Abstract
Seasonally breeding animals use a combination of photic (i.e., day length) and non-photic (e.g., food availability, temperature) cues to regulate their reproduction. How these environmental cues are integrated is not understood. To assess the potential role of two candidate neuropeptides, kisspeptin and RFamide-related peptide-3 (RFRP), we monitored regional changes in their gene expression in a seasonally breeding mammal exposed to moderate changes in photoperiod and food availability. Adult male Siberian hamsters (Phodopus sungorus) were housed in a long (16 h light/day; 16L) or intermediate (13.5L) photoperiod and fed ad libitum or a progressive food restriction schedule (FR; reduced to 80% of ad libitum) for 11 weeks. Gonadal regression occurred only in FR hamsters housed in 13.5L. Immunohistochemistry was used to identify diencephalic populations of kisspeptin- and RFRP-immunoreactive cells, and quantitative PCR was used to measure gene expression in adjacent coronal brain sections. Photoperiod but not food availability altered RFRP mRNA expression in the dorsomedial sections, whereas food availability but not photoperiod altered Kiss1 expression in the arcuate sections; intermediate photoperiods elevated RFRP expression, and food restriction suppressed Kiss1 expression. Regional- and neuropeptide-specific activity of RFamides may provide a mechanism for integration of multi-modal environmental information in the seasonal control of reproduction.
Keywords: RFamide, seasonality, energetics, reproduction, neuropeptides
INTRODUCTION
In environments where robust seasonal cycles of temperature and food availability prevail, seasonal cues regulate the phase of the geophysical cycle during which critical events in mammalian reproduction occur (puberty, ovulation, breeding, parturition, weaning) (Paul et al., 2008, Prendergast et al., 2009). In Siberian hamsters (Phodopus sungorus), as in other small photoperiodic rodents, summer photoperiods ≥14 h light/day (14L) stimulate, and winter photoperiods <12L inhibit, testicular development, gametogenesis, ovarian function, and reproductive behavior (Hoffmann, 1982, Park et al., 2004, Schlatt et al., 1995). The light-dark cycle entrains a circadian rhythm of nocturnal pineal melatonin secretion (Illnerová, 1991), the duration of which acts on pituitary, thalamic, and hypothalamic melatonin-sensitive target tissues to convey photoperiodic information to the reproductive axis (Badura & Goldman, 1992, Bartness et al., 1993, Carter & Goldman, 1983a, b, Glass & Lynch, 1982, Morgan & Hazlerigg, 2008).
Recent work has suggested that photoperiodic regulation of two hypothalamic RFamides (Arg-Phe-NH2), kisspeptin and RFamide-related peptide-3 (RFRP), may figure prominently in the transduction of melatonin signals to the HPG axis. Kisspeptin stimulates (Gottsch et al., 2004, Greives et al., 2007, Irwig et al., 2004, Messager et al., 2005), and RFRP inhibits (Kriegsfeld et al., 2006) LH secretion in mammals. In photoperiodic rodents, short photoperiods that trigger gonadal regression yield decreases in Kiss1 and RFRP mRNA and their respective protein expression in the anteroventral periventricular nucleus (AVPV; for kisspeptin) and mediobasal hypothalamus including the dorsomedial hypothalamus (DMH; for RFRP) (Greives et al., 2007, Mason et al., 2007, Revel et al., 2008, Revel et al., 2006). In Siberian hamsters, kisspeptin peptide and mRNA expression increase in the arcuate nucleus (ARC) after transfer to short photoperiods (Greives et al., 2007, Mason et al., 2007, Simonneaux et al., 2009); in Syrian hamsters, however, transfer to short photoperiods decreases kisspeptin expression in this nucleus (Revel et al., 2006). Kisspeptin and RFRP may act in concert to stimulate or inhibit the HPG axis during photoperiod-induced reproductive transitions (Kauffman et al., 2007, Smith et al., 2008).
Non-photic environmental cues, such as food availability, ambient temperature, and social interactions, also contribute to seasonal timekeeping, but their underlying neurobiology is not understood (reviewed in Ball, 1993, Paul et al., 2008). Recently, a model was developed whereby non-photic seasonal regulation can be studied under static photoperiods. Siberian hamsters housed in an intermediate day length (13.5 h light/day; 13.5L) from birth undergo gonadal growth. After this gonadal development occurs, mild food restriction or increased population density can trigger substantial reproductive inhibition in hamsters housed in 13.5L, but these non-photic cues are without effect on hamsters housed in a long photoperiod (16L; Paul et al., 2009). This model permits investigation into candidate neural substrates responsible for the integration of photoperiodic and non-photic cues for the reproductive axis (e.g., kisspeptin and RFRP). For example, if kisspeptin is one such site of environmental cue integration, then intermediate photoperiods and mild food restriction, neither of which elicits gonadal responses alone, should each induce partial changes in Kiss1 mRNA (e.g., moderate decreases in Kiss1 mRNA in the AVPV); when 13.5L and mild food restriction are presented in combination— and reproductive responses occur— larger Kiss1 mRNA responses would be expected. Alternatively, photoperiod and food restriction may be integrated via distinct kisspeptin neuronal populations: transfer to intermediate photoperiods may decrease Kiss1 mRNA in the AVPV and mild food restriction may decrease Kiss1 mRNA in the ARC. Similar predictions could be made for RFRP or for coordinated changes in both kisspeptin and RFRP signals among the AVPV, ARC, and DMH. On the other hand, if the integration of photoperiod and food restriction occurs upstream from the RFamides, then mRNA patterns in each nucleus should simply mirror gonadal responses: altered RFamide mRNA expression should only occur in hamsters challenged with both intermediate photoperiods and food restriction.
Here we describe an experiment that examined changes in Kiss1 and RFRP gene expression in response to modest changes in photoperiod and food availability, which combined, were sufficient to induce gonadal regression. The results suggest that these two RFamides respond to photic and non-photic cues in a peptide- and regionally-specific manner and may work in concert to integrate seasonal cues via distinct neuronal pathways.
MATERIALS and METHODS
Animals and housing conditions
Adult (>4 months) male Siberian hamsters (Phodopus sungorus) were obtained from 13.5:10.5 h light:dark cycle (13.5L) or 16L (lights off at 1230 h CST for both photoperiods) breeding colonies maintained at the University of Chicago and housed singly from weaning in polypropylene cages (28 × 17 × 12 cm) with wood shavings (Harlan Sani-Chips, Harlan Inc., Indianapolis, IN, USA) under their same natal photoperiods. Ambient temperature of the experimental rooms was 20 ± 0.5°C and relative humidity was maintained at 53 ± 2%. Food (Teklad Rodent Diet 8604, Harlan Inc.) and filtered tap water were provided ad libitum, except where otherwise indicated. The data reported here are derived from a subset of 23 animals in a recent study that described the efficacy of intermediate day lengths in unmasking morphological reproductive and hormonal responses to food restriction (Paul et al., 2009). All procedures conformed to the USDA Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Chicago.
Reproductive and somatic measures
The length and width of the left testis were measured (±0.1 mm) through the abdominal skin using calipers while hamsters were under light isoflurane anesthesia. The size of the testis was estimated as the product of the length × width2 (estimated testis volume; ETV), which is correlated with testis weight, circulating testosterone, and spermatogenesis (Gorman, 1995, Schlatt et al., 1995). Body masses (±0.1 g) were also recorded at the time of reproductive measurements.
Food restriction
Food restriction (FR) was accomplished by progressive reductions from mass-specific ad libitum intake (0.13 g food / g body mass; determined in a pilot study; data not shown) administered to each hamster in a single daily ration shortly after the onset of darkness (16L-FR: n=6; 13.5L-FR: n=6). From weeks 0 through 6, FR hamsters were initially provided with 90% of ad libitum daily intake (0.117 g/g), and thereafter (weeks 6 though 11) received 80% of ad libitum intake (0.104 g/g). This pattern of food restriction was designed to more closely simulate the progressive late-summer decrease in food availability that occurs in this species’ natural environment (Weiner, 1987). Control hamsters had free access to food throughout the study (AdLib; 16L-AdLib: n=5; 13.5L-AdLib: n=6).
Selection of regionally specific tissue samples for mRNA quantification
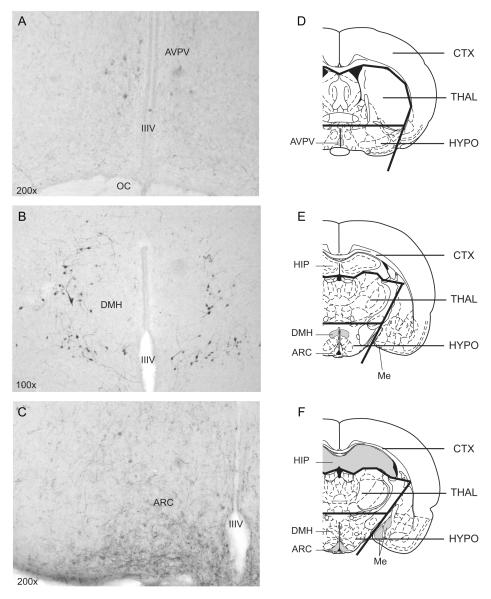
Given the opposite photoperiodic responses of kisspeptin peptide and mRNA expression in the AVPV and ARC of Siberian hamsters (Greives et al., 2007, Mason et al., 2007, Simonneaux et al., 2009), measures of whole hypothalamic Kiss1 expression is problematic. To achieve both quantitative and regionally specific RFamide data, a method combining immunohistochemistry (IHC) and quantitative PCR (qPCR) was used. One of two alternating sets of 30 μm coronal sections, rostral to caudal, was processed using IHC for RFamide immunoreactivity (RFamide-ir) in the anteroventral periventricular nucleus (AVPV), the arcuate nucleus (ARC), and the dorsomedial nucleus of the hypothalamus (DMH). The remaining set of sections was reserved for qPCR. Total RNA was extracted from the fixed sections adjacent to those that exhibited immunoreactivity in the AVPV, ARC, and DMH (3 sections per brain region). Sections were pooled within each brain region to generate 3 tissue samples for each hamster, 1 containing the AVPV, 1 containing the ARC, and 1 containing the DMH. Immunopositive perikarya were restricted to the AVPV, ARC, and DMH nuclei (Fig. 1A-C; cf. Gottsch et al., 2004). We further quantified Kiss1 and RFRP mRNA levels within the hypothalamus, thalamus, and cortex of a separate set of hamsters to validate claims that the changes in gene expression were in fact of hypothalamic origin.
Figure 1.
Representative photomicrographs of T-4771-ir cells in the (A) anteroventral periventriculr nucleus (AVPV), (B) dorsomedial hypothalamus (DMH), and (C) arcuate nucleus (ARC) of male Siberian hamsters. (D-F) Illustrations of cortical (CTX), thalamic (THAL), and hypothalamic (HYPO) microdissections of coronal sections for qPCR analysis at the level of the (D) AVPV, (E) DMH, and (F) ARC. IIIV = third ventricle; OC = optic chiasm; HIP = hippocampus; Me = medial amygdala. Shaded areas represent putative areas containing Kiss1 (in D and F) or RFRP mRNA (in E; see Discussion).
Immunohistochemical localization of RFamide-ir cells
FR and AdLib treatments continued through the day of sacrifice. At the midpoint of the light phase, hamsters were deeply anesthetized with an overdose of sodium pentobarbital (15 mg/animal, i.p.) and were perfused transcardially with 40 ml of 0.9% saline, followed by 40 ml of 4% paraformaldehyde in phosphate buffered saline (pH 7.4). Brains were postfixed for 2 days in fixative before cryoprotection with a mixture of buffered fixative and 30% sucrose for 2–3 days, and freezing at −80°C until sectioned.
Brains were sliced coronally at 30 μm on a freezing sliding microtome and free-floating sections were stored in cryoprotectant (Watson et al., 1986) at −20°C until processed for RFamide-ir. For each animal, alternating sections, rostral to caudal, were stained for using standard ABC immunocytochemistry, following the methods of Kramer et al. (2006). RFamide-ir cells were labeled using a rabbit anti-human kisspeptin antiserum diluted at 1:5000 (T-4771; Peninsula Laboratories Inc, Bachem, San Carlos, CA) raised against the following amino acids Tyr-Asn-Trp-Asn-Ser-Phe-Gly-Leu-Arg-Phe-NH2, corresponding to amino acids 4-13. Sections were examined under bright field illumination on a Nikon Eclipse 80i microscope.
In the Siberian hamster hypothalamus, the T-4771 antibody cross-reacts with kisspeptin and RFRP, detecting cells in the AVPV, the ARC, and the DMH. Pre-adsorption procedures have indicated that the immunoreactive cells in the AVPV and ARC express kisspeptin, whereas those in the DMH express RFRP (Greives et al., 2007). Nonetheless, concerns remain regarding the specificity of this antibody (Goodman et al., 2007, Mikkelsen & Simonneaux, 2009), and recent studies have indicated that a diffuse population of kisspeptin cells may also exist in the DMH, at least in the laboratory mouse (Clarkson et al., 2009). Given these concerns and the fact that IHC is only semi-quantitative by nature, we restricted our use of this staining for localization of RFamide expression within the target nuclei, allowing selection of sections containing the AVPV, ARC, or DMH for qPCR.
Quantification of Kiss1 and RFRP expression via qPCR
Extractions were performed according the manufacturer’s protocol (RNeasy FFPE kit, Qiagen, Valencia, CA, USA) with one exception: paraffin removal steps were skipped. Extracted RNA was suspended in 30μl RNase-free water and RNA concentration and quality were determined by spectrophotometer. All RNA samples were stored at −70° C until further analysis. cDNA was created via reverse transcription of 2μg of RNA from each sample with MMLV Reverse Transcriptase enzyme (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol.
To design primers and a probe for quantitative PCR with high specificity for this species, a portion of each gene of interest was sequenced. To sequence portions of these genes, semi-quantitative PCR was conducted on 1μl of pooled Siberian hamster hypothalamic cDNA with Taq DNA Polymerase enzyme (Invitrogen) according to the manufacturer’s protocol in a thermocycler for 40 cycles (Bio-Rad). Degenerate primers were designed based on conserved regions among multiple species with known gene sequences (GenBank) using PrimerExpress software (Applied Biosystems, Foster City, CA, USA). PCR gene product amplification was visualized on 2% TAE-agarose gels containing ethidium bromide using a CCD camera. To verify amplification of the correct gene, PCR products were purified (Centricon-100, Millipore, Billerica, MA, USA) and directly sequenced at the University of Chicago Cancer Research Center DNA Sequencing Facility. The resulting amplicon sequences for Siberian hamster Kiss1 and RFRP were >90% homologous to published sequences for Mus musculus Kiss1 and RFRP. Sequencing information was entered in the GENBANK database: RFRP (Accession # EU365871) and Kiss1 (Accession # EU365872).
After confirmation of gene products, primers and probes for quantitative PCR were designed using PrimerExpress. Primers and probes were synthesized as follows, with probes labeled with 6-FAM and MGB (non-fluorescent quencher) at the 5′ and 3′ ends, respectively: RFRP forward 5′-GCCCCTGCCAACAAAGTG-3′, RFRP reverse 5′-CAGGGTCCTCCCAAATCTCA-3′, RFRP probe 5′-CCCACTCAGCAGCCA-3′; Kiss1 forward 5′-AACTCATCAATGCCTGGGAAA-3′, Kiss1 reverse 5′-GCTCGCAGTCCTCCAGGTT-3′, Kiss1 probe 5′-CGGTGCGCAGAGAG-3′. A TaqMan 18S Ribosomal RNA primer and probe set (labeled with VIC; Applied Biosystems) was used as the control gene for relative quantification. Amplification was performed on an ABI 7900HT Sequencing Detection System by using Taqman® Universal PCR Master Mix. The universal two-step RT-PCR cycling conditions used were: 50° C for 2 min, 95° C for 10 min, followed by 40 cycles of 95° C for 15 sec and 60° C for 1 min. Relative gene expression of individual samples run in duplicate was calculated by comparison to relative standard curve consisting of serial dilutions of pooled P. sungorus hypothalamic cDNA (1:1, 1:10, 1:100, 1:1000, 1:10,000) followed by normalization to 18S rRNA gene expression. RNA quality for each sample was assessed via 260/280 ratio using a spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) before reverse transcription. All samples had acceptable ratios between 1.8 and 2.0 (mean: 1.98; range: 1.81-2.0).
Cerebral localization of mRNA signals
Hamsters (n=3) fed ad libitum were perfused in a manner identical to that described above for experimental animals. Brains were removed, post-fixed, frozen, and sectioned. Free-floating sections from each of three rostro-caudal brain regions (AVPV: bregma −0.3 mm; DMH: bregma −2.5 mm; ARC: bregma −3.3 mm) (Paxinos & Watson, 1998) were then dissected into cortex (including limbic structures), hypothalamus, and thalamus under a 4X dissecting microscope at 4°C (Fig. 1D-F). Between 10-12 microdissections from each brain region were pooled and expression levels of Kiss1 and RFRP were determined using qPCR as described above.
Statistics
Data are expressed as means ± SEM for each group. Repeated measures and factorial ANOVAs were used to measure variation within and between experimental groups, and differences between means were assessed by least significant difference tests and t-tests, where warranted by a significant F statistic. Significance level was set at P≤0.05 for all tests. Analyses were performed using Statview 5.0.1 for the PC (SAS Institute Inc., Cary, NC, USA).
RESULTS
Food restriction induces gonadal regression only in intermediate day lengths
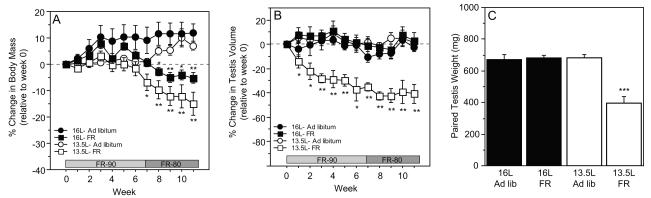
FR treatments inhibited body mass gains in both photoperiods (F=9.9; P=0.005; Fig. 2A); the magnitude of body mass inhibition tended to be greater in FR hamsters housed in 13.5L relative to those in 16L but fell short of significance (P<0.10). FR treatments and photoperiod interacted to affect testis size (F=24.4; P<0.0001); as in the parent population (Paul et al., 2009), significant gonadal regression only occurred in 13.5L hamsters (P<0.0001; Fig. 2B,C).
Figure 2.
Mean (±SEM) change in (A) body mass, (B) estimated testis volume, and (C) mean (+SEM) paired testis weights of hamsters singly housed in 16L or 13.5L and subsequently subjected to progressive food restriction (FR) or ad libitum feeding (AdLib) for 11 weeks, beginning on week 0. These data were derived from a subset of animals from a recent study in our laboratory (Paul et al., 2009). * P<0.05, ** P<0.005, ***P<0.0001 vs. AdLib-fed hamsters within photoperiod.
Kiss1 and RFRP mRNA expression
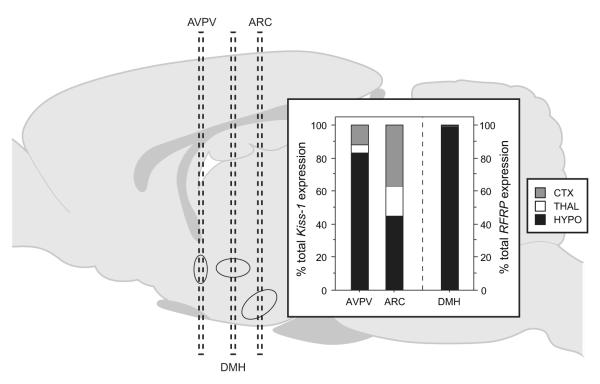
qPCR analysis of microdissections permitted evaluation of cortical, thalamic, and hypothalamic contributions to Kiss1 and RFRP gene expression obtained from whole coronal sections at each of the 3 rostro-caudal levels investigated (Kiss1: AVPV and ARC; RFRP: DMH; Fig. 3). At the level of AVPV, most of the Kiss1 signal (83%) was derived from hypothalamic cells; the remainder originated from cortex and thalamus. At the level of the ARC, the hypothalamus was the largest source of Kiss1 gene expression (45%), but a substantial Kiss1 signal (37%) was detected in cortex. At the level of the DMH, over 99% of the RFRP signal was hypothalamic in origin (Fig. 3).
Figure 3.
Relative contributions of the hypothalamus (HYPO), thalamus (THAL), and cortex (CTX) to total brain Kiss1 (left) and RFRP (right) mRNA expression in coronal sections at each of the three rostro-caudal levels analyzed (AVPV, ARC, and DMH).
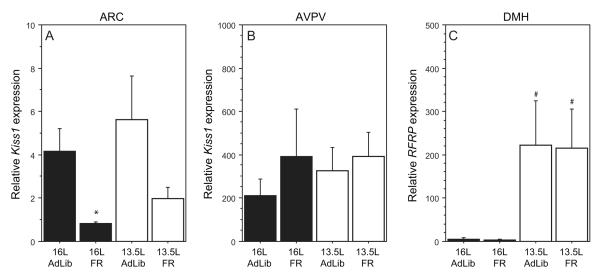
In sections containing the ARC, Kiss1 gene expression was significantly decreased in FR relative to AdLib hamsters (F=7.9; P=0.01; Fig. 4A) but was not affected by photoperiod (F=1.1; P>0.3); the interaction between these factors was not significant (F=0.2; P>0.8). In 16L hamsters, FR significantly inhibited Kiss1 expression at the level of the ARC (P<0.05); decreases in 13.5L FR hamsters fell just short of significance (P<0.06). At the level of the AVPV, neither photoperiod (F=0.2; P>0.6), nor FR (F=0.9; P>0.3) significantly affected Kiss1 gene expression (Fig. 4B); the interaction was also not significant (F=0.2; P>0.6).
Figure 4.
Mean (±SEM) relative expression of Kiss1 mRNA in coronal brain sections at the level of the (A) AVPV and (B) ARC of male Siberian hamsters; (C) mean (±SEM) relative expression of RFRP mRNA in sections at the level of the DMH. Kiss1 and RFRP mRNA levels were normalized to 18S rRNA gene expression. Photoperiod and feeding conditions as in Figure 2. The sample sizes for each group were 16L-AdLib: n=5; 16L-FR: n=6; 13.5L-AdLib: n=6; 13.5L-FR: n=6. * P<0.05 vs. AdLib hamsters within photoperiod; # P<0.05 13.5L vs. 16L within feeding condition.
In sections containing the DMH, RFRP expression was significantly greater in 13.5L relative to 16L hamsters (F=9.1; P<0.01), independent of food manipulations (F<0.1; P>0.9; Fig. 4C); again, there was no significant interaction between photoperiod and feeding (F<0.1; P>0.9). In both AdLib and FR hamsters, RFRP expression was ~40-fold higher in 13.5L relative to 16L hamsters (Fisher’s PLSD; P<0.05, both comparisons).
DISCUSSION
Intermediate day lengths render the reproductive axis of Siberian hamsters more responsive to non-photic, exteroceptive cues such that testicular regression occurs only in hamsters exposed to both food restriction and intermediate day lengths (Paul et al., 2009). The present work extended this finding to investigate neuroendocrine mechanisms of photic and non-photic cue integration.
At the level of the ARC, food restriction induced a ~5-fold decrease in Kiss1 expression but no main effect of photoperiod was detected. Thus, modest reductions in food availability decrease Kiss1 mRNA at the level of the ARC, either by downregulating Kiss1 transcription or by increasing post-transcriptional processing (e.g., translation and degradation). These data are consistent with reports in other non-photoperiodic species (Castellano et al., 2005, Luque et al., 2007, Smith et al., 2006). Given the well-established role of the ARC in regulating energy balance (Hill et al., 2008, Morgan et al., 2006), ARC kisspeptin neurons are ideally situated to monitor the metabolic state of the organism. In sections containing the AVPV, neither photoperiod nor food restriction affected Kiss1 mRNA expression. At the level of the DMH, food restriction did not alter RFRP gene expression; however, exposure to intermediate photoperiods increased RFRP mRNA in the DMH ~40-fold.
The current data provide novel insights into environmental influences on the RFamide system. Non-photic (reduced food availability) and photic (intermediate day lengths) stimuli inadequate to impact reproductive physiology, nonetheless alter Kiss1 and RFRP gene expression, respectively. Remarkably, in neither the ARC nor the DMH were any additive effects of food restriction or photoperiod evident. Gonadal responses were only associated with changes in both ARC Kiss1 and DMH RFRP. Such a pattern of responses is consistent with a role for the RFamide system as an integrator of multimodal seasonal cues (Kriegsfeld, 2006). Cue integration is a complex process and undoubtedly involves other neuropeptides as well. Nonetheless, a simple model in which environmental cues act on distinct GnRH input pathways, including kisspeptin and RFRP, is compatible with the present data.
Microdissections (cortex, thalamus, hypothalamus) of coronal brain sections indicated that at the level of the AVPV and the DMH the overwhelming majority of the Kiss1 (>80%) and RFRP (>99%) mRNA, respectively, originated in the hypothalamus. Given the highly localized patterns of kisspeptin-ir and RFRP-ir in adjacent sections, we are confident that the vast majority of the Kiss1 and RFRP mRNA obtained from whole coronal sections originated in the AVPV and DMH, and that the differences in mRNA expression between treatment groups reflect differences within these nuclei. Some caution is warranted, however, in interpreting data from the coronal sections at the rostro-caudal level of the ARC: although the strongest Kiss1 signal originated in the hypothalamus (45%), a substantial amount of Kiss1 mRNA was also expressed in cortex. Limbic structures (medial amygdala and hippocampus) are the likely source of this cortical Kiss1 (37% of total; cf. Arai, 2009, Gottsch et al., 2004). Although the efferent targets of these populations of kisspeptin neurons have not been identified, numerous amygdalo-hypothalamic projections exist that govern reproductive responses (e.g., pheromone-processing circuits; Baum, 2009). Amygdala Kiss1 may also participate in the regulation of gonadotrophin production by photoperiod or energetic cues. Future studies may attempt more precise neuroanatomical localization via in situ hybridization; however, in common with immunocytochemistry, such an approach is only semiquantitative. The approach we report here describes novel quantitative measurement of hypothalamic gene expression, in conjunction with neuroanatomical localization on adjacent sections, which may prove useful in future studies of brain gene expression.
A recent report indicated decreased RFRP mRNA in the mediobasal hypothalamus including the DMH following 10 weeks of exposure to a short photoperiod (Revel et al., 2008), but several methodological differences, most notably the use of intermediate photoperiods in the present report (cf. categorically short photoperiods in Revel et al., 2008), preclude direct reconciliation of these two studies. However, dynamic changes in the magnitude of RFRP restraint on GnRH neurons (Gibson et al., 2008, Kriegsfeld et al., 2006) may occur over time during photoperiod-induced gonadal regression. For example, an increase in RFRP expression may be required to initiate gonadal regression, but less critical for the maintenance of gonadal involution once regression is completed. Such a mechanism would be analogous to the dynamic stimulatory neuroendocrine events that evolve during photoperiod-induced gonadal growth in mammals and birds: robust, transient increases in GnRH expression and gonadotropin secretion initiate testicular development, but decline markedly thereafter, and are less critical to the maintenance of the long-day reproductive phenotype, once achieved (Bernard et al., 1999, Yellon & Goldman, 1984). Measurement of RFRP expression at several time points during the course of seasonal gonadal growth and regression should permit evaluation of this conjecture.
Gonadal steroids provide feedback regulation of kisspeptin and RFRP (Greives et al., 2008, Kriegsfeld et al., 2006, Smith et al., 2005a, Smith et al., 2005b). Because gonad-intact hamsters were used in the present study, gonadal steroid-dependent effects on Kiss1 and RFRP in the present report cannot be definitively excluded. However, it is unlikely that circulating steroid concentrations account for the broad patterns of altered gene expression for the following reasons. Testosterone concentrations would be expected to be lowest in the group that underwent gonadal regression. However, FR caused decreases in Kiss1 mRNA at the level of the ARC in hamsters maintained in both 16L and 13.5L, whereas gonadal regression occurred only in FR hamsters maintained in 13.5L. Similarly, exposure to 13.5L caused increases in DMH RFRP mRNA in both ad libitum and FR hamsters, despite gonadal regression only occurring in the latter group. In addition, circulating testosterone concentrations of ad libitum fed Siberian hamsters maintained in 13.5L or transferred to 16L do not differ (Paul et al., 2009), yet in the present investigation, RFRP mRNA was elevated in 16L hamsters compared to 13.5L hamsters. Lastly, the direction of these changes in Kiss1 and RFRP expression are opposite of what would be predicted by decreased gonadal steroids (Kriegsfeld et al., 2006, Smith et al., 2005b). Collectively, these arguments suggest that gene expression responses to photoperiod and food reported here are unlikely to be driven by altered gonadal steroid production. This conclusion is consistent with by several other studies which have demonstrated gonadal steroid-independent modulation of RFamide mRNA levels (Greives et al., 2008, Revel et al., 2008, Revel et al., 2006).
In nature, seasonal adaptations are regulated by both day length and non-photic cues. Early-spring and late-summer intermediate-duration day lengths usher an interval of heightened reproductive responsiveness to non-photic cues that ultimately govern the precise timing of seasonal reproductive transitions (Paul et al., 2009). The present results point to two hypothalamic RFamide peptides, kisspeptin and RFRP, in the mediation of photic and non-photic cues on the reproductive system, and suggest that they serve different roles. Intermediate day lengths increase DMH RFRP gene expression, and decreased food availability suppresses Kiss1 mRNA at the level of the ARC. Taken together, the data suggest regional- and neuropeptide-specific recruitment of the RFamide system in the integration of photic and non-photic cues for the control of reproduction.
ACKNOWLEDGEMENTS
The authors wish to thank Sean Bradley, August Kampf-Lassin, and Curtis Wilkerson for technical assistance. This work was supported by Grant AI-67406 from the NIAID (BJP), a Social Sciences Divisional Research Fund of the University of Chicago (BJP), Grant NS-58135 from the NINDS (MJP).
REFERENCES
- Arai AC. The role of kisspeptin and GPR54 in the hippocampus. Peptides. 2009;30:16–25. doi: 10.1016/j.peptides.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Badura LL, Goldman BD. Central sites mediating reproductive responses to melatonin in juvenile male Siberian hamsters. Brain Res. 1992;598:98–106. doi: 10.1016/0006-8993(92)90172-6. [DOI] [PubMed] [Google Scholar]
- Ball GF. The neural integration of environmental information by seasonally breeding birds. Amer Zool. 1993;33:185–199. [Google Scholar]
- Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Baum MJ. Sexual differentiation of pheromone processing: links to male-typical mating behavior and partner preference. Horm Behav. 2009;55:579–588. doi: 10.1016/j.yhbeh.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard DJ, Abuav-Nussbaum R, Horton TH, Turek FW. Photoperiodic effects on gonadotropin-releasing hormone (GnRH) content and the GnRH-immunoreactive neuronal system of male Siberian hamsters. Biol Reprod. 1999;60:272–276. doi: 10.1095/biolreprod60.2.272. [DOI] [PubMed] [Google Scholar]
- Carter DS, Goldman BD. Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): duration is the critical parameter. Endocrinology. 1983a;113:1261–1267. doi: 10.1210/endo-113-4-1261. [DOI] [PubMed] [Google Scholar]
- Carter DS, Goldman BD. Progonadal role of the pineal in the Djungarian hamster (Phodopus sungorus sungorus): mediation by melatonin. Endocrinology. 1983b;113:1268–1273. doi: 10.1210/endo-113-4-1268. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- Clarkson J, de Tassigny X d’Anglemont, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol. 2009;21:673–682. doi: 10.1111/j.1365-2826.2009.01892.x. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Humber SA, Jain S, Williams WP, 3rd, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149:4958–4969. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Lynch GR. Evidence for a brain site of melatonin action in the white-footed mouse, Peromyscus leucopus. Neuroendocrinology. 1982;34:1–6. doi: 10.1159/000123269. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- Gorman MR. Seasonal adaptations of Siberian hamsters. I. Accelerated gonadal and somatic development in increasing versus static long day lengths. Biol Reprod. 1995;53:110–115. doi: 10.1095/biolreprod53.1.110. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Greives TJ, Humber SA, Goldstein AN, Scotti MA, Demas GE, Kriegsfeld LJ. Photoperiod and testosterone interact to drive seasonal changes in kisspeptin expression in Siberian hamsters (Phodopus sungorus) J Neuroendocrinol. 2008;20:1339–1347. doi: 10.1111/j.1365-2826.2008.01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greives TJ, Mason AO, Scotti MA, Levine J, Ketterson ED, Kriegsfeld LJ, Demas GE. Environmental control of kisspeptin: implications for seasonal reproduction. Endocrinology. 2007;148:1158–1166. doi: 10.1210/en.2006-1249. [DOI] [PubMed] [Google Scholar]
- Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab. 2008;294:E827–832. doi: 10.1152/ajpendo.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K. The critical photoperiod in the Djungarian hamster Phodopus sungorus. In: Aschoff J, Daan S, Groos GA, editors. Vertebrate Circadian Systems. Springer-Verlag; Berlin: 1982. pp. 297–304. edn. Ed. [Google Scholar]
- Illnerová H. The suprachiasmatic nucleus and rhythmic pineal melatonin production. In: Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic Nucleus: The Mind’s Clock. Oxford University Press; New York: 1991. pp. 197–216. edn. Ed. [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin- GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007;30:504–511. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kramer KM, Choe C, Carter CS, Cushing BS. Developmental effects of oxytocin on neural activation and neuropeptide release in response to social stimuli. Horm Behav. 2006;49:206–214. doi: 10.1016/j.yhbeh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ. Driving reproduction: RFamide peptides behind the wheel. Horm Behav. 2006;50:655–666. doi: 10.1016/j.yhbeh.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A. 2006;103:2410–2415. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque RM, Kineman RD, Tena-Sempere M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: analyses using mouse models and a cell line. Endocrinology. 2007;148:4601–4611. doi: 10.1210/en.2007-0500. [DOI] [PubMed] [Google Scholar]
- Mason AO, Greives TJ, Scotti MA, Levine J, Frommeyer S, Ketterson ED, Demas GE, Kriegsfeld LJ. Suppression of kisspeptin expression and gonadotropic axis sensitivity following exposure to inhibitory day lengths in female Siberian hamsters. Horm Behav. 2007;52:492–498. doi: 10.1016/j.yhbeh.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen JD, Simonneaux V. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides. 2009;30:26–33. doi: 10.1016/j.peptides.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Morgan PJ, Hazlerigg DG. Photoperiodic signalling through the melatonin receptor turns full circle. J Neuroendocrinol. 2008;20:820–826. doi: 10.1111/j.1365-2826.2008.01724.x. [DOI] [PubMed] [Google Scholar]
- Morgan PJ, Ross AW, Mercer JG, Barrett P. What can we learn from seasonal animals about the regulation of energy balance? Prog Brain Res. 2006;153:325–337. doi: 10.1016/S0079-6123(06)53019-5. [DOI] [PubMed] [Google Scholar]
- Park JH, Takasu N, Alvarez MI, Clark K, Aimaq R, Zucker I. Long-term persistence of male copulatory behavior in castrated and photo-inhibited Siberian hamsters. Horm Behav. 2004;45:214–221. doi: 10.1016/j.yhbeh.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Zucker I, Schwartz WJ. Tracking the seasons: the internal calendars of vertebrates. Philos Trans R Soc Lond B Biol Sci. 2008;363:341–361. doi: 10.1098/rstb.2007.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Galang J, Schwartz WJ, Prendergast BJ. Intermediate-duration day lengths unmask reproductive responses to nonphotic environmental cues. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1613–1619. doi: 10.1152/ajpregu.91047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain: in stereotaxic coordinates. edn 4. Academic Press; San Diego: 1998. p.^pp Pages. [Google Scholar]
- Prendergast BJ, Nelson RJ, Zucker I. Mammalian seasonal rhythms: behavior and neuroendocrine substrates. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. edn 2nd Academic Press; San Diego: 2009. [Google Scholar]
- Revel FG, Saboureau M, Pevet P, Simonneaux V, Mikkelsen JD. RFamide-related peptide gene is a melatonin-driven photoperiodic gene. Endocrinology. 2008;149:902–912. doi: 10.1210/en.2007-0848. [DOI] [PubMed] [Google Scholar]
- Revel FG, Saboureau M, Masson-Pévet M, Pévet P, Mikkelsen JD, Simonneaux V. Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Curr Biol. 2006;16:1730–1735. doi: 10.1016/j.cub.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Schlatt S, De Geyter M, Kliesch S, Nieschlag E, Bergmann M. Spontaneous recrudescence of spermatogenesis in the photoinhibited male Djungarian hamster, Phodopus sungorus. Biol Reprod. 1995;53:1169–1177. doi: 10.1095/biolreprod53.5.1169. [DOI] [PubMed] [Google Scholar]
- Simonneaux V, Ansel L, Revel FG, Klosen P, Pevet P, Mikkelsen JD. Kisspeptin and the seasonal control of reproduction in hamsters. Peptides. 2009;30:146–153. doi: 10.1016/j.peptides.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005a;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005b;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, et al. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149:5770–5782. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RE, Jr., Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- Weiner J. Limits to energy budget and tactics in energy investments during reproduction in Djungarian hamster (Phodopus sungorus sungorus Pallas 1770) Symp zool Soc Lond. 1987;57:167–187. [Google Scholar]
- Yellon SM, Goldman BD. Photoperiod control of reproductive development in the male Djungarian hamster (Phodopus sungorus) Endocrinology. 1984;114:664–670. doi: 10.1210/endo-114-2-664. [DOI] [PubMed] [Google Scholar]