Abstract
Background
Antigen specificity and IgG subclass could be significant in the natural history of Chagas' disease. The relationship between the different stages of human Chagas' disease and the profiles of total IgG and its subclasses were thus analysed here; they were directed against a crude T. cruzi extract and three recombinant antigens: the T. cruzi kinetoplastid membrane protein-11 (rKMP-11), an internal fragment of the T. cruzi HSP-70 protein192-433, and the entire Trypanosoma rangeli HSP-70 protein.
Methods
Seventeen Brazilian acute chagasic patients, 50 Colombian chronic chagasic patients (21 indeterminate and 29 cardiopathic patients) and 30 healthy individuals were included. Total IgG and its subtypes directed against the above-mentioned recombinant antigens were determined by ELISA tests.
Results
The T. cruzi KMP-11 and T. rangeli HSP-70 recombinant proteins were able to distinguish both acute from chronic chagasic patients and infected people from healthy individuals. Specific antibodies to T. cruzi crude antigen in acute patients came from IgG3 and IgG4 subclasses whereas IgG1 and IgG3 were the prevalent isotypes in indeterminate and chronic chagasic patients. By contrast, the specific prominent antibodies in all disease stages against T. cruzi KMP-11 and T. rangeli HSP-70 recombinant antigens were the IgG1 subclass.
Conclusion
T. cruzi KMP-11 and the T. rangeli HSP-70 recombinant proteins may be explored together in the immunodiagnosis of Chagas' disease.
Polarising the IgG1 subclass of the IgG response to T. cruzi KMP-11 and T. rangeli HSP-70 recombinant proteins could have important biological effects, taking into account that this is a complement fixing antibody.
Background
Antibodies against several parasitic antigens are copious during blood-borne parasite infections such as malaria and Chagas' disease. These humoral immune responses have been used for diagnosis, following up individuals throughout the course of a natural infection, vaccination protocols and evaluating drug therapy efficacy. However, little is known about these antibodies' specific role or profile according to disease phases. Concerning humoral responses, it has been described that antibodies against repeat and/or evolutionary conserved sequences are highly predominant in parasitic infections such as that caused by Trypanosoma cruzi, the aetiological agent of Chagas' disease [1-3]. B lymphocytes and antigen specific antibodies seem to be crucial for controlling acute infection during the course of T. cruzi infection and could determine the fate of the disease's chronic phase [4]. Indeed, B lymphocyte-depleted rats and mice have succumbed to infection with a T. cruzi lethal strain [5,6].
Following human infection with T. cruzi, some individuals (mostly children) can develop a symptomatic acute phase. However, many individuals recover from acute infection and move on to the indeterminate phase where there are no symptoms and the parasite's persistence can only be indirectly detected by serological tests [7]. This stage of the disease could last several years or even decades. Some indeterminate chagasic individuals progress to chronic disease which unfolds in two major clinical settings compromising either cardiac or digestive tissues [8,9]. Chronic clinical output (cardiopathy or visceral enlargement) seems to be dependent of the host immune response and parasite genetics [8,10,11].
It is becoming apparent that an orchestrated humoral and cellular immune response is needed for controlling T. cruzi infection. CD4+ and CD8+ T lymphocytes are essential for eliminating the parasite during the intracellular stage (amastigotes) [10,11] and antibodies acting alone or in association with monocytes operate against extracellular stages (trypomastigotes) [12,13].
With the aim to understand some of the antigen-specific antibody-mediated responses, the profiles of IgG subclass antibody responses and their relationship with the different stages of human Chagas' disease were thus analysed. They were directed against a crude T. cruzi antigen and two parasite recombinant proteins: the kinetoplastid membrane protein-11 (KMP-11), a kinetoplastids conserved protein [14,15], and an internal fragment of the T. cruzi heat shock protein-70 protein192-433(HSP-70T), a highly conserved protein throughout divergent species' evolution [16,17]. The Trypanosoma rangeli homologous protein (GenBank accession ABL74477) was also selected to test a full length HSP-70 protein bearing the carboxy terminal GMPG motif which is highly immunogenic in rabbits [18]; it bears 14 copies of the GMPG motif, a higher number compared to the complete T. cruzi HSP70 protein (GenBank accession PO5456).
Methods
Parasite antigens
Epimastigote lysate was obtained from IRHO/CO/85/MTA Colombian T. cruzi I strain in exponential growth in 15% foetal calf serum supplemented-LIT medium (GIBCO, Invitrogen, USA) at 28°C. Parasites were washed twice with cold 1× phosphate-buffered saline (PBS), pH 7.0 and suspended at 1.5 × 107 parasites/μL in lysis buffer (50 mM Tris -HCl pH 7.0, 1% SDS, 2 mM PMSF, 1% NP40, 5 mM EDTA, 1% β-mercaptoethanol). After sample boiling at 100°C for 10 min and cooling at 4°C for 3 h, the sample was spun for 10 min at 13,000 g and the soluble antigen-containing supernatant was stored at -70°C until use. Protein lysate concentration was determined by Bradford assay and protein profile analysed by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining [19]. The T. cruzi KMP-11 recombinant protein (KMP-11r) [20] and an internal fragment of the T. cruzi HSP-70 protein (TcHSP-70T, corresponding to amino acids 192-433), which is conserved and induces functional maturation of murine and human dendritic cells [21,22], were purified as previously described [21]. The entire T. rangeli HSP-70 recombinant protein (TrHSP-70) was obtained by cloning in pQE30 plasmid (Qiagen, Hilden, Germany) the corresponding encoding region of the Colombian T. rangeli Tre strain which was PCR amplified using 70TreATG (5'-CTATAGGATCCATGACGTACGAGGGAGCCA-3') and 70TreTGA specific primers (5'CTATAAAGCTTTCAGTCAACCTCCTCCACCT-3'). Amplification reactions were performed in a final 50 μL volume containing 250 ng purified genomic DNA from the parasite, 1× reaction buffer (10 mM Tris-HCl, pH 9.0, 50 mM KCl, 0.1% Triton X-100), 2 mM MgCl2, 0.2 mM of each dNTP, 20 pmol of each primer and 2.6 units of expand high fidelity enzyme (Roche, Mannheim, Germany).
The reaction took place in a MJ Research PTC-100 DNA thermocycler running at 35 cycles having the following amplification profile: 94°C/1 min, 57°C/1 min and 72°C/2:30 min, with final incubation at 72°C for 10 min. Following ligation and transformation, the obtained clone was sequenced and the recombinant protein was over-expressed in Escherichia coli after incubation for 2 hours at 37°C with 0.02 mM IPTG. The soluble protein was purified on a Ni2+-NTA-agarose affinity column and eluted with phosphate buffer (50 mM NaHPO4, 300 mM NaCl) at pH 6.0.
Patient sera
Four groups of donors were enrolled in the study; all of them participated voluntarily and signed the informed consent form. Seventeen Brazilian acute chagasic (AC) patients from Santa Catarina state who were diagnosed by IgG and IgM immunofluorescence assays were included [23]. Fifty chronic chagasic patients were also enrolled who were positive for anti-T. cruzi antibodies in both immunofluorescence assay and ELISA test [24]. They were clinically evaluated at Fundación Abood Clínica Shaio and Instituto Nacional de Salud, Bogotá, Colombia and classified as being 21 indeterminate patients (IND), corresponding to patients with normal findings on electrocardiograms (ECGs) and chest radiographs, and 29 chronic chagasic cardiopathic patients (CCC) who presented abnormal findings on ECGs or/and chest radiographs. Healthy donors (HD) were also included, consisting of 30 people from Colombia who had always lived in non-endemic areas and had negative serology for T. cruzi. This study was approved by the Research and Ethics Committees from the Universidad Javeriana's Science Facultad de Ciencias, Pontificia Universidad Javeriana and Fundación Abood Clínica Shaio.
Detecting anti-T cruzi IgG antibodies by ELISA
The ELISA technique was standardised as follows: 96-well immunoassay plates (Nunc Maxisorp, Apogent, USA) were sensitised with 0.1 μg parasite lysate/well or 0.5 μg KMP-11r or 0.1 μg TcHSP-70T and TrHSP-70 recombinant proteins for 3 h at 37°C and subsequently overnight at 4°C. Plates were then washed with 0.05% PBS-Tween (PBS-T). The wells were incubated for 1 h at 37°C with blocking solution (5% nonfat dried milk powder in PBS-T) to avoid unspecific binding. After washing the wells three times with PBS-T sera at 1:100 dilution for lysate, KMP-11r and TrHSP-70 recombinant antigens and 1:50 dilution for TcHSP-70T protein, blocking solution was added to the wells and they were incubated for 2 h at 37°C. Wells were then washed four times with PBS-T and anti-human IgG (γ-chain specific) alkaline phosphatase conjugate (Sigma Chemical Co., St. Louis, MO) was added to blocking solution at 1:15,000 dilution for lysate and KMP-11r and 1:10,000 for TcHSP-70T and TrHSP-70 proteins. Plates were incubated for 1 h at 37°C. After four washes with PBS-T, p-nitrophenylphosphate substrate solution (Sigma Chemical Co., St. Louis, MO) diluted in diethanolamine buffer (pH 9.8) was added to each well. The reaction was developed in the dark at room temperature for 30 min and stopped by adding 0.5 N NaOH. Optical densities (OD) were determined on a Labsystems Multiskan plate reader (Vantaa, Finland) at 405 nm wavelength. Cut-off values were assessed as being mean optical density value in samples from non-chagasic donors plus 3 standard deviations.
Detecting anti-T cruzi IgG isotypes by ELISA
A 0.1 μg antigen/well concentration for lysate and 0.5 μg/well for KMP-11r and TrTHSP-70 was used in the ELISA technique for IgG1, IgG2, IgG3 and IgG4 subclass antibody detection (except for KMP-11r IgG3 detection where 0.1 μg/well was used).
Samples were diluted at 1:50 for all assays and anti-human IgG1, IgG2, IgG3 and IgG4 peroxidase conjugate (MP Biomedicals, Inc. Ohio, USA) were diluted at 1:500. Substrate solution was O-phenylendiamine dihydrochloride (OPD) (Sigma Chemical Co., St. Louis, MO, USA). Readings were taken at 492 nm wavelength.
Statistical analysis
Differences among groups were assessed by Student's t test when n ≥ 10 and by Mann-Whitney test when n < 10. GraphPad Prism 5.0 statistical software was used for both analyses. They were considered to be statistically significant when p < 0.05.
Results
Analysing recombinant proteins and parasite soluble proteins
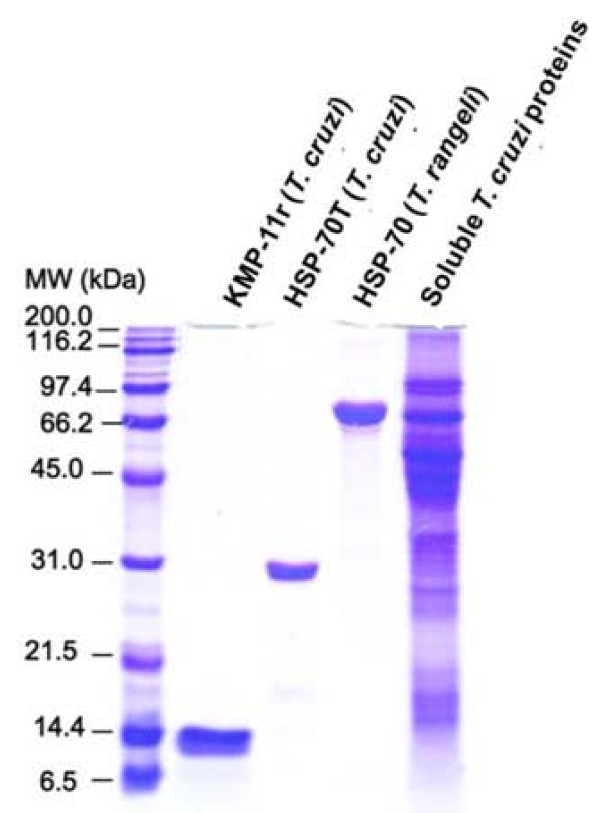
The KMP-11r, TcHSP-70T and TrHSP-70 recombinant proteins as well as T. cruzi total crude antigen from the parasite were analysed by SDS-PAGE (Figure 1). As expected, bands of around 14, 30, and 76 kDa were observed for KMP-11r, TcHSP-70, and TrHSP-70 recombinant antigens, respectively. Purity was >95% as assessed by Coomassie blue staining.
Figure 1.
Purifying recombinant proteins by Ni2+ affinity chromatography and extracting total soluble proteins from Trypanosoma cruzi. Purified T. cruzi KMP-11 recombinant protein (KMP-11r), Trypanosoma cruzi truncated HSP-70 protein (TcHSP-70T), Trypanosoma rangeli HSP-70 recombinant protein (TrHSP-70), and crude T. cruzi lysate were electrophoresed in 12% SDS-PAGE and stained with Coomassie blue. Lane MW corresponds to molecular weight markers shown in kDa.
Detecting anti-T. cruzi IgG antibodies
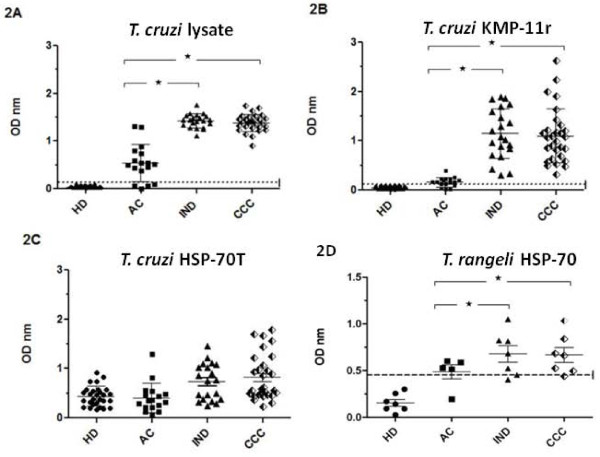
The results revealed that 76.5% (13 out of 17) of the patients in the acute phase and 100% of those in the indeterminate (No = 21) and cardiopathic (No = 29) chronic stages recognised T. cruzi lysate proteins. Healthy donors did not display reactivity against crude parasite antigen. There was a statistically significant higher mean OD for anti-T. cruzi IgG levels in sera from patients in both chronic stages (IND = 1.424 ± 0.14, and CCC = 1.384 ± 0.18) when compared to that from the acute group (0.539 ± 0.38), p < 0.0001, Figure 2A).
Figure 2.
ELISA for total IgG antibody levels from acute (AC), indeterminate (IND) and cardiac chronic (CCC) chagasic patients and healthy individuals (HD) against T. cruzi lysate (2A), KMP-11 (2B), truncated T. cruzi HSP-70 (TcHSP-70T) (2C), and T. rangeli HSP-70 (2D) recombinant proteins. Values are given as optical densities at 405 nm. The dotted line represents the cut-off values based on the mean of healthy individual values plus 3 standard deviations. Horizontal lines on each group represent mean and standard deviation values, the mean being the larger one. Statistically significant differences among chagasic groups are represented by an asterisk.
Figure 2B shows the reactivity of sera from all patient groups against the KMP-11 recombinant protein. Healthy donors did not recognise the KMP-11r protein. The percentage of chagasic patients presenting specific anti-KMP-11r protein IgG antibodies having a reactivity value above cut-off level was 64.7% (11 out of 17) in the acute group and 100% for both chronic indeterminate and cardiopathic groups. The mean OD value of IgG reactivity against the KMP-11r protein was higher in indeterminate patients (1.148 ± 0.508) than that found in the acute group (0.163 ± 0.096, p < 0.0001). Likewise, chronic chagasic cardiopathic patients also had a higher reactivity level (1.1 ± 0.55) than that observed in the acute group (p < 0.0001); 35.3% (6 out of 17) acute, 66.6% (14 out of 21) indeterminate and 86.2% (25 out of 29) chronic chagasic cardiopathic patients presented anti-TcHSP-70T reactivity values higher than the mean value for reactivity sera. However, it is worth mentioning that healthy donors recognised the T. cruzi HSP-70T recombinant protein, showing significant reactivity with absorbance values ranging from 0.161 to 0.922 (0.438 ± 0.201 mean) (Figure 2C).
The TrHSP-70 recombinant protein was assayed to test the recognition of full length HSP-70 by sera from healthy donors and chagasic patients at different stages of the sickness. Sera from 5 Brazilian acute and 14 Colombian chronic patients (7 indeterminate and 7 cardiopathic) were thus also tested for the presence of anti-TrHSP-70 protein antibodies (Figure 2D). The results showed that most patients in acute (4 out of 5), indeterminate (5 out of 7) and chronic (6 out 7) phases had specific anti-T. rangeli protein antibodies. Remarkably, sera from healthy donors did not recognise the T. rangeli HSP-70 protein at assayed sera dilution.
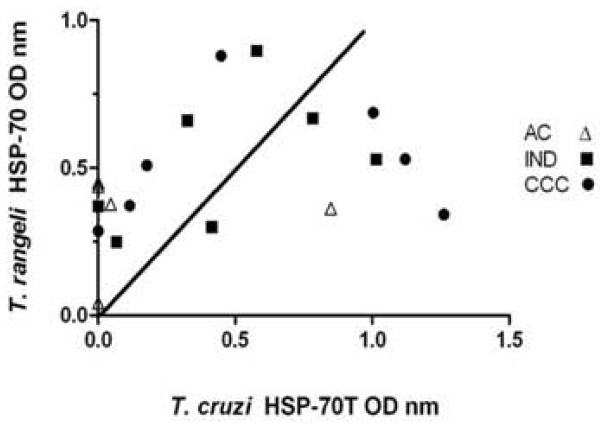
Their absorbance values were compared to analyse the relationship of sera reactivity against both T. cruzi and T. rangeli HSP-70 recombinant antigens. Absorbance values were thus normalised by subtracting healthy donors' mean OD values and comparing them on a graph. Figure 3 shows that 11 out of 19 chagasic patients had higher reactivity against TrHSP-70 than TcHSP-70. On the other hand, only 7 patients (1 out of 5 acute, 3 out of 7 indeterminate and 3 out of 7 cardiopathic groups) exhibited higher reactivity against TcHSP-70 than TrHSP-70.
Figure 3.
Comparing normalised optical densities (OD) from from sera evaluated for TcHSP-70T and TrHSP-70 recombinant proteins. The ELISA for the TcHSP-70T recombinant protein was performed with sera diluted 1/50 and 1/100 for the TrHSP-70 antigen. Data was normalised by subtracting the mean OD of the healthy group for each protein and, when mean OD obtained was lower than 0, it was fixed as 0.
Anti-T. cruzi antibody IgG subclass profile
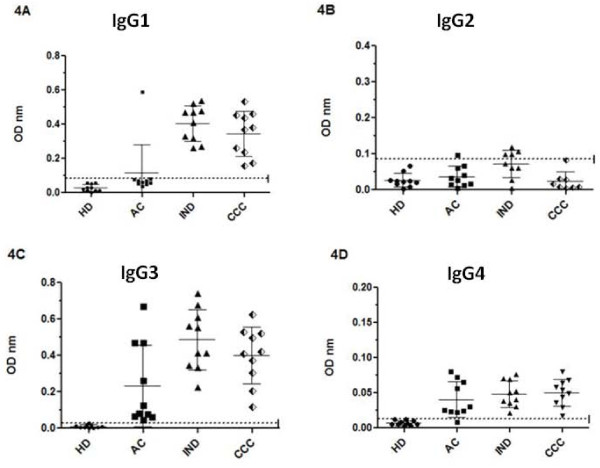
KMP-11 and TrHSP-70 recombinant proteins were measured in sera from ten patients who exhibited IgG levels higher than the cut-off value for all antigens used to help analyse IgG1, IgG2, IgG3 and IgG4 subclass levels against T. cruzi lysate (Figures 4, 5, 6).
Figure 4.
IgG isotype profile against T. cruzi lysate. IgG1 (4A), IgG2 (4B), IgG3 (4C) and IgG4 (4D) isotype levels for patients in acute (AC), indeterminate (IND) and cardiac chronic phase (CCC) and healthy individuals (HD). Values are given as optical densities at 492 nm. The dotted line represents the cut-off values based on the mean of healthy individual values plus 3 standard deviations. Horizontal lines on each group represent mean and standard deviation values, the mean being the larger one.
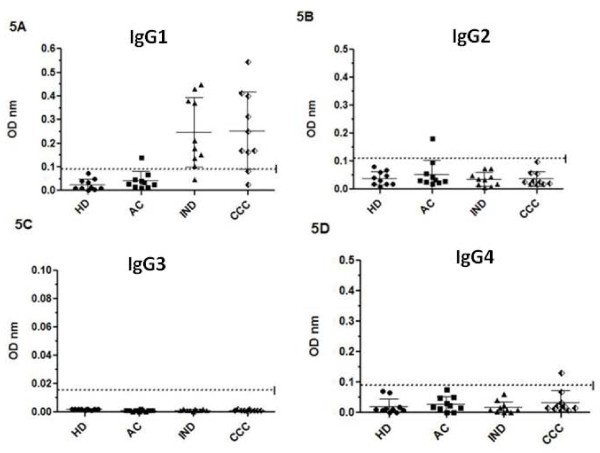
Figure 5.
IgG isotypes profile against T. cruzi KMP-11 recombinant protein. IgG1 (5A), IgG2 (5B), IgG3 (5C) and IgG4 (5D) isotype levels for patients in acute (AC), indeterminate (IND) and cardiac chronic phase (CCC) and healthy individuals (HD). Values are given as optical densities at 492 nm. The dotted line represents the cut-off values based on the mean of healthy individual values plus 3 standard deviations. Horizontal lines on each group represent the mean and standard deviation values, the mean being the larger one.
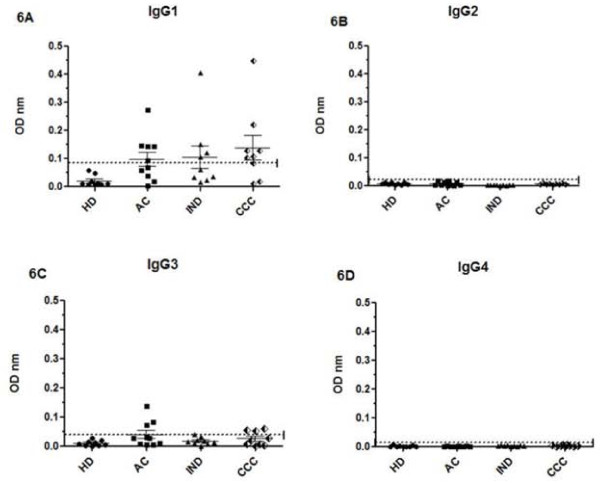
Figure 6.
IgG isotypes profile against T. rangeli HSP-70 recombinant protein. IgG1 (6A), IgG2 (6B), IgG3 (6C) and IgG4 (6D) isotype levels of patients in acute (AC), indeterminate (IND) and cardiac chronic phase (CCC) and healthy individuals (HD). Values are given as optical densities at 492 nm. The dotted line represents the cut-off values based on the mean of healthy individual values plus 3 standard deviations. Horizontal lines on each group represent the mean and standard deviation values, the mean being the larger one.
When the IgG antibody subtype was tested against parasite soluble proteins it was observed that IgG1 and IgG3 were the predominant subclasses in chagasic patients (Figure 4). Indeed, all sera from chagasic patients (acute, indeterminate and cardiopathic) presented IgG3 isotype antibodies against crude parasite lysate. Interestingly, IgG1 was detected in both groups of chronic chagasic patients (indeterminate and cardiopathic) having similar mean OD values (IND: 0.405 ± 0.1, CCC: 0.345 ± 0.13). These values were higher than those detected from patients in the acute phase (p = 0.0002 and p = 0.0016, respectively). A low IgG4 response was observed in all chagasic patients (0.08 maximum OD). Most chagasic patients presented an anti-lysate IgG2 reactivity OD below the cut-off value.
Figure 5 shows the IgG subclass profile for KMP-11 recombinant antigen. The results indicated that the antibodies generated against KMP-11r by chagasic patients were of the IgG1 subtype. There were no detectable anti-KMP-11 specific IgG2, IgG3 or IgG4 subclass antibodies with the exception of one serum from a patient in acute phase (subtype IgG2) and another in a chronic cardiopathic patient (subtype IgG4); the antibody titre proved low in both cases.
When the IgG subtype of anti-T. rangeli HSP-70 recombinant protein antibodies was studied it was observed that HSP-70 specific antibodies were predominantly of the IgG1 isotype in chronic chagasic patients (both indeterminate and cardiopathic) (Figure 6). IgG3 subclass antibodies were detected in some acute and chronic cardiopathic patients.
Discussion
Chagas' disease, before becoming confined to Latin-America, had reached the North-American and European continents through immigration, thus becoming a health problem in those non-endemic countries [25]. This is an intriguing parasitic disease due to its chronic natural evolution. However, only 30% of infected individuals during the indeterminate stage develop later heart or digestive tissue damage, suggesting an early infection control which may be related to the immune system and also to the parasite's strain and the host's genetic factors [8,26]. A specific antibody response seems to play an important role in controlling early parasite blood stages as demonstrated by passive transfer of immune serum [27], monoclonal antibodies [28] or B cells to depleted rats [5]. Dissecting protective antibody targets against parasite infection has proved difficult because of the large number of parasite genes (12,000 genes per haploid genome), from which at least 1,500 have been cloned [3].
The antibody profile of total IgG and IgG subclasses against parasite lysate and KMP-11 and HSP-70 recombinant proteins was evaluated to understand the course of antibody response during T. cruzi infection in acute, indeterminate and cardiopathic chronic chagasic patients. The results showed that the number of individuals recognising crude parasite antigen was significantly higher in patients in indeterminate and chronic phases than those in the acute phase. Moreover, indeterminate and chronic patient sera titre was also higher compared to that of acute patients. The consistently higher IgG response in chronic patients occurring with all the antigens used was also observed. The difference was even more notable with the response to the KMP-11 recombinant protein in which a very low response was found during the acute phase. K1 (an amino-terminal peptide from this protein) also induced significant reactivity in both groups of Colombian chronic chagasic patients [29]. The data observed here are consistent with that previously described in humans and animal models where high levels of total IgG specific response has been a hallmark of the sickness' chronic stages [30,31].
The acute sera tested in the present study were from patients involved in an outbreak of acute Chagas' disease due to ingesting sugar cane juice where T. cruzi TcII lineage was identified in nine patients [23]. It is possible that the low reactivity observed in the acute phase group might have been due to the early stage of infection (60 days) since all patients presented high anti-T. cruzi IgM antibody titres (1:320) in immunofluorescence test. Another explanation might be supported by the lineage of the T. cruzi strain implicated. It has been recently described that mice infection with T. cruzi TcI lineage, the main circulating lineage in Colombia [32], is correlated with higher IgG responses compared with the T. cruzi TcII ones [33].
The highly conserved chaperone HSP-70 is a major cloned sequence of pathogens which can induce cross-reactive responses [34]. A fast acute chagasic patient IgG response to TcHSP-70 may be explained by pre-existing specific memory T cells, elicited by other HSP-70 pathogens. Another non-exclusive explanation refers to the mitogenic capability of HSP-70 for B cells which has been reported for Leishmania infantum HSP-70 proteins in rodent models [35]. Similar to that reported by Requena et al., (1993) using the whole HSP-70 parasite protein, sera from healthy individuals in this study showed significant reactivity against the T. cruzi truncated protein (TcHSP-70T). This result was not surprising taking into account that the truncated version's identity with the same region in the human HSP-70 protein was a little bit higher (74%) than when comparing the whole protein (70%). Moreover, the truncated version lacks the C- terminal region in which some parasite specific epitopes are located [34].
It is particularly worth noting that the response against the complete T. rangeli HSP-70 protein was characterised by higher reactivity in most chagasic patient sera, along with the lack of it in healthy individuals.
This finding may have resulted from several GMPG motifs being present at the T. rangeli HSP-70 protein's C-terminal region. Besides, it has been demonstrated recently that some T. rangeli proteins can elicit a strong humoral response in chagasic patients [36]. Collectively, these results suggest that T. rangeli could be one of the pathogens which can boost antibody response to this antigen, the HSP-70 protein seems to be involved in cross-reaction between these trypanosomes and that the T. rangeli HSP-70 protein could be explored for diagnosis strategies.
IgG subclasses were then measured against total crude lysate and two recombinant proteins in all sera groups to determine the relationship with disease stage. The data showed that the specific antibodies in the acute patients were from subclasses IgG3 and IgG4 against crude T. cruzi antigen whereas IgG1 and IgG3 were the prevalent isotypes in indeterminate and chronic chagasic patients. These findings agreed with reported high prevalence for IgG3 and IgG1 isotypes against epimastigotes in all clinically severe stages of Argentinean and Brazilian chronic chagasic individuals [37,38]. By contrast, Mexican chagasic donors suffering cardiopathy had more IgG1 and IgG2 and less IgG3 [39]. Moreover, anti-T. cruzi IgG2 association with cardiac involvement or chronic cardiac disease severity has been reported in the Brazilian and Mexican chagasic population, respectively [38,39]. These apparently disagreeing results may have been due to differences in the circulating parasite strain in each area. Indeed, it has been shown recently that parasite genotype can affect IgG subclass profile [33].
The specific prominent antibodies in all disease stages against T. cruzi KMP-11 and T. rangeli HSP-70 recombinant antigens were only from the IgG1 subclass. These results were similar to those reported by Trujillo et al., (1999) using the Leishmania panamensis KMP-11 protein [40].
Likewise, when an epimastigote acidic antigenic fraction was used as antigen, IgG1 was the main antibody isotype detected by ELISA in all Argentinean chronic patients [37]. On the contrary, the distribution of IgG subclasses against epimastigote ribonucleoproteins and the cytoplasmic (CRA) and flagellar (FRA) recombinant repetitive antigens in chronic chagasic patients assessed by ELISA were shared by IgG1 and IgG3 [41,42]. Since, IgG1 is a complement fixing antibody which also mediates cooperative function with phagocytes through their Fc receptors [43], then this specific antibody's role in controlling the infection or the disease progressing in severity needs to be addressed.
Conclusion
The T. cruzi KMP-11 and the T. rangeli HSP-70 recombinant proteins may be explored together in Chagas' disease immunodiagnosis. Polarising the IgG response IgG1 subclass to T. cruzi KMP-11 and T. rangeli HSP-70 recombinant proteins could have important biological effects, taking into account that this is a complement fixing antibody.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
CP, JG, MCT, MCL and AC conceived and designed the study. ZC, FR and VV clinically evaluated the patients and controls. IDF performed the experiments. CP, JG, AC, MCT, MCL and MS interpreted the results and wrote the manuscript. All authors have read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Ivonne D Flechas, Email: iflechas@gmail.com.
Adriana Cuellar, Email: acuellar@javeriana.edu.co.
Zulma M Cucunubá, Email: zulmacp@ins.gov.co.
Fernando Rosas, Email: fernandorosas1994@gmail.com.
Víctor Velasco, Email: electrofisiologia@shaio.org.
Mario Steindel, Email: cccb1mst@ccb.ufsc.br.
María del Carmen Thomas, Email: mcthomas@ipb.csic.es.
Manuel Carlos López, Email: mclopez@ipb.csic.es.
John Mario González, Email: jmario_gonzalez@yahoo.com.
Concepción Judith Puerta, Email: cpuerta@javeriana.edu.co.
Acknowledgements
This work was supported by Colciencias Research project No. 1203-333-18692. IDF was supported by Colciencias and the Universidad Javeriana's Young Researcher 2008 Programme (Bogotá, Colombia). MCT and MCL were supported by P06-CTS-02242 Grant from PAI (Junta de Andalucia) and RICET-RD06/0021-0014, Spain. MS received financial support from the Brazilian agency - CNPq.
The authors are indebted to Claudia Cuervo from Laboratorio de Parasitología Molecular de la Pontificia Universidad Javeriana, for technical assistance in obtaining the TrHSP-70 protein.
References
- Goto Y, Carter D, Reed SG. Immunological dominance of Trypanosoma cruzi tandem repeat proteins. Infect Immun. 2008;76:3967–3974. doi: 10.1128/IAI.00604-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo VA, Buscaglia CA, Di Noia JM, Frasch AC. Immunocharacterization of the mucin-type proteins from the intracellular stage of Trypanosoma cruzi. Microbes Infect. 2006;8:401–409. doi: 10.1016/j.micinf.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Cooley G, Etheridge RD, Boehlke C, Bundy B, Weatherly DB, Minning T, Haney M, Postan M, Laucella S, Tarleton RL. High throughput selection of effective serodiagnostics for Trypanosoma cruzi infection. PLoS Negl Trop Dis. 2008;2:e316. doi: 10.1371/journal.pntd.0000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia V, Garg NJ. Previously unrecognized vaccine candidates control Trypanosoma cruzi infection and immunopathology in mice. Clin Vaccine Immunol. 2008;15:1158–1164. doi: 10.1128/CVI.00144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AM, Santoro F, Afchain D, Bazin H, Capron A. Trypanosoma cruzi infection in B-cell-deficient rats. Infect Immun. 1981;31:524–529. doi: 10.1128/iai.31.2.524-529.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MT. The nature of immunity against Trypanosoma cruzi in mice recovered from acute infection. Parasite Immunol. 1981;3:209–218. doi: 10.1111/j.1365-3024.1981.tb00400.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Control of Chagas' disease. WHO Technical Report Series. 2002;905:1–120. [PubMed] [Google Scholar]
- Dutra WO, Rocha MO, Teixeira MM. The clinical immunology of human Chagas disease. Trends Parasitol. 2005;21:581–587. doi: 10.1016/j.pt.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Marin-Neto JA, Cunha-Neto E, Maciel BC, Simões MV. Pathogenesis of chronic Chagas heart disease. Circulation. 2007;115:1109–1123. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- Tarleton RL. Immune system recognition of Trypanosoma cruzi. Curr Opin Immunol. 2007;19:430–434. doi: 10.1016/j.coi.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F. Where do we stand on the autoimmunity hypothesis of Chagas disease? Trends Parasitol. 2005;21:513–516. doi: 10.1016/j.pt.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Umekita L, Ramos D, Barbaro K, Mota I. Biological activity of chronic phase antibodies eluted from sensitized Trypanosoma cruzi trypomastigotes. Immunol Lett. 1999;10:73–76. doi: 10.1016/S0165-2478(99)00139-X. [DOI] [PubMed] [Google Scholar]
- Umekita LF, Mota I. How are antibodies involved in the protective mechanism of susceptible mice infected with T. cruzi? Braz J Med Biol Res. 2000;33:253–258. doi: 10.1590/S0100-879X2000000300001. [DOI] [PubMed] [Google Scholar]
- Stebeck CE, Beecroft RP, Singh BN, Jardim A, Olafson RW, Tuckey C, Prenevost KD, Pearson TW. Kinetoplastid membrane protein-11 (KMP-11) is differentially expressed during the life cycle of African trypanosomes and is found in a wide variety of kinetoplastid parasites. Mol Biochem Parasitol. 1995;71:1–13. doi: 10.1016/0166-6851(95)00022-S. [DOI] [PubMed] [Google Scholar]
- Thomas MC, García-Pérez JL, Alonso C, López MC. Molecular characterization of KMP11 from Trypanosoma cruzi: a cytoskeleton-associated protein regulated at the translational level. DNA Cell Biol. 2000;19:47–57. doi: 10.1089/104454900314708. [DOI] [PubMed] [Google Scholar]
- Olson CL, Nadeau KC, Sullivan MA, Winquist AG, Donelson JE, Walsh CT, Engman DM. Molecular and biochemical comparison of the 70-kDa heat shock proteins of Trypanosoma cruzi. J Biol Chem. 1994;269:3868–3874. [PubMed] [Google Scholar]
- Requena JM, López MC, Jimenez-Ruiz A, de la Torre JC, Alonso C. A head-to-tail tandem organization of hsp70 genes in Trypanosoma cruzi. Nucleic Acids Res. 1988;16:1393–1406. doi: 10.1093/nar/16.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena JM, Jimenez-Ruiz A, Soto M, Assiego R, Santarén JF, Lopez MC, Patarroyo ME, Alonso C. Regulation of hsp70 expression in Trypanosoma cruzi by temperature and growth phase. Mol Biochem Parasitol. 1992;53:201–211. doi: 10.1016/0166-6851(92)90022-C. [DOI] [PubMed] [Google Scholar]
- Sambrook JF, Russell DW. Molecular Cloning, A Laboratory Manual. 3. New York: Cold Spring Harbor Laboratory; 2001. [Google Scholar]
- Thomas MC, Longobardo MV, Carmelo E, Maranon C, Planelles L, Patarroyo ME, Alonso C, Lopez MC. Mapping of the antigenic determinants of the T. cruzi kinetoplastid membrane protein-11. Identification of a linear epitope specifically recognized by human Chagasic sera. Clin Exp Immunol. 2001;123:465–471. doi: 10.1046/j.1365-2249.2001.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planelles L, Thomas M, Pulgar M, Marañón C, Grabbe S, López MC. Trypanosoma cruzi heat-shock protein-70 kDa, alone or fused to the parasite KMP11 antigen, induces functional maturation of murine dendritic cells. Immunol Cell Biol. 2002;80:241–247. doi: 10.1046/j.1440-1711.2002.01081.x. [DOI] [PubMed] [Google Scholar]
- Cuellar A, Santander SP, Thomas MC, Guzmán F, Gómez A, López MC, Puerta CJ. Monocyte-derived dendritic cells from chagasic patients vs healthy donors secrete differential levels of IL-10 and IL-12 when stimulated with a protein fragment of Trypanosoma cruzi heat-shock protein-70. Immunol Cell Biol. 2008;86:255–260. doi: 10.1038/sj.icb.7100146. [DOI] [PubMed] [Google Scholar]
- Steindel M, Pacheco LK, Scholl D, Soares M, de Moraes MH, Kosmann C, Sincero TC, Stoco PH, Murta SMF, Carvalho-Pinto CJ, Grisard EC. Characterization of Trypanosoma cruzi isolated from humans and animal reservoirs following an outbreak of acute human Chagas disease in Santa Catarina, Brazil. Diagn Microbiol Infect Dis. 2008;60:25–32. doi: 10.1016/j.diagmicrobio.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Beltrán M, Duque S, Guhl F, Herrera CP, López MC, Moreno AL, Nicholls RS, Santacruz MM. In: Manual de procedimientos para el diagnóstico de la enfermedad de Chagas. Guhl F, Nicholls RS, editor. Bogotá:Ministerio de Salud; 2001. Prueba de ELISA y prueba de inmunofluorescencia indirecta (IFI) pp. 32–48. [Google Scholar]
- Milei J, Guerri-Guttenberg RA, Grana DR, Storino R. Prognostic impact of Chagas disease in the United States. Am Heart J. 2009;157:22–29. doi: 10.1016/j.ahj.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Llewellyn MS, Miles MA, Carrasco HJ, Lewis MD, Yeo M, Vargas J, Torrico F, Diosque P, Valente V, Valente SA, Gaunt MW. Genome-scale multilocus microsatellite typing of Trypanosoma cruzi discrete typing unit I reveals phylogeographic structure and specific genotypes linked to human infection. PLoS Pathog. 2009;5:e1000410. doi: 10.1371/journal.ppat.1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettli AU, Brener Z. Protective effects of specific antibodies in Trypanosoma cruzi infections. J Immunol. 1976;116:755–760. [PubMed] [Google Scholar]
- Franchin G, Pereira-Chioccola VL, Schenkman S, Rodrigues MM. Passive transfer of a monoclonal antibody specific for a sialic acid-dependent epitope on the surface of Trypanosoma cruzi trypomastigotes reduces infection in mice. Infect Immun. 1997;65:2548–2554. doi: 10.1128/iai.65.7.2548-2554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez H, Guzmán F, Alba MP, Cuéllar A, Thomas MC, López MC, Rosas F, Velasco V, González JM, Patarroyo ME, Puerta CJ. Immunological and structural characterization of an epitope from the Trypanosoma cruzi KMP-11 protein. Peptides. 2007;28:1520–1526. doi: 10.1016/j.peptides.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Umezawa ES, Nascimento MS, Kesper N Jr, Coura JR, Borges-Pereira J, Junqueira AC, Camargo ME. Immunoblot assay using excreted-secreted antigens of Trypanosoma cruzi in serodiagnosis of congenital, acute, and chronic Chagas' disease. J Clin Microbiol. 1996;34:2143–2147. doi: 10.1128/jcm.34.9.2143-2147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara HA, Da Silva AM, Brodskyn CI, Mota I. A comparative study of anti-Trypanosoma cruzi serum obtained in acute and chronic phase of infection in mice. Immunol Lett. 1989;23:81–85. doi: 10.1016/0165-2478(89)90117-X. [DOI] [PubMed] [Google Scholar]
- Mejía-Jaramillo AM, Peña VH, Triana-Chávez O. Trypanosma cruzi: biological characterization of lineages I and II supports the predominance of lineage I en Colombia. Exp Parasitol. 2009;121:83–91. doi: 10.1016/j.exppara.2008.10.002. [DOI] [PubMed] [Google Scholar]
- dos Santos DM, Talvani A, Guedes PM, Machado-Coelho GL, de Lana M, Bahia MT. Trypanosoma cruzi: Genetic diversity influences the profile of immunoglobulins during experimental infection. Exp Parasitol. 2009;121:8–14. doi: 10.1016/j.exppara.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Requena JM, Soto M, Guzman F, Maekelt A, Noya O, Patarroyo ME, Alonso C. Mapping of antigenic determinants of the T. cruzi hsp70 in chagasic and healthy individuals. Mol Immunol. 1993;30:1115–1121. doi: 10.1016/0161-5890(93)90158-8. [DOI] [PubMed] [Google Scholar]
- Rico AL, Gironès N, Fresno M, Alonso C, Requena JM. The heat shock proteins, Hsp70 and Hsp83, of Leishmania infantum are mitogens for mouse B cells. Cell Stress Chaperones. 2002;7:339–346. doi: 10.1379/1466-1268(2002)007<0339:THSPHA>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moraes MH, Guarneri AA, Girardi FP, Rodrigues JB, Eger I, Tyler KM, Steindel M, Grisard EC. Different serological cross-reactivity of Trypanosoma rangeli forms in Trypanosoma cruzi-infected patients sera. Parasit Vectors. 2008;1:20. doi: 10.1186/1756-3305-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerban FM, Gea S, Menso E, Vottero-Cima E. Chagas' disease: IgG isotypes against Trypanosoma cruzi cytosol acidic antigens in patients with different degrees of heart damage. Clin Immunol Immunopatho. 1993;67:25–30. doi: 10.1006/clin.1993.1041. [DOI] [PubMed] [Google Scholar]
- Morgan J, Pinto JC, Dias E, Bahia-Oliveira L, Correa-Oliveira R, Colley DG, Powell MR. Anti-Trypanosoma cruzi antibody isotype profiles in patients with different clinical manifestations of Chagas' disease. Am J Trop Med Hyg. 1996;55:355–359. doi: 10.4269/ajtmh.1996.55.355. [DOI] [PubMed] [Google Scholar]
- Hernández-Becerril N, Nava A, Reyes PA, Monteón VM. IgG subclass reactivity to Trypanosoma cruzi in chronic chagasic patients. Arch Cardiol Mex. . 2001;71:199–205. [PubMed] [Google Scholar]
- Trujillo C, Ramírez R, Vélez ID, Berberich C. The humoral immune response to the kinetoplastid membrane protein-11 in patients with American leishmaniasis and Chagas diseaseChagas' disease: prevalence of IgG subclasses and mapping of epitopes. Immunol Lett. 1999;70:203–209. doi: 10.1016/S0165-2478(99)00146-7. [DOI] [PubMed] [Google Scholar]
- Solana ME, Katzin AM, Umezawa ES, Miatello CS. High specificity of Trypanosoma cruzi epimastigote ribonucleoprotein as antigen in serodiagnosis of Chagas' disease. J Clin Microbiol. 1995;33:1456–1460. doi: 10.1128/jcm.33.6.1456-1460.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verçosa AF, Lorena VM, Carvalho CL, Melo MF, Cavalcanti MG, Silva ED, Ferreira AG, Pereira VR, Souza WV, Gomes YM. Chagas' disease: IgG isotypes against cytoplasmic (CRA) and flagellar (FRA) recombinant repetitive antigens of Trypanosoma cruzi in chronic Chagasic patients. J Clin Lab Anal. 2007;21:271–276. doi: 10.1002/jcla.20186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg HL. Biological activities of immunoglobulins of different classes and subclasses. Adv Immunol. 1974;19:259–294. doi: 10.1016/s0065-2776(08)60254-0. full_text. [DOI] [PubMed] [Google Scholar]