase-3 immunostaining in the ischemic cortex that was significantly reduced by a high soy diet. To further examine caspase activity, we measured the cleavage products of the cytoskeletal protein α-spectrin. Spectrin cleavage by caspase-3 leads to decreased cellular integrity (Davoli et al, 2002). Spectrin is also cleaved by calpain, a calcium-dependent protease that is widely distributed in neurons. Calpain and caspase-3 cleave spectrin at different sites once activated. The 120 kDa break-down product is caspase-3 mediated while the 150 kDa break down product is calpain mediated. Activation of calpain precedes that of caspase-3, LDH release, and DNA fragmentation (Zhang and Bhavnani, 2006). Estrogen decreases the caspase-mediated spectrin breakdown product 4 hr after MCAO in the ischemic cortex (Rau et al., 2003). Here, we show a similar reduction in caspase-mediated spectrin cleavage 22.5hr after tMCAO in the ischemic cortex in soy-fed rats. The increase in the calpain-mediated spectrin cleavage product suggests that soy is specifically downregulating caspase-3 mediated cell death.
While caspase-mediated cell death is important, it is not the only factor involved after ischemia. Indeed, inhibition of caspase-3 activity can delay, but not prevent, cell death in the hippocampus after transient global ischemia (Chen et al., 1998, Cao et al., 2003). The ubiquitous flavoprotein AIF has emerged as a caspase-independent factor that contributes to apoptosis following ischemia (Ferrer et al., 2003). Following induction of apoptosis, AIF translocates from the outer mitochondrial membrane to the nucleus, resulting in induction of nuclear chromatin condensation and large molecular weight DNA fragmentation in a caspase-independent manner (Daugas et al., 2000). Nuclear translocation of AIF has been shown to occur as early as 8 hr after ischemia (Zhao et al., 2004). Here, we demonstrate that rats fed a high soy diet have reduced nuclear translocation of AIF following tMCAO.
The bcl-2 family of proto-oncogenes encodes proteins that can either protect against or promote cell death. Anti-apoptotic members of the bcl-2 family (bcl-2 and bcl-xL) are associated with the mitochondrial outer membrane and can inhibit the release of cytochrome c into the cytosol, thereby inhibiting downstream caspase activation and free radical production (Ferrer and Planas, 2003). Also, bcl-2 and bcl-xL block the pro-apoptotic actions of other members of the bcl-2 family such as Bax and Bad (Merry and Korsmeyer, 1997). The anti-apoptotic function of bcl-2 is well demonstrated in models of cerebral ischemia (Zhao et al, 2003), and overexpression of bcl-2 leads to reduced infarct size (Martinou JC et al, 1994). Because bcl-2 is an estrogen-responsive gene and estrogen modulates bcl-2 expression in ischemic injury (Dubal et al., 1999), this has been suggested as one mechanism for estrogen-induced neuroprotection. Here, we compared the effect of a high soy diet on expression of bcl-2 and bcl-xL to that of estrogen. We have demonstrated that bcl-2 mRNA expression is decreased following tMCAO in control and high soy diet groups, but not in the rats treated with estradiol, similar to previously published data (Dubal et al., 1999, Won et al., 2006). Using IHC, we found that both bcl-xL and bcl-2 were localized to neurons and expression was increased in the ischemic hemisphere 22.5hr post-MCAO, consistent with previously published results (Alkayed et al., 2001). Using western blot analysis to compare protein expression in the ischemic cortex across treatment groups, we found that the ischemic cortex of SP rats had significantly more bcl-xL expression than the IFP group, whereas estrogen had no significant effect. In contrast, there was no significant difference in bcl-2 protein expression in the ischemic cortex among the groups. Therefore, a high soy diet appears to enhance the upregulation of bcl-xL in the ischemic cortex.
We suggest that soy-enhanced expression of bcl-xL is responsible for attenuating apoptosis following tMCAO, leading to reduced infarct size. Neuron-specific transgenic overexpression of bcl-xL in mice decreased lesion size after permanent MCAO (Wiessner et al., 1999). Postischemic infusion of a ginseng saponin that upregulates bcl-xL expression decreased infarct volume and prevented neuronal death in rats (Zhang et al., 2006a). Additionally, overexpression of bcl-xL protects neurons from acute ischemia-like stress in vitro (Panickar et al., 2005). Bcl-xL inhibits cytochrome c release and caspase activation induced by a variety of apoptotic insults in neurons and other cell types (Frankowski et al., 1995, Gonzalez-Garcia et al., 1995, Kharbanda et al., 1997, Shinoura et al., 2000). Indeed, bcl-xL is a potent inhibitor of AIF translocation (Cao et al., 2003). Therefore, bcl-xL potentially can prevent activation of both caspase-dependent and AIF dependent cell death pathways (Cao et al., 2003).
In conclusion, our data suggest that a high soy diet attenuates caspase-dependent and caspase-independent programmed cell death following tMCAO, leading to reduced infarct size. The induction of bcl-xL in the ischemic cortex may contribute to this soy-mediated neuroprotection. Induction of bcl-xL expression following tMCAO was not seen with estrogen in this study, suggesting that this effect may not be estrogenic. This is consistent with previous studies showing no effect of estradiol on bcl-xL expression following ischemia (Dubal et al., 1999, Won et al., 2006). While it remains to be determined whether soy is acting via estrogen receptors to exert anti-apoptotic effects, it appears that dietary soy may be a useful alternative to estrogen in protecting against stroke damage.
Acknowledgments
Sources of Support:
National Institutes of Health, National Center for Complementary and Alternative Medicine, R01AT001882 to D.A.S.; American Heart Association, Southeast Affiliate, AHA 0625626B to T.L.S.
List of Abbreviations
- MCAO
Middle cerebral artery occlusion
- tMCAO
transient middle cerebral artery occlusion
- IFP
isoflavone-reduced diet, placebo
- IFE
isoflavone-reduced diet, estrogen
- SP
high soy diet, placebo
- AIF
apoptosis inducing factor
- ER
estrogen receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkayed NJ, Goto S, Sugo N, Joh H-D, Klause J, Crain BJ, Bernard O, Traystman RJ, Hurn PD. Estrogen and Bcl-2: Gene induction and effect of transgene in experimental stroke. The Journal of Neuroscience. 2001;21:7543–7550. doi: 10.1523/JNEUROSCI.21-19-07543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso de Lecinana M, Egido JA. Estrogens as neuroprotectants against ischemic stroke. Cerebrovasc Dis. 2006;21 Suppl 2:48–53. doi: 10.1159/000091703. [DOI] [PubMed] [Google Scholar]
- Brass LM. Hormone replacement therapy and stroke: clinical trials review. Stroke. 2004;35:2644–2647. doi: 10.1161/01.STR.0000143218.20061.ac. [DOI] [PubMed] [Google Scholar]
- Burguete MC, Torregrosa G, Perez-Asensio FJ, Castello-Ruiz M, Salom JB, Gil JV, Alborch E. Dietary phytoestrogens improve stroke outcome after transient focal cerebral ischemia in rats. Eur J Neurosci. 2006;23:703–710. doi: 10.1111/j.1460-9568.2006.04599.x. [DOI] [PubMed] [Google Scholar]
- Cao G, Clark RS, Pei W, Yin W, Zhang F, Sun FY, Graham SH, Chen J. Translocation of apoptosis-inducing factor in vulnerable neurons after transient cerebral ischemia and in neuronal cultures after oxygen-glucose deprivation. J Cereb Blood Flow Metab. 2003;23:1137–1150. doi: 10.1097/01.WCB.0000087090.01171.E7. [DOI] [PubMed] [Google Scholar]
- Chen J, Nagayama T, Jin K, Stetler RA, Zhu RL, Graham SH, Simon RP. Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J Neurosci. 1998;18:4914–4928. doi: 10.1523/JNEUROSCI.18-13-04914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugas E, Nochy D, Ravagnan L, Loeffler M, Susin SA, Zamzami N, Kroemer G. Apoptosis-inducing factor (AIF): a ubiquitous mitochondrial oxidoreductase involved in apoptosis. FEBS Lett. 2000;476:118–123. doi: 10.1016/s0014-5793(00)01731-2. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. Journal of Neuroscience. 1999;19:6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T, Yang SH, Johnson E, Osteen B, Hayes R, Day AL, Simpkins JW. 17beta-Estradiol extends ischemic thresholds and exerts neuroprotective effects in cerebral subcortex against transient focal cerebral ischemia in rats. Brain Res. 2003;993:10–17. doi: 10.1016/j.brainres.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Friguls B, Dalfo E, Justicia C, Planas AM. Caspase-dependent and caspase-independent signalling of apoptosis in the penumbra following middle cerebral artery occlusion in the adult rat. Neuropathol Appl Neurobiol. 2003;29:472–481. doi: 10.1046/j.1365-2990.2003.00485.x. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol. 2003;62:329–339. doi: 10.1093/jnen/62.4.329. [DOI] [PubMed] [Google Scholar]
- Frankowski H, Missotten M, Fernandez PA, Martinou I, Michel P, Sadoul R, Martinou JC. Function and expression of the Bcl-x gene in the developing and adult nervous system. Neuroreport. 1995;6:1917–1921. doi: 10.1097/00001756-199510020-00023. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garcia M, Garcia I, Ding L, O’Shea S, Boise LH, Thompson CB, Nunez G. bcl-x is expressed in embryonic and postnatal neural tissues and functions to prevent neuronal cell death. Proc Natl Acad Sci U S A. 1995;92:4304–4308. doi: 10.1073/pnas.92.10.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield RH, Mendelow AD, Perry RH, Alvarez LM, Modha P. Triphenyltetrazolium chloride (TTC) as a marker for ischaemic changes in rat brain following permanent middle cerebral artery occlusion. Neuropathol Appl Neurobiol. 1991;17:61–67. doi: 10.1111/j.1365-2990.1991.tb00694.x. [DOI] [PubMed] [Google Scholar]
- Kharbanda S, Pandey P, Schofield L, Israels S, Roncinske R, Yoshida K, Bharti A, Yuan ZM, Saxena S, Weichselbaum R, Nalin C, Kufe D. Role for Bcl-xL as an inhibitor of cytosolic cytochrome C accumulation in DNA damage-induced apoptosis. Proc Natl Acad Sci U S A. 1997;94:6939–6942. doi: 10.1073/pnas.94.13.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzer MS. Phytoestrogen supplement use by women. J Nutr. 2003;133:1983S–1986S. doi: 10.1093/jn/133.6.1983S. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14:228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- Merry DE, Korsmeyer SJ. Bcl-2 gene family in the nervous system. Annu Rev Neurosci. 1997;20:245–267. doi: 10.1146/annurev.neuro.20.1.245. [DOI] [PubMed] [Google Scholar]
- Newton KM, Buist DS, Keenan NL, Anderson LA, LaCroix AZ. Use of alternative therapies for menopause symptoms: results of a population-based survey. Obstet Gynecol. 2002;100:18–25. doi: 10.1016/s0029-7844(02)02005-7. [DOI] [PubMed] [Google Scholar]
- Panickar KS, Nonner D, Barrett JN. Overexpression of Bcl-xl protects septal neurons from prolonged hypoglycemia and from acute ischemia-like stress. Neuroscience. 2005;135:73–80. doi: 10.1016/j.neuroscience.2005.02.052. [DOI] [PubMed] [Google Scholar]
- Rau SW, Dubal DB, Bottner M, Gerhold LM, Wise PM. Estradiol attenuates programmed cell death after stroke-like injury. J Neurosci. 2003;23:11420–11426. doi: 10.1523/JNEUROSCI.23-36-11420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. Jama. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Schreihofer DA, Do KD, Schreihofer AM. High-soy diet decreases infarct size after permanent middle cerebral artery occlusion in female rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R103–108. doi: 10.1152/ajpregu.00642.2004. [DOI] [PubMed] [Google Scholar]
- Setchell KD. Soy isoflavones--benefits and risks from nature’s selective estrogen receptor modulators (SERMs) J Am Coll Nutr. 2001;20:354S–362S. doi: 10.1080/07315724.2001.10719168. discussion 381S–383S. [DOI] [PubMed] [Google Scholar]
- Shinoura N, Koike H, Furitu T, Hashimoto M, Asai A, Kirino T, Hamada H. Adenovirus-mediated transfer of caspase-8 augments cell death in gliomas: implication for gene therapy. Hum Gene Ther. 2000;11:1123–1137. doi: 10.1089/10430340050015185. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Trieu VN, Uckun FM. Genistein is neuroprotective in murine models of familial amyotrophic lateral sclerosis and stroke. Biochem Biophys Res Commun. 1999;258:685–688. doi: 10.1006/bbrc.1999.0577. [DOI] [PubMed] [Google Scholar]
- Vaya J, Tamir S. The relation between the chemical structure of flavonoids and their estrogen-like activities. Curr Med Chem. 2004;11:1333–1343. doi: 10.2174/0929867043365251. [DOI] [PubMed] [Google Scholar]
- Wiessner C, Allegrini PR, Rupalla K, Sauer D, Oltersdorf T, McGregor AL, Bischoff S, Bottiger BW, van der Putten H. Neuron-specific transgene expression of Bcl-XL but not Bcl-2 genes reduced lesion size after permanent middle cerebral artery occlusion in mice. Neurosci Lett. 1999;268:119–122. doi: 10.1016/s0304-3940(99)00392-4. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Rau SW, Brown CM, Suzuki S. Are estrogens protective or risk factors in brain injury and neurodegeneration? Reevaluation after the Women’s health initiative. Endocr Rev. 2005;26:308–312. doi: 10.1210/er.2004-0014. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Wilson ME, Rau SW, Böttner M. Minreview: neuroprotective effects of estrogen - new insights into mechanisms of action. Endocrinology. 2001;142:969–973. doi: 10.1210/endo.142.3.8033. [DOI] [PubMed] [Google Scholar]
- Won CK, Kim MO, Koh PO. Estrogen modulates Bcl-2 family proteins in ischemic brain injury. J Vet Med Sci. 2006;68:277–280. doi: 10.1292/jvms.68.277. [DOI] [PubMed] [Google Scholar]
- Zhang B, Hata R, Zhu P, Sato K, Wen TC, Yang L, Fujita H, Mitsuda N, Tanaka J, Samukawa K, Maeda N, Sakanaka M. Prevention of ischemic neuronal death by intravenous infusion of a ginseng saponin, ginsenoside Rb(1), that upregulates Bcl-x(L) expression. J Cereb Blood Flow Metab. 2006a;26:708–721. doi: 10.1038/sj.jcbfm.9600225. [DOI] [PubMed] [Google Scholar]
- Zhang R, Xue YY, Lu SD, Wang Y, Zhang LM, Huang YL, Signore AP, Chen J, Sun FY. Bcl-2 enhances neurogenesis and inhibits apoptosis of newborn neurons in adult rat brain following a transient middle cerebral artery occlusion. Neurobiol Dis. 2006b;24:345–356. doi: 10.1016/j.nbd.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bhavnani BR. Glutamate-induced apoptosis in neuronal cells is mediated via caspase-dependent and independent mechanisms involving calpain and caspase-3 proteases as well as apoptosis inducing factor (AIF) and this process is inhibited by equine estrogens. BMC Neurosci. 2006;7:49. doi: 10.1186/1471-2202-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yenari MA, Cheng D, Barreto-Chang OL, Sapolsky RM, Steinberg GK. Bcl-2 transfection via herpes simplex virus blocks apoptosis-inducing factor translocation after focal ischemia in the rat. J Cereb Blood Flow Metab. 2004;24:681–692. doi: 10.1097/01.WCB.0000127161.89708.A5. [DOI] [PubMed] [Google Scholar]
- Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 2003;85:1026–1036. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]