Introduction
Two families of viruses contribute to lymphomagenesis in humans: herpesviruses and retroviruses. The two herpesviruses that are well established as contributing to lymphomagenesis are Epstein-Barr virus (EBV) and Kaposi’s sarcoma-associated herpesvirus (KSHV) which is also referred to as human herpesvirus-8 (HHV-8). Human T-cell leukemia virus-1 (HTLV-1) is a retrovirus which is associated with adult T-cell leukemia/lymphoma. This article will focus on EBV associated malignancies.
EBV infects the majority of the world’s population. Infection usually occurs in childhood, but if exposure is delayed until adolescence, infection can result in symptomatic infectious mononucleosis. EBV has a unique set of growth activating genes which are used to establish a latent infection of B lymphocytes. The growth of B cells latently infected with EBV is normally controlled by the host immune response, particularly virus-specific T cells. The great majority of people carry latent EBV all their lives without any symptoms, but in certain circumstances latent EBV infection is associated wiht EBV-positive malignancies, which include Burkitt’s lymphoma, B-cell lymphoproliferative diseases, Hodgkin’s lymphoma (HL), and T-cell lymphomas.
Following primary EBV infection, individuals remain lifelong carriers of the virus. In vivo, B lymphocytes infected with the EBV are initially controlled by natural killer (NK) cells and cytotoxic T lymphocytes (CTL) [Hislop 2007]. However, the initial CTL response does not remove all the EBV-infected B cells and a pool of memory B cells latently infected with EBV becomes established. Of the nearly 100 viral genes that are expressed during replication, none are expressed in most EBV-infected circulating B cells in healthy person [Rickinson and Kieff 2007]. When EBV is reactivated from latently infected cells, several viral proteins are expressed and the infected cells are recognized by CTLs and destroyed. Thus a balance is established and maintained between viral reactivation and host immune surveillance (reviewed in [Cohen 2000]).
The heterogeneous group of malignancies associated with EBV infection express some or all of the EBV latent proteins (Table 1). This paper will discuss the mechanisms of EBV lymphomagenesis and the therapeutic options that are available for these malignancies.
Table 1.
Expression of EBV latent genes
| Latency Type |
EBER | EBNA-1 | EBNA-2 | EBNA-3 | LMP1 | LMP2 |
BARTS |
Condition |
|---|---|---|---|---|---|---|---|---|
| 1 | + | + | + | Burkitt’s lymphoma | ||||
| 2 | + | + | + | + | + | HL, T-cell, PEL | ||
| 3 | + | + | + | + | + | + | + | LPD, AIDS, Mono |
| 4 | + | +/− | ? | Healthy carrier |
EBV, Epstein-Barr virus; EBNA, EBV nuclear antigen; LMP, latent membrane protein; EBER, EBV-encoded RNA; BARTS, BamHI A rightward transcripts; HL, Hodgkin’s lymphoma; T-cell, T-cell lymphoma; PEL, primary effusion lymphoma; LPD, lymphoproliferative disease; AIDS, acquired immunodeficiency syndrome with LPD; Mono, infectious mononucleosis.
Malignancies associated with EBV
Patients with primary immunodeficiencies such as Wiskott-Aldrich syndrome, severe combined immunodeficiency (SCID) or X-linked lymphoproliferative disease are prone to develop EBV-related lymphoproliferative disorders (LPDs). However, EBV-related LPDs are more frequently seen in patients with secondary immunodeficiencies. These include patients with AIDS and organ transplant recipients who receive immunosuppressive therapy. Post-transplant lymphoproliferative disorder (PTLD) occurs in up to 15% of solid organ transplant (SOT) recipients and is also a complication of hematopoietic stem cell transplantation (HSCT). The incidence of PTLD is dependent on the organ transplanted, the type and intensity of immunosuppression, and the EBV immune status of the donor and recipient [Lim 2006; Gottschalk 2006]. PTLD typically occurs after prolonged and profound immunosuppression, which decreases the pool of EBV-specific CTLs, resulting in uncontrolled EBV-induced B-cell proliferation [Penn 1994].
EBV-positive lymphomas can be divided into those occurring in immunodeficient individuals, which are true virally driven lymphomas, such as PTLD and HIV-associated immunoblastic lymphoma, and those occurring in immunocompetent individuals. The latter group includes endemic and sporadic Burkitt’s lymphoma, and some T-and NK-cell malignancies. In these malignancies occurring in immunocompetent individuals, EBV is a cofactor rather than the driving influence [Khanna 2005].
Endemic Burkitt’s lymphoma is most common in equatorial Africa and chronic infection with malaria is a key cofactor in oncogenesis. The malarial parasite is thought to act as a chronic immune stimulus to B cells, increasing the number of cells actively proliferating. When B cells are allowed to proliferate freely there is an increased risk of accidental c-myc translocation. EBV infection and c-myc translocation appear to be independent changes that together drive the B cell to malignancy [Rickinson and Kieff 2007].
EBV associated nasophayngeal carcinoma and certain forms of gastric carcinoma occur in immunocompetent individuals, particularly in southern China. EBV-associated T-cell and NK-cell lymphomas are especially prevalent in Southeast Asia. HL occurs worldwide, although EBV-positive HL is more common in children from less developed countries than children in Europe and the United States. [Khanna 2005; Rickinson and Kieff 2007].
To summarize, EBV-associated malignancies arise in both immunosuppressed and immunocompetent individuals. The malignancies are of variable cellular origin, but all involve the expression of either some or all of the EBV latent proteins. Some EBV-associated malignancies involve specific genetic lesions (reviewed in [Khanna 2005]).
Mechanisms of EBV-driven oncogenesis
When EBV infects a B lymphocyte, the linear viral genome becomes circular, and the virus persists within the cell as an episome. The virus can express genes in two distinct programs, the lytic cycle or the latent cycle. During the lytic cycle, viral genes encoding proteins involved in viral DNA replication and viral particle synthesis are expressed. In contrast, only a limited set of genes are expressed during latent cycle infection; these include EBV nuclear antigens (EBNAs) 1, 2, 3A, 3B, 3C and LP, and three latent membrane proteins (LMPs), which are associated with transforming activity [Kieff and Rickinson 2007; Rickinson and Kieff 2007].
The switch from latent to lytic infection in host cells requires activation of two EBV immediate–early genes, BZLF1 and BRLF1, which are not expressed during the latent form of infection. BZLF1 and BRLF1 both encode transcription activators and together these proteins induce transcription of the entire lytic viral gene program [Kieff and Rickinson 2007].
EBV-associated malignant diseases can be divided into three patterns of latency depending on the viral genes expressed. In type I, only EBNA-1 and non-translated EBV-encoded RNAs (EBERs) and BamH1 A rightward transcripts (BARTs) are expressed (e.g. Burkitt’s lymphoma); in type II, EBNA-1, LMP1, LMP2, EBERs, and BARTs are expressed (e.g. HL and nasopharyngeal carcinoma); and in type III, all latency genes are expressed (e.g. PTLD) [Cohen 2000; Rickinson and Kieff 2007]]. Type III EBV-associated malignancies, which express more EBV proteins, are more immunogenic than type II, which in turn are more immunogenic than type I (Table 1). Identification of the EBV proteins expressed in or on B cells, and their signaling activities, is important as this information could provide new targets for treatment of virus-associated malignancies [Khanna 2005].
LMP1 is the major transforming protein of EBV: it behaves as a classic oncogene in rodent fibroblast transformation assays and is essential for EBV-induced B-cell transformation in vitro [Wang 1985; Kaye 1995]. The expression of LMP1 also induces many changes associated with B-cell activation, including cell clumping, and increased expression of CD23, CD39, CD40, CD44, as well as cell adhesion molecules. LMP1 also upregulates expression of the anti-apoptotic proteins Bcl-2 and A20, and stimulates cytokine production (interleukin [IL]-6 and IL-8) [Kulwichit 1998; Young and Murray 2003].
LMP1, which functions as a constitutively activated member of the tumor necrosis factor receptor (TNFR) superfamily, activates signaling pathways in a ligand-independent manner [Gires 1997]. LMP1 resembles CD40 and can partially substitute for CD40 in vivo, stimulating growth and differentiation responses in B cells [Uchida 1999]. The nuclear factor (NF)-κB signaling cascade is also activated by LMP1 [Thorley-Lawson 2001]. The other LMP, LMP2, prevents reactivation of EBV in latently infected cells by blocking tyrosine kinase phosphorylation [Miller 1995].
EBNA-1 is a nuclear phosphoprotein that binds to viral DNA and allows the EBV genome to be maintained in the B cell as an episome [Yates 1984].
EBNA-2 is a viral transcription factor that induces expression of LMP1 and LMP2, as well as cellular proteins that enhance the growth and transformation of B cells [Johannsen 1995]. The EBNA-3s also upregulate expression of cellular proteins [Chen 2006]. EBERs, which do not encode proteins, may be important for oncogenesis and resistance to apoptosis [Komano 1999].
Thus, latent EBV infection is maintained through several mechanisms. EBNA-1 supports the EBV genome as a circular episome, EBNA-2 stimulates B-cell proliferation through up-regulation of LMP1, and prevents reactivation from latency by upregulating the expression of LMP2. In addition, EBV proteins also have properties that help them avoid host immune responses. EBV has at least two mechanisms of suppressing apoptosis. During the lytic cycle, BHRF1 (an early lytic cycle antigen, which is a member of the Bcl-2 family) suppresses apoptosis and thus protects cells replicating EBV from apoptosis [Young 1999]. During latent infection, EBV-infected B cells are protected form apoptosis by LMP1. LMP1 is a CD40 mimic, which upregulates cellular Bcl-2, A20, and other proteins that inhibit apoptosis and LMP1 activates the NF-κB signaling pathway to simulate cell growth.
Therapeutic options for EBV-associated PTLD and non-Hodgkin’s lymphoma (NHL)
Treatment strategies for EBV-associated PTLD include reduction of immunosuppression, which is often successful in SOT, use of anti-B-cell monoclonal antibody, conventional chemotherapy, and radiation. Newer approaches include infusions of donor derived lymphocytes for PTLD in HSCT recipients, or infusions of HLA matched or autologous EBV-specific CTLs in SOT recipients. Current therapies for EBV-associated NHL include chemotherapy and radiation therapy; newer approaches include the use of EBV-specific CTLs and inducing virus replication followed by treatment with ganciclovir to kill virus-infected cells
Reducing immunosuppression in transplant recipients
PTLD in transplant recipients is often treated by reducing immunosuppressive therapy, and allowing restoration of the EBV-specific CTL response. Reducing immunosuppression is usually first-line therapy for PTLD after SOT. The efficacy of this approach is greater in early PTLD than in later disease and in seropositive than in seronegative patients [Armitage 1991]. However, reducing immunosuppression increases the risk of graft rejection, so treatments must be carefully planned and monitored.
Anti-B cell therapy in transplant recipients
Antibodies directed against B-cell antigens, such as CD20, can deplete EBV-infected B-cells. The anti-CD20 monoclonal antibody rituximab has been used in patients with PTLD after matched sibling HSCT, after autologous HSCT following intensive chemotherapy. In these cases, donor-derived T-cell therapy was not a therapeutic option. Rituximab has been used to treat PTLD after HSCT [Kuehnle 2000] and SOT [Milpied 2000; Savoldo 2005]. Although rituximab results in responses in up to 75% of SOT patients with PTLD, recurrent disease frequently occurs in patients treated with rituximab alone. In contrast to T-cell therapies, while rituximab depletes B-cells, it does not restore T-cell immunity. Rituximab treatment is also associated with an increased risk of infection, such as cytomegalovirus (CMV) infection [Lee 2007].
Chemotherapy and radiation therapy
Chemotherapy is frequently used for patients with PTLD or HIV associated lymphomas who do not respond to immune modulation (see below) or rituximab therapy. While several regimens have been used, a dose dose-adjusted regimen using combination therapy such EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) with or without rituximab and suspending antiretroviral therapy during chemotherapy has often been very effective in patients with HIV-associated lymphomas (Little 2003). Radiation is often used for PTLD or HIV associated lymphomas involving the central nervous system.
Chemoimmunotherapy with cyclophosphamide/prednisone plus rituximab has also shown promising results in patients with PTLD following SOT [Orjuela 2003].
Immune-based treatments for type III latency malignancies including PTLD
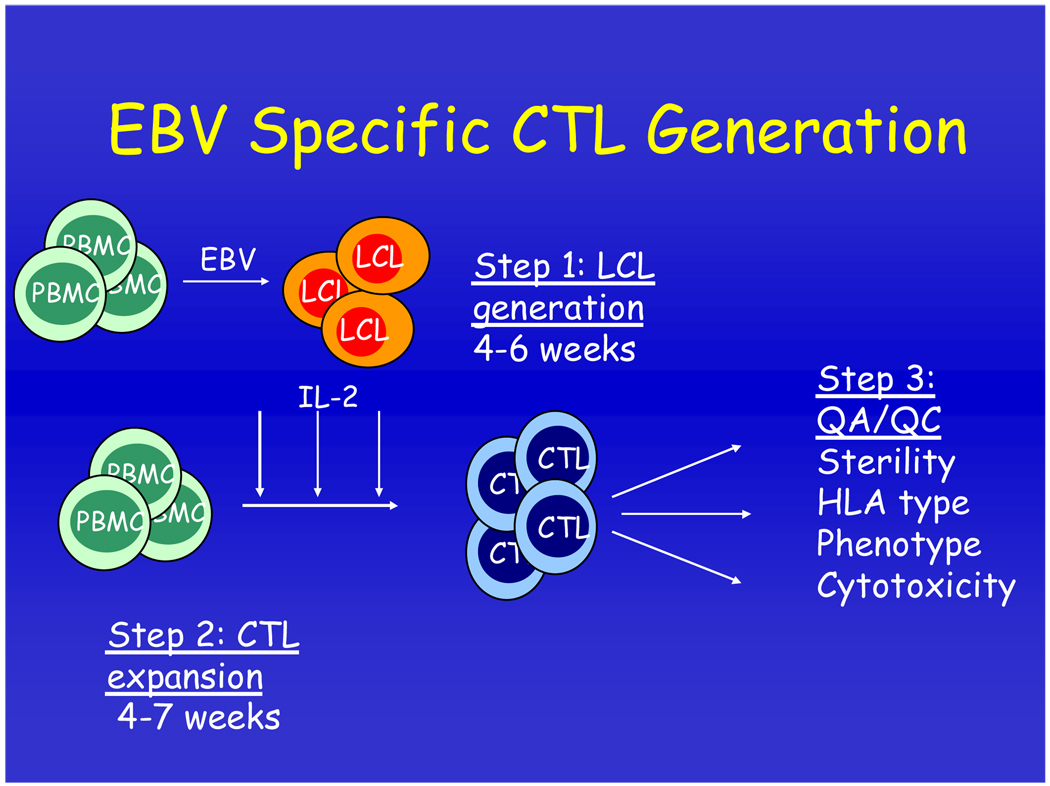
Early PTLD is characterized by an activated B-cell phenotype with high level expression of the immunodominant EBV latency proteins (reviewed in [Khanna 2005]); these virus-infected B cells are thus good targets for EBV-specific CTLs. EBV-infected LCLs generated by infecting normal peripheral blood cells with EBV can be used as stimulator cells to generate and expand EBV-specific CTLs ex vivo (Figure 1).
Figure 1. Generation of EBV-specific cytotoxic T cells.
Peripheral blood mononuclear cells (PBMCs) from donor blood are infected with a laboratory strain of EBV to set up a B-cell lymphoblastoid cell line (LCL). These cells show the same pattern of EBV gene expression as those derived from type III latency EBV malignancies such as post-transplant lymphoproliferative disease (PTLD). Irradiated LCLs are then used to stimulate another aliquot of PBMCs resulting in expanded EBV-specific cytotoxic T cells (CTLs).
Donor derived EBV-specific T cells have been adoptively transferred to bone marrow transplant recipients who had developed PTLD or were at high risk of developing PTLD. The adoptively transferred T cells promoted regression of established EBV-driven lymphomas, or prevented the development of tumors when administered prophylactically [Heslop 1996; Rooney 1998]. The study by Rooney et al. used a prophylactic approach in 39 patients who had received T cell depleted bone marrow transplants and were considered to be at high risk of EBV-induced lymphoma. Each patient received 2–4 intravenous infusions of donor-derived EBV-specific T lymphocytes. No PTLD occurred in treated patients, compared with 11% in historical controls [Rooney 1998].
EBV-specific T cells have also been transferred to patients who developed PTLD after SOT. Khanna et al. used autologous LCL-stimulated CTLs in a lung transplant recipient with PTLD who had regression of liver and lung lungs after treatment [Khanna 1999]. Haque et al. generated a bank of EBV-specific CTLs from healthy EBV-seropositive donors which were cryopreserved and subsequently infused into patients, based on the closest possible HLA match [Haque 2002]. In this study, 7 patients with established PTLD after SOT were treated. The outcomes were promising, with 3 complete remissions and 1 partial remission. However, these CTLs were cleared in 11–44 days, possibly due to the activity of alloreactive recipient T cells.
In another study, 10 SOT recipients at high risk for PTLD and 2 with preexisting PTLD received ex vivo expanded autologous CTL infusions, without toxicity [Savoldo 2006]. CTL injections were associated with a temporary increase in the plasma EBV load, suggesting lysis of EBV-infected cells. Interferon-gamma enzyme-linked immunospot assay and tetramer analysis showed an increase in the frequency of EBV-responsive T cells. None of the patients at risk for PTLD developed the disease, one patient with PTLD had a complete response, and one with PTLD had a partial response‥ However, this approach is highly labor-intensive as a specific autologous cell line has to be prepared for each patient [Savoldo 2006].
Although T-cell therapies have had some success, the ex vivo manipulations are time consuming, technically difficult and costly [Introna 2004]. Moreover, administering these treatments requires high-quality facilities concordant with good manufacturing practice and an advanced operational infrastructure.
Novel pharmacologic approaches
A potential therapeutic strategy would be to take advantage of the EBV genome in tumor cells and induce lytic EBV infection in tumor cells. This could be effected by targeting genes that switch the EBV-infected B cells from latent to lytic cycle, or by selectively activating latent viral genes.
EBV DNA encodes a thymidine kinase, but B cells latently infected with EBV do not express this viral transcript. As a result, these malignancies are resistant to the effect of nucleoside analogs, like ganciclovir, that can become incorporated in DNA resulting in growth arrest and apoptosis. However, lytic EBV gene expression can be induced in EBV-positive lymphoblastoid cell lines (LCLs) and in patient-derived tumor cell lines by argininine butyrate [Mentzer 1998]. In a recent study, Perrine et al. studied 15 patients with EBV-associated lymphoid malignancies. Fourteen of these patients had failed to respond to, or had become refractory to, aggressive combination chemotherapy and/or radiation therapy. Treatment of these patients with butyrate combined with the nucleoside analog ganciclovir effectively reduced or eliminated tumor growth in 10 of 15 patients [Perrine 2007]. Four patients achieved complete responses and 6 achieved partial responses.
Another potential pharmacologic strategy to treat virally driven lymphomas is the use of the proteasome inhibitor bortezomib, which is important in the intracellular degradation of proteins, including proteins involved in cell cycle regulation, transcription factor activation, apoptosis, and cell trafficking; notable among these is NF-κB [Paramore and Frantz 2003]. Bortezomib induces apoptosis of EBV-transformed LCLs in vitro by inducing cleavage of caspases 8 and 9 and by disrupting signaling through the NF-κB pathway [Zou 2007]. The dose of bortezomib (1 µM) that killed LCLs in vitro was equivalent to the peak plasma level achieved after a typical dose in humans. Burkitt’s lymphoma cells or EBV-negative B cells were less sensitive to bortezomib than EBV-transformed LCLs. Bortezomib also prolonged survival in mice inoculated with EBV-transformed B cells [Zou 2007].
Other drugs that inhibit the NF-κB pathway might also be used for PTLD. Simvastatin, which is used to control hypercholesterolemia and prevent cardiovascular disease, inhibits NF-κB activity. The drug also binds to leukocyte function antigen (LFA)-1 and inhibits LFA-1 activity including adhesion and co-stimulation of lymphocytes. High doses of simvastatin also induce apoptosis in LCLs in vitro and pretreatment of SCID mice with simvastatin prolongs survival after inoculation with LCLs and delays the development of EBV-associated lymphomas [Katano 2004]. Of note, the dose of simvastatin used in the mice results in plasma levels that are 4- to 8-fold higher than the mean plasma levels in humans after an 80 mg dose of the drug to treat hypercholesterolemia. These studies suggest that agents that inhibit the NF-κB pathway may have a role as adjuvant therapies for PTLD.
Hydroxyurea, can eradicate EBV episomes from virus-infected Burkitt lymphoma cells in vitro [Chodosh 1998]. In one report, two patients with AIDS who had central nervous system lymphomas had apparent responses to low dose hydroxyurea [Slobed].
Therapeutic options for immunocompetent patients with type II latency EBV-associated lymphomas
Chemotherapy is still the first choice for immunocompetent patients with EBV-associated Burkitt’s lymphoma, HL, NHL, or nasopharyngeal carcinoma [Gandhi 2004; Lin 2003]. These malignancies are relatively radiosensitive and chemosensitive in their early stages, but less so in later stages or following relapse
EBV-associated malignancies that develop in immunocompetent individuals present a challenge for immunotherapy as they do not express many of the immunodominant viral antigens. Burkitt’s lymphoma expresses only the EBNA-1 protein, and HL expresses EBNA-1, LMP1, and LMP2. LMP1 and LMP2 are less frequent targets of CD8+ T cells than the EBNA-3’s, while EBNA-1 is recognized by CD4 T cells (Hislop 2007). In addition, these malignancies possess a range of strategies to evade immune recognition.
Despite these obstacles, T cells specific for the weakly immunogenic LMP2 protein have been isolated and expanded from patients with EBV-associated lymphomas. After adoptive transfer, these LMP2-specific CTLs augmented T-cell responses, migrated to tumor deposits, and promoted tumor regression in a subset of patients with HL [Bollard 2004a]. The LMP2 CTLs persisted for up to 12 months in vivo and trafficked to tumor sites. Of 11 patients with measurable tumors, 2 had complete remissions, 1 a partial response, and 5 had stable disease after CTL therapy. Thus, the clinical responses were modest and only those with minimal residual disease showed a clinical response
In a follow-up study, gene transfer of LMP2 into antigen-presenting cells was used to markedly increase the frequency of CTLs that recognize LMP2. These LMP2 CTLs expanded and persisted in peripheral blood without adverse effects [Bollard 2007]. This study showed antitumor effects in 5 of 6 patients with active relapsed EBV-positive HL or NHL.
Another study generated LMP2A-specific CTLs from patients with relapsed HL [Bollard 2004b]. These patients had been heavily pretreated with cytotoxic agents, and therefore had reduced numbers of monocytes and lymphocytes, with some functional impairment. An adenoviral vector encoding LMP2 was used with patients’ peripheral blood to generate dendritic cells expressing LMP2. These dendritic cells were used to produce LMP2-specific CTLs, which in turn were then stimulated with LCLs overexpressing the same viral vector. As a result, large numbers of LMP2-specific CTLs were produced, including both CD4+ and CD8+ T cells, which would favor long-term persistence in vivo. In addition, the CTLs were specific for multiple LMP2 epitopes, thus minimizing the risk of expanding variants missing individual tumor antigens that can escape from CTL killing [Bollard 2004b].
Transplant recipients are also at risk of viral infections other than EBV, such as CMV and adenovirus. Traditionally these patients have been treated with antiviral drugs to prevent infections, but these drugs are expensive, have many toxic side effects, and need to be administered intravenously every day for several months. To give patients a better chance of recovery, Bollard and colleagues have recently reported the development of CTLs that could recognize all three viruses [Leen 2006]. An adenovirus was first engineered that produced the CMV protein pp65. This recombinant virus was then used to infect EBV-infected B cells, which in turn was used to stimulate the growth of CTLs. Overall, 14 CTL lines were developed from stem cell donors that responded to EBV, CMV, and adenoviruses. The CTLs were given to 11 immunocompromised individuals, who had undergone HSCT. None of the patients developed graft-versus-host-disease or other toxicities. All patients who showed signs of active CMV, EBV or adenoviral infection showed a relatively rapid reduction of disease symptoms. This coincided with the expansion of virus-specific CTLs [Leen 2006]. This was the first report of an immunotherapeutic approach targeting more than one virus type. Unlike antiviral drugs, which only control viruses, this immunotherapeutic treatment was able to improve the overall immune responses without generating toxic effects.
Conclusions
EBV is associated with a diverse range of malignancies. EBV often has a direct role in lymphomagenesis in patients with immunodeficiencies. EBV is a cofactor in oncogenesis in immunocompetent individuals. The presence of EBV in these malignancies offers prospects for therapeutic interventions such as adoptive immunotherapy, vaccination, or small molecules to target viral proteins or upregulate lytic antigens.
References
- Armitage JM, Kormos RL, Stuart RS, Fricker FJ, Griffith BP, Nalesnik M, Hardesty RL, Dummer JS. Posttransplant lymphoproliferative disease in thoracic organ transplant patients: ten years of cyclosporine-based immunosuppression. J Heart Lung Transplant. 1991;10:877–886. [PubMed] [Google Scholar]
- Bollard CM, Aguilar L, Straathof KC, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease. J Exp Med. 2004a;200:1623–1633. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollard CM, Gottschalk S, Leen AM, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110:2838–2845. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollard CM, Straathof KC, Huls MH, et al. The generation and characterization of LMP2-specific CTLs for use as adoptive transfer from patients with relapsed EBV-positive Hodgkin disease. J Immunother (1997) 2004b;27:317–327. doi: 10.1097/00002371-200407000-00008. [DOI] [PubMed] [Google Scholar]
- Chen A, Zhao B, Kieff E, Aster JC, Wang F. EBNA-3B-and EBNA-3C-regulated cellular genes in Epstein-Barr virus-immortalized lymphoblastoid cell lines. J Virol. 2006;80:10139–10150. doi: 10.1128/JVI.00854-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh J, Holder VP, Gan YJ, Belgaumi A, Sample J, Sixbey JW. Eradication of latent Epstein-Barr virus by hydroxyurea alters the growth-transformed cell phenotype. J Infect Dis. 1998;177:1194–1201. doi: 10.1086/515290. [DOI] [PubMed] [Google Scholar]
- Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000;343:481–492. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- Gandhi MK, Tellam JT, Khanna R. Epstein-Barr virus-associated Hodgkin's lymphoma. Br J Haematol. 2004;125:267–281. doi: 10.1111/j.1365-2141.2004.04902.x. [DOI] [PubMed] [Google Scholar]
- Gires O, Zimber-Strobl U, Gonnella R, et al. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J. 1997;16:6131–6140. doi: 10.1093/emboj/16.20.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk S, Rooney CM, Heslop HE. Post-transplant lymphoproliferative disorders. Annu Rev Med. 2005;56:29–44. doi: 10.1146/annurev.med.56.082103.104727. [DOI] [PubMed] [Google Scholar]
- Haque T, Wilkie GM, Taylor C, et al. Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet. 2002;360:436–442. doi: 10.1016/S0140-6736(02)09672-1. [DOI] [PubMed] [Google Scholar]
- Heslop HE, Ng CY, Li C, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- Introna M, Barbui AM, Golay J, et al. Innovative cell-based therapies in onco-hematology: what are the clinical facts ? Haematologica. 2004;89:1253–1260. [PubMed] [Google Scholar]
- Johannsen E, Koh E, Mosialos G, et al. Epstein-Barr virus nuclear protein 2 transactivation of the latent membrane protein 1 promoter is mediated by J kappa and PU.1. J Virol. 1995;69:253–262. doi: 10.1128/jvi.69.1.253-262.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano H, Pesnicak L, Cohen JI. Simvastatin induces apoptosis of Epstein-Barr virus (EBV)-transformed lymphoblastoid cell lines and delays development of EBV lymphomas. Proc Natl Acad Sci U S A. 2004;101:4960–4965. doi: 10.1073/pnas.0305149101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye KM, Izumi KM, Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci U S A. 1993;90:9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R, Bell S, Sherritt M, et al. Activation and adoptive transfer of Epstein-Barr virus-specific cytotoxic T cells in solid organ transplant patients with posttransplant lymphoproliferative disease. Proc Natl Acad Sci U S A. 1999;96:10391–10396. doi: 10.1073/pnas.96.18.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R, Moss D, Gandhi M. Technology insight: Applications of emerging immunotherapeutic strategies for Epstein-Barr virus-associated malignancies. Nat Clin Pract Oncol. 2005;2:138–149. doi: 10.1038/ncponc0107. [DOI] [PubMed] [Google Scholar]
- Kieff E, Rickinson AB. Epstein-Barr virus and its replication. In: Knipe DM, Howley PM, editors. Fields Virology. 5th ed. Lippincott: Williams and Wilkins; 2007. pp. 2603–2654. [Google Scholar]
- Komano J, Maruo S, Kurozumi K, et al. Oncogenic role of Epstein-Barr virus-encoded RNAs in Burkitt's lymphoma cell line Akata. J Virol. 1999;73:9827–9831. doi: 10.1128/jvi.73.12.9827-9831.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehnle I, Huls MH, Liu Z, et al. CD20 monoclonal antibody (rituximab) for therapy of Epstein-Barr virus lymphoma after hemopoietic stem-cell transplantation. Blood. 2000;95:1502–1505. [PubMed] [Google Scholar]
- Kulwichit W, Edwards RH, Davenport EM, et al. Expression of the Epstein-Barr virus latent membrane protein 1 induces B cell lymphoma in transgenic mice. Proc Natl Acad Sci U S A. 1998;95:11963–11968. doi: 10.1073/pnas.95.20.11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Chiou TJ, Hsiao LT, et al. Rituximab therapy increased post-transplant cytomegalovirus complications in Non-Hodgkin's lymphoma patients receiving autologous hematopoietic stem cell transplantation. Ann Hematol. 2007 doi: 10.1007/s00277-007-0397-0. [DOI] [PubMed] [Google Scholar]
- Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- Little RF, Pittaluga S, Grant N, Steinberg SM, Kavlick MF, Mitsuya H, Franchini G, Gutierrez M, Raffeld M, Jaffe ES, Shearer G, Yarchoan R, Wilson WH. Highly effective treatment of acquired immunodeficiency syndrome-related lymphoma with dose-adjusted EPOCH: impact of antiretroviral therapy suspension and tumor biology. Blood. 2003;101:4653–4659. doi: 10.1182/blood-2002-11-3589. [DOI] [PubMed] [Google Scholar]
- Lim WH, Russ GR, Coates PT. Review of Epstein-Barr virus and post-transplant lymphoproliferative disorder post-solid organ transplantation. Nephrology (Carlton) 2006;11:355–366. doi: 10.1111/j.1440-1797.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- Lin JC, Jan JS, Hsu CY, et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003;21:631–637. doi: 10.1200/JCO.2003.06.158. [DOI] [PubMed] [Google Scholar]
- Mentzer SJ, Fingeroth J, Reilly JJ, et al. Arginine butyrate-induced susceptibility to ganciclovir in an Epstein-Barr-virus-associated lymphoma. Blood Cells Mol Dis. 1998;24:114–123. doi: 10.1006/bcmd.1998.0178. [DOI] [PubMed] [Google Scholar]
- Miller CL, Burkhardt AL, Lee JH, et al. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity. 1995;2:155–166. doi: 10.1016/s1074-7613(95)80040-9. [DOI] [PubMed] [Google Scholar]
- Milpied N, Vasseur B, Parquet N, et al. Humanized anti-CD20 monoclonal antibody (Rituximab) in post transplant B-lymphoproliferative disorder: a retrospective analysis on 32 patients. Ann Oncol. 2000;11 Suppl 1:113–116. [PubMed] [Google Scholar]
- Orjuela M, Gross TG, Cheung YK, et al. A pilot study of chemoimmunotherapy (cyclophosphamide, prednisone, and rituximab) in patients with post-transplant lymphoproliferative disorder following solid organ transplantation. Clin Cancer Res. 2003;9:3945S–3952S. [PubMed] [Google Scholar]
- Paramore A, Frantz S. Bortezomib. Nat Rev Drug Discov. 2003;2:611–612. doi: 10.1038/nrd1159. [DOI] [PubMed] [Google Scholar]
- Penn I. The problem of cancer in organ transplant recipients: an overview. Transplant Sci. 1994;4:23–32. [PubMed] [Google Scholar]
- Perrine SP, Hermine O, Small T, et al. A phase 1/2 trial of arginine butyrate and ganciclovir in patients with Epstein-Barr virus-associated lymphoid malignancies. Blood. 2007;109:2571–2578. doi: 10.1182/blood-2006-01-024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickinson AB, Kieff E. Epstein-Barr virus. In: Knipe DM, Howley PM, editors. Fields Virology. 5th ed. Lippincott: Williams and Wilkins; 2007. pp. 2655–2700. [Google Scholar]
- Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- Savoldo B, Goss JA, Hammer MM, et al. Treatment of solid organ transplant recipients with autologous Epstein Barr virus-specific cytotoxic T lymphocytes (CTLs) Blood. 2006;108:2942–2949. doi: 10.1182/blood-2006-05-021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoldo B, Rooney CM, Quiros-Tejeira RE, et al. Cellular immunity to Epstein-Barr virus in liver transplant recipients treated with rituximab for post-transplant lymphoproliferative disease. Am J Transplant. 2005;5:566–572. doi: 10.1111/j.1600-6143.2004.00693.x. [DOI] [PubMed] [Google Scholar]
- Slobod KS, Taylor GH, Sandlund JT, Furth P, Helton KJ, Sixbey JW. Epstein-Barr virus-targeted therapy for AIDS-related primary lymphoma of the central nervous system. Lancet. 2000 Oct 28;356(9240):1493–1494. doi: 10.1016/S0140-6736(00)02879-8. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- Uchida J, Yasui T, Takaoka-Shichijo Y, et al. Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science. 1999;286:300–303. doi: 10.1126/science.286.5438.300. [DOI] [PubMed] [Google Scholar]
- Wang D, Liebowitz D, Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Yates J, Warren N, Reisman D, et al. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci U S A. 1984;81:3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LS, Dawson CW, Eliopoulos AG. Epstein-Barr virus and apoptosis: viral mimicry of cellular pathways. Biochem Soc Trans. 1999;27:807–812. doi: 10.1042/bst0270807. [DOI] [PubMed] [Google Scholar]
- Zou P, Kawada J, Pesnicak L, et al. Bortezomib induces apoptosis of Epstein-Barr virus (EBV)-transformed B cells and prolongs survival of mice inoculated with EBV-transformed B cells. J Virol. 2007;81:10029–10036. doi: 10.1128/JVI.02241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]