Abstract
Heat shock proteins (HSPs) are highly effective and versatile molecules in promoting anti-tumor immune responses. We tested whether a HSP-based DNA vaccine can induce effective immune response against Mage3, a cancer testis (CT) antigen frequently expressed in many human tumors, thereby controlling the Mage3-expressing tumor. The vaccine was constructed by linking human inducible HSP70 to the C-terminus of a modified Mage3 gene (sMage3) that was attached at its N-terminus with the signal leader sequence of the human RANTES for releasing the expressed fusion protein from the transduced cells. Intramuscular injection of sMage3Hsp DNA induced CD4+/CD8+ T cell and antibody responses. Vaccination with sMage3Hsp DNA was more effective in inhibiting Mage3-expressing TC-1 tumors. When we dissected the antitumor activity of CD4+ and CD8+ T cells by immunizing CD4+ and CD8+ knockout mice with sMage3Hsp DNA, we found that both CD8+ T and CD4+ T cells played a role in control of inoculated tumor, but did not constitute the whole of immune protection in the prophylactic immunization. Instead, depletion of natural killer (NK) cells led to a major loss of anti-tumor activity in the immunized mice. These results indicate that the HSP-based Mage3 DNA vaccine can more effectively inhibit tumor growth by inducing both the innate immune responses and Mage3-specific adaptive immune responses via the Hsp-associated adjuvant function.
INTRODUCTION
Cancer testis (CT) antigens are a category of tumor antigens with restricted expression in normal testis and active expression in various types of tumors due to disruption of gene regulation (1). Currently, approximately 20 CT antigens or antigen families have been identified by T cell epitope cloning, serological expression cloning, and differential mRNA expression analysis (2–7). Since CT antigens are immunogenic and their expression is highly restricted to tumors, they represent an ideal target for tumor immunotherapy.
Mage3 has been characterized as a more frequently expressed CT antigen in many tumors, including melanoma, non-small cell lung carcinoma, head and neck squamous cell carcinoma, and hepatocellular carcinoma (8). Several major histocompatibility (MHC) class I/II peptides have been identified in the protein (9–12). Clinical trials with synthetic peptides or peptide-pulsed dendritic cells (DCs) demonstrated that immune responses to Mage3 can be induced, and modest anti-tumor effects can be transiently achieved in some melanoma patients (13–15). Despite the clinical responses, however, anti-Mage cytotoxic T lymphocyte (CTL) responses were, in general, of low-level and rarely detectable by currently available tests, even in patients with clinical responses (14). These studies indicate that the efficacy of current CT antigen-based vaccines needs to be further improved.
Increasing evidence shows that heat shock proteins (HSPs), a group of conserved molecular chaperones throughout the evolution of prokaryotes and eukaryotes (16), are highly effective and versatile molecules in potentiating immune responses (17–20). HSPs have been used to elicit tumor antigen (Ag)-specific immune responses in vaccination with Ag-HSP fusion genes (21, 22), Ag-HSP fusion proteins (23, 24), or tumor tissue-derived HSP proteins (17, 25, 26). In a recent study, vaccination with an HSP70/Mage3 fusion protein enhanced Mage3-specific cellular and humoral immune responses in a murine tumor model (27). DNA vaccine represents a potent and convenient vaccination strategy, and a couple of DNA-based tumor vaccines have been in clinical trials for cancer patient. In the present study, we demonstrate that inclusion of HSP in a Mage-3-expressing DNA construct enhances the potency of the Mage3 DNA vaccine in control of Mage3-expressing tumor by induction of both innate immune responses and Mage3-specific, adaptive immune responses in immunized mice.
MATERIALS AND METHODS
Mice and cell lines
Six- to eight-week-old female C57BL/6 mice, CD4+ knockout (KO) mice, and CD8+ KO mice were purchased from Harlan Sprague Dawley, Taconic, and Jackson Laboratory, respectively. All mice were maintained in the animal facility of Baylor College of Medicine or University of Southern California with the approval of the Institutional Animal Care and Use Committee. The cell lines COS-1, 293T, and Sf9, and the tumor cell line TC-1 were purchased from ATCC. TC-1/Mage3 was generated by transfection of the plasmid pcDNA3.1-Mage3 using GenePORTER (GTS Inc.), and then selected in the presence of 800μg/ml Zeocin (Invitrogen). The Zeocin-resistant clones were subcloned and then screened for Mage3 expression by RT-PCR. The positive TC-1/Mage3 clones were maintained at 37°C in 5% CO2 in RPMI-1640 containing 10% heat-inactivated fetal bovine serum and 100μg/ml Zeocin.
Vector construction
Plasmids pcDNA3.1-MAGE3 (i.e., pc-Mage3) and pRC/CMV-sHSP70 (i.e., pc-sHsp) were constructed as described (21, 28). To construct plasmid pcDNA3.1-sMAGE3-HSP70 (i.e., pc-sMage3Hsp), in which a signal sequence derived from the human RANTES gene (21) was added to the 5′-end of the Mage3/HSP70 fusion gene, the signal-Mage3 fragment (sMage3) was generated by PCR amplification using the plasmid pFB-sMage3 (28) containing the sMage3 fragment as a template. The pair of primers for the PCR reaction were: 5′-primer (A), 5′-TTTGGTACCATGAAGGTCTCCGCGGCAGCCCT-3′, corresponding to the sequence of nucleotides 1–23 of the human RANTES gene with an additional KpnI restriction site, and 3′-primer (B), 5′-GCCGCGGCCGCCTCTTCCCCCTCTCTCAAAACCCA-3′, corresponding to nucleotides 921–945 of Mage3 with an additional NotI site. The human HSP70 cDNA was generated by PCR amplification using the plasmid pc-sHsp containing human HSP70 cDNA as a template. The pair of primers for the PCR reaction were: 5′-primer (C) 5′-GAGGCGGCCGCGGCCAAAGCCGCGGCGATCGGCAT-3′, corresponding to nucleotides 3–23 of the human HSP70 gene, with an additional NotI site, and 3′-primer (D) 5′-TTTCTAGACTCGAGCTAATCTACCTCCTCAATGGTGG-3′, corresponding to nucleotides 1904–1926 of the human HSP70 gene, with an additional XbaI site. The plasmid pc-sMage3Hsp was constructed by a three-way ligation of the KpnI/NotI-digested sMAGE3-containing fragment, the NotI/XbaI-digested HSP70 fragment, and the KpnI/XbaI-digested pcDNA3.1 vector. The resultant plasmid construction was identified by restriction enzyme analysis and confirmed by DNA sequencing. pcDNA3.1-sMAGE3 (pc-sMage3) which expresses the secretory Mage3 was constructed by using the Nhe1/EcoRI-digested fragment from pc-sMage3Hsp to replace the corresponding fragment in pc-Mage3 vector.
To construct the recombinant vector pFastBac-Mage3Fc for producing Mage3 protein, the Mage3Fc fusion gene was cut from plasmid pFB-Mage3Fc (28) with SalI/XmaI, and then cloned into SalI/XmaI digested pFastBacFc (there is an unique XmaI site within Fc gene) to create pFastBac-Mage3Fc.
Radiolabeling and immunoprecipitation
COS-1 cells were transfected with the various recombinant pcDNA3.1 vectors. Two days later, the transfected cells were incubated with 100μCi/ml 35S-Easytag Express (Perkin-Elmer) for 4 hr. The labeling media was harvested and the cells were lysed in TNT lysis buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, and 1% Triton X-100] with protease inhibitor cocktail tablets (Boehringer Mannheim). The clarified labeling media and cell lysates were submitted to immunoprecipitation with anti-Mage3 MAb57B (29) or anti-Hsp70 (Stressgen Biotechnologies) antibodies bound to protein A-Sepharose (Sigma-Aldrich). The immunocomplexes were analyzed by 4–15% SDS-PAGE reducing gels.
Recombinant protein production
Recombinant Mage3Fc fusion protein was produced in Sf9 insect cells by using a baculovirus expression system (Invitrogen), purified by using affinity binding to Protein A (Sigma-Aldrich), and tested by Western blot analysis and immunofluorescence analysis with an antibody to human IgG Fc (Sigma-Aldrich).
DNA preparation and immunization
DNA was isolated with an endotoxin-free purification kit (Qiagen) according to a standard protocol. DNA was resuspended in endotoxin-free PBS (Sigma-Aldrich) at a final concentration of 1 mg/ml. DNA was analyzed by restriction digestion and stored at −20°C. Mice were injected intramuscularly (i.m.) in the quadriceps with 100μg DNA in 100μl endotoxin-free PBS. At various times after immunization, spleens, blood, and other tissues were collected.
ELISA
Anti-Mage3 antibodies in the sera of immunized mice were detected by using ELISA. Briefly, microtiter plates coated with recombinant Mage3Fc proteins (50 ng per well) were incubated with serially-diluted sera in a blocking buffer (KPL, Inc.) at 37°C for 2 hr. Bound antibody was detected after incubation with a horseradish peroxidase (HRP)-conjugated antibody against mouse IgG (Sigma-Aldrich) diluted in the blocking buffer. MAb57B against Mage3 was used as a positive control, and normal mouse sera as a negative control. The background OD450 of normal mouse sera was less than 0.05. For detecting Mage3 secretion, MAB57B was used as a capture antibody, and rabbit polyclonal anti-Mage3 was used as a detection antibody (Santa Cruz Biotechnology, Inc.).
Elispot assay
The Elispot assay was used to detect the Mage3-specific T cell response. Briefly, Elispot plates (Millipore) were incubated with monoclonal anti-IFN-γ AN18 antibody (Mabtech AB) (75 ng/well) overnight and then blocked with RPMI media supplemented with 10% FBS. Effector cells (1×105, or 2×105) were co-cultured with protein (50 ng per well) or irradiated tumor cell lysate (5,000 cells per well) in the coated plate for 20 hr at 37°C. After washing, biotinylated anti-mouse IFN-γ antibody was added to the wells, and then incubated for 2 hr. After another wash, HRP-conjugated avidin was added to the wells and incubated for 1 hr. Finally, spots were developed by the addition of HRP substrate (Vectastain ABC Kit, Vector Laboratories). After spot development, the plate was rinsed thoroughly with ddH2O and allowed to dry. Spots were analyzed by Zellnet Consulting, Inc. (New York).
Preparation of DCs
Six- to eight-week-old C57BL/6 mice were sacrificed, and spleens were collected. DCs were positively isolated from spleen suspensions with anti-CD11c (N418) Micro-Beads (Miltenyi Biotec, Inc.), and cultured in RPMI-1640 media with 10% FBS, 20 ng/ml GM-CSF, 20 ng/ml IL-4, and 20 ng/ml TNFα as described (30–32). For preparation of antigen-pulsed DCs, on the second day of culture, DCs were incubated overnight with 50μg/ml Mage3Fc protein in culture media supplemented with the above concentrations of cytokines.
CD4+ T cell assay
To test the CD4+ T cell response, a separate set of immunized mice were sacrificed and spleens were collected. CD4+ T cells were positively isolated from spleen suspensions with anti-CD4 (L3T4) Micro-Beads. Freshly isolated CD4+ T cells were co-cultured with Mage3 protein-pulsed DCs (20:1) in RPMI-1640 supplemented with 10% FBS for 20 hr. The number of CD4+ T cells producing IFN-γ was determined by using the Elispot assay.
CTL cytotoxicity assay
CTL assay was used to detect a Mage3-specific CD8+ T cell response. Briefly, pooled splenocytes from immunized mice were restimulated in vitro in RPMI-1640 containing Mage3 protein (25μg/ml) for 6 d. TC-1/Mage3 was labeled with 150μCi of sodium 51Cr chromate solution (Amersham) for 90 min. Different numbers of effector cells were incubated with a constant number of target cells (1×104 cells/well) in 96-well U-bottom plates (200μl/well) for 5 hr at 37°C. The supernatants from triplicate cultures were collected. The percent lysis was defined as (experimental release − spontaneous release) / (maximum release − spontaneous release) × 100. Maximum release was determined by cell lysis using 1% Triton X-100.
Natural Killer cell depletion
For NK cell depletion, 250μg of anti-NK antibody (PK136) or control IgG in 250μl were intraperitoneally injected into each mouse on day −3 and −1 before tumor challenge, followed by three injections on days 2, 6, and 10 post-tumor injection. Depletion of NK cells was determined by using flow cytometry (33, 34).
Intracelluar IFN-γ staining
Splenocytes were stimulated with Mage3 protein for 12 h in 96-well round bottom plates (Costar) in the presence of the protein transport inhibitor GolgiPlug containing brefeldin A (BD Biosciences, Erembodgem, Belgium). After washing away culture supernatant, cells were stained for CD4 and CD8 membrane markers for 30 min at 4°C. Following fixation (Cytofix/Cytoperm; BD Bioscience) and permeabilization (Perm/Wash, BD Bioscience), pellets were stained at 4 °C for IFN-γ using specific monoclonal antibodies. Finally, cells were washed and acquired on a flow cytometer.
Tumor challenge studies
C57BL/6 mice were immunized by i.m. injection with 100 g of different recombinant plasmids twice at a two-week interval. Two weeks after the second DNA immunization, these mice were challenged with exponentially growing TC-1/Mage3 (5×104 cells) injected subcutaneously. For therapeutic purposes, mice were inoculated with 5×104 TC-1/Mage3. Three days later, the mice received an i.m. injection of 100μg of different recombinant DNA plasmid constructs twice at a 1-week interval. Tumor sizes were measured every three or four days, with tumor volumes calculated as follows: (longest diameter) x (shortest diameter)2 (28).
Statistical analyses
All data are presented as means and standard errors (s.e.). Analysis of variance was used to determine the level of differences between groups. Different groups were compared by using the Student-Newman-Keuls test with SigmaStat 2.03 software (SPSS Inc.), or a Chi-square test (be indicated in the text). P values were considered significant at 0.05.
RESULTS
Construction and characterization of DNA constructs
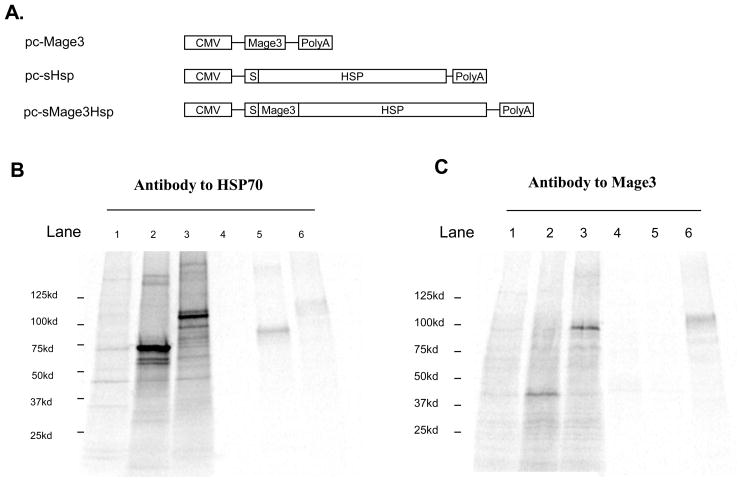
Owed to the promiscuous functions of HSPs in inducing innate and adaptive immunity (20, 35), human HSP70 has been used to enhance tumor antigen-specific immune responses in DNA vaccine strategies by immunization with tumor Ag-HSP fusion genes. Mage3 is a frequently-expressed CT antigen in many tumors, and therefore represents an ideal target for tumor immunotherapy. To assess whether human inducible HSP70 enhances the efficacy of a Mage3 DNA vaccine, Mage3 with a leader sequence (for secretion) was fused in-frame with the human HSP70 gene and then cloned into the pcDNA3.1 vector (pc-sMage3Hsp). Control vectors containing the Mage3 gene (pc-Mage3) or human HSP70 gene with the leader sequence (pc-sHsp) were also constructed (21, 28) (Fig. 1A). By using radiolabeling and immunoprecipitation/SDS-PAGE analyses, sMage3Hsp, with an estimated molecular mass (Mr) of ≈107 kDa, Mage3, with an estimated Mr of ≈ 40 kDa, and sHsp, with an estimated Mr of ≈72 kDa were found to efficiently express, and sMage3Hsp and sHsp were secreted from transfected cells due to the added RANTES signal sequence (Fig. 1B and 1C). Both intracellular and secreted sMage3Hsp proteins were recognized by anti-Mage3 and anti-HSP antibodies. The secreted HSP70 may be differentially glycosylated as HSP70 precipitated from culture medium migrated on SDS-PAGE as a band with a larger Mr than the protein that was immunoprecipitated from transfected cell lysates. The secreted sMage3Hsp displayed a similar Mr with its intracellular form. The expressed Mage3 protein has the same Mr as the reported.
Fig. 1. Construction and characterization of the pc-sMage3Hsp plasmid.
A, Schematic representation of recombinant pcDNA3.1 vectors. The sMage3Hsp fusion gene and control genes Mage3 and sHsp70 were cloned into the pcDNA3.1 vector under control of the CMV promoter. B and C, Expression and secretion of proteins expressed from recombinant pcDNA3.1 vectors. The transfected COS-1 cells were radiolabeled. Clarified cell lysates and culture media were then incubated with monoclonal anti-HSP70 (B) or anti-Mage3 (C) antibodies bound to protein A-Sepharose. The immune complexes were analyzed by 4–15% SDS-PAGE reducing gels. Lane 1, COS-1 cell lysate; lane 2, pc-sHsp (B) or pc-Mage3 (C) transfected COS-1 cell lysate; lane 3: pc-sMage3Hsp-transfected COS-1 cell lysate; lane 4: COS-1 cell culture medium; lane 5: pc-sHsp (B) or pc-Mage3 (C) transfected COS-1 cell culture medium; lane 6: pc-sMage3Hsp-transfected COS-1 cell culture medium.
Enhancement of Mage3-specific immune responses
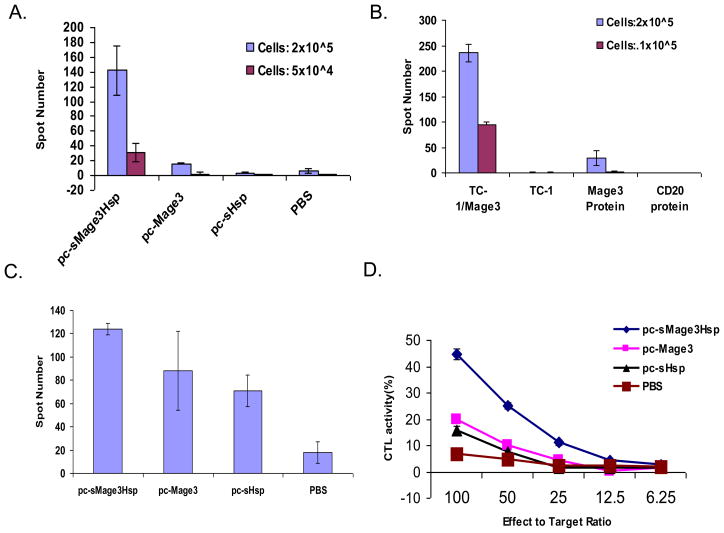
To evaluate whether inclusion of HSP70 enhances the efficacy of the Mage3 DNA vaccine, C57BL/6 mice were immunized with pc-sMage3Hsp, pc-Mage3, pc-sHsp, or PBS. The mice were sacrificed and peripheral blood, spleen, and other tissue samples were collected. First, we evaluated if Mage3-specific T cell responses could be induced in these mice. Splenocytes from DNA-immunized mice were isolated and incubated with a TC-1/Mage3 cell lysate. The frequency of T cells producing IFN-γ in response to Mage-3 was assessed by using Elispot assay. As shown in Fig. 2A, pc-sMage3Hsp-immunized mice showed an ≈7-fold increase in the number of T cells producing IFN-γ in response to stimulation of Mage3-expressing tumor cells relative to pc-Mage3-immunized mice (P < 0.05), while pc-sHsp and PBS-immunized mice only had background levels of T cells producing IFN-γ. The specificity of the T cells in response to Mage3 protein was evident in that the T cells from the pc-sMage3Hsp-immunized mice showed much lower activity in response to non-transfected TC-1 stimulation (P < 0.05) or stimulation with an irrelative protein (CD20) (P < 0.05) (Fig. 2B). These results indicate that vaccination with sMage3Hsp DNA elicited Mage3-specific immune responses with higher potency.
Fig. 2. Enhancement of Mage3-specific T cell immune responses.
Mice were immunized i.m. with 100 μg of different DNA constructs twice at a two-week interval. Data were mean +/− s.d. of triplicate wells. A, Splenocytes were harvested and incubated with TC-1/Mage3 cell lysate for 20 hr. The frequency of T cells producing IFN-γ was determined by using Elispot. B, Specificity of splenocytes from pc-sMage3Hsp-immunized mice responding to TC-1/Mage3 vs. parental TC-1 cells, and Mage3 protein vs. irrelevant CD20 protein was determined by using Elispot. C, Response of CD4+ T cells to Mage3-pulsed DCs was determined by using Elispot. D, CTL response of splenocytes to TC-1/Mage3 was determined by using a 51Cr-release assay.
To measure the Mage3-specific CD4+ T cell response, CD4+ T cells were isolated from splenocytes, co-cultured with CD11c+ DCs pulsed with Mage3 protein, and analyzed by using Elispot assay. As shown in Fig. 2C, a higher frequency of CD4+ T cells derived from pc-sMage3Hsp-immunized mice produced IFN-γ in response to stimulation of Mage3 protein-pulsed CD11c+ DCs (P < 0.05, pc-sMage3Hsp vs. pc-Mage3, by a Chi-square test). To determine the Mage3-specific CD8+ T cell response, we performed a 51Cr release assay. Splenocytes from a different set of immunized C57BL/6 mice were restimulated in vitro in medium containing Mage3 protein. The restimulated cells were then co-cultivated with 51Cr-labeled TC-1/Mage3 cells at various ratios to measure target cell killing. As shown in Fig. 2D, splenocytes from mice immunized with pc-sMage3Hsp DNA demonstrated significantly higher target cell killing than did those from mice immunized with pc-Mage3 (P < 0.05) or pc-sHsp DNA (P < 0.05) alone, or PBS (P < 0.01). The specificity of the killing was demonstrated by splenocytes from pc-sMage3Hsp-immunized mice that killed parental TC-1 target cells with a much lower potency (data not shown).
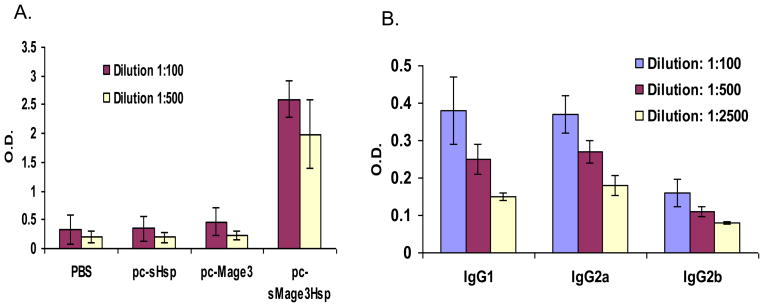
To determine whether immunization with DNA encoding sMage3Hsp can also enhance an antibody response, we measured the level of anti-Mage3 IgG in the sera of mice immunized with different DNA constructs by using ELISA. As shown in Fig. 3A, antibody levels detected in C57BL/6 mice immunized with pc-Mage3 or pc-sHsp DNA were markedly lower than those immunized with pc-sMage3Hsp DNA (P< 0.01). The specificity of the antibody response was demonstrated by the lack of antibody against an irrelative protein (human tyrosinase) in the immunized mice (data not shown). Analysis of IgG subtypes in sera from mice immunized with pc-sMage3Hsp DNA was then performed. As shown in Fig. 3B, IgG1 and IgG2a levels are similar and significantly higher than IgG2b, implying that pc-sMage3Hsp immunization might not polarize differentiation of either CD4+ T cell subset (T helper1 or T helper2) preferentially.
Fig. 3. Enhancement of antibody responses.
Mice were immunized with different DNA constructs twice at a two-week interval, and sera were harvested two weeks after the second immunization. The Mage3-specific IgG antibody levels from sera of different mouse groups were determined by ELISA. The mean OD450 values of sera from different groups were presented as mean +/− s.d. of three samples. The background OD450 of normal mouse sera was <0.05. A. IgG activity from different immunized C57BL6 mice. B. IgG subtypes analysis from C57BL/6 immunized with pc-sMage3Hsp.
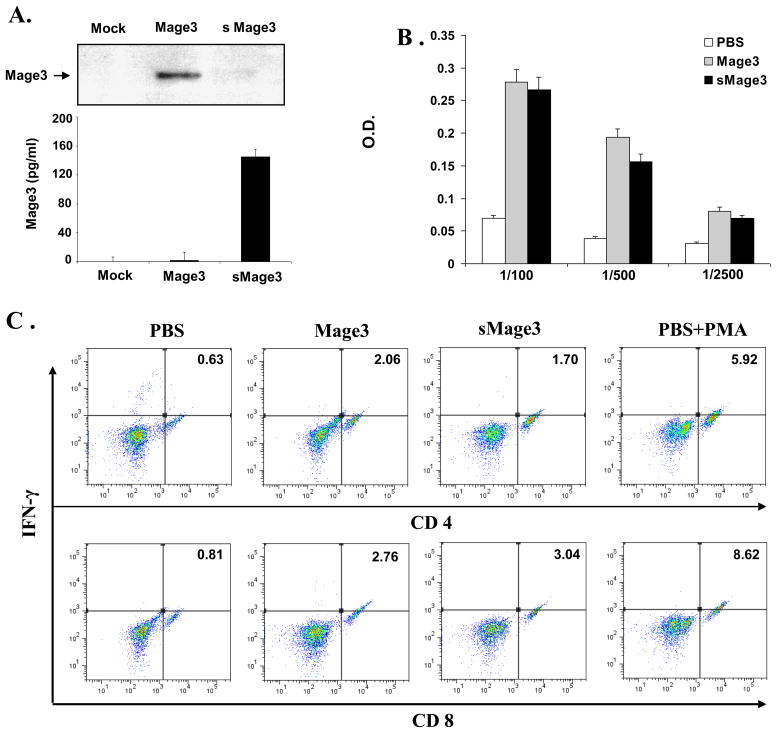
To rule out the possibility that the superior immunostimulatory function of pc-sMage3Hsp is derived from the secretion of the Mage3 fusion protein rather than the adjuvant function of Hsp protein, we constructed a secretory Mage3 (sMage3) expression vector pc-sMage3 in which the signal sequence of the human RANTES gene was in-framed fused to the N-terminus of Mage3 protein. Western blot assay showed that only a trace amount of sMage3 located inside the pc-sMage3 -transfected 293 T cells after being synthesized (Fig. 4A, the upper panel), and a majority of the protein was secreted, as manifested by analysis of the collected supernatants via ELISA (Fig. 4A, the lower panel). Furthermore, we compared immunostimulatory activity of pc-sMage3 and pc-Mage3 by immunization of C57BL6 mice with these expression vectors as described above. As shown in Fig. 4B, immunization with the vectors expressing different forms of Mage3 induced a comparable antibody response. Intracellular IFN-γ staining result showed that Mage3-specific CD4+ and CD8+ T cell responses were also equivalent (Fig. 4C). These results suggested that secretion of Mage3 from the originally transfected cells does not contribute to the immunostimulatory ability of Mage3, and the observed enhanced immunostimulation of pc-sMage3Hsp is derived from the Hsp-associated adjuvant function instead of the protein secretion from the transfected cells.
Fig. 4. Characterization and immunostimulatory effect of pc-sMage.
A, Expression and secretion of Mage3 from pc-Mage3 or pc-sMage3-transfected 293 T cells, tested by western blotting (the upper panel) or ELISA (the lower panel). B. Antibody reponse from pc-Mage3 or pc-sMage3 immunized mice evaluated by ELISA. C. CD4+ and CD8+ T cell responses from pc-Mage3 or pc-sMage3 immunized mice evaluated by intracellular IFN-γ staining. The data are the representative of the two independent experiments.
Antitumor effect of sMage3Hsp DNA immunization
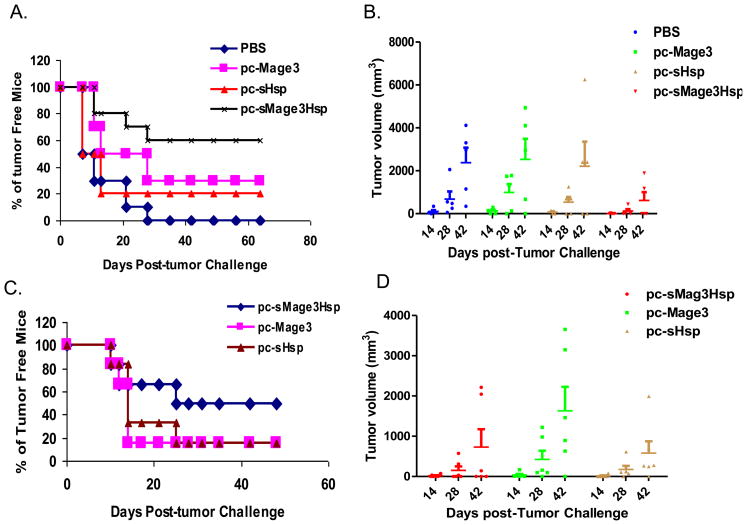
We developed a tumor challenge model that allows us to investigate anti-Mage3 immune responses induced by pc-sMage3Hsp DNA to control Mage3-expressing tumor cells in vivo. We used the tumor cell line TC-1 that grows rapidly in syngeneic mice (36) as the target cell line for challenge experiments. TC-1 clones transfected with the Mage3 expression vector were generated and shown to express Mage3 by RT-PCR (data not shown). When subcutaneously implanted into syngeneic C57BL/6 mice, TC-1/Mage3 cells showed aggressive tumor growth (albeit slightly slower than the parental TC-1 cells), producing visible tumors in mice by only 7–10 days after inoculation. To test if the enhanced anti-Mage3 immune response could lead to effective inhibition of TC-1/Mage3 growth in immunized mice, C57BL/6 mice were immunized with pc-sMage3Hsp, -Mage3, -sHsp DNA, or PBS, followed by challenge with TC-1/Mage3 cells. As shown in Fig. 5A, pc-sMage3Hsp immunization produced superior protection, with 60% of the mice tumor-free for the observed period (more than 12 weeks), and the total tumor volume only ≈1/3 that of the other groups (P < 0.05, pc-sMage3Hsp vs. pc-sHsp) (Fig. 5B). In contrast, immunization with the pc-Mage3 only inhibited tumor occurrence to 30% (P < 0.05, pc-sMage3Hsp vs. pc-Mage3, by a Chi-square test) pc-sHsp DNA inhibited to 20% (P < 0.05, pc-sMage3Hsp vs. pc-sHsp) and the PBS group had 100% tumor occurrence (P < 0.01, pc-sMage3Hsp vs. PBS). Repeated experiments showed similar results. Thus, the anti-tumor activity shown by immunization with pc-sMage3Hsp DNA correlated with the ability to induce immune responses.
Fig. 5. Antitumor effect of various DNA vaccines.
A & B, C57BL/6 mice were immunized by i.m. injection with 100 g of different expression constructs twice at a two-week interval. Two weeks after the second injection, the mice were inoculated s.c. with TC-1/Mage3 tumor cells. A, Tumor occurrence was monitored, and the percentage of tumor-free mice was calculated on the indicated days. B, Tumor size was measured for the tumor-bearing mice, and the data were reported as the tumor volume of each mouse per group and were from a representative experiment. C & D, C57BL/6 mice were inoculated s.c. with TC-1/Mage3 tumor cells. Three days later, the mice were immunized by i.m. injection with 100 μg of different constructs twice with a one-week interval. C, The percentage of tumor-free mice was calculated on the indicated days. D, Tumor size was measured on the indicated days. The data were reported as the tumor volumes of each mouse per group and were from a representative experiment.
One major challenge faced by tumor immunotherapy is how to control existing tumor in cancer patients. To test if the sMage3Hsp DNA vaccine has any efficacy in inhibiting existing tumor growth, C57BL/6 mice were first inoculated with TC-1/Mage3 cells. Three days later, the mice were treated with i.m. injection of 100 g of different DNA constructs. As shown in Fig. 5C, 50% of the mice were tumor-free following injection with sMage3Hsp DNA, while either Mage3 or sHsp DNA injection only protected 16.7% of the mice from tumor occurrence (P < 0.05). However, we were unable to detect any significant difference on the tumor volume between pc-sMage3Hsp and pc-sHsp immunization, although the tumor volumes in these two groups were apparently smaller than that in pc-Mage3 immunized group (Fig. 5D). We also noted that protection mediated by Mage3 DNA immunization only functions at an early stage of tumor development when tumor cell number is low, as the DNA vaccine either keeps mice tumor-free, or leaves tumor growing in the immunized mice with similar dynamics among the groups (Fig. 5D). This was further verified by inoculation of mice with a larger number of TC1/Mage3 cells (3×105) which led to all groups having tumor growth (data not shown). The result suggested that pc-sMage3Hsp immunization might only have a slight advantage to inhibit pre-existing tumor.
Role of different immune effector cells in sMage3Hsp DNA immunization
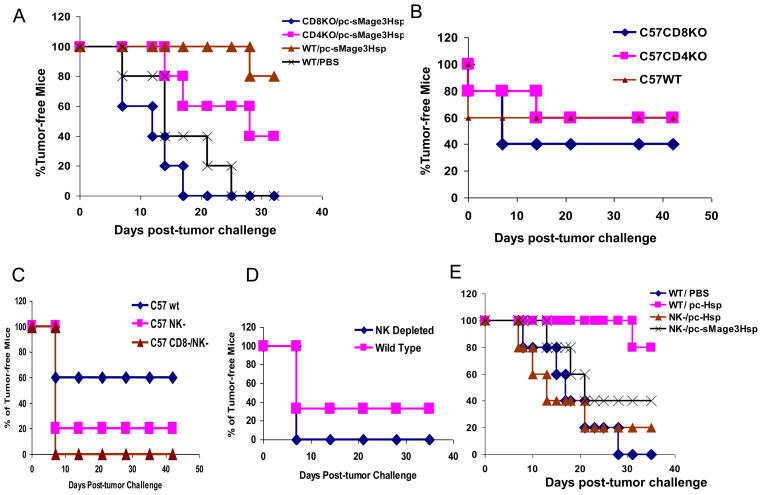
As described above, sMage3Hsp DNA immunization induced a potent CD4+ and CD8+ T immune response. To dissect the effect of CD4+ and CD8+ T cells in sMage3Hsp-mediated anti-tumor activity, we tested sMage3Hsp DNA immunization to control pre-existing tumor cells in CD4+ or CD8+ KO mice. Groups of mice including C57BL/6 wild type, CD8+KO, or CD4+KO mice were s.c. inoculated with TC-1/Mage3 tumor cells. Three days later, the mice immunized with 100ug of pc-sMage3Hsp twice at one week interval. One group of C57BL/6 wild type mice were injected with PBS to serve as a control. We failed to detect the function of CD8+ and CD4+ T cells in the experimental setting, i.e. all pc-sMage3Hsp DNA immunized mice displayed a similar and low level of tumor rejection (Data not shown) compared with PBS-injected group. In consideration of the possibility that cultured TC-1/Mage3 cells may gradually reduce Mage3 expression even in the presence of Zeocin (100ug/ml), which was later confirmed by RT-PCR (data not shown), we repeated the experiment by inoculation of newly generated TC-1/Mage3 instead of the in vitro maintained. Result showed that with no tumor-free mice found in non-immunized wild type group, pc-sMage3Hsp immunization led to 80% of wild type mice tumor-free, 40% of CD4+ KO mice tumor-free (P < 0.05, CD4+ KO vs. wild type, by a Chi-square test), and no tumor-free mice in CD8+ KO group after the observed period (32 days post-inoculation) (P < 0.01, CD8+ KO vs wild type) (Fig. 6A). The result implies that both CD4+ and CD8+ T cells play a role in pc-sMage3Hsp controlling pre-existing tumor cells, but CD8+ T cells are more critical for the pc-sMage3Hsp-mediated anti-tumor activity. The experimental results also suggest that pc-sMage3Hsp-induced adaptive immunity controls the pre-existing tumor cells when the tumor cells express the tumor antigen above a certain threshold.
Fig. 6. Function of different immune effector cells in Mage3Hsp DNA vaccination.
A C57BL/6 wild-type, CD4+ KO, or CD8+ KO mice were inoculated with newly generated TC-1/Mage3 cells. Three days later, the mice were immunized by i.m. injection with 100 μg of pc-sMage3Hsp DNA or PBS twice at a one-week interval.. The percentage of tumor-free mice was calculated on the indicated days. B. C57BL/6 wild-type, CD4+ KO, or CD8+ KO mice were immunized by i.m. injection with 100 μg of pc-sMage3Hsp DNA twice at a two-weeks interval. Two weeks after the second injection, the mice were inoculated s.c. with in vitro maintained TC-1/Mage3 tumor cells. The percentage of tumor-free mice was calculated on the indicated days. C. C57BL/6 wild-type and CD8+ KO mice were immunized by i.m. injection with 100 μg of pc-Mage3Hsp DNA twice at a two-week interval. Two weeks after the second injection, the mice were inoculated s.c. with TC-1/Mage3 tumor cells. The CD8+ KO mice and a half of the C57BL/6 wild-type mice received PK136 treatment prior to and after TC-1/Mage3 challenge. The remaining half of wild-type mice received control IgG administration. The percentage of tumor-free mice was calculated on the indicated days. D. C57BL/6 mice were immunized by i.m. injection with 100 g of pc-Mage3Hsp DNA twice at a two-week interval. Two weeks after the second injection, the mice were inoculated s.c. with TC-1 tumor cells. Half of mice received PK136 treatment, and half received control IgG administration. The percentage of tumor-free mice was calculated on the indicated days. E. C57BL/6 mice were immunized by i.m. injection with 100 μg of pc-sHsp, pc-Mage3Hsp DNA or PBS twice at a two-week interval. Two weeks after the second injection, the mice were inoculated s.c. with the newly generated TC-1/Mage3 tumor cells. Half of pc-sHsp-immunized mice and all pc-sMage3Hsp-immunized mice received PK136 treatment. The percentage of tumor-free mice was calculated on the indicated days.
We also tested the prophylactic immunization of pc-sMage3Hsp in these knockout mice. CD4+ and CD8+ T cell KO mice, in parallel with wild-type C57BL/6 mice, were immunized with sMage3Hsp DNA twice, and then challenged with 50,000 TC-1/Mage3 tumor cells (the in vitro maintained) two weeks later. We found that CD4+ T cells were dispensable, while CD8+ T cells played some (but not whole) role in the anti-tumor effect of pc-sMage3Hsp prophylactic immunization, as immunization with sMage3Hsp DNA resulted in 60% of the CD4+ KO mice or wild type mice tumor-free, and 40% of the CD8+ KO mice tumor-free (P < 0.05, CD8+ KO vs. wild-type, by a Chi-square test) (Fig. 6B).
To investigate whether other components of the immune system also play a role in pc-sMage3Hsp-induced prophylactic effect, we investigated contribution of NK cells to the anti-tumor effect by depletion of NK cells from pc-sMage3Hsp-immunized mice. For this study, three groups of mice were included: C57BL/6 wild-type; C57BL/6 with NK depletion; and CD8+ KO mice with NK depletion. The antibody PK136, which reacts with the NK-1.1 surface antigen expressed on NK cells (as well as NKT cells) of C57BL/6 mice, was used in the antibody-mediated NK depletion assay prior to and after tumor challenge. The results showed that immunization with sMage3Hsp DNA only protected 20% of the NK-depleted wild-type mice from TC-1/Mage3 challenge (P < 0.01, wild-type vs. NK-depleted, by a Chi-square test), and did not protect any NK-depleted CD8+ T cell KO mice from the tumor challenge (P < 0.01). In comparison with that sMage3Hsp protected 60% of the wild-type mice from tumor challenge (Fig. 6B), this result suggests that NK cells and CD8+ T cells synergically function for sMage3Hsp-mediated prophylactic effect.
To further confirm NK-mediated non-specific immunity in pc-sMage3Hsp prophylactic vaccination, C57BL/6 mice immunized with pc-sMage3Hsp were challenged with parental TC-1, not TC-1/Mage3 cells. Half of the mice received PK136 treatment, and half received control IgG prior to and after TC-1 tumor challenge. The results show that sMage3Hsp immunization protected 33.3% of the wild-type mice, but did not protect NK-depleted mice from parental TC-1 challenge (P < 0.05, NK depleted vs. wild type) (Fig. 6C). The result further supported that NK was important for the pc-sMage3Hsp-induced anti-tumor immunity.
To demonstrate activation of NK cells in the prophylactic immunization is owed to the Hsp component in pc-sMage3Hsp, we performed the NK depletion assay in mice immunized with pc-sHsp. Four groups of mice were included in the study: C57BL/6 injected with PBS; C57BL/6 immunized with pc-sHsp; C57BL/6 depleted of NK, immunized with pc-sHsp, and C57BL/6 depleted of NK, immunized with pc-sMage3Hsp. Two weeks after the 2nd immunization, the immunized mice were challenged with newly generated TC-1/Mage3, which expressed a higher level of Mage3. As expected, while no tumor-free mice were found in the non-immunized group, pc-sHsp immunization led to 80% of wild-type mice tumor-free, but only 20% of NK-depleted mice tumor-free (P < 0.05, NK depleted vs. wild type). As a control, pc-sMage3Hsp immunization led to 40% of NK-depleted mice tumor-free (Fig. 6E). The result confirms that Hsp has an adjuvant function in activation of NK-mediated innate immune response to control growth of the inoculated tumor. Of note, a big deviation was observed in the ability of pc-sHsp immunization to control tumor occurrence between the described experiment here (Fig. 6E) and that in Fig. 4A., which may be owed to the newly generated TC-1/Mage3 cells expressing a higher level of Mage3 and also possibly having a lower growth rate.
DISCUSSION
We and others have demonstrated the superior adjuvant ability of HSPs in DNA vaccination (21, 22). Here, we applied this strategy to a frequently-expressed CT antigen, Mage3, to test if HSP70 can improve the efficacy of a Mage3 DNA vaccine. Our results are consistent with other studies in that the HSP-based DNA vaccine induced both innate and adaptive immune responses for prevention of tumor occurrence in prophylactic models. Furthermore, we also demonstrated that vaccination with sMage3Hsp DNA overcame pre-existing tumor cells that was inoculated three days prior to vaccination with a higher efficiency. This finding is very significant for the clinical treatment of early stage cancer patients.
Anti-tumor immunity consists of the innate and adaptive immune responses. CD8+ and CD4+ T cells represent major adaptive cellular immune mechanisms: CD8+ T cells recognize tumor cells through an MHC I-dependent process, and then lyse cells harboring tumor-associated antigens by the secretion of perforin and granzymes; CD4+ T helper cells are activated by antigen presenting cells (APCs) via the MHC II/antigen complex. Activated CD4+ T helper cells produce different cytokines for polarization and optimization of immune responses. Administration of naked plasmid DNA only leads to a low level of protein expression in APCs in immunized animals (33). HSPs possess a superior ability to chaperone exogenous protein or peptide to access the MHC I pathway but also, albeit less efficiently, the MHC II pathway by cross-presentation to elicit CD8+ and CD4+ T cell responses (20, 34, 37–39). In support of the function of HSPs in mediating priming or cross-priming of CD4+/CD8+ T cells, our results showed that the sMage3Hsp DNA vaccine induced both CD4+ T cell responses and CTL immune responses more efficiently compared with the control groups, and the antitumor activity induced by pc-sMage3Hsp reduced in both CD4+ KO or CD8+ KO mice. The result supports that Hsp protein possesses an adjuvant function for DNA-based vaccination to trigger CD4+ and CD8+ T cell response.
The prophylactic immunization of pc-sMage3Hsp still preserved a certain antitumor immunity in either CD4+ KO or CD8+ KO mice, implying innate immune response might involve the antitumor activity of sMage3Hsp DNA. The innate immune system provides many ways to quickly resist tumor. The two best-studied mechanisms are the production of protective cytokines and the activation and expansion of innate lymphocytes (40). NK cells are a critical component of the innate immune response and can rapidly kill tumor or infected cells without prior activation (41, 42) by recognizing abnormal or reduced levels of MHC class I molecules (39–42) and/or ‘stress-induced’ proteins (47). In addition, NK cells also activate CD4+ and CD8+ T cells by production of cytokines and cross-talk with dendritic cells (48). As expected, NK depletion was found leading to a major loss of antitumor activity in pc-sMage3Hsp immunized mice, and pc-sMage3Hsp immunization also triggered antitumor activity against the parent TC-1 tumor which does not express Mage3 antigen in a NK cell-dependent manner. Furthermore, the pc-sHsp immunization induced potent tumor rejection in wild-type but not the NK-depleted mice, which supports activation of NK cell response is owed to the Hsp component of pc-sMage3Hsp. These studies reveal the adjuvant function of Hsp in activation of NK cells, also somehow support the recently proposed alternative Hsp working model (49), which suggests the role of Hsp in tumor immunity is activation of innate immune cells such as NK and/or NKT cells and γδT cells. These activated, innate immune effector cells non-specifically kill tumor which releases tumor antigens into the extracellular milieu. These tumor antigens can then be internalized and processed by DCs for the presentation of tumor-derived peptides and activation of tumor-specific CD4+and CD8+ T cells.
As above described, Mage3 is an ideal target for tumor immunotherapy given its immunogenicity and the tissue-restricted expression. However, although a mouse homolog of human MageA is reported (50), currently no mouse homologue of Mage3 has been defined, and neither has Mage3-transgenic mouse been developed, which greatly restricts development of Mage3-based tumor vaccine. It is certain that testing sMage3Hsp DNA vaccine in a mouse model would incur immune rejection against the human Mage3 antigen, as evidenced by that prophylactic immunization of pc-Mage3 resulted in 30% of immunized mice tumor-free in our present study. However, we noticed in the present study that all non-immunized mice had the inoculated TC-1/Mage3 tumor growth and all the immunized mice had the inoculated TC-1/Mage3 tumor growth when a larger number of TC-1/Mage3, e.g. 3–5×105 was given (data not shown), which implies the immune rejection response triggered by human Mage3 is limited in the mouse model, there is still a space in the mouse model to allow evaluation of Mage3-based vaccine strategy. Indeed, mouse model has been used for early preclinical study of human tumor-associated antigen (TAA)-based vaccine strategy. For instance, You et al tested immunization of mice with the transduced DCs expressing a human Mage3 fusion protein, and found the IgG-Fc fused to Mage3 can enhance ability of the transduced DCs to trigger Mage3-specific CD4 T cell immune response (28). Bae et al tested immunization of mice with DCs pulsed with human carcinoembryonic antigen (CEA), and found the human HIV transactivating Tat fused to CEA upregulated the pulsed DCs to induced CD4 Th1 immune response, and thereby enhanced protection from CEA-expressed tumor challenge (51). These studies testing human TAA in animal model, although don’t exactly reflect the genuine in human body, still provided important insights into developing better TAA-based tumor vaccines.
Even human Mage3 represents a foreign antigen, and Hsp enhanced activation of both innate and adaptive immune responses in the vaccine regimen, the pc-sMage3Hsp immunization is only effective to control a small number, Mage3-highly expressed, pre-existing tumor cells. The result suggested that tumor-mediated immune tolerance is developed by multiple mechanisms in addition to the central tolerance mechanism, which protects not only TAA-expressed tumor, also mutated Ag-expressed tumor from immune surveillance. Thus, further vaccine development necessitates incorporation of the component which can maximally overcome tumor-associated peripheral tolerance, such as myeloid-derived suppressor cells (52), tumor-associated macrophages (53), and regulatory T cells (54), etc. It is also worth pointing out that tumor cell might develop the mechanism to down-regulate expression of a mutated gene (here a foreign gene) to escape immune surveillance for the survival, as in vitro cultured TC-1/Mage3 was observed to gradually reduce Mage3 expression in the study. If the tumor-associated down-regulation mechanism is confirmed, it will throw an obstacle for immunotherapy to overcome tumor.
Finally, our experiment showed the secretory Mage3 and intracellularly expressed Mage3 have the same efficiency in induction of cellular and humoral immune response, which is consistent with our previously unpublished result. The reason may be that the vector expresses many proteins, which can function as antigens to invoke immune responses. Thus, the vector-transfected cells will possibly become a target for immune attack, and leak or release intracellular Mage3 for induction of both T cell and B cell immune response.
In summary, our results demonstrate that the sMage3Hsp DNA vaccine could inhibit tumor occurrence with a higher efficiency in a mouse model by inducing both the innate and adaptive immune responses. Future studies will need to test if the sMage3Hsp DNA vaccine can inhibit the occurrence and growth of tumor with a higher efficiency when Mage3 serves as a self tumor-associated antigen such as in syngeneic Mage3-transgenic mouse models or clinical trials.
Acknowledgments
We thank Drs. Lei Shen and Wenhong Ren for providing with helpful discussions and Pauline Pittman for excellent technical assistance. This work was supported by NIH grant (5 R01 CA100841) and DOD grant (W81XWH-04-1-0194).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 2.Van der Bruggen P, van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254(5038):1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 3.Traversari C, van der Bruggen P, Van den Eynde B, Hainaut P, Lemoine C, Ohta N, et al. Transfection and expression of a gene coding for a human melanoma antigen recognized by autologous cytolytic T lymphocytes. Immunogenetics. 1992;35(3):145–52. doi: 10.1007/BF00185107. [DOI] [PubMed] [Google Scholar]
- 4.Sahin U, Türeci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci USA. 1995;92(25):11810–3. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lethe B, Lucas S, Michaux L, De Smet C, Godelaine D, Serrano A, et al. LAGE-1, a new gene with tumor specificity. Int J Cancer. 1998;76(6):903–8. doi: 10.1002/(sici)1097-0215(19980610)76:6<903::aid-ijc22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Zendman AJ, Cornelissen IM, Weidle UH, Ruiter DJ, van Muijen GN. CTp11, a novel member of the family of human cancer/testis antigens. Cancer Res. 1999;59(24):6223–9. [PubMed] [Google Scholar]
- 7.De Wit N, Weidle UH, Ruitter DJ, van Muijen GNP. Expression profiling of MMA-1 and splice variant MMA-1B: new cancer/testis antigens identified in human melanoma. Int J Cancer. 2002;98(4):547–53. doi: 10.1002/ijc.10241. [DOI] [PubMed] [Google Scholar]
- 8.Van den Eynde BJ, van der Bruggen P. T cell defined tumor antigens. Curr Opin Immunol. 1997;9(5):684–93. doi: 10.1016/s0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 9.Van der Bruggen P, Bastin J, Gajewski T, Coulie PG, Boel P, De Smet C, et al. A peptide encoded by human gene MAGE-3 and presented by HLA-A2 induces cytolytic T lymphocytes that recognize tumor cells expressing MAGE-3. Eur J Immunol. 1994;24(12):3038–43. doi: 10.1002/eji.1830241218. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka F, Fujie T, Tahara K, Mori M, Takesako K, Sette A, et al. Induction of antitumor cytotoxic T lymphocytes with a MAGE-3-encoded synthetic peptide presented by human leukocytes antigen-A24. Cancer Res. 1997;57(20):4465–8. [PubMed] [Google Scholar]
- 11.Chaux P, Vantomme V, Stroobant V, Thielemans K, Corthals J, Luiten R, Eggermont AM, Boon T, van der Bruggen P. Identification of MAGE-3 epitopes presented by HLA-DR molecules to CD4(+) T lymphocytes. J Exp Med. 1999;189(5):767–78. doi: 10.1084/jem.189.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manici S, Sturniolo T, Imro MA, Hammer J, Sinigaglia F, Noppen C, et al. Melanoma cells present a MAGE-3 epitope to CD4(+) cytotoxic T cells in association with histocompatibility leukocyte antigen DR11. J Exp Med. 1999;189(5):871–6. doi: 10.1084/jem.189.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber JS, Hua FL, Spears L, Marty V, Kuniyoshi C, Celis E. A phase I trial of an HLA-A1 restricted MAGE-3 epitope peptide with incomplete Freund’s adjuvant in patients with resected high-risk melanoma. J Immunother. 1999;22(5):431–40. doi: 10.1097/00002371-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4(3):328–32. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds SR, Oratz R, Shapiro RL, Hao P, Yun Z, Fotino M, et al. Stimulation of CD8+ T cell responses to MAGE-3 and Melan A/MART-1 by immunization to a polyvalent melanoma vaccine. Int J Cancer. 1997;72(6):972–6. doi: 10.1002/(sici)1097-0215(19970917)72:6<972::aid-ijc9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 16.Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–91. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 17.Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278(5335):117–20. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 18.Srivastava PK. Immunotherapy of human cancer: lessons from mice. Nat Immunol. 2000;1(5):363–6. doi: 10.1038/808795. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 20.Binder RJ. Heat-shock protein-based vaccines for cancer and infectious disease. Expert Rev Vaccines. 2008;7(3):383–93. doi: 10.1586/14760584.7.3.383. [DOI] [PubMed] [Google Scholar]
- 21.Hauser H, Shen L, Gu QL, Krueger S, Chen SY. Secretory heat-shock protein as a dendritic cell-targeting. [DOI] [PubMed] [Google Scholar]
- 22.Hsu KF, Hung CF, Cheng WF, He L, Slater LA, Ling M, et al. Enhancement of suicidal DNA vaccine potency by linking Mycobacterium tuberculosis heat shock protein 70 to an antigen. Gene Ther. 2001;8(5):376–83. doi: 10.1038/sj.gt.3301408. [DOI] [PubMed] [Google Scholar]
- 23.Suzue K, Young RA. Adjuvant-free hsp70 fusion protein system elicits humoral and cellular immune responses to HIV-1 p24. J Immunol. 1996;156(2):873–9. [PubMed] [Google Scholar]
- 24.Suzue K, Zhou X, Eisen HN, Young RA. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci USA. 1997;94(24):13146–51. doi: 10.1073/pnas.94.24.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciupitu AM, Petersson M, Kono K, Charo J, Kiessling R. Immunization with heat shock protein 70 from methylcholanthrene-induced sarcomas induces tumor protection correlating with in vitro T cell responses. Cancer Immunol Immunother. 2002;51(3):163–70. doi: 10.1007/s00262-002-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janetzki S, Palla D, Rosenhauer V, Lochs H, Lewis JJ, Srivastava PK. Immunization of cancer patients with autologous cancer-derived heat shock protein gp96 preparations: a pilot study. Int J Cancer. 2000;88(2):232–8. doi: 10.1002/1097-0215(20001015)88:2<232::aid-ijc14>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Ma JH, Sui YF, Ye J, Huang YY, Li ZS, Chen GS, et al. Heat shock protein 70/MAGE-3 fusion protein vaccine can enhance cellular and humoral immune responses to MAGE-3 in vivo. Cancer Immunol Immunother. 2005;54(9):907–14. doi: 10.1007/s00262-004-0660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.You Z, Hester J, Rollins L, Spagnoli GC, van der Bruggen P, Chen SY. A retrogen strategy for presentation of an intracellular tumor antigen as an exogenous antigen by dendritic cells induces potent antitumor T helper and CTL responses. Cancer Res. 2001;61(1):197–205. [PubMed] [Google Scholar]
- 29.Kocher T, Schultz-Thater E, Gudat F, Schaefer C, Casorati G, Juretic A, et al. Identification and intracellular location of MAGE-3 gene product. Cancer Res. 1995;55(11):2236–9. [PubMed] [Google Scholar]
- 30.Inaba K, Steinman RM, Pack MW, Aya H, Inaba M, Sudo T, et al. Identification of proliferating dendritic cell precursors in mouse blood. J Exp Med. 1992;175(5):1157–67. doi: 10.1084/jem.175.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenzwajg M, Camus S, Guigon M, Gluckman JC. The influence of interleukin (IL)-4, IL-13, and Flt3 ligand on human dendritic cell differentiation from cord blood CD34+ progenitor cells. Exp Hematol. 1998;26(1):63–72. [PubMed] [Google Scholar]
- 32.Rougier N, Schmitt D, Vincent C. IL-4 addition during differentiation of CD34 progenitors delays maturation of dendritic cells while promoting their survival. Eur J Cell Biol. 1998;75(3):287–93. doi: 10.1016/S0171-9335(98)80124-6. [DOI] [PubMed] [Google Scholar]
- 33.Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997;186(10):1677–87. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang RF, Wang HY. Enhancement of antitumor immunity by prolonging antigen presentation on dendritic cells. Nat Biotechnol. 2002;20(2):149–54. doi: 10.1038/nbt0202-149. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2(3):185–94. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- 36.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56(1):21–6. [PubMed] [Google Scholar]
- 37.Beláková J, Horynová M, Krupka M, Weigl E, Raska M. DNA vaccines: are they still just a powerful tool for the future? Arch Immunol Ther Exp (Warsz) 2007;55(6):387–98. doi: 10.1007/s00005-007-0044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Multhoff G. Heat shock protein 70 (Hsp70): membrane location, export and immunological relevance. Methods. 2007;43(3):229–37. doi: 10.1016/j.ymeth.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Strbo N, Podack ER. Secreted heat shock protein gp96-Ig: an innovative vaccine approach. Am J Reprod Immunol. 2008;59(5):407–16. doi: 10.1111/j.1600-0897.2008.00594.x. [DOI] [PubMed] [Google Scholar]
- 40.Ullrich E, Ménard C, Flament C, Terme M, Mignot G, Bonmort M, et al. Dendritic cells and innate defense against tumor cells. Cytokine Growth Factor Rev. 2008;19(1):79–92. doi: 10.1016/j.cytogfr.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bloom BR. Natural killers to rescue immune surveillance? Nature. 1982;300(5889):214–5. doi: 10.1038/300214a0. [DOI] [PubMed] [Google Scholar]
- 43.Diefenbach A, Raulet DH. Strategies for target cell recognition by natural killer cells. Immunol Rev. 2001;181:170–84. doi: 10.1034/j.1600-065x.2001.1810114.x. [DOI] [PubMed] [Google Scholar]
- 44.Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319(6055):675–8. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 45.Liao NS, Bix M, Zijlstra M, Jaenisch R, Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991;253(5016):199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- 46.Bix M, Liao NS, Zijlstra M, Loring J, Jaenisch R, Raulet D. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 1991;349(6307):329–31. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- 47.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress- inducible MICA. Science. 1999;285(5428):727–9. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 48.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 49.Nicchitta CV. Re-evaluating the role of heat-shock protein-peptide interactions in tumour immunity. Nat Rev Immunol. 2003;3(5):427–32. doi: 10.1038/nri1089. [DOI] [PubMed] [Google Scholar]
- 50.Van Pel A, De Plaen E, Duffour MT, Warnier G, Uyttenhove C, Perricaudet M, et al. Induction of cytolytic T lymphocytes by immunization of mice with an adenovirus containing a mouse homolog of the human MAGE-A genes. Cancer Immunol Immunother. 2001;49(11):593–602. doi: 10.1007/s002620000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bae MY, Cho NH, Seong SY. Protective anti-tumour immune responses by murine dendritic cells pulsed with recombinant Tat-carcinoembryonic antigen derived from Escherichia coli. Clin Exp Immunol. 2009;157(1):128–38. doi: 10.1111/j.1365-2249.2009.03943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117(5):1155–66. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30(5):626–35. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]