Abstract
Background
Bcl-2 is a major regulator of neural plasticity and cellular resilience. A single nucleotide polymorphism (SNP) in the Bcl-2 gene, Bcl-2 rs956572, significantly modulates the expression of Bcl-2 protein and cellular vulnerability to apoptosis. We tested the hypothesis that this SNP would modulate gray matter (GM) volume in the limbic-cortical-striatal-pallidal-thalamic circuitry that plays major roles in mood regulation.
Methods
Forty-seven healthy subjects participated in this study (30 A carriers, 17 G homozygotes). Neuromorphometric differences between G homozygotes and A carriers were investigated using optimized voxel-based morphometry (VBM). Statistical significance was set at p <.05, corrected for multiple comparisons.
Results
A carriers showed less GM volume than G homozygotes in the left ventral striatum (pcorrected <.05).
Conclusions
Genetic variation in the Bcl-2 gene modulates GM volume in areas known to play key roles in the neurobiology of reward processes and emotion regulation and in the pathophysiology of mood disorders. Thus, the findings from the current study are noteworthy insofar as they converge with preclinical findings that Bcl-2 functions to enhance neuronal viability and might indirectly extend this evidence to humans.
Keywords: Accumbens, magnetic resonance imaging, neurogenetics, neuroplasticity, voxel-based morphometry (VBM)
Bcl-2 is a major antiapoptotic protein that inhibits apoptotic and necrotic cell death induced by a diverse set of adverse conditions (1). Bcl-2 also plays critical roles in neuronal morphogenesis and synaptic plasticity (2,3), and reduced Bcl-2 function is hypothesized to contribute to the impairment of cellular plasticity and resilience in patients with mood disorders (1).
A genetic variant in the rs956572 single nucleotide polymorphism (SNP) in the Bcl-2 gene has been recently associated with the risk for developing bipolar disorder (4); preclinical paradigms show that this SNP exerts functional effects on Bcl-2 expression, as the A homozygous genotype is associated with significantly lower Bcl-2 messenger RNA (mRNA) expression, ~ 50% lower Bcl-2 protein levels, and greater cellular sensitivity to stress-induced apoptosis (5). Increased vulnerability to apoptosis induced by physiological stressors might contribute to the pathophysiological mechanism underlying the reductions in regional cerebral volumes, neurons, and glia in patients with mood disorders (6).
Investigating the neural effects of Bcl-2 rs956572 SNP in healthy humans may elucidate the influence of this risk allele on normal brain development and morphology, without introducing the potentially confounding effects of diagnostic heterogeneity, illness progression, and previous treatment that are inherent in studying clinical samples. In this study, we investigate the effect of Bcl-2 rs956572 SNP on gray matter (GM) and white matter (WM) volumes in healthy humans using voxel-based morphometry (VBM). Voxel-based morphometry has been applied to investigate the morphometric effects of variation in other genes implicated in the pathophysiology of psychiatric disorders, including the serotonin transporter promoter region (7) and vascular endothelial growth factor gene polymorphisms (8). In the present study, we tested the hypothesis that subjects carrying the A allele would show decreased GM volume in structures of the limbic-cortical-striatal-pallidal-thalamic circuitry that plays major roles in mood regulation (9).
Methods and Materials
Subjects
Medically and neurologically healthy individuals (n = 47) ages 19 to 60 years with no personal or family history (in first-degree relatives) of a major psychiatric disorder participated. Subjects were recruited through advertisements posted in local newspapers and on the National Institutes of Health (NIH) Campus. Volunteers underwent psychiatric and medical evaluations in the outpatient psychiatry clinic of the NIH Clinical Center. Exclusion criteria included self-endorsed history of head trauma with loss of consciousness or followed by seizures, cognitive impairment, or personality change; substance abuse within 1 year; lifetime history of alcohol or drug dependence; and exposure to psychotropic drugs other than nicotine within 3 weeks. Subjects provided written consent as approved by the NIH Combined Neuroscience Institutional Review Board (IRB). Mental health status was evaluated by the Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Nonpatient Edition (SCID-I/NP) (10) and confirmed by an unstructured interview with a psychiatrist. The SCID-I/NP was administered by two research nurses who trained together with an interrater reliability coefficient of .87. Further exclusion criteria included major medical illnesses (i.e., conditions likely to affect brain anatomy and/or physiology), pregnancy, and structural brain abnormalities as assessed by a NIH Clinical Center neuroradiologist.
Genotype Analysis
The rs956572 SNP was genotyped using a modification of the 5′ nuclease (TaqMan, Applied Biosystems, Foster City, California) assay. Fluorophore-labeled polymerase chain reaction (PCR) primers were designed and verified by Applied Biosystems, based on the flanking sequence 5′-AAGTGCCTGGCAGCAAAGGCACTAA(A/G)ATTCAGTCATGACTCCCTCTCATTC-3′. This assay detects an A and a G allele, with frequencies of 42% and 58%, respectively, in persons of European ancestry (http://www.hapmap.org). Genotypes were scored using a clustering algorithm (courtesy by Sam Chen, Virginia Commonwealth University) that identifies data points that lie outside the genotype clusters, allowing manual review and recoding, which is done blind to phenotype. Clustering was consistently good for this SNP. Each plate of DNA samples contained negative (water blank) and positive (duplicate) control samples, and two plates were run twice. Negative control samples returned no genotypes, and all duplicates agreed exactly.
Magnetic Resonance Imaging
Neuromorphometric differences between G homozygotes (GG) and A carriers were investigated using VBM, a technique that allows investigation of inherent differences in GM and WM volume (11). Magnetic resonance imaging (MRI) scans were acquired on a General Electric 3-Tesla Signa scanner (Milwaukee, Wisconsin) using a T1-weighted gradient-echo pulse sequence (magnetization-prepared rapid acquisition gradient-echo [MP-RAGE]) optimized for GM/WM contrast (echo time [TE] = 2.1 msec, repetition time [TR] = 7.8 msec, prep time = 725 msec, flip angle = 6°, delay time = 1400 msec, voxel size = .85 mm × .85 mm × 1.2 mm).
Image Processing and Statistics
Data were analyzed using MATLAB 6.0 (MathWorks, Inc., Natick, Massachusetts; http://www.mathworks.com/products/matlab) and SPM2 (Wellcome Department of Cognitive Neurology, London, United Kingdom, http://www.fil.ion.ucl.ac.uk). Optimized VBM analysis (11) was run using the toolboxes implemented by the Structural Brain Mapping Group of the University of Jena (Jena, Germany, http://dbm.neuro.uni-jena.de/vbm). Images were first registered to a group-specific template and then extracted and segmented into GM, WM, and cerebrospinal fluid (CSF) images for each individual subject. The voxel resolution after normalization was 1 × 1 × 1 mm. As VBM segmentation is problematic in areas where the distinction between gray and white matter is less clear (e.g., thalamus, pallidum, cerebellum), segmented images were visually inspected to check the accuracy of the segmentation. To restore the prenormalization tissue volume, the voxel intensities were multiplied by the determinant of the spatial transformation matrix (modulation). Modulated GM and WM images were smoothed with a 9-mm full-width at half maximum (FWHM) kernel to compensate for the effects of interindividual anatomical variability. Loci where significant results were obtained were converted from Montreal Neurological Institute (MNI) coordinates to the stereotaxic array of Talairach and Tournoux (12) using a nonlinear transformation (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach).
Whole brain volumes were calculated by adding the GM and WM volumes.
An analysis of covariance (ANCOVA) model was designed using SPM2 software in which age, gender, and either total GM or WM volumes were used as covariates of no interest and Bcl-2 genotype was considered the factor of interest. The significance threshold was set at the voxel level p < .05 after applying the family-wise error correction for multiple comparisons (pcorrected ≤ .05).
Results
Demographics and Genotype
Of the 47 subjects, 30 were A carriers (mean age = 37.8 ± 11 years; 20 female subjects; 8 were AA genotype, 22 were AG; Hollingshead socioeconomic status [SES] score = 45 ± 11) and 17 were G homozygotes (mean age = 32.4 ± 12 years, 8 female subjects; Hollingshead SES score = 43 ± 8.3). Mean age, gender composition, and socioeconomic status did not differ significantly between groups (p > .1). Ethnic background did not differ across genotype groups (p > .1) (A carriers: 18 European American, 10 African American, 2 Asian/Pacific Islanders; G homozygotes: 10 European American, 7 African American).
Voxelwise Analysis of Whole Brain, GM, and WM
Whole brain volume did not differ across genotypes (1124.9 ± 96.25 mL and 1124.8 mL ± 123.97 mL for G homozygotes and A carriers, respectively).
A carriers showed less GM volume than G homozygotes in left ventral striatum. Peak voxel Z score was 5.20 (t = 6.24; pcorrected =.01; cluster size = 69 voxels) (Figure 1). At the peak voxel where the main effect of genotype was detected, the GM volume was 17.4% lower in A carriers than G homozygotes. Regional WM volume did not differ significantly between the two genotype groups.
Figure 1.
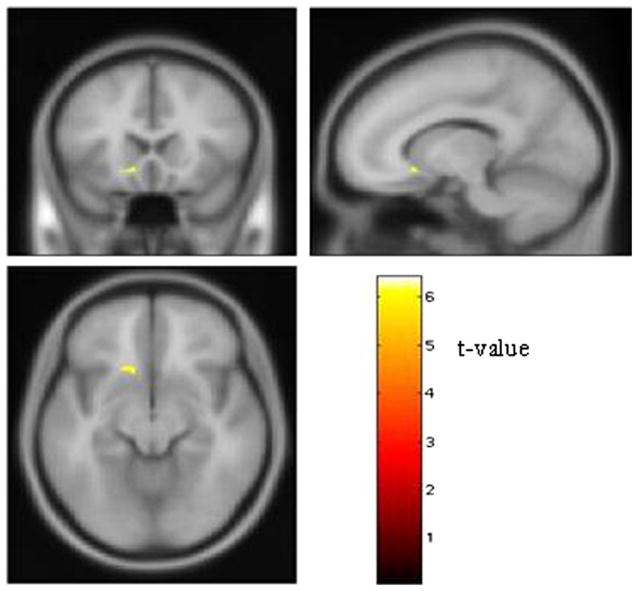
Effect of the Bcl-2 rs956572 SNP on left ventral striatal volume. Regions where GM volume was significantly larger in the G homozygous subjects (n = 17) than the A carriers (n = 30). The ventral striatal area where G homozygotes showed greater GM volume is shown on the canonical “avg152T1 template” provided with SPM2 software. The stereotaxic coordinates of the voxel containing the peak difference in GM volume localized to the anteroventral aspect of the accumbens area (x = −13, y = 20, z = −9); coordinates reflect the stereotaxic array of Talairach and Tournoux (12). Images thresholded at pcorrected < .05. GM, gray matter; SNP, single nucleotide polymorphism.
Discussion
In summary, we found that carriers of the A allele in the rs956572 Bcl-2 SNP showed lower GM volume in left ventral striatum than individuals with the G homozygous genotype. A secondary VBM analysis performed post hoc to compare the 8 A homozygotes versus the 17 G homozygotes showed similar results to those obtained in the whole group analysis, with the same peak voxel coordinates (t =6.39; pcorrected =.01; cluster size = 65 voxels).
The ventral striatum is known to play key roles in the neurobiology of reward processing and emotion regulation and in the pathophysiology of mood disorders (13). The ventral striatum receives afferent projections from the anterior cingulate cortex, hippocampus, and amygdala, which modulate cognitive and behavioral response patterns to reward-directed activities (14). The ventral striatal area affected specifically localized to the accumbens area, which plays crucial roles in anticipating and predicting rewards (13). The GM of this region has been shown to be reduced in bipolar disorder subjects studied postmortem (6).
The cellular and molecular determinants of the volumetric differences in the ventral striatum that we detected in A carriers versus G homozygotes remain unknown. Immunohistochemical studies in both nonhuman primates and humans provide evidence that Bcl-2 is expressed in this region (15,16). The preclinical data indicating that the A homozygote variant is associated with lower Bcl-2 mRNA and protein concentrations (5) suggest the hypothesis that decreased ventral striatal volume might reflect greater cellular sensitivity to stress-induced apoptosis and dendritic remodeling in A carrier variants (6).
Notably, stress-induced dendritic remodeling is hypothesized to underlie the GM reductions observed in humans with mood disorders (17), and lithium, which has mood-stabilizing effects in patients with bipolar disorder, upregulates Bcl-2 levels in areas of rodent striatum, frontal cortex, and hippocampus (18). This effect has been hypothesized to underlie lithium’s therapeutic mechanisms, as lithium treatment is associated with increased GM volume and N-acetyl aspartate levels in bipolar patients (19,20).
The findings from the current study thus are noteworthy insofar as they converge with preclinical evidence that Bcl-2 functions to enhance neuronal viability, by indicating that genetic variation in the Bcl-2 gene modulates GM volume in the human ventral striatum. Given the putative role of Bcl-2 in the pathophysiology of mood disorders and the molecular effects of mood stabilizers, future studies in larger sample sizes will need to address the brain structural effects of the rs956572 SNP or other variations in the Bcl-2 gene in patients with major depressive and bipolar disorders in the ventral striatum and other anatomically related areas, such as the anterior cingulate cortex, hippocampus, and amygdala.
Acknowledgments
This study was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health.
The author(s) declare that, except for income received from their primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- 1.Chen G, Manji HK. The extracellular signal-regulated kinase pathway: An emerging promising target for mood stabilizers. Curr Opin Psychiatry. 2006;19:313–323. doi: 10.1097/01.yco.0000218604.63463.cd. [DOI] [PubMed] [Google Scholar]
- 2.Chen DF, Schneider GE, Martinou JC, Tonegawa S. Bcl-2 promotes regeneration of severed axons in mammalian CNS. Nature. 1997;385:435–439. doi: 10.1038/385434a0. [DOI] [PubMed] [Google Scholar]
- 3.Jonas E. Bcl-xl regulates synaptic plasticity. Mol Interv. 2006;6:208–222. doi: 10.1124/mi.6.4.7. [DOI] [PubMed] [Google Scholar]
- 4.Manji HK. Bcl-2: A key regulator of affective resilience in the pathophysiology and treatment of severe mood disorders. Biol Psychiatry. 2008;63(suppl 1):243S. [Google Scholar]
- 5.Yuan P, Baum AE, Zhou R, Wang Y, Laje G, McMahon FJ, et al. Bcl-2 polymorphisms associated with mood disorders and antidepressant-responsiveness regulate Bcl-2 gene expression and cellular resilience in human lymphoblastoid cell lines. Biol Psychiatry. 2008;63(suppl 1):63S. [Google Scholar]
- 6.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frodl T, Koutsouleris N, Bottlender R, Born C, Jäger M, Mörgenthaler M, et al. Reduced gray matter brain volumes are associated with variants of the serotonin transporter gene in major depression. Mol Psychiatry. 2008;13:1093–1101. doi: 10.1038/mp.2008.62. [DOI] [PubMed] [Google Scholar]
- 8.Blumberg HP, Wang F, Chepenik LG, Kalmar JH, Edmiston E, Duman RS, et al. Influence of vascular endothelial growth factor variation on human hippocampus morphology. Biol Psychiatry. 2008;64:901–903. doi: 10.1016/j.biopsych.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamo-cortical circuits: Parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- 10.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition. New York: Biometrics Research Department, New York State Psychiatric Institute; 2002. [Google Scholar]
- 11.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 12.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System—An Approach to Cerebral Imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 13.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Grace AA, Floresco SB, Goto Y, Lodge D. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Fudge JL, Haber SN. Defining the caudal ventral striatum in primates: Cellular and histochemical features. J Neurosci. 2002;22:10078–10082. doi: 10.1523/JNEUROSCI.22-23-10078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vyas S, Javoy-Agid F, Herrero MT, Strada O, Boissiere F, Hibner U, et al. Expression of Bcl-2 in adult human brain regions with special reference to neurodegenerative disorders. J Neurochem. 1997;69:223–231. doi: 10.1046/j.1471-4159.1997.69010223.x. [DOI] [PubMed] [Google Scholar]
- 17.Glantz LA, Gilmore JH, Lieberman JA, Jarskog LF. Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophr Res. 2006;81:47–63. doi: 10.1016/j.schres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Chen G, Zeng WZ, Yuan PX, Huang LD, Jiang YM, Zhao ZH, et al. The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein Bcl-2 in the CNS. J Neurochem. 1999;72:879–882. doi: 10.1046/j.1471-4159.1999.720879.x. [DOI] [PubMed] [Google Scholar]
- 19.Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK. Lithium-induced increase in human brain grey matter. Lancet. 2000;356:1141–1142. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- 20.Moore GJ, Bebchuk JM, Hasanat K, Chen G, Seraji-Bozorgzad N, Wilds IB, et al. Lithium increases N-acetyl-aspartate in the human brain: In vivo evidence in support of Bcl-2’s neurotrophic effects? Biol Psychiatry. 2000;48:1–8. doi: 10.1016/s0006-3223(00)00252-3. [DOI] [PubMed] [Google Scholar]


