Abstract
Alpha-particles are suitable to treat cancer micrometastases because of their short range and very high linear energy transfer. Alpha-particle-emitter 213Bi based radioimmunotherapy has shown efficacy in a variety of metastatic animal cancer models, such as breast, ovarian, prostate cancer and leukemia. Its clinical implementation, however, is challenging due to the limited supply of 225Ac, the high technical requirement to prepare radioimmunoconjugate with very short half-life (T1/2=45.6 mins) on site and prohibitive cost. In this study, we investigated the efficacy of the alpha-particle-emitter 225Ac, parent of 213Bi, in a mouse model of breast cancer metastases. A single administration of 225Ac (400 nCi) labeled anti-rat HER-2/neu monoclonal antibody (7.16.4) completely eradicated breast cancer lung micrometastases in about 67% of HER-2/neu transgenic mice and led to long-term survival of these mice for up to one year. Treatment with 225Ac-7.16.4 is significantly more effective than 213Bi-7.16.4 (120 μCi) (median survival = 61 days, P=0.001), and 90Y-7.16.4 (120 μCi) (median survival = 50 days, P<0.001), as well as untreated control (median survival = 41 days, P=0.0001). Dosimetric analysis showed that 225Ac treated metastases received a total dose of 9.6 Gy, significantly higher than 2.0 Gy from 213Bi and 2.4 Gy from 90Y. Biodistribution studies revealed that 225Ac daughters, 221Fr and 213Bi, accumulated in kidneys and probably contributed to the long-term renal toxicity observed in surviving mice. These data suggest 225Ac labeled anti-HER-2/neu monoclonal antibody could significantly prolong survival in HER-2/neu-positive metastatic breast cancer patients.
Keywords: alpha-particle, 225Ac, 213Bi, radioimmunotherapy, metastasis, rat HER-2/neu
INTRODUCTION
Radioimmunotherapy of metastatic cancer using alpha-particle-emitter-labeled monoclonal antibodies (mAb) is promising because alpha-particles can deliver highly focused energy along their short path length (1). The high-energy alpha radiation typically causes complex DNA double strand breaks (DSBs) that are difficult to repair, thus leading to effective tumor cell kill. Preclinical studies using 213Bi labeled mAbs have shown substantial efficacy in various tumor models including leukemia (2), prostate (3), ovarian (4), and colon cancer (5). We have also shown that 213Bi labeled anti-HER-2/neu mAb (7.16.4) is effective in prolonging the survival of HER-2/neu transgenic mice that if left untreated develop widespread metastases, including bone and liver metastases (6). Clinical trials using 213Bi labeled anti-CD33 mAb to treat myeloid leukemia have shown feasibility and safety (7,8). However, the short half-life of 213Bi complicates the preparation of the radioimmunoconjugates for clinical use and demands a large amount of 213Bi activity that is limited by worldwide availability of its parent, 225Ac. As a result, the maximum tolerated dose was not reached in the clinical trial at the largest administered activity of 37 MBq/kg (8).
To overcome the short half-life of 213Bi, McDevitt et al. (9) proposed the concept of an in vivo 225Ac (T1/2=10 days) generator which would deliver 4 alpha-particles to the target site per decay of 225Ac. This compares to one alpha from 213Bi, making, 225Ac much more potent. Indeed, 225Ac-labeled mAbs have greatly improved the survival in lymphoma and ovarian cancer models (9,10). More recently, 225Ac-labeled anti-vascular endothelial cadherin mAb targeting tumor neovasculature has been shown to inhibit tumor growth in a prostate cancer (LNCaP) model especially when it is combined with sequential chemotherapy (11). The in vivo generator concept has also been investigated in other alpha-particle-emitters. 212Pb (T1/2=10.6 hr, alpha-particle-emitting daughter 212Bi) in vivo generator (212Pb-Trastuzumab) has prolonged survival in a colon cancer xenograft model (12). Dahle et al. showed that a single injection of 227Th-Rituximab (T1/2=18.7 days, alpha-particle-emitting daughters 223Ra, 213Rn, 215Po and 211Bi) is able to completely eradicate 60% of B-cell lymphoma xenografts (13). Since 223Ra, the first daughter of 227Th has a 10-day half-life and rapidly localizes to bone, alpha-particles are mainly delivered from 227Th itself and the studies have demonstrated that subsequent daughters do not lead to toxicity.
Several studies have been published to compare the efficacy of alpha and beta-radiation directly in metastatic tumor models. Behr et al. (14) found that 213Bi is therapeutically more effective than the beta emitter, 90Y, in a metastatic colon cancer model. Few studies, however, directly compared the efficacy of in vivo alpha-particle generators with that of conventional alpha and beta particle-emitters. In this work, we compare the efficacy of targeted therapy using the 225Ac in vivo generator with targeted 213Bi and 90Y in a syngeneic HER-2/neu metastatic breast cancer model.
HER-2/neu is a tumor cell surface tyrosine kinase associated with aggressive phenotype and poor prognosis (15). Targeting HER-2/neu with Trastuzumab has shown significant clinical benefit in patients with metastatic breast cancer (16). In this study, we demonstrate the efficacy of 225Ac-7.16.4 in targeting rat HER-2/neu positive pulmonary metastases. Rat HER-2/neu is also expressed on normal lung tissue in this mouse model as determined by Western blot (17). This allows for evaluating efficacy and toxicity of 225Ac-7.16.4 in a model that closely mimics clinical cases where cross reactivity of tumor antigen expressed on normal organs is common.
MATERIALS AND METHODS
Mice, cell line and monoclonal antibodies
neu-N transgenic mice, age 6 to 8 wk, expressing rat HER-2/neu under the mouse mammary tumor virus (MMTV) promoter were obtained from Harlan (Harlan Lab., Madison, WI). All experiments involving the use of mice were conducted with the approval of the Animal Care and Use Committee of The Johns Hopkins University School of Medicine. NT2.5, a rat HER-2/neu expressing mouse mammary tumor cell line, was established from spontaneous mammary tumors (18). The NT2.5 cells are maintained in RPMI media containing 20% fetal bovine serum, 0.5% penicillin/streptomycin (Invitrogen, Carlsbad, CA), 1% L-glutamine, 1% nonessential amino acids, 1% sodium pyruvate, 0.02% gentamicin, and 0.2% insulin (Sigma, St. Louis, MO) at 37°C in 5% CO2. 7.16.4, a mouse anti-rat HER-2/neu mAb was purified from the ascites of athymic mice. The hybridoma cell line was kindly provided by Dr. Mark Greene (University of Pennsylvania). Rituximab (IDEC Pharmaceuticals Corp.), an anti-human CD20 monoclonal antibody, was used as a negative control.
Radiolabeling of antibody with 213Bi, 90Y and 225Ac
7.16.4 was conjugated to SCN-CHX-A”-DTPA following published protocol (19). 90Y was purchased from PerkinElmer (Waltham, MA) and labeled to 7.16.4-Chx-A”-DTPA (10 mCi/mg) at 37°C for 30 mins in acetate buffer (pH=4.5). 225Ac/213Bi (Institute for Transuranium Elements) generator was constructed and 213Bi was labeled to 7.16.4-Chx-A”-DTPA (10 mCi/mg) as described previously (6). Both 90Y-, and 213Bi-labeled 7.16.4 radioimmunoconjugates were purified by MicroSpin G-25 column (GE BioSciences, Pittsburgh, PA).
225Ac was purchased from Curative Technologies Corporation (Richland, WA). 225Ac was labeled to mAb in a two-step reaction following McDevitt et al (20). First, 225Ac (0.15-0.2 mCi in 20-80μL) was chelated to 1μL (10mg/mL) p-SCN-Bn-DOTA (Macrocyclics, Richardson, TX) at 56°C for 1 hr. Ascorbic acid (1μL, 150mg/mL) was added as a radio-protectant and 2M Sodium acetate (40 to 60 μL) was added to raise the pH to 6.5. The efficiency of 225Ac chelation to DOTA was determined by Sephadex C-25 column (GE Bioscience). Second, 100μg mAb (~20μL, 5mg/mL) was incubated with p-SCN-Bn-DOTA-225Ac at 37°C for 45 mins (pH=8.5). 225Ac-labeled mAb was purified with a Centricon centrifuge filter unit (YM-10, Millipore).
The reaction efficiency and purity of the radioimmunoconjugates were determined with instant thin layer chromatography (ITLC) using silica gel impregnated paper (Gelman Science Inc., Ann Arbor, MI). ITLC paper strips were counted the next day with a gamma counter (LKB Wallac, Perkin-Elmer) to allow 225Ac to reach equilibrium. 225Ac-7.16.4 immunoreactivity was determined by incubating 5 ng of 225Ac-7.16.4 with excess antigen binding sites (1×107 NT2.5 cells) twice on ice for 30 mins each time. Immunoreactivity was calculated as the percentage of 225Ac-7.16.4 bound to the cells. Stability of 225Ac-7.16.4 was measured by incubating 225Ac-7.16.4 in cell culture media containing 20% FBS for 30 days and the fraction of 225Ac chelated to DOTA was measured with Sephadex C-25 column and ITLC. To determine internalization of 7.16.4, NT2.5 cells (2×106 cells/ml) were incubated with 111In labeled 7.16.4 (1μg/ml) at 37 °C. After 0, 10, 20, 30, 60, 120, 240, 360, 480 mins (two samples for each time point) of incubation, reaction was stopped and NT2.5 cells were washed three times with cold PBS. Cell pellet was then incubated with 1 ml NaCl 150 mM/50 mM Glycine (pH=2.0) for 10 mins at room temperature and washed twice with PBS. Both pellet and supernatant were collected and counted with gamma counter. The activity fraction of the cell pellet was the fraction of internalized 7.16.4 (10).
Specific cell kill in vitro by 225Ac-7.16.4
Specific cell kill in vitro was determined by colony formation assay. NT2.5 cells were seeded into 96 well plates at 2.0×103 cells/well. NT2.5 cells were then treated with serially diluted 225Ac-7.16.4 or 225Ac-Rituximab (from 0.2 to 20 nCi/ml at a specific activity of 0.10 μCi/μg). After incubation for either 3 or 5 days, NT2.5 cells were trypsinized and re-plated on cell culture petri dishes for colony growth. Blocking of cell kill by 225Ac-7.16.4 using unconjugated 7.16.4 (50 μg/ml) was also tested.
Biodistribution of 225Ac-, 213Bi- and 111In- 7.16.4
neu-N mice (3 mice/group) bearing s.c. tumors in the mammary fat pad were injected intravenously with 400 nCi 225Ac-7.16.4. At 1, 6, 24, 72, 144, 288 hrs after injection, mice were sacrificed and major organs including blood, heart, lung, liver, spleen, kidney, stomach, intestine, femur, tumor were collected. 225Ac in each organ was counted the next day using an energy window of 150-500 keV. To determine the distribution of free 221Fr (T1/2=4.9 mins) and 213Bi that was released after 225Ac decay, the organs were counted right away repeatedly for 221Fr or 213Bi using the 190-250 keV and the 400-480 keV energy window, respectively. An exponential expression was fitted to the decay curves thus obtained. The fitted activity coefficient at time zero is the activity concentration at the time of sacrifice. Differences in 221Fr and 213Bi activity at the time of sacrifice and the levels from equilibrium of 225Ac reflect clearance or accumulation of free 221Fr and 213Bi. The time activity curves of free 213Bi and 221Fr in each organ were then constructed with free 221Fr, 213Bi activities at multiple sacrifice time points. Injectates with equivalent injected activity were counted as standards for decay correction. In a separate series of animals, the biodistribution of 225Ac-, 213Bi-, and 111In-7.16.4 to lung metastases were also measured and compared. neu-N mice (3 mice/group) were injected with either 225Ac-, 213Bi-, or 111In-labeled 7.16.4 and sacrificed at 0.5, 1.0, 3.0, and 6.0 hr after injection; 111In radionuclide was used as a surrogate for 90Y (21). Lung metastases were collected and counted for 225Ac, free 221Fr and 213Bi.
Efficacy of 225Ac-, 213Bi-, 90Y-labeled anti-rat HER-2/neu mAb to treat lung metastases
The maximum tolerated dose (MTD) was determined as the highest administered activity that allows 100% survival with no significant body weight loss (>15%). Healthy neu-N mice were injected (five mice/group) with 100, 200, 400, 500, 600, 700, or 1,000 nCi 225Ac-7.16.4. Mice were weighed twice per week for 90 days. To evaluate the efficacy of radiolabeled mAbs to treat early stage micrometastases, three days after neu-N mice were injected with 1×105 NT2.5 cells (i.v.), mice were treated intravenously with (a) 400 nCi 225Ac-7.16.4, n=12; (b) 120 μCi 213Bi-7.16.4, n=10; (c) 120 μCi 90Y-7.16.4, n=5; (d) 200 nCi 225Ac-7.16.4, n=5; (e) 200 nCi + 200 nCi 225Ac-7.16.4 (injected one week apart), n=5; (f) 100 μg 7.16.4, unlabeled control, n=5; (g) 400 nCi 225Ac-Rituximab, nonspecific control, n=5; (h) untreated control, n=10. To evaluate the efficacy of treating late stage lung metastases, at eighteen days after tumor cell inoculation, neu-N mice were treated with (a) 400 nCi 225Ac-7.16.4, n=5; (b) 120 μCi 213Bi-7.16.4, n=5; (c) 120 μCi 90Y-7.16.4, n=5. Mice were monitored and weighed three times a week and were euthanized when significant body weight loss (>15%) or breathing difficulties developed. In survival and long-term toxicity studies, animals were followed for up to one year.
At time of sacrifice, all major organs were collected for histopathological examination. The number of visible lung metastases was counted. Lung metastases were snap frozen in liquid nitrogen and sectioned with a cryotome into 8 μm thick slices. The section was fixed in acetone and immunostained with 7.16.4 and a biotinylated rat anti-mouse IgG2a antibody (BD Pharmingen). The stain was developed with a Vectastain ABC Kit (Vector Lab, Burlingame, CA) according to manufacturer’s instruction.
Dosimetry
Organ and tumor absorbed doses for 225Ac were calculated based on measured biodistribution data (22). Time activity curves in each organ and in s.c. tumors and small lung metastases were measured and integrated over time to obtain the total disintegration of 225Ac, free 221Fr and 213Bi. 213Po and 217At, with half-lives of 4.2μs and 32.3ms, were assumed to decay at the same position as 213Bi or 221Fr. Absorbed doses for three tumor geometries were calculated: subcutaneous (s.c.) tumor (approximately 1 cm-diameter), small lung metastases (300 μm diameter - i.e., day 18) and single cells (day 3). In the s.c. tumor calculation, the alpha-particle and electron energies of 225Ac were assumed completely absorbed within the tumor. Photon doses were not included since they typically account for less than 1% of the total absorbed dose. Absorbed doses for normal organs and the s.c. tumor were thus calculated as ; ; D= Dα + De, wherein Dα, De and D are the alpha-particle, electron, and summed alpha and electron absorbed dose, respectively, is the total number of disintegrations in an organ/tumor, Δα, Δe are the mean energy emitted per nuclear transition for alpha-particles and electrons, and M is the weight of the organ/tumor. The total absorbed doses were calculated as the sum of doses from 225Ac at equilibrium and its free daughters. If an organ is accumulating or depleting free daughters, the dose from free daughters is added or subtracted from the 225Ac doses at equilibrium. Absorbed doses for single tumor cells (day 3) and small lung metastases (day 18) were also calculated and compared for 225Ac-, 213Bi-, and 90Y- 7.16.4. Bi-213 and In-111 (surrogate for Y-90) biodistribution data for small lung metastases were used for Bi-213 and Y-90 dosimetry. The absorbed fractions of electron and alpha-particle emissions in small lung metastases were calculated using Monte Carlo; these calculations accounted for the range of all of the betas and alphas (23). Absorbed doses to single cells were obtained using MIRD cellular S values (24,25). In the single-cell calculations, daughter radionuclides resulting from the decay of 7.16.4-conjuagated 225Ac that was tumor-cell bound but not internalized were assumed to diffuse away and not contribute to the target cell absorbed dose. The fraction of internalized 225Ac-7.16.4 was obtained from the internalization assay.
Statistical Analysis
Kaplan-Meier survival analysis or statistical comparisons between groups (Student t-test) were performed with MedCalc (MedCalc Software, Belgium). For all studies, the level of statistical significance is set at P <0.05.
RESULTS
Radiolabeling, immunoreactivity and antibody internalization
Both 90Y, and 213Bi labeling efficiencies were about 90% and purities reached ~98% after size exclusion purification. The two-step 225Ac labeling efficiency was 12.0% ± 3.8% (n=12). After purification, the purity of 225Ac-7.16.4 was 97.0% ± 1.8% (n=12). The specific activity of 225Ac-7.16.4 was between 0.06 to 0.10 μCi/μg. The immunoreactivity of 225Ac-7.16.4 was 62.4% ± 5.9% (n=3), worse than that of 111In- or 213Bi-7.16.4 (~80%). After incubation in 20% BSA at 37°C for 30 days, purity of 225Ac-7.16.4 became 80.9% ± 8.2% and 65.9% ± 3.9% as measured by Sephadex C-25 column and ITLC (n=3), respectively. 7.16.4 internalizes after binding to NT2.5 cells. The internalized 111In-7.16.4 increased from 1.55 × 10-2 μg/106 cells at 5 mins after binding started to 3.05 × 10-2 μg/106 cells at 120 mins and plateaued afterwards to 3.25 × 10-2 μg/106 cells at 1440 mins.
In vitro cell kill
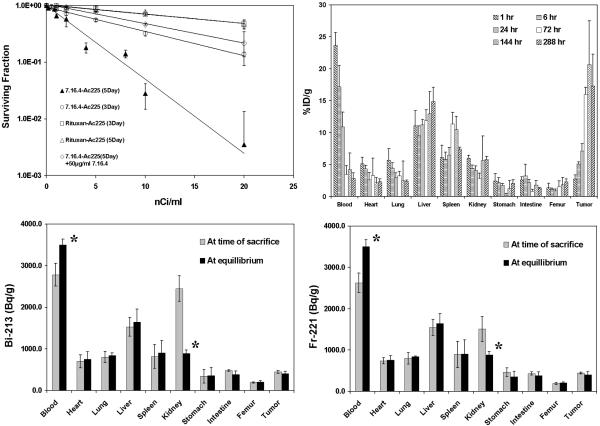
225Ac-7.16.4 was very effective in killing rat HER-2/neu expressing NT2.5 cells (Fig. 1A). The effective dose concentration of 225Ac-7.16.4 that can kill 50% of the NT2.5 cells (ED50) was 2.3 nCi/ml and 6.4 nCi/ml for 5-day or 3-day incubation, respectively. When 50 μg/ml unlabeled 7.16.4 was used to block 225Ac-7.16.4, the ED50 increased to 9.4 nCi/ml (5-day incubation). The ED50 for 225Ac-Rituximab was 19.0 nCi/ml and 18.8 nCi/ml when it was incubated with NT2.5 cells for 3 or 5 days. In comparison, the ED50 for 213Bi-7.16.4 was 940 nCi/ml.
Figure 1.
A, In vitro kill of NT2.5 breast cancer cells by 225Ac-7.16.4. NT2.5 cells were treated with 225Ac-7.16.4 for 3 days (open circle), 5 days (closed triangle), or with the presence of 50 μg/ml unconjugated 7.16.4 (open diamond). 225Ac-Rituximab was used as non-specific control to treat NT2.5 cells for 5 (open triangle) or 3 (open square) days. Survival fraction was calculated by dividing the number of colonies in treatment groups over that in the untreated control group.
B, Biodistribution of 225Ac-7.16.4 in neu-N mice at decay equilibrium; data are presented as percent injected dose per gram (%ID/g±SD). Biodistributions of free 213Bi (C) and 221Fr (D) in major organs (at 1 hr) are shown at the time of sacrifice and decay equilibrium. Star (*) marks the difference that is statistically significant; data are expressed as Bq per gram (Bq/g±SD).
Biodistribution of 225Ac-, 213Bi- and 111In-7.16.4
The biodistribution of 225Ac-7.16.4 at equilibrium in (s.c. mammary fat-pad) tumor bearing neu-N mice is shown in Fig. 1B. The effective half-life of antibody clearance from blood in the first 72 hr was 26.8 hr, similar to that of 111In-labeled 7.16.4, 26.3 hr. 225Ac-7.16.4 targeting to tumors increased continuously and accumulation peaked at 20.6%/g 6 days after injection. Antibody localization in the tumor was 17.3%ID/g 12 days post-injection. The peak tumor uptake of 225Ac-7.16.4, however, was lower than that of 111In-7.16.4 (~38%ID/g), probably reflecting impaired immunoreactivity of 225Ac-7.16.4.
The biodistributions of free 213Bi, 221Fr at 1hr after 225Ac-7.16.4 injection are shown in Fig. 1C and 1D. For 213Bi (Fig. 1C), the activity concentration in blood increased from 2779.9 Bq/g at time of sacrifice to 3502.1 Bq/g at equilibrium (P=0.03), while the kidneys activity decreased from 2449.8 Bq/g at time of sacrifice to 887.0 Bq/g at equilibrium (P=0.005). Similar pattern was observed for 221Fr (Fig. 1D). These data strongly suggest that both free 213Bi and 221Fr cleared from blood through kidneys. The fractions of free 213Bi and 221Fr in blood that ended up in kidneys were 41.1% and 18.7%, suggesting a higher kidney retention rate for 213Bi, which has been attributed to the higher positive charge of 213Bi (Bi3+) that interacts with the negatively charged basement membrane of the glomerulus. In biodistribution study of lung metastases, 225Ac-7.16.4 was found to be 13.7±1.5%ID/g at 144 hr, lower than 30.8±1.5%ID/g of 213Bi-7.16.4 at 6 hrs.
Treating rat HER-2/neu expressing lung metastases with 225Ac-, 213Bi- and 90Y-7.16.4
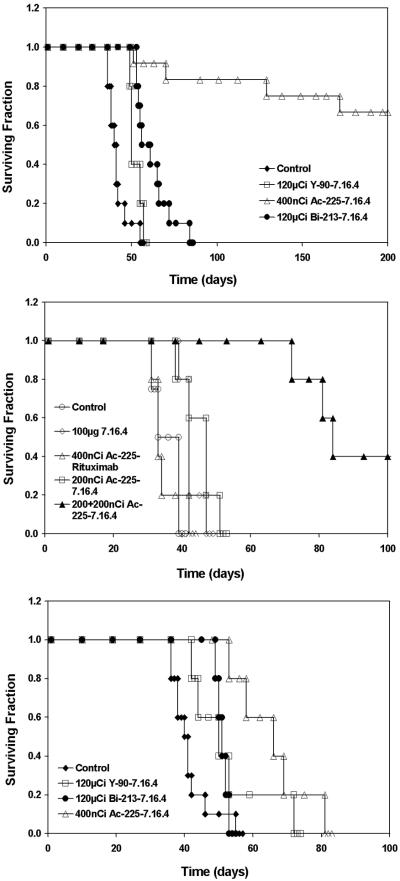
The maximum tolerated dose of 225Ac-7.16.4 in neu-N mice was found to be 400 nCi in a single injection. MTD of 213Bi-7.16.4 (120 μCi) and 90Y-7.16.4 (120 μCi) were obtained from literature and confirmed in the neu-N model (6,26). Kaplan-Meier survival curves of neu-N mice bearing lung metastases after treatment by radiolabeled anti-rat HER-2/neu mAb are shown in Fig. 2A. All untreated mice (100%) developed pulmonary metastases, showing signs of labored breath and stress at later stages. Median survival improved to 50 days in 90Y-7.16.4 group (P=0.013), 61 days in 213Bi-7.16.4 group (P=0.0001) compared to untreated mice (41 days). Eight of twelve mice in 225Ac-7.16.4 treated group achieved long-term (one-year) survival (P<0.0001). Both 213Bi-(P=0.025) and 225Ac-labeled (P<0.001) 7.16.4 treated mice lived significantly longer than the mice treated by 90Y-7.16.4. 225Ac-7.16.4 also improved survival of neu-N mice compared to alpha emitter 213Bi-7.16.4 (P=0.001). 225Ac-Rituximab did not demonstrate efficacy in these models with median survivals of only 33 days (P=0.99). Unlabeled 7.16.4 (single i.v. dose 100μg/mouse) slightly improved median survival to 42 days (P=0.014) and the median survival of neu-N mice treated with 200 nCi 225Ac-7.16.4 improved to 47 days (P=0.029). Notably, when a second dose of 200 nCi 225Ac-7.16.4 was administered one week after the first 200 nCi injection, median survival improved to 84 days (P=0.004) with 2 out 5 mice achieved long-term survival for up to one year (Fig. 2B).
Figure. 2.
Therapeutic efficacy of 225Ac-, 213Bi- and 90Y-7.16.4. A, Kaplan-Meier survival curves of neu-N transgenic mice treated 3 days after i.v. injection of 1×105 NT2.5 cells. Neu-N mice were treated with 400 nCi 225Ac-7.16.4 (open triangle, n=12), 120 μCi 213Bi-7.16.4 (closed circle, n=10), 120 μCi 90Y-7.16.4 (open square, n=5) or untreated (closed diamond, n=10). B, Neu-N mice were treated 3 days after tumor cell inoculation with 100 μg unlabeled 7.16.4 (open diamond, n=5), 400 nCi 225Ac-Rituximab (open triangle, n=5), 200 nCi 225Ac-7.16.4 (open square, n=5), 200 nCi + 200 nCi 225Ac-7.16.4 (closed triangle, n=5) or untreated (open circle, n=4) C, neu-N mice were treated 18 days after tumor cell inoculation with 400 nCi 225Ac-7.16.4 (open triangle, n=5), 120 μCi 213Bi-7.16.4 (closed circle, n=5), 120 μCi 90Y-7.16.4 (open square, n=5) or untreated (closed diamond, n=10).
To examine the efficacy of 225Ac-7.16.4 on later stage metastases, neu-N mice were treated 18 days after tumor cell injection (Fig. 2C), where the average diameter of lung metastases was 295.7 ± 93.6 μm (n=29). 213Bi-7.16.4 treated mice have a median survival of 51 days, not a statistically significant improvement over 90Y-7.16.4 with a median survival of 50 days (P=0.76). Although mice treated with 213Bi-7.16.4 at 18 days had a significantly shorter survival compared to those treated at 3 days (P=0.0001), mice treated with 90Y-7.16.4 at 18 days and 3 days have about the same median survival of 50 days (P=0.96), suggesting that targeting with the 90Y is less sensitive to the size of the metastases. The median survival decreased to 66 days for mice treated with 225Ac-7.16.4 at 18 days compared to 3 days (P=0.0005). Nevertheless, 225Ac-7.16.4 treated mice still survived significantly longer compared to 213Bi-7.16.4 (P=0.004).
Number of lung metastases
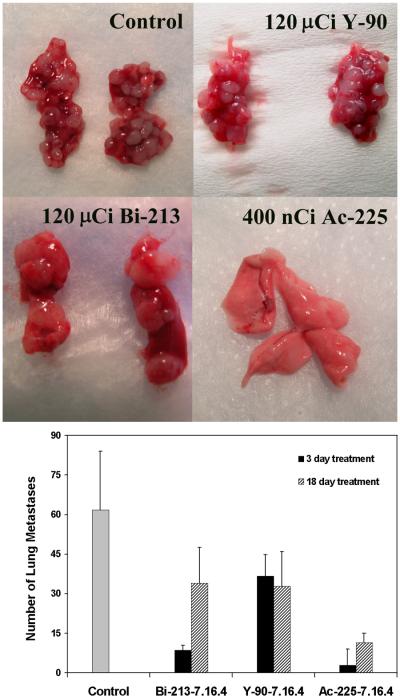
The numbers of visible metastases on the lungs of neu-N mice and representative images are shown in Fig. 3. All treated mice have reduced number of lung metastases compared to untreated mice. When treatment was initiated at 3 days, mice treated with 213Bi-7.16.4 and 225Ac-7.16.4 had 8.5±1.8 (P<0.0001) and 2.9±6.1 (P<0.0001) lung metastases, significantly less than 90Y-7.16.4 treated mice with 36.7±8.1 metastases. No lung metastases were found in neu-N mice surviving 225Ac-7.16.4 treatment (Fig. 3A, bottom right). It is also evident that the sizes of the metastases were much larger in 213Bi-7.16.4 treated mice (Fig. 3A, bottom left) than those from 90Y-7.16.4 treated mice (Fig. 3A, top right), which suggests 213Bi-7.16.4 is able to eliminate more metastases initiating cells compared to 90Y-7.16.4 and it takes longer for the surviving tumor cells to grow to a lethal tumor burden. When treatment was initiated at 18 days, the number of metastases found on 90Y-7.16.4 treated mice (32.8±13.2) was similar to that obtained for mice treated with 90Y-7.16.4 at 3 days. More metastases (34.0±13.5) were found on mice treated with 213Bi-7.16.4 at 18 days compared to 3 days (P<0.0001), indicating reduced efficacy of 213Bi to treat later stage metastases. 225Ac-7.16.4 treated mice again presented with the fewest number of lung metastases, 11.5±3.5, compared to the other two radioisotopes (Fig. 3B).
Figure 3.
A, Representative photographs show lungs bearing metastases from neu-N mice after treatment by 225Ac-, 213Bi-, 90Y- 7.16.4. These mice were treated at 3 days after tumor cell injection with untreated (top left), 120 μCi 90Y-7.16.4 (top right), 120 μCi 213Bi-7.16.4 (bottom left), 400 nCi 225Ac-7.16.4 (bottom right). B, Number of lung metastases from mice treated at 3 days (filled column) or 18 (striped column) days after tumor cell inoculation was counted. Data are presented as average number of metastases±SD.
Histopathology and immunohistostaining of rat HER-2/neu
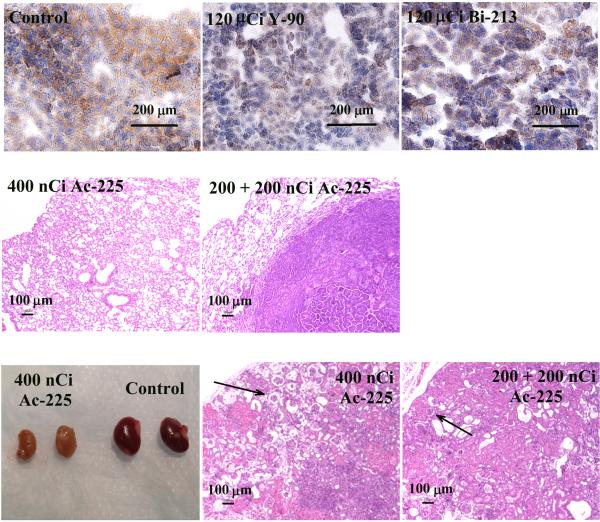
Immunohistostaining showed that the HER-2/neu expression level decreased on the metastases surviving the treatment of 90Y-7.16.4 (Fig. 4A, middle) and 213Bi-7.16.4 (Fig. 4A, right), compared to untreated tumors (Fig. 4A, left). At one year after treatment, no residual tumor cells were found on the lungs of mice treated with 400 nCi 225Ac-7.16.4 (Fig. 4B, left) and a single metastatic tumor nodule was found on one of the lungs from a mouse treated with 200 +200nCi 225Ac-7.16.4 (Fig. 4B, right). No damage to the normal lung tissue was observed in groups treated with 225Ac-7.16.4. Examination of kidneys from these mice, however, revealed shrunken and pale kidneys (Fig. 4C, left). H&E staining showed wide-spread loss of tubular epithelium in the kidney cortex (Fig. 4C, middle). In the medulla there is extensive loss of tubules and replacement with marked fibrosis and a mixed inflammatory infiltrate composed mainly of mononuclear cells and lesser numbers of neutrophils. Scattered dilated cystic structures are visible throughout the cortex and medulla. These observations are also present but less evident in kidneys treated with 200 + 200 nCi 225Ac-7.16.4 (Fig. 4C, right).
Figure 4.
A, Immunohistostaining of rat HER-2/neu expression on lung metastases. Untreated control (left); treated with 120 μCi 90Y-7.16.4, (middle) or with 120 μCi 213Bi-7.16.4 (right). B, H&E staining of lungs from mice treated with 400 nCi 225Ac-7.16.4 that showed no sign of tumor cells or lung tissue damage (left) and of a single metastasis found on the lungs of a mouse treated with 200 + 200nCi 225Ac-7.16.4 (right). C, left, Representative photographs of kidneys from a neu-N mouse surviving one year after treatment with 400 nCi 225Ac-7.16.4 (left pair) and a healthy neu-N mouse (right pair). H&E staining of kidneys from neu-N mice surviving one year after treatment with 400 nCi (middle) or 200 nCi + 200 nCi 225Ac-7.16.4 (right). Arrows point to collapse of cortical tissue due to loss of tubular epithelium in the kidney cortex. Bar indicates 100 μm.
Dosimetry
The absorbed doses to major organs and tumors are shown in Table 1. Reductions or increases in the absorbed dose from 225Ac at equilibrium with its free daughters are indicated by negative or positive dose contributions. The dose calculations show that free 221Fr and 213Bi are cleared from the blood, lungs, liver and spleen while kidney, stomach, and intestine are accumulating these daughters. A 400 nCi administration of 225Ac-7.16.4 gives a tumor absorbed dose of 10.49 Gy; no significant depletion or accumulation of free daughters is observed. Absorbed doses of 225Ac-, 213Bi-, 90Y-7.16.4 to single tumor cells and small lung metastases (~300μm in diameter) are shown in Table 2. The 225Ac absorbed dose to small metastases is 9.6 Gy, much higher than that of 2.0 Gy from 213Bi and 2.4 Gy from 90Y. Similarly, the 25.3 Gy 225Ac dose to single tumor cells, is also higher than that of 213Bi at 2.6 Gy and 90Y at 0.6 Gy. When no antibody-receptor internalization or 100% internalization was considered, 225Ac dose to single cells became 12.0 Gy or 38.6 Gy, respectively.
Table 1.
Absorbed doses (Gy) from 225Ac (400 nCi) and its free daughters 213Bi and 221Fr
| Organs |
225Ac at Equilibriuma |
Free 213Bib | Free 221Fr c | Total Alpha Dosed | Total Beta Dosed |
|---|---|---|---|---|---|
| Blood | 2.71 | -0.12 | -0.25 | 2.29 | 0.05 |
| Heart | 1.07 | 0.00 | 0.00 | 1.04 | 0.02 |
| Lung | 0.43 | -0.01 | -0.01 | 0.39 | 0.01 |
| Liver | 8.25 | -0.01 | -0.05 | 8.00 | 0.19 |
| Spleen | 7.42 | -0.01 | 0.00 | 7.24 | 0.17 |
| Kidney | 1.20 | 0.54 | 0.23 | 1.91 | 0.07 |
| Stomach | 0.17 | 0.00 | 0.04 | 0.20 | 0.00 |
| Intestine | 0.52 | 0.00 | 0.01 | 0.52 | 0.01 |
| Femur | 0.56 | 0.00 | 0.00 | 0.54 | 0.01 |
| Tumor | 10.49 | 0.00 | 0.00 | 10.24 | 0.25 |
Includes doses from alphas and electrons of 225Ac and daughters in equilibrium.
Includes doses from alphas and electrons of 213Bi daughters, 213Po, 209Ti and 209Pb.
Includes alphas from 221Fr daughter, 217At.
Total doses are presented as doses from alpha-particle and beta-particle emissions, respectively.
Table 2.
Absorbed doses to lung metastases or single tumor cells from 90Y-, 213Bi-, 225Ac-7.16.4
| Lung metastases (Gy) a | Single tumor cells (Gy) b | |
|---|---|---|
| 90Y-7.16.4 | 2.4 | 0.6 |
| 213Bi-7.16.4 | 2.0 | 2.6 |
| 225Ac-7.16.4 | 9.6 | 25.3 |
|
225Ac-7.16.4, 0% internalized |
-- | 12.0 |
|
225Ac-7.16.4, 100% internalized |
-- | 38.6 |
Electron doses are calculated with a sphere of 300 μm in diameter. Alpha doses are assumed to be deposited within the sphere. Cross-fire doses are not included.
Absorbed dose to single cells are calculated with 59.2% internalization fraction and specific activities of 0.1, 10, 10μCi/μg for 225Ac, 213Bi, 90Y, respectively.
DISCUSSION
We demonstrated that alpha-particle-emitter 225Ac-labeled anti-rat HER-2/neu mAb is effective in eliminating breast cancer lung metastases, leading to long-term animal survival but also renal toxicity most likely caused by the free daughters of 225Ac (27).
The improved efficacy of alpha emitter 225Ac over 213Bi and 90Y can partially be attributed to the higher radiation doses that micrometastases receive from 225Ac. 225Ac, emits four alpha-particles along its decay chain, deposits a total energy of 4.50×10-12 J/Bq-s, 3.2 and 30.0 times higher than that by 213Bi and 90Y. Furthermore, the majority of alpha radiation will be absorbed locally, while most of the beta-particle-energy from 90Y will be deposited outside of micrometastases. An important finding from the biodistribution studies is that only a small fraction of 221Fr and 213Bi was released from major organs/tumors, suggesting that these daughters are either retained in the cells due to antibody internalization or there is simply not enough time for a substantial fraction of the daughters to diffuse out of the organs/tumors. The long half-life of 225Ac also helps to deliver more doses to the lung metastases than 213Bi. The biodistribution studies showed that antibody localization to s.c. tumors peaked at least 24 hrs after injection. For lung metastases, antibody localization is much faster, reaching peak at about 6 hrs after injection. Nevertheless, for 213Bi, a substantial fraction (>90%) of the decays will have already occurred, greatly reducing the number of alpha-particles that can be delivered.
The higher potency and longer half-life of 225Ac overcame the ~35% lower immunoreactivity of 225Ac- compared to 213Bi- 7.16.4. The immunoreactivity of 225Ac-labeled mAbs seems to vary significantly among different antibodies (20), independent of the labeling procedure. When the incubation time in the second step of the reaction was reduced to 30 mins or 15 mins, the immunoreactivity of 225Ac-7.16.4 did not improve in the biodistribution study, with a 16.2%ID/g and 15.4%ID/g tumor localization at 72 hr after injection (3 mice/group). Furthermore, stability assay suggested slow but significant release of 225Ac from the antibody, which could also contribute to the lower tumor localization. More studies are needed to understand and improve the immunoreactivity and stability of 225Ac-7.16.4.
The main concern of radioimmunotherapy with alpha emitter 225Ac-7.16.4 is the long-term renal toxicity caused by free 221Fr and 213Bi (27). Furosemide or chlorothiazide and competitive metal blockade were found to be effective in reducing renal uptake of the free daughters (22). The extent to which such interventions are needed will likely depend upon the residence time of the antibody in circulation and the internalization properties of the antibody-antigen complex. In a recent update of results from a clinical trial of 225Ac-HuM195 antibody in leukemia patients, no acute toxicity was seen and no evidence of radiation nephritis has been seen, with follow-up to 10 months in patients receiving up to 4 μCi/kg (28). Liposomal, encapsulation of 225Ac (29) has also been proposed to trap free 225Ac daughters. Extracorporeal metal chelation of blood 221Fr, 213Bi could also be used to reduce renal toxicity (30). Interestingly, 225Ac labeled anti-thrombomodulin mAb (201B, targeting lung vasculature) caused lethal toxicity to the lungs while it was able to inhibit metastatic growth (31). No evident damage to lung tissue was observed when rat HER-2/neu was targeted in this study, demonstrating the importance of selecting tumor antigen for targeting to reduce toxicity.
The reduced efficacy when treating micrometastases at 18 day after cancer cell inoculation was expected and may be partially explained by increased tumor burden and increased tumor heterogeneity leading to non-uniform dose distributions (32). Monte Carlo modeling has shown that, when treating a micrometastases containing 1,000 cancer cells, the tumor control probability could drop from 93% to 0% when a 50% variance of antigen expression was introduced to a uniform expression (33). A recent clinical trial has shown that consolidation of chemotherapy regimen with 213Bi-HuM195 in acute myeloid leukemia patients is highly effective (34). The efficacy data from day 18 and day 3 metastatic models suggest that 225Ac based radioimmunotherapy should be tested ideally in patients undergoing remission after first line chemotherapy treatments.
In conclusion, we have demonstrated that alpha-particle-emitter 225Ac-labeled mAb is very effective, better than the alpha emitter, 213Bi, and the beta emitter, 90Y, in prolonging the survival of neu-N transgenic mice bearing lung metastases. Long-term renal toxicity was observed and approaches designed to mitigate such toxicity (22) may need to be implemented in clinical studies.
ACKNOWLEDGMENTS
Grant support: National Cancer Institute grant R01 CA 113797 and Department of Defense Fellowship BC044176 (to H.S.).
Reference List
- 1.McDevitt MR, Sgouros G, Finn RD, Humm JL, Jurcic JG, Larson SM, et al. Radioimmunotherapy with alpha-emitting nuclides. Eur J Nucl Med. 1998;25:1341–51. doi: 10.1007/s002590050306. [DOI] [PubMed] [Google Scholar]
- 2.Nikula TK, McDevitt MR, Finn RD, Wu C, Kozak RW, Garmestani K, et al. Alpha-emitting bismuth cyclohexylbenzyl DTPA constructs of recombinant humanized anti-CD33 antibodies: pharmacokinetics, bioactivity, toxicity and chemistry. J Nucl Med. 1999;40:166–76. [PubMed] [Google Scholar]
- 3.McDevitt MR, Barendswaard E, Ma D, Lai L, Curcio MJ, Sgouros G, et al. An alpha-particle emitting bismuth-213 labeled antibody (J591) to the external domain of prostate specific membrane antigen. Cancer Res. 2000;60:6095–100. [PubMed] [Google Scholar]
- 4.Song EY, Qu CF, Rizvi SMA, Raja C, Beretov J, Morgenstern A, et al. Bismuth-213 radioimmunotherapy with C595 anti-MUC1 monoclonal antibody in an ovarian cancer ascites model. Cancer Biology & Therapy. 2008;7:76–80. doi: 10.4161/cbt.7.1.5132. [DOI] [PubMed] [Google Scholar]
- 5.Milenic DE, Garmestani K, Brady ED, Albert PS, Ma DS, Abdulla A, et al. Targeting of HER2 antigen for the treatment of disseminated peritoneal disease. Clinical Cancer Research. 2004;10:7834–41. doi: 10.1158/1078-0432.CCR-04-1226. [DOI] [PubMed] [Google Scholar]
- 6.Song H, Shahverdi K, Huso DL, Esaias C, Fox J, Liedy A, et al. Bi-213 (alpha-emitter)-antibody targeting of breast cancer metastases in the neu-N transgenic mouse model. Cancer Research. 2008;68:3873–80. doi: 10.1158/0008-5472.CAN-07-6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jurcic JG, Caron PC, Nikula TK, Papadopoulos EB, Finn RD, Gansow OA, et al. Radiolabeled anti-CD33 monoclonal antibody M195 for myeloid leukemias. Cancer Res. 1995;55:5908s–10s. [PubMed] [Google Scholar]
- 8.Jurcic JG, Larson SM, Sgouros G, McDevitt MR, Finn RD, Divgi CR, et al. Targeted at particle immunotherapy for myeloid leukemia. Blood. 2002;100:1233–9. [PubMed] [Google Scholar]
- 9.McDevitt MR, Ma D, Lai LT, Simon J, Borchardt P, Frank RK, et al. Tumor therapy with targeted atomic nanogenerators. Science. 2001;294:1537–40. doi: 10.1126/science.1064126. [DOI] [PubMed] [Google Scholar]
- 10.Borchardt PE, Yuan RR, Miederer M, McDevitt MR, Scheinberg DA. Targeted actinium-225 in vivo generators for therapy of ovarian cancer. Cancer Research. 2003;63:5084–90. [PubMed] [Google Scholar]
- 11.Singh JJ, Henke E, Seshan SV, Kappel BJ, Chattopadhyay D, May C, et al. Selective alpha-particle mediated depletion of tumor vasculature with vascular normalization. PLoS One. 2007;2:e267. doi: 10.1371/journal.pone.0000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milenic DE, Garmestani K, Brady ED, Albert PS, Abdulla A, Flynn J, et al. Potentiation of high-LET radiation by gemcitabine: Targeting HER2 with trastuzumab to treat disseminated peritoneal disease. Clinical Cancer Research. 2007;13:1926–35. doi: 10.1158/1078-0432.CCR-06-2300. [DOI] [PubMed] [Google Scholar]
- 13.Dahle J, Borrebaek J, Jonasdottir TJ, Hjelmerud AK, Melhus KB, Bruland OS, et al. Targeted cancer therapy with a novel low-dose rate alpha-emitting radioimmunoconjugate. Blood. 2007;110:2049–56. doi: 10.1182/blood-2007-01-066803. [DOI] [PubMed] [Google Scholar]
- 14.Behr TM, Behe M, Stabin MG, Wehrmann E, Apostolidis C, Molinet R, et al. High-linear energy transfer (LET) alpha versus low-LET beta emitters in radioimmunotherapy of solid tumors: therapeutic efficacy and dose-limiting toxicity of 213Bi- versus 90Y-labeled CO17-1A Fab’ fragments in a human colonic cancer model. Cancer Res. 1999;59:2635–43. [PubMed] [Google Scholar]
- 15.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, Mcguire WL. Human-Breast Cancer - Correlation of Relapse and Survival with Amplification of the Her-2 Neu Oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 16.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 17.Song H, Shahverdi K, Huso DL, Wang Y, Fox JJ, Hobbs RF, et al. An Immunotolerant HER-2/neu Transgenic Mouse Model of Metastatic Breast Cancer. Clinical Cancer Research. 2008;14:6116–24. doi: 10.1158/1078-0432.CCR-07-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reilly RT, Gottlieb MBC, Ercolini AM, Machiels JPH, Kane CE, Okoye FI, et al. HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res. 2000;60:3569–76. [PubMed] [Google Scholar]
- 19.Brechbiel MW, Pippin CG, Mcmurry TJ, Milenic D, Roselli M, Colcher D, et al. An Effective Chelating Agent for Labeling of Monoclonal-Antibody with Bi-212 for Alpha-Particle Mediated Radioimmunotherapy. J Chem Soc Chem Commun. 1991:1169–70. [Google Scholar]
- 20.McDevitt MR, Ma D, Simon J, Frank RK, Scheinberg DA. Design and synthesis of 225Ac radioimmunopharmaceuticals. Applied Radiation and Isotopes. 2002;57:841–7. doi: 10.1016/s0969-8043(02)00167-7. [DOI] [PubMed] [Google Scholar]
- 21.Carrasquillo JA, White JD, Paik CH, Raubitschek A, Le N, Rotman M, et al. Similarities and differences in In-111- and Y-90-labeled 1B4M-DTPA antiTac monoclonal antibody distribution. J Nucl Med. 1999;40:268–76. [PubMed] [Google Scholar]
- 22.Jaggi JS, Kappel BJ, McDevitt MR, Sgouros G, Flombaum CD, Cabassa C, et al. Efforts to control the errant products of a targeted in vivo generator. Cancer Research. 2005;65:4888–95. doi: 10.1158/0008-5472.CAN-04-3096. [DOI] [PubMed] [Google Scholar]
- 23.Song H, Du Y, Sgouros G, Prideaux A, Frey E, Wahl RL. Therapeutic potential of Y-90- and I-131-labeled anti-CD20 monoclonal antibody in treating non-Hodgkin’s lymphoma with pulmonary involvement: A Monte Carlo-based dosimetric analysis. Journal of Nuclear Medicine. 2007;48:150–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Hamacher KA, Den RB, Den EI, Sgouros G. Cellular dose conversion factors for alpha-particle-emitting radionuclides of interest in radionuclide therapy. J Nucl Med. 2001;42:1216–21. [PubMed] [Google Scholar]
- 25.Goddu SM, Howell RL, Bouchet LG, Bolch WE, Rao DV. MIRD Cellular S Values. Society of Nuclear Medicine; Reston VA: 1997. [Google Scholar]
- 26.Stein R, Govindan SV, Chen S, Reed L, Richel H, Griffiths GL, et al. Radioimmunotherapy of a human lung cancer xenograft with monoclonal antibody RS7: Evaluation of Lu-177 and comparison of its efficacy with that of Y-90 and residualizing I-131. Journal of Nuclear Medicine. 2001;42:967–74. [PubMed] [Google Scholar]
- 27.Miederer M, McDevitt MR, Sgouros G, Kramer K, Cheung NK, Scheinberg DA. Pharmacokinetics, dosimetry, and toxicity of the targetable atomic generator, 225Ac-HuM195, in nonhuman primates. J Nucl Med. 2004;45:129–37. [PubMed] [Google Scholar]
- 28.Scheinberg DA, McDevitt MR, Larson SM, Jurcic JG, Villa C, EScorcia F. Alpha-Particle Immunotherapy with Bi-213 and Ac-225-Antibodies; 6th Symposium on Alpha-Emitting Radionuclides in Therapy; Toronto, Canada. June 13-14, 2009. [Google Scholar]
- 29.Sofou S, Thomas JL, Lin Hy, McDevitt MR, Scheinberg DA, Sgouros G. Engineered Liposomes for Potential {alpha}-Particle Therapy of Metastatic Cancer. J Nucl Med. 2004;45:253–60. [PubMed] [Google Scholar]
- 30.Wang Z, Garkavij M, Tennvall JG, Ohlsson T, Strand SE, Sjogren HO. Application of extracorporeal immunoadsorption to reduce circulating blood radioactivity after intraperitoneal administration of indium-111- HMFG1-biotin. Cancer. 2002;94:1287–92. doi: 10.1002/cncr.10298. [DOI] [PubMed] [Google Scholar]
- 31.Kennel SJ, Chappell LL, Dadachova K, Brechbiel MW, Lankford TK, Davis IA, et al. Evaluation of 225Ac for vascular targeted radioimmunotherapy of lung tumors. Cancer Biother Radiopharm. 2000;15:235–44. doi: 10.1089/108497800414329. [DOI] [PubMed] [Google Scholar]
- 32.Braun S, Hepp F, Sommer HL, Pantel K. Tumor-antigen heterogeneity of disseminated breast cancer cells: Implications for immunotherapy of minimal residual disease. International Journal of Cancer. 1999;84:1–5. doi: 10.1002/(sici)1097-0215(19990219)84:1<1::aid-ijc1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 33.Sgouros G, Song H. Cancer stem cell targeting using the alpha-particle emitter, Bi-213: Mathematical modeling and feasibility analysis. Cancer Biotherapy and Radiopharmaceuticals. 2008;23:74–81. doi: 10.1089/cbr.2007.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenblat T, McDevitt MR, Mulford DA, Pandit-Taskar N, Weiss MA, Heaney ML, et al. Sequential Cytarabine and Alpha-Particle Immunotherapy with Bismuth-213 (Bi-213)-Labeled-HuM195 (Lintuzumab) for Acute Myeloid Leukemia (AML) Blood. 2008;112:1025. doi: 10.1158/1078-0432.CCR-10-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]