Abstract
We have developed a four-part protocol to differentiate human embryonic stem cells (hESCs) to oligodendrocyte progenitor cells (OPCs) according to developmental principles. In the first 2 weeks, hESCs are induced to differentiate into neuroepithelial cells, which form neural tube–like rosettes. In the following 10 d, these neuroepithelial cells are specified to OLIG2-expressing progenitors in the presence of retinoic acid (RA) and sonic hedgehog (SHH). Upon treatment with fibroblast growth factor 2 (FGF2) for another 10 d, these progenitors convert to OLIG2 and NKX2.2-expressing pre-OPCs. Finally, the pre-OPCs take 8–9 weeks to differentiate into OPCs, which express additional markers of oligodendrocytes, such as SOX10, platelet-derived growth factor receptor alpha (PDGFRα) and NG2. The unique aspects of the protocol are the use of FGF2 to promote the differentiation of gliogenic pre-OPCs in the third part and the removal of FGF2 during the transition of pre-OPCs to OPCs. This 3-month differentiation protocol consistently yields OPCs of high purity capable of producing myelin sheaths in vivo.
INTRODUCTION
Oligodendrocytes are the glial cells that produce myelin sheaths around nerve fibers in the central nervous system. Most of the oligodendrocytes originate from neuroepithelial cells in the ventral neural tube in a sonic hedgehog (SHH)-dependent way1,2. In the spinal cord, the neural progenitors in the motor neuron progenitor (pMN) domain express OLIG2 and can give rise to either motoneurons or oligodendrocyte precursor cells (OPCs) in a time-dependent manner. During the neurogenic phase, the OLIG2 progenitors co-express neuronogenic transcription factors, such as neurogenin 2 (NGN2) and differentiate to motoneurons3,4. After this time, the OLIG2-expressing progenitors become OPCs by switching off neuronogenic transcription factors and turning on oligodendroglial transcription factors, such as NKX2.2 and SOX105–8. These OPCs also express platelet-derived growth factor receptor alpha (PDGFRα) and the membrane proteoglycan NG2 (ref. 9).
These developmental principles have been reproduced in vitro by differentiating mouse embryonic stem cells (ESCs) or rodent neural stem cells (NSCs) to OPCs10–13. SHH potently induces mouse ESCs to differentiate into OPCs by activating the transcriptional network described above11,12. Other soluble factors such as fibroblast growth factor 2 (FGF2) and PDGF also enhance oligodendrocyte differentiation from rodent neural stem/progenitor cells10,14. However, differentiation of human NSCs to OPCs by many laboratories over the past decade has not been successful15–17. Moreover, differentiation of human ESCs to OPCs is inconsistent18,19. By analyzing the transcriptional networks in response to extracellular signals during human embryonic stem cell (hESC) differentiation to OPCs, we found that human OPC specification relies on the similar SHH-dependent transcriptional network to mouse ESCs20. However, we discovered that in the human OPC differentiation process FGF2, a commonly used mitogen for neural progenitors, blocks OPC specification from NSCs by repressing hedgehog signaling20. In addition, the human OPC differentiation takes a long time in comparison to experiments with rodent cells, mainly because of the protracted period for the generation of OPCs from OLIG2 progenitors20, which nevertheless coincides with the OPC development in vivo. By incorporating these new findings, we have devised a four-part protocol for differentiating OPCs from hESCs.
Overview of the protocol
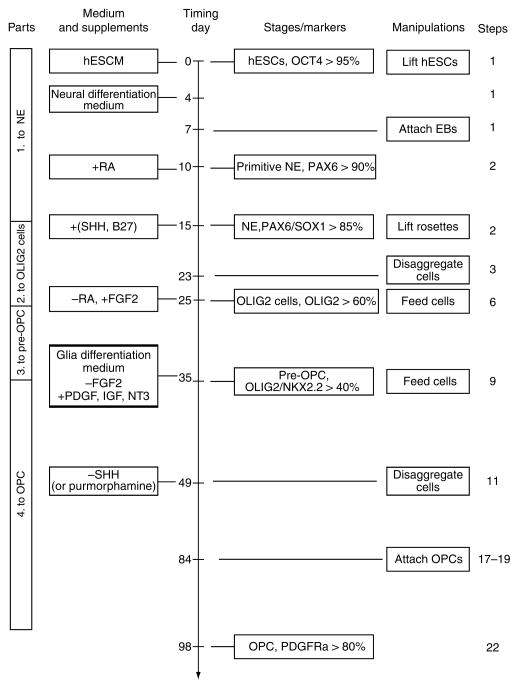
The protocol comprises the induction of neuroepithelia (or NSCs), patterning of OLIG2 progenitors, differentiation of OLIG2/NKX2.2-expressing pre-OPCs and generation of OPCs (Fig. 1). In the first part, hESCs are directed toward the neuroectoderm fate under a chemically defined condition in the absence of growth factors for 2 weeks. Neuroepithelial differentiation can be easily and reliably identified by the characteristic columnar epithelial cells that form neural tube-like rosettes5 and the expression of neuroectoderm transcription factors, including PAX6 and SOX121,22. The second part is to pattern the neuroepithelia to ventral spinal progenitors with the caudalizing factor retinoic acid (RA) and the ventralizing morphogen SHH in the following 10 d. The identity of the progenitors is defined by their expression of OLIG2. These OLIG2 cells can then differentiate into spinal motoneurons. To prevent the differentiation to motoneurons and promote the generation of OPCs, we use FGF2 in the third part of the protocol for 10 d. By day 35, the OLIG2 progenitors co-express NKX2.2 and no longer give rise to motoneurons. The co-expression of OLIG2 and NKX2.2 generally indicates the precursor of OPCs in mouse and chick6,8,23,24. However, these hESC-derived OLIG2/NKX2.2-expressing cells do not possess morphological, biochemical and functional characteristics of OPCs. We therefore refer to these cells as pre-OPCs20. Finally, the pre-OPCs are cultured in a glia medium containing triiodothyronine (T3), neurotrophin 3 (NT3), PDGF, cAMP, IGF-1 and biotin, which individually or synergistically can promote the survival and proliferation of the hESC derived OPCs, for another 8 weeks to generate OPCs. These OPCs are bipolar or multipolar, express OLIG2, NKX2.2, SOX10 and PDGFRα, become motile, and can further differentiate to mature oliogodendrocytes20.
Figure 1.
Flowchart of the four-part oligodendrocyte progenitor cell (OPC) differentiation from human embryonic stem cells (hESCs). Part one differentiates the hESCs to PAX6+ neuroepithelial cells in the serum free medium without morphogens for 2 weeks. Part two patterns the neureopithelia to OLIG2+ ventral progenitors by retinoic acid (RA) and sonic hedgehog (SHH) (or purmorphamine). In part three, the neurogenic potential of the OLIG2 progenitors is repressed by removal of RA and addition of fibroblast growth factor 2 (FGF2) for 10 d. The OLIG2 progenitors now co-express NKX2.2 and become gliogenic, which we refer to as pre-OPCs. Finally, the pre-OPCs are differentiated to OPCs in the glia differentiation medium, which lacks FGF2, and contains platelet-derived growth factor (PDGF), insulin like growth factor (IGF) and neurotrophin 3 (NT3). This fourth part takes 8–9 weeks. The OPCs express SOX10, platelet-derived growth factor receptor alpha (PDGFRα), NG2 in addition to OLIG2 and NKX2.2. This chart is modified from Figure 1 of our motoneuron differentiation protocol25. hESCM, human embryonic stem cell medium; IGF, insulin like growth factor; NE, neuroepithelial cells; PDGF, platelet derived growth factor; NT3, neurotrophin 3. Percentage denotes the promotion of positive cells among total progenies from the starting hESCs.
Applications of the protocol
This 3-month differentiation protocol, corresponding to the developmental program of oligodendrocytes in the human spinal cord, consistently yields OPCs of high purity capable of producing myelin sheaths. It is, thus, useful to study the regulation of human oligodendrocyte development. The OPCs possess robust myelinating potential, therefore they are a potential source of cells for future clinical uses. We have also found that OPCs can be differentiated from human induced pluripotent stem cells (B.-Y.H. and S.-C.Z., unpublished observations). Hence, this protocol will allow the generation of OPCs from diseased stem cells for pathological analysis and drug screening.
Limitations of the protocol
The protracted protocol could present technical difficulty to some investigators, particularly because the mitogen FGF2 needs to be avoided during the 8-week transition period from pre-OPCs to OPCs. Hence, the yield of OPCs is relatively low while the purity is high. We attempted to increase the yield of OPCs by using epidermal growth factor (EGF), but this treatment decreased the purity of OPCs. It thus opens possibilities to explore novel ways to shorten the duration of differentiation and increase the yield without losing purity.
Experimental design
Quality of hESCs
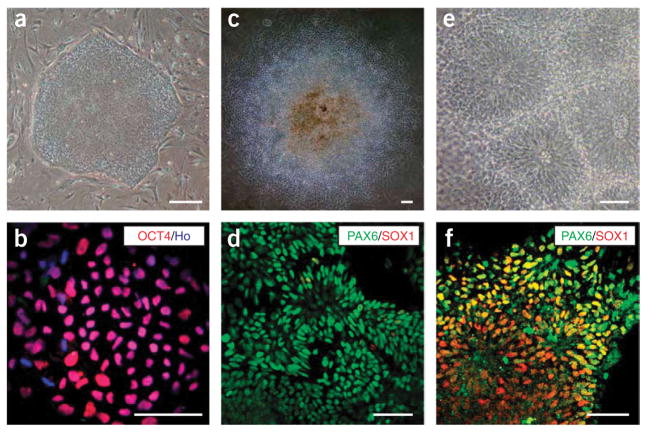
The neuroectoderm differentiation (first part) of the protocol critically depends on the quality of hESCs, which in turn relies on the quality of the mouse embryonic fibroblast (MEF) feeder. We recommend that the MEF cells be tested with at least two hESC lines for no less than three passages. The hESCs should exhibit a uniform undifferentiated phenotype (Figs. 2a,b). Partially differentiated hESCs will decrease the neural differentiation efficiency. This protocol has been reproduced in hESC lines H1,H9 (ref.20) andH14 (B.-Y.H. and S.-C.Z., unpublished observations) between passages 30 and 50.
Figure 2.
Induction of neuroepithelial cells. Human embryonic stem cells (hESCs) growing on mouse embryonic fibroblast (MEF) feeder cells as a uniform colony (a). All cells are positively stained for OCT4 (b). On day 10, columnar epithelial cells appear and organize into rosettes in the colony (c). These cells express PAX6 but not SOX1 (d). At day 15, neural tube-like rosettes are obvious (e) and cells within these rosettes are stained for both PAX6 and SOX1 (f). Scale bar, 50 μm. Ho: Hoechst 33258.
Induction of neuroepithelia
The first two parts of the protocol are similar to the protocol we described for spinal motoneuron differentiation from hESCs25, except we need to briefly disaggregate the cells before treating with FGF2, as the cells grow much faster in mediums containing FGF2. To initiate differentiation, hESCs are separated from the MEF feeder cells to form aggregates in suspension (also known as embryoid bodies). In regular cell culture vessels, the hESC aggregates usually do not attach to the bottom of the flasks/plates, and thus can be grown as spheres in suspension. The contaminating MEFs, however, will attach within 12 h such that the floating spheres can be removed and transferred to a new flask. The differentiation process does not require exogenous growth factors, such as FGFs and Noggin (a BMP inhibitor), as these factors are produced by the differentiating cells themselves26. In cases where hESCs are partially differentiated, addition of these factors can help the neural differentiation process.
The neuroepithelial differentiation part of the protocol takes place in adherent colony culture. After 6 d of floating, the hESC aggregates adhere to the laminin-coated surface to form individual flattened colonies. Such a design has two purposes, one is to allow continuous observation of morphological changes and the other is to allow the enrichment of neuroepithelial colonies by manually scraping off the non-neural colonies. The columnar epithelial cells line up radially like rosettes on day 10, which further form neural tube-like structures by days 14–17. These neural epithelial cells initially express PAX6 and then PAX6 and SOX1 (Figs. 2c–f). Thus, it takes about 2 weeks for hESCs to differentiate into neural epithelial cells.
Patterning of OLIG2 progenitors
The hESC-derived neural epithelial cells carry a dorsal anterior identity and express PAX6, OTX2 and BF121,22. For differentiation to OPCs, which originate from the ventral neural tube, it is necessary to pattern the neuroepithelia to ventral progenitors that express OLIG2. RA (100 nM), a caudalizing factor and SHH (100 ng ml−1), a ventralizing morphogen, are used to pattern the cells to the ventral spinal progenitor fate from day 10 to day 21. The identity of the progenitors is marked by OLIG2 expression. Purmorphamine, a small molecule that activates the hedgehog signaling pathway, can replace SHH in differentiating hESCs into OPCs20. Within its narrow working concentration range (0.5–1.5 μM), we found 1 μM of purmorphamine can efficiently direct hESCs derived NE into OLIG2 expressing progenitors20,27.
Differentiation of pre-OPCs
OLIG2-expressing progenitors appear in culture at the beginning of the fourth week of culture, and most of these cells become motorneurons within the next 10 d (day 25 through 35) following our protocol for motoneuron differentiation25. We discovered that during this period, removal of RA and addition of FGF2 (10 ng ml−1) almost completely blocks the OLIG2-expressing cells from differentiating into motoneurons20. We dissociate the cell aggregates at day 23 so that on day 24 individual cells re-aggregate into clusters, at which size FGF2 can easily diffuse through. FGF2 promotes proliferation of the neural progenitors so that there is no obvious change in cell morphology during this period. We normally change 1/3 to 1/2 of the culture medium, so that the culture environment is not drastically altered during each feeding. When culture condition changes from the previous steps (e.g., factors are removed from or added to the culture medium), we usually pellet the cells by a brief centrifugation step, followed by replacing the old medium with the same amount of fresh medium but with a new recipe. In the steps following FGF2 removal, cAMP, PDGF-AA, IGF and NT3 are supplemented in the culture to allow for better survival of the pre-OPCs or OPCs. In addition, biotin in the medium can fulfill the increasing demands of the oligodendrocyte lineage for synthesizing lipids.
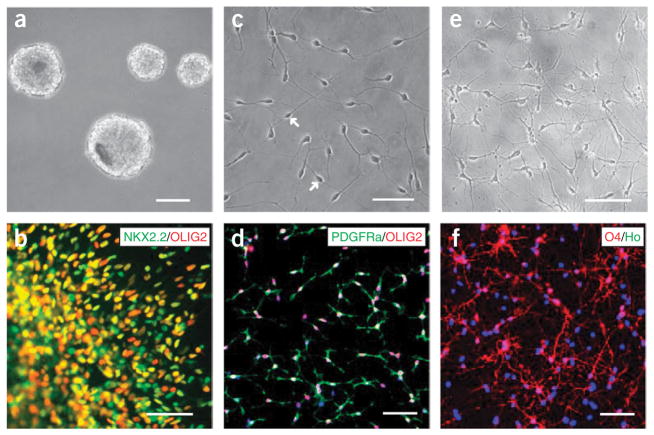
Consequently, the populations of OLIG2-expressing cells, which now also express NKX2.2, increase significantly by day 35. It should be noted that only the OLIG2 and NKX2.2 double positive cells can give rise to OPCs. Hence, cells that co-express OLIG2 and NKX2.2 should be quantified by either counting or FACS analysis20. Different from those in rodents and birds, the hESC derived OLIG2 NKX2.2-expressing cells do not express other markers of OPCs. Thus, we refer to these cells as pre-OPCs (Figs. 3a,b).
Figure 3.
Generation of pre-oligodendrocyte progenitor cells (OPCs) and OPCs. The pre-OPCs grow as spheres in suspension at day 35 (a) and express both OLIG2 and NKX2.2 (b). Dissociated OPCs after 14 weeks of human embryonic stem cell (hESC) differentiation exhibit a bipolar morphology (c, arrows), and co-express OLIG2 and platelet-derived growth factor receptor alpha (PDGFRα) (d). At 4 weeks after growing on substrate for differentiation, OPCs become multipolar immature oligodendrocytes (e), and many of these cells express 04 (f). Scale bar, 50 μm. Ho denotes Hoechst 33258.
Generation of OPCs
Transition from pre-OPCs to OPCs is a protracted step, taking about 8–9 weeks. This process is still SHH-dependent, as inhibition of hedgehog signaling blocks OPC differentiation. Nevertheless, addition of exogenous SHH does not significantly increase the yield of OPCs. Thus, we do not continue using SHH or purmorphamine after day 50 of differentiation. We attempted to speed up the process by adding growth factors known to enhance oligodendrogliogenesis in other vertebrates (e.g., SHH, neuregulin, for proliferation or PDGF-AA, NT3 and IGF for survival), or by removing differentiation factors such as T3 or blocking FGF signaling. However, none of these treatments altered the timing of the differentiation process. Another unique aspect of this part of the protocol is that the expansion of progenitors with media containing FGF2 will block OPC generation. Hence, we culture the pre-OPCs in suspension for the next several weeks without mitogens. The suspension culture helps in preventing the progenitors from differentiating into neurons or astrocytes before they are specified to OPCs. During this process, the progenitors do not proliferate substantially. However, over the long period of culture, the large clusters need to be regularly disaggregated to smaller ones, so that the cells inside the clusters can be better nourished. Another mitogen, EGF (10 ng ml−1) can promote the proliferation of the pre-OPCs. However, the proportion of OPCs decreases after expansion of pre-OPCs in the presence of EGF, thus we exclude it.
Oligodendrocyte progenitor cells can be identified by their characteristic bipolar morphology on laminin or ornithine substrates (Fig. 3c). These substrates are ideal for motile OPCs to migrate and extend processes. The OPC clusters may be dissociated into individual cells or plated as clusters; the latter is technically easier and allows measurement of the distance the cells have migrated. To confirm the identity of OPCs, we stain them for the transcription factor SOX10 in addition to OLIG2 and NKX2.2, and the cell surface markers PDGFRα and NG2 (Fig. 3d). The co-expression of OLIG2 and NKX2.2, which is used to define OPCs in other vertebrates, is not sufficient for marking OPCs, as described above. A bona fide human OPC should express all of these markers simultaneously. The OPCs begin to appear during the tenth week of differentiation, but in a small number (< 3%). The population increases gradually in the next 3 weeks, but bursts in the fourteenth week, reaching 80% in the total population. This time course is likely related to the intrinsic program of human oligodendrocyte development although the exact mechanism is unknown.
Further differentiation of OPCs results in branching of the process, exhibiting extensively ramified processes like a spider web (Fig. 3e). The oligodendrocyte identity can be marked by a surface antibody called O4, which specifically labels maturing oligodendrocytes28 (Fig. 3f). For some membrane proteins, such as O4, NG2 and PDGFRα, we usually incubate live cells with antibodies against these proteins before the cells are fixed20. Staining after regular fixation, which permeabilizes the cells, will give non-continuous membrane staining. Under the serum-free culture condition and in the absence of neurons (neurites), the majority of O4 + immature oligodendrocytes fail to mature and produce myelin sheathes around nerve fibers, and hence usually do not survive for a long period. Consequently, the O4 + proportion is usually around 40% of the total population. As PDGF, IGF and NT3 (10 ngml−1) do not promote the O4 + oligodendrocytes to proliferate at this stage, only moderate amounts (5 ng ml−1) of these components are supplemented for a better survival of the cells. A small number of oligodendrocytes, which do survive under such a condition, will mature and express myelin basic protein (MBP), which is required for producing compact myelin sheath. The functional identity of the hESC-derived OPCs can be best confirmed by their ability to migrate, mature and produce myelin sheaths around axons after transplantation into the brain of the dysmyelinating shiverer mice20.
MATERIALS
REAGENTS
ACCUTASE (Innovative Cell Technology, cat. no. AT 104)
B27 supplement without vitamin A 50× (Gibco-BRL, cat. no. 12587-010)
Brain derived neurotrophic factor (BDNF, PeproTech, cat. no. 450-02)
β-Mercaptoethanol (14.3 M) (Sigma, cat. no. M7522) ! CAUTION Combustible, corrosive and toxic if ingested and absorbed through the skin. Avoid direct contact or exposure to ignition sources.
Fibroblast growth factor 2 (FGF2, R&D, cat. no. 233-FB)
Biotin (Sigma, cat no. B4639)
Bovine serum albumin (BSA) (Sigma, cat. no. A-7906)
Cyclic AMP (Sigma, cat. no. D-0260)
Dispase (Gibco-BRL, cat. no. 17105-041)
Dulbecco’s modified Eagle’s medium:Nutrient mixture F-12 1:1 (DMEM: F-12, Gibco-BRL, cat. no. 11330)
Heparin (Sigma, cat. no. H3149)
Insulin-like growth factor 1 (IGF1, PeproTech, cat. no. 100-11)
Knockout serum replacer (Gibco-BRL, cat. no. 10828) ▲CRITICAL Store stock at − 80 °C. Make aliquots of 50 ml and store at − 20 °C if the whole stock cannot be used within a week of thawing.
Leibovitz’s L-15 Medium (Gibco-BRL, cat. no. 11415)
L-Glutamine solution (Gibco-BRL, cat. no. 25030)
Laminin from human placenta (Sigma, cat. no. L6274)
MEM non-essential amino acids solution (Gibco-BRL, cat. no. 11140)
N1 supplement 100× (Sigma)
N2 supplement 100× (Gibco-BRL, cat. no. 17502-048)
Paraformaldehyde (PFA, Sigma-Aldrich, cat. no. P6148)! CAUTION Toxic if inhaled. Harmful if absorbed through skin and may cause skin irritation. Use PFA in a fume hood and avoid contact with skin and eyes.
Platelet-derived growth factor (PDGF-AA, R&D, cat. no. 221-AA)
Poly-L-ornithine (Sigma, cat. no. P3655)
Purmorphamine (Calbiochem, cat. no. 540220)
Retinoic acid (Sigma, cat. no. R2625)
Sonic hedgehog (SHH, R&D, cat. no. 1845-SH)
Antibodies (see Table 1)
TABLE 1.
Antibodies used for staining cells to verify their identity.
| Antibody | Isotype | Source | Cat. no. | Dilution |
|---|---|---|---|---|
| OCT4 | Mouse IgG | Santa Cruz | sc-5279 | 1:1000 |
| PAX6 | Mouse IgG | DSHB | PAX6 | 1:5000 |
| SOX1 | Goat IgG | R&D | AF3366 | 1:1000 |
| OLIG2 | Goat IgG | Santa Cruz | SC-19969 | 1:500 |
| NKX2.2 | Mouse IgG | DSHB | 74.5A5 | 1:50 |
| NG2 | Mouse IgG | BD Pharmingen | 554275 | 1:400 |
| PDGFRα* | Rabbit IgG | Santa Cruz | SC-338 | 1:400 |
| 04* | Mouse IgM | Chemicon | MAB345 | 1:50 |
PDGFRα, platelet-derived growth factor receptor alpha. After fixation with 4% (wt/vol) paraformaldehyde (PFA) for 10 min and penetrating with 0.1% (vol/vol) Triton-X, the cells are incubated with serum (donkey or goat) for 1 h to block the unspecific staining and with primary antibodies overnight at 4 °C, followed by thorough rinsing with phosphate-buffered saline (PBS) and incubation with fluorophore labeled secondary antibodies for 30 min at room temperature.
The cells on coverslips are incubated with primary antibodies in L15 medium for 15–20 min at room temperature before fixation, and then stained with secondary antibodies.
EQUIPMENT
15-ml and 50-ml conical tubes (BD Biosciences, cat. no. 352095 and 352073)
30-mm Petri dishes (Fisher Scientific, cat. no. 08-757-13A)
6-well and 24-well plates (Nunc, cat. no. 140675 and 142475)
9″ Pasteur pipettes (Fisher Scientific, cat. no. 13-678-20D)
Cover slips (diameter 12 mm, Bellco Glass, cat. no. 1943–10012)
Micropipette puller (model 720, David KOPF instrument)
Laboratory plastic tubing (0.02–0.04″. inner diameter)
Stericup filtration system (Millipore, cat. no. SCGPU05RE).
Steriflip filtration system (Millipore, cat. no. SCGP00525)
T25 and T75 flasks (Nunc, cat. no. 136196 and 178891)
Desktop centrifuge (Centrifuge 5702, Eppendorf)
REAGENT SETUP
Human ESC growth medium (500 ml)
Under sterile conditions combine 392.5 ml of DMEM:F-12,100 ml of Knockout serum replacer, 5 ml of MEM non-essential amino acids solution, 2.5 ml of 200 mM L-glutamine solution (final concentration of 1 mM) and 3.5 μl of 14.3 M β-mercaptoethanol (final concentration of 0.1 mM). The medium can be stored at 4 °C for up to 7–10 d.! CAUTION β-Mercaptoethanol is combustible, corrosive and toxic if ingested and absorbed through the skin. Avoid ingestion, direct contact or direct exposure to ignition.
Neural differentiation medium (DMEM:F-12/N2, 500 ml)
Under sterile conditions combine 489 ml of DMEM:F-12, 5 ml of N2 supplement, 5 ml of MEM non-essential amino acids solution and 1 ml of 1 mg ml−1 heparin. The medium can be stored at 4 °C for up to 2 weeks.
Glia differentiation medium
Under sterile conditions combine 479 ml of DMEM:F-12, 5 ml of N1 supplement, 10 ml of B27 supplement, 5 ml of MEM non-essential amino acids solution, 0.3 ml of 100 mg ml−1 T3, 0.5 ml of 1 mM cAMP and 50 μl of 1 mg ml−1 biotin. The medium can be stored at 4 °C for up to 2 weeks.
Dispase (1 U ml−1)
Dissolve 50 U dispase in 50 ml of DMEM:F-12. Warm at 37 °C for 15 min. Dissolve it completely and filter with a 50 ml Steri-flip. Can be stored at 4 °C for up to 2 weeks. ▲CRITICAL Be aware that the amount of 50 U dispase varies between lots.
Heparin (1 mgml−1)
Dissolve 1 mg of heparin in 10 ml DMEM medium, aliquot and store at − 80 °C for up to 3 months.
FGF2 (100 μg ml−1)
Dissolve 100 μg of bFGF in 1 ml of sterilized PBS with 0.1% (wt/vol) BSA, aliquot and store at −80 °C for up to 3 months.
IGF-1, PDGF-AA (100 μg ml−1)
Dissolve 100 μg of growth factor in 1 ml sterilized distilled water or PBS with 0.1% (wt/vol) BSA, aliquot and store at − 80 °C for up to 3 months.
SHH (100 μg ml−1)
Dissolve 100 μg of SHH in 1 ml of sterilized PBS with 0.1% (wt/vol) BSA. Aliquot 100 μl into sterilized tubes and store at −80 °C for up to 3 months.
Purmorphamine (10 mM)
Dissolve 5 mg of purmorphamine in 480 μl ethanol and 480 μl DMSO, aliquot and store at − 20 °C for up to 8 weeks. ▲CRITICAL The working concentration range of purmorphamine is very narrow. Prepare the stock solution as accurately as possible. When adding stock solution into the culture medium, use the smallest tip and at well-calibrated pipetteman.
RA (100 mM)
Dissolve 50 mg of RA in 1.67 ml of DMSO. Aliquot 50 μl into brown microtubes and store at − 80 °C. ▲CRITICAL RA is extremely sensitive to UV light, air and oxidizing agents, especially in solution. It is recommended to use all the powder immediately after opening the ampoule. Dilute each aliquot with 4.95 ml ethanol and store at − 20 °C as a working stock solution. Try not to use working stock solution older than 2 weeks.
Cyclic AMP (1 mM)
Dissolve 4.914 mg of cyclic AMP in 10 ml of sterilized water. Aliquot and store at − 80 °C for up to 3 months.
Biotin (1 mg ml−1)
Disolve 100 mg of biotin in 10 ml of 1N NaOH, aliquot in 1 ml each and store at − 80 ° for up to 6 months. Dilute 1 ml of concentrated stock solution in 10 ml of DMEM:F-12 and filter through a 0.22 μm teflon filter, store at 4 °C for up to 4 weeks.
Boric acid buffer (pH 8.4)
In 100 ml of distilled water add 0.927 g H3BO3 and 0.6 g NaOH. Adjust pH to 8.4 by adding HCl. Store at room temperature (20–25 °C) for up to 3 months.
10× Poly-L-ornithine (1 mg ml−1)
Add 0.1 g of poly-L-ornithine to 100 ml of boric acid buffer pH 8.4. Filter through a 0.22 μm teflon filter. Store at room temperature (20–25 °C) for up to 3 months.
4% PFA (wt/vol) (100 ml)
In a fume hood add 4 g of PFA with a stirring bar to the water. Keep stirring while heating up to 60 °C. Add 1–2 drops of 2N NaOH until the solution becomes clear. Let the solution cool down to room temperature. In a separate beaker slowly add 0.22 g of sodium phosphate monobasic and 1.22 g of sodium phosphate dibasic into 30 ml of water and stir to help to dissolve them. Mix the sodium phosphate buffer and PFA solutions and adjust the pH of the solution to 7.2–7.4 with HCl. Add water to 100 ml and filter. Store at 4 °C and use the fixative within a week. ! CAUTION Toxic if inhaled. Harmful if absorbed through skin and may cause skin irritation. Avoid contact with skin and eyes. ▲CRITICAL Be careful not to overheat the PFA solution.
Antibodies Refer to Table 1 for details.
EQUIPMENT SETUP
Poly-L-ornithine coated coverslips
In a sterile hood, put one sterilized coverslip in each well of a 24-well plate. Add 75 μl of 0.1 mg ml−1 poly-L-ornithine onto each coverslip. Incubate the plates at 37 °C overnight. The next day, aspirate poly-L-ornithine and let the coverslips dry for ~30 min. Wash three times with 1 ml of sterile water for each well. Leave the plate open in the hood until the coverslips dry completely. Cover the plates, wrap in foil and label with the date. Plates can be stored at −20 °C for 2 weeks.
Laminin coated 6-well plate
Dilute laminin with fresh neural differentiation medium to a final concentration of 20 μg ml−1. Add 300 μl of laminin solution into each well of a 6-well plate. Let the medium hold as a big drop and spread within the central area of the well. Do not let the medium drain to the edge. Incubate the plate at 37 °C for 1 h. Prepare the plates for prompt use only. ▲CRITICAL Laminin is very easily absorbed by plastic and tends to form aggregates at room temperature. Store laminin at −80 °C and thaw at 4 °C before using. Do not aliquot laminin to plastic tubes from the original glass vial.
PROCEDURE
Induction of primitive neuroepithelial cells ● TIMING 10 d (days 0–10)
1. Induce the primitive neuroepithelial cells according to Steps 1–26 of our protocol for motoneuron differentiation from hESCs25.
Specification of Olig2-expressing progenitors ● TIMING 15 d (days 10–25)
2. Differentiate OLIG2-expressing progenitors following Steps 27–39 of our motor neuron protocol25. Cells from 1 to 2 6-well plates should be cultured in one T-75 flask in 35 ml of neural differentiation medium containing B27, 0.1 μM of RA and 100 ng ml−1 SHH (or purmorphamine at 1 μM).
3. On day 21 feed the cells one more time as described in Step 39 of our previous protocol25. On day 23, stand the flask for 3 min so that clusters larger than 200 μm will settle down to the bottom and the small clusters remain in suspension. Disaggregate the larger clusters using ACCUTASE or a glass pipette as described in Step 40 of our protocol of motoneuron differentiation from hESC25. Transfer the smaller clusters that remained in suspension into a 50 ml conical tube. Centrifuge the tube at 80g for 2 min, room temperature. Remove the old medium and re-suspend the pellet with 5 ml of the neural differentiation medium described in Step 2.
4. Collect all the cells and transfer into a T-75 flask and incubate at 37 °C, 5% CO2 in a total of 35 ml of the fresh neural differentiation medium described in Step 2.
5. On day 24, follow the procedures described in Box 1 of our motoneuron differentiation protocol25 to plate 4–5 clusters of cells (about 100 μm in diameter measured with an objective ruler on the microscope) onto poly-L-ornithine and laminin coated coverslips in 50 μl of neural differentiation medium described in Step 2. Immunostain the attached clusters for OLIG220.
Generation of pre-OPCs ● TIMING 10 d (days 25–35)
-
6. On day 25, collect the cells from Step 4 into a 50 ml conical tube and let the cells settle down for 5 min. Remove most of the old medium but leave 2–3 ml in the tube so that the cell clusters will not be accidentally sucked out. Resuspend the cell clusters with 35 ml of the neural differentiation medium described in Step 2, except replacing RA with FGF2 at a final ▲concentration of 10 ng ml−1. Culture the cells in an incubator at 37 °C, 5% CO2.
▲CRITICAL STEP Remember to replace RA with FGF2 from this step onwards.
7. On days 27, 29, 31 and 33, stand the flask at a angle of 45 degree to collect the cells to one corner, remove half (about 20 ml) of the medium and replenish with the neural differentiation medium described in Step 6. Culture the cells in an incubator at 37 °C, 5% CO2.
8. On days 34–35, plate 4–5 clusters of cells, usually 150–200 μm in diameter, onto poly-L-ornithine and laminin coated coverslips as described in Box 1 of our protocol for motoneuron differentiation from hESCs (ref. 25). Once the cells have attached, double stain the cells for OLIG2 and NKX2.2 (ref. 20).
Transition from pre-OPCs to OPCs in suspension ● TIMING 49 d (days 35–84)
9. On day 35 (fifth week), settle down the clusters from Step 7 and remove the medium as described in Step 6. Resuspend the cells with 35 ml of glia differentiation medium (DMEM/F12, 1X Nl supplement, 1 μM purmorphamine, 60 ng ml−1 T3, 100 ng ml−1 biotin and 1 μM cAMP), supplemented with an additional cocktail of cytokines and growth factors consisting of PDGF-AA, IGF1 and NT3 (all at 10 ng ml−1). Culture the cells in an incubator at 37 °C, 5% CO2. Culture the cells for 2 weeks. ▲CRITICAL STEP FGF2 is removed from the medium at this step.
10. On days 38, 41, 44 and 47, settle down the clusters using the procedure described in Step 7. Change half of the medium and replenish with the medium described in Step 9.
-
11. On day 49 (seventh week), disaggregate the big progenitor spheres into smaller cell clusters using the same procedures described in Step 40 of our previous protocol25. Transfer the disaggregated cells into a T-75 flask in 35 ml of the glia differentiation medium described in Step 9, except that purmorphamine is no longer needed from this step on.
▲CRITICAL STEP Purmorphamine is not needed from this step on.
12. Continue feeding the cells as described in Step 10 but ensure that purmorphamine is removed from the medium.
-
13. On day 70 (tenth week), disaggregate the big clusters one more time using the procedures described in Step 40 of our previous protocol25.
▲CRITICAL STEP Briefly disaggregate the cells to loosen clusters, but do not let the cells dissociate to single cells.
14. Culture the cells in a T-75 flask and in 35 ml of the glia differentiation medium described in Step 11, let the cells aggregate for 3 d.
15. On day 73, stand the flask and let the cell clusters sink down to the bottom as described in Step 7. Remove most of the medium containing dead cells and debris leaving the last 2–3 ml behind. Feed the cell clusters with 35 ml of the glia differentiation medium described in Step 11.
16. On days 77 and 82, feed the floating cell clusters using the same procedures as described in Step 7, but with the glia differentiation medium described in Step 11.
Differentiate OPCs on substrate ● TIMING 48 d (days 84–112)
17. On day 84 (twelfth week), transfer about 50 clusters of cells into a 60-mm petri-dish in 5 ml of the glia differentiation medium described in Step 11. Continue culturing the remaining clusters in suspension in 35 ml of the same medium in a T-75 flask. Feed the cells twice a week as described in Step 7. These cells can be used for additional maturation and functional analysis, or for transplantation within the next 4 weeks (ref. 20).
-
18. Pick up 4–5 clusters of cells from the petri-dish and plate them onto glass coverslips that are coated with poly-ornithine and laminin in 50 μl of the glia differentiation medium.
? TROUBLESHOOTING
-
19. Incubate the cells at 37 °C, 5% CO2 overnight. The next day, feed the cells with 0.5 ml of glia differentiation medium supplemented with PDGF-AA, IGF1 and NT3, all at 5 ng ml−1.
? TROUBLESHOOTING
20. After 24 h of culture (day 85), check the culture and observe the bi-polar or multi-polar OPCs migrating out from the clusters.
-
21. Feed the cells every other day by removing 0.3–0.4 ml of the old medium and adding back the same volume of the fresh glia differentiation medium described in Step 19 for the next 10 d.
▲CRITICAL STEP Feed the cells with 2/3 fresh medium in order not to disturb the attached cells.
-
22. On day 98 (fourteenth week), immunostain the cells for OLIG2, NKX2.2, SOX10, PDGFRα and NG2 (ref. 20). Refer to Table 1.
? TROUBLESHOOTING
-
23. On day 112 (sixteenth week), immunostain the cells with 04 antibodies20.
? TROUBLESHOOTING
● TIMING
Step 1, induction of primitive neuroepithelial cells: 10 d
Steps 2–5, specification of Olig2-expressing progenitors: 15 d
Steps 6–8, generation of pre-OPCs: 10 d
Steps 9–16, transition from pre-OPCs to OPCs in suspension: 49 d
Steps 17–23, differentiation of oligodendrocytes on substrate: 48 d
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 2.
TABLE 2.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 18 | 50 μl of medium does not stay on coverslips | The coverslips do not recover to room temperature and are still wet | Leave the plates at room temperature longer or leave the lid half open for quick evaporation |
| 19 | The coverslips dry out | The drop of medium has drained off the coverslip | When moving and transferring the plate, make sure this is done gently. Ensure that the incubator door is opened and closed gently |
| 22 | No PDGFRα stained | The cells are overfixed or not fixed properly | Stain for the membrane protein before fixation as described in the notes of Table 1 |
| 23 | No 04+ cells | 04 cells died and detached; or inappropriate staining procedure | Adding 0.5% (vol/vol) fetal bovine serum into the culture medium may help the survival of 04+ cells. Incubate the cells with 04 antibody for 15 min before fixing the cells with 4% PFA (wt/vol) |
PDGFRα, platelet-derived growth factor receptor alpha; PFA, paraformaldehyde.
ANTICIPATED RESULTS
The four-part OPC differentiation from hESCs mirrors in vivo development of the neural plate/tube (induction of neuro-epithelia), patterning of the pMN domain in the ventral neural tube (specification of OLIG2 progenitors), differentiation of pre-OPCs (differentiation of OLIG2/NKX2.2-expressing progenitors), and generation of OPCs (Fig. 1). In the first part, which takes 10–14 d (Steps 1–26 (ref. 25)), we expect that at least 90–95% of the differentiated progenies will be PAX6 positive neuroepithelial cells (Fig. 2)21, 22. In the second part, the combination of RA and SHH will induce about 60% of the progenitors to be OLIG2 positive ventral progenitors. When SHH is replaced with purmorphamine, we expect a higher proportion of OLIG2 progenitors, up to 80%. By using FGF2 and SHH on the floating cell spheres in the third part, we predict that ~40% of the OLIG2 cells also express NKX2.2, indicative of pre-OPCs (Figs. 3a,b). The last part of the protocol is very long and requires the avoidance of FGF2. Hence, there is limited cell expansion even though the cells continue to divide. By the end of the fourteenth week, the PDGFRα + cells will account for ~80% of the total population and most cells co-express OLIG2, NKX2.2, SOX10 or NG2 (Figs. 3c,d). Further differentiation of the OPCs will result in maturation of the progenitors, as indicated by 04 in immature oligodendrocytes (Figs. 3e,f) and MBP in mature oligodendrocytes20. After transplantation into the brain of shiverer mice, the hESC derived OPCs usually migrate in the corpus callosum. These cells express MBP and form myelin sheaths around nerve fibers20.
Acknowledgments
This study was supported by the National Institute of Neurological Disorders and Stroke (R01 NS045926), the National Multiple Sclerosis Society (NMSS TR-3761), a gift from the Busta family and the Bleser family, and partly by a core grant to the Waisman Center from the National Institute of Child Health and Human Development (P30 HD03352).
Footnotes
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
AUTHOR CONTRIBUTIONS B.-Y.H. and Z.-W.D. designed and carried out experiments, analyzed data and wrote the paper; S.-C.Z. coordinated the study, designed experiments, analyzed data, wrote and finally approved the paper.
References
- 1.Lu QR, et al. Sonic hedgehog-regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25:331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]
- 3.Sugimori M, et al. Combinatorial actions of patterning and HLH transcription factors in the spatiotemporal control of neurogenesis and gliogenesis in the developing spinal cord. Development. 2007;134:1617–1629. doi: 10.1242/dev.001255. [DOI] [PubMed] [Google Scholar]
- 4.Lee SK, Lee B, Ruiz EC, Pfaff SL. Olig2 and Ngn2 function in opposition to modulate gene expression in motor neuron progenitor cells. Genes Dev. 2005;19:282–294. doi: 10.1101/gad.1257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marquardt T, Pfaff SL. Cracking the transcriptional code for cell specification in the neural tube. Cell. 2001;106:651–654. doi: 10.1016/s0092-8674(01)00499-8. [DOI] [PubMed] [Google Scholar]
- 6.Qi Y, et al. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;128:2723–2733. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- 7.Stolt CC, Lommes P, Friedrich RP, Wegner M. Transcription factors Sox8 and Sox10 perform non-equivalent roles during oligodendrocyte development despite functional redundancy. Development. 2004;131:2349–2358. doi: 10.1242/dev.01114. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Q, Choi G, Anderson DJ. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. 2001;31:791–807. doi: 10.1016/s0896-6273(01)00414-7. [DOI] [PubMed] [Google Scholar]
- 9.Zhang SC. Defining glial cells during CNS development. Nat Rev Neurosci. 2001;2:840–843. doi: 10.1038/35097593. [DOI] [PubMed] [Google Scholar]
- 10.Avellana-Adalid V, Nait-Oumesmar B, Lachapelle F, Baron-Van Evercooren A. Expansion of rat oligodendrocyte progenitors into proliferative “oligospheres” that retain differentiation potential. J Neurosci Res. 1996;45:558–570. doi: 10.1002/(SICI)1097-4547(19960901)45:5<558::AID-JNR6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 11.Billon N, Jolicoeur C, Ying QL, Smith A, Raff M. Normal timing of oligodendrocyte development from genetically engineered, lineage-selectable mouse ES cells. J Cell Sci. 2002;115:3657–3665. doi: 10.1242/jcs.00049. [DOI] [PubMed] [Google Scholar]
- 12.Du ZW, Li XJ, Nguyen GD, Zhang SC. Induced expression of Olig2 is sufficient for oligodendrocyte specification but not for motoneuron specification and astrocyte repression. Mol Cell Neurosci. 2006;33:371–380. doi: 10.1016/j.mcn.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Samanta J, Kessler JA. Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development. 2004;131:4131–4142. doi: 10.1242/dev.01273. [DOI] [PubMed] [Google Scholar]
- 14.Zhang SC, Lundberg C, Lipsitz D, O’Connor LT, Duncan ID. Generation of oligodendroglial progenitors from neural stem cells. J Neurocytol. 1998;27:475–489. doi: 10.1023/a:1006953023845. [DOI] [PubMed] [Google Scholar]
- 15.Chandran S, et al. FGF-dependent generation of oligodendrocytes by a hedgehog-independent pathway. Development. 2003;130:6599–6609. doi: 10.1242/dev.00871. [DOI] [PubMed] [Google Scholar]
- 16.Roy NS, et al. Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J Neurosci. 1999;19:9986–9995. doi: 10.1523/JNEUROSCI.19-22-09986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang SC, Ge B, Duncan ID. Tracing human oligodendroglial development in vitro. J Neurosci Res. 2000;59:421–429. doi: 10.1002/(SICI)1097-4547(20000201)59:3<421::AID-JNR17>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 18.Izrael M, et al. Human oligodendrocytes derived from embryonic stem cells: effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol Cell Neurosci. 2007;34:310–323. doi: 10.1016/j.mcn.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Kang SM, et al. Efficient induction of oligodendrocytes from human embryonic stem cells. Stem Cells. 2007;25:419–424. doi: 10.1634/stemcells.2005-0482. [DOI] [PubMed] [Google Scholar]
- 20.Hu BY, Du ZW, Li XJ, Ayala M, Zhang SC. Human oligo dendrocytes from embryonic stem cells: conserved SHH signaling networks and divergent FGF effects. Development. 2009;136:1443–1452. doi: 10.1242/dev.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li XJ, et al. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 22.Pankratz MT, et al. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007;25:1511–1520. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu H, et al. Dual origin of spinal oligodendrocyte progenitors and evidence for the cooperative role of Olig2 and Nkx2.2 in the control of oligodendrocyte differentiation. Development. 2002;129:681–693. doi: 10.1242/dev.129.3.681. [DOI] [PubMed] [Google Scholar]
- 24.Vallstedt A, Klos JM, Ericson J. Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron. 2005;45:55–67. doi: 10.1016/j.neuron.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 25.Hu BY, Zhang SC. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat Protoc. 2009;4:1295–1304. doi: 10.1038/nprot.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavaute TM, et al. Regulation of neural specification from human embryonic stem cells by BMP and FGF. Stem Cells. 2009;27:1741–1749. doi: 10.1002/stem.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li XJ, et al. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. 2008;26:886–893. doi: 10.1634/stemcells.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sommer I, Schachner M. Monoclonal antibodies (01 to 04) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981;83:311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]