Abstract
We have devised a reproducible protocol by which human embryonic stem cells (hESCs) or inducible pluripotent stem cells (iPSCs) are efficiently differentiated to functional spinal motor neurons. This protocol comprises four major steps. Pluripotent stem cells are induced to form neuroepithelial (NE) cells that form neural tube-like rosettes in the absence of morphogens in the first 2 weeks. The NE cells are then specified to OLIG2-expressing motoneuron progenitors in the presence of retinoic acid (RA) and sonic hedgehog (SHH) or purmorphamine in the next 2 weeks. These progenitor cells further generate post- mitotic, HB9-expressing motoneurons at the 5th week and mature to functional motor neurons thereafter. It typically takes 5 weeks to generate the post-mitotic motoneurons and 8–10 weeks for the production of functional mature motoneurons. In comparison with other methods, our protocol does not use feeder cells, has a minimum dependence on proteins (purmorphamine replacing SHH), has controllable adherent selection and is adaptable for scalable suspension culture.
INTRODUCTION
Directed differentiation of specific lineages from human embryonic stem cells (hESCs) is the first critical step toward the use of hESCs in understanding early human development and potential future application in the clinic. In the past decade, various protocols have been developed to differentiate hESCs to cells of the neural lineage, including neuroepithelial (NE) cells1, spinal motor neurons2,3, midbrain dopamine neurons4–6, cerebral glutamate and γ-butyric acid (GABA) neurons7,8, astrocytes and oligodendrocytes9,10. These protocols vary considerably in the state of the starting hESCs, use of feeders for co-culture, presence of unknown factors (e.g., sera and conditioned media), differentiation efficiency and purity of the target cell type11. Hence, these protocols may be useful for certain applications but not others. The series of neural differentiation protocols we have developed over the past decade, including the one described here, were devised to fulfill several objectives: creating a model for dissecting the cellular and molecular interactions underlying early human brain development, producing enriched/pure populations of functional neural cell types for the development of therapeutics and modeling diseases with human-induced pluripotent stem (iPS) cell-derived motoneurons for investigating the pathogenesis and screening of drugs. We therefore follow the developmental principles and use chemically defined media for controllability and reproducibility of cell culture. This protocol almost always leads to a high efficiency in production of the target cell type without losing the simplicity of the culture system and medium components.
In the mouse, spinal motor neurons are differentiated from NE cells in a very narrow band of the ventral neural tube known as the pMN domain. These progenitor cells express the helix–loop–helix transcription factor Olig2. The specification of the Olig2-expressing motoneuron progenitors is strictly dependent on a particular amount of sonic hedgehog (SHH) that is secreted from the notochord12,13. Through interaction with neurogenic transcription factors including Ngn2 and PAX6, theOlig2-expressing progenitors differentiate to post-mitotic motor neurons during the neurogenesis phase. These post-mitotic motor neurons express motoneuron-specific transcription factors such as HB9 and Isl1, whereas Olig2 is downregulated14–17. HB9-expressing motoneuorns further differentiate and express choline acetyltransferase (ChAT), an enzyme catalyzing acetylcholine synthesis for signal conduction through the neuromuscular junctions. As for humans, it is generally predicted that human motor neurons are generated following a similar process. Based on the evidence from limited studies on human embryos, it can be inferred that spinal motoneuron progenitors appear in the developing human spinal cord at the end of the 5th gestation week and motor neurons in the ventral horn in the following week18,19.
The generation of spinal motoneurons from hESCs follows the same basic steps as shown in the development of the spinal cord (Fig. 1). The hESCs are initially suspended in ESC growth medium for 4 d to support cell survival and to initiate the differentiation process. The cells are then transferred to a serum-free neural differentiation medium in order to guide the cells toward the neuroectoderm fate in the next 10 d (ref. 20). During the neural induction phase, the hESC aggregates are reseeded fromday 7 onto a culture surface without feeder cells to form individual colonies of monolayer cells. This allows even exposure of the cells to the medium for a synchronized differentiation and easy observation of morphological changes over time. The adherent nature of the cells also allows for easy purification in case there are non-neural colonies. By 2 weeks of differentiation (days 14–17), NE cells develop, which can be easily and reliably identified by the presence of neural tube-like rosettes1 and by the expression of a host of neuroectoderm transcription factors including PAX6 and SOX1. Interestingly, by default, almost all of the NE cells carry an anterior phenotype by expressing OTX2 (ref. 20). Therefore, the second step is to treat the anterior NE cells with retinoic acid (RA) and SHH in the next 2 weeks, in order to produce OLIG2-expressing ventral spinal progenitors. We discovered that the early NE cells at day 10, also referred to as primitive NE cells20, are much more responsive to RA; thus, RA is added at day 10 in our protocol2,21. These OLIG2-expressing cells then differentiate to spinal motoneurons in the 5th week and express transcription factors such as HB9 and Isl1. These motoneurons, when further cultured in the presence of neurotrophic factors, extend long axonal projections, express ChAT and become electrophysiologically active. When co-cultured with myoblasts, these hESC-derived motoneurons form neuromuscular junctions.
Figure 1.
Scheme of differentiation of spinal cord motoneurons (MNs) from hESCs. The starting hESCs are positive for OCT4. After lifting from MEF feeder cells, the hESCs form aggregates (resemble to embryoid bodies, EBs) in suspension. They are cultured for 4 d in hESC medium and in neural differentiation medium thereafter. In the 2nd week, the aggregates that are attached to the surface of culture dishes and possess features of neuroepithelial (NE) rosettes can be enriched for by manual selection. The primitive NE cells (day 10, PAX6-positive) are patterned with retinoic acid (RA) for 5 d. The cells are then PAX6/SOX1-positive and are lifted off the culture surface on day 15 and then cultured with RA and sonic hedgehog (SHH) (or purmorphamine) for the subsequent 2 weeks. The OLIG2-expressing motoneuron progenitors are present in the 4th week. From day 28, these motoneuron progenitors can be differentiated to post-mitotic motoneurons on the substrate in the presence of neurotrophic factors and reduced concentrations of SHH and RA. HB-9-positive neurons form within another week of culture. Except during the initial transition period using the hESC growth medium, the entire process uses a simple serum-free neural differentiation medium. The adherent culture during the neural induction phase is uniquely designed for direct visualization of NE differentiation and for potential purification of the NE cells.
The protocol described here is optimized from our earlier reports2,21. We have made a number of modifications to streamline the procedure and improve the yield owing to the availability of the more potent recombinant SHH (resulting from a mutation at the N terminus) and the discovery of small molecules for activating the hedgehog pathway that work in human cells. The optimized protocol typically generates over 50% of HB9-expressing motoneurons from the original hESC progenies. This protocol has also been shown to be effective formotoneuron differentiation from iPS cells22. This protocol is thus useful for dissecting molecular interactions underlying human motoneuron specification under a defined condition, following pathological changes in motoneuorns (that are derived from transgenic stem cells and disease iPS cells), developing motoneuron templates for drug screening and generating functional motoneurons for potential cell therapy in diseases involving motoneurons.
Experimental design
This protocol critically depends on the quality of hESCs. The starting hESCs (lines H1 and H9), maintained on mouse embryonic fibroblast (MEF) feeder, should exhibit a uniform undifferentiated phenotype (Fig. 2a,b). hESCs expanded in feeder-free conditions and partially differentiated hESCs generally result in a lower neural differentiation efficiency. Quality MEFs are essential for maintaining the undifferentiated state of hESCs. Before use, it is necessary to test each batch of MEFs on at least two hESC lines for no less than three passages.
Figure 2.
Induction of neuroepithelial cells. hESCs growing on MEF feeders form uniform colonies (a); all cells are positive for OCT4 (red) and negative for PAX6 (green) (b). Columnar epithelial cells at day 10 organize into rosettes in the colony (c); these cells express PAX6 (green) but not SOX1 (red) (d). Neural tube-like rosettes at day 15 (e) stain positive for both PAX6 (green) and SOX1 (red) (f). Bar = 50 µm, Ho shown as blue denotes Hoschest33258-stained nuclei.
The neural differentiation process begins with the lifting of hESC colonies from MEF feeder layers. The detachment of hESCs from MEFs and the formation of aggregates in suspension (also known as embryoid bodies) initiate the differentiation process. The hESC aggregates are usually grown as free-floating spheres in regular cell culture dishes or flasks so that any contaminating MEFs will attach to the flask. These fibroblasts can then be easily removed by transferring the floating ESC aggregates to a new culture vessel. The differentiation process does not require exogenous growth factors such as FGFs or noggin, a BMP antagonist, as these factors are produced by the differentiating cells23. The addition of noggin could help the neural differentiation, if the hESCs are partially differentiated.
A unique aspect of the differentiation protocol is the adherence of the hESC aggregates to the culture surface from day 7. The attached aggregates form individual monolayer colonies, permitting continual observation of morphological differentiation. This also allows enrichment of NE colonies by manually scraping off the non-neural colonies. Although the hESC aggregates can attach to the culture surface, coating the culture surface with laminin enhances the adherent process. The cells should be seeded so that after 7 d of growth individual colonies remain separated from each other. Using a higher cell density will compromise the neural differentiation, as autocrine and/or paracrine signaling through the TGFβ signaling will inhibit neural differentiation.
The first sign of neural differentiation is the appearance of columnar epithelial cells that line up radially like rosettes at around 10 d of hESC differentiation1,2,20. Surrounding the neural epithelial rosettes are round spreading cells that are likely to be of the neural crest lineage (Fig. 2c). The NE cells at this stage express PAX6 but not SOX1 (Fig. 2d). We refer to these cells as primitive NE cells, as these cells can be patterned to versatile neural cell types1,2,20.
The columnar NE cells proliferate quickly and form neural tube-like rosettes after an additional 4 d of culture (Fig. 2e). We refer to these cells as definitive NE cells1,2,20. Immunostaining will indicate that these cells express both PAX6 and SOX1 (Fig. 2f). Thus, it takes about 2 weeks for hESCs to differentiate to NE cells. At this time point, cells grow into many layers and multiple neural tube-like rosettes appear in individual colonies. The cells in the form of neural tube-like rosettes attach to the substrate loosely, whereas the flat cells in the surrounding area attach more tightly. Therefore, gentle pipetting of the colony will blow the neural rosettes off but leave the surrounding flat cells behind. Colonies that do not possess rosettes are usually non-neural colonies. Those colonies can be observed under a microscope and scraped off using a pipette tip. This will minimize, if not eliminate, the contamination of non-neural cells.
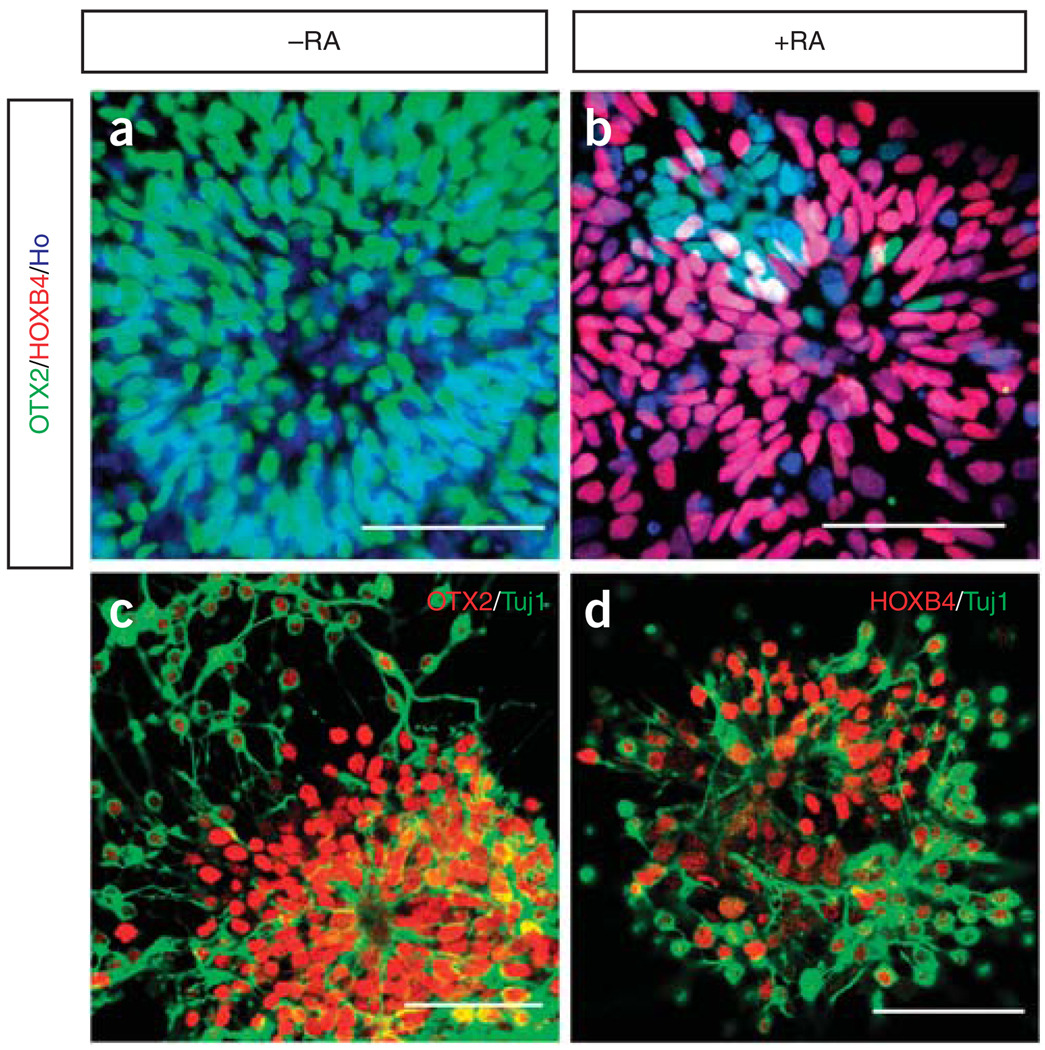
The NE cells differentiated from hESCs in the absence of morphogens bear an anterior identity and express forebrain and midbrain markers such as OTX2 and BF1 (refs. 2,20). Hence, we choose RA, a caudalizing factor, and SHH, a ventralizing morphogen, for inducing the ventral spinal progenitor fate24,25. We have discovered that the primitive but not the definitive NE cells are responsive to morphogens for regional patterning2. We therefore treat the NE cells with RA at day 10 for motoneuron specification2. By carefully titrating the concentrations, we found that RA at 100 nM is sufficient to pattern OTX2-expressing primitive NE cells to progenitors with upper spinal cord identity, which express HOXB42 (Fig. 3a–d). From day 15, after lifting off rosettes, the spinal cord progenitors are then directed to a ventral fate with recombinant SHH (100 ng ml−1), which can be marked by OLIG2 expression. Recently, we discovered that purmorphamine, a small molecule targeting smoothened of the SHH signaling pathway, can replace SHH for motor neuron specification. Purmorphamine has a narrow working concentration range (0.5–1.5 µM) and 1 µM of purmorphamine can efficiently induce OLIG2 expression.
Figure 3.
Patterning of spinal cord progenitors. The hESC-derived neuroepithelial cells cultured without RA are positive for OTX2 (green) (a). When the cells are cultured in the presence of RA (100 nM) for 1 week, the majority of the cells express HOXB4 (red) (b). (c,d) Following a further week of culture, these progenitors retain the rostral (c) or caudal identity (d) when they differentiate to tubulin+ (Tuj1) (green) neurons. Both OTX2 and HOXB4 are represent as red in c and d. Bar µ 50 µm.
From day 15, the cells grow quickly in suspension and form large NE spheres. In order for growth factors and morphorgens to penetrate into the compact clusters, those clusters larger than 300 µm need to be broken into smaller ones. We usually triturate the clusters using a curved Pasteur pipette without dissociating them to single cells. Dissociation of the progenitor clusters into single cells will significantly reduce the yield of post-mitotic motor neurons. This requires the preparation of the Pasteur pipette to a right angle with an appropriate aperture26. A brief digestion with Accutase, that contains proteolytic and collagenolytic enzymes, can also achieve the dissociation of large clusters to smaller ones (Fig. 4a).
Figure 4.
Differentiation and maturation of hESC-derived motoneurons. (a) The motoneuron progenitors are cultured in suspension before they are attached for differentiation. (b) Extensive axonal projections project from the clusters a week after adherent culture. At the 4th week of culture, a large proportion of the cells express OLIG2 (red), whereas a smaller population of HB9-expressing (green) motoneurons is present (c). HB9-positive motorneurons (red) are also positively stained for neuronal marker Tuj1+ (green) at the 5th week (d). At the 6th week of culture, some cells are positive for ChAT (red), a few of the cells co-label with HB9 (green) (e). The hESC-derived motoneurons express synapsin (green) and contact with myotubes in culture (f). The contacts induce clustering of the acetylcholine receptor on myotubes, as shown by bungarotoxin (BTX) (red) staining. Bar = 50 µm in a–e, 20 µm in f.
On day 28, OLIG2-expressing motoneuron progenitors appear. For differentiation of post-mitotic motoneurons, the OLIG2-expressing progenitor clusters are grown on substrates such as laminin. Two days after plating, the spheres spread out and neurites extend from the spheres (Fig. 4b). Neurotrophic factors, brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF), insulin-like growth factor-1 (IGF1), and cAMP are added to support the survival and process outgrowth of motoneurons. The concentration of SHH and RA is reduced as high concentrations of SHH or RA inhibit motoneuron differentiation at this stage.
At day 35, HB9-expressing motoneurons increase in number, whereas the OLIG2-expressing progenitors decrease (Fig. 4c). The HB9+ neurons also express βIII-tubulin (Fig. 4d) and are positive for the LIM domain transcription factor ISL1/2. Under the optimal condition, this protocol yields ~50% HB9+ motoneuron among the total differentiated progenies. It should be noted that some commercially available antibodies against HB9 are not specific and cannot be reliably used as a marker of spinal cord motoneurons. Hence, multiple motoneuron-related markers such as ISL1 and LHX3 should be investigated as well as HB9. In general, cells positive for both HB9 and ISL1 are considered to be motoneurons. As a positive control, embryonic spinal cord tissues can be reliably used for ascertaining the specificity of the HB9 antibodies. In our hands, MNR2 (HB9, monoclonal antibody, Developmental Studies Hybridoma Bank (DSHB; ref. 21), 81.5C10) specifically labels motoneurons in vitro and in vivo. Both OLIG2- and HB9-stained cells can also be quantitated with fluorescence-activated flow cytometry.
ChAT catalyzes acetylcholine synthesis in cholinergic neurons, which in the ventral spinal cord is exclusively expressed in motoneurons. In the hESC-derived motoneurons, ChAT-positive motor-neurons begin to appear at around the 6th week (days 40–42) and increase in number overtime; meanwhile, HB9-expressing cells decrease. During the 6th and 7th weeks, some cells express HB9 in the nucleus and ChAT in the cytoplasm and neurites (Fig. 4e). The available ChAT antibodies usually give rise to a high background when staining cultured cells that are fixed with paraformaldehyde (PFA) only. The addition of 0.2% (vol/vol) picric acid in the fixative buffer will fix the enzyme better and minimize the background.
The motoneurons growing on the substrate can be maintained for several weeks. When co-cultured with myocytes, the motoneuron axons induce acetylcholine receptor clustering, which can be visualized by fluorescently labeled α-bungarotoxin (Fig. 4f).
MATERIALS
REAGENTS
Antibodies; refer to Table 1 for details.
Accutase (Innovative Cell Technology, cat. no. AT104)
Ascorbic acid (Sigma, cat. no. A4403)
B27 supplement without vitamin A 50× (Gibco-BRL, cat. no. 12587-010)
BDNF (PeproTech, cat. no. 450-02)
β-Mercaptoethanol (2-ME, 14.3 M, Sigma, cat. no. M7522)
 2-ME is combustible, corrosive and toxic in case of ingestion and skin absorption; keep away from sources of ignition; and avoid direct contact.
2-ME is combustible, corrosive and toxic in case of ingestion and skin absorption; keep away from sources of ignition; and avoid direct contact.Bovine serum albumin (BSA) (Sigma, cat. no. A-7906)
Boric acid (H3BO3; Sigma, cat. no. B1934)
C2C12 myoblasts (ATCC, CRL-1772)
Cyclic AMP (Sigma, cat. no. D-0260)
Dispase (Gibco-BRL, cat. no. 17105-041)
DMSO (Sigma, cat. no. D8418)
Dulbecco’s modified eagle medium/Nutrient mixture F-12 (DMEM/F12) (1:1) (Gibco-BRL, cat. no. 11330)
DMEM (Gibco-BRL, cat. no. 11965)
Dulbecco’s phosphate-buffered saline (DPBS) (Gibco-BRL, cat. No. 14190)
Ethyle alcohol (Ethanol; Pharmco-AAPER, cat. no. 111USP200)
 Ethanol is flammable. Avoid exposure to ignition.
Ethanol is flammable. Avoid exposure to ignition.Fetal bovine serum (FBS) (Gibco-BRL, cat. no. 10437)
Fibroblast growth factor 2 (FGF2) (R&D, cat. no. 233-FB)
GDNF (PeproTech, cat. no. 450-10)
Glass coverslips (Bellco, cat. no. 1943-10012)
Heparin (Sigma, cat. no. H3149)
HCl (Fisher Scientific, cat. no. A144)
 HCl is corrosive. Manipulate in a fume hood. Wear personal protective equipment such as rubber gloves.
HCl is corrosive. Manipulate in a fume hood. Wear personal protective equipment such as rubber gloves.hESC lines H1 and H9 (National Stem Cell Bank, cat. nos. WA01 and WA09)
IGF1 (PeproTech, cat. no. 100-11)
KnockOut Serum Replacement (Gibco-BRL, cat. no. 10828)
l-Glutamine solution (Gibco-BRL, cat. no. 25030)
Laminin from the human placenta (Sigma, cat. no. L6274)
MEM non-essential amino acids solution (Gibco-BRL, cat. no. 11140)
NaOH (Fisher Scientific, cat. no. S318)
 NaOH is corrosive. Wear rubber gloves and protective eye goggles.
NaOH is corrosive. Wear rubber gloves and protective eye goggles.N2 supplement 10× (Gibco-BRL, cat. no. 17502-048)
PFA (Sigma, cat. no. P9148)
 PFA is toxic. Manipulate in a fume hood. Avoid direct contact.
PFA is toxic. Manipulate in a fume hood. Avoid direct contact.Poly-l-ornithine (Sigma, cat. no. P3655)
Purmorphamine (CALBIOCHEM, cat. no. 540220)
RA (Sigma, cat. no. R2625)
Sodium hydroxide (Sigma, cat. no. S8045)
SHH (R&D, cat. no. 1845-SH)
Trypsin inhibitor (Gibco-BRL, cat. no. 17075-029)
Trypsin–EDTA solution (1×, Gibco-BRL, cat. no. 25300)
TABLE 1.
Antibodies and probes used for determining the neural differentiation of hES cells.
| Antibody | Isotype | Source | Cat. no. | Dilution |
|---|---|---|---|---|
| Oct4 | Mouse IgG | Santa Cruz Biotechnology | sc-5279 | 1:1,000 |
| Pax6 | Mouse IgG | DSHB | NA | 1:5,000 |
| Sox1 | Goat IgG | R&D | AF3366 | 1:1,000 |
| Otx2 | Goat IgG | R&D | AF1979 | 1:2,000 |
| HoxB4 | Rat IgG | DSHB | 112 anti-Hoxb4 | 1:50 |
| Olig2 | Goat IgG | Santa Cruz Biotechnology | SC-19969 | 1:500 |
| MNR2 (HB9) | Mouse IgG | DSHB | 81.5C10 | 1:50 |
| ChAT | Goat IgG | Chemicon | AB144P | 1:200 |
| βIII-tubulin | Rabbit IgG | Covance | PRB-435P | 1:5,000 |
| Synapsin | Rabbit IgG | CALBIOCHEM | 574777 | 1:250 |
| α-bungarotoxin | NA | Molecular Probe | B13423 | 1:500 |
NA, not applicable.
EQUIPMENT
15- and 50-ml conical tubes (BD Biosciences, cat. nos. 352095 and 352073)
30-mm Petri dishes (Fisher Scientific, cat. no. 08-757-13A)
6- and 24-well plates (Nunc, cat. nos. 140675 and 142475)
9" Pasteur pipettes (Fisher Scientific, cat. no. 13-678-20D)
Humidified tissue culture incubator (37 °C, 5% CO2)
Inverted phase contrast microscope (Nikon, ECLIPSE TS100)
Serological pipettes 5-, 10- and 25-ml (Fisher Scientific, cat. nos. 13-678-11D, 13-678-11E and 13-678-11)
Stericup filtration system (Millipore, cat. no. SCGPU05RE, 500 ml)
Steriflip filtration system (Millipore, cat. no. SCGP00525, 50 ml)
REAGENT SETUP
hESC growth medium (500 ml)
In a sterile environment, mix 392.5 ml of DMEM-F12, 100 ml of KnockOut serum replacement, 5 ml of MEM non-essential amino acids solution, 2.5 ml of 200 mM l-glutamine solution (final concentration of 1 mM) and 3.5 µl of 14.3 M of 2-ME (final concentration of 0.1 mM). The medium can be stored at 4 °C for up to 7–10 d.  2-ME is combustible, corrosive and toxic in case of ingestion and skin absorption; keep away from sources of ignition; avoid direct contact.
2-ME is combustible, corrosive and toxic in case of ingestion and skin absorption; keep away from sources of ignition; avoid direct contact.
KnockOut serum replacement
Store the 500-ml stock at −80 °C. Use up the thawed serum replacement within a week. If it cannot be used up, then prepare aliquots of 50 ml and store at −20 °C for up to 4 weeks.
Neural differentiation medium (DMEM/F12/N2, 500 ml)
In a sterile environment, mix 489 ml of DMEM/F12, 5 ml of N2 supplement, 5 ml of MEM non-essential amino acids solution and 1ml of 1 mg ml−1 heparin. The medium can be stored at 4 °C for up to 2 weeks. For neuronal differentiation, add cAMP (1:10,000), ascorbic acid (1:1,000), BDNF (1:10,000), GDNF (1:10,000) and IGF-1 (1:10,000) immediately before use.  The neuronal differentiation medium should be made up fresh each day.
The neuronal differentiation medium should be made up fresh each day.
Dispase (1 Uml−1)
Dissolve 50 U of dispase in 50 ml of F12/DMEM medium; warm at 37 °C for 15 min; and filter using a 50-ml Steriflip. The solution can be stored at 4 °C for up to 2 weeks.  Note that the amount of dispase (U ml−1) varies among lots.
Note that the amount of dispase (U ml−1) varies among lots.
Trypsin inhibitor (1 mg ml−1)
Dissolve 50 mg of trypsin inhibitor in 50 ml of DMEM/F12 and filter through a 50-ml Steriflip. Store at 4 °C and use within 2 weeks.
Heparin (1mg ml−1)
Dissolve 10 mg of heparin in 10 ml of DMEM medium, aliquot and store at −80 °C for up to 3 months.
FGF2 (100 µg ml−1)
Dissolve 100 µg of bFGF in 1 ml of sterilized PBS with 0.1% BSA (wt/vol). Store at −80 °C for up to 3 months.
BDNF, GDNF and IGF1 (100 µg ml−1)
Dissolve 100 µg of growth factor in 1 ml of sterilized distilled water, aliquot and store at −80 °C for up to 3 months.
SHH (100 µg ml−1)
Dissolve 100 µg of SHH in 1 ml of sterilized PBS with 0.1% BSA (wt/vol). Aliquot 100 µl into sterilized tubes and store at −80 °C for up to 3 months.
Purmorphamine (10 mM)
Dissolve 5 mg of purmorphamine in 480 µl of 100% ethanol and 480 µl of DMSO, aliquot and store at −20 °C for up to 8 weeks.  Ethanol is flammable. Avoid exposure to ignition.
Ethanol is flammable. Avoid exposure to ignition.  The working concentration range of purmorphamine is very narrow. Prepare the stock solution as accurately as possible. When adding the stock solution into the culture medium, use the smallest tip and a well-calibrated pipette.
The working concentration range of purmorphamine is very narrow. Prepare the stock solution as accurately as possible. When adding the stock solution into the culture medium, use the smallest tip and a well-calibrated pipette.
RA (100 mM)
Dissolve 50 mg of RA in 1.67 ml of DMSO. Aliquot 50 µl into brown microtubes. The concentrated stock can be stored at −80 °C for up to 6 weeks. Dilute each concentrated aliquot with 4.95ml of 100%ethanol as a working stock solution. This can be stored at −20 °C for up to 2 weeks.  RA is extremely sensitive to UV light, air and oxidizing agents, especially in solution. It is recommended to use all of the powder in the ampule immediately after opening. Do not use the working stock solution older than 2 weeks.
RA is extremely sensitive to UV light, air and oxidizing agents, especially in solution. It is recommended to use all of the powder in the ampule immediately after opening. Do not use the working stock solution older than 2 weeks.
cAMP (1mM)
Dissolve 4.914 mg of cAMP in 10 ml of sterilized water. Aliquot and store at −80 °C for up to 3 months.
Ascorbic acid (200 µg ml−1)
Dissolve 2 mg of ascorbic acid in 10 ml of PBS. Aliquot and store at −80 °C for up to 3 months.
Boric acid buffer (pH 8.4)
In 100 ml distilled water, add 0.927 g of H3BO3 and 0.6 g of NaOH. Adjust pH to 8.4 by adding HCl and testing aliquots. Store at room temperature (20–25 °C) for up to 3 months.  NaOH and HCl are corrosive. Wear rubber gloves and protective eye goggles.
NaOH and HCl are corrosive. Wear rubber gloves and protective eye goggles.
10× Poly-l-ornithine (1 mg ml−1)
Add 0.1 g of poly-l-ornithine to 100 ml of boric acid buffer (pH 8.4). Filter through a 0.22-µm Teflon filter. Store at room temperature for up to 3 months.
Myocytes complete growth medium
DMEM containing 10% FBS (vol/vol). Store at 4 °C and use the medium within 2 weeks.
4% PFA (wt/vol) (100 ml)
Heat 60 ml of water in a beaker to 60 °C. In a fume hood, add 4 g of PFA using a stir bar to the water. Keep stirring the solution on a heat plate at 60 °C. Add 1–2 drops of 2 N NaOH using a Pasteur pipette until the solution becomes clear. Remove from the heat and let the solution cool down. In a separate beaker outside the fume hood, slowly add 0.22 g of sodium phosphate monobasic and 1.22 g of sodium phosphate dibasic into 30 ml and stir until the solution is clear. In a fume hood, mix the sodium phosphate buffer and PFA solutions. Adjust the pH of the solution to 7.2–7.4 with HCl, add water to 100 ml and filter. Use up the fixative within a week.  PFA is toxic. Operate in a fume hood. Wear rubber gloves and protective eye goggles.
PFA is toxic. Operate in a fume hood. Wear rubber gloves and protective eye goggles.  Note that the PFA solution should not be overheated.
Note that the PFA solution should not be overheated.
EQUIPMENT SETUP
Poly-l-ornithine-coated coverslips
In a sterile hood, put one sterilized coverslip in each well of a 24-well plate. Add 75 µl of 0.1 mg ml−1 poly-l-ornithine onto each coverslip. Incubate the plates at 37 °C overnight. The next day, aspirate the poly-l-ornithine and let the coverslips dry at roomtemperature for ~30 min. Wash three times using 1 ml of sterile water for each well. Leave the plate open in the hood until completely dry. Cover the plates, wrap in foil, label with date and store at −20 °C for up to 4 weeks.
Laminin-coated six-well plate
Dilute laminin with fresh neural differentiation medium to a final concentration of 20 µg ml−1. Add 300 µl of laminin solution to each well of a six-well plate. Allow the medium to hold as a big drop and spread within the central area of the well. Do not let the medium drain to the edge. Incubate the plate at 37 µC for 1 h.  Laminin easily absorbs to plastic and tends to form aggregates at room temperature. Store laminin at −80 °C and thaw at 4 °C before using. Avoid aliquotting laminin into plastic tubes from the original glass vial.
Laminin easily absorbs to plastic and tends to form aggregates at room temperature. Store laminin at −80 °C and thaw at 4 °C before using. Avoid aliquotting laminin into plastic tubes from the original glass vial.
PROCEDURE
Induction of primitive NE cells  10 d (days 0–10)
10 d (days 0–10)
Grow hESCs in a six-well MEF plate with 2.5 ml of hESC medium containing 4 ng ml−1 of FGF2 until they form colonies, express OCT4 uniformly1 and are ready to be subcultured (Fig. 2a and b). Incubate the cells at 37 °C, 5% CO2.
Warm DMEM/12 and dispase to 37 °C for 10 min.
Remove the old medium from the cells and rinse each well of hESCs with 2 ml of DMEM/F12 for 2 min.
Remove the DMEM/F12 medium.
-
Add 1 ml of freshly prepared dispase (1 U ml−1) to each well of a six-well plate; incubate the cultures at 37 °C for 3–5 min.
 Inspect the cells every 3 min. Do not incubate the cells in dispase for too long.
Inspect the cells every 3 min. Do not incubate the cells in dispase for too long. Aspirate the dispase from the cells when the edge of the hESC colonies begin to curl.
-
Gently rinse the cells with 2 ml of DMEM/F12 medium.
 The hESC colonies are now loosely attached and are very easy to be washed away. Remove the medium carefully without disturbing the colonies.
The hESC colonies are now loosely attached and are very easy to be washed away. Remove the medium carefully without disturbing the colonies. Add 2 ml of fresh hESC medium to each well.
Lift the colonies off by gently swirling the plate.
-
Gently blow off the colonies remaining attached to the plate using a 5-ml serological pipette or a 1,000-µl pipette tip. Pipette the hESC colonies to the size of 50–100 µm.
 Do not pipette the cells more than five times.
Do not pipette the cells more than five times. Collect the colonies into a 15-ml conical tube and centrifuge for 1 min, at 50g at room temperature. Alternatively, let the hESC colonies sink by leaving the tube to stand for 3 min.
Aspirate the medium gently without disturbing the pellet or colonies.
Re-suspend the hESC colonies with 5 ml of fresh hESC medium, and wash the cells once by repeating Steps 11 and 12.
Re-suspend the hESC colonies with 5 ml of hESC medium without FGF2.
Transfer the cells to a T75 culture flask. Cells from one six-well plate should be transferred to one T75 flask with 40 ml of hESC medium.
Record the date when hESC colonies are lifted off from feeder cells as day 0 of hESC differentiation and culture the cells for 24 h at 37 °C, 5% CO2.
The following day (day 1), allow the flask to stand and let the aggregates sink for 5 min. The hESC colonies generally round up as individual spheres with some individual cell debris floating in the culture. Debris attaching to the cell clusters can be stripped off by pipetting the culture using a 10-ml serological pipette.
Remove the old medium and re-suspend the hESC aggregates with 40 ml of fresh hESC medium.
Transfer the culture to a new T75 flask and culture the cells in suspension at 37 °C, 5% CO2.
-
Observe the cells daily. Feed the cells every day by replacing the old medium with fresh hESC medium using the same procedure described in Steps 17–19.

-
On day 4, switch the culture medium to the neural differentiation medium and feed the cells repeating Steps 17–19 every other day.

On day 7, collect the hESC aggregates into a 50-ml conical tube, centrifuge for 2 min at 50g at room temperature. An additional wash with DMEM/F12 medium is optional to remove the dead cells and facilitate the attachment of the clusters to the culture surface.
Aspirate the medium and re-suspend the hESC aggregates with 5 ml of neural differentiation medium.
Transfer the cells to a 60-mm Petri dish.
-
Carefully pick the hESC aggregates from the Petri dish using a 200-µl pipette. Seed 20–25 clusters of cells evenly to each well of a laminin-coated six-well plate in 300 µl of neural differentiation medium. Do not let the medium drain to the edge of the well. Incubate the culture at 37 °C, 5% CO2 overnight.
 Seed the colonies evenly.
Seed the colonies evenly. -
On day 8, observe the attachment of cell aggregates under a microscope and add 2 ml of neural differentiation medium. Incubate the culture at 37 °C, 5% CO2 for 48 h.

Specification of Olig2-expressing motoneuron progenitors
 18 d (days 10–28)
18 d (days 10–28) On day 10, observe the cultures under the microscope. Evaluate the differentiation efficiency by counting the clusters with obvious early rosettes (Fig. 2e).
Replace the old medium in each well with 2 ml of fresh neural differentiation medium containing 0.1 µM of RA (1:10,000 dilution of the stock solution). Incubate the culture at 37 °C, 5% CO2 for 48 h.
On days 12 and 14, feed the culture with the same medium and procedures as those described in Step 28.
-
On day 15, carefully observe the cells for neural tube-like rosettes (Fig. 2e); mark out those colonies that do not possess typical rosettes using a microscope object marker.

Gently swirl the plate and then remove the old medium.
Add 2 ml of fresh neural differentiation medium to each well of the six-well plate.
-
Gently blow the clusters using a 1-ml pipette to detach the neural tube-like rosettes in the colonies.
 Keep the pipette tip within the medium to avoid bubbles when pipetting. Flat cells at the peripheral part of the colony should remain attached. Avoid lifting those colonies marked in Step 30.
Keep the pipette tip within the medium to avoid bubbles when pipetting. Flat cells at the peripheral part of the colony should remain attached. Avoid lifting those colonies marked in Step 30. Transfer the cell clusters into a 15-ml conical tube using a 1-ml pipette.
-
Put a 10-ml pipette in the conical tube; let the pipette tip gently touch the bottom. Pipette the cell clusters by taking in all the clusters and then blowing them out. Pipette two or three times.
 Do not break the clumps to single cells or clusters smaller than 100 µm.
Do not break the clumps to single cells or clusters smaller than 100 µm. Centrifuge the cell clusters for 2 min at 50g, room temperature.
Aspirate the old medium from the tube; add 5 ml of fresh neural differentiation medium containing B27, SHH (100 ng ml−1) and RA (0.1 µM). As an option, purmorphamine at 1 µM can replace SHH from this step onward.
Transfer the cell clusters from 1–2 plates into a T75 flask (alternatively, cells from three wells may be added to one T25 flask). Culture the cells at 37 °C, 5% CO2 for 48 h.
-
On days 17 and 19, allow the flask to stand for 2–3 min and let the cell clusters settle to one corner. Aspirate 30 ml of the supernatant without disturbing the clusters. Add 30 ml of fresh neural differentiation medium described in Step 37.

-
On day 20, break the spheres bigger than 300 µm using either option A a fire polished Pasteur pipette or Option B, by incubating large clusters (>300 µm) with 1 ml of Accutase at 37 °C for 3 min followed by gentle pipetting.
-
Passaging NE spheres using polished Pasteur pipette
 10 min
10 minFeed the cells as described in Step 39 the day before splitting.
Fire polish the tip of a Pasteur pipette and narrow down the diameter of the inner lumen to around 200 µm (ref. 26).
Briefly heat the pipette 2 cm from the tip on the flame. Gently bend the pipette to a 145–150° angle.
Cool down the pipette to room temperature. Keep the pipette sterile.
Lean the flask at 45° to gather the clusters to a corner.
Before placing the pipette into the culture, briefly rinse the pipette with the medium three times to prevent the cell clusters from sticking onto the pipette.
-
Take all the clusters into the glass pipette using the pipette aid. Blow out the spheres with force into the medium in the flask.

Culture the cells at 37 °C, 5% CO2 for 48 h.
Replace 25 ml of old medium with the same volume of fresh medium using the medium and procedures described in Step 39.
-
Splitting big neurospheres using Accutase
 30 min
30 minCollect the big clusters into a 15- or 50-ml conical tube.
Centrifuge for 2 min at 50g, room temperature to pellet the cells.
Remove the medium from the tube.
Add 1 ml of Accutase to each tube. Resuspend the pellet by gentle shaking or tapping.
Incubate the clusters in Accutase for 3–5 min at 37 °C. Examine the solution for cloudiness and gently shake the tubes every 2 min.
When the clusters look loose and/or the solution appears cloudy add 9 ml of the medium described in Step 37. Centrifuge for 2 min at 50g, at room temperature.
Remove the medium containing Accutase without disturbing the pellet.
Add 800 µl of the medium described in Step 37 to the tube. Pipette the clusters up and down gently <5 times with a 1-ml tip.
Let the tube stand for 2 min. Transfer the medium containing the single cells and small clusters to a flask pre-filled with 30 ml of fresh medium described in Step 37. Leave the big clusters in the tube.
Repeat Step 40B (viii) and (ix) to further break the rest of the large clusters.
The next day, using the medium and procedures described in Step 39, remove 10 ml of old medium and add back 20 ml of fresh medium.
-
-
On day 23, plate some clusters onto a coverslip (Box 1) to immunostain for OLIG22. On days 24 and 26, feed the cell clusters in suspension using the same medium and procedures as described in Step 39.
Generation of spinal motor neurons by differentiation on the substrate
 7 d (days 28–35)
7 d (days 28–35) On day 28, using the procedures described in Box 1, plate 3–5 spheres onto a glass coverslip coated with poly-l-ornithine and laminin. Culture the cells in 50 µl of neural differentiation medium supplemented with BDNF, GDNF, IGF1 (each at 10 ng ml−1), cAMP (1 µM) and ascorbic acid (AA, 200ng ml−1). RA and SHH concentrations are now reduced to 50 nM and 50 ng ml−1, respectively. Culture the rest of the motoneurons using the procedures described in Step 39 until day 35 for other assays such as fluorescence-activated flow cytometry or reverse transcription-polymerase chain reaction (RT-PCR) (ref. 21).
Incubate at 37 °C, 5% CO2 overnight.
-
On days 29, 31 and 33, feed the attached spheres with 0.5 ml of neural differentiation medium described in Step 42.

-
On day 35, immunostain some of the attached cells for HB9 (ref. 2).

Maturation of spinal motor neurons on the substrate
 14 d (days 42–56)
14 d (days 42–56) On days 36, 38 and 40, feed the remaining clusters that are attached on coverslips with the neural differentiation medium described in Step 42.
-
On day 42, evaluate the differentiation by immunostaining the cells for ChAT.

Continue feeding the cultures every 2–3 d using the procedures described in Step 44. On day 56, immunostain for sysnapsin2.
BOX 1 PLATE CELLS ONTO THE LAMININ SUBSTRATE ON POLY-l-ORNITHINE-COATED GLASS COVERSLIPS  100 MIN
100 MIN
Take out the 24-well plate containing the coverslips pre-coated with poly-l-ornithine from the freezer. Leave the plates at room temperature for 20 min.
Dilute the laminin with neural differentiation medium to a final concentration of 20 µg ml−1.
Add 50 µl of the medium containing laminin and spread evenly on top of the coverslip pre-coated with poly-l-ornithine. Leave the plate at 37 °C in an incubator for 1 h.
Remove the medium.
Transfer the motoneuron progenitor clusters to a Petri dish (from Steps 41 and 42, pick 3–5 small clusters) and seed them in 50 µl of the medium described in Step 42 onto the pre-coated coverslips.
Incubate at 37 °C for 2 h. Once the cells have attached, add 500 µl of the medium to each well.
For long-term culture (8–10 weeks), feed the cells every other day with 0.5 ml of the medium described in Step 42. For immunostaining, fix the cells on ice with 0.5 ml of 4% PFA (wt/vol) in each well for 10 min.
Steps 1–26, Induction of primitive NE cells: 10 d (days 0–10)
Steps 27–41, Specification of Olig2-expressing motoneuron progenitors: 18 d (days 10–28)
Steps 42–45, Generation of spinal motor neurons by differentiating on the substrate: 7 d (days 28–35)
Steps 46–48, Maturation of spinal motor neurons on the substrate: 14 d (days 42–56)
Box 1, Plating cells onto laminin substrate on poly-l-ornithine coated glass coverslip: 100 min
Troubleshooting advice can be found in Table 2.
TABLE 2.
Troubleshooting table.
| Step | Problem | Possible reasons | Solution |
|---|---|---|---|
| 20, 21 | Attachment of hESC aggregates (embryoid bodies) in suspension culture | Feeder fibroblasts lifted together with hESCs | Transfer the hESCs aggregates to a new flask once the fibroblasts have adhered to the plastic |
| 26 | Difficulties in attaching hESC aggregates to the plastic surface in the serum-free medium at day 7 | Too many dead cells or poor neural differentiation | Wash the aggregates with neural induction medium and plate them again onto a new plate coated with laminin (20 µg ml−1). Alternatively, addition of 10% (vol/vol) FBS into the culture overnight will promote the attachment of the aggregates |
| 30 | No typical rosettes | Early RA treatment, or hESCs are partially differentiated or the cells are damaged in the Steps 1–16 in the development process of EBs | Add RA from day 10. Avoid over pipetting in manipulating hESCs or hESC-derived progenitors |
| 39 | Non-neural cell contamination | Partially differentiated hESCs that will produce ‘bad colonies’ | Identify the ‘bad colonies’ and manually remove them in the rosettes formation in Step 30 |
| 40A (vii) | Hard to break the big spheres | The Pasteur pipette is not appropriately narrowed and banded | Make several sets of glass pipettes with different tip lumen sizes |
| 44 | Poor survival of motor neuron progenitors | Cell damage in enzymatic disaggregation of neuroepithelial spheres | Plating cells at a high density (30,000 cells per 11-mm coverslip), or seeding small clusters (100–200 µm) will help. Partially dissociate progenitor clusters with Accutase (for 3–5 min) before plating will facilitate the formation of monolayer cells |
| 45 | Nonspecific HB9 staining | HB9 antibody is not specific | Use the suggested monoclonal antibody |
| 47 | The available ChAT antibody presents strong background in vitro | Non-ideal fixation of the enzyme | Use of picric acid buffer for fixation and dilution of the antibody to 1:500 or more will reduce the background. Try to use the goat IgG ChAT antibody from Chemicon (AB144P) |
ANTICIPATED RESULTS
Before starting the differentiation protocol, well-maintained hESCs should be uniformly positive for pluripotent markers such as OCT4 (Fig. 2b). In the neural induction period (first 2 weeks), at least 90% of the total differentiated cells are NE cells that express PAX6 and later PAX6 and SOX1 (Fig. 2c–f). This is based on our study on multiple hESC lines over the past decade, including H1 and H9. If the non-neural colonies are scraped before collecting and assessing the neural population (Steps 30–38), the PAX6+ cell population should be 95–99%. NE differentiation efficiency below 90% is almost always caused by the poor quality of the starting hESCs.
The primitive NE cells cultured in the presence of RA are caudalized to HOXB4-expressing spinal progenitors, whereas those without RA retain an anterior identity by expressing OTX2 (Fig. 3). On day 15, the cells are lifted off and grown in suspension as spheres (Fig. 4a). About 50% of the total cells will be positive for OLIG2 at the end of the 4th week when SHH is used in the entire protocol. When purmorphamine is used in this protocol, the proportion of OLIG2-expressing cells is 60–80% and the population peaks in the middle of the 4th week.
The OLIG2-expressing motoneuron progenitors grown on substrate differentiate to HB9-expressing post-mitotic motor neurons in the 5th week. By the end of the 5th week, the cells protrude long processes (Fig. 4b), and the HB9-expressing cells account for 30–40% of the total population, whereas OLIG2 cells decrease to <30% (Fig. 4c). Most of the HB9 cells are also Tuj1+ (Fig. 4d), indicating that these cells exit the cell cycle and commit to the neuronal lineage. The HB9-expressing cells generally remain in clusters, rarely migrating away from the cluster (Fig. 4d). Dissociation of the OLIG2-expressing progenitor spheres often results in a reduced population of motor neurons.
After the 5th week, the motor neurons can be further cultured for several weeks or months depending on the applications. This will lead to expression of mature motoneuron markers such as ChAT (Fig. 4e). When co-cultured with myoblasts (C2C12, ATCC CRL-1772) for 2 weeks, the motoneurons begin to express synapsin, a vesicle protein required for transmitter release and appropriate signal transduction across synapses (Fig. 4f). The myotubes in contact with the neurites often express clusters of acetylcholine receptors, which can be detected through their binding to fluorephore-conjugated α-bungarotoxin (Fig. 4f).
ACKNOWLEDGMENTS
This study was supported by the National Institutes of Neurological Diseases and Stroke (NS045926 and NS057778), the ALS Association and partly by a core grant to the Waisman Center from the National Institute of Child Health and Human Development (P30 HD03352).
Footnotes
AUTHOR CONTRIBUTIONS B.-Y.H. designed and performed experiments, analyzed data and wrote the paper; S.-C.Z. supervised the project, designed experiments, analyzed data, wrote and approved the final paper.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 2.Li XJ, et al. Specification of motoneurons from human embryonic stem cells. Nat. Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 3.Singh Roy N, et al. Enhancer-specified GFP-based FACS purification of human spinal motor neurons from embryonic stem cells. Exp. Neurol. 2005;196:224–234. doi: 10.1016/j.expneurol.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Perrier AL, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan Y, et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy NS, et al. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat. Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe K, et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat. Neurosci. 2005;8:288–296. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
- 8.Johnson MA, Weick JP, Pearce RA, Zhang SC. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J. Neurosci. 2007;27:3069–3077. doi: 10.1523/JNEUROSCI.4562-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu BY, Du ZW, Li XJ, Ayala M, Zhang SC. Human oligodendrocytes from embryonic stem cells: conserved SHH signaling networks and divergent FGF effects. Development. 2009;136:1443–1452. doi: 10.1242/dev.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 11.Zhang SC. Neural subtype specification from embryonic stem cells. Brain Pathol. 2006;16:132–142. doi: 10.1111/j.1750-3639.2006.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu QR, et al. Sonic hedgehog-regulated oligodendrocyte lineage genes encoding bHLH proteins in themammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage-specific basic helix–loop–helix transcription factors. Neuron. 2000;25:331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]
- 14.Ericson J, et al. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- 15.Mizuguchi R, et al. Combinatorial roles of olig2 and neurogenin2 in the coordinated induction of pan-neuronal and subtype-specific properties of motoneurons. Neuron. 2001;31:757–771. doi: 10.1016/s0896-6273(01)00413-5. [DOI] [PubMed] [Google Scholar]
- 16.Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 17.Lee SK, Lee B, Ruiz EC, Pfaff SL. Olig2 and Ngn2 function in opposition to modulate gene expression in motor neuron progenitor cells. Genes Dev. 2005;19:282–294. doi: 10.1101/gad.1257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakovcevski I, Zecevic N. Olig transcription factors are expressed in oligod-endrocyte and neuronal cells in human fetal CNS. J. Neurosci. 2005;25:10064–10073. doi: 10.1523/JNEUROSCI.2324-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marti E, et al. Ontogeny of peptide- and amine-containing neurones in motor, sensory, and autonomic regions of rat and human spinal cord, dorsal root ganglia, and rat skin. J. Comp. Neurol. 1987;266:332–359. doi: 10.1002/cne.902660304. [DOI] [PubMed] [Google Scholar]
- 20.Pankratz MT, et al. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007;25:1511–1520. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li XJ, et al. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. 2008;26:886–893. doi: 10.1634/stemcells.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saravanan Karumbayaram BGN. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells. 2009;27:806–811. doi: 10.1002/stem.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaVaute TM, et al. Regulation of neural specification from human embryonic stem cells by BMP and FGF. Stem Cells. 2009 doi: 10.1002/stem.99. doi: 10.1002/stem.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okada Y, Shimazaki T, Sobue G, Okano H. Retinoic-acid-concentration-dependent acquisition of neural cell identity during in vitro differentiation of mouse embryonic stem cells. Dev. Biol. 2004;275:124–142. doi: 10.1016/j.ydbio.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 25.Mizuseki K, et al. Generation of neural crest-derived peripheral neurons and floor plate cells from mouse and primate embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2003;100:5828–5833. doi: 10.1073/pnas.1037282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia X, Zhang SC. Differentiation of neuroepithelia from human embryonic stem cells. In: Scolding NJ, Gordon D, editors. Methods in Molecular Biology. Vol. 549. New York: Humana Press; 2009. pp. 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]