Abstract
The kisspeptin/Gpr54 signaling pathway plays a critical role in reproduction by stimulating the secretion of GnRH, yet mice carrying mutations in Kiss1 (which encodes kisspeptin) or Gpr54 exhibit partial sexual maturation. For instance, a proportion of female Kiss1−/− and Gpr54−/− mice exhibit vaginal oestrus, and some male Kiss1−/− and Gpr54−/− mice exhibit spermatogenesis.
To characterise this partial sexual maturation, we examined the vaginal cytology of female Kiss1−/− and Gpr54−/− mice over time. Nearly all mutant mice eventually enter oestrus, then spontaneously transition from oestrus to dioestrus and back to oestrus again. These transitions are not associated with ovulation, and the frequency of these transitions increases with age. The oestrus exhibited by female Kiss1−/− and Gpr54−/− mice was disrupted by administration of the competitive GnRH antagonist acyline, which also resulted in lower uterine weights and, in Kiss1−/− mice, lower serum FSH and LH concentrations. Similarly, male Kiss1−/− and Gpr54−/− mice treated with acyline had smaller testicular sizes and absence of mature sperm.
In addition to examining intact Kiss1−/− and Gpr54−/− mice, we also assessed the effects of acyline on gonadotrophin concentrations in gonadectomised mice. Gonadectomy resulted in a significant increase in serum FSH concentrations in male Gpr54−/− and Kiss1−/− mice. Acyline administration to gonadectomised Kiss1−/− and Gpr54−/− male mice lowered serum FSH and LH concentrations significantly. In contrast to males, gonadectomy did not result in significant gonadotrophin changes in female Kiss1−/− and Gpr54−/− mice, but acyline administration was followed by a decrease in LH concentrations.
These results demonstrate that, while kisspeptin signaling is critical for the high levels of GnRH activity required for normal sexual maturation and for ovulation, Kiss1−/− and Gpr54−/− mice retain some degree of GnRH activity. This GnRH activity is sufficient to produce significant effects on vaginal cytology and uterine weights in female mice and on spermatogenesis and testicular weights in male mice.
Keywords: metastin, Kiss1r, LHRH
Introduction
Signaling by the neuropeptide kisspeptin through its receptor Gpr54 (also called Kiss1r) is the most potent stimulus for GnRH-induced gonadotrophin release identified to date (reviewed in refs. 1–2). Both humans and mice with mutations in GPR54/Gpr54 exhibit failure of sexual maturation, impaired gametogenesis, and infertility (3–6). Three groups, including our own, have characterised mice with targeted deletions of Gpr54 and have determined that their reproductive phenotypes are due to low levels of sex steroids and of the pituitary gonadotrophins FSH and LH, that is, hypogonadotropic hypogonadism (4–6). Recently, mice carrying mutations in Kiss1 have been reported to have similar defects (7–8), emphasizing the importance of this receptor-ligand pair for reproduction.
However, the hypogonadism of Kiss1−/− and Gpr54−/− mice is not complete. While all animals are infertile, we and others have observed that testes from male Kiss1−/− and Gpr54−/− mice sometimes contain mature sperm (7–8). In addition, we have observed that a subset of female Kiss1−/− and Gpr54−/− mice exhibit normal uterine weights, vaginal cornification, and folliculogenesis but no evidence of ovulation (8). Vaginal cornification is a characteristic of the oestrus phase of the female oestrous cycle and reflects the action of oestrogen (9). Therefore, despite their failure of sexual maturation and infertility, some Kiss1−/− and Gpr54−/− animals have findings consistent with weak activity of the reproductive endocrine axis.
Our previous finding that only some female Kiss1−/− and Gpr54−/− mice exhibit partial sexual maturation could be due to a subset of mice never achieving sexual maturation, or alternatively could be due to cross-sectional observations of a dynamic phenotype that changes over time. To distinguish between these possibilities, we examined the vaginal cytology of Kiss1−/− and Gpr54−/− mice longitudinally. We also sought to determine whether the differences in vaginal cytology in Gpr54−/− mice were due to differences in hypothalamic Gnrh1 mRNA expression.
We then sought to demonstrate and quantify GnRH activity in Kiss1−/− and Gpr54−/− mice. To demonstrate that the partial sexual maturation of Kiss1−/− and Gpr54−/− mice is due to GnRH activity, we administered the competitive GnRH antagonist acyline and observed its effect on vaginal cytology, uterine weight, and reproductive hormone levels in female mice, and on testicular size, spermatogenesis, and hormone levels in male mice. We also assessed the effects of acyline administration on gonadotrophin levels in Kiss1−/− and Gpr54−/− mice that had been gonadectomised to remove negative feedback inhibition of gonadotrophin secretion.
Materials and Methods
Animals and animal care
Kiss1−/− and Gpr54−/− mice were generated as previously described (8). Animals were housed in the Massachusetts General Hospital (MGH) Center for Comparative Medicine at controlled temperature on a 12-hour light-dark cycle. All protocols were approved by the MGH Subcommittee on Research Animal Care.
Vaginal smears
To examine vaginal cytology, calcium alginate swabs were wetted with PBS and inserted in the vaginal opening. The swab was smeared on a glass slide, which was then stained using the Hema 3 system (Fisher). The cellular appearance of the smears was used to assign an oestrous cycle phase: dioestrus (predominantly neutrophils), prooestrus (predominantly nucleated epithelial cells), oestrus (abundant cornified epithelial cells), or metoestrus (mixture of cell types, predominantly neutrophils).
Histology
Dissected tissues were fixed in Bouin’s solution. Fixed tissues were embedded in paraffin, sectioned at 4 μm, mounted on slides, and stained with hematoxylin and eosin by the Harvard Medical School Rodent Histopathology Core.
Hormone assays
FSH concentrations for Experiments 2, 3, and 4 and LH and testosterone concentrations for all experiments were measured by RIA performed by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core. The measurable range for FSH was 2.6–21.7 ng/mL, for LH 0.04–37.4 ng/mL, and for testosterone 6.2–695.8 ng/dL. Intraassay and interassay coefficients of variation were 6.7% and 8.4% for FSH, 3.9% and 6.7% for LH, and 3.4% and 8.1% for testosterone, respectively. FSH concentrations for Experiment 5 were measured using the FSH (Rat) EIA kit (Alpco Diagnostics). The measurable range was 0.89–57.0 ng/mL, and intraassay and interassay coefficients of variation were 5.5% and 7.7%, respectively. There was not sufficient serum for all hormones to be measured in all animals.
Experiment 1: Longitudinal assessment of vaginal smears
Daily vaginal smears were obtained for two cohorts of Kiss1−/− and Gpr54−/− mice. A younger cohort of 7 Kiss1−/− and 11 Gpr54−/− mice was followed from weaning on postnatal day 21 for up to 9 months; an older cohort of 5 Kiss1−/− and 6 Gpr54−/− mice was followed for 9 months starting from 2–12 months of age. Male bedding was added weekly to cages to prevent entry into anoestrus. Four Kiss1−/− mice that had undergone an oestrus-to-dioestrus transition were sacrificed for ovarian histology, with sections every 100 μm mounted and examined for the presence of corpora lutea.
Experiment 2: Gnrh1 mRNA levels in Gpr54−/− females in oestrus and dioestrus
Gpr54−/− female mice 24–58 weeks of age were sacrificed, brains rapidly removed, and the basal forebrain dissected with cuts at the posterior edges of the olfactory bulbs, at the anterior edge of the optic chiasm, extended from the lateral hypothalamic sulci, and at a depth of 2 mm. The dissected tissue was immediately frozen and stored at −80°C. RNA was extracted using the RNeasy Lipid Kit (Qiagen), treated with DNase I (Invitrogen), and used for cDNA synthesis with the Superscript III First-Strand Synthesis kit (Invitrogen). Quantitative PCR was performed with the TaqMan 2X Universal PCR Mix (Applied Biosystems) on an ABI 7000 Real-Time PCR system using the following primers at 200 nM concentration: for Gnrh1, forward primer 5′-GAACCCCAGCACTTCGAATGTA-3′, reverse primer 5′-TGGCTTCCTCTTCAATCAGACTTT-3′, probe 5′-6-FAM-TCCACTGGCCCCGTTCACCCCTC-BHQ-1-3′; for Gadph, forward primer 5′-CAATGTGTCCGTCGTGGATCT-3′, reverse primer 5′-AGAGTGGGAGTTGCTGTTGAAG-3′, probe 5′-HEX-CGTGCCGCCTGGAGAAACCTGCC-BHQ-1-3′. The accompanying software was used to calculate Ct’s, and relative expression levels calculated by normalizing Ct’s to standard curves generated from serial dilutions of a reference sample. Expression levels were analyzed unadjusted, normalized to Gapdh expression, and normalized to the weight of the dissected tissue. No amplification was seen with samples from which reverse transcriptase was omitted (data not shown).
Experiment 3: Effects of acyline on female reproductive phenotypes in intact mice
Acyline was a generous gift of R. Blye, Eunice Kennedy Shriver National Institute of Child Health and Human Development. Female mice (17–59 weeks of age) exhibiting vaginal estrus were given two daily subcutaneous injections of acyline 50 μg (1 mg/mL in PBS; 13 Kiss1−/−, 8 Gpr54−/−) or PBS alone (17 Kiss1−/−, 7 Gpr54−/−), and vaginal smears were obtained for five days. After five days, a subset of these animals as well as WT animals treated with acyline or PBS were sacrificed, blood was collected by cardiac puncture, and ovaries and uteri were dissected, weighed, and fixed.
Experiment 4: Effects of acyline on male reproductive phenotypes in intact mice
Male mice 14–24 weeks in age received subcutaneous injections of acyline 50 μg or PBS alone on days 1, 2, 8, 15, 22, and 23 of the study. After the final injection, mice were sacrificed, blood was collected by cardiac puncture, and testes were dissected, weighed, and fixed.
Experiment 5: Effects of acyline on gonadotrophin levels in gonadectomised mice
Paired subcutaneous injections of acyline or PBS were given 24 h and 1 h before each blood collection, which was performed by submandibular puncture using Goldenrod lancets (Medipoint, Inc.). Male mice 9–24 weeks and female mice 10–39 weeks were used. For most animals, pre-gonadectomy gonadotrophin levels were obtained after injection with PBS. Animals were then anaesthetised with an intraperitoneal injection of ketamine 118 mg/kg and xylazine 11.8 mg/kg. The abdomen was shaved and opened with midline incisions through the skin and peritoneum. The gonads were identified, ligated, and resected (this step was omitted for animals undergoing sham surgery). The peritoneum was closed with a running chromic gut suture (Ethilon) and the skin closed with simple interrupted nylon sutures (Ethilon). After at least one week for recovery, paired PBS injections were given and post-gonadectomy blood samples obtained. After another week for recovery, paired injections of acyline 50 μg were given, followed by blood collection and sacrifice.
Statistics
Changes in vaginal smears after acyline or PBS injections were compared using Fisher’s two-tailed exact test. Length of oestrous phases, RT-PCR results, and levels of hormones were compared with unpaired, two-tailed t-tests, except for comparisons of FSH and LH levels pre-and post-acyline, for which paired t-tests were used. Because most LH levels were close to the detection limits of the assay, the sign test (binomial probability) was also used to analyze directions of change in LH levels. For quantitative calculations, assay results that were below the measurable range were set at the lower limit of the measurable range. Data are presented as mean ± SEM.
Results
Longitudinal examination of vaginal smears in Kiss1−/− and Gpr54−/− mice
We examined daily vaginal smears of Kiss1−/− and Gpr54−/− mice over time to characterise the oestrous cyclicity of mice lacking kisspeptin/Gpr54 signaling (Figure 1). Kiss1−/− mice first exhibited oestrus at a median of 130 days of life (n=7), whereas Gpr54−/− mice first exhibited oestrus at a median of 265 days (n=11). Nearly all Kiss1−/− and Gpr54−/− mice of both genotypes eventually exhibited oestrus, though some Gpr54−/− females were never seen to enter oestrus. Indeed, rare mice in our Gpr54−/− colony failed to exhibit even vaginal opening by 2 years of age (data not shown).
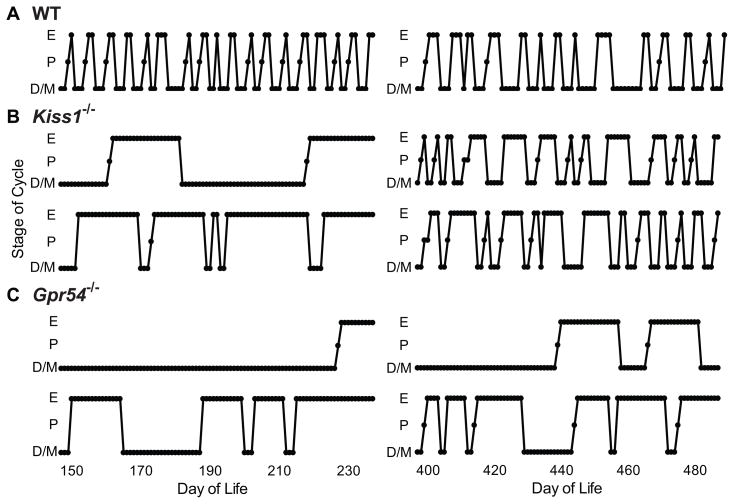
Figure 1.
Representative patterns of vaginal cytology of Kiss1−/− and Gpr54−/− female mice. A, wild-type mice; B, Kiss1−/− mice; C, Gpr54−/− mice. D/M, dioestrus/metoestrus; P, prooestrus; E, oestrus.
Mice that had entered oestrus were observed to spontaneously exit oestrus and enter a state of prolonged dioestrus, then enter oestrus once again (Figure 1). The frequency of these transitions increased with age. In Kiss1−/− mice 2–12 months of age, oestrus lasted 17.9 ± 2.2 days (n=12) and dioestrus 28.1 ± 6.2 days (n=13), whereas in Kiss1−/− mice 12–24 months of age oestrus lasted 7.7 ± 1.0 days (n=43, P < 0.001 compared to younger mice) and dioestrus lasted 7.6 ± 1.2 days (n=34, P = 0.007). In Gpr54−/− mice 2–12 months of age, oestrus lasted 23.1 ± 4.7 days (n= 14) and dioestrus 13.6 ± 4.5 days (n=11), and in Gpr54−/− mice 12–24 months of age oestrus lasted 15.3 ± 3.4 days (n=13, P = 0.19 compared to younger mice) and dioestrus 11.6 ± 3.1 days (n=19, P = 0.71).
To determine whether these oestrus-to-dioestrus transitions were the result of ovulation, four Kiss1−/− mice were sacrificed and their ovaries examined histologically. Follicles at all stages of development and large numbers of atretic follicles were present in Kiss1−/− mice, as we had observed previously (8), but no corpora lutea were seen on serial sections through the entirety of these ovaries (data not shown). Thus, even though older Kiss1−/− mice transitioned from dioestrus to oestrus and back again at a frequency approaching that of wild-type animals, these “cycles” were in fact an ovulatory.
Gnrh1 mRNA expression in female Gpr54−/− mice in oestrus and dioestrus
To determine whether the changes in vaginal cytology were due to changes in Gnrh1 expression, Gnrh1 mRNA levels in the basal forebrain of Gpr54−/− mice were measured by quantitative RT-PCR. No difference was seen in Gnrh1 expression in mice in oestrus compared to mice not in oestrus (Supplemental Table 1).
Effects of acyline on reproductive phenotypes of female Kiss1−/− and Gpr54−/− mice
To determine whether the persistent vaginal oestrus seen in Kiss1−/− and Gpr54−/− mice is due to GnRH activity, mice exhibiting vaginal oestrus were given two daily injections of the competitive GnRH antagonist acyline or vehicle (PBS). Of the 13 Kiss1−/− mice that received acyline, 12 left oestrus within 4 days, whereas only 2 of 17 mice that received vehicle left oestrus (Figure 2A, P < 0.001). Similarly, 7 of 8 Gpr54−/− mice that received acyline left oestrus compared to 1 of 7 that received vehicle (Figure 2A, P = 0.01).
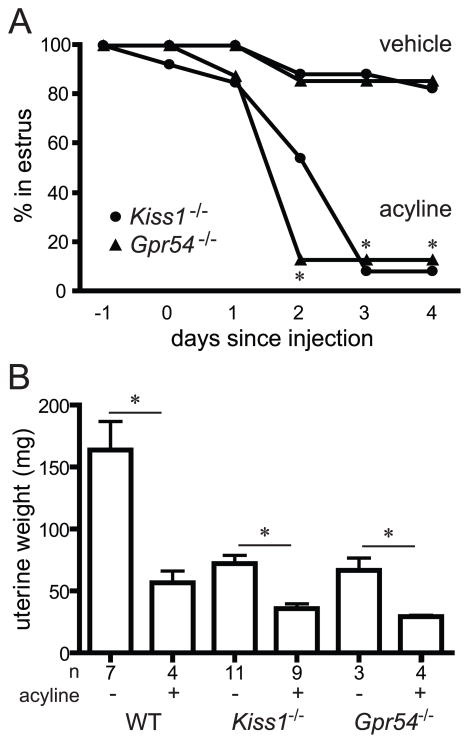
Figure 2.
Effects of acyline administration on oestrus and uterine weights in female Kiss1−/− and Gpr54−/− mice. A, female mice remaining in oestrus after administration of two doses of acyline 50 μg or vehicle (PBS) on days 0 and 1. B, uterine weights on day 4 after administration of acyline or vehicle. Asterisks, P < 0.05 compared to vehicle-treated mice.
Five days after acyline administration, a subset of Kiss1−/− and Gpr54−/− females was sacrificed to examine internal sex organs. Uterine weights of Kiss1−/− and Gpr54−/− females treated with acyline were significantly lower than those of females treated with vehicle (Figure 2; Kiss1−/− after acyline 35.8 ± 3.9 mg, after vehicle 72.1 ± 6.7 mg, P = 0.0003; Gpr54−/− after acyline 29.2 ± 1.2 mg, after vehicle 66.7 ± 9.8 mg, P = 0.007). No differences in ovarian histology were seen after this relatively short exposure to acyline (data not shown).
Serum LH concentrations were significantly lower in Kiss1−/− females treated with acyline (Table 1, acyline 0.05 ± 0.01 ng/mL, vehicle 0.61 ± 0.17 ng/mL, P = 0.01), with a similar pattern in Gpr54−/− mice (acyline 0.04 ± 0 ng/mL, vehicle 0.96 ± 0.06 ng/mL, P = 0.26). Similarly, FSH concentrations were lower in Kiss1−/− females treated with acyline (acyline 2.6 ± 0 ng/mL, vehicle 3.4 ± 0.2 ng/mL, P = 0.007). Thus, treatment with a GnRH antagonist resulted in disruption of vaginal oestrus and lower uterine weights in female Kiss1−/− and Gpr54−/− mice and lower FSH and LH concentrations in female Kiss1−/− mice.
Table 1.
Hormone levels (mean ± SEM) in intact wild-type, Kiss1−/−, and Gpr54−/− mice treated with acyline or vehicle (PBS). P values are for differences between acyline and vehicle treatment. The lower end of the measurable range was 2.6 ng/mL for FSH, 0.04 ng/mL for LH, and 6.2 ng/dL for testosterone.
| female | wild-type | Kiss1−/− | Gpr54−/− | ||||||
|---|---|---|---|---|---|---|---|---|---|
| vehicle | acyline | P | vehicle | acyline | P | vehicle | acyline | P | |
| FSH (ng/mL) | 5.7 ± 2.6 (n=7) | 2.7 ± 0.1 (n=4) | 0.18 | 3.4 ± 0.2 (n=8) | 2.6 ± 0 * (n=8) | 0.007 | 3.4 ± 0.1 (n=3) | 2.9 ± 0.5 (n=4) | 0.53 |
| LH (ng/mL) | 0.62 ± 0.25 (n=7) | 0.07 ± 0.02 (n=4) | 0.07 | 0.61 ± 0.17 (n=10) | 0.05 ± 0.01 (n=9) | 0.01 | 0.96 ± 0.06 (n=3) | 0.04 ± 0 * (n=4) | 0.26 |
| male | |||||||||
| FSH (ng/mL) | 12.6 +/− 2.2 (n=8) | 2.6 ± 0 * (n=8) | 0.002 | 2.6 ± 0 * (n=7) | 2.6 ± 0 * (n=6) | ND | 2.6 ± 0 * (n=7) | 2.6 ± 0 * (n=5) | ND |
| LH (ng/mL) | 0.78 ± 0.46 (n=8) | 0.04 ± 0 * (n=8) | 0.15 | 0.73 ± 0.58 (n=7) | 0.04 ± 0 * (n=6) | 0.28 | 0.34 ± 0.14 (n=7) | 0.04 ± 0 * (n=5) | 0.07 |
| testosterone (ng/dL) | 90.4 ± 51.0 (n=7) | 11.1 ± 2.6 (n=8) | 0.17 | 79.0 ± 36.2 (n=7) | 10.4 ± 2.6 (n=6) | 0.11 | 22.1 ± 2.6 (n=7) | 33.3 ± 12.4 (n=5) | 0.42 |
Asterisks, all measurements were below the reportable range; ND, not determinable.
Effects of acyline on reproductive phenotypes of male Kiss1−/− and Gpr54−/− mice
To determine whether the spermatogenesis seen in male Kiss1−/− and Gpr54−/− mice is also due to GnRH activity, male mice were treated with acyline or vehicle for three weeks. As expected, wild-type male mice treated with acyline had testicular weights lower than those of mice treated with vehicle (Figure 3; acyline 59.7 ± 5.4 mg, vehicle 95.3 ± 2.8 mg, P = 0.0007). Similarly, male Kiss1−/− mice treated with acyline had significantly smaller testes than Kiss1−/− mice treated with vehicle (acyline 8.2 ± 0.8 mg, vehicle 40.5 ± 4.6 mg, P = 0.004), and the same was seen for male Gpr54−/− mice (acyline 4.3 ± 0.3 mg, vehicle 16.5 ± 3.9 mg, P = 0.02).
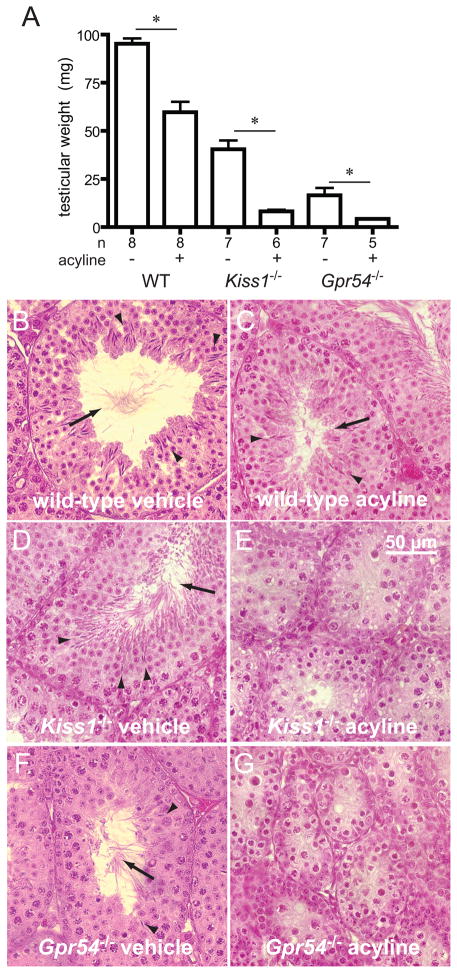
Figure 3.
Effects of acyline administration on male wild-type, Kiss1−/− and Gpr54−/− mice. A, testicular weights, and B–G, testicular histology after three weeks of weekly doses of acyline 50 μg or vehicle. Asterisks, P < 0.05; arrowheads, sperm heads; arrows, sperm tails.
Histological examination of testes from Kiss1−/− and Gpr54−/− mice treated with vehicle showed varying degrees of spermatogenesis (Figure 3), as we and others have previously observed (7–8), ranging from arrest at meiosis II to appearance of mature sperm. In contrast, no mature sperm were seen in Kiss1−/− and Gpr54−/− males treated with acyline (Figure 3), with arrest of spermatogenesis at meiosis II. No significant changes in FSH, LH, or testosterone concentrations were seen with acyline treatment of male Kiss1−/− and Gpr54−/− mice; many of these measurements were near or below the measurable range of the assays employed (Table 1).
Effects of gonadectomy on gonadotrophin concentrations in Kiss1−/− and Gpr54−/− mice
Because changes in hormone levels with acyline treatment were difficult to demonstrate consistently in intact Kiss1−/− and Gpr54−/− mice, we examined gonadotrophin levels in animals that were gonadectomised to remove feedback inhibition of GnRH and gonadotrophin secretion. Gonadectomy of wild-type mice resulted in marked increases in serum FSH and LH concentrations, as expected (Figure 4).
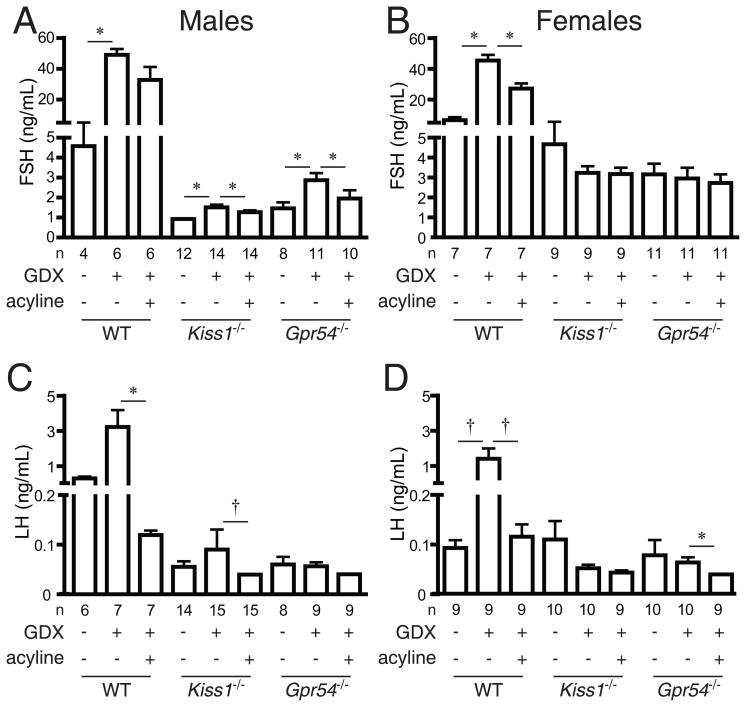
Figure 4.
Gonadotrophin levels after gonadectomy and acyline administration. A, FSH levels in male mice; B, FSH levels in female mice; C, LH levels in male mice; D, LH levels in female mice. WT, wild-type; GDX, gonadectomy. Asterisks, P < 0.05 by paired t-test; crosses, P < 0.05 by sign test.
FSH concentrations rose in male mice of both lines after gonadectomy. After gonadectomy, FSH concentrations in Kiss1−/− males (Figure 4A, 1.51 ± 0.13 ng/mL) were significantly higher than at baseline (0.93 ± 0.02 ng/mL, P = 0.002). Similarly, FSH concentrations in Gpr54−/− mice after gonadectomy were significantly higher (Figure 4A, mean ± SD 2.87 ± 0.36 ng/mL) than FSH concentrations before gonadectomy (1.46 ± 0.30 ng/mL, P = 0.008) and were also higher than FSH concentrations of mice that underwent sham surgery (1.25 ± 0.26 ng/mL, P = 0.003). In contrast to male mice, no significant changes in FSH concentrations were seen after gonadectomy in Kiss1−/− and Gpr54−/− female mice (Figure 4B), and no significant changes in LH concentrations were seen after gonadectomy in Kiss1−/− or Gpr54−/− male or female mice (Figure 4C and D).
Effects of acyline on gonadotrophin concentrations in Kiss1−/− and Gpr54−/− mice
Acyline administration resulted in significant reductions in gonadotrophin concentrations in gonadectomised wild-type, Kiss1−/−, and Gpr54−/− mice of both sexes. Specifically, administration of acyline to gonadectomised Kiss1−/− and Gpr54−/− male mice resulted in significant decreases in FSH concentrations (Figure 4A; Kiss1−/− pre-acyline 1.51 ± 0.13 ng/mL, post-acyline 1.27 ± 0.09 ng/mL, P = 0.002; Gpr54−/− pre-acyline 2.87 ± 0.36 ng/mL, post-acyline 1.95 ± 0.42 ng/mL, P = 0.007). In female Kiss1−/− and Gpr54−/− mice, there was no detectable change in FSH concentrations after acyline administration (Figure 4B).
Mean LH concentrations were not significantly different before and after acyline in gonadectomized Kiss1−/− and Gpr54−/− males, with most LH concentrations near the lower end of the measurable range of the assay (Figure 4C; Kiss1−/− pre-acyline 0.090 ± 0.040 ng/mL, post-acyline 0.04 ± 0 ng/mL, P = 0.23; Gpr54−/− pre-acyline 0.057 ± 0.008 ng/mL, post-acyline 0.040 ± 0.0004 ng/mL, P = 0.08). However, in the 8 Kiss1−/− males in which a change in LH concentration could be determined, LH concentrations decreased in all 8 cases, more frequently than would be expected by chance alone (P = 0.002 by sign test); a similar trend was seen in Gpr54−/− males (5 of 5 mice with decreases in LH concentrations, P = 0.06). Mean LH concentrations decreased significantly in Gpr54−/− females after acyline administration (Figure 4D, pre-acyline 0.065 ± 0.010 ng/mL, post-acyline 0.041 ± 0.001 ng/mL, P = 0.04 by paired t-test, P = 0.008 by sign test), with a similar trend in Kiss1−/− females (pre-acyline 0.053 ± 0.007 ng/mL, post-acyline 0.044 ± 0.004 ng/mL, P = 0.12 by sign test).
Discussion
Multiple groups have shown that Kiss1−/− and Gpr54−/− mice have abnormal sexual maturation, with gonadal underdevelopment, impaired gametogenesis, low sex steroids and gonadotrophins, and infertility (4–8). Despite these impairments in reproductive maturation and function, we have now shown persistent GnRH activity in Kiss1−/− and Gpr54−/− mice of both sexes. Kiss1−/− and Gpr54−/− females alternate between periods of prolonged dioestrus and prolonged oestrus. These transitions increase in frequency with increasing age, are not due to ovulation, and are not associated with changes in hypothalamic Gnrh1 mRNA expression. We further demonstrated that this oestrus is disrupted by administration of the competitive GnRH antagonist acyline, which also resulted in lower uterine weights. Similarly, acyline administration to male Kiss1−/− and Gpr54−/− mice results in lower testicular weights and blockade of spermatogenesis. Finally, we found that acyline lowers gonadotrophins in gonadectomised Kiss1−/− and Gpr54−/− mice. These results demonstrate the presence of low-level GnRH activity in Kiss1−/− and Gpr54−/− mice and suggest that this activity is sufficient to cause partial sexual maturation.
The persistence of GnRH activity in the absence of kisspeptin/Gpr54 signaling has been suggested by several previous reports, although never formally explored. First, as noted above, Kiss1−/− and Gpr54−/− mice exhibit partial sexual maturation (7–8). Second, human patients with severe loss-of-function mutations in GPR54 exhibit low-amplitude LH pulses (4, 10). Third, administration of an oestrogen regimen to Gpr54−/− mice can induce a GnRH-dependent LH surge (11), although this effect has not been observed by all investigators (12). Fourth, rhesus macaques undergoing pharmacologic desensitization of Gpr54 exhibit low-amplitude LH pulses at night, though these pulses did not a meet statistically significant threshold (13). Fifth, administration of kisspeptin to rats does not alter the rhythmic electrophysiological activity of the arcuate nucleus, which is associated with the pulsatile release of GnRH (14). Lastly, physiologically distinct subpopulations of GnRH neurones have recently been identified: one subpopulation responds to kisspeptin but not to 3,5-dihydroxyphenylglycine (DHPG), an agonist of group 1 metabotropic glutamate receptors, while the other subpopulation responds to DHPG but not to kisspeptin (15). Our findings add to these studies to provide the most direct evidence to date that physiologically significant GnRH activity persists in the absence of kisspeptin/Gpr54 signaling.
Illustrating the known importance of kisspeptin signaling for GnRH secretion, gonadotrophin levels were markedly lower in gonadectomised Kiss1−/− and Gpr54−/− mice compared to gonadectomised wild-type mice. Our findings are consistent with those of Dungan et al. (11), who observed low LH concentrations in gonadectomised Gpr54−/− mice that were not higher than those of intact mice, evidence that kisspeptin signaling is an essential mediator of the negative feedback effects of sex steroids. We similarly did not see an effect of gonadectomy on LH, but we did find that FSH increased in Kiss1−/− and Gpr54−/− males after gonadectomy; this can be attributed to loss of gonadal inhibins, which restrain FSH but not LH secretion (16). It is unclear why FSH concentrations in female Kiss1−/− and Gpr54−/− mice did not change after gonadectomy or acyline treatment, particularly since LH levels were lower with acyline treatment. This may reflect a greater contribution of signals other than GnRH (e.g., activins) to FSH secretion in female than in male mice, or may be due to lack of specificity of the FSH assay at low FSH concentrations in female mice.
Our results provide an indirect measure of kisspeptin-dependent versus kisspeptin-independent GnRH activity. For example, mean FSH levels of gonadectomised wild-type male mice were 54 ng/mL, whereas those of gonadectomised Gpr54−/− male mice were 2.9 ng/mL. In contrast, administration of acyline to gonadectomised Gpr54−/− male mice resulted in a decrease in FSH levels from 2.9 to 2.0 ng/mL. A rough calculation shows that kisspeptin-dependent GnRH activity is ~50-fold greater than kisspeptin-independent GnRH activity in the context of gonadectomised mice. Nevertheless, even this modest amount of GnRH and gonadotrophin activity is sufficiently potent to induce spermatogenesis and uterine hypertrophy.
Kisspeptin-independent GnRH activity could be due to low-level constitutive activity of GnRH neurones. Indeed, cultured GnRH neurones spontaneously produce synchronous pulses of GnRH secretion in the absence of external inputs (17). However, it is unclear how this could explain the increasing frequency of oestrus-to-dioestrus transitions exhibited by female Kiss1−/− and Gpr54−/− mice with advancing age. Alternatively or additionally, the GnRH activity in Kiss1−/− and Gpr54−/− mice could be induced by one or more of the many neuroendocrine pathways that modulate GnRH neuronal activity and secretion (18,19).
Indeed, decreases in glutamate and increases in GABA inputs have been proposed to produce the neuroendocrine changes with reproductive senescence in female rodents (19,20). The effects of these inputs may superimpose on the residual GnRH activity in Kiss1−/− and Gpr54−/− mice to accelerate entry into oestrus, thereby causing the increasing frequency of dioestrus-to-oestrus transitions we observed in these mutant mice. The milder phenotypes of Kiss1−/− mice compared to Gpr54−/− mice suggests a higher basal GnRH “tone” that results in a lower threshold for entry into oestrus, which could account for the more dynamic pattern of vaginal smears seen in female Kiss1−/− mice.
A potential alternative, technical explanation for our findings is that the mutations in our Kiss1−/− and Gpr54−/− mice are not null. We had previously demonstrated that no Gpr54 transcript could be detected by RT-PCR in the Gpr54−/− mice and no kisspeptin peptide could be detected by immunohistochemistry in the Kiss1−/− mice (8), and so this possibility appears unlikely. We are further reassured that low-level GnRH activity was observedin both Kiss1−/− and Gpr54−/− mice.
Because we used traditional, global targeted deletions, Kiss1 and Gpr54 activity was absent from our mutant mice throughout development. It is therefore possible that alternative pathways emerged to compensate for the absence of Kiss1/Gpr54 activity and that our results underestimate the contribution of kisspeptin signaling to the stimulation of GnRH activity in normal animals. It is also possible that kisspeptin/Gpr54 signaling is required for the proper development of the reproductive endocrine system, independent of the need for Kiss1/Gpr54 activity during sexual maturation and in adulthood. The identification and use of specific kisspeptin/Gpr54 antagonists (21) would allow acute blockade of kisspeptin/Gpr54 signaling and more direct determination of the extent to which kisspeptin-dependent and kisspeptin-independent pathways contribute to GnRH activity in wild-type mice.
To date, most studies of the reproductive role of kisspeptin/Gpr54 signaling have emphasized the importance and potency of this pathway for GnRH secretion. Our results demonstrate the presence of low-level GnRH activity in Kiss1−/− and Gpr54−/− mice and emphasize the importance of the contribution of other pathways to the regulation of GnRH secretion. Further study of Kiss1−/− and Gpr54−/− mice will determine which of these pathways are responsible for inducing sexual maturation and oestrous changes, how these pathways change with increasing age, and whether they can be manipulated to either enhance or overcome the hypogonadism caused by deficits in kisspeptin/Gpr54 signaling.
Supplementary Material
Acknowledgments
This work was supported by NIH U54 HD028138. Y.-M.C. received support from NIH T32 HD07369, F32 HD056759, and a Novo Nordisk Research Fellowship Award from the Lawson Wilkins Pediatric Endocrine Society. The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by NIH U54-HD28934. We thank members of the Harvard Reproductive Sciences Center for valuable discussion of this work. S.B.-F. is currently at the New York University School of Medicine, and K.M.W. is currently at the University of Amsterdam Medical School.
References
- 1.Roseweir AK, Millar RP. The role of kisspeptin in the control of gonadotrophin secretion. Hum Reprod Update. 2009;15:203–212. doi: 10.1093/humupd/dmn058. [DOI] [PubMed] [Google Scholar]
- 2.Dhillo WS. Kisspeptin: a novel regulator of reproductive function. J Neuroendocrinol. 2008;20:963–970. doi: 10.1111/j.1365-2826.2008.01753.x. [DOI] [PubMed] [Google Scholar]
- 3.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 5.Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 6.Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, Pavlova MN, Rohde AD, Clifton DK, Steiner RA, Rissman EF. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci. 2007;27:8826–8835. doi: 10.1523/JNEUROSCI.2099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.d’Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lapatto R, Pallais JC, Zhang D, Chan Y-M, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- 9.Robson JM, Adler J. Site of action of oestrogens. Nature. 1940;146:60. [Google Scholar]
- 10.Tenenbaum-Rakover Y, Commenges-Ducos M, Iovane A, Aumas C, Admoni O, de Roux N. Neuroendocrine phenotype analysis in five patients with isolated hypogonadotropic hypogonadism due to a L102P inactivating mutation of GPR54. J Clin Endocrinol Metab. 2007;92:1137–1144. doi: 10.1210/jc.2006-2147. [DOI] [PubMed] [Google Scholar]
- 11.Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci. 2007;27:12088–12095. doi: 10.1523/JNEUROSCI.2748-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;27:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seminara SB, DiPietro MJ, Ramaswamy S, Crowley WF, Jr, Plant TM. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male rhesus monkey Macaca mulatta: a finding with therapeutic implications. Endocrinology. 2006;147:2122–2126. doi: 10.1210/en.2005-1550. [DOI] [PubMed] [Google Scholar]
- 14.Kinsey-Jones JS, Li XF, Luckman SM, O’Byrne KT. Effects of kisspeptin-10 on the electrophysiological manifestation of gonadotropin-releasing hormone pulse generator activity in the female rat. Endocrinology. 2008;149:1004–1008. doi: 10.1210/en.2007-1505. [DOI] [PubMed] [Google Scholar]
- 15.Dumalska I, Wu M, Morozova E, Liu R, van den Pol A, Alreja M. Excitatory effects of the puberty-initiating peptide kisspeptin and group I metabotropic glutamate agonists differentiate two distinct subpopulations of gonadotropin-releasing hormone neurons. J Neurosci. 2008;28:8003–8013. doi: 10.1523/JNEUROSCI.1225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall JE. Neuroendocrine control of the menstrual cycle. In: Strauss JF III, Barbieri RL, editors. Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 6. Philadelphia: Elsevier Saunders; 2009. pp. 139–154. [Google Scholar]
- 17.Martinez de la Escalara G, Choi ALH, Weiner RI. Generation and synchronization of gonadotropin-releasing hormone pulses: intrinsic properties of the GT1-1 GnRH neuronal cell line. Proc Natl Acad Sci USA. 1992;89:1852–1855. doi: 10.1073/pnas.89.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clifton DK, Steiner DA. Neuroendocrinology of reproduction. In: Strauss JF III, Barbieri RL, editors. Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 6. Philadelphia: Elsevier Saunders; 2009. pp. 3–33. [Google Scholar]
- 19.Maffucci JA, Gore AC. Hypothalamic neural systems controlling the female reproductive life cycle: gonadotropin-releasing hormone, glutamate, and GABA. Int Rev Cell Mol Biol. 2009;274:69–127. doi: 10.1016/S1937-6448(08)02002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neal-Perry GS, Zeevalk GD, Shu J, Etgen AM. Restoration of the luteinizing hormone surge in middle-aged female rats by altering the balance of GABA and glutamate transmission in the medial preoptic area. Biol Reprod. 2008;79:878–888. doi: 10.1095/biolreprod.108.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29:3920–3929. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.