1. Structure
Mammary Serine Protease Inhibitor or maspin (GenBank Accession Number: U04313; RefSeq ID: NM_002639; PDB ID: 1xqj, 1xqg, 1wz9, 1xu8) is a 42 kDa, non-classical, non-inhibitory member of the ovalbumin clade of serine protease inhibitors (serpins). In humans, the gene encoding maspin is located on chromosome 18 in a cluster containing genes for other serpins such as squamous cell carcinoma antigen (SCCA) 1 and 2 and plasminogen activator inhibitor type 2 (PAI-2). Maspin was identified from subtractive hybridization studies using cDNAs produced from mRNA of normal and malignant mammary epithelial cells (Zou et al. 1994). While myoepithelial cells of normal mammary tissue expressed maspin, its expression was down-regulated in metastatic mammary tumor cells. Maspin was classified as a class II tumor suppressor gene in human mammary epithelial cells because although its expression is downregulated in many metastatic carcinoma cells, it is not mutated. Although maspin resembles classical inhibitory serpins in structure, the reactive site (or center) loop (RSL or RCL) possesses a non-standard hinge region, on the N-terminus of the RSL, which prevents it from undergoing a critical structural change from the stressed (S) to relaxed (R) state (Fig. 1). This prevents maspin from being able to trap and inhibit serine proteases.
Figure.
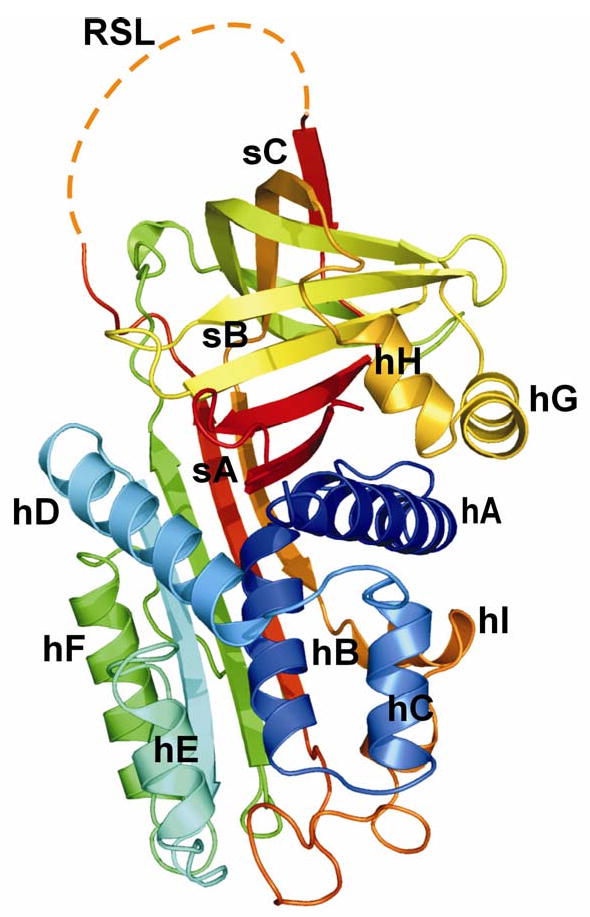
Cartoon representation of human maspin in the closed form (PDB ID: 1xu8, modified from Law et al., 2005). Maspin adopts the native serpin fold consisting of nine α-helices (hA-hI) and three β-sheets (sA-sC). The RSL of maspin lacked electron density due to its high degree of mobility, and is outlined here in dashes.
Maspin consists of nine α-helices (helix A- I) and three β-sheets (sheet A-C) and adopts the native serpin fold with the RSL fully expelled from the body of the protein (Fig. 1). The RSL of maspin is unique in length, structure and position. Although the RSL is exposed and cleaved by some proteases, it functions in the uncleaved form. The RSL makes very little contact with the rest of the molecule and is very flexible as observed by low electron density. An unusual bulge is caused by a buried salt bridge in the region of the D and E α-helices, an area shown in many other serpins to be important for co-factor recognition. Additionally, intact maspin can undergo a major conformational change in the G α-helix and adjacent sequences, switching between an ‘open’ and ‘closed’ conformation thereby altering the electrostatic properties of a presumptive co-factor binding surface of the molecule, which may affect the function of the molecule (Law et al. 2005).
2. Function
Maspin localizes to the cytoplasm and nucleus of cells, and is secreted. In the cytoplasm, it partitions into the ER, Golgi and secretory vesicles, and is present at the cell surface. It is expressed by most epithelial cells including mammary myoepithelial cells, but not others such as pancreatic and ovarian epithelial cells. In the cornea, maspin is expressed not only by the corneal epithelial cells, but also by stromal keratocytes and endothelial cells.
Maspin increases corneal stromal cell adhesion to collagen types I and IV, fibronectin and laminin (Ngamkitidechakul et al. 2003). Replacement of the RSL and C-terminus or RSL alone of maspin with that of ovalbumin resulted in loss of enhanced adhesion of cultured corneal stromal fibroblast cells and of MDA-MB-231 mammary carcinoma cells to ECM molecules. The RSL peptide alone induced increased cell-matrix adhesion of corneal stromal fibroblast cells and mammary carcinoma cells, and inhibited invasion of the carcinoma cells. Substitution of the RSL of ovalbumin with that of maspin resulted in conversion of ovalbumin in to a fully active molecule. Sufficiency of the RSL alone for induction of increased adhesion and inhibition of invasion suggests the serpin mechanism of protease inactivation may not be involved in regulating cell-matrix adhesion and invasion.
Maspin is an effective inhibitor of angiogenesis both in vivo and in vitro. Maspin blocked neovascularization in the rat cornea pocket model, and acted directly on cultured vascular endothelial cells to limit their migration towards basic fibroblast growth factor (FGF-2) and vascular endothelial growth factor (VEGF), mitogenesis and tube formation. RSL mutants of maspin retained their anti-angiogenic activity suggesting that regions of the molecule outside the RSL are involved in inhibiting angiogenesis. It is possible that the anti-angiogenic property of maspin contributes significantly to the tumor suppressor activity of maspin and to the avascular nature of the cornea.
3. Disease Involvement
The importance of maspin in regulation of properties associated with malignant cells was shown in experiments in which maspin constructs were transfected into mammary carcinoma cells and then injected into nude mice (Zou et al. 1994). These cells produced much smaller tumors than vector controls. Additionally, the metastatic and invasive potential of the tumor cells was significantly reduced. Exogenous addition of maspin or transfection of the maspin gene increased adhesion and inhibited migration of mammary cancer cells.
Although maspin is low or absent in human mammary carcinoma cells, the maspin gene is rarely rearranged or deleted. The down-regulation of maspin expression in human mammary carcinoma cells is due to aberrant cytosine methylation and chromatin condensation of the maspin promoter. Complete methylation of this promoter was observed in maspin-negative normal peripheral blood lymphocytes. Chromatin in the region of the maspin gene was in a closed conformation. Treatment with 5-aza-2′-deoxycytidine, a DNA demethylating agent, did re-activate low levels of maspin expression. In some cells a histone deacetylase inhibitor, trichostatin A, also stimulated maspin expression.
Maspin expression is downregulated by wound healing phenotypes in the corneal stroma due to a combination of promoter and histone methylation (Horswill et al. 2008). In the event of a wound in the cornea, keratocytes proximal to the wound undergo apoptosis creating an acellular zone near the wound. In the meantime, keratocytes distal from the wound differentiate to produce the wound healing phenotypes, fibroblasts and myofibroblasts. While the epithelial cells of the cornea appear to constitutively express and secrete maspin, the keratocytes akin to metastatic mammary cancer cells down-regulate the expression of maspin as they differentiate into fibroblasts and myofibroblasts. The loss of maspin expression in fibroblasts and myofibroblasts allows them to be motile and migrate to the area of the wound. These cells can respond to maspin secreted by the epithelium by up-regulating their adhesion to the ECM. Additionally, maspin increases extracellular levels of the tissue-type and urokinase plasminogen activators (tPA and uPA) secreted by the fibroblasts and myofibroblasts, respectively. This leads to increased activation of plasminogen to plasmin, which cleaves fibrin laid down in the area of the wound and generates angiostatin, an anti-angiogenic molecule, by cleavage of plasminogen (unpublished results). Thus, maspin functions in multiple modes by a) increasing adhesion of the corneal stromal cells at the wound, b) stimulating ECM remodeling via the plasminogen system in the wound area, and c) inducing the production of angiostatin and/or by inhibiting vascular endothelial cell migration and tube formation to keep angiogenesis in check.
4. Future Studies
The cornea is the only ocular tissue where maspin has been studied. Much remains to be understood about the molecular mechanism of action of maspin in the cornea and other tissues. While the RSL of the molecule appears to be involved in increasing adhesion of cells to the ECM, an alternate site on the molecule is thought to be the area that inhibits angiogenesis. Studies are ongoing to determine the post-translational modifications of maspin such as phosphorylation and acetylation, which may help elucidate the role of maspin in signaling pathways relevant to cell adhesion and angiogenesis. The ability of maspin and its post-translationally modified forms to act as effective therapeutic agents in the cornea is also being pursued.
Acknowledgments
Supported by Research grants RO1 EY12731, RO1 14168 and P30 EY01931 from the National Eye Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Horswill MA, Narayan M, Warejcka DJ, Cirillo LA, Twining SS. Epigenetic silencing of maspin expression occurs early in the conversion of keratocytes to fibroblasts. Exp Eye Res. 2008;86:586–600. doi: 10.1016/j.exer.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law RH, Irving JA, Buckle AM, Ruzyla K, Buzza M, Bashtannyk-Puhalovich TA, Beddoe TC, Nguyen K, Worrall DM, Bottomley SP, Bird PI, Rossjohn J, Whisstock JC. The high resolution crystal structure of the human tumor suppressor maspin reveals a novel conformational switch in the G-helix. J Biol Chem. 2005;280:22356–22364. doi: 10.1074/jbc.M412043200. [DOI] [PubMed] [Google Scholar]
- Ngamkitidechakul C, Warejcka DJ, Burke JM, O'Brien WJ, Twining SS. Sufficiency of the reactive site loop of maspin for induction of cell-matrix adhesion and inhibition of cell invasion. Conversion of ovalbumin to a maspin-like molecule. J Biol Chem. 2003;278:31796–31806. doi: 10.1074/jbc.M302408200. [DOI] [PubMed] [Google Scholar]
- Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, Rafidi K, Seftor E, Sager R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]


