Abstract
Toxicity and liver tumor promotion of cyanotoxins microcystins have been extensively studied. However, recent studies document that other metabolites present in the complex cyanobacterial water blooms may also have adverse health effects. In this study we used rat liver epithelial stem-like cells (WB-F344) to examine the effects of cyanobacterial extracts on two established markers of tumor promotion, inhibition of gap-junctional intercellular communication (GJIC) and activation of mitogen-activated protein kinases (MAPKs) – ERK1/2. Extracts of cyanobacteria (laboratory cultures of Microcystis aeruginosa and Aphanizomenon flos-aquae and water blooms dominated by these species) inhibited GJIC and activated MAPKs in a dose-dependent manner (effective concentrations ranging 0.5 - 5 mg d.w./mL). Effects were independent of the microcystin content and the strongest responses were elicited by the extracts of Aphanizomenon sp. Neither pure microcystin-LR nor cylindrospermopsin inhibited GJIC or activated MAPKs. Modulations of GJIC and MAPKs appeared to be specific to cyanobacterial extracts since extracts from green alga Chlamydomonas reinhardtii, heterotrophic bacterium Klebsiella terrigena, and isolated bacterial lipopolysaccharides had no comparable effects. Our study provides the first evidence on the existence of unknown cyanobacterial toxic metabolites that affect in vitro biomarkers of tumor promotion, i.e. inhibition of GJIC and activation of MAPKs.
Keywords: liver tumor promotion, gap-junctional intercellular communication, mitogen-activated protein kinases, microcystin, cylindrospermopsin, cyanobacteria
1. Introduction
Cyanobacteria are an important component of aquatic as well as terrestrial ecosystems and they are known to produce numerous bioactive compounds. Various human activities resulted in the eutrophication of waters with occurrence of massive water blooms dominated by cyanobacteria. Consequent production of toxic metabolites (cyanotoxins) has become a health and ecological problem worldwide (Carmichael, 2001). The increased prevalence of hepatocellular or colorectal cancer associated with consumption of drinking water contaminated with cyanobacterial blooms as reported in China (Yu, 1995; Zhou et al., 2002) is considered to be one of the most severe chronic consequences of cyanobacterial bloom development and cyanotoxin production. One major class of cyanotoxins are the cyclic heptapeptides, microcystins, which are known to induce hepatotoxic and liver tumor-promoting activities that has attracted broad scientific and regulatory attention (Chorus and Bartram, 1999; Codd et al., 2005). Although the toxicity and ecotoxicity of microcystins have been investigated in detail, several recent studies indicate that cyanobacterial water blooms also contain many unidentified components that may evoke toxic effects that could be more pronounced than those of microcystins or other chemically characterized cyanotoxins (Pietsch et al., 2001; Buryskova et al., 2006).
Cyanotoxins act via multiple mechanisms resulting in various adverse in vivo effects. For example, microcystins and nodularins are known inhibitors of regulatory protein phosphatases 1 and 2A, a mechanism considered the most important for their in vivo toxicities, such as acute liver necroses or chronic liver tumor promotions (Nishiwaki-Matsushima et al., 1992; Ohta et al., 1994). Although phosphatases have been implicated in the cancer process and microcystin-LR has been recently classified by the International Agency for Research on Cancer as “possibly carcinogenic to humans” (group 2B) (Grosse et al., 2006), other mechanisms also play important roles in cancer. In particular, the downregulation of gap-junctional intercellular communication (GJIC) and the activation of mitogen-activated protein kinases (MAPKs), specifically extracellular receptor kinases 1 and 2 (ERK 1 and ERK 2), have been strongly linked to the tumor promoting phase of cancer (Trosko and Ruch, 2002; Trosko and Upham, 2005). GJIC is an important mechanism controlling homeostasis in normal tissue, and its malfunction promotes a growth of transformed cells (King, 2004). Most cancer cells are known to be defective in GJIC, chemical tumor promoters and oncogenes inhibit GJIC, while tumor suppressor genes and chemopreventive compounds enhance GJIC (Trosko and Ruch, 2002; Trosko and Upham, 2005). MAPK pathways are the major intracellular signaling mechanisms by which a cell activates transcription factors involved in the cell proliferation (Denhardt, 1996; Wright et al., 1999), and a subclass of MAPKs, extracellular receptor kinases (ERKs), has been extensively characterized (Denhardt, 1996). Both parameters, i.e. downregulation of GJIC and activation of MAPKs by chemicals, were recognized as important in vitro biomarkers of in vivo tumor promoting potencies of carcinogenic chemicals (Rosenkrantz et al., 2000).
In this study, we focused on potencies of toxic cyanobacteria to modulate GJIC (using a scrape-loading dye transfer assay) and to activate ERK1/2 (determination of phosphorylated ERK1/2 by Western blotting) in rat liver epithelial WB-F344 cells, which is a normal diploid, non-tumorigenic and pluripotent (stem-like) cell line (Tsao et al., 1984). This cell line has been thoroughly characterized for its expressed gap junction genes, and extensively used for studying the effects of tumor promoters, growth factors, tumor suppressor genes and oncogenes on GJIC (Trosko and Ruch, 2002). To discriminate between cyanobacteria-specific and non-specific effects; we assessed different cyanobacterial metabolites and extracts including pure microcystin-LR, cylindrospermopsin, extracts from laboratory cultures of the most prevalent cyanobacteria (Microcystis aeruginosa and Aphanizomenon flos-aquae), extracts from a series of complex natural water blooms (dominated by Microcystis aeruginosa, Aphanizomenon flos-aquae, Woronichinia naegeliana or Planktothrix aghardii), and we compared these results to those obtained from the extracts of a heterotrophic bacterium Klebsiella terrigena or a eukaryotic green alga Chlamydomonas reinhardtii, and a lipopolysaccharide (LPS) isolated from bacterium Salmonella typhimurium. In summary, the data indicated the presence of yet unknown non-microcystin tumor promoting metabolites specific to cyanobacteria that modulated GJIC and MAPKs.
2. Material and methods
Chemicals
Microcystin-LR and cylindrospermopsin were obtained from Alexis Biochemicals (Läufelfingen, Switzerland), lipopolysaccharides (LPS) from Salmonella enterica serotype typhimurium and epidermal growth factor (EGF) were from Sigma-Aldrich (St. Louis, MO).
Microorganisms
Laboratory cultures of cyanobacteria Microcystis aeruginosa PCC 7806 and Aphanizomenon flos-aquae CCALA008 and green alga Chlamydomonas reinhardtii UTEX 2246 were obtained from the Culture Collection of Algal Laboratory (Institute of Botany, Czech Academy of Sciences, Třeboň, Czech Republic). Organisms were grown at 22°C under continuous light (cool white fluorescent tubes, 3000 lux) in cultivation medium with following composition: mixture of Zehnder medium (Schlosser, 1994), Bristol (modified Bold) medium (Stein, 1975) and distilled water (1:1:2, v/v). Cultures were aerated with ambient air sterilized by 0.22 μm filter. Bacterium Klebsiella terrigena CCM 3568 were obtained from the Czech Collection of Microorganisms (Masaryk University, Brno, Czech Republic), cultured in beef-peptone B1 medium at 30°C for 3-4 days under sterile conditions. Biomasses of laboratory cultures of cyanobacteria, bacterium and green alga were harvested by centrifugation at 2500× g for 10 min and then lyophilized. Natural cyanobacterial water blooms were collected with plankton net (20 μm) from reservoirs in the Czech Republic (Table 1) and lyophilized.
Table 1.
Characterization of the studied samples with concentrations of microcystins (MCs) and effects on gap junctional intercellular communication after 15 minutes (GJIC).
| Samples (cultures / locality & date) |
Description / Dominant species | Content of microcystins (MCs μg/g d.w.) |
Inhibition of GJIC (IC50; mg d.w./mL) |
|---|---|---|---|
|
Microcystis aeruginosa PCC 7806 |
laboratory culture - cyanobacteria | 1946 μg/g d.w. (MC-LR 1240, unidentified MC 706) | 3.5 (2.2 - 4.8)a |
|
Aphanizomenon flos-aquae CCALA008 |
laboratory culture - cyanobacteria | NDb | 3.3 (2.1 - 4.5) |
|
Chlamydomonas reinhardtii UTEX 2246 |
laboratory culture - green alga | ND | > 12.5 |
|
Klebsiella terrigena CCM 3568 |
laboratory culture - heterotrophic bacteria | ND | > 12.5 |
| Loudilka (04/10/2004) |
water bloom - Microcystis aeruginosa (98%) | 3662 μg/g d.w. (MC-LR 1361, MC-YR 289, MC-RR 2012) | 4.4 (2.7 - 5.1) |
| Dolní Heřmanice (24/8/2004) |
water bloom - Aphanizomenon flos-aquae (95%) | ND | 0.8 (0.4 - 1.2) |
| Dubice (08/09/2004) |
water bloom - Planktothrix agardhii (95%) | 2602 μg/g d.w. (unidentified MCs) | 2.1 (1.7 - 2.6) |
| Stanovice (13/09/2004) |
water bloom - Woronichinia naegaliana (75%), Microcystis sp.(25%) | ND | 7.8 (6.7- 8.5) |
95% confidence interval for IC50 in parenthesses
ND - not detected (reliable detection limit of the method was 1 μg/g d.w.)
Extract preparations
Dry microorganism biomasses were homogenized in methanol (equivalents of 200 mg d.w. per 1 mL), sonicated (ultrasonic probe Bandelin Sonopuls HD2070, Bandelin Electronics, Berlin, Germany), decanted by centrifugation (14000× g, 10 min) and extracts evaporated under vacuum. Desired concentrations were prepared prior to testing by diluting in 50% methanol. Concentrations were expressed as dry weight of extracted biomass per volume (mg d.w./mL).
Analyses of microcystins
Microcystin content in samples was analyzed according to established methods (Babica et al., 2006), using HPLC Agilent 1100 Series coupled with photodiode array detector (Agilent Technologies, Waldbronn, Germany). Microcystins were separated on a Supelcosil ABZ+ Plus column (150 × 4.6 mm, 5 μm, Supelco, Bellefonte, PA) at 30°C. Mobile phases were water and acetonitrile, both containing 0.1% (v/v) trifluoroacetic acid. Chromatographic separation was achieved at a flow rate 1 mL/min using a linear gradient starting at 20% aqueous acetonitrile increasing to 59% over the next 30 min. UV spectra were recorded from 200 to 300 nm and chromatograms were evaluated at 238 nm. Microcystins were identified by comparison of UV spectra and retention time with standards of microcystin-LR, -LF, -LW, -RR (Alexis Biochemicals) or microcystin-YR (Sigma-Aldrich). Microcystins were quantified using microcystin-LR as standard. Reliable detection limit of the method for the individual microcystin variants was 1 μg/g d.w.
Cell Culture
The WB-F344 rat liver epithelial cell line was obtained from Drs. J. W. Grisham and M. S. Tsao of the University of North Carolina (Chapel Hill, NC) (Tsao et al., 1984), and cultured in D-medium (Formula No. 78-5470EF, GIBCO Laboratories, Grand Island, NY) on 35 mm tissue culture plates (Corning Inc., Corning, NY), supplemented with 5% fetal bovine serum (GIBCO Laboratories), and incubated at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air. Bioassays were conducted with confluent cultures obtained after two or three days of growth.
Bioassay of GJIC
The scrape loading-dye transfer (SL-DT) technique was adapted after the method of El-Fouly et al. (1987). The test samples were added directly to the cell culture medium from concentrated stock solutions. Appropriate solvent controls (maximum 3% methanol, v/v, in the case of cyanobacterial extracts) were run in each experiment and did not induce responses significantly different from non-treated control. After the exposure (15, 30 and 120 min), cells were washed with phosphate buffered saline (PBS) followed by the addition of 1 mg/mL of Lucifer-Yellow (Sigma-Aldrich) dissolved in PBS. The dye was introduced into the cells with three scrapes through the monolayer of confluent cells using a surgical steel scalpel blade. The transfer of dye through gap junction channels was allowed for three minutes, followed by a thorough rinse of cells with PBS to remove extracellular dye, and then fixation with a 4% formaldehyde solution in PBS. Migration of the dye in the cells was observed at 200-times magnification using a Nikon epifluorescence microscope equipped with a Nikon Cool Snap EZ CCD camera and the images were acquired by Nikon NIS-Elements F2.2 imaging system. The fluorescence area of the dye migration from the scrape line was quantified using ‘Gel Expert’ image analysis program (NucleoTech Corp, San Mateo, CA). The results were expressed as fraction of the solvent control. To evaluate time-dependent effects and recovery (known to vary among tumor promoters acting via different mechanisms), the cells were exposed for 30 min, washed with PBS and samples were replaced with the fresh serum-free culture medium for another 90 min. Each SL-DT experiment was performed three times independently.
Western Blot Analysis
Confluent cells were incubated in serum-free medium for 4-5 h before an experiment and then exposed to the test samples for 30, 60 and 120 minutes under the same conditions as those used in the SL-DT assay. Cells exposed to EGF (5 ng/mL) for 30 min were used as a positive control for ERK1/2 activation. Appropriate solvent controls (maximum 1.25% methanol, v/v, in the case of cyanobacterial extracts) were run in each experiment and did not induce responses significantly different from non-treated control. The proteins from the cells were extracted with 20% SDS solution containing 1 mM phenylmethylsulfonyl fluoride, 100 μM Na3VO4, 100 nM aprotinin, 1.0 μM leupeptin and 1.0 μM antipain. The proteins (15 μg, determined by the DC protein kit Bio-Rad Laboratories Inc., Hercules, CA) were separated by 12.5% SDS-PAGE (Bio-Rad Laboratories Inc.) and then electrophoretically transferred from the gel to PVDF membranes (Millipore Corp., Billerica, MA). To visualize activated, i.e phosphorylated ERK1/2, we used phospho-specific polyclonal antibodies directed to ERK 1 phosphorylated at Thr 202/Tyr204, and ERK 2 directed to phosphorylated Thr185/Tyr187 (New England Biolabs, Ipswich, MA). Levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a house keeping protein were determined with mouse anti-GAPDH antibody (Chemicon; currently Millipore). Secondary anti-rabbit or anti-mouse IgG conjugated with horse radish peroxidase, ECL detection kit and HyperFilm™-MP (Amersham Life Science) were used for detection of signals for ERK1/2 and GAPDH. Each Western blotting experiment was repeated twice independently.
Data analysis
The mean values ± standard deviations from three independent experiments were evaluated by one-way ANOVA followed by Dunnett's post hoc test. P values less than 0.05 were considered statistically significant. The IC50 values and 95% confidence intervals were calculated using non-linear regression. Calculations were performed in Statistica 8.0 (StatSoft, Tulsa, OK, USA).
3. Results
Potencies of extracts from cyanobacteria, a bacterium and a green alga to inhibit GJIC in WB-F344 cells are shown in Fig. 1 and Table 1. After short 15 or 30 min exposures, extracts of cyanobacteria (both laboratory cultures and water blooms) significantly inhibited GJIC in a dose dependent manner with IC50 values ranging from 0.8 to 7.8 mg d.w./mL (Fig. 1 and Table 1). The effects of cyanobacterial extracts on GJIC decreased in the following order: A. flos-aquae (bloom) > P. aghardii (bloom) > A. flos-aquae (culture) ∼ M. aeruginosa (culture) > M. aeruginosa (bloom) > W. naegeliana (bloom). To the contrary, weak or no inhibitory effects on GJIC were observed in cells exposed to extracts of the heterotrophic bacterium K. terrigena or the green alga C. reinhardtii (Fig. 1A and Table 1). The highest concentration of microcystins was found in the water bloom of M. aeruginosa, followed by the bloom sample of P. aghardii and the laboratory culture of M. aeruginosa, but no microcystins were detected in the samples extracted from A. flos-aquae, which were extracts that induced strong inhibition of GJIC. These results indicate that inhibition of GJIC was independent of microcystin content (Table 1 and Fig. 2). In addition, purified LPS, microcystin-LR and cylindrospermopsin did not inhibit GJIC in WB-F344 cells (Fig. 2).
Fig. 1.
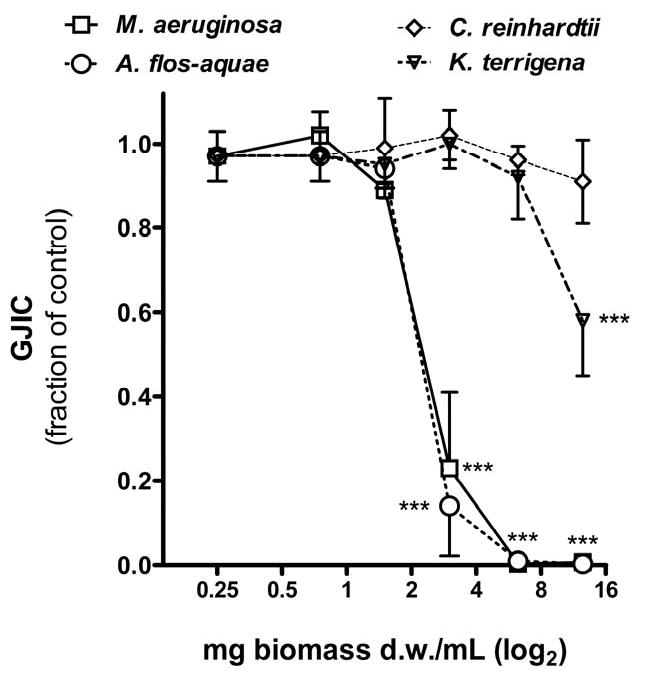
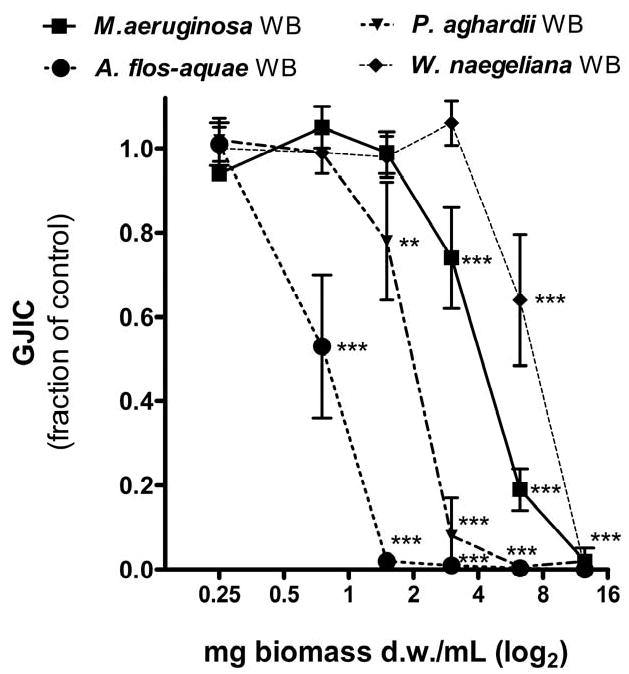
Effects of tested samples (0.25-12.5 mg d.w./mL) on gap junctional intercellular communication (GJIC) in WB-F344 cells after 30 min exposure. A - concentration-response effects of laboratory cultures of cyanobacteria, bacterium and green alga; B - effects of water bloom (WB) samples dominated by various cyanobacterial species. Data are means ± standard deviations of three independent experiments. Significant differences from the solvent control are indicated at 0.001≤ P <0.01 (**) or P < 0.001 (***) as determined by one-way ANOVA followed by Dunnett's post hoc test.
Fig. 2.
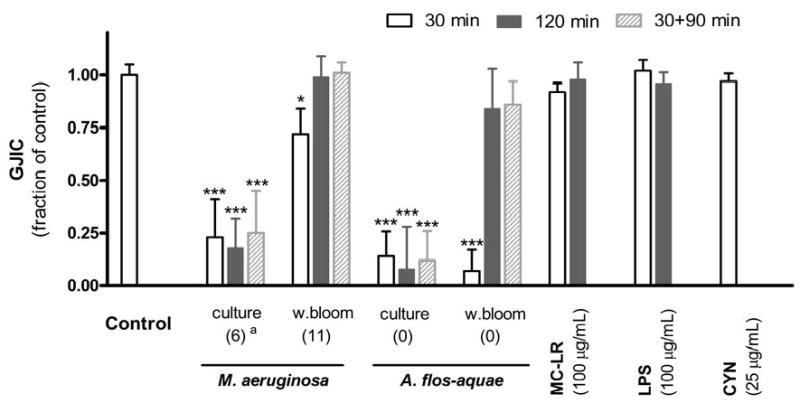
Modulation of GJIC by cyanobacterial samples (3 mg d.w./mL of laboratory cultures and water blooms of M.aeruginosa and A. flos-aquae) after 30 or 120 min continual exposure or after 30 min incubation with the sample followed by 90 min incubation in fresh serum-free medium (30+90 min). For comparison, presented are also effects of 100 μg/mL of microcystin-LR (MC-LR), Salmonella lipopolysaccharide (LPS) and 25 μg/mL of cylindrospermopsin (CYN). Data are means ± standard deviations of three independent experiments. Significant differences from the solvent control are indicated at 0.01 ≤ P < 0.05 (*) or P < 0.001 (***) as determined by one-way ANOVA followed by Dunnett's post hoc test.a Values in parentheses at cyanobacterial samples indicate actual concentrations of microcystins in tested samples (μg/mL).
Time-responses of GJIC varied among cyanobacterial samples (Fig. 2). Unlike the extracts from the blooms where GJIC was inhibited for the first 30 min followed by a complete recovery from inhibition at 120 min, the extracts from laboratory cultures of both M. aeruginosa and A. flos-aquae continued inhibiting GJIC throughout the 120 min exposure period. An additional experiment was done in which the cells were exposed to the extracts for 30 min and then rinsed with PBS and incubated for an additional 90 min in fresh serum-free medium containing no extracts (Fig. 2). Again, inhibition of GJIC was maintained for the total 120 min time period in cells that were exposed to the extracts from the cyanobacterial cultures but not the blooms.
Extracts from both M. aeruginosa and A. flos-aquae cyanobacteria (cultures and water blooms) induced phosphorylation of ERK1/2 (Fig. 3A). The most pronounced effects were observed at both A. flos-aquae samples, where phosphorylation and activation of ERK1/2 was sustained during the entire 30 to 120 min exposure periods, whereas the activation induced by M. aeruginosa extracts was weaker and diminished after incubation for 120 min (Fig. 3A). Neither extracts from bacterium K. terrigena, alga C. reinhardtii (Fig. 3B), isolated bacterial LPS, microcystin-LR nor cylindrospermopsin induced significant effects on the activation of ERK1/2 (Fig. 3C).
Fig. 3.
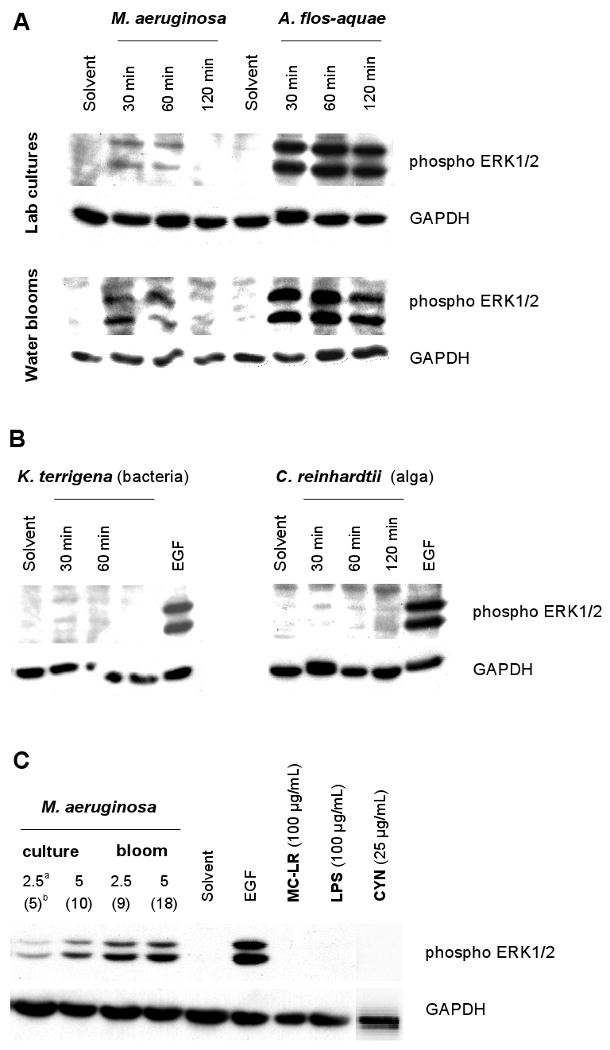
Activation of a subclass of mitogen-activated protein kinasese (MAPKs) - extracellular receptor kinases 1 and 2 (ERK1/2) by tested samples as determined by Western blotting (glyceraldehyde 3-phosphate dehydrogenase, GAPDH, was assayed as a house keeping protein). EGF (5 ng/mL, 30 min) was used as a positive control.
A - Time-dependent effects of extracts from water blooms and laboratory cultures of cyanobacteria M. aeruginosa (2.5 mg d.w./mL) and A. flos-aquae (0.75 mg d.w./mL).
B - Effects of extracts of bacterium K. terrigena and alga C. reinhardtii (2.5 mg d.w./mL both)
C - No effects (30 min exposures) of microcystin-LR (MC-LR), Salmonella lipopolysacharide (LPS) and cylindrospermopsin (CYN) compared with the effects of M. aeruginosa (2.5 and 5 mg d.w./mL).
a Concentration of cyanobacterial extract (mg d.w./mL)
b Values in parentheses indicate actual concentrations of microcystins in tested samples (μg/mL).
4. Discussion
This is the first study documenting the effects of extracts from toxic cyanobacteria on the downregulation of GJIC and activation of ERK1/2, which are two important in vitro biomarkers of tumor promotion. We focused in detail on Microcystis sp., as this is the most often studied and reported taxa dominating cyanobacterial blooms in North America and Europe, including Czech Republic (Codd et al., 2005; Blahova et al., 2007). Microcystis strains are also potent producers of the most studied cyanobacterial toxins - microcystins (Chorus and Bartram, 1999). Previous toxicological research focused mostly on microcystins, which might elevate MAPK activities by inhibiting protein phosphatase 2A (MacKintosh et al., 1990), which is the phosphatase that deactivates MAPKs (Junttila et al., 2008), or via induction of oxidative stress (Dietrich and Hoeger, 2005), which is also known to regulate MAPK activity (McCubrey et al., 2006). For instance, microcystin-LR was reported to activate MAPKs ERK1/2, SAP/JNK and p38 in HEK293 cells transfected with OATP1B3 transporter (Komatsu et al., 2007). Gene expression of JNK and p38 was also upregulated at the mRNA-level in the microcystin-LR–transformed and conditionally immortalized normal human colorectal crypt epithelial cell line NCC (Zhu et al., 2005). However, in our study there were no apparent effects on GJIC and MAPKs related to the microcystin content in cyanobacterial biomass (Table 1, Fig. 2 and 3). Pure microcystin-LR also did not affect GJIC (Fig. 2) or ERK1/2 (Fig. 3C), even in concentration much higher (100 μg/mL) than were the actual concentrations of microcystins in the tested dilutions of cyanobacterial extracts (≤46 μg/mL). One of the explanations could be possible absence of OATP-transporters in WB-F344 cells used in our experiments but this issue remains to be studied in detail.
Our study showed that the effects of cyanobacterial samples on the inhibition of GJIC and activation of ERK1/2 did not correlate with microcystin concentrations, and the strongest responses were induced by extracts of Aphanizomenon sp. samples that did not contain microcystins (Fig. 1-3, Table 1). These results are important considering that a toxicity of the water blooms dominated by Microcystis, Planktothrix or Anabaena sp. has been extensively studied because of their ability to produce microcystins (Codd et al., 2005), and less attention has been paid to the possible hazards of other cyanobacteria that do not produce microcystins such as Aphanizomenon sp. Regulations concerning cyanobacterial toxins has revolved around the determination of microcystin content. Aphanizomenon sp. may be of concern due to the possible production of hepatotoxic and potentially carcinogenic cylindrospermopsin (Rucker et al., 2007; Blahova et al., 2008). Since we did not analyze cylindrospermopsin content in the tested samples, we can only speculate about concentrations of cylindrospermopsin in A. flos-aquae extracts. Concentrations of this cyanotoxin in Aphaninzomenon sp. biomass are known to range from several tens μg/g d.w. in water blooms (Fastner et al., 2007; Blahova et al., 2009) to 5000-6600 μg/g d.w. in isolated cultures (Bacsi et al., 2006; Preussel et al., 2006). If cylindrospermopsin content in our A. flos-aquae samples had been at the higher level reported from the literature (5000 μg/g d.w.), then the cyanobacterial extract at the dose 2.5 mg of d.w./mL (which induced significant effects on both GJIC and ERK1/2) would have contained cylindrospermopsin at concentration 12.5 μg/mL. However, pure cylindrospermopsin in concentration 25 μg/mL affected neither GJIC nor ERK1/2 activities (Fig. 2 and 3C). Moreover, extracts of M. aeruginosa or P. aghardii inhibited GJIC (Fig. 1), and M. aeruginosa also activated ERK1/2 (Fig. 3A, C), although these species are not known to produce cylindrospermopsin (Codd et al., 2005). Thus, the strong effects of cyanobacterial extracts on GJIC and ERK1/2 were probably microcystin- and cylindrospermopsin-independent, which indicates the existence and presence of unidentified toxins that could potentially contribute to tumor promotion.
Apparently, these not-yet-identified substances may induce tumor promotion-related effects in other types of liver cells independent from differentiated hepatocytes, which are the primary targets for known cyanobacterial hepatotoxins such as microcystins and cylindrospermopsin. WB-F344 stem-like cell line used in our study have the characteristics of oval cells, which have been implicated as the target cell type in liver cancer (Sell, 1993; Ruch and Trosko, 1999) and their proliferation is often seen in the early stages of diseased states that lead to cancer not only in rodents but also humans (Roskams et al., 1998; Lowes et al., 1999). Also, the effects of cyanobacterial extracts on GJIC and ERK1/2 in WB-F344 cells are very similar to the effects of many well-recognized nongenotoxic carcinogens and tumor promoters such as phorbolesters (Madhukar et al., 1996), polycyclic aromatic hydrocarbons (Blaha et al., 2002), polychlorinated biphenyls, PCBs (Kang et al., 1996; Machala et al., 2003), organochlorine pesticides (Trosko et al., 1987; Upham et al., 1997; Sai et al., 1998; Masten et al., 2001), perfluorinated fatty acids (Upham et al., 1998a) or organic peroxides (Upham et al., 2007). Thus, our results suggest not only an existence of so far uncharacterized tumor promoting chemicals of cyanobacterial origin and an involvement of GJIC and MAPK activation in cyanobacteria-associated tumor promotion, but also a possible role of adult stem or stem-like cells in cyanobacteria-induced carcinogenesis.
Interestingly, there were significant differences in the time-dependent effects on GJIC between cyanobacterial extracts from water blooms and laboratory cultures (Fig. 2). Rapid inhibition of GJIC followed by almost complete recovery of communication after prolonged exposures was observed from the water bloom extracts of both M. aeruginosa and A. flos-aquae, even in the case of continual incubation with the extracts. These results are similar to those of EGF or tumor promoting phorbol ester, where the inhibition of GJIC has a transient character (Madhukar et al., 1996; Rivedal and Opsahl, 2001). On the contrary, the inhibition of GJIC by extracts from laboratory cultures persisted for at least 2 h. Persistent inhibition of GJIC was reported from experiments with PCB 153 (Machala et al., 2003) or perfluorooctanoic acid (Upham et al., 2009), where intercellular communication was not restored even after 24-h or longer incubation in the presence of inhibiting factor. However, inhibition of GJIC by extracts from laboratory cultures continued for 90 min after the cells were transferred to toxin-free medium, although the removal of GJIC inhibitor usually results in recovery of communication. For instance, GJIC is completely restored within periods ranging from 30 min for fluorinated fatty acids (Upham et al., 1998a) to 2 h for polycylic aromatic hydrocarbons (Upham et al., 1998b), pesticides (Masten et al., 2001) or organic peroxides (Upham et al., 2007), and partial but significant recovery of GJIC occurs actually in 60 min or less. Since tested concentrations of extracts (based on the dry weight of biomass) were similar for all samples, it seems that the active compound(s) and/or their concentrations had to be different between laboratory cultures and bloom samples considering that there were significant differences in both dose and time responses, and particularly in the ability to recover from the inhibition of GJIC. Moreover, the effects of cyanopbacterial extracts on GJIC did not always correlate with the effects on ERK1/2. Both extracts from cultures of A. flos-aque and M. aeruginosa inhibited GJIC with similar potencies (Fig. 1A), but A. flos-aquae extract induced more pronounced activation of ERK1/2 (Fig. 3A). Also, M. aeruginosa bloom extract was a weaker inhibitor of GJIC (Fig. 1) but stronger activator of ERK1/2 (Fig. 3A, C) than the extract from M. aeruginosa culture. In the case of A. flos-aquae, both bloom and culture extract elicited similar activation of ERK1/2 (Fig. 3A), but the extract from water bloom inhibited GJIC more significantly than the extract from the culture (Fig. 1). It is therefore possible that the effects on GJIC and ERK1/2 could be elicited by different compounds of cyanobacterial origin or that they could be synergistically/antagonistically modulated by other bioactive chemicals present in cyanobacterial extracts.
To explore the selectivity of the in vitro tumor promoting effects of cyanobacteria, we compared these results to the extracts from a model eukaryotic green alga, C. reinhardtii, and from a heterotrophic gram-negative bacterium, K. terrigena. Cyanobacteria belong among gram-negative bacteria by the composition of the cell wall containing lipopolysaccharides (Papageorgiou et al., 2004; Bernardova et al., 2008). LPS have been reported to activate ERKs in some cell types (Schumann et al., 1996; Watters et al., 2002), but no such effects were observed in our experiments with the rat liver epithelial WB-F344 cells, even when we used LPS isolated from Salmonella typhimurium, a serotype producing LPS known to induce significant biological responses. Both pure LPS and bacterial extracts caused only weak and temporal downregulations of GJIC that were not accompanied by activations of ERK1/2 (Fig. 1-3). Considering also no effects of green algal extracts (Fig. 1A and 3B), our results indicate that observed effects are really specific to metabolites present dominantly in cyanobacterial cells.
In summary, cyanobacteria seem to produce specific metabolites that modulate two important in vitro biomarkers of tumor promotion, i.e. inhibition of GJIC and activation of ERK1/2. Our study demonstrates significant effects especially for the Aphanizomenon extracts and we have confirmed that these effects were independent of microcystins and cylindrospermopsin. Tumor promoting and/or carcinogenic effects of cyanobatcerial blooms therefore should not be simply attributed to known tumor promoters like microcystins or potential human carcinogens like cylindrospermopsin. Further efforts should focus on the identification and chemical characterization of biologically active compounds, determination of the biochemical effectors that are upstream and downstream of ERKs and GJIC, and linking these molecular signaling events with cellular responses such as proliferation or apoptosis.
Acknowledgments
The research is supported by the Czech Science Foundation grant No. GACR 524/08/0496 to Blaha; and by NIEHS grant #R01 ES013268-01A2 to Upham and its content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS; and by the Center of Water Science at Michigan State University.
Footnotes
Conflict of interest statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babica P, Kohoutek J, Blaha L, Adamovsky O, Marsalek B. Evaluation of extraction approaches linked to ELISA and HPLC for analyses of microcystin-LR, -RR and -YR in freshwater sediments with different organic material contents. Analytical and Bioanalytical Chemistry. 2006;285(8):1545–1551. doi: 10.1007/s00216-006-0545-8. [DOI] [PubMed] [Google Scholar]
- Bacsi I, Vasas G, Suranyi G, Hamvas M, Mathe C, Toth E, Grigorszy I, Gaspar A, Toth S, Borbely G. Alteration of cylindrospermopsin production in sulfate- or phosphate-starved cyanobacterium Aphanizomenon ovalisporum. Fems Microbiology Letters. 2006;259(2):303–310. doi: 10.1111/j.1574-6968.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- Bernardova K, Babica P, Marsalek B, Blaha L. Isolation and endotoxin activities of lipopolysaccharides from cyanobacterial cultures and complex water blooms and comparison with effects of heterotrophic bacteria and green alga. Journal of Applied Toxicology. 2008;28(1):72–77. doi: 10.1002/jat.1257. [DOI] [PubMed] [Google Scholar]
- Blaha L, Kapplova P, Vondracek J, Upham BL, Machala M. Inhibition of gap-junctional intercellular communication by environmentally occurring polycyclic aromatic hydrocarbons. Toxicological Sciences. 2002;65(1):43–51. doi: 10.1093/toxsci/65.1.43. [DOI] [PubMed] [Google Scholar]
- Blahova L, Babica P, Marsalkova E, Marsalek B, Blaha L. Concentrations and seasonal trends of extracellular microcystins in freshwaters of the Czech Republic results of the national monitoring program. Clean-Soil Air Water. 2007;35(4):348–354. [Google Scholar]
- Blahova L, Babica P, Adamovsky O, Kohoutek J, Marsalek B, Blaha L. Analyses of cyanobacterial toxins (microcystins, cylindrospermopsin) in the reservoirs of the Czech Republic and evaluation of health risks. Environmental Chemistry Letters. 2008;6(4):223–227. [Google Scholar]
- Blahova L, Oravec M, Marsalek B, Sejnohova L, Simek Z, Blaha L. The first occurrence of the cyanobacterial alkaloid toxin cylindrospermopsin in the Czech Republic as determined by immunochemical and LC/MS methods. Toxicon. 2009;53(5):519–524. doi: 10.1016/j.toxicon.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Buryskova B, Hilscherova K, Babica P, Vrskova D, Marsalek B, Blaha L. Toxicity of complex cyanobacterial samples and their fractions in Xenopus laevis embryos and the role of microcystins. Aquatic Toxicology. 2006;80(4):346–354. doi: 10.1016/j.aquatox.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Carmichael WW. Health effects of toxin-producing cyanobacteria: “The CyanoHABs”. Human and Ecological Risk Assessment. 2001;7(5):1393–1407. [Google Scholar]
- Chorus I, Bartram J, editors. Toxic Cyanobacteria in Water: A guide to their public health consequences, monitoring and management. E&FN Spon; London: 1999. p. 432. [Google Scholar]
- Codd GA, Morrison LF, Metcalf JS. Cyanobacterial toxins: risk management for health protection. Toxicology and Applied Pharmacology. 2005;203(3):264–272. doi: 10.1016/j.taap.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Denhardt DT. Signal-transducing protein phosphorylation cascades mediated by Ras/Rho proteins in the mammalian cell: the potential for multiplex signalling. Biochemical Journal. 1996;318(Pt 3):729–747. doi: 10.1042/bj3180729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich D, Hoeger S. Guidance values for microcystins in water and cyanobacterial supplement products (blue-green algal supplements): a reasonable or misguided approach? Toxicology and Applied Pharmacology. 2005;203(3):273–289. doi: 10.1016/j.taap.2004.09.005. [DOI] [PubMed] [Google Scholar]
- El-Fouly MH, Trosko JE, Chang CC. Scrape-loading and dye transfer: a rapid and simple technique to study gap junctional intercellular communication. Experimental Cell Research. 1987;168(2):422–430. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]
- Fastner J, Rucker J, Stuken A, Preussel K, Nixdorf B, Chorus I, Kohler A, Wiedner C. Occurrence of the cyanobacterial toxin cylindrospermopsin in northeast Germany. Environmental Toxicology. 2007;22(1):26–32. doi: 10.1002/tox.20230. [DOI] [PubMed] [Google Scholar]
- Grosse Y, Baan R, Straif K, Secretan B, El Ghissassi F, Cogliano V. Carcinogenicity of nitrate, nitrite, and cyanobacterial peptide toxins. Lancet Oncology. 2006;7(8):628–629. doi: 10.1016/s1470-2045(06)70789-6. [DOI] [PubMed] [Google Scholar]
- Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signalling pathways in the regulation of cell survival. FASEB Journal. 2008;22(4):954–965. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- Kang KS, Wilson MR, Hayashi T, Chang CC, Trosko JE. Inhibition of gap junctional intercellular communication in normal human breast epithelial cells after treatment with pesticides, PCBs, and PBBs, alone or in mixtures. Environmental Health Perspectives. 1996;104(2):192–200. doi: 10.1289/ehp.96104192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King TJ. Mice deficient for the gap junction protein Connexin32 exhibit increased radiation-induced tumorigenesis associated with elevated mitogenactivated protein kinase (p44/Erk1, p42/Erk2) activation. Carcinogenesis. 2004;25(5):669–680. doi: 10.1093/carcin/bgh071. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Furukawa T, Ikeda R, Takumi S, Nong Q, Aoyama K, Akiyama SI, Keppler D, Takeuchi T. Involvement of mitogen-activated protein kinase signaling pathways in microcystin-LR-induced apoptosis after its selective uptake mediated by OATP1B1 and OATP1B3. Toxicological Sciences. 2007;97(2):407–416. doi: 10.1093/toxsci/kfm054. [DOI] [PubMed] [Google Scholar]
- Lowes KN, Brennan BA, Yeoh GC, Olynyk JK. Oval cell numbers in human chronic liver diseases are directly related to disease severity. American Journal of Pathology. 1999;154(2):537–541. doi: 10.1016/S0002-9440(10)65299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machala M, Blaha L, Vondracek J, Trosko JE, Scott J, Upham BL. Inhibition of gap junctional intercellular communication by noncoplanar polychlorinated biphenyls: Inhibitory potencies and screening for potential mode(s) of action. Toxicological Sciences. 2003;76(1):102–111. doi: 10.1093/toxsci/kfg209. [DOI] [PubMed] [Google Scholar]
- MacKintosh C, Beattie K, Klumpp S, Cohen C, Codd GA. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Letters. 1990;264(2):187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- Madhukar BV, de Feijter-Rupp HL, Trosko JE. Pulse treatment with the tumor promoter TPA delays the onset of desensitization response and prolongs the inhibitory effect on gap junctional intercellular communication of a rat liver epithelial cell line WB F-344. Cancer Letters. 1996;106(1):117–123. doi: 10.1016/0304-3835(96)04315-7. [DOI] [PubMed] [Google Scholar]
- Masten SJ, Tian M, Upham BL, Trosko JE. Effect of selected pesticides and their ozonation by-products on gap junctional intercellular communication using rat liver epithelial cell lines. Chemosphere. 2001;44(3):457–465. doi: 10.1016/s0045-6535(00)00296-4. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, LaHair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxidants & Redox Signaling. 2006;8(910):1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- Nishiwaki-Matsushima R, Ohta T, Nishiwaki S, Suganuma M, Kohyama K, Ishikawa T, Carmichael WW, Fujiki H. Liver tumor promotion by the cyanobacterial cyclic peptide toxin microcystin-LR. Journal of Cancer Research and Clinical Oncology. 1992;118(6):420–424. doi: 10.1007/BF01629424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T, Sueoka E, Iida N, Komori A, Suganuma M, Nishiwaki R, Tatematsu M, Kim SJ, Carmichael WW, Fujiki H. Nodularin, a potent inhibitor of protein phosphatase-1 and phosphatase-2A, is a new environmental carcinogen in male F344 rat-liver. Cancer Research. 1994;54(24):6402–6406. [PubMed] [Google Scholar]
- Papageorgiou J, Linke TA, Kapralos C, Nicholson BC, Steffensen DA. Extraction of cyanobacterial endotoxin. Environmental Toxicology. 2004;19(1):82–87. doi: 10.1002/tox.10152. [DOI] [PubMed] [Google Scholar]
- Pietsch C, Wiegand C, Ame MV, Nicklisch A, Wunderlin D, Pflugmacher S. The effects of a cyanobacterial crude extract on different aquatic organisms: Evidence for cyanobacterial toxin modulating factors. Environmental Toxicology. 2001;16(6):535–542. doi: 10.1002/tox.10014. [DOI] [PubMed] [Google Scholar]
- Preussel K, Stuken A, Wiedner C, Chorus I, Fastner J. First report on cylindrospermopsin producing Aphanizomenon flos-aquae (Cyanobacteria) isolated from two German lakes. Toxicon. 2006;47(2):156–162. doi: 10.1016/j.toxicon.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Rivedal E, Opsahl H. Role of PKC and MAP kinase in EGF- and TPA-induced connexin43 phosphorylation and inhibition of gap junction intercellular communication in rat liver epithelial cells. Carcinogenesis. 2001;22(9):1543–1550. doi: 10.1093/carcin/22.9.1543. [DOI] [PubMed] [Google Scholar]
- Rosenkrantz HS, Pollack N, Cunningham AR. Exploring the relationship between the inhibition of gap junctional intercellular communication and other biological phenomena. Carcinogenesis. 2000;21(5):1007–1011. doi: 10.1093/carcin/21.5.1007. [DOI] [PubMed] [Google Scholar]
- Roskams T, De Vos R, Van Eyken P, Myazaki H, Van Damme B, Desmet V. Hepatic OV-6 expression in human liver disease and rat experiments: evidence for hepatic progenitor cells in man. Journal of Hepatology. 1998;29(3):455–463. doi: 10.1016/s0168-8278(98)80065-2. [DOI] [PubMed] [Google Scholar]
- Ruch RJ, Trosko JE. The role of oval cells and gap junctional intercellular communication in hepatocarcinogenesis. Anticancer Research. 1999;19(6A):4831–4838. [PubMed] [Google Scholar]
- Rucker J, Stuken A, Nixdorf B, Fastner J, Chorus I, Wiedner C. Concentrations of particulate and dissolved cylindrospermopsin in 21 Aphanizomenon-dominated temperate lakes. Toxicon. 2007;50(6):800–809. doi: 10.1016/j.toxicon.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Sai K, Upham BL, Kang KS, Hasegawa R, Inoue T, Trosko JE. Inhibitory effect of pentachlorophenol on gap junctional intercellular communication in rat liver epithelial cells in vitro. Cancer Letters. 1998;130(12):9–17. doi: 10.1016/s0304-3835(98)00082-2. [DOI] [PubMed] [Google Scholar]
- Schlosser UG. SAG - Sammlung von Algenkulturen at the University of Gottingen - Catalog of Strains 1994. Botanica Acta. 1994;107(3):113–186. [Google Scholar]
- Schumann R, Pfeil D, Lamping N, Kirschning C, Scherzinger G, Schlag P, Karawajew L, Herrmann F. Lipopolysaccharide induces the rapid tyrosine phosphorylation of the mitogen-activated protein kinases erk-1 and p38 in cultured human vascular endothelial cells requiring the presence of soluble CD14. Blood. 1996;87(7):2805–14. [PubMed] [Google Scholar]
- Sell S. The role of determined stem-cells in the cellular lineage of hepatocellular-carcinoma. International Journal of Developmental Biology. 1993;37(1):189–201. [PubMed] [Google Scholar]
- Stein J. Handbook of phycological methods: culture methods and growth measurements. Cambridge University Press; Cambridge: 1975. p. 477. [Google Scholar]
- Trosko JE, Jone C, Chang CC. Inhibition of gap junctional-mediated intercellular communication in vitro by aldrin, dieldrin, and toxaphene: a possible cellular mechanism for their tumor-promoting and neurotoxic effects. Molecular Toxicology. 1987;1(1):83–93. [PubMed] [Google Scholar]
- Trosko JE, Ruch RJ. Gap junctions as targets for cancer chemoprevention and chemotherapy. Current Drug Targets. 2002;3(6):465–482. doi: 10.2174/1389450023347371. [DOI] [PubMed] [Google Scholar]
- Trosko JE, Upham BL. The emperor wears no clothes in the field of carcinogen risk assessment: ignored concepts in cancer risk assessment. Mutagenesis. 2005;20(2):81–92. doi: 10.1093/mutage/gei017. [DOI] [PubMed] [Google Scholar]
- Tsao MS, Smith JD, Nelson KG, Grisham JW. A diploid epithelial cell line from normal adult rat liver with phenotypic properties of ‘oval’ cells. Experimental Cell Research. 1984;154(1):38–52. doi: 10.1016/0014-4827(84)90666-9. [DOI] [PubMed] [Google Scholar]
- Upham BL, Boddy B, Xing XS, Trosko JE, Masten SJ. Non-genotoxic effects of selected pesticides and their disinfection by-products on gap junctional intercellular communication. Ozone-Science & Engineering. 1997;19(4):351–369. [Google Scholar]
- Upham BL, Deocampo ND, Wurl B, Trosko JE. Inhibition of gap junctional intercellular communication by perfluorinated fatty acids is dependent on the chain length of the fluorinated tail. International Journal of Cancer. 1998a;78(4):491–495. doi: 10.1002/(sici)1097-0215(19981109)78:4<491::aid-ijc16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Upham BL, Weis LM, Trosko JE. Modulated gap junctional intercellular communication as a biomarker of PAH epigenetic toxicity: structure-function relationship. Environmental Health Perspectives. 1998b;106(4):975–981. doi: 10.1289/ehp.98106s4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upham BL, Guzvic M, Scott J, Carbone JM, Blaha L, Coe C, Li LL, Rummel AM, Trosko JE. Inhibition of gap junctional intercellular communication and activation of mitogen-activated protein kinase by tumor-promoting organic peroxides and protection by resveratrol. Nutrition and Cancer. 2007;57(1):38–47. doi: 10.1080/01635580701268188. [DOI] [PubMed] [Google Scholar]
- Upham BL, Park JS, Babica P, Sovadinova I, Rummel AM, Trosko JE, Hirose A, Hasegawa R, Kanno J, Sai K. Structure-activity-dependent regulation of cell communication by perfluorinated fatty acids using in vivo and in vitro model systems. Environmental Health Perspectives. 2009;117(4):545–551. doi: 10.1289/ehp.11728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters J, Sommer J, Pfeiffer Z, Prabhu U, Guerra A, Bertics P. A differential role for the mitogen-activated protein kinases in lipopolysaccharide signaling - The MEK/ERK pathway is not essential for nitric oxide and interleukin 10 production. Journal of Biological Chemistry. 2002;277(11):9077–9087. doi: 10.1074/jbc.M104385200. [DOI] [PubMed] [Google Scholar]
- Wright JH, Munar E, Jameson DR, Andreassen PR, Margolis RL, Seger R, Krebs E. Mitogen-activated protein kinase activity is required for the G(2)/M transition of the cell cycle in mammalian fibroblasts. Proceedings of the National Academy of Sciences USA. 1999;96(20):11335–11340. doi: 10.1073/pnas.96.20.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SZ. Primary prevention of hepatocellular carcinoma. Journal of Gastroenterology and Hepatology. 1995;10(6):674–682. doi: 10.1111/j.1440-1746.1995.tb01370.x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Yu H, Chen K. Relationship between microcystin in drinking water and colorectal cancer. Biomedical and Environmental Sciences. 2002;15(2):166–171. [PubMed] [Google Scholar]
- Zhu YL, Zhong X, Zheng S, Ge Z, Du Q, Zhang SZ. Transformation of immortalized colorectal crypt cells by microcystin involving constitutive activation of Akt and MAPK cascade. Carcinogenesis. 2005;26(7):1207–1214. doi: 10.1093/carcin/bgi069. [DOI] [PubMed] [Google Scholar]


