Abstract
Interactions between innate and adaptive immune receptors are critical for an optimal immune response, but the role played by antigen receptors in modulating innate receptor functions is less clear. TLRs are a family of pattern recognition receptors that play crucial roles in detecting microbial pathogens and subsequent development of immune responses. However, chronic stimulation through TLRs render immune cells hyporesponsive to subsequent stimulation with TLR ligands; a phenomenon known as TLR tolerance, well characterized in myeloid cells. However, it has not been studied in detail in B lymphocytes. In addition to the BCR, B cells express almost all known TLRs and respond robustly to many TLR ligands. Thus, B cells may receive signals through both TLRs and BCR during an infection and may respond differently to TLR stimulation than myeloid cells. We tested this possibility by stimulating repeatedly through either TLR alone or through both TLR and BCR. Prestimulation through TLR7 resulted in reduced B cell proliferation, cytokine production and IgM secretion upon subsequent TLR7 restimulation. The hyporesponsiveness to TLR7 restimulation was associated with reduced NF-κB and MAP kinase activation and defective c-Jun phosphorylation. However, simultaneous BCR signaling prevented or reversed TLR7 tolerance in both mouse and human B cells. Importantly, BCR signaling also rescued B cells from TLR7-mediated TLR9 tolerance. Additionally, the reversal of TLR7-mediated JNK activation was dependent on phosphatidylinositol 3-kinase activation. Together these results present a novel mechanism to prevent and reverse TLR tolerance in B cells.
Keywords: B cells, Tolerance, Human, cytokines, kinases
INTRODUCTION
Signals through innate immune receptors have an important influence upon the outcome of antigen engagement by the BCR (1–3). Of particular interest is a family of pattern recognition receptors called TLR (4). TLRs are expressed on a variety of cell types where they recognize molecular patterns unique to or enriched in microbes and alert the immune system to the presence of an invading pathogen (5).
However, TLR responses must be tightly regulated as uncontrolled production of cytokines can result in immunopathology. Thus, repeated or chronic stimulation through TLRs can render immune cells unresponsive to the same or different TLR ligands. This phenomenon, known as TLR tolerance, is well characterized in macrophages especially in the case of TLR4 and its ligand LPS (6–8). However, TLR tolerance is not restricted to TLR4 as prestimulation with other TLR ligands has also been shown to induce refractoriness in immune cells in response to subsequent challenge with the same or different TLR ligands (9–12). The tolerant state in macrophages is associated with reduced activation of NF-κB and mitogen-activated protein kinases (MAPK) and suppression of various cytokines such as TNF, IL-6 and IL-12 (13). However, other macrophage functions such as bacterial phagocytosis (14) or nitric oxide and IL-10 production (15) are not affected in tolerant macrophages. Although a number of mechanisms are thought to be involved in induction of tolerance, such as down regulation of surface receptors, transcriptional induction of negative regulators such as IRAK-M, SOCS-1 and SHIP and production of anti-inflammatory cytokines such as TGF-β and IL-10 (16), the exact mechanism by which tolerance is induced is not clear. Because not all TLR-mediated functions are affected in tolerant macrophages a recent study proposed that the regulation could be at the level of individual promoters. According to this model, gene-specific control mechanisms will allow transient inactivation of certain genes following initial TLR stimulation while maintaining normal induction of others upon subsequent TLR stimulation (17).
Although a wealth of information is available on macrophage and dendritic cell TLR responses, B cell-intrinsic TLR functions are only beginning to be appreciated and it is not clear whether TLR responses are regulated similarly in B cells and myeloid cells. We have previously shown that R848, a synthetic low-molecular weight compound belonging to the imidazoquinoline family, can induce NF-κB and MAPK activation in B cells, activate B cells to proliferate, produce cytokines and antibody, and express increased levels of costimulatory molecules (1, 18, 19). Additionally, R848 has been shown to cooperate synergistically with the BCR and CD40 to induce increased amounts of cytokines and antibody production (1). Results from our lab further showed that the synergestic IL-6 production in B cells stimulated through TLR7 and CD40 is through enhanced c- Jun kinase (JNK) signaling and AP-1 activity (20). However, it is not known how these B cell responses are regulated or the effect of repeated or prolonged stimulation through TLR7 to subsequent stimulation through TLR7 or other TLRs, especially TLR9 which recognizes unmethylated CpG motifs of bacterial and viral DNA. This is an important issue to be addressed not only to understand B cell TLR responses but also because a number of synthetic ligands for this TLR subfamily are under consideration as vaccine adjuvants (21). A better understanding of how B cell responses are regulated following stimulation through TLRs that recognize microbial nucleic acids will be useful in developing improved vaccines. In contrast to myeloid cells, B cells express both germline-encoded pattern recognition receptors and clonally rearranged antigen specific receptors on their surfaces. Thus, it is likely that during an infection B cells will receive signals through both TLRs and the BCR. In this study we sought to investigate the effects of repeated stimulation through TLR7 on B cell responses and how these responses are modulated in the presence of concurrent BCR signaling. The results suggest a novel mechanism to prevent and reverse TLR7 tolerance in B cells.
MATERIALS AND METHODS
Mouse and human B cells
Resting high density splenic B cells were isolated from C57BL/6 mice as described previously with slight modifications (22). Briefly, splenocytes were depleted of T cells by treatment with anti-Thy-1.2 Ab, followed by complement lysis; the resultant cells were subjected to density gradient centrifugation and high density B cells were recovered from the 65% to 85% interface. In some experiments, high density resting splenocytes were first isolated by density gradient centrifugation through a 60%:65%:85% Percoll gradient. Resting lymphocytes at the interface between 65% and 85% were then further purified by negative selection with anti-mouse CD43 magnetic beads (Miltenyi Biotec) according to the manufacturer's protocols to obtain resting high density splenic B cells. Cell preparations were >90% pure, as determined by FACS analysis with FITC-anti-B220 staining. Use of normal mouse cells in this study followed a protocol approved by The University of Iowa Animal Care and Use Committee. Normal human PBMC were isolated from buffy coats of healthy adult blood donors by Ficoll-Hypaque density sedimentation. B cells were isolated from PBMC suspensions by negative selection using a B cell isolation kit according to manufacturer’s instruction (StemCell Technologies Inc., Vancouver, Canada). B cell purity as determined by CD19 staining was >95%. The use of normal human B cells in this study was approved by The University of Iowa IRB. Cells were cultured in RPMI 1640 with 10% heat-inactivated fetal calf serum (FCS; Atlanta Biologicals, Norcross, GA), 10 µM 2-ME (GIBCO, Grand Island, NY), 2 mM L-glutamine, penicillin and streptomycin.
Reagents
Polyclonal rabbit antibodies (Ab) against phospho-JNK, phospho-p38, p38, c-Jun, and AKT were from Cell Signaling Technology (Beverly, MA). Rabbit anti-JNK1/2 (FL) Ab was from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal anti-phospho ERK (p44/42 MAPK) and rabbit monoclonal anti-phospho-Akt (ser 473) Abs were purchased from Biosource/Invitrogen (Carlsbad, CA). Ab specific for phospho-c-Jun was from Upstate Biotechnologies (Lake Placid, NY). Mouse anti-actin Ab (C4) was purchased from Chemicon International (Temecula, CA). Affinity purified (Fab')2 goat anti-mouse and anti-human IgM (µ-chain specific) Ab, goat IgG (Fab')2 Ab and peroxidase-labeled goat anti-rabbit and goat anti-mouse IgG Abs were from Jackson ImmunoResearch Laboratories (West Grove, PA). LY294002 and BAY11-7082 was from Calbiochem Inc. (San Diego, CA). Anti-mouse and anti-human IL-6 mAbs (coating and biotinylated) and anti-TNF mAbs (coating and biotinylated) were from eBioscience (San Diego, CA). Multiplex assay (Mouse 20-plex panel) was from Biosource/Invitrogen (Carlsbad, CA). Recombinant mouse IL-6 and TNF-α were purchased from PeproTech. Streptavidin-HRP was purchased from Jackson ImmunoResearch Laboratories. ELISA TMB peroxidase substrate was purchased from KPL. Anti-mouse IgM Abs for ELISA (coating, biotinylated, and standards) were purchased from Southern Biotechnology Associates. CellTrace™ CFSE cell proliferation kit was obtained from Invitrogen Life technologies. CpG ODN 1826 (TCCATGACGTTCCTGACGTT) was from IDT (Coralville, IA) and R848 was from Alexis Biochemicals (San Diego, CA).
B cell stimulation
Mouse high density splenic B cells or human peripheral blood B cells (1 × 106/ml) stimulated with R848 for 24h were washed and restimulated with R848 or R848 and anti-IgM for another 24h. Cell culture supernatants were collected and stored at −20°C until used for ELISA assays to determine cytokine production. Control B cells were incubated for 1hr (mouse splenic B cells) or for 24h (human peripheral blood B cells) in medium and then stimulated with R848 for 24h. Goat IgG (Fab')2 Ab stimulation was used as a control to confirm the specificity of the anti-IgM response. Mouse high density resting splenic B cells prestimulated with R848 and anti-IgM Ab were used to determine the effect of dual stimulation on tolerance induction. In these experiments, after 24h stimulation, cells were washed and restimulated with R848 alone for another 24h.
Western blots
Purified splenic B cells (2 ×106) were treated as indicated in Figures. The cells were then pelleted by centrifugation, dissolved in 2X SDS samples loading buffer and sonicated. Total cell lysates were fractionated by SDS-PAGE and blotted to polyvinylidene difluoride membranes. After blotting with the appropriate Abs bands were visualized on Western blots using a chemiluminescent detection reagent (Pierce, Rockford, IL). Images of blots were recorded with a low-light imaging system (LAS-3000, Fujifilm Medical Systems USA, Stamford, CT). Immunoblots were stripped and reprobed with control Ab to verify equal protein loading in each lane. Chemiluminesence was subsequently quantified using ImageGauge software (Fujifilm). Values were then used to calculate ratios of phosphorylated:total protein (phospho-protein:control protein).
CFSE assays
Naïve high density resting splenic B cells isolated from mouse spleen or B cells prestimulated with R848 or R848 and anti-IgM antibody for 24h were labelled with CFSE (0.5µM) following manufacturer’s instruction. Cells were then stimulated with or without R848 in the presence or absence of anti-IgM antibody for four days. Proliferation was determined by CFSE dilution via flow cytometry.
ELISA
Mouse or human primary B cells (1 × 106/ml) were stimulated as described. Supernatants were collected and stored at −20°C. Cytokine levels in the culture supernatants were measured by sandwich ELISA, using mAb from eBiosciences following the manufacturer's instructions. Plates were read at 450 nm by a SpectraMax 250 Reader (Molecular Devices). Data were analyzed with SoftMax Pro software (Molecular Devices). Luminex-based multiplex (mouse 20-plex) was performed according to the manufacturer’s protocol. Mouse IgM specific ELISA was performed according to the protocol provided by Southern Biotech. Plates were read at 405 nm by a SpectraMax 250 Reader. Data were analyzed with SoftMax Pro software (Molecular Devices);
RESULTS
Effect of repeated stimulation through TLR7 on B cell cytokine production
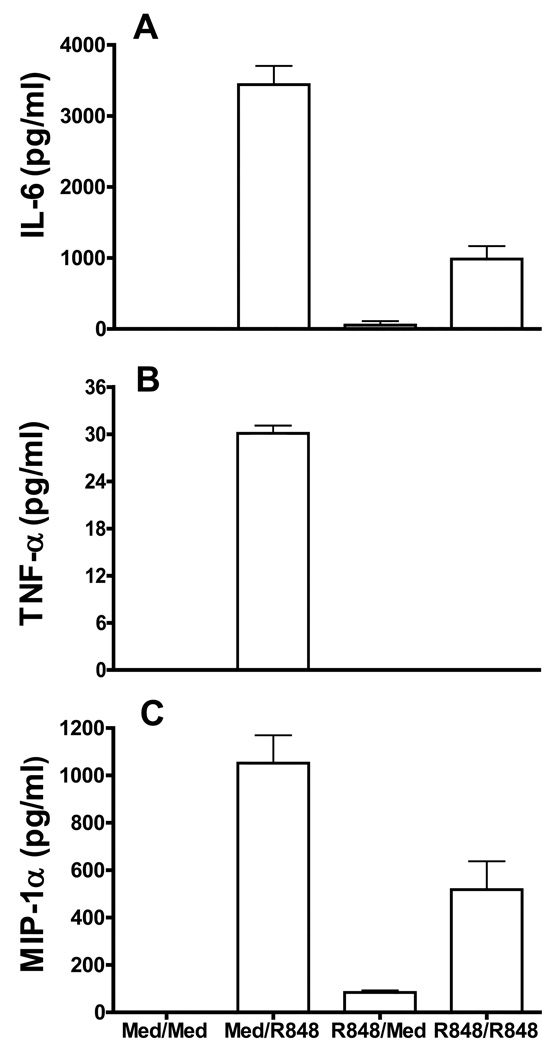
We have previously shown that R848, a synthetic TLR7 ligand, is a potent activator of B cells. R848 stimulation can initiate B cell proliferation, upregulation of costimulatory molecule expression and antibody production, and this synthetic compound is currently under consideration as a vaccine adjuvant (1, 18). Although B cell activating properties of R848 have been well characterized, how these responses are regulated or how B cell functions are modulated in the context of repeated or chronic stimulation through TLR7 is not clear. To address this, high density resting B cells isolated from mouse spleen were stimulated with R848 for 24 h. Cells were washed extensively and restimulated with R848 for another 24h. Cell culture supernatants were then assayed for cytokine production by ELISA. Naïve splenic B cells responded robustly to R848 stimulation by producing large amounts of cytokines IL-6, TNF-α and a chemokine MIP-1α (Fig.1A, B and C) in cell culture supernatants. In contrast, cytokine production was greatly reduced in B ells prestimulated with R848 in response to second R848 stimulation. However, IL-10 level was not different between TLR7 prestimulated and control B cells (data not shown). This suggests that, similar to macrophages (23), repeated stimulation through TLR7 can induce a refractory state in B cells. We focused on IL-6 production in subsequent experiments as this is one of the most striking effects of TLR7 stimulation on B cells.
Figure 1. Effect of repeated stimulation through TLR7 on B cell cytokine production.
High density resting B cells isolated from mouse spleen (1 × 106 /ml) were treated with R848 (1µg/ml) for 24h. Cells were then washed and restimulated with R848 (1µg/ml) for another 24h. Cell culture supernatants were assayed for IL-6 (A), TNF-α (B) and MIP-1α (C) production by ELISA/Multiplex assay. Naïve B cells incubated in medium (Med) for 1h and then stimulated with R848 for 24h were used as controls. Results are representative of 3 independent experiments for IL-6 and TNF-α and two independent experiments for MIP-1α.
The induction of R848-mediated tolerance was dependent on the concentration of the ligand as well as duration of the initial stimulation. Prestimulation with as little as 0.01µg/ml of R848 was able to induce tolerance in B cells (Supplementary Fig. 1A). Additionally, tolerance was induced only after prestimulation with R848 for greater than 12h (Supplementary Fig. 1B).
Effect of TLR7 tolerance on B cell proliferation and IgM secretion
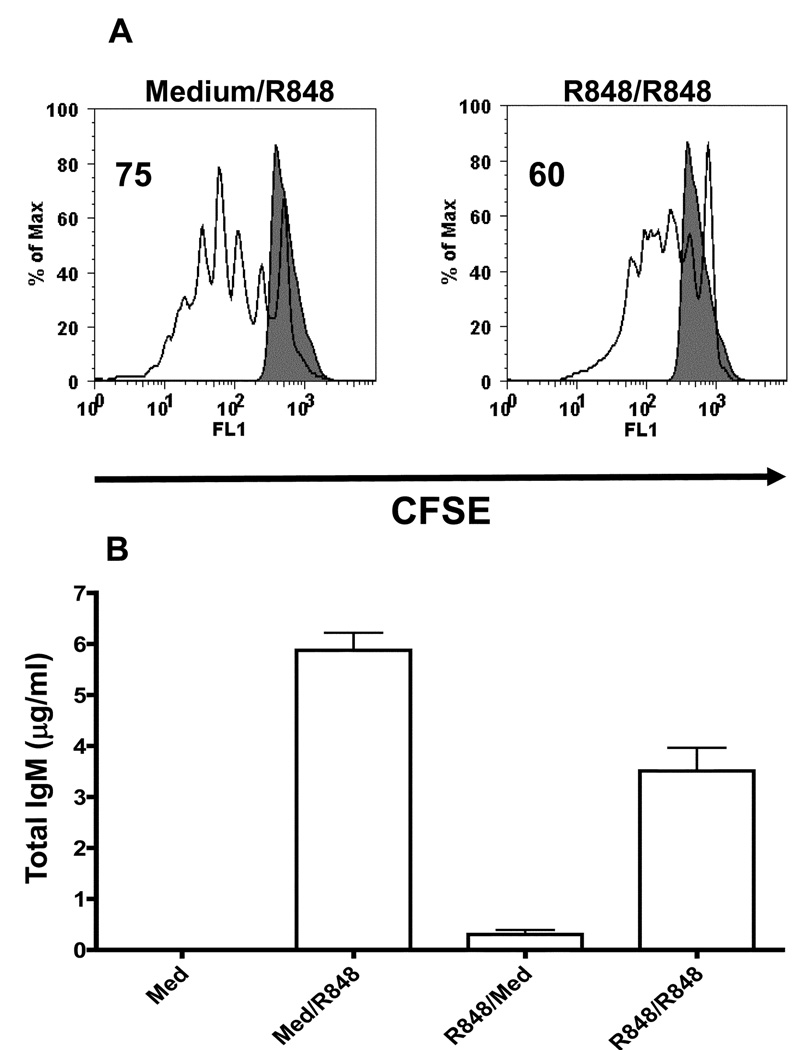
R848 has also been shown to induce B cell proliferation, upregulation of costimulatory molecules and antibody secretion (19). So we next tested whether TLR7 tolerance is associated with a defect in any of the above mentioned B cell functions. To determine the effect of TLR7 tolerance on B cell proliferation, naïve B cells isolated from mouse spleen or B cells prestimulated with R848 for 24h were labelled with CFSE and cultured with or without R848 for four days. CFSE dilution was subsequently determined by flow cytometry. As shown previously (19), R848 stimulation induced robust proliferation in naïve B cells (Fig. 2A). Although B cells prestimulated through TLR7 also exhibited proliferative responses upon subsequent restimulation with R848, the percentage of cells undergoing proliferation was reduced compared to naïve B cells. Because R848 can induce B cell differentiation and antibody secretion, we next investigated the effect of TLR7 tolerance on antibody secretion. For this, naïve B cells or B cells prestimulated with R84 for 24h were stimulated with R848 for four days. Total IgM in the cell culture supernatants was determined by IgM specific ELISA. As shown in Figure 2B, TLR7 tolerant B cells secreted less IgM in culture supernatants when compared to control B cells stimulated with R848. We also tested the effect of TLR7 tolerance on costimulatory molecule upregulation. However, CD80, CD86 and MHC class II expression were not different between TLR7 tolerant and control B cells (data not shown).
Figure 2. Effect of TLR7 tolerance on B cell proliferation and IgM secretion.
Naïve high density resting B cells or B cells prestimulated with R848 for 24h were labelled with CFSE. Cells were then stimulated with R848 for 4 days and analyzed by flow cytometry (A). Filled histogram represents CFSE labelled naïve B cells incubated with medium alone for 4 days. Results are representative of three independent experiments. B. R848-mediated IgM secretion in TLR7 tolerant B cells. Mouse high density resting B cells or B cells prestimulated with R848 for 24h were stimulated with R848 for 4 days. Cell culture supernatants were tested for IgM secretion by mouse IgM specific ELISA. Results are representative of two independent experiments
Defective MAP kinase activation in TLR7 tolerant B cells
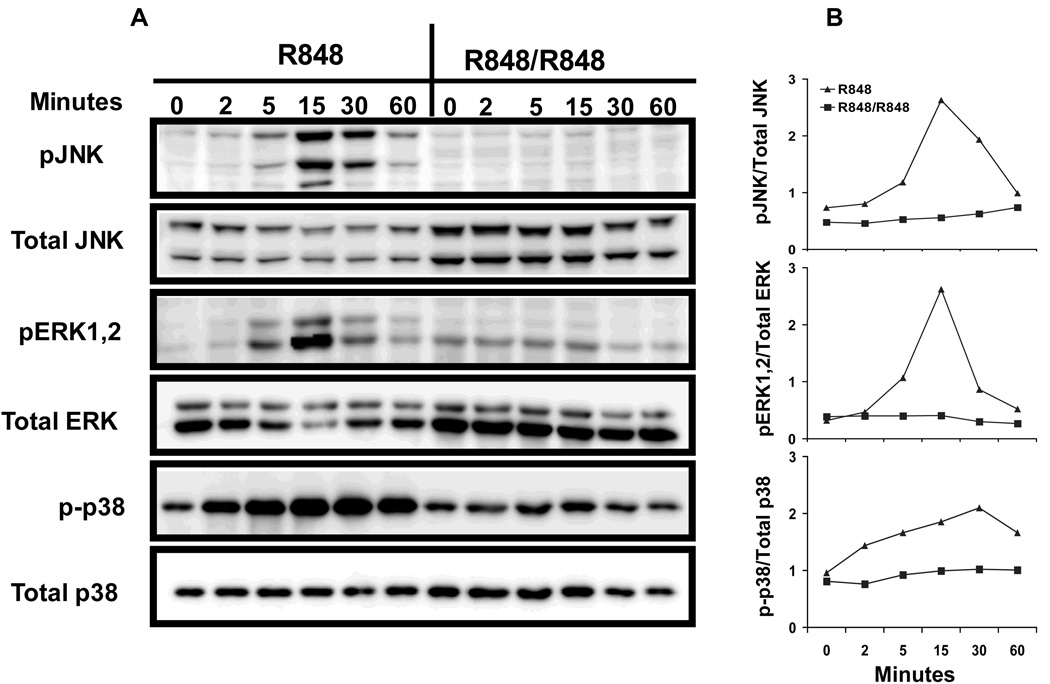
MAP kinase activation, one of the earliest events initiated by R848 stimulation in B cells (18), has been shown to be important for many B cell functions including IL-6 production (24). Thus, we next studied whether the reduced IL-6 production in TLR7 tolerant B cells is associated with any defects in MAP kinase activation. Mouse primary B cells were stimulated with R848 for 24h and after washing, cells were stimulated with R848 for indicated time points. Cellular extracts were then subjected to Western blotting and stained with antibodies specific for phoshorylated forms of JNK, ERK 1,2 and p38 kinase. Cellular extracts from naïve B cells stimulated with R848 for indicated time points were used as controls. R848 stimulation induced robust MAP kinase activation in control B cells with phosphorylation of JNK, ERK1,2 and p38 evident as early as 5 minutes in control B cells (Fig. 3) and peak response at ~15min. In contrast, MAP kinase activation was severely impaired in R848 prestimulated cells in response to subsequent R848 stimulation. The effect was even more striking for JNK. Phosphorylation of JNK was almost completely absent in TLR7-prestimulated B cells in response to second R848 stimulation. Phosphorylation of ERK1,2 and p38 was also greatly reduced in prestimulated B cells compared to control B cells.
Figure 3. MAP kinase activation in TLR7 tolerant B cells.
A. Mouse high density resting B cells stimulated with R848 (1µg/ml) for 24h were washed and restimulated with R848 (1µg/ml) for indicated time points. Control B cells were incubated at 37°C for 1h in culture medium and then stimulated with R848 for times indicated. Cells were lysed and whole cell extracts were subjected to Western blot analysis as described in Materials and Methods for pJNK, pERK1,2 and p-p38. Blots were stripped and reprobed for total JNK, ERK1,2 and p38. B. Quantitation of MAP kinase activation. The amount of phospho and total JNK, ERK1,2 and p38 in each lane was quantified using ImageGauge software. The amount of pJNK, pERK1,2 and p-p38 in each lane was normalized to the intensity of corresponding total JNK, ERK1,2 or p38 bands and the results are presented graphically. Results are representative of 3 independent experiments.
NF-κB activation and c-Jun phosphorylation in TLR7 tolerant B cells
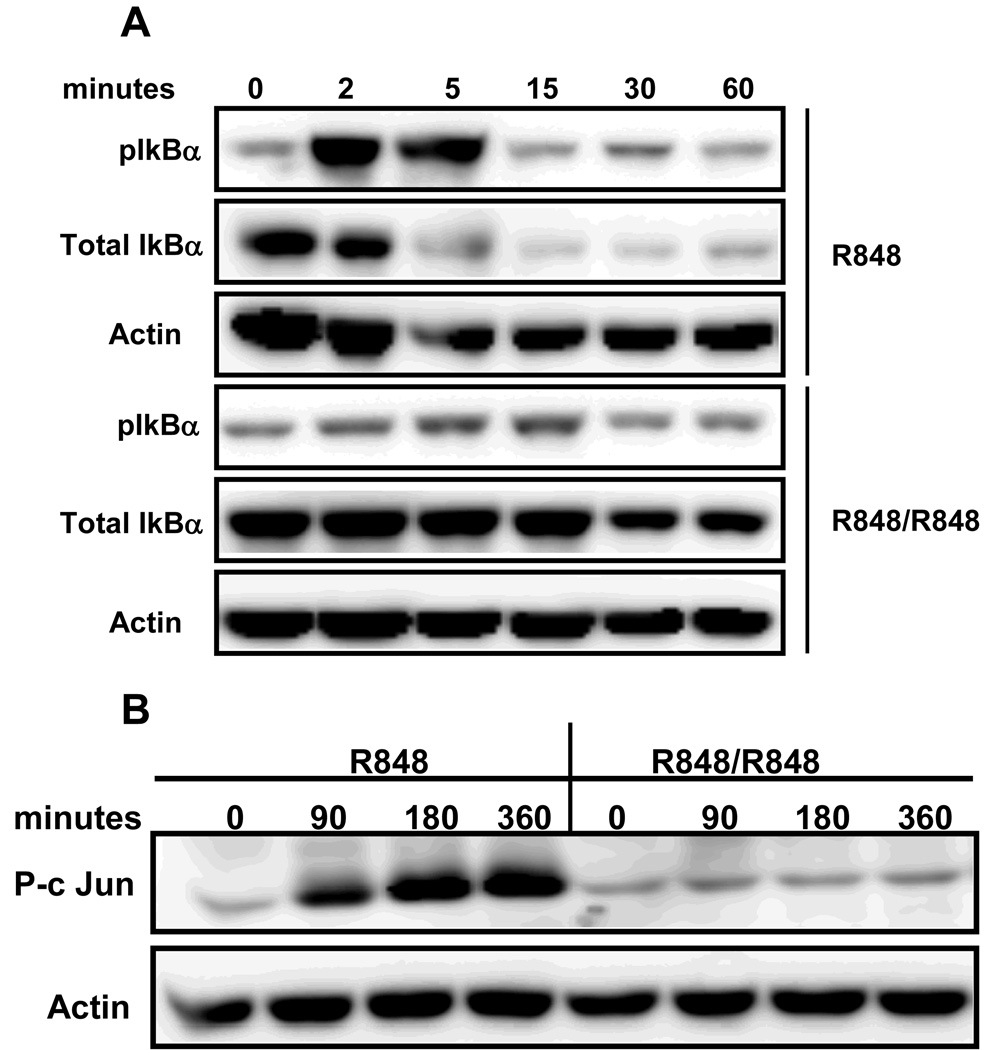
Defective NF-κB activation is a well known feature of LPS tolerance in macrophages (13). To test whether NF-κB activation is affected in TLR7 tolerized B cells, IκB-α phosphorylation and degradation was determined in R848 prestimulated and control B cells in response to subsequent R848 stimulation. As shown in Fig. 4A, R848 induced IκB-α phosphorylation in naïve B cells as early as 2 minutes with peak response at 5min after R848 stimulation. R848 also induced IκB-α degradation in naïve B cells, evident by 15min. IκB-α phosphorylation or degradation was markedly reduced in B cells prestimulated with R848 in response to subsequent R848 stimulation.
Figure 4. NF-κB activation and c-Jun phosphorylation in TLR7 tolerant B cells.
Mouse high density resting B cells stimulated with R848 (1µg/ml) for 24h were washed and restimulated with R848 (1µg/ml) for indicated time points. Control B cells were incubated at 37°C for 1h in medium and then stimulated with R848 for times indicated. Cells were lysed and subjected to Western blot analysis for phospho and total IkBα (A) or p-c Jun (B). Blots were stripped and reprobed for actin as a lane-loading control.
B cell activation properties of R848 and CD154/CD40 ligand are in some ways remarkably similar (1) and CD40-mediated IL-6 production in B cells has been shown to dependent on factors downstream of JNK signaling (24). Because R848-mediated JNK phosphorylation was severely impaired in TLR7 tolerant B cells, we investigated whether phosphorylation of its substrate, c-Jun, is also affected. Indeed, phosphorylation of c-Jun was severely impaired in B cells prestimulated with R848 in response to a second R848 stimulation (Fig. 4B). As above, naïve B cells stimulated with R848 exhibited a progressive increase in c-Jun phosphorylation. These results indicate that repeated stimulation through TLR7 can induce a state of refractoriness in B cells characterized by severe defects in major signaling pathways.
Effect of combined BCR and TLR7 stimulation on TLR7 tolerance in B cells
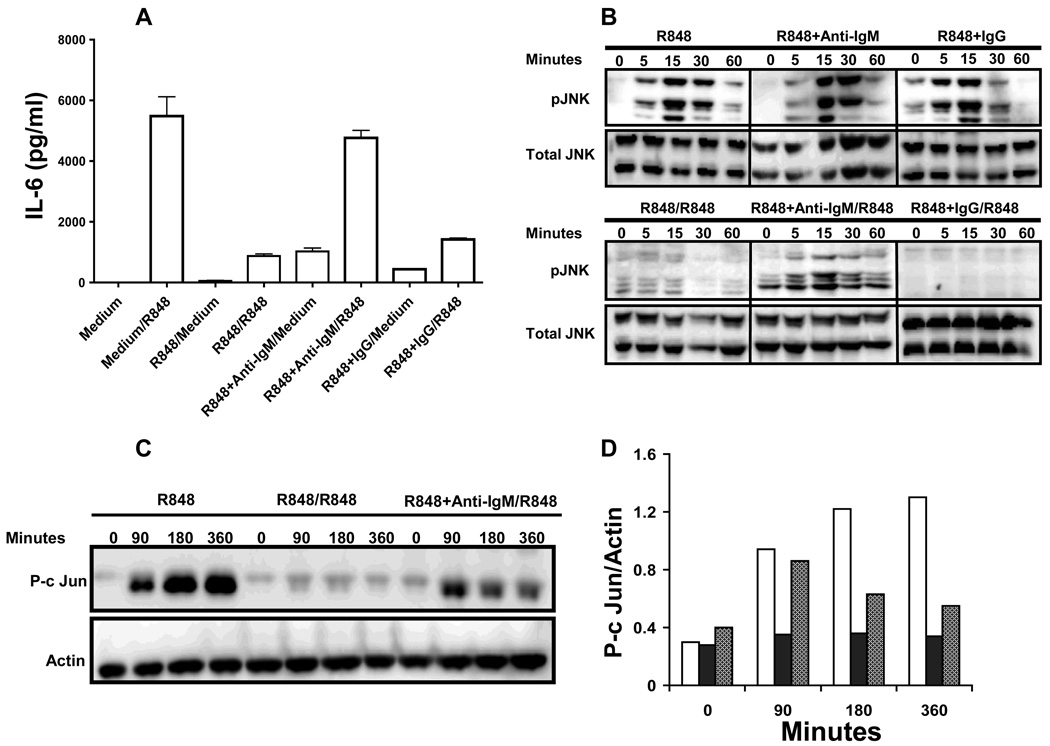
Most of our understanding about TLR tolerance to date is from studies conducted using myeloid cells. However an important difference between myeloid and B cells is the presence of a specific antigen receptor on the surface of the latter. Thus it is possible that B cells may receive signals simultaneously through both the BCR and TLR. However, the effect of combined signaling through BCR and TLR7 on TLR7 tolerance is not known. To address this, B cells were stimulated either with R848 alone or with R848 and anti-IgM antibody for 24h, after which cells were washed extensively and stimulated with R848 for another 24h and cell culture supernatants were tested for IL-6 production. Consistent with above findings, R848 stimulation induced high amounts of IL-6 production by naïve B cells and markedly reduced IL-6 production in TLR7 tolerant B cells (Fig. 5A). Interestingly, B cells prestimulated with both R848 and anti-IgM Ab produced markedly higher amounts of IL-6 following subsequent R848 stimulation suggesting that BCR signals can prevent TLR tolerance in B cells. Importantly, control IgG Ab treatment was not effective in preventing TLR7 tolerance. Additionally anti-IgM Ab stimulation alone did not induce any IL-6 production (data not shown).
Figure 5. Effect of prestimulation through BCR and TLR7 on subsequent TLR7-mediated JNK activation, c-Jun phosphorylation and IL-6 production in B cells.
(A) Mouse high density splenic B cells were stimulated with R848 (1µg/ml) or R848 and F(ab')2 fragments of goat anti-mouse IgM Ab at 10 µg/ml (anti-IgM) or R848 and F(ab')2 fragments of goat IgG (10µg/ml) (IgG) for 24h. Cells were then washed and restimulated with R848 (1µg/ml) for 24h. IL-6 production in cell culture supernatants was determined by ELISA. Results are compared with B cells cultured in medium for 1h and then stimulated with R848 for 24h. Results shown are representative of three independent experiments. (B–D) Mouse high density resting B cells stimulated with R848 (1µg/ml) (filled bars), R848 and anti-IgM (10µg/ml) (grey bars) or R848 and IgG (10µg/ml) for 24h were washed and restimulated with R848 (1µg/ml) for indicated time points. B cells incubated at 37°C for 1h in medium and then stimulated with R848 (1µg/ml) (open bars) for times indicated were used as controls. Cells were lysed and whole cell extracts were subjected to Western blot analysis for phospho JNK (B) or p-c Jun (C). Blots were stripped and reprobed for total JNK (B) or actin (C). (D) Quantitation of p-c Jun. The intensity of p-c Jun in each lane was normalized to the intensity of corresponding actin band and the results are presented graphically.
Because TLR7 tolerance was associated with reduced MAP kinase activation in B cells, we next investigated whether combined BCR and TLR7 stimulation can ameliorate the impaired R848-mediated MAP kinase activation seen in TLR7 tolerant B cells (Fig. 5B). Combined BCR and TLR7 stimulation induced enhanced JNK phosphorylation in naïve B cells and more importantly preserved R848-mediated JNK phosphorylation in prestimulated B cells. Simultaneous BCR ligation also rescued defective ERK1,2 and p38 phosphorylation in TLR7 tolerant B cells (data not shown). Furthermore, B cells prestimulated with R848 and anti-IgM antibody exhibited higher amounts of c-Jun phosphorylation compared to B cells prestimulated with R848 alone in response to subsequent R848 stimulation (Fig. 5C and D). To our understanding this is the first report of prevention of TLR7 tolerance by a physiological antigen receptor signal. Importantly, antigen signals are frequently delivered to B cells together with TLR signals during an infection or vaccination.
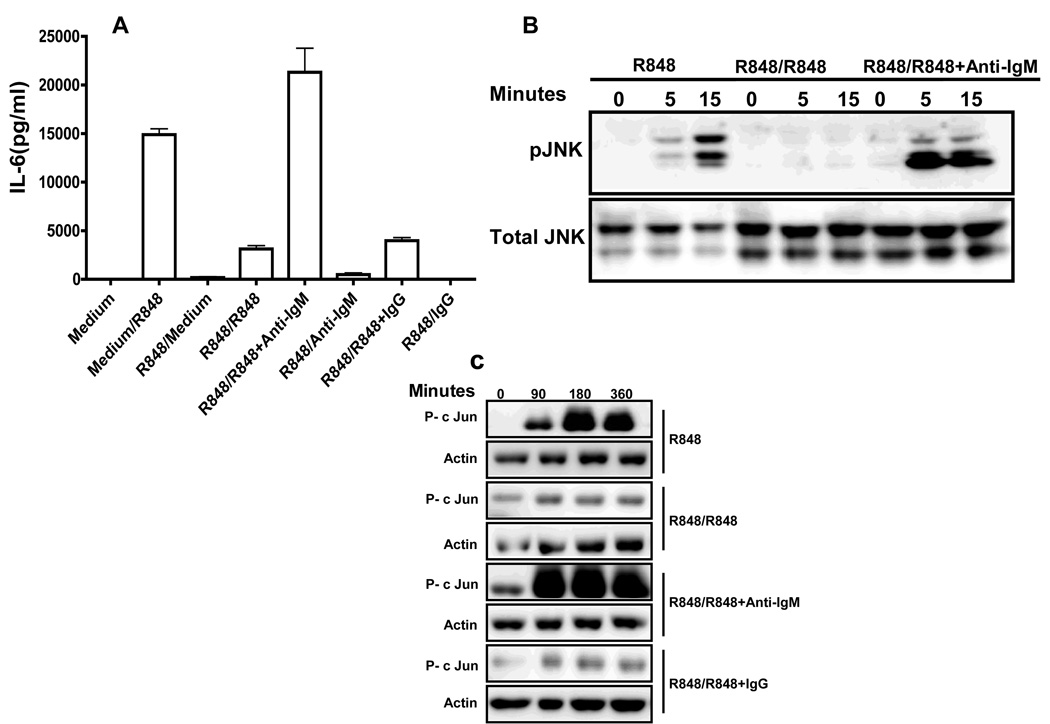
To determine whether TLR tolerance can be reversed if BCR signals are provided during the second stimulation B cells were prestimulated with R848 for 24h to induce TLR7 tolerance. After washing, cells were restimulated with R848 or R848 and anti-IgM Ab for another 24h. B cells prestimulated with R848 produced significantly higher amounts of IL-6 in response to second R848 stimulation if simultaneously triggered through BCR compared to B cells restimulated with R848 and IgG control, showing that BCR signals can not only prevent but also rescue B cells from TLR7 tolerance (Fig. 6A). We also found that as little as 1µg/ml of anti-IgM can reverse TLR7 tolerance in B cells (Supplementary Fig 2). BCR signals also reversed defective JNK activation (Fig. 6B) and c-Jun phosphorylation (Fig. 6C) in TLR7 tolerant B cells.
Figure 6. JNK activation, c-Jun phosphorylation and IL-6 production in TLR7 tolerant B cells restimulated through both BCR and TLR.
(A) Mouse high density splenic B cells were stimulated with R848 (1µg/ml) for 24h. Cells were then washed and restimulated with R848, R848 and anti- IgM at 10 µg/ml or R848 and IgG (10µg/ml) for 24h. Control cells were incubated in medium for 1hr and then stimulated with R848 for 24h. IL-6 production in cell culture supernatants was assayed by ELISA. (B) Mouse high density resting B cells stimulated with R848 (1µg/ml) for 24h were washed and restimulated with R848 (1µg/ml) or R848 and anti-IgM at 10 µg/ml for indicated time points. B cells incubated at 37°C for 1h in medium and then stimulated with R848 were used as controls. Cells were lysed and whole cell extracts were subjected to Western blot analysis for phospho JNK (B) or p-c Jun (C). Blots were stripped and reprobed for total JNK (B) or actin (C). Results from one of three comparable experiments are shown for each panel.
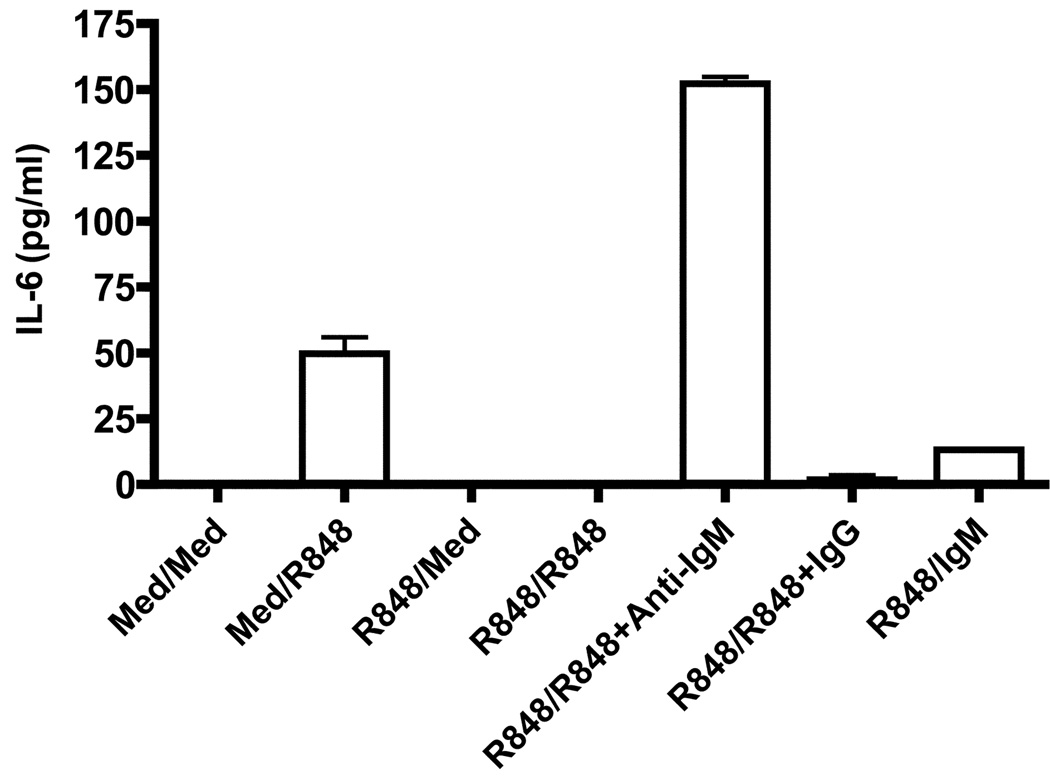
Effect of repeated TLR7 stimulation on human peripheral blood B cells
Because human and mouse B cells can respond differentially to some TLR ligands, we investigated whether repeated stimulation with R848 also induced tolerance in human peripheral blood B cells and whether BCR signals can rescue human B cells from TLR7 tolerance. To address this, CD19+ B cells isolated from the peripheral blood of healthy volunteers by negative selection were stimulated with R848 for 24 h. After extensive washing, cells were stimulated either with R848 alone or with R848 and anti-IgM Ab or R848 and IgG control. Human B cells prestimulated with medium robustly produced IL-6 in response to subsequent R848 stimulation (Fig. 7). In contrast, IL-6 production was markedly reduced in R848 prestimulated B cells when restimulated with R848. Thus, as in the case of mouse B cells, repeated stimulation through TLR7 induced tolerance in human B cells. Importantly, B cells stimulated with R848 and anti-IgM Ab produced IL-6 in equal or greater amounts compared to control B cells upon subsequent R848 restimulation, demonstrating that BCR prevention and rescue effects on TLR tolerance were also valid for human B cells.
Figure 7. Effect of repeated stimulation through TLR7 on human peripheral blood B cells.
Human peripheral blood CD19+ B cells isolated by negative selection were stimulated with R848 for 24h. Cells were then washed and restimulated with R848 or with R848 and anti-IgM (goat anti-human IgM F(ab’)2) at 10µg/ml or R848 and IgG for another 24h. B cells cultured in medium for 24h and then washed and stimulated with R848 for 24h were used as controls. IL-6 production in cell culture supernatant was determined by ELISA. Results are representative of experiments performed with peripheral blood B cells isolated from three different donors.
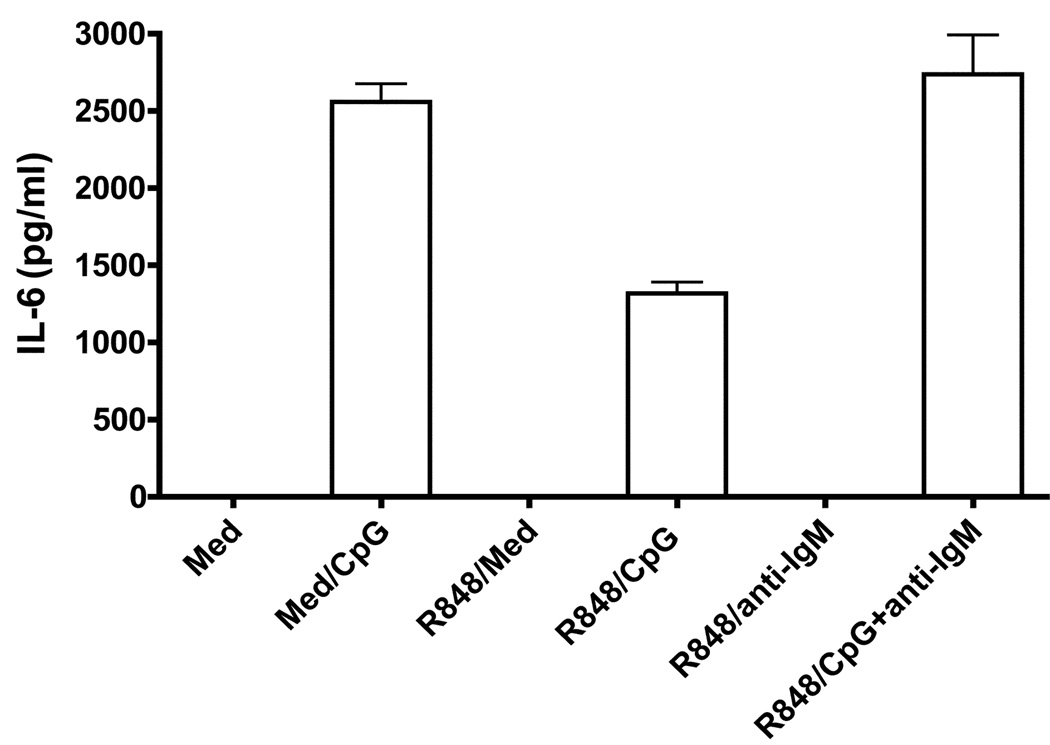
Effect of prestimulation through TLR7 on B cell IL-6 production upon subsequent TLR9 stimulation
Prestimulation through TLR7 has also been shown to affect subsequent responses to other TLR ligands in myeloid cells (25, 26). Because B cells are known to express TLR9 and respond robustly to CpG stimulation (2), we next investigated whether prestimulation through TLR7 has any effect on B cell IL-6 production upon subsequent restimulation through TLR9. Naïve B cells stimulated through TLR9 produced large amounts of IL-6 (Fig 8). However, B cells prestimulated through TLR7 exhibited a marked reduction in IL-6 production in response to subsequent CpG stimulation suggesting the induction of heterotolerance as described in macrophages. Importantly, BCR signals were able to reverse TLR7-mediated hyporesponsiveness to TLR9 stimulation in B cells. Because TLR7 stimulation has also been shown to affect subsequent TLR4 responses in macrophages (26), we also tested the effect of prestimulation through TLR7 upon subsequent restimulation through TLR4 in B cells. However, IL-6 production in TLR4 stimulated naïve B cells were either very low or absent and TLR7 prestimulation had no effect on subsequent TLR4-mediated IL-6 production (data not shown).
Figure 8. Effect of prestimulation through TLR7 on subsequent TLR9 mediated IL-6 production.
Mouse high density splenic B cells were stimulated with R848 (1µg/ml) for 24h. Cells were then washed and restimulated with CpG (100nM/ml) or CpG and F(ab')2 fragments of goat anti-mouse IgM Ab at 10µg/ml (anti-IgM) for 24h. IL-6 production in cell culture supernatants was determined by ELISA. Results are compared with B cells cultured in medium for 1h and then stimulated with CpG for 24h. Results shown are representative of two independent experiments.
Role of PI-3 kinase in BCR-mediated reversal of TLR7 tolerance
The molecular basis for TLR7 tolerance induction and BCR-mediated reversal of TLR tolerance is not clear. It is possible that initial TLR7 signaling may induce the expression of negative regulators shown to play roles in TLR tolerance induction (16) and BCR-mediated reversal of TLR tolerance could be through downregulation of these negative regulators. Because induction of TLR7 tolerance requires > 12hrs, this suggests a potential role for newly synthesized proteins in development of tolerance. Unfortunately, various inhibitors of protein synthesis employed to test this possibility were highly toxic to B cells over the period of time required to induce TLR7 tolerance (data not shown). We also investigated the expression of various negative regulators of TLR tolerance such as MyD88s, IRAK-M and SOCS-3 in tolerant B cells, and observed no induction of any of these molecules (data not shown). The expression of IRAK-4 did not differ between B cells prestimulated through TLR7 alone or through both TLR7 and BCR (data not shown).
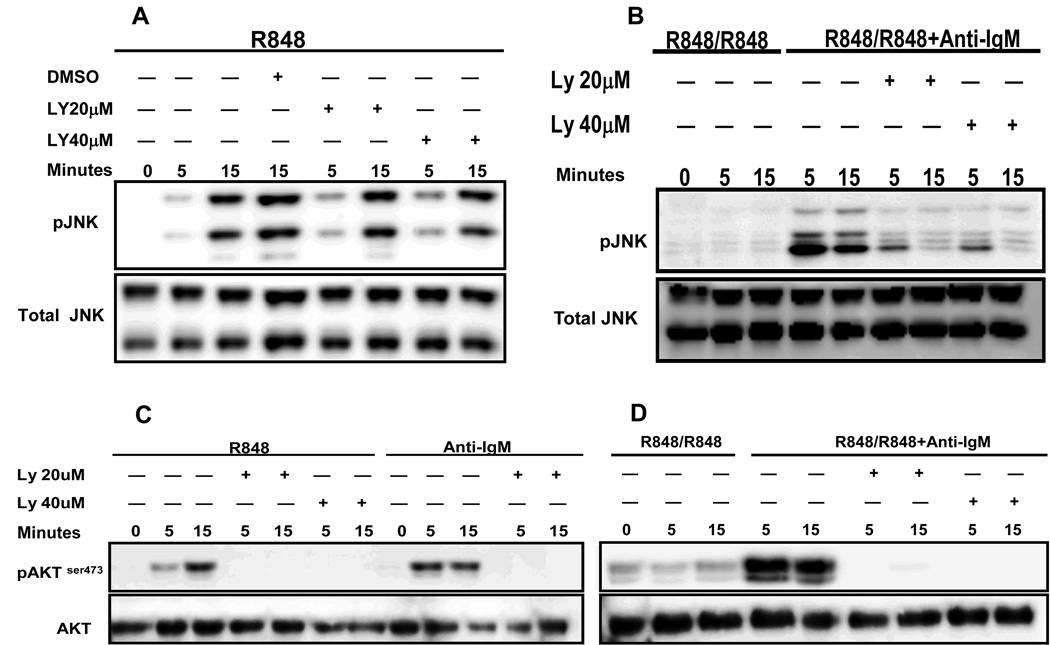
Phosphatidyl inositol-3 kinase (PI-3K) is one of the key signaling molecules activated early during BCR signaling (27). PI-3K activation has also been reported in myeloid cells in response to a variety of TLR ligands. Selective inhibition of PI-3K increases TLR-mediated IL-12 production in dendritic cells and macrophages, suggesting a potential role for PI-3K as a negative regulator of TLR-mediated inflammatory responses in these cells (28). However, the responses of B cells vs myeloid cells to various TLR stimuli do not always coincide. To address the role of PI-3K in B cells, mouse high density B cells were stimulated with R848 for 24h. After washing, cells were treated with different concentrations of the PI-3K inhibitor LY294002 and then stimulated with R848 or R848 and anti-IgM antibody for time points indicated. As shown in Fig. 9A, treatment with PI-3K inhibitor had no effect on R848-mediated JNK phosphorylation in naïve B cells. However, BCR-mediated reversal of TLR7 tolerance-induced decrease in JNK phosphorylation was markedly blocked at 5 and 15 min in B cells treated with PI-3K inhibitor (Fig. 9B) suggesting that this pathway is important for BCR-mediated reversal of TLR7 tolerance in B cells. Importantly, the concentrations of the inhibitor used in this study completely prevented the phosphorylation of Akt, a substrate of PI-3K, in R848 (Fig. 9C) and R848 and anti-IgM (Fig. 9D) stimulated B cells. Additionally, pretreatment with PI-3K inhibitor also blocked BCR-mediated reversal of ERK1/2, p38 and IκB-α phosphorylation and IκB-α degradation in TLR7 tolerant B cells (data not shown). Because BCR crosslinking can also trigger NF-κB signaling we investigated whether BCR-mediated NF-κB activation plays a role in reversal of TLR7 tolerance. For this, TLR7 prestimulated B cells were stimulated with R848 and anti-IgM in the presence or absence of two different concentrations of an IκB inhibitor for 24h. However, the concentrations of IκB inhbitor used did not block the anti-IgM mediated reversal of IL-6 production in TLR7 tolerant B cells (Supplementary Fig. 3).
Figure 9. Role of PI3 kinase (PI-3K) activation in BCR- mediated reversal of JNK activation in TLR7 tolerant B cells.
Mouse high density resting B cells stimulated with R848 (1µg/ml) for 24h were washed and restimulated with R848 (1µg/ml), R848 and anti-IgM at 10 µg/ml for indicated time points. B cells incubated at 37°C for 1h in medium and then stimulated with R848 for indicated times were used as controls. Cells were exposed to different concentrations of PI-3K inhibitor, LY294002, or diluent control DMSO starting 60 min before addition of stimuli. Cells were lysed and whole cell extracts were subjected to Western blot analysis for phospho JNK (A and B) or pAkt (C and D). Blots were stripped and reprobed for total JNK (A and B) or Akt (C and D). Results shown are representative of two comparable experiments.
DISCUSSION
TLR tolerance is a well characterized phenomenon in cells of myeloid lineage and is defined as a temporary hyporesponsiveness of immune cells to TLR ligands following repeated or chronic stimulation through the same or different TLRs (12). However, our understanding of TLR tolerance in B cells is fragmentary. In addition to the clonally rearranged antigen specific receptor that they use to recognize, bind and internalize specific antigens, B cells also express almost all known TLRs and respond robustly to many TLR ligands (2, 29–31). Accordingly, B cells can receive signals simultaneously through both BCR and TLRs, and thus may respond differently to TLR stimulation compared to myeloid cells. In this report we tested this possibility by stimulating B cells repeatedly through TLR alone or through both TLR and BCR.
Consistent with previous reports (1, 20), naïve B cells isolated from mouse spleen responded robustly to TLR7 stimulation by producing high amounts of IL-6, TNF-α and the chemokine MIP1-α. However prestimulation with R848 resulted in a drastic reduction in the ability of B cells to produce these cytokines upon subsequent restimulation through TLR7. Additionally, TLR7 tolerance also impacted B cell proliferative responses and their ability to secrete IgM antibody in response to subsequent R848 restimulation. This state of refractoriness was associated with reduced MAPK and NF-κB activation and c-Jun phosphorylation. These findings are consistent with TLR7 tolerance reported in macrophages and in human B-chronic lymphocytic leukemia (B-CLL) cells (23, 32). Reduced proinflammatory cytokine production associated with defective MAPK signaling and impaired activation of transcription factors NF-κB and AP-1 are also well documented features of endotoxin tolerant macrophages (13). However, B cell IL-10 production or costimulatory molecule expression was not altered in TLR7 tolerant B cells, which was again consistent with similar reports in TLR tolerant macrophages and dendritic cells of selective inhibition of certain functions while preserving others in (14, 15, 33).
IL-6 production in B cells following CD40 or combined TLR7 and CD40 stimulation was shown to be dependent on JNK activation and phosphorylation of the transcription factor c -Jun (20, 24). Additionally, the TNF-α promoter contains both AP-1 and NF-κB sites (34). Thus, defective activation of MAPK and transcription factors that play crucial roles in governing the expression of various cytokines could be contributing to the defective cytokine production in TLR7 tolerant B cells. Because both IL-6 and TNF-α play important roles in B cell differentiation, the reduced IgM production in TLR7 tolerant B cells could be due to defective IL-6 and TNF-α production by these cells (35).
Although the dampened B cell responses to repeated TLR7 stimulation could be a potential regulatory mechanism to control immunopathology, this may also make individuals more susceptible to infection, as reported in septic shock patients (36). Although macrophages and dendritic cells are the major cell types affected in endotoxin tolerance (37, 38), tolerance can be induced through other TLRs and this may affect the functions of other cell types, especially B cells that express almost all known TLRs (39), although this has not been studied in detail. Triggering TLR7/8 responses by R848 or HIV ssRNA has been shown to prevent HIV replication in lymphoid tissues and this anti-HIV activity was absent when B cells were depleted from lymphoid tissues (40) suggesting a potential role for B cells activated through TLR7 in preventing HIV replication. Furthermore, a recent study showed that both naïve and memory B cells from HIV infected individuals are defective in their proliferative responses to TLR9 stimulation (41). In this context, it is interesting to note that in our study TLR9 response, as determined by IL-6 production, were also defective in TLR7 prestimulated B cells. Thus it is possible that in HIV infected individuals, repeated stimulation through TLR7 by HIV ssRNA could be making their B cells unresponsive to subsequent TLR9 stimulation. Cross tolerance between various TLR ligands has been demonstrated in macrophages (10) and concurrent TLR7 signaling has been shown to negatively regulate TLR9 mediated IFN-α production by plasmacytoid dendritic cells (25, 33).
Although the TLR7 tolerance phenotype in B cells resembled that reported in macrophages, B cell responses were entirely different when TLR7 stimulation was combined with simultaneous BCR ligation. While anti-IgM alone was not effective in inducing IL-6 production in naïve or R848 prestimulated B cells, when BCR ligation was combined with R848 stimulation, it greatly enhanced IL-6 production in naïve B cells and more importantly, prevented and reversed TLR7 homotolerance and TLR7 mediated TLR9 tolerance in B cells. It is important to note that BCR stimulation reversed TLR7 tolerance in human as well as mouse B cells. Because human and mouse B cells are known to respond differently in some situations to certain TLR agonists, species-specific differences must be considered when interpreting TLR responses (42).
Prevention or reversal of endotoxin tolerance by certain cytokines has been demonstrated in human monocytes (43) and is thought to be mediated through modulating IRAK expression and its association with MyD88 in tolerant cells (44). However, type1 IFNs were not effective in reversing TLR7 tolerance in B-CLL cells (32). Reversal of TLR tolerance by adaptive immune receptor signals has not been reported before. Interestingly, we found that reversal of TLR tolerance by signaling through an immunoreceptor tyrosine based activation motif (ITAM) containing receptor is not unique to B cells. Our preliminary results showed that TLR7 tolerance can be prevented or reversed in a macrophage cell line, RAW 264.7, by simultaneous cross linking of Fcγ receptors (FcγR) (data not shown). We are currently investigating whether stimulation through FcγR can prevent endotoxin tolerance in macrophages.
Although it was known for several years that TLR7 can synergize with antigen-specific signals (1), how these two signaling pathways interact is not clear. To begin to address the molecular mechanisms involved in BCR-mediated reversal of tolerance, we investigated the role of PI-3K, a key signaling molecule activated following BCR engagement that is also thought to be involved in TLR signaling (45, 46). Engagement of antigen receptor has been shown to initiate a multitude of signaling pathways (47) and PI-3K activation is one of the earliest events (48, 49). PI-3K may function as an important initiator of downstream pathways through recruitment to the membrane of a number of PH-domain containing signaling molecules (50, 51). The PI-3K/Akt pathway has also been shown to play an important role in TLR3-mediated IRF-3 phosphorylation and activation (52). Additionally, B cells deficient in PI-3K subunits have been shown to exhibit reduced proliferation following LPS stimulation (53). Our findings indicate that although PI-3K activation was not required for R848-mediated JNK activation in naïve B cells, PI-3K activation was essential for BCR-mediated reversal of TLR7 tolerance. Although PI-3K has been shown to play a role in BCR-mediated ERK phosphorylation and activation (54), its role in JNK activation following BCR crosslinking is not clear. It is possible that PI-3K may interact with signaling molecules upstream of JNK that are initiated following triggering through TLR7. Interestingly, PI-3K has been shown to interact with MyD88 following LPS stimulation in macrophages and is shown to play a role in LPS mediated IL-1β production (55). Moreover, anti-IgM stimulation has been shown to increase MyD88 protein and gene expression in B cells (56). Although none of the chemical inhibitors are absolutely specific, treatment of B cells with LY294002 completely inhibited anti-IgM or R848-mediated phosphorylation of Akt in both R848 or R848 and anti-IgM stimulated B cells. Additionally, the drastic difference in JNK activation between control and LY294002 treated cells further argues for a potential role for this molecule in BCR-mediated reversal of TLR tolerance.
It has been shown that CpG- mediated TLR9 signals can synergize with BCR signals in enhancing p38 and JNK phosphorylation and NF-κB activation (57) and that the BCR recruits TLR9 containing endosomes to autophagosomes where hyperactivation of MAPKs occurs (58). Interestingly, the recruitment of TLR9 to autophagosome like compartments was not observed in B cells stimulated in the presence of PI-3K inhibitors (58). In the study presented here, we have also observed enhanced JNK, p38 and ERK1,2 phosphorylation in B cells stimulated through both R848 and anti-IgM. Similar to TLR9, TLR7 is localized to endosomes (59) and it is possible that BCR stimulation may recruit TLR7 to autophagosomes. We are currently investigating whether BCR crosslinking recruits TLR7 to autophagosomes and whether signaling from different intracellular compartments in R848 vs R848 and anti-IgM stimulated cells could explain the reversal of tolerance observed in BCR stimulated cells.
In conclusion, we present a novel mechanism to prevent or reverse TLR tolerance in mouse or human B cells. Identifying the molecular mechanisms involved in B cell intrinsic TLR responses and how they are modulated in the context of simultaneous signaling through adaptive immune receptors could greatly improve our ability to develop better vaccines. Because BCR-TLR7 synergy has also been shown to play a role in autoimmune disorders, a better understanding of the interaction between these two receptors as well as the regulation of their responses will be helpful when in identifying potential therapeutic targets.
Supplementary Material
Acknowledgments
This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and by grant from the National Institute of Allergy and Infectious Disease (to G.A.B)
Abbreviations used in this paper
- B-CLL
B-chronic lymphocytic leukemia.
References
- 1.Bishop GA, Ramirez LM, Baccam M, Busch LK, Pederson LK, Tomai MA. The immune response modifier resiquimod mimics CD40-induced B cell activation. Cell Immunol. 2001;208:9–17. doi: 10.1006/cimm.2001.1769. [DOI] [PubMed] [Google Scholar]
- 2.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 3.Haxhinasto SA, Bishop GA. Synergistic B cell activation by CD40 and the B cell antigen receptor: role of B lymphocyte antigen receptor-mediated kinase activation and tumor necrosis factor receptor-associated factor regulation. J Biol Chem. 2004;279:2575–2582. doi: 10.1074/jbc.M310628200. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10:233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West MA, Heagy W. Endotoxin tolerance: A review. Crit Care Med. 2002;30:S64–S73. [PubMed] [Google Scholar]
- 8.Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda K, Akira S. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164:3476–3479. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- 9.Sato S, Nomura F, Kawai T, Takeuchi O, Muhlradt PF, Takeda K, Akira S. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J Immunol. 2000;165:7096–7101. doi: 10.4049/jimmunol.165.12.7096. [DOI] [PubMed] [Google Scholar]
- 10.Yeo SJ, Yoon JG, Hong SC, Yi AK. CpG DNA induces self and cross-hyporesponsiveness of RAW264.7 cells in response to CpG DNA and lipopolysaccharide: alterations in IL-1 receptor-associated kinase expression. J Immunol. 2003;170:1052–1061. doi: 10.4049/jimmunol.170.2.1052. [DOI] [PubMed] [Google Scholar]
- 11.Mizel SB, Snipes JA. Gram-negative flagellin-induced self-tolerance is associated with a block in interleukin-1 receptor-associated kinase release from toll-like receptor 5. J Biol Chem. 2002;277:22414–22420. doi: 10.1074/jbc.M201762200. [DOI] [PubMed] [Google Scholar]
- 12.Broad A, Jones DE, Kirby JA. Toll-like receptor (TLR) response tolerance: a key physiological "damage limitation" effect and an important potential opportunity for therapy. Curr Med Chem. 2006;13:2487–2502. doi: 10.2174/092986706778201675. [DOI] [PubMed] [Google Scholar]
- 13.Medvedev AE, Kopydlowski KM, Vogel SN. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J Immunol. 2000;164:5564–5574. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- 14.Varma TK, Durham M, Murphey ED, Cui W, Huang Z, Lin CY, Toliver-Kinsky T, Sherwood ER. Endotoxin priming improves clearance of Pseudomonas aeruginosa in wild-type and interleukin-10 knockout mice. Infect Immun. 2005;73:7340–7347. doi: 10.1128/IAI.73.11.7340-7347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002;4:903–914. doi: 10.1016/s1286-4579(02)01613-1. [DOI] [PubMed] [Google Scholar]
- 16.Liew FY, Xu D, Brint EK, O'Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 17.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 18.Bishop GA, Hsing Y, Hostager BS, Jalukar SV, Ramirez LM, Tomai MA. Molecular mechanisms of B lymphocyte activation by the immune response modifier R-848. J Immunol. 2000;165:5552–5557. doi: 10.4049/jimmunol.165.10.5552. [DOI] [PubMed] [Google Scholar]
- 19.Tomai MA, Imbertson LM, Stanczak TL, Tygrett LT, Waldschmidt TJ. The immune response modifiers imiquimod and R-848 are potent activators of B lymphocytes. Cell Immunol. 2000;203:55–65. doi: 10.1006/cimm.2000.1673. [DOI] [PubMed] [Google Scholar]
- 20.Vanden Bush TJ, Bishop GA. TLR7 and CD40 cooperate in IL-6 production via enhanced JNK and AP-1 activation. Eur J Immunol. 2008;38:400–409. doi: 10.1002/eji.200737602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomai MA, Miller RL, Lipson KE, Kieper WC, Zarraga IE, Vasilakos JP. Resiquimod and other immune response modifiers as vaccine adjuvants. Expert Rev Vaccines. 2007;6:835–847. doi: 10.1586/14760584.6.5.835. [DOI] [PubMed] [Google Scholar]
- 22.Baccam M, Bishop GA. Membrane-bound CD154, but not CD40-specific antibody, mediates NF-kappaB-independent IL-6 production in B cells. Eur J Immunol. 1999;29:3855–3866. doi: 10.1002/(SICI)1521-4141(199912)29:12<3855::AID-IMMU3855>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 23.Tsukada K, Kitazawa T, Fukushima A, Okugawa S, Yanagimoto S, Tatsuno K, Koike K, Nagase H, Hirai K, Ota Y. Macrophage tolerance induced by stimulation with Toll-like receptor 7/8 ligands. Immunol Lett. 2007;111:51–56. doi: 10.1016/j.imlet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Baccam M, Woo SY, Vinson C, Bishop GA. CD40-mediated transcriptional regulation of the IL-6 gene in B lymphocytes: involvement of NF-kappa B, AP-1, and C/EBP. J Immunol. 2003;170:3099–3108. doi: 10.4049/jimmunol.170.6.3099. [DOI] [PubMed] [Google Scholar]
- 25.Berghofer B, Haley G, Frommer T, Bein G, Hackstein H. Natural and synthetic TLR7 ligands inhibit CpG-A- and CpG-C-oligodeoxynucleotide-induced IFN-alpha production. J Immunol. 2007;178:4072–4079. doi: 10.4049/jimmunol.178.7.4072. [DOI] [PubMed] [Google Scholar]
- 26.Sato S, Takeuchi O, Fujita T, Tomizawa H, Takeda K, Akira S. A variety of microbial components induce tolerance to lipopolysaccharide by differentially affecting MyD88-dependent and -independent pathways. Int Immunol. 2002;14:783–791. doi: 10.1093/intimm/dxf046. [DOI] [PubMed] [Google Scholar]
- 27.Marshall A, Niiro JH, Yun TJ, Clark EA. Regulation of B-cell activation and differentiation by the phosphatidylinositol 3-kinase and phospholipase Cgamma pathway. Immunol Rev. 2000;176:30–46. doi: 10.1034/j.1600-065x.2000.00611.x. [DOI] [PubMed] [Google Scholar]
- 28.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- 29.Ogata H, Su I, Miyake K, Nagai Y, Akashi S, Mecklenbrauker I, Rajewsky K, Kimoto M, Tarakhovsky A. The toll-like receptor protein RP105 regulates lipopolysaccharide signaling in B cells. J Exp Med. 2000;192:23–29. doi: 10.1084/jem.192.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barr TA, Brown S, Ryan G, Zhao J, Gray D. TLR-mediated stimulation of APC: Distinct cytokine responses of B cells and dendritic cells. Eur J Immunol. 2007;37:3040–3053. doi: 10.1002/eji.200636483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102:956–963. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- 32.Shi Y, White D, He L, Miller RL, Spaner DE. Toll-like receptor-7 tolerizes malignant B cells and enhances killing by cytotoxic agents. Cancer Res. 2007;67:1823–1831. doi: 10.1158/0008-5472.CAN-06-2381. [DOI] [PubMed] [Google Scholar]
- 33.Marshall JD, Heeke DS, Gesner ML, Livingston B, Van Nest G. Negative regulation of TLR9-mediated IFN-alpha induction by a small-molecule, synthetic TLR7 ligand. J Leukoc Biol. 2007;82:497–508. doi: 10.1189/jlb.0906575. [DOI] [PubMed] [Google Scholar]
- 34.Spriggs DR, Deutsch S, Kufe DW. Genomic structure, induction, and production of TNF-alpha. Immunol Ser. 1992;56:3–34. [PubMed] [Google Scholar]
- 35.Rieckmann P, D'Alessandro F, Nordan RP, Fauci AS, Kehrl JH. IL-6 and tumor necrosis factor-alpha. Autocrine and paracrine cytokines involved in B cell function. J Immunol. 1991;146:3462–3468. [PubMed] [Google Scholar]
- 36.Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 37.Freudenberg MA, Galanos C. Induction of tolerance to lipopolysaccharide (LPS)-D-galactosamine lethality by pretreatment with LPS is mediated by macrophages. Infect Immun. 1988;56:1352–1357. doi: 10.1128/iai.56.5.1352-1357.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wysocka M, Robertson S, Riemann H, Caamano J, Hunter C, Mackiewicz A, Montaner LJ, Trinchieri G, Karp CL. IL-12 suppression during experimental endotoxin tolerance: dendritic cell loss and macrophage hyporesponsiveness. J Immunol. 2001;166:7504–7513. doi: 10.4049/jimmunol.166.12.7504. [DOI] [PubMed] [Google Scholar]
- 39.Gururajan M, Jacob J, Pulendran B. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS ONE. 2007;2:e863. doi: 10.1371/journal.pone.0000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlaepfer E, Audige A, Joller H, Speck RF. TLR7/8 triggering exerts opposing effects in acute versus latent HIV infection. J Immunol. 2006;176:2888–2895. doi: 10.4049/jimmunol.176.5.2888. [DOI] [PubMed] [Google Scholar]
- 41.Jiang W, Lederman MM, Mohner RJ, Rodriguez B, Nedrich TM, Harding CV, Sieg SF. Impaired naive and memory B-cell responsiveness to TLR9 stimulation in human immunodeficiency virus infection. J Virol. 2008;82:7837–7845. doi: 10.1128/JVI.00660-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, Lipford G, Bauer S. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 43.Randow F, Docke WD, Bundschuh DS, Hartung T, Wendel A, Volk HD. In vitro prevention and reversal of lipopolysaccharide desensitization by IFN-gamma, IL-12, and granulocyte-macrophage colony-stimulating factor. J Immunol. 1997;158:2911–2918. [PubMed] [Google Scholar]
- 44.Adib-Conquy M, Cavaillon JM. Gamma interferon and granulocyte/monocyte colony-stimulating factor prevent endotoxin tolerance in human monocytes by promoting interleukin-1 receptor-associated kinase expression and its association to MyD88 and not by modulating TLR4 expression. J Biol Chem. 2002;277:27927–27934. doi: 10.1074/jbc.M200705200. [DOI] [PubMed] [Google Scholar]
- 45.Lee J, Mira-Arbibe L, Ulevitch RJ. TAK1 regulates multiple protein kinase cascades activated by bacterial lipopolysaccharide. J Leukoc Biol. 2000;68:909–915. [PubMed] [Google Scholar]
- 46.Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, Godowski PJ, Ulevitch RJ, Knaus UG. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 47.Bishop GA, Haxhinasto SA, Stunz LL, Hostager BS. Antigen-specific B-lymphocyte activation. Crit Rev Immunol. 2003;23:149–197. doi: 10.1615/critrevimmunol.v23.i3.10. [DOI] [PubMed] [Google Scholar]
- 48.Gold MR, Chan VW, Turck CW, DeFranco AL. Membrane Ig cross-linking regulates phosphatidylinositol 3-kinase in B lymphocytes. J Immunol. 1992;148:2012–2022. [PubMed] [Google Scholar]
- 49.Gold MR, Aebersold R. Both phosphatidylinositol 3-kinase and phosphatidylinositol 4-kinase products are increased by antigen receptor signaling in B cells. J Immunol. 1994;152:42–50. [PubMed] [Google Scholar]
- 50.Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 51.Isakoff SJ, Cardozo T, Andreev J, Li Z, Ferguson KM, Abagyan R, Lemmon MA, Aronheim A, Skolnik EY. Identification and analysis of PH domain-containing targets of phosphatidylinositol 3-kinase using a novel in vivo assay in yeast. Embo J. 1998;17:5374–5387. doi: 10.1093/emboj/17.18.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarkar SN, Peters KL, Elco CP, Sakamoto S, Pal S, Sen GC. Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat Struct Mol Biol. 2004;11:1060–1067. doi: 10.1038/nsmb847. [DOI] [PubMed] [Google Scholar]
- 53.Fruman DA, Snapper SB, Yballe CM, Davidson L, Yu JY, Alt FW, Cantley LC. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85alpha. Science. 1999;283:393–397. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- 54.Jacob A, Cooney D, Pradhan M, Coggeshall KM. Convergence of signaling pathways on the activation of ERK in B cells. J Biol Chem. 2002;277:23420–23426. doi: 10.1074/jbc.M202485200. [DOI] [PubMed] [Google Scholar]
- 55.Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol. 2003;33:597–605. doi: 10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- 56.Meyer-Bahlburg A, Khim S, Rawlings DJ. B cell intrinsic TLR signals amplify but are not required for humoral immunity. J Exp Med. 2007;204:3095–3101. doi: 10.1084/jem.20071250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yi AK, Yoon JG, Krieg AM. Convergence of CpG DNA- and BCR-mediated signals at the c-Jun N-terminal kinase and NF-kappaB activation pathways: regulation by mitogen-activated protein kinases. Int Immunol. 2003;15:577–591. doi: 10.1093/intimm/dxg058. [DOI] [PubMed] [Google Scholar]
- 58.Chaturvedi A, Dorward D, Pierce SK. The B cell receptor governs the subcellular location of Toll-like receptor 9 leading to hyperresponses to DNA-containing antigens. Immunity. 2008;28:799–809. doi: 10.1016/j.immuni.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heil F, Ahmad-Nejad P, Hemmi H, Hochrein H, Ampenberger F, Gellert T, Dietrich H, Lipford G, Takeda K, Akira S, Wagner H, Bauer S. The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur J Immunol. 2003;33:2987–2997. doi: 10.1002/eji.200324238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.