Abstract
Platinum based drugs continue to be the mainstay of therapy for ovarian cancer. Along with adverse effects, chemoresistance (intrinsic or acquired) has become a major limitation in the management of recurrent disease. Even though much is known about the effects of platinum drugs on cancer cells, the mechanisms underlying resistance are poorly understood. In this review, we summarize the current data on chemoresistance and discuss novel strategies to reverse resistance to platinum-based drugs. The most important targets highlighted here include Aurora kinases, PARP, ATP7B, and ERCC1. Furthermore, we discuss the implications of these novel approaches for ovarian cancer treatment.
Keywords: ATP7B, Cisplatin, Chemoresistance, Drug transport, Kinases, MK-0457, Ovarian cancer, siRNA, Tumor stroma, BRCA1
1. Introduction
Epithelial ovarian cancer is initially responsive to platinum-based therapy; however, recurrent disease is often refractory to treatment and is associated with high mortality rates (McGuire et al., 1996; Ozols and Young, 1984; Sood and Buller, 1998). It has been more than three decades since the discovery of the anticancer activity of cisplatin (Gottlieb and Drewinko, 1975; Rosenberg et al., 1965; Rosenberg et al., 1969), but we are just starting to discover the mechanisms that cause resistance. Platinum based drugs generally work by forming intra- or inter-strand cross-links in DNA that begins the process of cell-cycle arrest and results in tumor cell apoptosis (Kartalou and Essigmann, 2001; Pinto and Lippard, 1985). However, once the DNA is damaged, repair mechanisms are triggered resulting in improved cell survival (Brabec and Kasparkova, 2005).
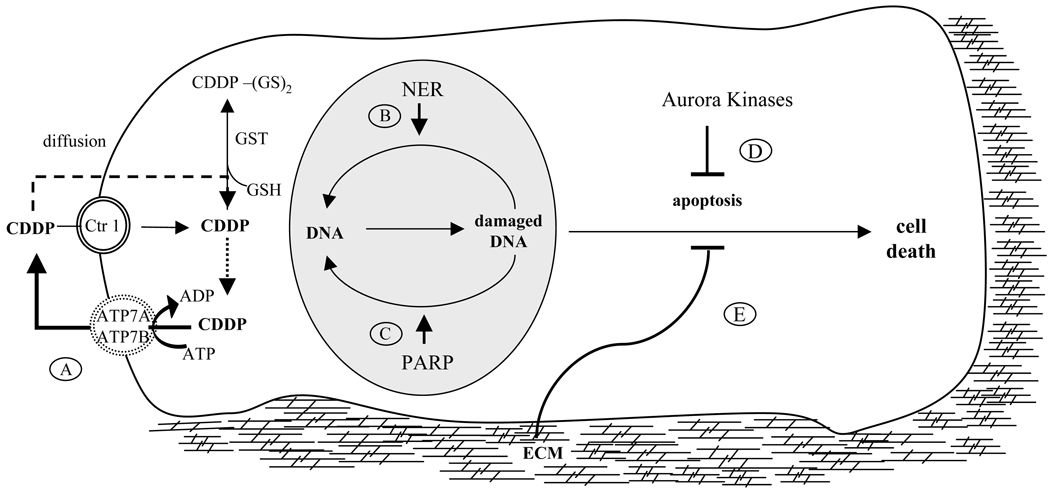
Mechanisms that underlie platinum resistance are poorly understood, and are most likely multifactorial in origin (Agarwal and Kaye, 2003; Perez, 1998; Richardson and Kaye, 2005; Stewart, 2007; Vasey, 2003; Stordal and Davey, 2009). Broadly, resistance to anticancer platinum agents can be classified into two categories (Fojo and Bates, 2003; Stewart, 2007): first, due to inadequate delivery, platinum compounds may not reach intracellular levels needed for response. For example, poor or altered vascularization (van Hensbergen et al., 2004), reduced uptake, enhanced efflux of drug from endothelium, or increased metabolism of drugs in the tumor cell may all compromise drug levels in the tumor. Second, increased DNA damage repair mechanisms or prevention of apoptosis may lead to increased viability, and hence resistance (Figure 1).
Fig. 1. Mechanisms of platinum resistance in cancer cells.
Cisplatin (CDDP) enters cell by Ctr1 or diffusion and leads to DNA damage and cell death. Cisplatin resistance may evolve through the following pathways: A) cisplatin can be pumped out of the cell by ATP7B. B) Nucleotide excision repair pathway (NER) and C) PARP repair of DNA damage. D) Aurora kinases may protect cells against chemotherapy by blocking apoptosis; E) ECM produces favorable changes in tumor microenvironment that inhibit chemotherapy-induced apoptosis
Early on, it became evident that resistance to cis-platinum and nephrotoxicity will present as major obstacles in achieving sustainable responses. Therefore, studies started to focus on genetic and molecular mechanisms that mediate resistance. Recently, the role of microenvironment, especially, of extracellular matrix in platinum resistance is being explored and novel targets are being identified (Sherman-Baust et al., 2003; Morin, 2003). Additionally, the ability to identify new genes and proteins involved in drug resistance and to silence them using RNA interference (RNAi), small molecule inhibitors or specific antibodies offers hopes for reversing platinum resistance (Mangala et al., 2009). In this review, we will focus on some of these approaches, including those related to RNAi.
2. Strategies for Reversing Platinum Resistance
2.1. Targeting the tumor microenvironment
A decade ago, Sethi et al. reported that the extracellular matrix (ECM) protects tumor cells from chemotherapy induced apoptosis (Sethi et al., 1999). The interactions between the tumor cells and the stroma may result in conditions that generate favorable ECM interactions for protecting tumor cells from chemotherapy induced apoptosis (Hazlehurst and Dalton, 2001; Sethi et al., 1999; Meads et al., 2009). One of the critical fallouts of these interactions is the reorganization of the ECM in chemotherapy resistance. Sherman-Baust and colleagues reported that production of collagen VI by the tumor cells results in resistance to therapy (Sherman-Baust et al., 2003). In this study, many of the genes increased in the resistant cells were related to ECM. For example, COL6A3 was one of the most expressed genes. Propagation of cisplatin-sensitive cells in collagen VI containing conditions prompted resistance to platinum-based treatment. Staining of tumors with collagen VI antibody not only confirmed its expression, but also suggested reorganization of ECM around the tumor. Such remodeling may promote tumor cell survival when targeted with chemotherapeutic agents. Therefore, components of the ECM may represent novel targets for cancer therapy. Given the absence of conventional approaches to address many novel targets, RNAi may represent a promising new strategy. Landen and colleagues have demonstrated successful delivery of non-targeted small-interfering RNA (siRNA) incorporated into nanoparticles (neutral liposomal-DOPC) in orthotopic mouse models of ovarian carcinoma (Landen et al., 2005). More recently, Pan and associates applied quantitative proteomic approaches and analyzed tumor tissues that were harvested during primary cytoreductive surgery prior to the start of chemotherapy (Pan et al., 2009). They identified 44 proteins that were overexpressed and 34 proteins that had lower expression in chemosensitive compared to chemoresistant tumors. More than 25% of the overexpressed proteins belonged to the ECM. Expression of these proteins may be useful for assessing and following response to therapy.
2.2. Nucleotide excision repair pathway
Nucleotide excision repair is highly conserved and plays an instrumental role in mediating resistance to platinum compounds (Rabik and Dolan, 2007). Lesions that result in alterations in the helical structure of the DNA or interfere with its replication and transcription are repaired by this pathway. The excision repair cross-complementation group1 (ERCC1) is a protein that plays a major role in mediating nucleotide excision repair. The complex dimerization of ERCC1 with pigmentosum complementation group F is required to excise the damaged DNA. In vitro exposure to cisplatin has been shown to result in platinum resistance and was associated with increased ERCC1 expression (Ferry et al., 2000). Additionally, ovarian cancer cell lines known to be resistant to platinum compounds had increased sensitivity after silencing ERCC1 expression using RNA interference (Selvakumaran et al., 2003). Chen and coworkers report that DNA hypermethylation of the ERCC1 promoter was inversely correlated to its mRNA expression and this association resulted in enhanced cisplatin chemosensitivity in a cohort of 32 glioma samples (Chen et al., 2009). ERCC1 mRNA levels evaluated in tumors from patients in clinical trials of non-small cell lung (Lord et al., 2002), colorectal (Shirota et al., 2001), and ovarian cancers (Kang et al., 2006) also showed an inverse association with survival or response to platinum compounds. Interestingly, clear cell ovarian carcinoma (known to have poor prognosis and being relatively platinum resistant) has significantly higher ERCC1 levels (Reed et al., 2003), which may reflect its role in protection from chemotherapy. Among 101 ovarian cancer patients who received carboplatin-paclitaxel combination therapy, almost 14% of the tumors had positive expression of ERCC1 (Steffensen et al., 2009). Seventy-five percent of the patients with chemoresistant cancer had positive ERCC1 expression in the tumor compared to 27% with negative expression. Additionally, ERCC1 expression was inversely correlated with progression-free survival. These findings offer opportunities to utilize RNAi or other approaches to target ERCC1 in patients that are prone to have resistance to platinum-based compounds to enhance therapeutic response.
2.3. Poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitor combinations
In cells, DNA is constantly subjected to damage, however, many pathways work in a synchronized effort to repair the damage and preserve genomic integrity, thereby leading to improved cell survival (Hoeijmakers, 2001; Jackson, 2001; Lindahl, 1993). PARPs make up a large family of multifunctional enzymes and the most abundant enzyme in this group is PARP1. Playing a major role in repair of single strand DNA breaks, PARP1 repairs base excisions (Ame et al., 2004; Dantzer et al., 2000). Inactivity of PARP1 results in accumulation of single strand DNA breaks, eventually leading to double strand breaks. Homologous-recombination double-strand DNA repair pathway normally repairs these breaks and BRCA1 and BRCA2 are the key components of this repair mechanism. Rottenberg and colleagues reported a genetically engineered mouse model (GEMM) for BRCA1 associated breast cancer (Rottenberg et al., 2008; Borst et al., 2008) where the combination of a PARP inhibitor (AZD2281) with cisplatin or carboplatin resulted in increased recurrence-free and overall survival. In this study, PARP inhibition potentiated the effects of DNA damaging agents.
Sakai and colleagues have recently reported secondary mutations as a mechanism of cisplatin resistance in tumors that have BRCA2 mutations (Sakai et al., 2008; Sakai et al., 2009). They demonstrated that the acquired resistance to cisplatin was secondary to restoration of the wild-type BRCA2 reading frame. Additionally, Swisher and associates reported that in ovarian tumors that contain BRCA1 mutations, secondary mutations in BRCA1 can also mediate resistance to platinum-based therapy (Swisher et al., 2008). These reports demonstrate that BRCA1/2 mutations that are known to predispose to cancer also play a vital role in determining clinical response to therapy. They suggest that restoration of these genes results in augmented repair of the DNA damage caused by platinum compounds, which contributes to chemoresistance.
To date, there are over 30 ongoing or recently completed studies that are evaluating PARP inhibition in combination with several different chemotherapeutic agents. Fong and coworkers reported a phase 1 trial of the PARP1 inhibitor, olaparib (AZD2281), in patients with specific DNA-repair defects, including those with BRCA1 or BRCA2 mutations. In this dose escalation study, 60 patients with advanced solid tumors were enrolled and treated with olaparib 10 mg daily to 600 mg twice daily. Additionally, a cohort of 22 patients who were carriers of BRCA1 or BRCA2 mutations were treated with olaparib at a dose of 200 mg twice daily. PARP1 inhibition was greater then 90% compared with baseline values. In patients with BRCA1 or BRCA2 mutations, 63% of the patients had clinical benefit and stabilization of disease (Fong et al., 2009).
2.4. Targeting the Aurora family of kinases
Aurora kinases play essential functions in mitotic integrity and cell cycle (Carvajal et al., 2006; Harrington et al., 2004; Katayama et al., 2003; Keen and Taylor, 2004). Landen and colleagues reported that Aurora-A is overexpressed in 83% of human epithelial ovarian cancers and predicts poor clinical outcome (Landen et al., 2007). Aurora-A kinase has also been implicated in protecting cells from apoptosis induced by chemotherapeutic agents such as cisplatin and paclitaxel by activation of AKT and checkpoint dysregulation (Anand et al., 2003; Yang et al., 2006). Sun and associates have recently demonstrated that inhibition of Aurora kinase can sensitize cells to chemotherapy by down-regulating NF-κB (Sun et al., 2007).
In a recent study, Lin and associates tested the effects of a pan-Aurora kinase inhibitor, MK-0457, in both platinum sensitive and resistant mouse models (Lin et al., 2008). Aurora kinase inhibition in the platinum-resistant A2780-cp20 model as monotherapy resulted in 78% reduction, and in combination with cisplatin resulted in 92% reduction in tumor growth compared to control. Furthermore, cisplatin plus MK-0457 treatment resulted in 91% reduction in tumor growth compared with cisplatin therapy alone. Combination of the Aurora kinase inhibitor and cisplatin significantly reduced tumor metastasis compared to cisplatin alone. These data were supported by favorable changes in the tumor microenvironment (tumor cell proliferation and apoptosis) that resulted from the addition of MK-0457. Additionally, therapeutic inhibition of Aurora kinases in taxane-resistant orthotopic models resulted in reduced tumor growth, which was related to increased apoptosis. This further emphasizes apoptosis as a key anti-tumor mechanism of Aurora kinase inhibition. Interestingly, several protease-related genes (CPB1, CTRB1 and ELA2A) were found to have increased expression in response to therapy. Increased expression of these degenerative genes may also be important in decreasing tumor growth. Recently, Kiat and coworkers described an Aurora-A kinase interacting protein (AIP) that works as an endogenous negative regulator of Aurora-A kinase (Kiat et al., 2002). Here, authors showed specificity and efficacy of Aurora-A down-regulation that is proteasome-dependent; however, the clinical potential of this pathway remains to be demonstrated.
There is growing interest in the role of Aurora kinases in chemoresistance. In pancreatic cancer models, Aurora-A kinase silencing with siRNA enhanced paclitaxel sensitivity (Hata et al., 2005). MK-0457 reported by Lin and associates also has high specificity for Aurora kinase (Lin et al., 2008). This study demonstrated potent antitumor activity in platinum-resistant orthotopic ovarian cancer models. The mechanisms described in this study provide the preclinical rationale for clinical trials focusing on targeting Aurora kinase.
2.5. Targeting platinum transport mechanisms
Recent data indicate that copper uptake and efflux transporter may also regulate the cellular pharmacodynamics of cisplatin (Katano et al., 2003; Ooi et al., 1996). In particular, ATP7A and ATP7B (copper transporters) have higher expression levels in platinum-resistant cells (Katano et al., 2002; Katano et al., 2004; Qian et al., 1995; Samimi et al., 2004). These copper transporters have also been functionally implicated in resistance to several platinum compounds, including cisplatin, carboplatin and oxaliplatin (Samimi et al., 2004). Among the solid tumors, ATP7B is shown to be overexpressed in several tumor types including gastric, breast, esophageal, hepatocellular, colorectal, uterine, and oral squamous cell carcinomas (Higashimoto et al., 2003; Kanzaki et al., 2002; Miyashita et al., 2003; Nakayama et al., 2004; Ohbu et al., 2003; Sugeno et al., 2004). Primary function of ATP7A and ATP7B is to transport copper into the lumen of trans-Golgi network (TGN) to facilitate biosynthesis of copper-dependent enzymes. The copper transporters also facilitate export of excess copper by sequestering copper into exocytic vesicles (Lutsenko et al., 2007). Cu-ATPases are known to bind copper at their NH2-terminal domain and transfer copper across cell membranes using energy generated by ATP hydrolysis. Interestingly, elevated copper levels induce Cu-ATPase activity, leading to intracellular trafficking of ATP7A and ATP7B from the TGN to exocytic vesicles.
Until recently, the ability to target the transporters involved in cisplatin chemoresistance was limited. Mangala and colleagues have now utilized RNAi approaches to specifically target a key cisplatin resistance gene (ATP7B). First, using quantitative real-time RT-PCR, higher ATP7B expression levels were reported in platinum-resistant cells compared with sensitive cell lines (Mangala et al., 2009). They further showed that in vitro targeting ATP7B using RNAi approaches resulted in a 2.5-fold reduction in the IC50 levels of cisplatin and there was a 30% increase in DNA adduct formation in cisplatin-resistant cells. This may represent enhanced nuclear availability of cisplatin in cells where ATB7B was specifically targeted. Additionally, a highly efficient method for systemic delivery of siRNA was utilized (Halder et al., 2006; Landen et al., 2005), and demonstrated a 70–80% reduction in tumor growth when cisplatin therapy was combined with ATP7B gene silencing in orthotopic mouse models of ovarian carcinoma known to be resistant to cisplatin (A2780-CP20). The anti-tumor activity was accompanied by reductions in proliferation, cell survival, and angiogenesis. Leonhardt and colleagues (Leonhardt et al., 2009) provided additional evidence that supports the role of the NH2-terminal metal binding domain in functional interactions between cisplatin and ATP7B. Data in this study provide a new understanding of cisplatin resistance in cancer cells and may have implications for therapeutic reversal of drug resistance.
3. Conclusion
One of the major obstacles to achieving the desired responses to treatment is resistance to therapy. In this review, we have summarized current data on cisplatin resistance and have described novel strategies that may be effective in reversing cisplatin resistance. Particularly, the potential benefits of targeting ATP7B using RNAi approaches, targeting Aurora family of kinases using small molecule inhibitors as well as PARP inhibitor combinations are discussed (see Table 1). These are promising targets that may hold the key to reversing platinum resistance in patients and should be explored further. Some of the targets involved in chemoresistance, however, may also play normal physiological functions. Therefore, therapeutic strategies aimed at reversing resistance should be pursued with caution to avoid potential toxicities.
Table1.
Current studies exploring reversal of cisplatin resistance by targeting PARP, Aurora Kinase, ERCC1 or ATP7B
| Target | Model | Study Design | Compound | Target/use | Reference |
|---|---|---|---|---|---|
| PARP | Ovarian | Phase II | KU-0059436 | PARP inhibition | www.clinicaltrials.gov |
| IGF1R | Ovarian | Preclinical | BMS-536924 | PARPI sensitization | (Beauchamp et al., 2009) |
| PARP | Ovarian | Phase I | AZD2281 | BRCA mutation | (Fong et al., 2009) |
| PARP | Breast | Preclinical | AZD2281 | BRCA2 mutation/P53 | (Hay et al, 2009) |
| PARP | Triple (−) Breast cancer | Phase II | BSI201 | PARP inhibition | www.clinicaltrials.gov |
| Aurora Kinase | Tumor cell lines | Preclinical | R763/AS703569 | Anti-tumor activity | (McLaughlin et al., 2009) |
| Aurora Kinase | Ovarian | Preclinical | MK-0457 | Reversal of platinum resistance |
(Mangala et al., 2009) |
| Aurora Kinase | Multiple | Preclinical | SNS-314 | Anti-tumor activity | (Arbitrario et al., 2009) |
| Multikinase | Leukemia | Preclinical | KW-2449 | Anti-tumor activity | (Shiotsu et al., 2009) |
| ERCC1 | No data | No data | - | - | - |
| ATP7B | Ovarian | Preclinical | siRNA/DOPC | Reversal of platinum resistance |
(Mangala et al., 2009) |
Acknowledgements
MMS is supported by the GCF-Molly Cade ovarian cancer research grant and the NIH/NICHD Baylor WRHR scholarship grant (HD050128). This research was funded in part by a Program Project Development Grant from the Ovarian Cancer Research Fund, Inc., the Zarrow Foundation, NIH grants (CA109298 and CA110793), the Marcus Foundation, the U.T.M.D. Anderson Cancer Center SPORE in Ovarian Cancer (P50 CA083639), the EIF Foundation, and the Betty Ann Asche Murray Distinguished Professorship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat. Rev. Cancer. 2003;3:502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26:882–893. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3:51–62. doi: 10.1016/s1535-6108(02)00235-0. [DOI] [PubMed] [Google Scholar]
- Arbitrario JP, Belmont BJ, Evanchik MJ, Flanagan WM, Funcini RV, Hansen SK, Harris SO, Hashash A, Hoch U, Hogan NJ, Howlett AR, Jacobs JW, Lam JW, Ritchie SC, Romanowski MJ, Silverman JA, Stockett DE, Teague JN, Zimmerman MK, Taverna P. SNS-314, a pan-Aurora kinase inhibitor, shows potent anti-tumor activity and dosing flexibility in vivo. Cancer Chemother Pharmacol. 2009 doi: 10.1007/s00280-009-1076-8. doi: 10.1007/s00280-009-1076-8. [DOI] [PubMed] [Google Scholar]
- Beauchamp MC, Knafo A, Yasmeen A, Carboni JM, Gottardis MM, Pollak MN, Gotlieb WH. BSM-536924 sensitizes human epithalial ovarian cancer cells to the PARP inhibitor, 3-amniobenzamide. Gynecol. Oncol. 2009 doi: 10.1016/j.ygyno.2009.07.009. doi:10.1016/j.ygyno.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Borst P, Rottenberg S, Jonkers J. How do real tumors become resistant to platinum? Cell Cycle. 2008;7:1353–1359. doi: 10.4161/cc.7.10.5930. [DOI] [PubMed] [Google Scholar]
- Brabec V, Kasparkova J. Modifications of DNA by platinum complexes. Relation to resistance of tumors to platinum antitumor drugs. Drug Resist. Updat. 2005;8:131–146. doi: 10.1016/j.drup.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Carvajal RD, Tse A, Schwartz GK. Aurora kinases: new targets for cancer therapy. Clin. Cancer Res. 2006;12:6869–6875. doi: 10.1158/1078-0432.CCR-06-1405. [DOI] [PubMed] [Google Scholar]
- Chen HY, Shao CJ, Chen FR, Kwan AL, Chen ZP. Role of ERCC1 promoter hypermethylation in drug resistance to cisplatin in human gliomas. Int. J. Cancer. 2009 doi: 10.1002/ijc.24772. doi: 10.1002/ijc.24772. [DOI] [PubMed] [Google Scholar]
- Dantzer F, de La Rubia G, Menissier-De Murcia J, Hostomsky Z, de Murcia G, Schreiber V. Base excision repair is impaired in mammalian cells lacking Poly(ADP-ribose) polymerase-1. Biochemistry. 2000;39:7559–7569. doi: 10.1021/bi0003442. [DOI] [PubMed] [Google Scholar]
- Ferry KV, Hamilton TC, Johnson SW. Increased nucleotide excision repair in cisplatin-resistant ovarian cancer cells: role of ERCC1-XPF. Biochem. Pharmacol. 2000;60:1305–1313. doi: 10.1016/s0006-2952(00)00441-x. [DOI] [PubMed] [Google Scholar]
- Fojo T, Bates S. Strategies for reversing drug resistance. Oncogene. 2003;22:7512–7523. doi: 10.1038/sj.onc.1206951. [DOI] [PubMed] [Google Scholar]
- Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. New Engl. J. Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- Gottlieb JA, Drewinko B. Review of the current clinical status of platinum coordination complexes in cancer chemotherapy. Cancer Chemother. Rep. 1975;59:621–628. [PubMed] [Google Scholar]
- Halder J, Kamat AA, Landen CN, Jr., Han LY, Lutgendorf SK, Lin YG, Merritt WM, Jennings NB, Chavez-Reyes A, Coleman RL, Gershenson DM, Schmandt R, Cole SW, Lopez-Berestein G, Sood AK. Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy. Clin. Cancer Res. 2006;12:4916–4924. doi: 10.1158/1078-0432.CCR-06-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, Graham JA, Demur C, Hercend T, Diu-Hercend A, Su M, Golec JM, Miller KM. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat. Med. 2004;10:262–267. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- Hata T, Furukawa T, Sunamura M, Egawa S, Motoi F, Ohmura N, Marumoto T, Saya H, Horii A. RNA interference targeting aurora kinase a suppresses tumor growth and enhances the taxane chemosensitivity in human pancreatic cancer cells. Cancer Res. 2005;65:2899–2905. doi: 10.1158/0008-5472.CAN-04-3981. [DOI] [PubMed] [Google Scholar]
- Hay T, Mathews JR, Pietzka L, Lau A, Cranston A, Nygren AO, Douglas-Jones A, Smith GC, Martin NM, O'Connor M, Clarke AR. Poly(ADP-ribose) polymerase-1 inhibitor treatment regresses autochthonous Brca2/p53-mutant mammary tumors in vivo and delays tumor relapse in combination with carboplatin. Cancer Res. 2009;69:3850–3855. doi: 10.1158/0008-5472.CAN-08-2388. [DOI] [PubMed] [Google Scholar]
- Hazlehurst LA, Dalton WS. Mechanisms associated with cell adhesion mediated drug resistance (CAM-DR) in hematopoietic malignancies. Cancer Metastasis Rev. 2001;20:43–50. doi: 10.1023/a:1013156407224. [DOI] [PubMed] [Google Scholar]
- Higashimoto M, Kanzaki A, Shimakawa T, Konno S, Naritaka Y, Nitta Y, Mori S, Shirata S, Yoshida A, Terada K, Sugiyama T, Ogawa K, Takebayashi Y. Expression of copper-transporting P-type adenosine triphosphatase in human esophageal carcinoma. Int. J. Mol. Med. 2003;11:337–341. [PubMed] [Google Scholar]
- Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- Jackson SP. Detecting, signalling and repairing DNA double-strand breaks. Biochem. Soc. Trans. 2001;29:655–661. doi: 10.1042/0300-5127:0290655. [DOI] [PubMed] [Google Scholar]
- Kang S, Ju W, Kim JW, Park NH, Song YS, Kim SC, Park SY, Kang SB, Lee HP. Association between excision repair cross-complementation group 1 polymorphism and clinical outcome of platinum-based chemotherapy in patients with epithelial ovarian cancer. Exp. Mo.l Med. 2006;38:320–324. doi: 10.1038/emm.2006.38. [DOI] [PubMed] [Google Scholar]
- Kanzaki A, Toi M, Neamati N, Miyashita H, Oubu M, Nakayama K, Bando H, Ogawa K, Mutoh M, Mori S, Terada K, Sugiyama T, Fukumoto M, Takebayashi Y. Copper-transporting P-type adenosine triphosphatase (ATP7B) is expressed in human breast carcinoma. Jpn. J. Cancer Res. 2002;93:70–77. doi: 10.1111/j.1349-7006.2002.tb01202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutat. Res. 2001;478:23–43. doi: 10.1016/s0027-5107(01)00141-5. [DOI] [PubMed] [Google Scholar]
- Katano K, Kondo A, Safaei S, Holzer A, Samimi G, Mishima M, Kuo YM, Rochdi M, Howell SB. Acquisition of resistance to cisplatin is accompanied by changes in the cellular pharmacology of copper. Cancer Res. 2002;62:6559–6565. [PubMed] [Google Scholar]
- Katano K, Safaei R, Samimi G, Holzer A, Rochdi M, Howell SB. The copper export pump ATP7B modulates the cellular pharmacology of carboplatin in ovarian carcinoma cells. Mol. Pharmacol. 2003;64:466–473. doi: 10.1124/mol.64.2.466. [DOI] [PubMed] [Google Scholar]
- Katano K, Safaei R, Samimi G, Holzer A, Tomioka M, Goodman M, Howell SB. Confocal microscopic analysis of the interaction between cisplatin and the copper transporter ATP7B in human ovarian carcinoma cells. Clin. Cancer Res. 2004;10:4578–4588. doi: 10.1158/1078-0432.CCR-03-0689. [DOI] [PubMed] [Google Scholar]
- Katayama H, Brinkley WR, Sen S. The Aurora kinases: role in cell transformation and tumorigenesis. Cancer Metastasis Rev. 2003;22:451–464. doi: 10.1023/a:1023789416385. [DOI] [PubMed] [Google Scholar]
- Keen N, Taylor S. Aurora-kinase inhibitors as anticancer agents. Nat. Rev. Cancer. 2004;4:927–936. doi: 10.1038/nrc1502. [DOI] [PubMed] [Google Scholar]
- Kiat LS, Hui KM, Gopalan G. Aurora-A kinase interacting protein (AIP), a novel negative regulator of human Aurora-A kinase. J. Biol. Chem. 2002;277:45558–45565. doi: 10.1074/jbc.M206820200. [DOI] [PubMed] [Google Scholar]
- Landen CN, Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, Sood AK. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65:6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- Landen CN, Jr, Lin YG, Immaneni A, Deavers MT, Merritt WM, Spannuth WA, Bodurka DC, Gershenson DM, Brinkley WR, Sood AK. Overexpression of the centrosomal protein Aurora-A kinase is associated with poor prognosis in epithelial ovarian cancer patients. Clin. Cancer Res. 2007;13:4098–4104. doi: 10.1158/1078-0432.CCR-07-0431. [DOI] [PubMed] [Google Scholar]
- Leonhardt K, Gebhardt R, Mössner J, Lutsenko S, Huster D. Funtional interactions of Cu-ATPase ATP7B with cisplatin and the role of ATP7B in the resistance to the drug. J. Biol. Chem. 2009;284:7793–7802. doi: 10.1074/jbc.M805145200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YG, Immaneni A, Merritt WM, Mangala LS, Kim SW, Shahzad MM, Tsang YT, Armaiz-Pena GN, Lu C, Kamat AA, Han LY, Spannuth WA, Nick AM, Landen CN, Jr, Wong KK, Gray MJ, Coleman RL, Bodurka DC, Brinkley WR, Sood AK. Targeting aurora kinase with MK-0457 inhibits ovarian cancer growth. Clin. Cancer Res. 2008;14:5437–5446. doi: 10.1158/1078-0432.CCR-07-4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- Lord RV, Brabender J, Gandara D, Alberola V, Camps C, Domine M, Cardenal F, Sanchez JM, Gumerlock PH, Taron M, Sanchez JM, Danenberg KD, Danenberg PV, Rosell R. Low ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin. Cancer Res. 2002;8:2286–2291. [PubMed] [Google Scholar]
- Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol. Rev. 2007;87:1011–1046. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- Mangala LS, Zuzel V, Schmandt R, Leshane ES, Halder JB, Armaiz-Pena GN, Spannuth WA, Tanaka T, Shahzad MM, Lin YG, Nick AM, Danes CG, Lee JW, Jennings NB, Vivas-Mejia PE, Wolf JK, Coleman RL, Siddik ZH, Lopez-Berestein G, Lutsenko S, Sood AK. Therapeutic targeting of ATP7B in ovarian carcinoma. Clin. Cancer Res. 2009;15:3770–3780. doi: 10.1158/1078-0432.CCR-08-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. New Engl. J. Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, Markovtsov V, Li H, Wong S, Gelman M, Zhu Y, Franci C, Lang DW, Pali E, Lasaga J, Low C, Zhao F, Chang B, Gururaja TL, Xu W, Baluom M, Sweeny D, Carrol D, Sran A, Thota S, Parmer M, Romane A, Clemens G, Grossbard E, Qu K, Jenkins T, Kinoshita T, Taylor V, Holland SJ, Argade A, Singh R, Pine P, Payan DG, Hitoshi Y. Preclinical characterization of Aurora kinase inhibitor R763/AS703569 indentified through an image-based phenotypic screen. J Cancer Res. Clin. Oncol. 2009 doi: 10.1007/s00432-009-0641-1. doi: 10.1007/s00432-009-0641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat. Rev. Cancer. 2009;9:665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- Miyashita H, Uchida T, Mori S, Echigo S, Motegi K. Expression status of Pin1 and cyclins in oral squamous cell carcinoma: Pin1 correlates with Cyclin D1 mRNA expression and clinical significance of cyclins. Oncol. Rep. 2003;10:1045–1048. [PubMed] [Google Scholar]
- Morin PJ. Drug resistance and the microenvironment: nature and nurture. Drug Resist. Updat. 2003;6:169–172. doi: 10.1016/s1368-7646(03)00059-1. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Kanzaki A, Terada K, Mutoh M, Ogawa K, Sugiyama T, Takenoshita S, Itoh K, Yaegashi N, Miyazaki K, Neamati N, Takebayashi Y. Prognostic value of the Cu-transporting ATPase in ovarian carcinoma patients receiving cisplatin-based chemotherapy. Clin. Cancer Res. 2004;10:2804–2811. doi: 10.1158/1078-0432.ccr-03-0454. [DOI] [PubMed] [Google Scholar]
- Ohbu M, Ogawa K, Konno S, Kanzaki A, Terada K, Sugiyama T, Takebayashi Y. Copper-transporting P-type adenosine triphosphatase (ATP7B) is expressed in human gastric carcinoma. Cancer Lett. 2003;189:33–38. doi: 10.1016/s0304-3835(02)00462-7. [DOI] [PubMed] [Google Scholar]
- Ooi CE, Rabinovich E, Dancis A, Bonifacino JS, Klausner RD. Copper-dependent degradation of the Saccharomyces cerevisiae plasma membrane copper transporter Ctr1p in the apparent absence of endocytosis. EMBO J. 1996;15:3515–3523. [PMC free article] [PubMed] [Google Scholar]
- Ozols RF, Young RC. Chemotherapy of ovarian cancer. Semin. Oncol. 1984;11:251–263. [PubMed] [Google Scholar]
- Pan S, Cheng L, White JT, Lu W, Utleg AG, Yan X, Urban ND, Drescher CW, Hood L, Lin B. Quantitative proteomics analysis integrated with microarray data reveals that extracellular matrix proteins, catenins, and p53 binding protein 1 are important for chemotherapy response in ovarian cancers. OMICS. 2009;13:345–354. doi: 10.1089/omi.2009.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez RP. Cellular and molecular determinants of cisplatin resistance. Eur. J. Cancer. 1998;34:1535–1542. doi: 10.1016/s0959-8049(98)00227-5. [DOI] [PubMed] [Google Scholar]
- Pinto AL, Lippard SJ. Binding of the antitumor drug cis-diamminedichloroplatinum(II) (cisplatin) to DNA. Biochim. Biophys. Acta. 1985;780:167–180. doi: 10.1016/0304-419x(85)90001-0. [DOI] [PubMed] [Google Scholar]
- Qian Y, Tiffany-Castiglioni E, Harris ED. Copper transport and kinetics in cultured C6 rat glioma cells. Am. J .Physiol. 1995;269:C892–C898. doi: 10.1152/ajpcell.1995.269.4.C892. [DOI] [PubMed] [Google Scholar]
- Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treatm. Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed E, Yu JJ, Davies A, Gannon J, Armentrout SL. Clear cell tumors have higher mRNA levels of ERCC1 and XPB than other histological types of epithelial ovarian cancer. Clin. Cancer Res. 2003;9:5299–5305. [PubMed] [Google Scholar]
- Richardson A, Kaye SB. Drug resistance in ovarian cancer: the emerging importance of genetranscription and spatio-temporal regulation of resistance. Drug Resist. Updat. 2005;8:311–321. doi: 10.1016/j.drup.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Rosenberg B, Vancamp L, Krigas T. Inhibition of cell division in Escherichia Coli by electrolysis products from a platinum electrode. Nature. 1965;205:698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg B, VanCamp L, Trosko JE, Mansour VH. Platinum compounds: a new class of potent antitumour agents. Nature. 1969;222:385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, Derksen PW, de Bruin M, Zevenhoven J, Lau A, Boulter R, Cranston A, O'Connor MJ, Martin NM, Borst P, Jonkers J. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl. Acad. Sci. USA. 2008;105:17079–17084. doi: 10.1073/pnas.0806092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai W, Swisher EM, Jacquemont C, Chandramohan KV, Couch FJ, Langdon SP, Wurz K, Higgins J, Villegas E, Taniguchi T. Functional restoration of BRCA2 protein by secobdary mutatuions in BRCA2-mutated ovarian carcinoma. Cancer Res. 2009;69:6381–6386. doi: 10.1158/0008-5472.CAN-09-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai W, Swisher EM, Karlan BY, Agarwal MK, Higgins J, Friedman C, Villegas E, Jacquemont C, Farrugia DJ, Couch FJ, Urban N, Taniguchi T. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451:1116–1120. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samimi G, Safaei R, Katano K, Holzer AK, Rochdi M, Tomioka M, Goodman M, Howell SB. Increased expression of the copper efflux transporter ATP7A mediates resistance to cisplatin, carboplatin, and oxaliplatin in ovarian cancer cells. Clin. Cancer Res. 2004;10:4661–4669. doi: 10.1158/1078-0432.CCR-04-0137. [DOI] [PubMed] [Google Scholar]
- Selvakumaran M, Pisarcik DA, Bao R, Yeung AT, Hamilton TC. Enhanced cisplatin cytotoxicity by disturbing the nucleotide excision repair pathway in ovarian cancer cell lines. Cancer Res. 2003;63:1311–1316. [PubMed] [Google Scholar]
- Sethi T, Rintoul RC, Moore SM, MacKinnon AC, Salter D, Choo C, Chilvers ER, Dransfield I, Donnelly SC, Strieter R, Haslett C. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat. Med. 1999;5:662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- Sherman-Baust CA, Weeraratna AT, Rangel LB, Pizer ES, Cho KR, Schwartz DR, Shock T, Morin PJ. Remodeling of the extracellular matrix through overexpression of collagen VI contributes to cisplatin resistance in ovarian cancer cells. Cancer Cell. 2003;3:377–386. doi: 10.1016/s1535-6108(03)00058-8. [DOI] [PubMed] [Google Scholar]
- Shiotsu Y, Kiyoi H, Ishikawa Y, Tanizaki R, Shimizu M, Umehara H, Ishii K, Mori Y, Ozeki K, Minami Y, Abe A, Maeda H, Akiyama T, Kanda Y, Sato Y, Akinaga S, Naoe T. KW-2449, a novel multikinase inhibitor, suppresses the growth of leukemia cells with FLT3 mutations or T3151-mutated BCR/ABL translocation. Blood. 2009;114:1607–1617. doi: 10.1182/blood-2009-01-199307. [DOI] [PubMed] [Google Scholar]
- Shirota Y, Stoehlmacher J, Brabender J, Xiong YP, Uetake H, Danenberg KD, Groshen S, Tsao-Wei DD, Danenberg PV, Lenz HJ. ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J. Clin. Oncol. 2001;19:4298–4304. doi: 10.1200/JCO.2001.19.23.4298. [DOI] [PubMed] [Google Scholar]
- Sood AK, Buller RE. Drug resistance in ovarian cancer: from the laboratory to the clinic. Obstet. Gynecol. 1998;92:312–319. doi: 10.1016/s0029-7844(98)00184-7. [DOI] [PubMed] [Google Scholar]
- Steffensen KD, Waldstrom M, Jakobsen A. The relationship of platinum resistance and ERCC1 protein expression in epithelial ovarian cancer. Int. J. Gynecol. Cancer. 2009;19:820–825. doi: 10.1111/IGC.0b013e3181a12e09. [DOI] [PubMed] [Google Scholar]
- Stewart DJ. Mechanisms of resistance to cisplatin and carboplatin. Crit. Rev. Oncol. Hematol. 2007;63:12–31. doi: 10.1016/j.critrevonc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Stordal B, Davey R. A systematic review of genes involved in the inverse resistance relationship between cisplatin and paclitaxel chemotherapy: Role of BRCA1. Current Cancer Drug Targets. 2009;9:354–365. doi: 10.2174/156800909788166592. [DOI] [PubMed] [Google Scholar]
- Sugeno H, Takebayashi Y, Higashimoto M, Ogura Y, Shibukawa G, Kanzaki A, et al. Expression of copper-transporting P-type adenosine triphosphatase (ATP7B) in human hepatocellular carcinoma. Anticancer Res. 2004;24:1045–1048. [PubMed] [Google Scholar]
- Sun C, Chan F, Briassouli P, Linardopoulos S. Aurora kinase inhibition downregulates NF-kappaB and sensitises tumour cells to chemotherapeutic agents. Biochem. Biophys. Res. Commun. 2007;352:220–225. doi: 10.1016/j.bbrc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Swisher EM, Sakai W, Karlan BY, Wurz K, Urban N, Taniguchi T. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 2008;68:2581–2586. doi: 10.1158/0008-5472.CAN-08-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hensbergen Y, Broxterman HJ, Rana S, van Diest PJ, Duyndam MC, Hoekman K, Pinedo HM, Boven E. Reduced growth, increased vasccular area, and reduced response to cisplatin in CD13-overexpressing human ovarian cancer xenografts. Clin. Cancer Res. 2004;10:1180–1191. doi: 10.1158/1078-0432.ccr-0482-3. [DOI] [PubMed] [Google Scholar]
- Vasey PA. Resistance to chemotherapy in advanced ovarian cancer: mechanisms and current strategies. Br. J. Cancer. 2003;89 Suppl 3:S23–S28. doi: 10.1038/sj.bjc.6601497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, He L, Kruk P, Nicosia SV, Cheng JQ. Aurora-A induces cell survival and chemoresistance by activation of Akt through a p53-dependent manner in ovarian cancer cells. Int. J. Cancer. 2006;119:2304–2312. doi: 10.1002/ijc.22154. [DOI] [PubMed] [Google Scholar]