Summary
Collagens IV, XV and XVIII are major components of various basement membranes. In addition to the collagen-specific triple helix, these collagens are characterized by the presence of several non-collagenous domains. It is clear now that these ubiquitous collagen molecules are involved in more subtle and sophisticated functions than just the molecular architecture of basement membranes, particularly in the context of extracellular matrix degradation. Degradation of the basement membrane collagens occurs during numerous physiological and pathological processes such as embryonic development or tumorigenesis and generates collagen fragments. These fragments are involved in the regulation of functions differing from those of their original intact molecules. The non-collagenous C-terminal fragment NC1 of collagen IV, XV and XVIII have been recently highlighted in the literature because of their potential in reducing angiogenesis and tumorigenesis, but it is clear that their biological functions are not limited to these processes. Proteolytic release of soluble NC1 fragments stimulates migration, proliferation, apoptosis or survival of different cell types and suppresses various morphogenetic events.
Keywords: Basement membrane collagens, NC1 fragments, Angiogenesis, Morphogenesis
Introduction
The development of an extracellular matrix (ECM), a complex mesh of various proteins, was a crucial event in the emergence of multicellular organisms. Providing a mechanical support for the cells, the ECM also influences cell behavior. Its remodeling during physiological or pathological processes generates new signals, particularly between cells and the basement membranes (BMs). BMs are thin layers of specialized ECM associated closely with epithelial or endothelial cells, muscle fibers, adipocytes and peripheral nerves. Collagens type IV, XV and XVIII, together with laminins, nidogens, heparan sulfate proteoglycans (HSPG), fibulins, dystroglycan and other glycoproteins, are major constituents of BM (for a review, see Erickson and Couchman, 2000; Miosge, 2001; Schwarzbauer, 1999). Numerous mutations of the genes encoding these collagens are involved in human diseases (for a review, see Myllyharju and Kivirikko, 2001). Recently, the non-collagenous C-terminal domain (NC1), of these collagens has been the focus of numerous studies investigating its role in the molecular architecture of the BM as well as more subtle and sophisticated biological functions distinct from those of their original collagen, such as angiogenesis or branching morphogenesis.
Angiogenesis is a complex and invasive process by which neovascularization occurs in adults. Because of its crucial role in tumorigenesis, much effort has been focused on developing and identifying anti-angiogenic molecules in the past decade with the aim of producing potential medical treatments. A search for endogenous angiogenesis inhibitors led to the discovery of endostatin, which is a non-collagenous C-terminal fragment of collagen XVIII (O’Reilly et al., 1997). These data emphasized the role of collagen fragments in regulating cell behavior and increased the interest around their potential biological functions. To date, half of the endogenous angiogenesis inhibitors described are cryptic fragments issued from proteolysis of large proteins, including angiostatin, a fragment of plasminogen, or vasostatin, a fragment of calreticulin, and six of them derive from collagens (for a review, see Cao, 2001; Marneros and Olsen, 2001). Besides angiogenesis, some other functions have been described for collagen fragments (Table 1).
Table 1.
Biological functions of various collagen fragments
| Collagens | Fragments | Biological functions |
|---|---|---|
| Col I | Trimer carboxyl propeptide |
Chemotactic for endothelial cells (Palmieri et al., 2000) |
| Various fragments | Chemotactic for dermal fibroblasts and peripheral monocytes (Albini and Adelmann-Grill, 1985; Malone et al., 1991) |
|
| Col II | Various fragments | Inhibit collagen synthesis and MMP secretion by chondrocytes (Jennings et al., 2001) |
| C-propeptide or chondrocalcin |
Cartilage calcification (Alini et al., 1992; Van der Rest et al., 1986) | |
| Col IV | NC1 and 7S domains | Inhibit morphogenesis in Hydra vulgaris (Sarras et al., 1993) |
| NC1 domain | Promote axonal growth (Lein et al., 1991) | |
| α1 NC1, arresten | Anti-angiogenic (Colorado et al., 2000) | |
| α2 NC1, canstatin | Anti-angiogenic (Kamphaus et al., 2000; Petitclerc et al., 2000) | |
| α3 NC1, tumstatin | Anti-angiogenic (Maeshima et al., 2000; Petitclerc et al., 2000) | |
| α6 NC1 | Anti-angiogenic (Petitclerc et al., 2000) | |
| Col VIII | NC1 domain vastatin | Angiogenic (Xu et al., 2001) |
| Col XIV | N-terminal fragment | Chemotactic for neutrophils (Nakagawa et al., 1999) |
| Col XV | NC1 | Anti-angiogenic (Sasaki et al., 2000) |
| Restin | Anti-angiogenic (Ramchandran et al., 1999; Sasaki et al., 2000) | |
| Col XVIII | NC1 | Induce migration of neural and non-neural cells in C. elegans (Ackley et al., 2001) |
| Endostatin | Anti-angiogenic (O’Reilly et al., 1997) | |
| Inhibit branching morphogenesis (Karihaloo et al., 2001) |
Here, we focus on collagens IV, XV and XVIII because they have been recently highlighted in the literature. After a brief description of these collagens (for a review, see Olsen and Ninomiya, 1999; Sado et al., 1998), we discuss what is known about the biological functions of their NC1 fragments not only in angiogenesis but also in other processes. Indeed, although these fragments have become a focal point in tumor biology, it is clear that they are involved in other fundamental morphogenetic events.
Collagen type IV, XV and XVIII: genes, structure and physiological functions
Collagen type IV
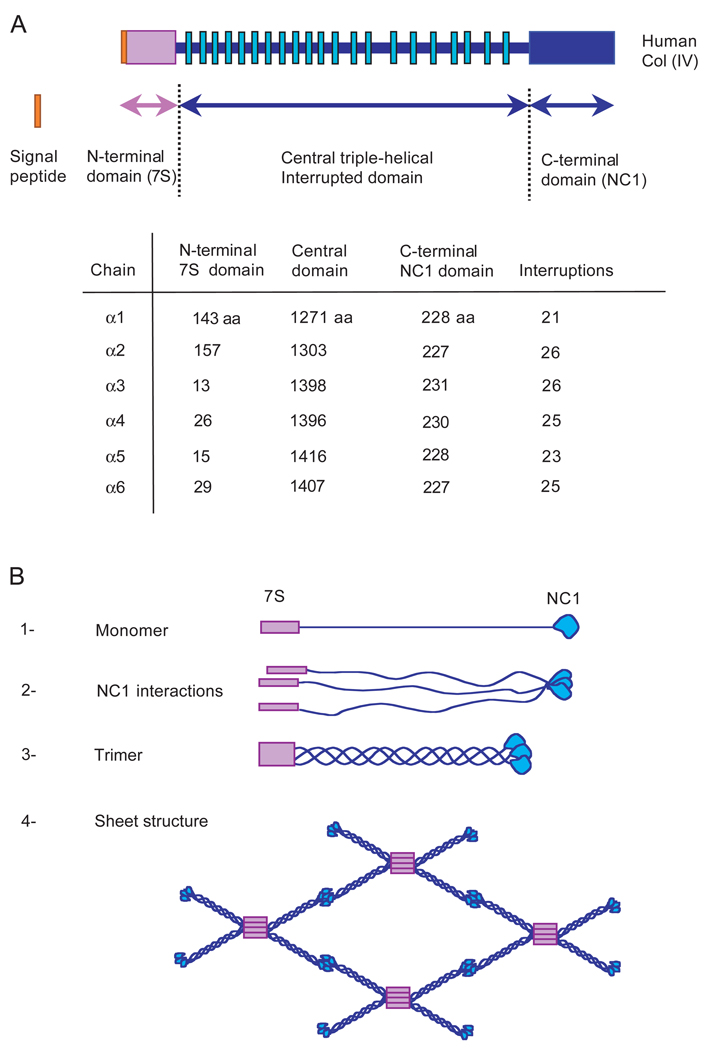
Collagen IV regulates cell adhesion and migration and is highly conserved in vertebrates and invertebrates in terms of both its structure and its functional role in BM architecture (Blumberg et al., 1987; Netzer et al., 1998; Sarras et al., 1993). Six different polypeptides, α1 to α6, encoded by three sets of genes form collagen IV heterotrimers† (Filie et al., 1995; Momota et al., 1998; Soininen et al., 1988; Sugimoto et al., 1994).
Each α-chain is composed of three domains, a cysteine-rich N-terminal 7S domain, a central triple-helical domain and a globular C-terminal non-collagenous NC1 domain (Fig. 1A). The NC1 domain is involved in the assembly of α-chains to form the heterotrimers and the 7S domain is involved in the covalent assembly of four heterotrimers in a spider-shaped structure (Boutaud et al., 2000; Dolz et al., 1988; Tsilibary et al., 1990). Thus, collagen IV forms a complex branching network that serves as a scaffold for the BM (Yurchenco et al., 1987) (Fig. 1B). Although α1 and α2 chains are widely expressed and colocalize in numerous tissues, there is a temporal and spatial regulation of α3, α4, α5 and α6 expression in physiological as well as pathological processes (Dehan et al., 1997; Fleischmajer et al., 1997; Miner and Sanes, 1994; Shen et al., 1990; Tanaka et al., 1997).
Fig. 1.
(A) Linear structure of human collagen IV α chains. Six different genes encode collagen IV α chains; each polypeptide is composed of three distinct domains: a cysteine-rich N-terminal 7S domain, a central triple-helical domain with multiple small interruptions (green boxes) and a globular C-terminal non-collagenous NC1 domain. The NC1 and central triple-helical domains are of an equivalent size, whereas the 7S domains are shorter in the case of α3, α4, α5 and α6 compared with α1 and α2. On the basis of sequence homology these different chains can be divided in two groups, the α1-like (α1, α3, α5) and the α2-like (α2, α4, α6). Assembly of collagen IV α chains. (B) The assembly of trimers is dependent first on the association of the NC1 domains, then the triple-helical structure forms and 7S domains are covalently associated. Four trimers interact through their 7S domains in a spider-shaped structure, and two trimers interact head to head through their NC1 domains, forming a sheet structure. Several trimers can also lace together along their triple-helical domain, thickening the structure (Timpl et al., 1981).
Several human genetic diseases have provided insights into the physiological role of collagen IV. Mutations in Col4a5, Col4a3 and Col4a4 are involved in Alport syndrome, which is characterized by a defective glomerular BM and the subsequent development of glomerulonephritis (Mochizuki et al., 1994), whereas some deletions spanning the 5′ regions of the Col4a5/Col4a6 cluster have been associated with Alport syndrome and diffuse leiomyomatosis, a benign smooth muscle tumor (Heidet et al., 1997; Zhou et al., 1993). The C-terminal region of the α3 chain has been identified as an autoantigen involved in Goodpasture syndrome, an immune disease characterized by glomerulonephritis and pulmonary hemorrhage (for a review, see Hudson et al., 1993; Kalluri, 1999). Mouse models for autosomal Alport syndrome have been developed and phenocopy human disease (Cosgrove et al., 1996; Cosgrove et al., 1998; Lu et al., 1999; Miner and Sanes, 1996).
The existence of different α chains for collagen IV and their restricted tissue distribution determine the structural and functional specificity of BM. Moreover, the extremely high conservation of this molecule from lowest metazoans up to vertebrates identifies collagen IV as a key regulator of morphogenesis that is critical for the regulation of adhesion, migration and survival of different cell types.
Collagen type XV and XVIII
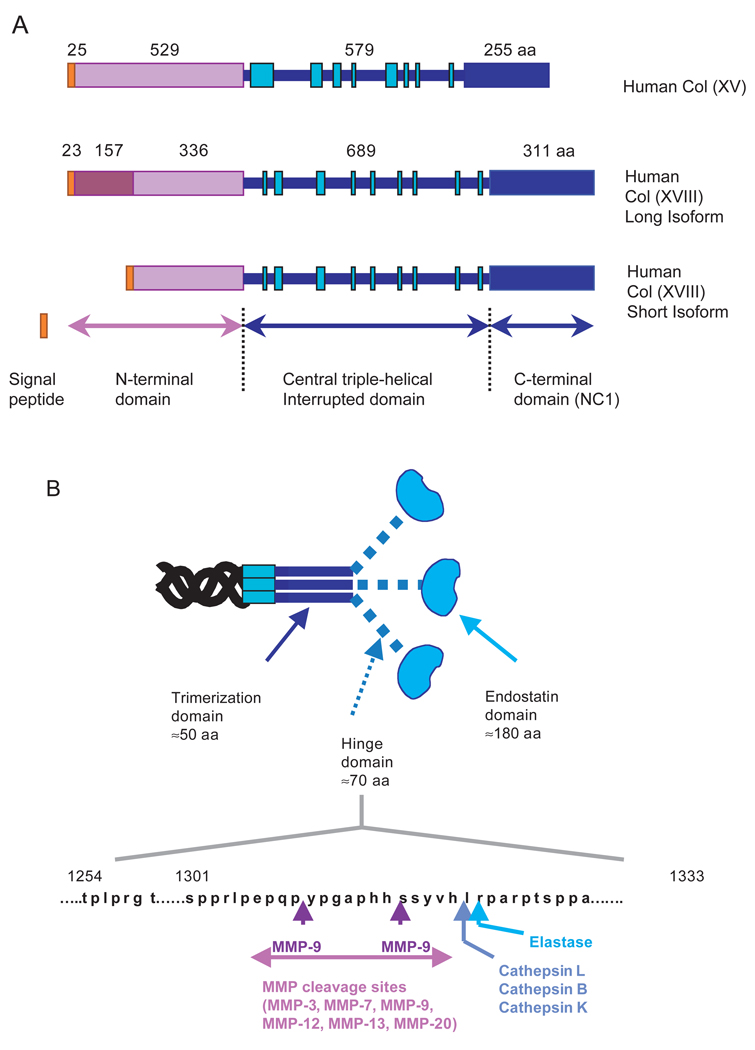
Collagens type XV and XVIII, identified as a chondroitin sulfate and heparan sulfate proteoglycan, respectively (Halfter et al., 1998; Li et al., 2000), are closely related non-fibrillar collagens that define the multiplexin subfamily (multiple triple helix domains with interruptions) (Abe et al., 1993; Oh et al., 1994a; Oh et al., 1994b; Rehn and Pihlajaniemi, 1994; Rehn et al., 1994). α1 (XV) and α1 (XVIII) chains organize as homotrimers. Each chain is divided in three subdomains that, as in collagen IV, include a C-terminal NC1 domain‡ (Fig. 2A). The genes encoding α1 chains of collagens XV and XVIII have been cloned and mapped to human chromosomes 9 and 21 and mice chromosomes 4 and 10, respectively (Hagg et al., 1997a; Huebner et al., 1992; Oh et al., 1994a).
Fig. 2.
(A) Linear structure of human collagen XV and XVIII α1 chains. The α1 chains of collagen XV and XVIII are structurally homologous; they define a new collagen subfamily, the multiplexin family, on the basis of their central triple-helical domain with multiple long interruptions (green boxes). They are also characterized by a long non-collagenous N-terminal-domain-containing Thrombospondin sequence motif with two splicing variants in human collagen XVIII and a long non-collagenous globular C-terminal domain or NC1 domain. (B) Functional sub-domains of human NC1(XVIII) and protease cleavage sites. The NC1 domain contains three functionally different subdomains: these domains consist of a N-terminal non-covalent trimerization domain necessary for the association of trimers, a hinge domain containing multiple sites sensitive to different proteases and an endostatin globular domain covering a fragment of 20 kDa with anti-angiogenic and anti-branching morphogenesis activities. Numerous enzymes can generate fragments containing endostatin. Cathepsin L and elastase are the most efficient, but in contrast to MMP cleavage leading to accumulation of endostatin, cathepsin L and B degrade the molecule [cleavage sites are indicated according to the data published by Ferreras (Ferreras et al., 2000)].
Collagen XV is highly expressed in heart, skeletal muscles and the placenta and moderately expressed in the adrenal gland, kidney and pancreas (Kivirikko et al., 1995; Muragaki et al., 1994). Its expression is associated with vascular, neuronal, mesenchymal and some epithelial BM, indicating a probable function in adhesion between BM and the underlying connective tissue stroma (Myers et al., 1996). Highly regulated during kidney, heart and lung development in the embryo, collagen XV colocalizes with collagen IV and is a component of continuous or fenestrated capillary BM, with the exception of the blood-brain barrier and liver and spleen sinusoids (Hagg et al., 1997b; Muona et al., 2002). For collagen XVIII several splicing variants exist: two isoforms have been described in humans (Saarela et al., 1998a) and three in mice (Rehn and Pihlajaniemi, 1995). Interestingly, these variants have specific expression patterns. The short isoform is expressed in various organs, whereas the long isoform is more specifically expressed in liver sinusoids and hepatocytes (Musso et al., 2001; Rehn et al., 1994; Saarela et al., 1998a; Saarela et al., 1998b). Transcription of collagen XVIII also occurs during adipogenesis (Inoue-Murayama et al., 2000) and a strong expression is observed in developing and post-natal eyes in various BM, except Descemet’s membrane (Fukai et al., 2002).
The physiological roles of collagens XV and XVIII are not well understood. Mice lacking collagen XV show a higher sensitivity to exercise-induced muscle injury and progressive degeneration of skeletal muscles with collapsed capillaries and endothelial cell degeneration. Thus collagen XV might be involved in the survival and stabilization of muscle fibers and endothelial cells on the subjacent BM (Eklund et al., 2001). For collagen XVIII, a mutation affecting the short isoform has been recently associated with Knobloch syndrome, an autosomal recessive disorder characterized by high myopia, vitreoretinal degeneration with retinal detachment, macular abnormalities and occipital defects (Sertie et al., 2000). Interestingly, mice lacking collagen XVIII develop the same ocular abnormalities (Fukai et al., 2002). Thus, collagens XV and XVIII regulate critical functions within specialized BMs.
Structure, localization and receptors for C-terminal NC1 fragments of collagen IV, XV and XVIII
The NC1 domains of collagens IV, XV and XVIII have been isolated from invertebrate and vertebrate BM and identified as circulating fragments in serum. Collagen XV and XVIII share similar structures within their NC1 domains, which are organized into three different subdomains (Fig. 2B) (Sasaki et al., 1998; Sazaki et al., 2000). Endostatin, the C-terminal subdomain of NC1 (XVIII), was the first endogenous collagen fragment characterized with anti-angiogenic properties (O’Reilly et al., 1997). On the basis of its high degree of sequence similarity with collagen XVIII, the C-terminal subdomain of NC1 (XV), named restin (Ramchandran et al., 1999) or endostatin-like§ (Sasaki et al., 2000), was also identified as an endogenous angiogenesis inhibitor.
For collagen IV, the NC1 domains from α1, α2 and α3 chains have also been identified as inhibitors of angiogenesis and named arresten, canstatin and tumstatin, respectively (Colorado et al., 2000; Kamphaus et al., 2000; Maeshima et al., 2000b). These NC1 domains have been implicated in the self-association of the heterotrimers (Boutaud et al., 2000; Timpl and Brown, 1996; Tsilibary et al., 1990). The recent crystal structure of the collagen IV NC1 domain showed that NC1 monomers fold into a novel tertiary structure comprising β-strands and two homologous subdomains, N and C. The trimers are assembled through unique three-dimensional domain swapping (Sundaramoorthy et al., 2002), and two trimers can be stabilized head to head by an uncharacterized covalent crosslink (Than et al., 2002).
The crystal structure of endostatin revealed a compact fold with a zinc-binding site and an extensive basic patch of 11 arginine residues, which explains the high affinity of endostatin for heparin. The overall structure of the endostatin domain is related to the C-type lectin carbohydrate-recognition domain, and the domains are present as dimers in the crystals (Ding et al., 1998; Hohenester et al., 1998; Hohenester et al., 2000). The structure of endostatin-like is very similar to that of endostatin (60% sequence identity) but lacks the zinc and heparin-binding sites (Sasaki et al., 2000).
Endostatin and endostatin-like fragments colocalize with collagen XV and XVIII in the BM of numerous organs, with the exception of the liver sinusoids, where endostatin-like but not collagen type XV staining is present (Miosge et al., 1999; Sasaki et al., 2000; Tomono et al., 2002).
What are the receptors for these fragments? Numerous integrins have been identified as major cellular receptors for NC1 fragments (Table 2). For endostatin two cell surface binding sites with Kd values of 18 pM and 200 pM have been described. The low-affinity receptor corresponds to glypicans, whereas the high-affinity receptor has not yet been identified. These receptors are not specific to endothelial cells; they are also present on epithelial cells (Karumanchi et al., 2001). Recently it has been shown that endostatin binds to VEGF-R2, a receptor involved in proliferation of endothelial cells. Besides integrins, two other types of receptors have been described for collagens, glycoprotein VI in platelets and discoidin domain receptors (DDR) in various cell types (for a review, see Vogel, 1999). But whether or not these interactions occur through NC1 domains of collagen IV, XV and XVIII is not known. Furthermore, several ECM proteins interact with these fragments (Table 2).
Table 2.
Interaction of specific receptors and other proteins with NC1 fragments of collagens IV, XV and XVIII
| Protein interactions | NC1 fragments |
|---|---|
| Receptors | |
| Integrins | |
| α1β1 and α2β1 | All NC1 (IV) fragments |
| Α5β1, αvβ5 and αvβ3 | Endostatin (Rehn et al., 2001) |
| α6β1 and αvβ3 | α3NC1(IV) or Tumstatin (Maeshima et al., 2000b) |
| α3β1 and αvβ3 | Canstatin (Kamphaus et al., 2000) |
| Glypican3 | Endostatin (Karumanchi et al., 2001) |
| VEGF-R2 | Endostatin (Kim et al., 2002) |
| ECM proteins | |
| Fibulin1, fibulin2, nidogen2 |
Endostatin and endostatin-like (Sasaki et al., 2000) |
| Perlecan, laminin1 | NC1(XV) and NC1(XVIII) (Sasaki et al., 2000) |
| MMP-2 | Endostatin (Kim et al., 2000; Lee et al., 2002) |
| Intracellular proteins | |
| Tropomyosin | Endostatin (MacDonald et al., 2001) |
Taken together, these data suggest that NC1 fragments might be involved not only in the regulation of angiogenesis but also in other morphogenetic processes.
Proteolytic pathways generating NC1 fragments
For collagen IV the proteolytic pathways involved in generation of NC1 fragments are not clear, even though different fragments are detected in the serum. For collagens XV and XVIII, several different NC1 fragments have been extracted from tissue homogenates, and circulating forms have been isolated from human blood filtrates, suggesting that these fragments exist as physiological cleavage products (John et al., 1999; Sasaki et al., 1998; Standker et al., 1997). Endostatin originally was purified from conditioned medium of a murine hemangioendothelioma cell line (EOMA) as a 20 kDa fragment that binds to heparin (O’Reilly et al., 1997). In vitro studies with the same cell line have shown that the NC1 hinge domain contains cleavage sites for matrix metalloproteinases (MMPs) and cathepsin L. MMPs generate fragments of 30 kDa containing the endostatin domain, whereas cathepsin L directly and specifically releases the 20 kDa endostatin domain (Felbor et al., 2000). Other in vitro studies confirmed these results and shown that generation of endostatin is also mediated by elastase after a first processing of NC1 by MMPs (Ferreras et al., 2000; Lin et al., 2001; Wen et al., 1999). In corneal epithelial cells, MMP-7 generates a 28 kDa NC1 (collagen XVIII) fragment (Lin et al., 2001). Thus, several distinct proteolytic pathways may be involved in the generation of endostatin in various tissues. Although we have some clues for in vitro processing, the physiological pathways still need to be more extensively investigated (Fig. 2B).
Biological functions of NC1 fragments and morphogenesis
Collagens IV, XV and XVIII, which are ubiquitously present in vascular and epithelial BM, are well conserved among different phyla. Interestingly, the biological functions of the NC1 domain also seem to be conserved throughout evolution. In the primitive invertebrate Hydra vulgaris, addition of NC1 (IV) alters morphogenesis, blocking cell aggregate development (Sarras et al., 1993; Zhang et al., 1994). In vitro, NC1 (IV) promotes axonal but not dendritic growth in sympathetic neurons from rat embryos (Lein et al., 1991), and hexameric NC1 supports attachment and migration of chicken neural crest cells. By contrast, intact dimers of collagen IV do not (Perris et al., 1993). These results imply that biological functions supported by NC1 domains are conformation dependent; proteolytic digestion of collagen IV may induce appearance of cryptic sites involved in new signals between cells and BMs.
A collagen homologous to type XV–XVIII collagens has been identified in C. elegans. Deletion of the NC1-encoding region of this gene (cle-1) causes defects in axon guidance and migration of neural and non-neural cells. This phenotype can be rescued by ectopic expression of NC1, but not endostatin (Ackley et al., 2001). These functional differences could be explained by NC1 being trimeric, whereas endostatin is monomeric. In analogous data, NC1 (XVIII) inhibits endothelial tube formation in Matrigel and stimulates cell motility of endothelial and non-endothelial cells. Monomeric endostatin has no effect by itself, but blocks the migration induced by NC1. The artificial oligomerization of endostatin stimulates cell motility (Kuo et al., 2001), indicating that proteolytic processes may regulate endogenous NC1 functions. These effects were not observed with the NC1 (XV) and endostatin-like domain. This lack of activity may not be surprising, since these molecules may have different spectra of activity depending on the identity of the motility signals.
More recently, murine endostatin has been shown to inhibit HGF-induced migration and branching morphogenesis of renal epithelial cells and the ureteric bud. These processes are dependent on the presence of glypican3. Ureteric bud expresses endostatin, and addition of neutralizing anti-endostatin antibodies enhances ureteric bud outgrowth and branching (Karihaloo et al., 2001).
Thus, high levels of endostatin or endostatin-like molecule may interfere with different pathways, downregulating morphogenetic processes. They may act as dominant-negative ligands that interact with the same receptors as their native molecule and, thus, inhibit proliferation and migration, or they may interact with different receptors and induce apoptosis. They may also have a mechanical effect in interacting with the original collagen trimers and disrupt them, leading to the loss of anchors between BMs and cells. Differential degradation of NC1 thus constitutes a negative feedback loop.
Biological functions of NC1 fragments in angiogenesis and tumorigenesis
Tumstatin and other collagen IV NC1 fragments
Biological functions of collagen IV NC1 fragments have recently been extensively studied in mammals. A synthetic peptide encompassing residues 183–205 of α3 chain NC1 domain and containing an SNS triplet unique to α3 specifically inhibits activation of polymorphonuclear leukocytes (Monboisse et al., 1994). This peptide binds to a CD47/αvβ3 integrin complex, promotes adhesion and chemotaxis and inhibits proliferation of various human cancer cell lines (Han et al., 1997; Shahan et al., 1999a; Shahan et al., 1999b; Shahan et al., 2000). Indeed, direct interaction between α3NC1 and β3 integrin, independently of CD47, stimulates focal adhesion kinase (FAK) and PI 3-kinase phosphorylation (Pasco et al., 2000a). Furthermore, the inhibition of migration observed with melanoma and fibrosarcoma cells on native collagen IV or on the 185–205 α3NC1 peptide correlates with a decrease in expression of MT1-MMP and the β3 integrin subunit and a decrease in the level of activated membrane-bound MMP-2 (Pasco et al., 2000b). MMP-2 is involved in tumor progression and metastasis and its activation depends on MT1-MMP/TIMP-2 complexes (Itoh et al., 1998; Kinoshita et al., 1998). In vitro, α3NC1 fragment decreases the expression of MT1-MMP in a bronchial tumor cell line and the 185–205 α3NC1 peptide inhibits their invasion through [α1(IV)]2 α2(IV) collagen (Martinella-Catusse et al., 2001). Altogether, these data indicate that the ability of collagen IV to inhibit proliferation and regulate cellular adhesion and motility resides in the NC1 domain. The α3(IV) chain has a specific interaction with invasive cancer cells, and, in contrast to α1 and α2 chains, which favor migration, α3 limits the invasive phenotype. Thus, in the context of tumor progression and metastasis, the presence of either the α3(IV) collagen chain or the α3NC1 fragment may negatively regulate the invasion process. Interestingly, in the lung, where α1, α2, α3, α4 and α5 (IV) collagen chains are expressed in normal alveolar BM, development of bronchioalveolar carcinoma correlates with a loss of α3, α4 and α5 chain expression and an increase in α1 and α2 chain expression (Nakano et al., 2001). The heterotrimer [α1(IV)]2 α2 is permissive for the invasion of different cancer cell lines and mediates pro-MMP-2 activation (Maquoi et al., 2000). By contrast, α1NC1 or α2NC1 monomers have some structural similarities to TIMP-1¶ and inhibit MMP-2 and MMP-3 activity towards small synthetic peptide substrates in vitro (Netzer et al., 1998). Thus, the presence of different collagen chains or NC1 fragments during tumorigenesis might represent a key regulatory mechanism for the acquisition of an invasive phenotype.
Synthesis of collagen IV by vascular BM is a prerequisite for angiogenesis (Maragoudakis et al., 1993; Haralabopoulos et al., 1994). Moreover, α1 and α2 NC1 induce adhesion and spreading of endothelial cells (Koliakos et al., 1989; Tsilibary et al., 1990). On the basis of these observations and the data previously described, several groups have focused their attention on potential anti-angiogenic properties of NC1 fragments. Kalluri’s group identified anti-angiogenic activities for α1, α2 and α3 NC1, named arresten, canstatin and tumstatin, respectively (Colorado et al., 2000; Kamphaus et al., 2000; Maeshima et al., 2000b). In vitro, these molecules inhibit endothelial cell proliferation and migration. Tumstatin seems to be the most efficient. None of the whole NC1 fragments inhibited the proliferation of cancer cell lines, as observed with the 185–205 α3NC1 peptide, which indicated that this effect is dependent on partial degradation of the NC1 domain. Arresten, canstatin and tumstatin molecules inhibit angiogenesis: in vitro, they block the formation of tubular structures by mouse aortic endothelial cells embedded in Matrigel; in vivo, they block the recruitment of capillaries in Matrigel plugs and inhibit the growth of large and small tumors in mouse xenograft models. Brooks’ group generated similar NC1 fragments and described the anti-angiogenic effects of α2, α3 and α6 NC1 in chorioallantoic membrane (CAM) assays (Petitclerc et al., 2000). However, none of these NC1 fragments inhibited proliferation of cancer cell lines or endothelial cells in vitro.
In contrast to the results shown for arresten, no inhibitory effects were observed for the α1NC1 fragment. Although identical mammalian expression systems were used for the production of the fragments in both cases, these discrepancies may be attributed to the use of different endothelial cell types and different tumorigenesis models (xenografts in mice versus development of tumor on chorioallantoic membranes).
What are the cell surface receptors involved in these functions? It is clear that integrins are key targets of NC1 (Table 2). Tumstatin binds αvβ3 integrin in an RGD-independent manner and interacts through two different sites, one site, composed of tumstatin residues 54–132, is involved in the anti-angiogenic effect, whereas the other, composed of residues 185–203, is involved in the anti-proliferative activity on cancer cell lines (Maeshima et al., 2000a; Maeshima et al., 2001b; Shahan et al., 1999b). Adhesion of endothelial cells (HUVEC or C-PAE) to tumstatin also seems to occur through α6β1 integrin binding (Maeshima et al., 2000b). Although it is well known that the central triple-helical domain, as well as the NC1 domain of collagen type IV, interacts with cells via α1β1 and α2β1 integrins (Eble et al., 1993; Setty et al., 1998), these new results indicate that NC1 domains support novel integrin-mediated cellular interactions involved in the regulation of angiogenesis. Interestingly, collagen IV also contains cryptic integrin-binding sites. During angiogenesis these sites are exposed and induce a switch in integrin recognition, with a loss of α1β1 binding and a gain of αvβ3 binding (Xu et al., 2001), which might be due to denaturation and concomitant degradation of collagen IV by MMPs such as MMP-2 (Eble et al., 1996).
In the case of tumstatin, a peptide composed of residues 45–132 of α3NC1 fragment is sufficient to inhibit in vitro and in vivo angiogenesis by increasing apoptosis of endothelial cells and is tenfold more active than endostatin. Because of the involvement of this fragment in Goodpasture syndrome, deletion of the Goodpasture epitope (residues 45–54) has been done, and the anti-angiogenic properties are preserved (Maeshima et al., 2000a). The effects of tumstatin are independent of disulfide bonds and are located in a 25-residue peptide (residues 74–98) (Maeshima et al., 2001a; Maeshima et al., 2001b). Apoptosis induced specifically in endothelial cells by this peptide is associated with inhibition of cap-dependent translation through negative regulation of mTOR signaling and depends on the presence of β3 integrin (Maeshima et al., 2002).
Thus, NC1 domains of collagen type IV exhibit specific regulatory subdomains controlling adhesion, proliferation or apoptosis of various cells. The specificity of these subdomains for endothelial or cancer cells is very interesting, particularly in the case of α3NC1. Indeed, the recently published crystal structure of the collagen IV NC1 domain reveals a 3D structure with two homologous subdomains, N and C, with the major difference between these subdomains for each chain occurring in the region composed of residues 86–95 in the N subdomain and 196–209 in the C subdomain. Curiously these regions overlap two sequences identified previously as having anti-angiogenic activity and cancer cell anti-proliferative effects, respectively. It will be interesting to identify more precisely the integrin-binding sites on these NC1 domains. Indeed, their interactions with integrins might be involved in the disruption of the contacts between endothelial cells or tumor cells and the basement membrane, leading to apoptosis of these cells. They might be also involved in the disruption of the C-terminal association that occurs during the assembly of a collagen IV network and induce disorganization of this matrix, thus disturbing migration, proliferation or survival of the cells. Moreover, the unique properties of the tumstatin NC1 domain in regulating neovascularization are very interesting and suggest a promising new family of integrin-dependent angiogenesis inhibitors.
NC1(XV), NC1(XVIII), endostatin and endostatin-like fragments
Since its discovery by O’Reilly and co-workers in 1997, endostatin has been the object of extensive research, trials and controversy in the angiogenesis field. In vitro, endostatin inhibits proliferation of bovine capillary endothelial cells but not cancer cells. In vivo, it inhibits angiogenesis on CAM assays and growth of various primary tumors. Moreover, no signs of toxicity, drug resistance or regrowth of tumors are observed as long as mice are treated (O’Reilly et al., 1997). Another striking effect is that endostatin when administrated on repeated cycles allowing the tumor to re-grow between each of them is still efficient and induces a prolonged dormancy of the tumor without resistance after two to six cycles, depending on the tumor model (Boehm et al., 1997). Thus far, multiple studies have demonstrated anti-angiogenic properties of endostatin in pathological models of tumorigenesis (Bergers et al., 1999; Blezinger et al., 1999; Boehle et al., 2001; Dhanabal et al., 1999a; Kisker et al., 2001; Sorensen et al., 2002; Yoon et al., 1999), choroidal neovascularization (Mori et al., 2001) and arthritis (Matsuno et al., 2002; Yin et al., 2002). However, in a controlled angiogenic process such as wound healing, endostatin does not affect the overall neovascularization. Ultrastructural analysis demonstrated some abnormalities in vessel maturation, but the blood vessel density is not affected (Bloch et al., 2000; Berger et al., 2000).
Tumorigenesis is not affected in mice lacking collagen XVIII, indicating that even if endostatin is detected as a circulating molecule, the physiological levels may not be sufficient to decrease tumor progression (Fukai et al., 2002). By contrast, collagen XVIII is required for normal regression of hyaloid vessels and for anchoring vitreal collagen fibrils to the retina inner limiting membrane. Thus, collagen XVIII may act as a gatekeeper, inducing regression of vessels in non-permissive territory for angiogenesis. Although endostatin is efficient in reducing choroidal neovascularization, a role for endogenous endostatin has still to be fully demonstrated.
Ex-vivo, endostatin decreases and stabilizes microvessel formation in rat aortic or human vein ring angiogenesis assays (Kruger et al., 2000; Ergun et al., 2001). In vitro, endostatin inhibits basal and FGF2 or VEGF-induced proliferation and migration of different endothelial cell types (Dhanabal et al., 1999b; O’Reilly et al., 1997; Taddei et al., 1999; Yamaguchi et al., 1999; You et al., 1999). The circulating form purified from human plasma lacks 12 N-terminal residues and does not inhibit proliferation (Standker et al., 1997); so the anti-proliferative activity of endostatin requires the full-length fragment but is independent of its zinc- or heparin-binding capacity (Yamaguchi et al., 1999). Soluble endostatin induces endothelial cell apoptosis by altering Bcl-2 expression (Dhanabal et al., 1999c) and inducing tyrosine phosphorylation of the protein adaptor Shb (Dixelius et al., 2000).
The molecular targets of endostatin are not yet clear. In endothelial cells growing exponentially, endostatin mimics serum deprivation in downregulating the transcription of genes involved in proliferation, apoptosis and cell migration. In the presence of serum, endostatin affects only migration of endothelial cells (Hanai et al., 2002; Shirichi and Hirata, 2001).
Inhibition of migration and survival are the most constant functions reported for endostatin. It is clear that migration of endothelial cells involves assembly and disassembly of focal adhesions in concert with integrin signaling, and endostatin seems to interfere effectively with both systems. Immobilized endostatin promotes and soluble endostatin inhibits α5β1 and αvβ3 integrin-dependent endothelial cell migration and survival (Rehn et al., 2001). Depending on the cell type and the growth factor environment, inhibition of migration with soluble endostatin correlates with an increase or a decrease in focal adhesion and actin stress fiber formation (Dixelius et al., 2002; Wickstrom et al., 2001). Inhibition of VEGF-induced migration induces eNOS dephosphorylation (Urbich et al., 2002). Furthermore, endostatin induces a downregulation of the urokinase plasminogen activator system (Wickstrom et al., 2001), indicating that the signals induced by endostatin not only modify the cytoskeletal architecture and survival signals, but also affect pericellular proteolytic activity.
Interestingly, as observed in the case of collagen IV NC1 fragments, some functions of endostatin may interfere with MMP signaling (for a review, see Egeblad and Werb, 2002). Indeed, endostatin has been reported to inhibit endothelial and cancer cell invasion through Matrigel. This effect seems to be mediated by its association with pro-MMP-2, which inhibits MMP-2 activation (Kim et al., 2000). Recent data have shown a direct interaction of endostatin with the catalytic domain of MMP-2 (Lee et al., 2002). MMP-2 is very inefficient in generating endostatin fragments from collagen XVIII (Ferreras et al., 2000), but it may be one of the endostatin key targets for downregulating expression or activation of other MMPs and proteases.
A direct interaction between endostatin and VEGFR2 has been described previously (Kim et al., 2002). This interaction may be due to the basic character of endostatin, similar to the interaction demonstrated between VEGFR2 and the transactivator protein Tat of HIV-1 (Albini et al., 1996). Thus endostatin may act essentially by interfering with the binding of VEGF to its receptors, VEGF-R2 as well as VEGF-R1**.
Molecular data are more limited for the related endostatin-like molecule. Inhibition of FGF2-induced migration, but not proliferation, of endothelial cells has been reported. In a model of renal cell carcinoma xenograft, a reduction of the tumor growth was observed but, in contrast with endostatin, endostatin-like did not induce any regression (Ramchandran et al., 1999). However, in CAM assays, only endostatin-like and NC1(XV) inhibited angiogenesis induced by VEGF, whereas angiogenesis induced by FGF2 was inhibited only by endostatin and NC1(XV) (Sasaki et al., 2000). Thus these fragments might have different inhibition properties depending on their angiogenic environment and motility signaling, as discussed in the previous paragraph. These data correlate with previous observations showing that VEGF and FGF2 mediate their effects through different integrins, αvβ5 and αvβ3, respectively (Friedlander et al., 1995).
Conclusions and perspectives
The detailed biological functions of these different NC1 collagen fragments are not completely identified, but the similarities within the pathways they affect are troubling (Table 3). As immobilized substrates, NC1 fragments from collagen IV, XV or XVIII can induce migration, proliferation or survival depending on the cell type; however, soluble forms act in the opposite way, inhibiting proliferation, migration and inducing apoptosis (Fig. 3). This balance between negative and positive pathways may be part of a highly controlled regulatory mechanism. In the context of an invasive process such as angiogenesis or branching morphogenesis, the cells need to migrate and proliferate, and these processes are dependent on protease activity. In the first steps of these processes, partial degradation of the matrix may unmask some cryptic sites exposing NC1 immobilized domains that bind to cell surface integrins and activate different pathways depending on the integrin pattern. Sustained proteolysis of the extracellular matrix may release high local concentration of soluble fragments that then act as dominant-negative molecules. They can inhibit proliferation and migration, allowing stabilization of new vessels or tubular structures or induce complete regression of these new tubules through the activation of apoptotic pathways.
Table 3.
Functions of NC1 and NC1 fragments of collagen IV, XV and XVIII
| Induction | Inhibition | |
|---|---|---|
| Collagen IV | ||
| Immobilized NC1 | Adhesion and chemotaxis of cancer cell lines (α3) (Han et al., 1997; Shahan et al., 1999a; Shahan et al., 2000) | |
| FAK and PI3K phosphorylation (α3) (Pasco et al., 2000a) | ||
| Soluble NC1 | (α1 and α2 chains) Axonal growth of sympathetic neurons (Lein et al., 1991) | Hydra regeneration (Sarras et al., 1993) Activation of leukocytes (a3) (Monboisse et al., 1994) |
| Adhesion and migration of chicken neural crest cells (α1 and α2) (Perris et al., 1993) | Proliferation and migration of cancer cell lines (α3) (Han et al., 1997; Shahan et al., 1999a; Shahan et al., 2000; Pasco et al., 2000b) | |
| Adhesion and spreading of endothelial cell (α1 and α2) (Koliakos et al., 1989; Tsilibary et al., 1990) | MMP-2 and MMP-3 activation (α1, α2 and α3) (Netzer et al., 1998; Pasco et al., 2000b) | |
| Apoptosis of endothelial cells (54–132 aa peptide α3) (Maeshima et al., 2001a) | Proliferation and migration of endothelial cells (α1, α2 and α3) (Colorado et al., 2000; Kamphaus et al., 2000; Maeshima et al., 2000a; Maeshima et al., 2001a) | |
| In vitro angiogenesis (α1, α2 and α3) (Colorado et al., 2000; Kamphaus et al., 2000; Maeshima et al., 2000a) | ||
| Chorioallantoic membrane angiogenesis FGF2-induced (α1, α2 and α6) or VEGF-induced (α2) (Petitclerc et al., 2000) | ||
| Tumor angiogenesis (α1, α2 and α3) (Colorado et al., 2000; Kamphaus et al., 2000; Maeshima et al., 2000a; Petitclerc et al., 2000) | ||
| Protein synthesis cap-dependent (54–132 aa peptide α3) (Maeshima et al., 2002) | ||
| Collagen XV | ||
| Soluble monomeric endostatin-like | Reduction of tumor growth (Ramchandran et al., 1999) | Migration of endothelial cells FGF2-induced (Ramchandran et al., 1999) |
| Chorioallantoic membrane angiogenesis VEGF-induced (Sasaki et al., 2000) | ||
| NC1 trimer | Chorioallantoic membrane angiogenesis FGF2 and VEGF-induced (Sasaki et al., 2000) | |
| Collagen XVIII | ||
| Soluble endostatin | Apoptosis of endothelial cells (Dhanabal et al., 1999b) | Proliferation and migration of endothelial cells FGF2 or VEGF-induced (O’Reilly et al., 1997; Yamaguchi et al., 1999; You et al., 1999; Taddei et al., 1999; Dhanabal et al., 1999a) |
| Tumor regression (O’Reilly et al., 1997; Boehm et al., 1997) | Tumor angiogenesis (O’Reilly et al., 1997; Boehm et al., 1997; Bergers et al., 1999; Blezinger et al., 1999; Boehle et al., 2001; Dhanabel et al., 1999b; Kisker et al., 2001; Sorensen et al., 2002; Yoon et al., 1999) | |
| Down-regulation of gene transcription (Shirichi and Hirata, 2001; Hanai et al., 2002) | Choroidal neovascularization (Mori et al., 2001) | |
| G1 arrest of endothelial cells (Hanai et al., 2002) | Arthritis (Matsuno et al., 2002; Yin et al., 2002) | |
| In vitro stabilization of microvessels (Ergun et al., 2001) | Microvessel formation in rat aortic or human vein ring angiogenesis assay (Kruger et al., 2000; Ergun et al., 2001) | |
| Chorioallantoic membrane angiogenesis FGF2-induced (Sasaki et al., 2000) | ||
| Migration of neural and non-neural cells in C. elegans (Ackley et al., 2001) | ||
| Migration and branching morphogenesis of renal epithelial cells (Karihaloo et al., 2001) | ||
| FAK phosphorylation, focal adhesion and actin stress fibers formation growth factor induced (Dixelius et al., 2002; Wickstrom et al., 2001) | ||
| Endothelial and cancer cell invasion through Matrigel (Kim et al., 2000) | ||
| Immobilized | ||
| Monomeric endostatin | Endothelial cell spreading, FAK phosphorylation (Rehn et al., 2001) | |
| NC1 trimer | Cell motility of endothelial and non-endothelial cells (Kuo et al., 2001) | Endothelial tube formation in Matrigel (Kuo et al., 2001) |
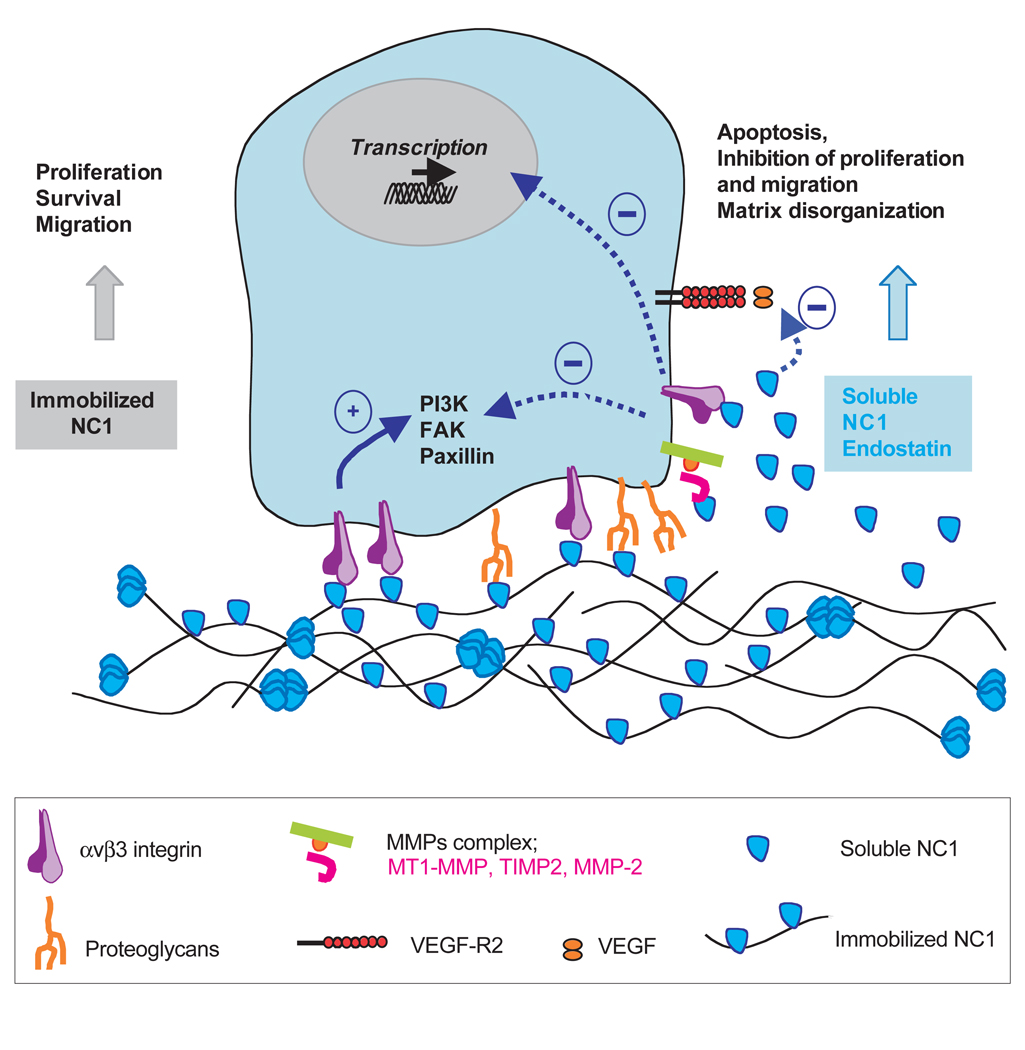
Fig. 3.
Biological activities of immobilized versus soluble NC1 and endostatin fragments. Immobilized NC1 domains from collagen IV, XV and XVIII induce proliferation, survival and migration of different cell types. These effects correlate with an increase in PI3K, FAK and paxillin phosphorylation. By contrast, soluble NC1 or endostatin fragments bind to various receptors on the surface of the cells and decrease phosphorylation of PI3K, FAK and paxillin. Endostatin as well as NC1(IV) induces a decrease in the transcription of different genes. Endostatin interacts with VEGF-R2 and thus decreases the binding of VEGF to its receptor; endostatin also interacts directly with MMP-2, inhibiting the activation of the enzyme. Taken together, these soluble fragments act in the opposite way to their original molecules, negatively regulating the proliferation and the migration of different cell types and inducing apoptosis and extracellular matrix disorganization.
Interestingly, the activities of these fragments are dependent on the activation of integrins or proteoglycans, such as glypicans in the case of endostatin. The integrin αvβ3 is a common ligand for collagen IV, XV and XVIII NC1 fragments and endostatin. In mice lacking β3 integrin, tumor angiogenesis as well as VEGF or hypoxia-induced angiogenesis are enhanced, suggesting a role for this integrin in limiting angiogenesis in vivo (Reynolds et al., 2002). The anti-angiogenic activities of different NC1 fragments dependent on binding to β3 integrin might also support this hypothesis. In that case, the NC1-fragment–β3-integrin interaction might be a key regulator of this negative feedback. Another feature in the biological activities of these collagen fragments is the downregulation of gene expression: the collagen IV α3NC1 domain as well as endostatin decreases the expression of various genes involved in cell cycle regulation, migration and survival. However these activities seem to be correlated with environmental factors, and the anti-angiogenic activities of various fragments may depend on the specificity and the concentrations of growth factors locally released.
Besides β3 integrin, another common target emerging for these fragments is MMP-2. A direct interaction with the catalytic domain has been shown in the case of endostatin. For endostatin-like and the collagen IV α3NC1 domain, such an interaction has not been demonstrated, but the three molecules induce a decrease in MMP-2 membrane-bound activity, which might be part of the negative feedback loop mentioned previously. A decrease in the basal level of pro-MMP-2 activity is also observed in collagen-XV knockout mice (Eklund et al., 2001). Moreover, several MMPs are involved in the generation of the fragments themselves. Thus, in a process such as angiogenesis, proteolytic activity of MMPs might be part of a biphasic regulation: proangiogenic in early steps, critical for the rupture of basement membrane and migration of the endothelial cells, and anti-angiogenic in late steps, generating endogenous inhibitor fragments.
Endogenous inhibitors or activators derived from larger precursor proteins now appear to be a common theme in the context of remodeling processes. If endostatin or tumstatin have received increased attention recently because of their strong anti-angiogenic potential, it is clear that their activity is not specific for microvascular endothelial cells, but this work has opened new interest in the potential cryptic biological functions of these ubiquitous collagen molecules. Thus, these studies might be interesting not only in the context of cancer but also, considering the large number of diseases linked to collagens, in numerous other human disorders.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NS39278, CA72006, AR46238), funds from the Sandler Family Sustaining Foundation and a fellowship from the Osteogenesis Imperfecta Foundation. We would like to apologize for our failure to cite many of the important and relevant studies in this field because of space limitations. We are grateful to Bryony Wiseman for critical reading of the manuscript.
Footnotes
α1 to α6 polypeptides are encoded by three sets of genes, Col4a1/Col4a2, Col4a3/Col4a4 and Col4a5/Col4a6, mapping to human chromosomes 13, 2 and X, and mouse chromosomes 8, 1 and X respectively. Each pair of genes is organized head-to-head on the chromosome, and their expression is regulated by bidirectional promoters localized between the genes.
As proposed by Olsen and Ninomiya (Olsen and Ninomiya, 1999), non-collagenous domains for collagen XV and XVIII are numbered as for collagen IV, starting from the C-terminal domain.
For consistency, we will use the terms ‘endostatin’ in the case of collagen XVIII and ‘endostatin-like’ for collagen XV.
The homology of NC1 domain and TIMP-1 has been described using sequence-based approaches (Netzer et al., 1998) but it should be noted that recent X-ray crystallography data, which found that the structure of NC1 domain is unlike any other protein of known structure (Sundaramoorthy et al., 2002; Than et al., 2002), does not report this homology.
The authors mentioned also an interaction with VEGF-R1 (Kim et al., 2002).
References
- Abe N, Muragaki Y, Yoshioka H, Inoue H, Ninomiya Y. Identification of a novel collagen chain represented by extensive interruptions in the triple-helical region. Biochem. Biophys. Res. Commun. 1993;196:576–582. doi: 10.1006/bbrc.1993.2288. [DOI] [PubMed] [Google Scholar]
- Ackley BD, Crew JR, Elamaa H, Pihlajaniemi T, Kuo CJ, Kramer JM. The NC1/Endostatin domain of Caenorhabditis elegans type XVIII collagen affects cell migration and axon guidance. J. Cell Biol. 2001;152:1219–1232. doi: 10.1083/jcb.152.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albini A, Soldi R, Giunciuglio D, Giraudo E, Benelli R, Primo L, Noonan D, Salio M, Camussi G, Rockl W, Bussolino F. The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat. Med. 2:1371–1375. doi: 10.1038/nm1296-1371. [DOI] [PubMed] [Google Scholar]
- Alini M, Matsui Y, Dodge GR, Poole AR. The extracellular matrix of cartilage in the growth plate before and during calcification: changes in composition and degradation of type II collagen. Calcif. Tissue Int. 1992;50:327–535. doi: 10.1007/BF00301630. [DOI] [PubMed] [Google Scholar]
- Albini A, Adelmann-Grill BC. Collagenolytic cleavage products of collagen type I as chemoattractants for human dermal fibroblasts. Eur. J. Cell Biol. 1985;36:104–107. [PubMed] [Google Scholar]
- Berger AC, Feldman AL, Gnant MF, Kruger EA, Hewitt S, Figg WD, Alexander HR, Libutti SK. The angiogenesis inhibitor, endostatin, does not affect murine cutaneous wound healing. J. Surg. Res. 2000;91:26–31. doi: 10.1006/jsre.2000.5890. [DOI] [PubMed] [Google Scholar]
- Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808–812. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- Blezinger P, Wang J, Gondo M, Quezada A, Mehrens D, French M, Singhal A, Sullivan S, Rolland A, Ralston R, Min W. Systemic inhibition of tumor growth and tumor metastases by intramuscular administration of the endostatin gene. Nat. Biotechnol. 1999;17:343–348. doi: 10.1038/7895. [DOI] [PubMed] [Google Scholar]
- Bloch W, Huggel K, Sasaki T, Grose R, Bugnon P, Addicks K, Timpl R, Werner S. The angiogenesis inhibitor endostatin impairs blood vessel maturation during wound healing. FASEB J. 2000;14:2373–2376. doi: 10.1096/fj.00-0490fje. [DOI] [PubMed] [Google Scholar]
- Blumberg B, MacKrell AJ, Olson PF, Kurkinen M, Monson JM, Natzle JE, Fessler JH. Basement membrane procollagen IV and its specialized carboxyl domain are conserved in Drosophila, mouse, and human. J. Biol. Chem. 1987;262:5947–5950. [PubMed] [Google Scholar]
- Boehle AS, Kurdow R, Schulze M, Kliche U, Sipos B, Soondrum K, Ebrahimnejad A, Dorhmann P, Kalthoff H, Henne-bruns D, Neumaier M. Human endostatin inhibits growth of human non-small-cell lung cancer in a murine xenotransplant model. Int. J. cancer. 2001;94:420–428. doi: 10.1002/ijc.1471. [DOI] [PubMed] [Google Scholar]
- Boehm T, Folkman J, Browder D, O’Reilly MR. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404–407. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]
- Boutaud A, Borza DB, Bondar O, Gunwar S, Netzer KO, Singh N, Ninomiya Y, Sado Y, Noelken ME, Hudson BG. Type IV collagen of the glomerular basement membrane. Evidence that the chain specificity of network assembly is encoded by the noncollagenous NC1 domain. J. Biol. Chem. 2000;275:30716–30724. doi: 10.1074/jbc.M004569200. [DOI] [PubMed] [Google Scholar]
- Cao Y. Endogenous angiogenesis inhibitors and their therapeutic implications. Int. J. Biochem. Cell. Biol. 2001;33:357–369. doi: 10.1016/s1357-2725(01)00023-1. [DOI] [PubMed] [Google Scholar]
- Colorado PC, Torre A, Kamphaus G, Maeshima Y, Hopfer H, Takahashi K, Volk R, Zamborsky ED, Herman S, Sarkar PK, et al. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res. 2000;60:2520–2526. [PubMed] [Google Scholar]
- Cosgrove D, Meehan DT, Grunkemeyer JA, Kornak JM, Sayers R, Hunter WJ, Samuelson GC. Collagen COL4A3 knockout: a mouse model for autosomal Alport syndrome. Genes Dev. 1996;10:2981–2992. doi: 10.1101/gad.10.23.2981. [DOI] [PubMed] [Google Scholar]
- Cosgrove D, Samuelson G, Meehan DT, Miller C, McGee J, Walsh EJ, Siegel M. Ultrastructural, physiological, and molecular defects in the inner ear of a gene-knockout mouse model for autosomal Alport syndrome. Hear. Res. 1998;121:84–98. doi: 10.1016/s0378-5955(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Dehan P, Waltregny D, Beschin A, Noel A, Castronovo V, Tryggvason K, de Leval J, Foidart JM. Loss of type IV collagen alpha 5 and alpha 6 chains in human invasive prostate carcinomas. Am. J. Pathol. 1997;151:1097–1104. [PMC free article] [PubMed] [Google Scholar]
- Dhanabal M, Volk R, Ramchandran R, Simons M, Sukhatme VP. Cloning, expression, and in vitro activity of human endostatin. Biochem. Biophys. Res. Commun. 1999a;258:345–352. doi: 10.1006/bbrc.1999.0595. [DOI] [PubMed] [Google Scholar]
- Dhanabal M, Ramchandran R, Volk R, Stillman IE, Lombardo M, Iruela-Arispe ML, Simons M, Sukhatme VP. Endostatin: yeast production, mutants, and antitumor effect in renal cell carcinoma. Cancer Res. 1999b;59:189–197. [PubMed] [Google Scholar]
- Dhanabal M, Ramchandran R, Waterman MJF, Lu H, Knebelmann B, Segal M, Sukhatme VP. Endostatin induces endothelial cell apoptosis. J. Biol. Chem. 1999c;274:11721–11726. doi: 10.1074/jbc.274.17.11721. [DOI] [PubMed] [Google Scholar]
- Ding Y-H, Javaherian K, Lo K-M, Chopra R, Boehm T, Lanciotti J, Harris BA, Li Y, Shapiro R, Hohenester E, Timpl R, Folkman J, Wiley DC. Zinc-dependent dimmers observed in crystals of human endostatin. Proc. Natl. Acad. Sci. USA. 1998;95:10443–10448. doi: 10.1073/pnas.95.18.10443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixelius J, Larsson H, Sasaki T, Holmqvist K, Lu L, Engstrom A, Timpl R, Welsh M, Claesson-Welsh L. Endostatin-induced tyrosine kinase signaling through the Shb adaptor protein regulates endothelial cell apoptosis. Blood. 2000;95:3403–3411. [PubMed] [Google Scholar]
- Dixelius J, Cross M, Matsumoto T, Sasaki T, Timpl R, Claesson-Welsh L. Endostatin regulates endothelial cell adhesion and cytoskeletal organization. Cancer Res. 2002;62:1944–1947. [PubMed] [Google Scholar]
- Dolz R, Engel J, Kuhn K. Folding of collagen IV. Eur. J. Biochem. 1988;178:357–366. doi: 10.1111/j.1432-1033.1988.tb14458.x. [DOI] [PubMed] [Google Scholar]
- Eble JA, Golbik R, Mann K, Kuhn K. The alpha 1 beta 1 integrin recognition site of the basement membrane collagen molecule [alpha 1(IV)]2 alpha 2(IV) EMBO J. 1993;12:4795–4802. doi: 10.1002/j.1460-2075.1993.tb06168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble JA, Ries A, Lichy A, Mann K, Stanton H, Gavrilovic J, Murphy G, Kuhn K. The recognition sites of the integrins alpha1beta1 and alpha2beta1 within collagen IV are protected against gelatinase Aattack in the native protein. J. Biol. Chem. 1996;271:30964–30970. doi: 10.1074/jbc.271.48.30964. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Eklund L, Piuhola J, Komulainen J, Sormunen R, Ongvarrasopone C, Fässler R, Muona A, Ilves M, Ruskoaho H, Takala TES, Pihlajaniemi T. Lack of type XV collagen causes a skeletal myopathy and cardiovascular defects in mice. Proc. Natl. Acad. Sci. USA. 2001;98:1194–1199. doi: 10.1073/pnas.031444798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergun S, Kilic N, Wurmbach JH, Ebrahimnejad A, Fernando M, Sevinc S, Kilic E, Chalajour F, Fiedler W, Lauke H, et al. Endostatin inhibits angiogenesis by stabilization of newly formed endothelial tubes. Angiogenesis. 2001;4:193–206. doi: 10.1023/a:1014027218980. [DOI] [PubMed] [Google Scholar]
- Erickson AC, Couchman JR. Still more complexity in mammalian basement membranes. J. Histochem. Cytochem. 2000;48:1291–1306. doi: 10.1177/002215540004801001. [DOI] [PubMed] [Google Scholar]
- Felbor U, Dreier L, Bryant RAR, Ploegh HL, Olsen BR, Mothes W. Secreted cathepsin L generates endostatin from collagen XVIII. EMBO J. 2000;19:1187–1194. doi: 10.1093/emboj/19.6.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreras M, Felbor U, Lenhard T, Olsen BR, Delaisse J-M. Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 2000;486:247–251. doi: 10.1016/s0014-5793(00)02249-3. [DOI] [PubMed] [Google Scholar]
- Filie JD, Burbelo PD, Kozak CA. Genetic mapping of the alpha 1 and alpha 2 (IV) collagen genes to mouse chromosome 8. Mamm. Genome. 1995;6:487. doi: 10.1007/BF00360662. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R, Kuhn K, Sato Y, MacDonald ED, 2nd, Perlish JS, Pan TC, Chu ML, Kishiro Y, Oohashi T, Bernier SM, Yamada Y, Ninomiya Y. There is temporal and spatial expression of alpha1 (IV), alpha2 (IV), alpha5 (IV), alpha6 (IV) collagen chains and beta1 integrins during the development of the basal lamina in an “in vitro” skin model. J. Invest. Dermatol. 1997;109:527–533. doi: 10.1111/1523-1747.ep12336696. [DOI] [PubMed] [Google Scholar]
- Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- Fukai N, Eklund L, Marneros AG, Tamarkin L, Niemela M, Ilves M, Li E, Pihlajaniemi T, Olsen BR. Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J. 2002;21:1535–1544. doi: 10.1093/emboj/21.7.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg PM, Horelli-Kuitunen N, Eklund L, Palotie A, Pihlajaniemi T. Cloning of mouse type XV collagen sequences and mapping of the corresponding gene to 4B1-3. Comparison of mouse and human alpha 1 (XV) collagen sequences indicates divergence in the number of small collagenous domains. Genomics. 1997a;45:31–41. doi: 10.1006/geno.1997.4884. [DOI] [PubMed] [Google Scholar]
- Hagg PM, Hagg PO, Peltonen S, Autio-Harmainen H, Pihlajaniemi T. Location of type XV collagen in human tissues and its accumulation in the interstitial matrix of the fibrotic kidney. Am. J. Pathol. 1997b;150:2075–2086. [PMC free article] [PubMed] [Google Scholar]
- Halfter W, Dong S, Schurer B, Cole GJ. Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J. Biol. Chem. 1998;273:25404–25412. doi: 10.1074/jbc.273.39.25404. [DOI] [PubMed] [Google Scholar]
- Han J, Ohno N, Pasco S, Monboisse JC, Borel JP, Kefalides NA. A cell binding domain from the α3 chain of type IV collagen inhibits proliferation of melanoma cells. J. Biol. Chem. 1997;272:20395–20401. doi: 10.1074/jbc.272.33.20395. [DOI] [PubMed] [Google Scholar]
- Hanai J-I, Dhanabal M, Karumanchi SA, Albanese C, Waterman M, Chan B, Ramchandran R, Pestell R, Sukhatme VP. Endostatin causes G1 arrest of endothelial cells through inhibition of cyclin D1. J. Biol. Chem. 2002;277:16464–16469. doi: 10.1074/jbc.M112274200. [DOI] [PubMed] [Google Scholar]
- Haralabopoulos GC, Grant DS, Kleinman HK, Lelkes PI, Papaioannou SP, Maragoudakis ME. Inhibitors of basement membrane collagen synthesis prevent endothelial cell alignement in matrigel in vitro and angiogenesis in vivo. Lab. Invest. 1994;71:575–582. [PubMed] [Google Scholar]
- Heidet L, Cohen-Solal L, Boye E, Thorner P, Kemper MJ, David A, Larget Piet L, Zhou J, Flinter F, Zhang X, Gubler MC, Antignac C. Novel COL4A5/COL4A6 deletions and further characterization of the diffuse leiomyomatosis-Alport syndrome (DL-AS) locus define the DL critical region. Cytogenet. Cell. Genet. 1997;78:240–246. doi: 10.1159/000134666. [DOI] [PubMed] [Google Scholar]
- Hohenester E, Sasaki T, Olsen BR, Timpl R. Crystal structure of the angiogenesis inhibitor endostatin at 1.5Å resolution. EMBO J. 1998;17:1656–1664. doi: 10.1093/emboj/17.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenester E, Sasaki T, Mann K, Timpl R. Variable zinc coordination in endostatin. J. Mol. Biol. 2000;297:1–6. doi: 10.1006/jmbi.2000.3553. [DOI] [PubMed] [Google Scholar]
- Hudson BG, Reeders ST, Tryggvason K. Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J. Biol. Chem. 1993;268:26033–26036. [PubMed] [Google Scholar]
- Huebner K, Cannizzaro LA, Jabs EW, Kivirikko S, Manzone H, Pihlajaniemi T, Myers JC. Chromosomal assignement of a gene encoding a new collagen type (COL15A1) to 9q21 → q22. Genomics. 1992;14:220–224. doi: 10.1016/s0888-7543(05)80209-5. [DOI] [PubMed] [Google Scholar]
- Inoue-Murayama M, Sugimoto Y, Niimi Y, Aso H. Type XVIII collagen is newly transcribed during bovine adipogenesis. Differentiation. 2000;65:281–285. doi: 10.1046/j.1432-0436.2000.6550281.x. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Ito A, Iwata K, Tanzawa K, Mori Y, Nagase H. Plasma membrane-bound tissue inhibitor of metalloproteinases (TIMP)-2 specifically inhibits matrix metalloproteinase 2 (gelatinase A) activated on the cell surface. J. Biol. Chem. 1998;273:24360–24367. doi: 10.1074/jbc.273.38.24360. [DOI] [PubMed] [Google Scholar]
- Jennings L, Wu L, King KB, Hammerle H, Cs-Szabo G, Mollenhauer J. The effects of collagen fragments on the extracellular matrix metabolism of bovine and human chondrocytes. Connect. Tissue Res. 2001;42:71–86. doi: 10.3109/03008200109014250. [DOI] [PubMed] [Google Scholar]
- John H, Preissner KT, Forssman WG, Standker L. Novel glycosylated forms of human plasma endostatin and circulating endostatin-related fragments of collagen XV. Biochemistry. 1999;38:10217–10224. doi: 10.1021/bi990787+. [DOI] [PubMed] [Google Scholar]
- Kalluri R. Goodpasture syndrome. Kidney Int. 1999;55:1120–1122. doi: 10.1046/j.1523-1755.1999.0550031120.x. [DOI] [PubMed] [Google Scholar]
- Kamphaus GD, Colorado PC, Panka DJ, Hopfer H, Ramchandran R, Torre A, Maeshima Y, Mier JW, Sukhatme VP, Kalluri R. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J. Biol. Chem. 2000;275:1209–1215. doi: 10.1074/jbc.275.2.1209. [DOI] [PubMed] [Google Scholar]
- Karihaloo A, Karumanchi SA, Barasch J, Jha V, Nickel CH, Yang J, Grisaru S, Bush KT, Nigam S, Rosenblum ND, et al. Endostatin regulates branching morphogenesis of renal epithelial cells and ureteric bud. Proc. Natl. Acad. Sci. USA. 2001;98:12509–12514. doi: 10.1073/pnas.221205198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karumanchi SA, Jha V, Ramchandran R, Karihaloo A, Tsiokas L, Chan B, Dhanabal M, Hanai J-I, Venkataraman G, Shriver Z, et al. Cell surface Glypicans are low-affinity endostatin receptors. Mol. Cell. 2001;7:811–822. doi: 10.1016/s1097-2765(01)00225-8. [DOI] [PubMed] [Google Scholar]
- Kim YM, Jang JW, Lee OH, Yeon J, Choi EY, Kim KW, Lee ST, Kwon YG. Endostatin inhibits endothelial and tumor cellular invasion by blocking the activation and catalytic activity of matrix metalloproteinase. Cancer Res. 2000;60:5410–5413. [PubMed] [Google Scholar]
- Kim YM, Hwang S, Kim YM, Pyun BJ, Kim TY, Lee ST, Gho YS, Kwon YG. Endostatin blocks VEGF-mediated signaling via direct interaction with KDR/Flk-1. J. Biol. Chem. 2002;277:27872–27879. doi: 10.1074/jbc.M202771200. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Sato H, Okada A, Ohuchi E, Imai K, Okada Y, Seiki M. TIMP-2 promotes activation of progelatinase A by membrane-type 1 matrix metalloproteinase immobilized on agarose beads. J. Biol. Chem. 1998;273:16098–16103. doi: 10.1074/jbc.273.26.16098. [DOI] [PubMed] [Google Scholar]
- Kisker O, Becker CM, Prox D, Fannon M, D’Amato R, Flynn E, Fogler WE, Sim BK, Allred EN, Pirie-Shepherd SR, Folkman J. Continuous administration of endostatin by intraperitoneally implanted osmotic pump improves the efficacy and potency of therapy in a mouse xenograft tumor model. Cancer Res. 2001;61:7669–7674. [PubMed] [Google Scholar]
- Kivirikko S, Saarela J, Myers JC, Autio-Harmainen H, Pihlajaniemi T. Distribution of type XV collagen transcripts in human tissue and their production by muscle cells and fibroblasts. Am. J. Pathol. 1995;147:1500–1509. [PMC free article] [PubMed] [Google Scholar]
- Koliakos GG, Kouzi-Koliakos K, Furcht LT, Reger LA, Tsilibary EC. The binding of heparin to type IV collagen: domain specificity with identification of peptides sequences from the alpha 1(IV) and alpha 2(IV) which preferentially bind heparin. J. Biol. Chem. 1989;264:2313–2323. [PubMed] [Google Scholar]
- Kruger EA, Duray PH, Tsokos MG, Venzon DJ, Libutti SK, Dixon SC, Rudek MA, Pluda J, Allegra C, Figg WD. Endostatin inhibits microvessel formation in the ex vivo rat aortic ring angiogenesis assay. Biochem. Biophys. Res. Commun. 2000;268:183–191. doi: 10.1006/bbrc.1999.2018. [DOI] [PubMed] [Google Scholar]
- Kuo CJ, LaMontagne KR, Jr, Garcia-Cardeña G, Ackley BD, Kalman D, Park S, Christofferson R, Kamihara J, Ding Y-H, Lo K-M, Gillies S, et al. Oligomerization-dependent regulation of motility and morphogenesis by the collagen XVIII NC1/Endostatin domain. J. Cell Biol. 2001;152:1233–1246. doi: 10.1083/jcb.152.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Jang JW, Kim YM, Lee HI, Jeon JY, Kwon YG, Lee ST. Endostatin binds to the catalytic domain of matrix metalloproteinase-2. FEBS Lett. 2002;519:147–152. doi: 10.1016/s0014-5793(02)02742-4. [DOI] [PubMed] [Google Scholar]
- Lein PJ, Turner DC, Flier LA, Terranova VP. The NC1 domain of type IV collagen promotes axonal growth in sympathetic neurons through interaction with the alpha 1 beta 1 integrin. J. Cell Biol. 1991;113:417–428. doi: 10.1083/jcb.113.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Clark CC, Myers JC. Basement membrane zone type XV collagen is a disulfide-bonded chondroitin sulfate proteoglycan in human tissues and cultured cells. J. Biol. Chem. 2000;275:22339–22347. doi: 10.1074/jbc.M000519200. [DOI] [PubMed] [Google Scholar]
- Lin H-C, Chang J-H, Jain S, Gabison EE, Kure T, Kato T, Fukai N, Azar DT. Matrilysin cleavage of corneal collagen type XVIII NC1 domain and generation of a 28-kDa fragment. Invest. Ophtalmol. Vis. Sci. 2001;42:2517–2524. [PubMed] [Google Scholar]
- Lu W, Phillips CL, Killen PD, Hlaing T, Harrison WR, Elder FF, Miner JH, Overbeek PA, Meisler MH. Insertional mutation of the collagen genes Col4a3 and Col4a4 in a mouse model of Alport syndrome. Genomics. 1999;61:113–124. doi: 10.1006/geno.1999.5943. [DOI] [PubMed] [Google Scholar]
- MacDonald NJ, Shivers WY, Narum DL, Plum SM, Wingard JN, Furhmann SR, Liang H, Holland-Linn J, Chen DHT, Sim KL. Endostatin binds tropomyosin. J. Biol. Chem. 2001;276:25190–25196. doi: 10.1074/jbc.M100743200. [DOI] [PubMed] [Google Scholar]
- Maeshima Y, Colorado PC, Torre A, Holthaus KA, Grunkemeyer JA, Ericksen MB, Hopfer H, Xiao Y, Stillman IE, Kalluri R. Distinct anti-tumor properties of a type IV collagen domain derived from basement membrane. J. Biol. Chem. 2000a;275:21340–21348. doi: 10.1074/jbc.M001956200. [DOI] [PubMed] [Google Scholar]
- Maeshima Y, Colorado PC, Kalluri R. Two RGD-independent αvβ3 integrin binding sites on Tumstatin regulate distinct antitumor properties. J. Biol. Chem. 2000b;275:23745–23750. doi: 10.1074/jbc.C000186200. [DOI] [PubMed] [Google Scholar]
- Maeshima Y, Yerramalla UL, Dhanabal M, Holthaus KA, Barbashov S, Kharbanda S, Reimer C, Manfredi M, Dickerson WM, Kalluri R. Extracellular matrix-derived peptide binds to avb3 integrin and inhibits angiogenesis. J. Biol. Chem. 2001a;276:31959–31968. doi: 10.1074/jbc.M103024200. [DOI] [PubMed] [Google Scholar]
- Maeshima Y, Manfredi M, Reimer C, Holthaus KA, Hopfer H, Chandamuri BR, Kharbanda S, Kalluri R. Identification of the anti-angiogenic site within vascular basement membrane-derived Tumstatin. J. Biol. Chem. 2001b;276:15240–15248. doi: 10.1074/jbc.M007764200. [DOI] [PubMed] [Google Scholar]
- Maeshima Y, Sudhakar A, Lively JC, Ueki K, Kharbanda S, Kahn CR, Sonenberg N, Hynes RO, Kalluri R. Tumstatin, an endothelial cell-specific inhibitor of protein synthesis. Science. 2002;295:140–143. doi: 10.1126/science.1065298. [DOI] [PubMed] [Google Scholar]
- Malone JD, Richards M, Jeffrey JJ. Recruitment of peripheral mononuclear cells by mammalian collagenase digests of type I collagen. Matrix. 1991;11:289–295. doi: 10.1016/s0934-8832(11)80237-4. [DOI] [PubMed] [Google Scholar]
- Maquoi E, Frankenne F, Noël A, Krell H-W, Grams F, Foidart J-M. Type IV collagen induces matrix metalloproteinase 2 activation in HT1080 fibrosarcoma cells. Exp. Cell Res. 2000;261:348–359. doi: 10.1006/excr.2000.5063. [DOI] [PubMed] [Google Scholar]
- Maragoudakis ME, Missirlis E, Karakiulakis GD, Sarmonica M, Bastakis M, Tsopanoglou N. Basement membrane biosynthesis as a target for developing inhibitors of angiogenesis with anti-tumor properties. Kidney Int. 1993;43:147–150. doi: 10.1038/ki.1993.24. [DOI] [PubMed] [Google Scholar]
- Marneros AG, Olsen BR. The role of collagen-derived proteolytic fragments in angiogenesis. Matrix Biol. 2001;20:337–345. doi: 10.1016/s0945-053x(01)00151-2. [DOI] [PubMed] [Google Scholar]
- Martinella-Catusse C, Polette M, Noël A, Gilles C, Dehan P, Munaut C, Colige A, Volders L, Monboisse J-C, Foidart J-M, Birembaut P. Down-regulation of MT1-MMP expression by the α3 chain of type IV collagen inhibits bronchial tumor cell line invasion. Lab. Invest. 2001;81:167–175. doi: 10.1038/labinvest.3780224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno H, Yudoh K, Uzuki M, Nakazawa F, Sawai T, Yamaguchi N, Olsen BR, Kimura T. Treatment with the angiogenesis inhibitor endostatin: anovel therapy in rheumatoid arthritis. J. Rheumatol. 2002;29:890–895. [PubMed] [Google Scholar]
- Miner JH, Sanes JR. Collagen IV alpha 3, alpha 4, and alpha 5 chains in rodent basal laminae: sequence, distribution, association with laminins, and developmental switches. J. Cell Biol. 1994;127:879–891. doi: 10.1083/jcb.127.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Sanes JR. Molecular and functional defects in kidneys of mice lacking collagen alpha 3(IV): implications for Alport syndrome. J. Cell Biol. 1996;135:1403–1413. doi: 10.1083/jcb.135.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miosge N, Sasaki T, Timpl R. Angiogenesis inhibitor endostatin is a distinct component of elastic fibers in vessel walls. FASEB J. 1999;13:1743–1750. doi: 10.1096/fasebj.13.13.1743. [DOI] [PubMed] [Google Scholar]
- Miosge N. The ultrastructural composition of basement membranes in vivo. Histol. Histopathol. 2001;16:1239–1248. doi: 10.14670/HH-16.1239. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Lemmink HH, Mariyama M, Antignac C, Gubler MC, Pirson Y, Verellen-Dumoulin C, Chan B, Schroder CH, Smeets HJ, et al. Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat. Genet. 1994;8:77–81. doi: 10.1038/ng0994-77. [DOI] [PubMed] [Google Scholar]
- Momota R, Sugimoto M, Oohashi T, Kigasawa K, Yoshioka H, Ninomiya Y. Two genes, COL4A3 and COL4A4 coding for the human alpha3(IV) and alpha4(IV) collagen chains are arranged head-tohead on chromosome 2q36. FEBS Lett. 1998;424:11–16. doi: 10.1016/s0014-5793(98)00128-8. [DOI] [PubMed] [Google Scholar]
- Monboisse JC, Garnotel R, Bellon G, Ohno N, Perreau C, Borel JP, Kefalides NA. The alpha 3 chain of type IV collagen prevents activation of human polymorphonuclear leukocytes. J. Biol. Chem. 1994;269:25475–25482. [PubMed] [Google Scholar]
- Mori K, Ando A, Gehlbach P, Nesbitt D, Takahashi K, Goldsteen D, Penn M, Chen CT, Mori K, Melia M, et al. Inhibition of choroidal neovascularization by intravenous injection of adenoviral vectors expressing secretable endostatin. Am. J. Pathol. 2001;159:313–320. doi: 10.1016/S0002-9440(10)61697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muona A, Eklund L, Väisänen T, Pihlajaniemi T. Developmentally regulated expression of type XV collagen correlates with abnormalities in col15a1−/− mice. Matrix Biol. 2002;21:89–102. doi: 10.1016/s0945-053x(01)00187-1. [DOI] [PubMed] [Google Scholar]
- Muragaki Y, Abe N, Ninomiya Y, Olsen BR, Ooshima A. The human alpha 1 (XV) collagen chain contains a large amino-terminal non-triple helical domain with a tandem repeat structure and homology to alpha 1 (XVIII) collagen. J. Biol. Chem. 1994;269:4042–4046. [PubMed] [Google Scholar]
- Musso O, Theret N, Heljasvaara R, Rehn M, Turlin B, Campion JP, Pihlajaniemi T, Clement B. Tumor hepatocytes and basement membrane-Producing cells specifically express two different forms of the endostatin precursor, collagen XVIII, in human liver cancers. Hepatology. 2001;33:868–876. doi: 10.1053/jhep.2001.23189. [DOI] [PubMed] [Google Scholar]
- Myers JC, Dion AS, Abraham V, Amenta PS. Type XV collagen exhibits a widespread distribution in human tissues but a distinct localization in basement membrane zones. Cell. Tissue Res. 1996;286:493–505. doi: 10.1007/s004410050719. [DOI] [PubMed] [Google Scholar]
- Myllyharju J, Kivirikko KI. Collagens and collagen-related diseases. Ann. Med. 2001;33:7–21. doi: 10.3109/07853890109002055. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Takano K, Kuzumaki H. A 16-kDa fragment of collagen type XIV is a novel neutrophil chemotactic factor purified from rat granulation tissue. Biochem. Biophys. Res. Commun. 1999;256:642–645. doi: 10.1006/bbrc.1999.0393. [DOI] [PubMed] [Google Scholar]
- Nakano KY, Iyama KI, Mori T, Yoshioka M, Sado Y, Ninomiya Y. Loss of alveolar basement membrane type IV collagen alpha 3, alpha 4, and alpha 5 chains in bronchioalveolar carcinoma of the lung. J. Pathol. 2001;194:420–427. doi: 10.1002/path.928. [DOI] [PubMed] [Google Scholar]
- Netzer KO, Suzuki K, Itoh Y, Hudson BG, Khalifah RG. Comparative analysis of the noncollagenous NC1 domain of type IV collagen: identification of structural features important for assembly, function, and pathogenesis. Protein. Sci. 1998;7:1340–1351. doi: 10.1002/pro.5560070610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SP, Warman ML, Seldin MF, Cheng SD, Knoll JH, Timmons S, Olsen BR. Cloning of cDNA and genomic DNA encoding human type XVIII collagen and localization of the alpha 1 (XVIII) collagen gene to mouse chromosome 10 and human chromosome 10. Genomics. 1994a;19:494–499. doi: 10.1006/geno.1994.1098. [DOI] [PubMed] [Google Scholar]
- Oh PS, Kamagata Y, Muragaki Y, Timmons S, Ooshima A, Olsen BR. Isolation and sequencing of cDNAs for proteins with multiple domains of Gly-Xaa-Yaa repeats identify a distinct family of collagenous proteins. Proc. Natl. Acad. Sci. USA. 1994b;91:4229–4233. doi: 10.1073/pnas.91.10.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen BR, Ninomiya Y. In: Guidebook to the Extracellular matrix, anchor, and adhesion proteins. Kreis T, Vale R, editors. Oxford: Oxford University Press; 1999. pp. 380–404. [Google Scholar]
- O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- Palmieri D, Camardella L, Ulivi V, Guasco G, Manduca P. Trimer carboxyl propeptide of collagen I produced by mature osteoblasts is chemotactic for endothelial cells. J Biol. Chem. 2000;275:32658–32663. doi: 10.1074/jbc.M002698200. [DOI] [PubMed] [Google Scholar]
- Pasco S, Monboisse JC, Kieffer N. The alpha 3(IV)185–206 peptide from non collagenous domain 1 of type IV collagen interacts with a novel binding site on the beta 3 subunit of integrin alpha V beta 3 and stimulates focal adhesion kinase and phosphatidylinositol 3-kinase phosphorylation. J. Biol. Chem. 2000a;275:32999–33007. doi: 10.1074/jbc.M005235200. [DOI] [PubMed] [Google Scholar]
- Pasco S, Han J, Gillery P, Bellon G, Maquart FX, Borel JP, Kefalides NA, Monboisse JC. A specific sequence of the non collagenous domain of the a3(IV) chain of type IV collagen inhibits expression and activation of matrix metalloproteinases by tumor cells. Cancer Res. 2000b;60:467–473. [PubMed] [Google Scholar]
- Perris R, Syfrig J, Paulsson M, Bronner-Fraser M. Molecular mechanisms of neural crest cell attachment and migration on type I and IV collagen. J. Cell Sci. 1993;106:1357–1368. doi: 10.1242/jcs.106.4.1357. [DOI] [PubMed] [Google Scholar]
- Petitclerc E, Boutaud A, Prestayko A, Xu J, Sado Y, Ninomiya Y, Sarras MP, Jr, Hudson BG, Brooks P. New functions for non-collagenous domains of human collagen type IV. J. Biol. Chem. 2000;275:8051–8061. doi: 10.1074/jbc.275.11.8051. [DOI] [PubMed] [Google Scholar]
- Ramchandran R, Dhanabal M, Volk R, Waterman MJF, Segal M, Lu H, Knebelmann B, Sukhatme VP. Antiangiogenic activity of Restin, NC10 domain of human collagen XV: comparison to endostatin. Biochem. Biophys. Res. Commun. 1999;255:735–739. doi: 10.1006/bbrc.1999.0248. [DOI] [PubMed] [Google Scholar]
- Rehn M, Pihlajaniemi T. α1(XVIII), a collagen chain with frequent interruptions in the collagenous sequence, a distinct tissue distribution, and homology with type XV collagen. Proc. Natl. Acad. Sci. USA. 1994;91:4234–4238. doi: 10.1073/pnas.91.10.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehn M, Pihlajaniemi T. Identification of three N-terminal ends of type XVIII collagen chains and tissue-specific differences in the expression of the corresponding transcripts. J. Biol. Chem. 1995;270:4705–4711. doi: 10.1074/jbc.270.9.4705. [DOI] [PubMed] [Google Scholar]
- Rehn M, Hintikka E, Pihlajaniemi T. Primary structure of the alpha 1 chain of mouse type XVIII collagen, partial structure of the corresponding gene, and comparison of the alpha 1 (XVIII) chain with its homologue, the alpha 1 (XV) collagen chain. J. Biol. Chem. 1994;269:13929–13935. [PubMed] [Google Scholar]
- Rehn M, Veikkola T, Kukk-Valdre E, Nakamura H, Ilmonen M, Lombardo CR, Pihlajaniemi T, Alitalo K, Vuori K. Interaction of endostatin with integrins implicated in angiogenesis. Proc. Natl. Acad. Sci. USA. 2001;98:1024–1029. doi: 10.1073/pnas.031564998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM. Enhanced pathological angiogenesis in mice lacking β3 integrin or β3 and β5 integrins. Nat. Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- Saarela J, Ylikarppa R, Rehn M, Purmonen S, Pihlajaniemi T. Complete primary structure of two variant forms of human type XVIII collagen and tissue-specific differences in the expression of the corresponding transcripts. Matrix Biol. 1998a;16:319–328. doi: 10.1016/s0945-053x(98)90003-8. [DOI] [PubMed] [Google Scholar]
- Saarela J, Rehn M, Oikarinen A, Autio-Harmainen H, Pihlajaniemi T. The short and long forms of type XVIII collagen show clear tissue specificities in their expression and location in basement membrane zones in humans. Am. J. Pathol. 1998b;153:611–626. doi: 10.1016/S0002-9440(10)65603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sado Y, Kagawa M, Naito I, Ueki Y, Seki T, Momota R, Oohashi T, Ninomiya Y. Organization and expression of basement membrane collagen IV genes and their roles in human disorders. J. Biochem. 1998;123:767–776. doi: 10.1093/oxfordjournals.jbchem.a022003. [DOI] [PubMed] [Google Scholar]
- Sarras MP, Jr, Zhang X, Huff JK, Accavitti MA, St John PL, Abrahamson DR. Extracellular matrix (mesoglea) of Hydra vulgaris III. Formation and function during morphogenesis of hydra cell aggregates. Dev. Biol. 1993;157:383–398. doi: 10.1006/dbio.1993.1143. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Fukai N, Mann K, Göhring W, Olsen BR, Timpl R. Structure, function and tissue forms of the C-terminal globular domain of collagen XVIII containing the angiogenesis inhibitor endostatin. EMBO J. 1998;17:4249–4256. doi: 10.1093/emboj/17.15.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Larsson H, Tisi D, Claesson-Welsh L, Hohenester E, Timpl R. Endostatins derived from collagens XV and XVIII differ in structural and binding properties, tissue distribution and anti-angiogenic activity. J. Mol. Biol. 2000;301:1179–1190. doi: 10.1006/jmbi.2000.3996. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. Basement membrane: Putting up the barriers. Curr. Biol. 1999;9:R242–R244. doi: 10.1016/s0960-9822(99)80153-5. [DOI] [PubMed] [Google Scholar]
- Setty S, Kim Y, Fields GB, Clegg DO, Wayner EA, Tsilibary EC. Interactions of type IV collagen and its domains with human mesangial cells. J. Biol. Chem. 1998;273:12244–12249. doi: 10.1074/jbc.273.20.12244. [DOI] [PubMed] [Google Scholar]
- Sertie AL, Sossi V, Camargo AA, Zatz M, Brahe C, Passos-Bueno MR. Collagen XVIII, containing an endogenous inhibitor of angiogenesis and tumor growth, plays a critical role in the maintenance of retinal structure and in neural tube closure (Knobloch syndrome) Hum. Mol. Genet. 2000;9:2051–2058. doi: 10.1093/hmg/9.13.2051. [DOI] [PubMed] [Google Scholar]
- Shahan TA, Ohno N, Pasco S, Borel JP, Monboisse JC, Kefalides NA. Inhibition of tumor cell proliferation by type IV collagen requires increased levels of camp. Connect. Tissue Res. 1999a;40:221–232. doi: 10.3109/03008209909005285. [DOI] [PubMed] [Google Scholar]
- Shahan TA, Ziaie Z, Pasco S, Fawzi A, Bellon G, Monboisse JC, Kefalides NA. Identification of CD47/integrin-associated protein and alpha(v)beta3 as two receptors for the alpha3(IV) chain of type IV collagen on tumor cells. Cancer Res. 1999b;59:4584–4590. [PubMed] [Google Scholar]
- Shahan TA, Fawzi A, Bellon G, Monboisse JC, Kefalides NA. Regulation of tumor cell chemotaxis by type IV collagen is mediated by a Ca(2+)-dependant mechanism requiring CD47 and the integrin alpha(v)beta(3) J. Biol. Chem. 2000;275:4796–4802. doi: 10.1074/jbc.275.7.4796. [DOI] [PubMed] [Google Scholar]
- Shen GQ, Butkowski R, Cheng T, Wieslander J, Katz A, Cass J, Fish AJ. Comparison of non-collagenous type IV collagen subunits in human glomerular basement membrane, alveolar basement membrane, and placenta. Connect. Tissue Res. 1990;24:289–301. doi: 10.3109/03008209009152156. [DOI] [PubMed] [Google Scholar]
- Shichiri M, Hirata Y. Antiangiogenesis signals by endostatin. FASEB J. 2001;15:1044–1053. doi: 10.1096/fj.99-1083com. [DOI] [PubMed] [Google Scholar]
- Soininen R, Huotari M, Hostikka SL, Prockop DJ, Tryggvason K. The structural genes for alpha 1 and alpha 2 chains of human type IV collagen are divergently encoded on opposite DNA strands and have an overlapping promoter region. J. Biol. Chem. 1988;263:17217–17220. [PubMed] [Google Scholar]
- Sorensen DR, Read TA, Porwol T, Olsen BR, Timpl R, Sasaki T, Iversen PO, Benestad HB, Sim BK, Bjerkvig R. Endostatin reduces vascularization, blood flow, and growth in a rat gliosarcoma. Neuro-Oncol. 2002;4:1–8. doi: 10.1215/15228517-4-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standker L, Schrader M, Kanse SM, Jurgens M, Forssmann WG, Preissner KT. Isolation and characterization of the circulating form of human endostatin. FEBS. Lett. 1997;420:129–133. doi: 10.1016/s0014-5793(97)01503-2. [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Oohashi T, Ninomiya Y. The genes COL4A5 and COL4A6, coding for basement membrane collagen chains alpha 5(IV) and alpha 6(IV), are located head-to-head in close proximity on human chromosome Xq22 and COL4A6 is transcribed from two alternative promoters. Proc. Natl. Acad. Sci. USA. 1994;91:11679–11683. doi: 10.1073/pnas.91.24.11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaramoorthy M, Meiyappan M, Todd P, Hudson BG. Crystal structure of NC1 domains: Structural basis for type IV collagen assembly in basement membranes. J. Biol. Chem. 2002;277:31142–31153. doi: 10.1074/jbc.M201740200. [DOI] [PubMed] [Google Scholar]
- Taddei L, Chiarugi P, Brogelli L, Cirri P, Magnelli L, Raugei G, Ziche M, Granger HJ, Chiarugi V, Ramponi G. Inhibitory effect of full-length human endostatin on in vitro angiogenesis. Biochem. Biophys. Res. Commun. 1999;263:340–345. doi: 10.1006/bbrc.1999.1342. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Iyama K, Kitaoka M, Ninomiya Y, Oohashi T, Sado Y, Ono T. Differential expression of alpha 1(IV), alpha 2(IV), alpha 5(IV) and alpha 6(IV) collagen chains in the basement membrane of basal cell carcinoma. Histochem. J. 1997;29:563–570. doi: 10.1023/a:1026428010104. [DOI] [PubMed] [Google Scholar]
- Than ME, Henrich S, Huber R, Ries A, Mann K, Kühn K, Timpl R, Bourenkov GP, Bartunik HD, Bode W. The 1.9-Å crystal structure of the noncollagenous (NC1) domain of human placenta collagen IV shows stabilization via a novel type of covalent Met-Lys crosslink. Proc. Natl. Acad. Sci. USA. 2002;99:6607–6612. doi: 10.1073/pnas.062183499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R, Wiedemann H, van Delden V, Furthmayr H, Kuhn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur. J. Biochem. 1981;120:203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- Timpl R, Brown JC. Supramolecular assembly of basement membranes. Bioessays. 1996;18:123–132. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- Tomono Y, Naito I, Ando K, Yonezawa T, Sado Y, Hirakawa S, Arata J, Okigaki T, Ninomiya Y. Epitope-defined monoclonal antibodies against multiplexin collagens demonstrate that type XV and XVIII collagens are expressed in specialized basement membranes. Cell. Struct. Funct. 2002;27:9–20. doi: 10.1247/csf.27.9. [DOI] [PubMed] [Google Scholar]
- Tsilibary EC, Reger LA, Vogel AM, Koliakos GG, Anderson SS, Charonis AS, Alegre JN, Furcht LT. Identification of a multifunctional, cell-binding peptide sequence from the a1 (NC1) of type IV collagen. J. Cell. Biol. 1990;111:1583–1591. doi: 10.1083/jcb.111.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbich C, Reissner A, Chavakis E, Dernbach E, Haendeler J, Fleming I, Zeiher AM, Kaszkin M, Dimmeler S. Dephosphorylation of endothelial nitric oxide synthase contributes to the anti-angiogenic effects of endostatin. FASEB J. 2002;16:706–708. doi: 10.1096/fj.01-0637fje. [DOI] [PubMed] [Google Scholar]
- Van der Rest M, Rosenberg LC, Olsen BR, Poole AR. Chondrocalcin is identical with the C-propeptide of type II procollagen. Biochem. J. 1986;237:923–925. doi: 10.1042/bj2370923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel W. Discoidin domain receptors: structural relations and functional implications. FASEB J. 1999;13:S77–S82. doi: 10.1096/fasebj.13.9001.s77. [DOI] [PubMed] [Google Scholar]
- Wen W, Moses MA, Wiederschain D, Arbiser JL, Folkman J. The generation of endostatin is mediated by elastase. Cancer Res. 1999;59:6052–6056. [PubMed] [Google Scholar]
- Wickström SA, Veikkola T, Rehn M, Pihlajaniemi T, Alitalo K, Keski-Oja J. Endostatin-induced modulation of plasminogen activation with concomitant loss of focal adhesions and actin stress fibers in cultured human endothelial cells. Cancer Res. 2001;61:6511–6516. [PubMed] [Google Scholar]
- Xu J, Rodriguez D, Petitclerc E, Kim JJ, Hangai M, Yuen SM, Davis GE, Brooks P. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J. Cell Biol. 2001;154:1069–1079. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N, Anad-Apte B, Lee M, Sasaki T, Fukai N, Shapiro R, Que I, Lowik C, Timpl R, Olsen BR. Endostatin inhibits VEGF-induced endothelial cell migration and tumor growth independently of zinc binding. EMBO J. 1999;18:4414–4423. doi: 10.1093/emboj/18.16.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin G, Liu W, An P, Li P, Ding I, Planelles V, Schwarz EM, Min W. Endostatin gene transfer inhibits joint angiogenesis and pannus formation in inflammatory arthritis. Mol. Ther. 2002;5:547–554. doi: 10.1006/mthe.2002.0590. [DOI] [PubMed] [Google Scholar]
- Yoon SS, Eto H, Lin CM, Nakamura H, Pawlik TM, Song SU, Tanabe KK. Mouse endostatin inhibits the formation of lung and liver metastases. Cancer Res. 1999;59:6251–6256. [PubMed] [Google Scholar]
- You WK, So SH, Lee H, Park SY, Yoon MR, Chang SI, Kim HK, Joe YA, Hong YK, Chung SI. Purification and characterization of recombinant murine endostatin in E. coli. Exp. Mol. Med. 1999;31:197–202. doi: 10.1038/emm.1999.32. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Ruben GC. Basement membrane structure in situ: evidence for lateral associations in the type IV collagen networks. J. Cell Biol. 1987;105:2559–2568. doi: 10.1083/jcb.105.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Hudson BG, Sarras MP., Jr Hydra cell aggregate development is blocked by selective fragments of fibronectin and type IV collagen. Dev. Biol. 1994;164:10–23. doi: 10.1006/dbio.1994.1176. [DOI] [PubMed] [Google Scholar]
- Zhou J, Mochizuki T, Smeets H, Antignac C, Laurila P, de Paepe A, Tryggvason K, Reeders ST. Deletion of the paired alpha 5(IV) and alpha 6(IV) collagen genes in inherited smooth muscle tumors. Science. 1993;261:1167–1169. doi: 10.1126/science.8356449. [DOI] [PubMed] [Google Scholar]