Abstract
The purpose of the study was to determine whether novel, selective antagonists of human A3 adenosine receptors (ARs) derived from the A3-selective agonist Cl-IB-MECA lower intraocular pressure (IOP) and act across species. IOP was measured invasively with a micropipette by the Servo-Null Micropipette System (SNMS) and by non-invasive pneumotonometry during topical drug application. Antagonist efficacy was also assayed by measuring inhibition of adenosine-triggered shrinkage of native bovine nonpigmented ciliary epithelial (NPE) cells. Five agonist-based A3AR antagonists lowered mouse IOP measured with SNMS tonometry by 3–5 mm Hg within minutes of topical application. Of the five agonist derivatives, LJ 1251 was the only antagonist to lower IOP measured by pneumotonometry. No effect was detected pneumotonometrically over 30 min following application of the other four compounds, consonant with slower, smaller responses previously measured non-invasively following topical application of A3AR agonists and the dihydropyridine A3AR antagonist MRS 1191. Latanoprost similarly lowered SNMS-measured IOP, but not IOP measured non-invasively over 30 minutes. Like MRS 1191, agonist-based A3AR antagonists applied to native bovine NPE cells inhibited adenosine-triggered shrinkage. In summary, the results indicate that antagonists of human A3ARs derived from the potent, selective A3 agonist Cl-IB-MECA display efficacy in mouse and bovine cells, as well. When intraocular delivery was enhanced by measuring mouse IOP invasively, five derivatives of the A3AR agonist Cl-IB-MECA lowered IOP but only one rapidly reduced IOP measured non-invasively after topical application. We conclude that derivatives of the highly selective A3AR agonist Cl-IB-MECA can reduce IOP upon reaching their intraocular target, and that nucleoside-based derivatives are promising A3 antagonists for study in multiple animal models.
Keywords: Aqueous humor, Servo-Null Micropipette System, pneumotonometry, nucleoside-based antagonists, bovine nonpigmented ciliary epithelial cells
1. Introduction
Intraocular pressure (IOP) is commonly elevated in glaucoma, leading to death of retinal ganglion cells and optic nerve atrophy. Reducing IOP is the only intervention known to delay the onset and slow progression of blindness, even in patients with normotensive disease (Collaborative Normal-Tension Glaucoma Study Group, 1998a; Collaborative Normal-Tension Glaucoma Study Group, 1998b; Kass et al., 2002; The AGIS investigators, 2000). IOP can be reduced by lowering either the rate of inflow or the resistance to outflow of aqueous humor.
Among novel strategies for lowering IOP, focus on adenosine receptors (ARs) has seemed promising because knockout of A3-subtype ARs reduces IOP in the living mouse (Avila et al., 2002a), likely through a reduction in inflow. Several observations obtained with isolated cells have suggested that A3ARs physiologically increase inflow of aqueous humor by activating Cl− channels of the nonpigmented ciliary epithelial (NPE) cells at the aqueous surface of the ciliary epithelium (Carré et al., 1997; Carré et al., 2000; Mitchell et al., 1999). In contrast to the robust effects of A3AR agonists on isolated cells from the inflow pathway, A3AR agonists have been found to exert relatively modest actions on whole-cell currents of cells cultured from the trabecular meshwork (Fleischhauer et al., 2003)] and from Schlemm’s canal inner wall (Karl et al., 2005).
Based on the results obtained with isolated NPE cells, adenosine and selective A3AR agonists would be expected to increase inflow and IOP, and A3AR antagonists would be expected to exert opposite effects. The predicted changes in IOP triggered by A3AR agonists and antagonists have been confirmed in the living mouse (Avila et al., 2001b; Avila et al., 2002b; Yang et al., 2005). While these responses in mice suggest a potential relevance of A3AR-selective antagonists to humans, the binding affinities of these antagonists display substantial species variation (Jacobson et al., 1997; Linden, 2001). For instance, the binding inhibition constants (Ki) of some antagonists can vary by more than 30,000-times between rat and human A3ARs (Yang et al., 2005). Interestingly, the responses of A3ARs to selective agonists are much more highly conserved across species(Yang et al., 2005). We previously tested one A3AR-selective antagonist, MRS 1292 (Yang et al., 2005), generated by modifying the A3AR agonist IB-MECA (Gao et al., 2002), and observed antagonist activity in both the living mouse and immortalized human NPE cells (Yang et al., 2005).
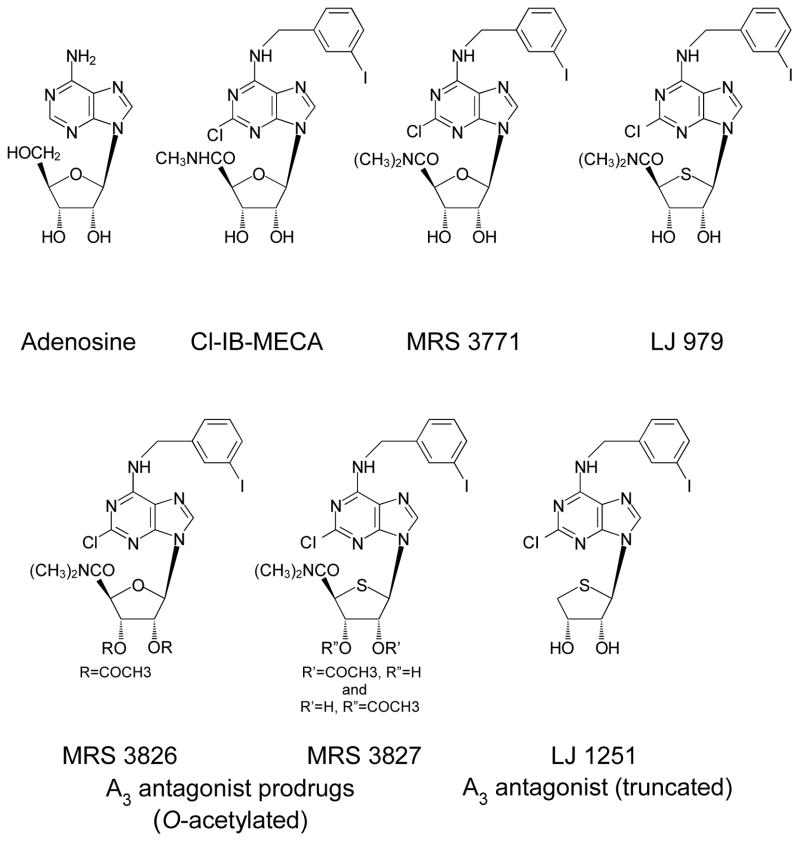
Compared to the parent agonist previously modified (IB-MECA), the A3AR agonist Cl-IB-MECA displays 3–4-fold greater potency and a 50-fold greater selectivity for A3 receptors than for A1 and A2A receptors in rat brain (Kim et al., 1994). In the present work, we have examined the effects of five new A3AR-selective antagonists constructed (Gao et al., 2006; Jeong et al., 2007) by modifying the far more selective agonist Cl-IB-MECA (Figure 1), with the hope that this strategy might lead to the generation of even more selective A3AR antagonists that are active across species. Measurements of mouse IOP have been conducted with both invasive SNMS (Avila et al., 2001a) and non-invasive pneumotonometric techniques (Avila et al., 2005). Similar baseline IOP values are obtained by applying the two techniques to the same eyes (Avila et al., 2005). However, under experimental conditions, the measurements can be complementary, rather than identical, because the intact mouse eye can present a substantial barrier to drug penetration (Wang et al., 2007). The fine-tipped micropipettes used for SNMS tonometry enhance entry of topically-applied drugs into the mouse eye (Wang et al., 2007), so that drug efficacy can be detected even if topical drug permeation is too slow to alter IOP measured non-invasively over similar periods.
Figure 1.
Structures of the physiologic agonist adenosine, the A3-selective agonist Cl-IB-MECA and the A3-antagonist nucleoside derivatives, MRS 3771, LJ 979, MRS 3826, MRS 3827 and LJ 1251.
2. Methods
2.1. Mice and anesthesia
Black Swiss outbred mice of mixed sex, 25–30 g in weight and 7–9 weeks old (Taconic Inc., Germantown, NY, USA) were maintained under a 12:12-h light/dark illumination cycle with unrestricted access to food and water. Animals were anesthetized with intraperitoneal ketamine (250 mg kg−1), complemented with 0.5% topical proparacaine HCl (Allergan, Bausch & Lomb). Anesthetized mice were secured in a surgical stereotaxic device (David Kopf Instruments, Tujunga, CA), and body temperature was maintained with a heating pad at 37°C (Delta Phase Isothermal Pad, Braintree Scientific, Braintree, MA) (Avila et al., 2001a). As previously described (Wang et al., 2007), IOP was measured invasively with the Servo-Null Micropipette System (SNMS) and non-invasively by pneumotonometry in separate animals. The rate of cardiac contraction was monitored with a pressure transducer wrapped around the mouse tail (MLT1010, Adinstruments, USA). All procedures were performed in adherence with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research.
2.2. Measurement of IOP invasively by SNMS tonometry
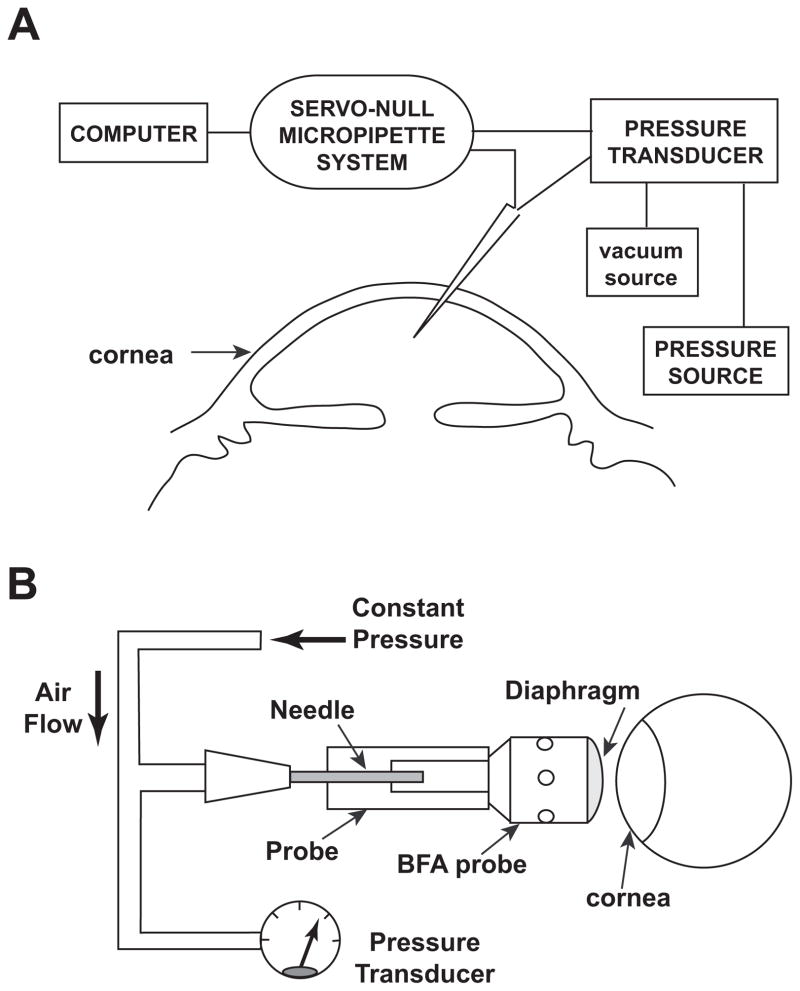
With this electrophysiologic approach (Avila et al., 2001a), an exploring micropipette, 5–10 μm in outer diameter, is filled with a highly conducting solution and advanced through the cornea into the anterior chamber (Fig. 2A). To enhance stability and reduce background noise, our current micropipettes are filled with 2M NaCl and display resistances of 0.1–0.3 MΩ (Wang et al., 2006; Wang et al., 2007). Upon advancing the micropipette into the anterior chamber of the eye, the IOP forces aqueous humor into the tip. The aqueous humor has a much lower conductance than the original filling solution, increasing the micropipette resistance. The IOP is measured as the hydrostatic pressure necessary to restore the position of the column of filling solution and returning the resistance to its initial value. Measurements are conducted at 1 kHz for tens of minutes during the course of drug applications.
Figure 2.
Measurement of mouse IOP by (A) invasive Servo-Null Micropipette System (SNMS) and by (B) non-invasive pneumotonometry.
2.3. Measurement of IOP non-invasively by pneumotonometry
Non-invasive measurements were conducted with a pneumotonometric technique adapted and validated for measuring mouse IOP (Avila et al., 2005; Wang et al., 2006; Wang et al., 2007) (Fig. 2B). The technique incorporates a commercially available tip of the ocular blood tomography pneumotonometer (Blood Flow Analyzer [BFA] probe tip; Paradigm Medical Industries Inc.) that is fit to a custom-built mount.
A constant pressure source provides air flow to the diaphragm at the end of the BFA tip. The diaphragm is distended by the air flow, enabling air to escape through holes in the probe-tip wall. Pressure at the base of the BFA tip is monitored through a T-connection to a transducer. The probe is advanced to the cornea with a micromanipulator until a shift in the baseline output reading indicates that contact is made. The tip is then visually displaced from the tear film and the output readjusted to zero before advancing the probe again. Contacting the cornea depresses the diaphragm of the BFA tip, reducing access of the air flow to the escape holes, and thereby increasing the pressure at the tip base. The probe is then advanced in ~10 steps of ~50 μm each at intervals of ~10 s. Elevation in pressure with probe advance displays a relative plateau or inflection region, taken to be the endpoint for the IOP (Wang et al., 2006; Wang et al., 2007). The endpoint is considered technically acceptable if the pressure recording also displays pressure oscillations synchronous with heart rate (Avila et al., 2005; Wang et al., 2006; Wang et al., 2007). The position along the axis of advance is recorded, and the probe is withdrawn to avoid possible artifacts arising from prolonged corneal pressure. Measurements are then conducted at 10-minute intervals by advancing the probe to the same position (Wang et al., 2007). As illustrated by the control trace of Figs. 4A, measurements with this protocol were stable for more than 30 min.
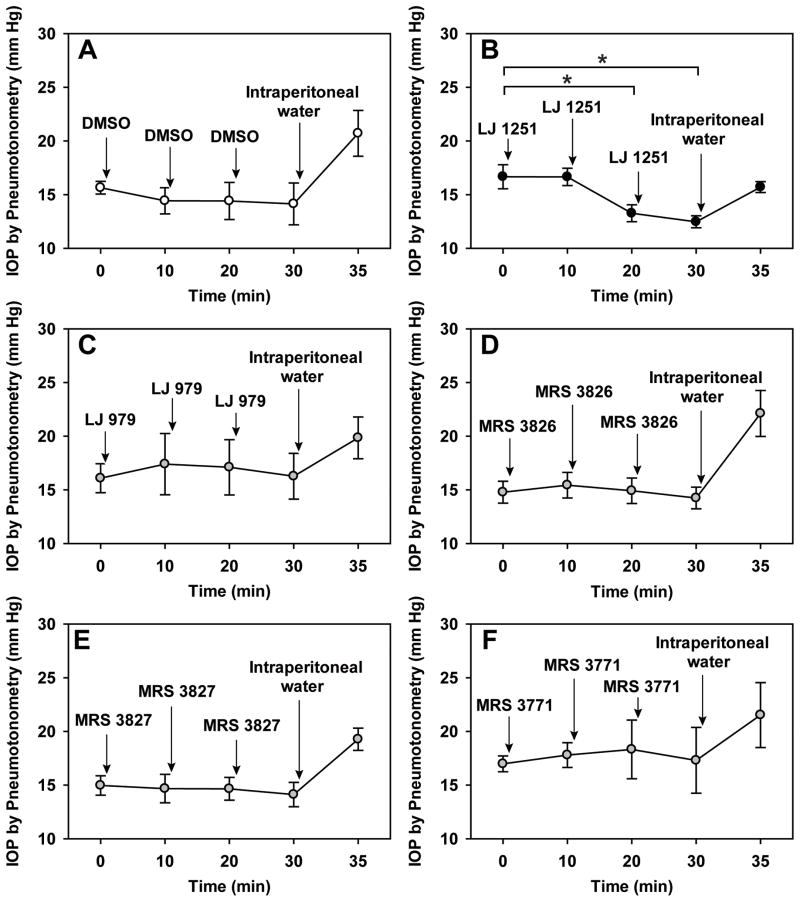
Figure 4.
Effects of the five A3-antagonist nucleoside derivatives measured non-invasively. At the conclusion of the experiments, water was introduced intraperitoneally to verify that expected changes in IOP could be detected. As indicated by the asterisks, Measured by pneumotonometry, LJ 1251 did reduce IOP (B). Analyzed by one-way ANOVA with the Bonferroni post-test, the IOP was significantly reduced at times 20 min (P<0.05) and 30 min (P=0.01) at times 20 and 30 min. However, no significant changes in IOP were noted following: topical addition of vehicle alone (2% DMSO, A), LJ 979 (C), MRS 3826 (D), MRS 3827 (E) and MRS 3771 (F). Further details are provided in Table 2.
2.4. Reduction of measurements with living mouse
As previously described (Wang et al., 2006), IOP and heart rate signals were band-pass filtered (1–100 Hz), amplified using a signal conditioner (CyberAmp 380, Axon Instruments, Inc., USA) and digitized at 1 kHz with an analog-to-digital converter (MiniDigi 1A two-channel acquisition system, Axon Instruments, Inc., USA) in the gap-free mode. The resulting digital files were analyzed offline using Clampfit 9 (Axon Instruments).
2.5. Preparation of native bovine NPE cells
Fresh bovine eyes were obtained from a local abattoir. Native bovine NPE cells were dissociated by enzymatic digestion (Do et al., 2006). Ciliary processes were excised and washed with Dulbecco’s phosphate-buffered saline (PBS) (GIBCO, Grand Island, NY). Cells were dissociated by incubating the preparation with 0.25% trypsin in a shaker for 30–35 min (250 rpm) at 37°C. Cells were subsequently rinsed twice with PBS and plated on coverslips in medium 199 for ≥ 2 h in 5% CO2 at 37°C. The medium included 10% fetal bovine serum and 0.1% gentamicin (GIBCO-BRL). The cells were prepared after a post-mortem time of about 1.5 h.
2.6. Cell volume measurements
Cell volume was monitored continually by measuring both calcein fluorescence quenching (Do et al., 2006; Karl et al., 2007) and cell area (Do et al., 2004; Mitchell et al., 2002). Coverslips containing the cells were placed in a chamber connected to a Nikon Diaphot microscope. Cells were loaded with 2 μM calcein-AM and 0.02% Pluronic for 30–40 min, and then perfused with isotonic Tyrode’s solution for 30 min at room temperature. Bleaching was minimized by exciting calcein at intervals of 20 s at 488 nm; light emitted at 520 nm was collected with an IC-200 charge-coupled device (CCD) camera (Photon Technology International, Princeton, NJ). Data were analyzed with Imagemaster software (Photon Technology International).
The cell volume was measured specifically of NPE cells. Although pigmented ciliary epithelial (PE) cells were also present in the dissociated cell preparations, those cells were readily distinguished by their prominent cell granules. The presence of the granules has permitted measurement and differentiation of the electrophysiologic and volume-regulatory properties of the NPE and PE cells, even in mixed harvested preparations (Do et al., 2005; Do et al., 2004; Zhang & Jacob, 1997). Regions for analysis were chosen to include nonconfluent cells with similar diameter and loading, and usually included one to four cells. In measuring volume through fluorescence quenching, total calcein fluorescence was monitored (Do et al., 2006). Calcein fluorescence quenching is increased by shrinkage and lowered by swelling. In monitoring volume by cell area, the number of pixels above threshold within the region of interest was determined using Imagemaster software (Photon Technology International) (Mitchell et al., 2002).
2.7. Solutions and pharmacologic agents
LJ 979 and LJ 1251 were synthesized at Ewha Womans University (Jeong et al., 2007) and MRS 3771 (Gao et al., 2006), as well as MRS 3827 and MRS 3826 (Besada et al., 2006), were prepared at the National Institute of Diabetes and Digestive and Kidney Diseases. Figure 1 provides the structures of the purinergic drugs used in the study, and Table 1 presents the pharmacologic potencies. Ketamine HCl was purchased from Phoenix Pharmaceutical, Inc. (St. Joseph, MO), and other drugs were obtained from Sigma Chemical (St. Louis, MO).
Table 1.
Potency of a series of agonists and agonist-derived antagonists at the four subtypes of human adenosine receptors.
| Action | Drug | Potency, nM | Source (Ref.) | |||
|---|---|---|---|---|---|---|
| A1 | A2A | A2B | A3 | |||
| Nonselective agonist | Adenosine | 310 | 700 | 24,000 | 290 | (Fredholm et al., 2001) |
| A3 agonist | Cl-IB-MECA | 222±22 | 5360 ± 2470 | >10,000 | 1.4 ± 0.3 | (Gao et al., 2002; Tchilibon et al., 2005) |
| A3 antagonist | MRS 3771 | 6,220±640 | >10,000 | >10,000 | 29.0±4.9 | (Gao et al., 2006) |
| A3 antagonist | LJ 979 | >10,000 | >10,000 | >10,000 | 15.5±3.1 | (Gao et al., 2006) |
| A3 antagonist | MRS 3826 | >10,000 | >10,000 | ND | 910±330 | (Besada et al., 2006) |
| A3 antagonist | MRS 3827 | ND | ND | ND | ND | - |
| A3 antagonist | LJ 1251 | 2,490±940 | 341±75 | >10,000 | 4.16±0.50 | (Jeong et al., 2007) |
ND: not determined because MRS 3827 is not a pure compound, but a mixture of two isomers (in a 3:2 ratio) of MRS 3771. In MRS 3827, the two free OH groups in MRS3771 were incompletely acetylated. The completely acetylated form is MRS3826.
Drugs were dissolved in DMSO and applied topically. The droplets contained ≤2% DMSO at an osmolality of 295–300 mOsm. This vehicle solution did not significantly affect mouse IOP (N=6, Table 2), consistent with previous measurements (Avila et al., 2002a). Drug actions on IOP were evaluated with invasive measurements 10 min after topical application (Table 2) since the continued presence of the micropipette can be associated with a downward drift of the IOP after 10 min. However, smaller droplets (5 μl rather than 10 μl) were topically applied more frequently during the course of the non-invasive measurements to prevent drying the eye with the airflow from the pneumotonometer.
Table 2.
Effects of topically-added drugs on IOP measured by pneumotonometry and SNMS tonometry.
| Drug | Class | Concentration | Pneumotonometry |
SNMS |
||||
|---|---|---|---|---|---|---|---|---|
| N | Δ(IOP) | P | N | Δ(IOP) | P | |||
| Control | ||||||||
| DMSO | Vehicle | 2% | 9 | −1.4±1.5 | >0.3 | 6 | −0.5±0.6 | >0.4 |
| A3 antagonists | ||||||||
| MRS 3771 | A3 antagonist | 2.5mM (2% DMSO) | 4 | +0.3±2.6 | >0.9 | |||
| 250μM (2% DMSO) | 9 | +0.4±0.6 | >0.5 | 9 | −3.0±1.1 | <0.05 | ||
| LJ 979 | A3 antagonist | 250μM (2% DMSO) | 6 | +0.2±1.1 | >0.8 | 10 | −4.2±1.2 | <0.01 |
| MRS 3826 | di-acetyl ester of LJ 979 | 250μM (2% DMSO) | 6 | −0.5±0.8 | >0.5 | 4 | −4.0±0.8 | <0.05 |
| MRS 3827 | mono-acetyl ester of LJ 979 | 250μM (2% DMSO) | 9 | −0.8±1.1 | >0.4 | 6 | −4.4±1.3 | <0.05 |
| LJ 1251 | modified from LJ 979 (rat/human A3 antagonist) | 250μM (2% DMSO) | 6 | −4.2±0.7 | <0.002 | 7 | −1.5±0.6 | <0.05 |
| 75μM (0.6% DMSO) | 6 | −0.3±0.8 | >0.7 | 6 | −3.1±1.4 | <0.08 | ||
| 25μM (0.2% DMSO) | 6 | −0.6±1.5 | >0.7 | 6 | −4.9±1.7 | <0.05 | ||
| 5μM (0.2% DMSO) | 6 | −2.2±0.7 | <0.03 | |||||
| 500nM (0.2% DMSO) | 6 | −0.09±3.4 | >0.8 | |||||
| Outflow Enhancer | ||||||||
| Latanoprost | prostaglandin analogue | 116μM | 6 | −0.1±1.6 | >0.9 | 6 | −3.8±0.8 | <0.01 |
Concentrations provided are for the drugs in topically-applied droplets. Values are means ±SE. N is the number of mice in each protocol; only one eye was studied in each mouse. P is the probability of the null hypothesis.
2.8. Statistical analysis
Results are provided as means ± SEM. Significance was evaluated between two sets of data with Student’s t-test (paired or unpaired). Groups of three or more measurements were evaluated with one- or two-way analysis of variance (ANOVA), as appropriate, followed by the Bonferroni post-test. Differences were considered significant if the probability of the null hypothesis (P) was <0.05.
3. Results
3.1. Measurements of IOP conducted by SNMS tonometry
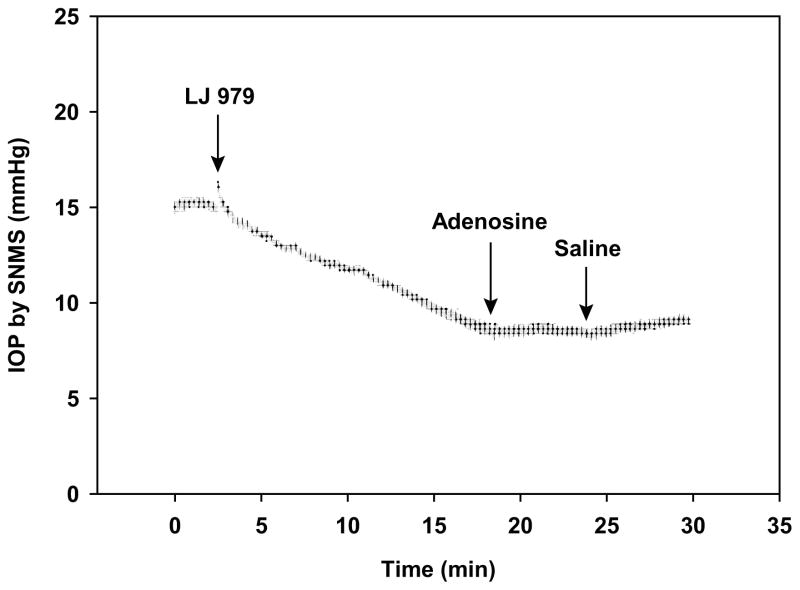
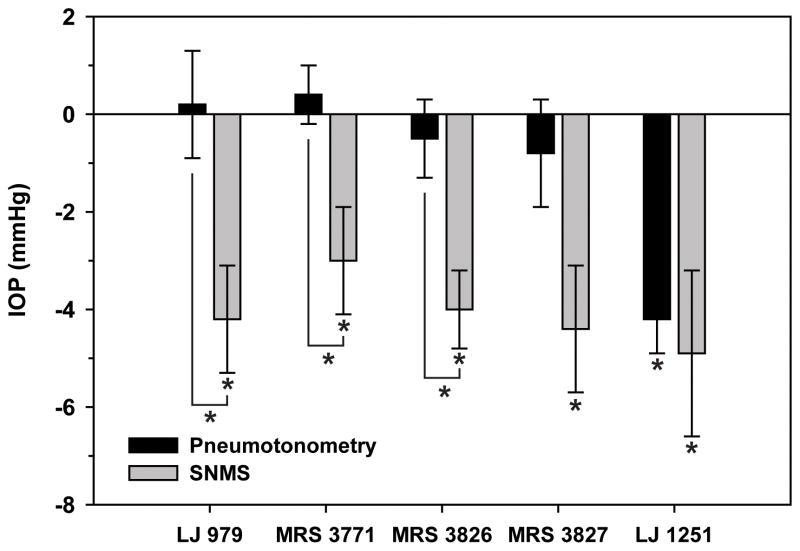
At a concentration of 250 μM in a 10-μL droplet (1.44 μg dose), topical application of the nucleoside-derived antagonist LJ 979 reduced IOP within minutes, when measured by invasive SNMS tonometry (Fig. 3). The relationship between drug concentration in the applied droplet and aqueous humor is considered in the Discussion. The maximal fall in IOP was 4.2 ±1.2 mm Hg (mean ±SEM, N=10, P<0.01). Subsequent application of 10 mM adenosine (26.4 μg) in a 10-μL droplet did not elicit the increase in IOP (Fig. 3) characteristically observed after adding adenosine without drug pretreatment (Avila et al., 2001b; Avila et al., 2002b). Each of the other four nucleoside-derived antagonists described in Table 1 and Fig. 1 also reduced invasive-measured IOP. The reductions ranged from 3.0 ±1.1 mm Hg [MRS 3771, droplet concentration (dose) of 250 μM (1.4 μg, N=9, P<0.05] to 4.9 ±1.7 mm Hg [LJ 1251, droplet concentration (dose) of 25 μM (0.13 μg), N=6, P<0.05] (Table 2, Fig. 5). For purposes of comparison, mouse IOP was also monitored by SNMS tonometry after topical application of the prostaglandin analogue latanoprost (Table 2). Consistent with our previous findings (Avila et al., 2001a), latanoprost lowered SNMS-measured IOP by 3.8 ±0.8 (N=6, P<0.01).
Figure 3.
Effect of LJ 979 on IOP measured invasively IOP by SNMS tonometry. The SNMS-measured IOP began to fall almost immediately upon applying a droplet concentration of 250 μM. Following LJ 979, the agonist adenosine exerted a negligible effect on IOP, at a droplet concentration of 10 mM.
Figure 5.
Comparison of the effects of the five A3-antagonist nucleoside derivatives on mouse IOP measured non-invasively (Pneumotonometry) and invasively (SNMS). Each of the drugs significantly lowered IOP measured by SNMS tonometry, but only LJ 1251 significantly reduced IOP measure by pneumotonometry. The probabilities that the differences were random between the two techniques were estimated by the unpaired t-test: (LJ 979) P<0.02, (MRS 3771) P<0.02, (MRS 3826) P<0.02, (MRS 3827) P=0.056, and (LJ 1251) P>0.7. Asterisks indicate differences with a null probability of <0.05. Further details are presented in Table 2.
3.2. Measurements of IOP by pneumotonometry
The single nucleoside derivative that lowered IOP by pneumotonometry as well as by SNMS tonometry was LJ 1251 (Fig. 4B). At a droplet concentration of 250 μM and droplet dose of 0.630 μg, LJ 1251 lowered IOP measured non-invasively by 4.2±0.7 mmHg (P<0.002, Table 2). As a positive control, 0.1 mL water g−1 was introduced intraperitoneally at the conclusion of the measurements (Figs. 4A–F). The increase in IOP characteristically triggered by systemic hypotonicity was uniformly observed by non-invasive pneumotonometry.
In contrast to LJ 1251, neither the vehicle alone (2% DMSO, Fig. 4A) nor the other four nucleoside derivatives significantly affected IOP measured with pneumotonometry. The four other derivatives were LJ 979 (Fig. 4C), MRS 3826 (Fig. 4D), MRS 3827 (Fig. 4E) and MRS 3771 (Fig. 4F). The mean measured changes, droplet concentrations, numbers of experiments and probabilities are specified in Table 2, with summary results after 30 min shown in Fig. 5.
The IOP was also monitored non-invasively after topical application of the prostaglandin analogue latanoprost (Table 2). In agreement with our earlier pneumotonometric observations (Wang et al., 2007), latanoprost had no significant effect within 30 min (N=6, P>0.9).
3.3. Effects of nucleoside derivatives on bovine ciliary epithelial cell volume
The potencies of the antagonists studied have previously been quantified against human adenosine receptors (Table 1), and the whole-animal work of the present study was conducted with mice. We also tested the effects of Cl-IB-MECA-derived antagonists, three of the putative cross-species nucleoside derivatives, by studying cells from an additional species, the cow. The cell volume of native bovine nonpigmented ciliary epithelial (NPE) cells was monitored by measuring both cell area (Do et al., 2004) and calcein-fluorescence quenching (Do et al., 2006).
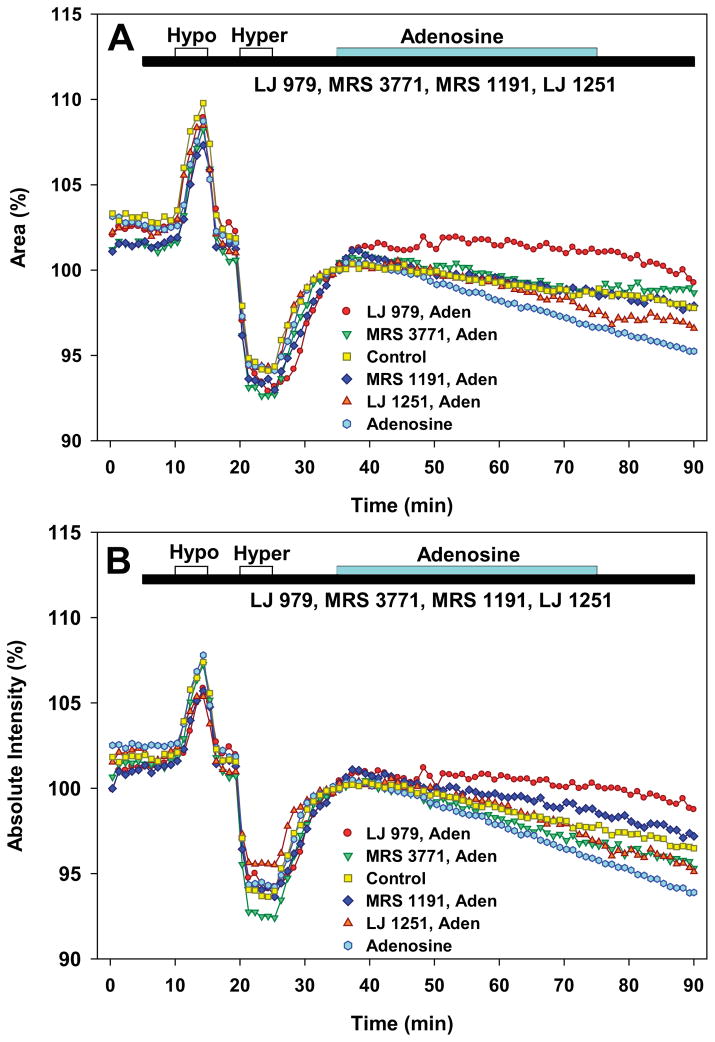
After an initial baseline period, cells were sequentially and briefly perfused with 25% hypotonic, isotonic, 25% hypertonic and isotonic solutions (Fig. 6). These initial perfusions served as controls to test whether the measured isotonic cell volume responded appropriately by swelling and shrinking during exposure to the hypo- and hypertonic challenges, respectively. Cells were perfused at ~2 mL min−1, leading to application of 24–48 nmol of derivative in each experiment. Sufficient material was synthesized to test the first two derivatives developed, LJ 979 and MRS 3771, and also LJ 1251, the derivative that reduced mouse IOP measured either invasively or non-invasively (Table 2, Figs. 4B and 5). For purposes of comparison, the previously studied dihydropyridine A3 antagonist, MRS 1191, was also tested. The values displayed in Fig. 6 were normalized to the area or fluorescence measured just before perfusing with the test solution, at t=35 min.
Figure 6.
Effects of A3 antagonists on cell volume of native bovine nonpigmented ciliary epithelial cells. Volume was simultaneously monitored as area (A) and calcein fluorescence quenching (B). For purposes of clarity, the standard errors have been omitted and the data points are averages of the three measurements obtained each minute. Results for each series of measurements are presented as means normalized to the average of the three points measured just before application of adenosine at 35 min. Brief initial perfusions with 25% hypotonic and 25% hypertonic solutions produced swelling and shrinkage, respectively. Adenosine (300 μM, Aden) produced cell shrinkage that was partially/completely prevented by perfusion with 300 nM LJ 979, 300 nM MRS 3771, 300 nM MRS 1191 or 150 nM LJ 1251. Data were analyzed by two-way ANOVA with the Bonferroni post-test.
After the initial control perfusions with isosmotic and anisosmotic solutions, adenosine with or without an antagonist was perfused for 40 min, 35 min after beginning the experiment. Perfusion with adenosine, the physiologic AR agonist, triggered a concentration-dependent shrinkage (light blue symbols, Fig. 6) in comparison to controls perfused with 0.1% DMSO alone (N=10, yellow symbols, Fig. 6). We previously found that the physiologic A3 agonist, adenosine, is less potent in activating Cl− channels of native bovine nonpigmented ciliary epithelial (NPE) cells than of immortalized human NPE cell lines (Carré et al., 1997). Consistent with the previous finding, we found that the EC50 for adenosine-triggered shrinkage was ~250 μM, based on perfusion with adenosine concentrations ranging from 10 μM to 1 mM (N=30, data not shown). This observation guided our choice of studying the effects of antagonists on cell shrinkage elicited by 300 μM adenosine. At 100 nM, the selective A3 agonist Cl-IB-MECA (Table 1) produced shrinkage (N=19, data not shown) insignificantly different from that elicited by high concentrations of adenosine, tested either by area (P>0.7) or fluorescence quenching (P>0.05, one-way ANOVA with Bonferroni post-test).
As illustrated by Fig. 6, the A3 antagonists significantly inhibited adenosine-triggered cell shrinkage (two-way ANOVA with Bonferroni post-test). MRS 3771 (N=5, P<0.001) significantly inhibited the action of adenosine (N=14), monitored as cell area. LJ 979 (N=5), LJ 1251 (N=8) and MRS 1191 (N=5) significantly inhibited adenosine-produced cell shrinkage, monitored both by cell area and by calcein fluorescence quenching; significance was attained at the 0.001 level, with the exception of the inhibition of the adenosine-triggered reduction in area by LJ 1251 (P=0.006). Ranking the antagonists according to the mean cell area at the conclusion of the test period (t=75 min), the ranking of antagonist efficacy was: LJ 979 > MRS 1191 ~ MRS 3771 > LJ 1251. Monitored as calcein fluorescence quenching, the ranking was similar, with a single inversion: LJ 979 > MRS 1191 > LJ 1251 > MRS 3771. The effect of LJ 979 in stabilizing cell volume was particularly striking (red symbols, Fig. 6), significantly reducing even the baseline rate of shrinkage noted under control conditions. This stabilizing action may have reflected inhibition of baseline A3AR activity since the LJ 979 had no effect on the time course or magnitude of swelling or shrinkage produced by anisosmotic solutions in the control period (ending at t=35 min, Fig. 6).
4. Discussion
The salient results of the present study are that: (1) five derivatives of the selective A3AR agonist Cl-IB-MECA lowered IOP, measured by SNMS tonometry; (2) of these derivatives, only LJ 1251 rapidly reduced IOP, measured non-invasively after topical application; and (3) the three nucleoside derivatives applied to native bovine nonpigmented ciliary epithelial cells inhibited adenosine-triggered shrinkage.
4.1. Nucleoside-derived A3 antagonists
The five antagonists of the human A3AR were modifications of the parent nucleoside agonist Cl-IB-MECA (Fig. 1). The chemical strategy was two-fold. Choosing an A3 agonist as the parent compound enhanced the probability that the derived antagonist would be effective against many mammalian species [Table 1, (Yang et al., 2005)]. Second, the specific modifications were aimed at reducing the H-bond-donating capability associated with activation of the 5′-uronamide of the full agonist, Cl-IB-MECA. This was achieved by adding an additional methyl group to the 5′-uronamide, with (LJ 979) or without (MRS 3771) concomitant 4′-thio substitution (Gao et al., 2006). The derivatives MRS 3826 and MRS 3827 are esters of MRS 3771 and LJ 979, respectively. The fifth derivative LJ 1251 was developed by removing the 5′-uronamide, entirely, in order to eliminate H-bonding at that position, accompanied by the 4′-thio substitution (Jeong et al., 2007).
4.2. Effects of nucleoside-derived A3 antagonists on IOP measured by SNMS
All five of the derivative compounds reduced invasively-measured IOP by 4–5 mm Hg within minutes after topical addition (Figs. 3 and 5, Table 2). We have previously observed that corneal impalement of mice with the exploring micropipette used in SNMS tonometry, enhances delivery of drugs to their intraocular targets (Wang et al., 2007). This increased delivery likely reflects enhanced permeation of the cornea around the tips of the small micropipettes, ~5–10 μm in diameter. We do not know the drug concentration actually delivered to the intraocular aqueous humor following topical application. However, comparison of the droplet concentrations with known potencies of 13 oculotensive drugs or drug combinations led us to estimate that the concentration of drug topically delivered to the aqueous humor during SNMS tonometry is no more than 1%, and for some drugs may be closer to 0.1–0.2%, of the concentration within a 10-μL droplet (Avila et al., 2001b). This approximation is consonant with the commonly-applied assumption of ~1% penetration of drugs topically applied to rabbits (Honjo et al., 2001) and primates (Asseff et al., 1973).
Comparison of the delivered concentrations, calculated as 1% of the effective droplet concentrations (Table 2), with the known potencies at hARs (Table 1) is consistent with the view that the derivatives lowered IOP by acting selectively on A3ARs.
4.3. Effects of nucleoside-derived A3 antagonists on non-invasively measured IOP
The measurements by SNMS tonometry indicate that all five agonist-based A3AR antagonists can reduce IOP upon reaching their intraocular target. LJ 1251 was the only one of the derivatives that lowered non-invasively-measured IOP at any of the droplet concentrations during the durations of the current studies. The other four derivatives had no effect on non-invasively-measured IOP at any of the droplet concentrations during the durations of the current studies (Figs. 4–5, Table 2). Similarly, topical application of latanoprost in the current study and Cl-IB-MECA in a prior work (Wang et al., 2007) did not significantly alter IOP measured by pneumotonometry over ~30 min.
The foregoing observations indicate that delivery of multiple drugs was substantially reduced by the ocular coats of the mouse eye. Corneal impalement by even fine-tipped micropipettes can significantly improve the entry of topically-applied drugs into the mouse aqueous humor. We speculate that this enhancement is mediated either by direct diffusion around the tip or by a more complex impalement-triggered change in ocular barrier properties. SNMS tonometry and noninvasive pneumotonometry can be complementary in facilitating study of ocular hypotensive drugs. The SNMS provides a tool for measuring IOP with accuracy, prolonged stability and excellent time resolution, while the noninvasive pneumotonometry gives the opportunity to estimate the efficiency of topical drug delivery.
It is unclear why the apparent permeability of LJ 1251 was different from the other four agonist-based A3AR antagonists. Relative hydrophobicity of the derivatives can in general play a role. However, we have previously observed that both the agonist adenosine and the dihydropyridine antagonist MRS 1191 also alter IOP measured non-invasively, although the responses to topical application of these drugs was slower and smaller than those measured with SNMS tonometry (Wang et al., 2007). Table 3 presents calculated values of calculated log concentration ratios (cLogP) of drugs in octanol:water (a measure of relative hydrophobicity) (Lipinski et al., 2001). The most hydrophilic (adenosine), most hydrophobic (MRS 1191) and the moderately hydrophobic derivative (LJ 1251) were all apparently able to permeate the eye’s ocular coats. Thus, relative hydrophobicity does not provide a ready explanation for the differential drug effects.
Table 3.
Calculated log concentration ratios (cLogP) of drugs in octanol:water to estimate relative hydrophobicity.
| COMPOUND | cLog P |
|---|---|
| Adenosine | −2.16 |
| Cl-IB-MECA | 1.20 |
| MRS 3771 | 1.69 (Gao et al., 2006) |
| LJ 979 (MRS 3642) | 1.73 (Gao et al., 2006) |
| LJ 1251 (MRS 3820) | 2.25 |
| MRS 3826 | 3.40 |
| MRS 1191 | 6.86 (Gao et al., 2006) |
4.4. Effects on Isolated Bovine Ciliary Epithelial Cells
The strategy of developing antagonists by modifying nucleoside agonists addressed the need to obtain drugs inhibiting A3ARs of many mammalian species with high selectivity, potency and efficacy (Gao et al., 2002). The nucleoside derivatives of the present study have been found to be highly potent and selective in studies of human ARs (Table 1). The current measurements of IOP by SNMS tonometry demonstrate the functional efficacy of these antagonists in the mouse.
The effects of three of the derivatives were also studied on the adenosine-triggered shrinkage of native bovine nonpigmented ciliary epithelial cells. This shrinkage is thought to reflect A3AR-mediated activation of Cl− channels, leading to release of cellular Cl−, accompanied by K+ and water (Carré et al., 1997; Carré et al., 2000; Mitchell et al., 1999). The drugs displayed differing, but significant inhibitions of the shrinkage, suggesting that these novel antagonists are efficacious against bovine, as well as mouse and human A3AR receptors.
4.5. Summary
Agonists of A3ARs stimulate Cl− channels in the basolateral membranes of nonpigmented ciliary epithelial cells, accelerating the rate of aqueous humor formation and increasing IOP. Antagonists of A3ARs inhibit these effects, thereby reducing IOP. Investigation of A3AR antagonists has been complicated by their highly variable binding affinities in different mammalian species. This issue has been addressed by modifying the highly-selective full agonist Cl-IB-MECA to form A3AR antagonists designed to act across species. The five derivatives studied all lower IOP in the living mouse, and the three derivatives applied to isolated bovine cells inhibit A3AR-mediated effects. One of the derivatives, LJ 1251, appeared to permeate the cornea particularly rapidly, but identification of the underlying mechanism will require further study.
Acknowledgments
Supported in part by research grant EY13624 (MMC) and core grant EY01583 from the National Institutes of Health, the Paul and Evanina Bell Mackall Foundation Trust (RAS) and Research to Prevent Blindness (RAS). BVJ and KAJ acknowledge support from the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Disclosure: Z. Wang, P; C.W. Do, P; M.Y. Avila, P; K. Peterson-Yantorno, N; R.A. Stone, P; Z. Gao, P; B. Joshi, P; L.S. Jeong, P; K.A. Jacobson, P; M.M. Civan, P.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asseff CF, Weisman RL, Podos SM, Becker B. Ocular penetration of pilocarpine in primates. Am J Ophthalmol. 1973;75:212–215. doi: 10.1016/0002-9394(73)91015-5. [DOI] [PubMed] [Google Scholar]
- Avila MY, Carré DA, Stone RA, Civan MM. Reliable measurement of mouse intraocular pressure by a servo-null micropipette system. Invest Ophthalmol Vis Sci. 2001a;42:1841–1846. [PubMed] [Google Scholar]
- Avila MY, Múnera A, Guzmán A, Do CW, Wang Z, Stone RA, Civan MM. Noninvasive intraocular pressure measurements in mice by pneumotonometry. Invest Ophthalmol Vis Sci. 2005;46:3274–3280. doi: 10.1167/iovs.04-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila MY, Seidler RW, Stone RA, Civan MM. Inhibitors of NHE-1 Na+/H+ exchange reduce mouse intraocular pressure. Invest Ophthalmol Vis Sci. 2002a;43:1897–1902. [PubMed] [Google Scholar]
- Avila MY, Stone RA, Civan MM. A(1)-, A(2A)- and A(3)-subtype adenosine receptors modulate intraocular pressure in the mouse. Br J Pharmacol. 2001b;134:241–245. doi: 10.1038/sj.bjp.0704267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila MY, Stone RA, Civan MM. Knockout of A(3) adenosine receptors reduces mouse intraocular pressure. Invest Ophthalmol Vis Sci. 2002b;43:3021–3026. [PubMed] [Google Scholar]
- Besada P, Mamedova LK, Palaniappan KK, Gao ZG, Joshi BV, Jeong LS, Civan MM, Jacobson KA. Nucleoside prodrugs of A3 adenosine receptor agonists and antagonists. Coll Czech Chem Comm. 2006;71:912–928. doi: 10.1135/cccc20060912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré DA, Mitchell CH, Peterson-Yantorno K, Coca-Prados M, Civan MM. Adenosine stimulates Cl− channels of nonpigmented ciliary epithelial cells. Am J Physiol. 1997;273:C1354–1361. doi: 10.1152/ajpcell.1997.273.4.C1354. [DOI] [PubMed] [Google Scholar]
- Carré DA, Mitchell CH, Peterson-Yantorno K, Coca-Prados M, Civan MM. Similarity of A(3)-adenosine and swelling-activated Cl(−) channels in nonpigmented ciliary epithelial cells. Am J Physiol Cell Physiol. 2000;279:C440–451. doi: 10.1152/ajpcell.2000.279.2.C440. [DOI] [PubMed] [Google Scholar]
- Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998a;126:487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998b;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- Do CW, Lu W, Mitchell CH, Civan MM. Inhibition of swelling-activated Cl− currents by functional anti-ClC-3 antibody in native bovine non-pigmented ciliary epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:948–955. doi: 10.1167/iovs.04-1004. [DOI] [PubMed] [Google Scholar]
- Do CW, Peterson-Yantorno K, Civan MM. Swelling-activated Cl− channels support Cl− secretion by bovine ciliary epithelium. Invest Ophthalmol Vis Sci. 2006;47:2576–2582. doi: 10.1167/iovs.05-0851. [DOI] [PubMed] [Google Scholar]
- Do CW, Peterson-Yantorno K, Mitchell CH, Civan MM. cAMP-activated maxi-Cl− channels in native bovine pigmented ciliary epithelial cells. Am J Physiol Cell Physiol. 2004;287:C1003–1011. doi: 10.1152/ajpcell.00175.2004. [DOI] [PubMed] [Google Scholar]
- Fleischhauer JC, Mitchell CH, Stamer WD, Karl MO, Peterson-Yantorno K, Civan MM. Common actions of adenosine receptor agonists in modulating human trabecular meshwork cell transport. J Membr Biol. 2003;193:121–136. doi: 10.1007/s00232-002-2013-5. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443–448. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- Gao ZG, Joshi BV, Klutz AM, Kim SK, Lee HW, Kim HO, Jeong LS, Jacobson KA. Conversion of A3 adenosine receptor agonists into selective antagonists by modification of the 5′-ribofuran-uronamide moiety. Bioorg Med Chem Lett. 2006;16:596–601. doi: 10.1016/j.bmcl.2005.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Kim SK, Biadatti T, Chen W, Lee K, Barak D, Kim SG, Johnson CR, Jacobson KA. Structural determinants of A3 adenosine receptor activation: nucleoside ligands at the agonist/antagonist boundary. J Med Chem. 2002;45:4471–4484. doi: 10.1021/jm020211+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo M, Tanihara H, Inatani M, Kido N, Sawamura T, Yue BY, Narumiya S, Honda Y. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci. 2001;42:137–144. [PubMed] [Google Scholar]
- Jacobson KA, Park KS, Jiang JL, Kim YC, Olah ME, Stiles GL, Ji XD. Pharmacological characterization of novel A3 adenosine receptor-selective antagonists. Neuropharmacology. 1997;36:1157–1165. doi: 10.1016/s0028-3908(97)00104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong LS, Choe SA, Gunaga P, Kim HO, Lee HW, Lee SK, Tosh DK, Patel A, Palaniappan KK, Gao ZG, Jacobson KA, Moon HR. Discovery of a new nucleoside template for human A3 adenosine receptor ligands: D-4′-thioadenosine derivatives without 4′-hydroxymethyl group as highly potent and selective antagonists. J Med Chem. 2007;50:3159–3162. doi: 10.1021/jm070259t. [DOI] [PubMed] [Google Scholar]
- Karl MO, Fleischhauer JC, Stamer WD, Peterson-Yantorno K, Mitchell CH, Stone RA, Civan MM. Differential P1-purinergic modulation of human Schlemm’s canal inner-wall cells. Am J Physiol Cell Physiol. 2005;288:C784–794. doi: 10.1152/ajpcell.00333.2004. [DOI] [PubMed] [Google Scholar]
- Karl MO, Peterson-Yantorno K, Civan MM. Cell-specific differential modulation of human trabecular meshwork cells by selective adenosine receptor agonists. Exp Eye Res. 2007;84:126–134. doi: 10.1016/j.exer.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Gordon MO. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. discussion 829–730. [DOI] [PubMed] [Google Scholar]
- Kim HO, Ji XD, Siddiqi SM, Olah ME, Stiles GL, Jacobson KA. 2-Substitution of N6-benzyladenosine-5′-uronamides enhances selectivity for A3 adenosine receptors. J Med Chem. 1994;37:3614–3621. doi: 10.1021/jm00047a018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Mitchell CH, Fleischhauer JC, Stamer WD, Peterson-Yantorno K, Civan MM. Human trabecular meshwork cell volume regulation. Am J Physiol Cell Physiol. 2002;283:C315–326. doi: 10.1152/ajpcell.00544.2001. [DOI] [PubMed] [Google Scholar]
- Mitchell CH, Peterson-Yantorno K, Carré DA, McGlinn AM, Coca-Prados M, Stone RA, Civan MM. A3 adenosine receptors regulate Cl− channels of nonpigmented ciliary epithelial cells. Am J Physiol. 1999;276:C659–666. doi: 10.1152/ajpcell.1999.276.3.C659. [DOI] [PubMed] [Google Scholar]
- Tchilibon S, Joshi BV, Kim SK, Duong HT, Gao ZG, Jacobson KA. (N)-methanocarba 2,N6-disubstituted adenine nucleosides as highly potent and selective A3 adenosine receptor agonists. J Med Chem. 2005;48:1745–1758. doi: 10.1021/jm049580r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The AGIS investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- Wang Z, Do CW, Avila MY, Stone RA, Civan MM. Refinements of invasive and non-invasive mouse intraocular pressure measurements (letter) Invest Ophthalmol Vis Sci 2006 [Google Scholar]
- Wang Z, Do CW, Avila MY, Stone RA, Jacobson KA, Civan MM. Barrier qualities of the mouse eye to topically applied drugs. Exp Eye Res. 2007;85:105–112. doi: 10.1016/j.exer.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Avila MY, Peterson-Yantorno K, Coca-Prados M, Stone RA, Jacobson KA, Civan MM. The cross-species A3 adenosine-receptor antagonist MRS 1292 inhibits adenosine-triggered human nonpigmented ciliary epithelial cell fluid release and reduces mouse intraocular pressure. Curr Eye Res. 2005;30:747–754. doi: 10.1080/02713680590953147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JJ, Jacob TJ. Three different Cl− channels in the bovine ciliary epithelium activated by hypotonic stress. J Physiol. 1997;499(Pt 2):379–389. doi: 10.1113/jphysiol.1997.sp021935. [DOI] [PMC free article] [PubMed] [Google Scholar]