Abstract
Based on our previous observation, the whole Scutellaria baicalensis extract (SbE) did not show significant breast cancer cell inhibitory effect. In this study, we isolated a baicalin-deprived-fraction (SbF1) of Scutellaria baicalensis, and baicalin-fraction (SbF3), and evaluated their anti-breast cancer properties using MCF-7 cells. The content of four flavonoids in extract/fractions were determined using high performance liquid chromatography. Analytical data showed that in SbF1, the major constituents are baicalein and wogonin, while SbF3 only contains baicalin. The antiproliferative effects of fractions and SbE were assayed using modified trichrome stain method. SbF1 showed significant antiproliferative effect. Treated with 100 µg/ml of SbF1 for 72 h inhibited MCF-7 cell growth by 81.6%, while in the same treatment concentration, SbF3 increased cell growth by 22.6%. SbF1 was recognized as an active fraction of SbE. The effects of four flavonoids in SbE, scutellarin, baicalin, baicalein and wogonin, were determined, and data showed that baicalein and wogonin significantly inhibited MCF-7 cell growth. In contrast, in certain concentrations, scutellarin and baicalin increased cancer cell growth. The effects of SbF1 on cell cycle and apoptosis were assayed using flow cytometry. SbF1 arrested MCF-7 cells in S- and G2/M-phases, and significantly increased induction of cell apoptosis. These combined phytochemical and biological data provide evidence for further chemopreventive studies of the baicalin-deprived SbE on breast cancer.
Keywords: Scutellaria baicalensis, Breast cancer, Apoptosis, Baicalin, Baicalein, Wogonin
Introduction
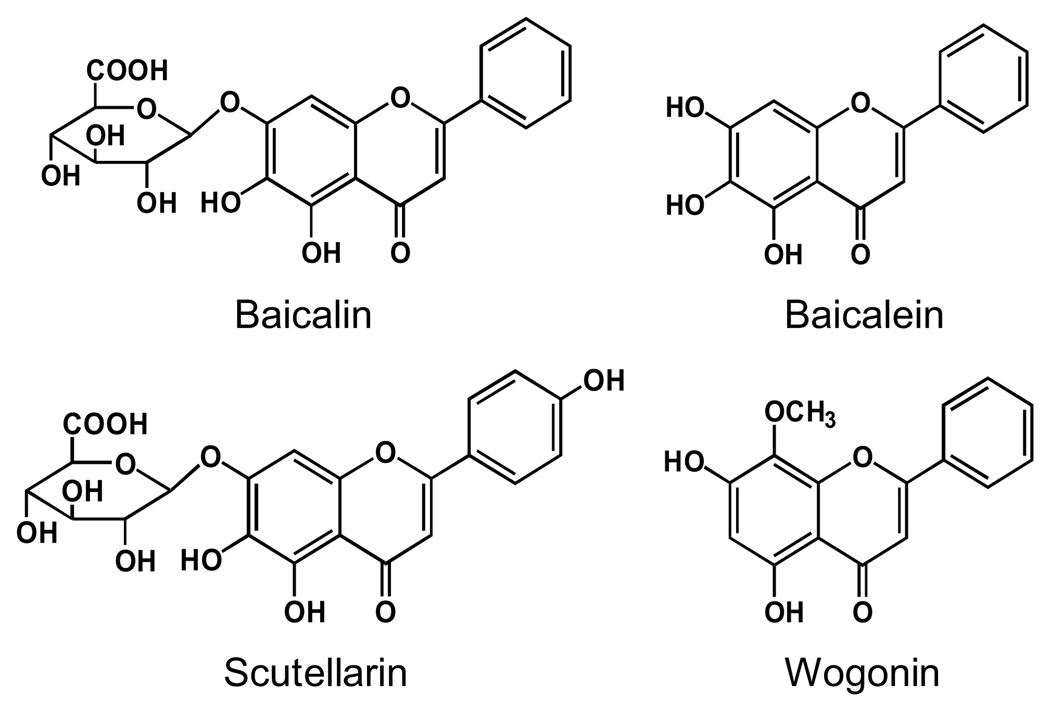
The root of Scutellaria baicalensis Georgi (Labiatae) is a widely used herb in the traditional medical systems of China and Japan, where it has been used as an ingredient in botanical formulations for many years with positive results for inflammatory diseases, allergies, hyperlipemia, and arteriosclerosis (Bruno et al. 2002; Wang et al. 2007; Huang et al. 2008). The representative constituents of S. baicalensis are a group of flavonoids that include baicalin, scutellarin, baicalein, and wogonin (Fig. 1) (Ye et al. 2004; Wang et al. 2007).
Fig. 1.
Chemical structures of four flavonoids in Scutellaria baicalensis.
In recent years, anticancer activities of S. baicalensis extract (SbE) and its constituents were evaluated (Himeji et al. 2007; Li-Weber 2009). The effects of baicalin on human LNCaP prostate cancer and SK-Hep1 hepatoma cells were observed and results showed that the inhibition of cell growth by 50% (IC50) were 60.8 µM and 25 µM, respectively (Chen et al. 2001; Chang et al. 2002; Li-Weber 2009). However, another major flavonoid in this herb, baicalein, possessed a stronger cell growth inhibitory effect than baicalin in these two cell lines. The IC50 of baicalein was 29.8 µM and 9.1 µM, respectively (Chen et al. 2001; Chang et al. 2002; Li-Weber 2009). The difference in chemical structure between these two components is that in baicalin, the 7-hydroxy group of baicalein was replaced by a glucuronyloxy group (Li et al. 2004). It is considered that this structure difference between baicalin and baicalein contributes to the difference in their pharmacological activities (Hong et al. 2002).
To date, effects of S. baicalensis on human breast cancer are largely unknown. Limited data showed some inhibitory effects of baicalein and wogonin on breast cancer cells (Po et al. 2002; Chung et al. 2008; Li-Weber 2009). However, Ikezoe et al. observed that baicalin was insensitive in the inhibition of MCF-7 breast cancer cell growth (Ikezoe et al. 2001). Thus, such reports suggest that the limited inhibition activity of SbE on breast cancer may be due to the insensitivity of baicalin on breast cancer cells.
In this study, in order to remove baicalin from SbE, we isolated the SbE into three fractions. In fraction 1, baicalin was successfully removed. The content of representative flavonoids in fractions and SbE were determined by high performance liquid chromatography (HPLC). The activities of baicalin-deprived-fraction on MCF-7 breast cancer cell growth, cell cycle and apoptosis were evaluated, and data were compared to that of other fractions and SbE.
Materials and methods
Chemicals
HPLC grade methanol, ethanol, n-butanol and acetonitrile were obtained from Fisher Scientific (Pittsburgh, PA, USA). Mili Q water was supplied by a water purification system (U.S. Filter, Palm Desert, CA, USA). Flavonoid standards baicalin and baicalein were obtained from Sigma (St. Louis, MO, USA); scutellarin and wogonin were obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). All standards were of biochemical-reagent grade and at least 95% pure as confirmed by HPLC. Trypsin, DMEM medium, fetal bovine serum (FBS), and penicillin-streptomycin solution (200×) were obtained from Mediatech, Inc. (Herndon, VA, USA). A CellTiter 96 Aqueous One Solution Cell Proliferation Assay kit was obtained from Promega (Madison, WI, USA). Propidium iodide (PI) and RNase were obtained from Sigma (St. Louis, MO, USA). Annexin V-FITC Apoptosis Detection Kit was obtained from BD Biosciences (San Diego, CA, USA).
Plant materials, extraction and isolation
The roots of Scutellaria baicalensis were obtained from Chengde (Hebei, China). The voucher samples were deposited at the Tang Center for Herbal Medicine Research at The University of Chicago. Dried S. baicalensis roots were ground to powder, and the powdered roots were extracted with 70% ethanol for 2 h. The extraction method was boiling under reflux. The filtrate was collected and the extraction procedure was repeated one more time on the residue. The combined filtrate was condensed under vacuum and lyophilized to yield dried S. baicalensis extract (SbE). The separation of SbE was performed using liquid-liquid extraction. The SbE powder was distributed in water, and then extracted serially with ethyl acetate (fraction 1, SbF1), 1-butanol (fraction 2, SbF2), and water (fraction 3, SbF3). Following extraction, the fractions were evaporated under vacuum and lyophilized.
HPLC analysis
The HPLC system was a Waters 2960 instrument (Milford, MA, USA) with a quaternary pump, an automatic injector, a photodiode array detector (Model 996), and a Waters Millennium 32 software for peak identification and integration (Wang et al. 2009). The separations were carried out on a Phenomenex Prodigy ODS(2) column (150×3.2 mm, 5 µm). A binary gradient solvent system of acetonitrile (eluent A) - 0.03% (v/v) phosphoric acid in water (eluent B) was used as follows: 15% A and 85% B (0 min), 28% A and 72% B (12 min), 35% A and 65% B (23 min), 50% A and 50% B (30 min), 95% A and 5% B (32–34 min), 15% A and 85% B (37–42 min). The flow-rate of 0.8 ml/min was used and absorbance was detected at 280 nm. All tested solutions were filtered through Millex 0.2-µm nylon membrane syringe filters before use. The contents of the constituents were calculated using standard curves of flavonoids.
Cell culture
MCF-7 human breast cancer cells (ATCC, Manassas, VA) were cultured in DMEM medium, supplemented with 10% fetal bovine serum and penicillin-streptomycin (50 units/ml). Cells were maintained at 37 °C in a humidified atmosphere, with 5% carbon dioxide and 95% air.
Cell proliferation assay using modified trichrome stain (MTS) method
Cells were seeded in 96-well plates. After 1 day, various concentrations of extract/constituents were added to the wells. The final concentration of DMSO was 0.5%. Controls were exposed to culture medium containing 0.5% DMSO without drugs. All experiments were performed in triplicate and repeated 2 times. Cell proliferation was evaluated using an MTS assay according to the manufacturer’s instructions. Briefly, at the end of the drug exposure period, the medium was replaced with 100 µl of fresh medium and 20 µl of MTS reagent (CellTiter 96 Aqueous Solution) in each well, and the plate was returned to the incubator for 1–2 h. A 60-µl aliquot of medium from each well was transferred to an ELISA 96-well plate and its absorbance at 490 nm was recorded. Results were expressed as a percentage of the control (DMSO vehicles set at 100%).
Cell cycle analysis after staining with propidium iodide
Cells were seeded in 24-well tissue culture plates. On the second day, the medium was changed, and cells were treated with 50 µg/ml of SbE, SbF1, SbF2 and SbF3. Cells were incubated for 24, 48 and 72 h before harvesting. The cells were fixed gently by adding 80% ethanol and placing them in a freezer for 2 h. They were then treated with 0.25% Triton X-100 for 5 min in an ice bath. The cells were resuspended in 300 µl of PBS containing 40 µg/ml propidium iodide (PI) and 0.1 mg/ml RNase. Cells were incubated in a dark room for 20 min at room temperature then subjected to cell cycle analysis using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA, USA) and FlowJo 7.1.0 software (Tree Star, Ashland, OR, USA). For each measurement, at least 20,000 cells were counted.
Apoptosis analysis after staining by annexin V/propidium iodide
Cells were seeded in 24-well tissue culture plates. After 1 day, the medium was changed and SbE, SbF1, SbF2 and SbF3 were added. After treatment for 48 h, cells floating in the medium were collected. The adherent cells were detached with 0.05% trypsin. Then culture medium containing FBS (and floating cells) was added to inactivate trypsin. After being pipetted gently, the cells were centrifuged for 5 min at 1500 g. The supernatant was removed and cells were stained with annexin V-FITC and propidium iodide (PI) according to the manufacturer’s instructions. Untreated cells were used as a control for double staining. The cells were analyzed immediately after staining using a FACScan flow cytometer. For each measurement, at least 20,000 cells were counted.
Antiproliferative effect of scutellarin, baicalin, baicalein and wogonin
The antiproliferative effects of four single compounds in SbE were assayed. Scutellarin, baicalin, baicalein and wogonin were dissolved in DMSO. Cells were seeded in 96-well plates. Controls were exposed to culture medium containing the same quantity of DMSO without drugs. The treatment time was 72 h and the cell proliferation analysis process was similar to that of SbE and fractions.
Statistical analysis
All cell proliferation, flow cytometry experiments were performed in triplicate. The data are presented as mean ± standard error (SE). A one-way ANOVA determined whether the results had statistical significance. In some cases, the Student's t-test was used for comparing two groups. The level of statistical significance was set at p<0.05.
Results
HPLC analysis of SbE and three fractions
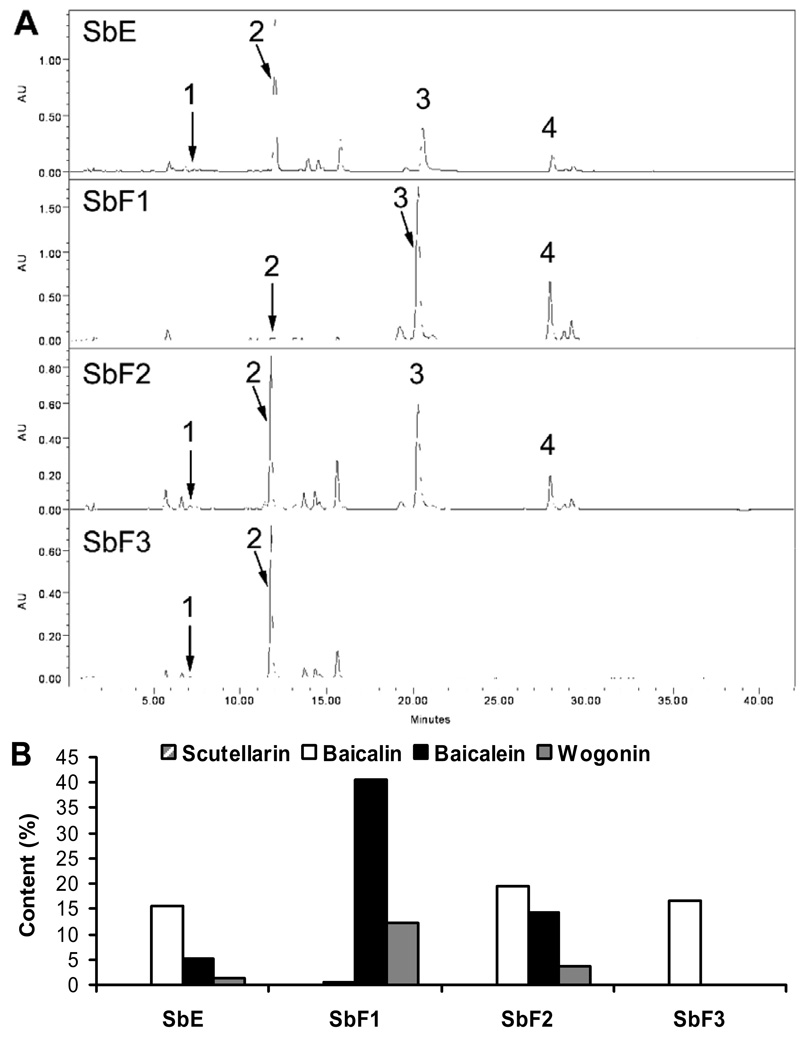
The HPLC chromatograms of baicalin-deprived-fraction (SbF1), SbE, and the other two fractions (SbF2 and SbF3) were shown in Fig. 2A. The main constituents in SbE were baicalin, baicalein and wogonin. In SbF1, baicalein and wogonin were the major constituents. On the other hand, the main constituents in SbF2 were baicalin, baicalein and wogonin, while in SbF3, the only main constituent present was baicalin (Fig. 2A). The contents of the four determined flavonoids in SbE and its three fractions are shown in Fig. 2B. In the baicalin-deprived-fraction (SbF1), the content of baicalein and wogonin were 40.54% and 12.35%, while baicalin was in trace content (0.51%). The content of scutellarin, baicalin, baicalein and wogonin in SbE were 0.03%, 15.67%, 5.19%, 1.42%; in SbF2 were 0.08%, 19.58%, 14.24%, 3.61%; and in SbF3 were 0.01%, 16.68%, 0%, 0%, respectively (Fig. 2B). Baicalein and wogonin were not detected in SbF3.
Fig. 2.
HPLC chromatograms of SbE, SbF1, SbF2 and SbF3 recorded at 280 nm (A), and percentage content of flavonoids in SbE and three fractions (B). Flavonoid peaks: (1) scutellarin, (2) baicalin, (3) baicalein, (4) wogonin. Peak numbers are not shown if flavonoids were not detected.
Antiproliferative effect of baicalin-deprived-fraction and SbE
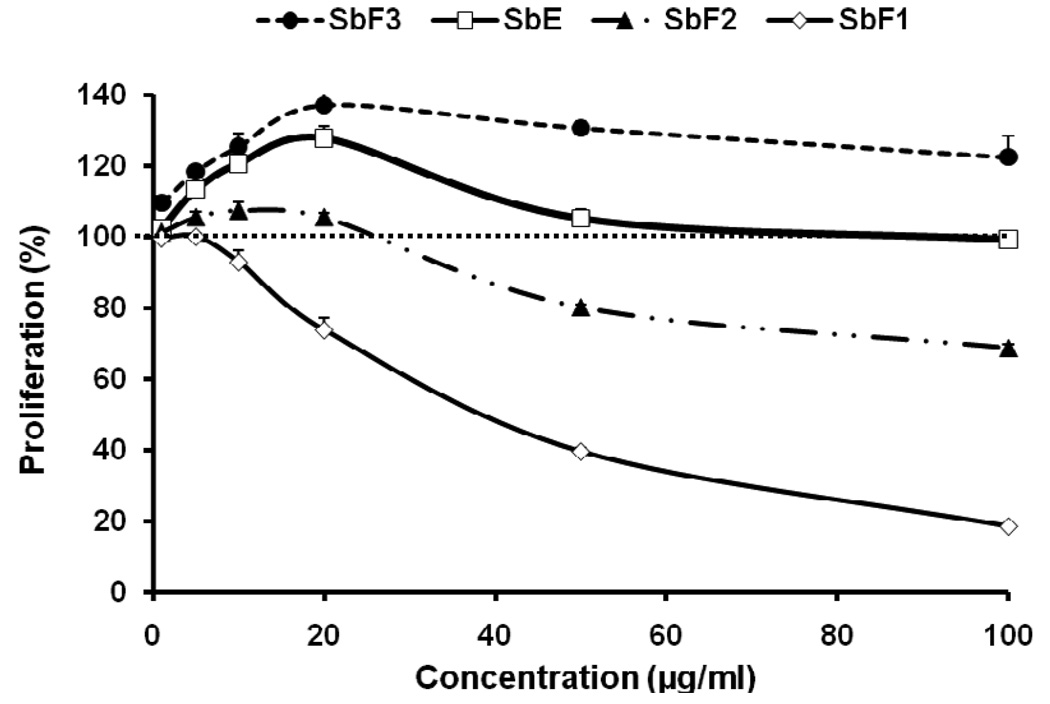
Using human breast cancer cell line MCF-7, the antiproliferative effects of baicalin-deprived-fraction (SbF1), SbE, and other fractions were evaluated. As shown in Fig. 3, using the whole extract, SbE did not inhibit cell growth at the concentrations of 1–100 µg/ml. Interestingly, at certain concentrations, SbE actually increased cell growth. We then observed the effects of the three fractions on the proliferation of MCF-7 cells. The SbF3, which contains baicalin, increased cell growth. Compared to the control, at 20, 50 and 100 µg/ml, SbF3 increased MCF-7 cell growth by 37.3%, 30.8% and 22.6% (all p<0.01). In contrast, the baicalin-deprived-fraction, SbF1, showed the most potent antiproliferative effect. At 20, 50 and 100 µg/ml, SbF1 inhibited cell growth by 26.2%, 60.5%, and 81.6%, respectively (all p<0.01 vs. control). SbF2 showed some antiproliferative effect at the concentrations of 50 and 100 µg/ml, but less than that of SbF1. This result suggested that the effect of SbE was reduced by the existence of SbF3, which mainly contains baicalin. Thus, it is shown that SbF1, the baicalin-deprived-fraction, is an active antiproliferative fraction in SbE.
Fig. 3.
Effects of different fractions and SbE on proliferation of MCF-7 human breast cancer cells. Cells were treated with 1, 5, 10, 20, 50 and 100 µg/ml of SbF1 (baicalin-deprived-fraction), SbF2, SbF3 (baicalin-fraction), and SbE for 72 h, and then assayed by MTS method. Data are shown as means ± standard error of three independent experiments.
Effect of baicalin-deprived-fraction on MCF-7 cell cycle
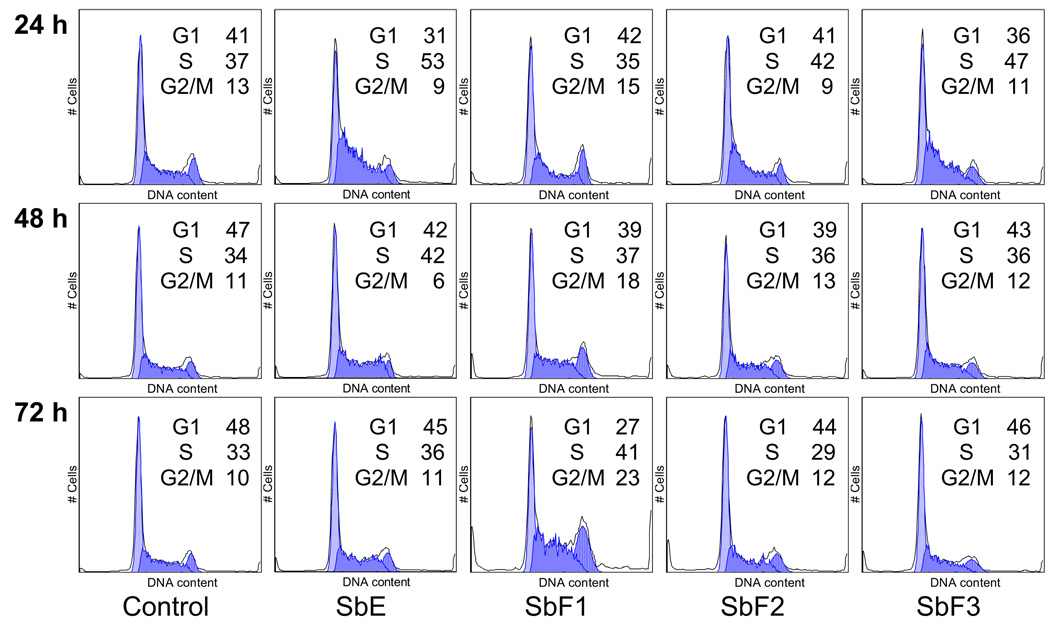
To explore the potential mechanism by which baicalin-deprived-fraction (SbF1) inhibits cell growth, the cell cycle profile was assayed by flow cytometry after staining with PI, and the assay data of SbF1 were compared with SbF2, SbF3, and the whole SbE. Based on the MTS assay data, significant antiproliferative effect was observed by treatment with 50 µg/ml of SbF1. Thus, we used the treatment concentration of 50 µg/ml to evaluate the effect of SbF1 on MCF-7 cell cycle. As shown in Fig. 4, compared to the control, 24 h treatment did not change the cell cycle profile. However, after 48 h treatment, cell cycle profile was changed. SbF1 reduced the percentage of G1-phase cells, and increased S- and G2/M-phase cells. After 72 h treatment, more obvious changes were observed. Compared to the control (48% of G1-phase, 33% of M-phase, and 10% of G2/M-phase), treatment with 50 µg/ml of SbF1 for 72 h, 27% of cells were in G-phase, 41% of cells were in M-phase, and 23% of cells were in G2/M-phase. SbF1 increased MCF-7 cells in S- and G2/M-phases. On the other hand, the other fractions (SbF2 and SbF3) and the whole SbE did not significantly change the cell cycle profile at 48 and 72 h. We also treated cells With 100 µg/ml of SbF1, however, since there were not enough viable cells, the cell cycle profile of 100 µg/ml of SbF1 was not evaluated.
Fig. 4.
Cell cycle analysis of MCF-7 cells using flow cytometry after staining with propidium iodide (PI). MCF-7 cells were treated with 50 µg/ml of SbF1 (baicalin-deprived-fraction), SbF2, SbF3, and SbE for 24, 48 and 72 h. The percentage of cells in G1-, S- and G2/M- phases are indicated.
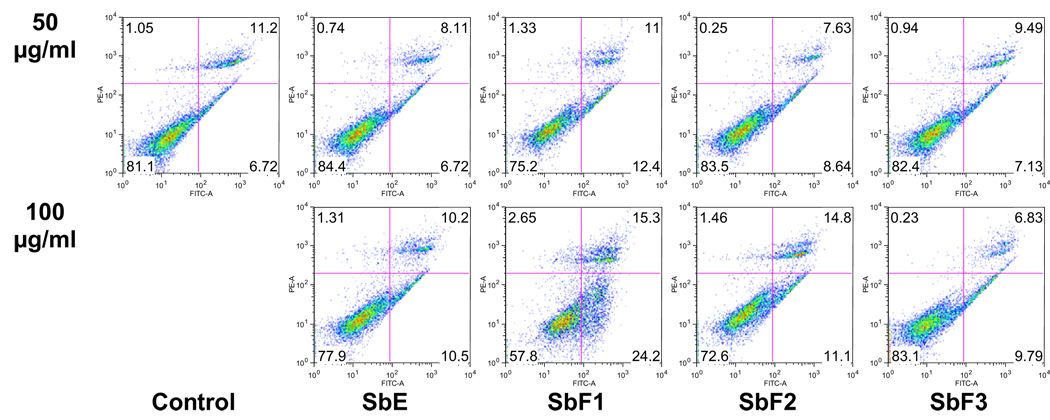
Apoptotic effect of baicalin-deprived-fraction on MCF-7 cells
The cell proliferation assay results suggested that after treatment with baicalin-deprived-fraction (SbF1), the viable cell number was decreased in a dose-dependent manner (Fig. 3). In the cell cycle assay, we also observed that viable cells were decreased by the increasing of SbF1 treatment concentration. To further characterize the potential mechanism of SbF1’s anticancer activity, we carried out an apoptotic assay by flow cytometry after staining with annexin V and PI. Annexin V can be detected in both the early and late stages of apoptosis. PI enters the cell in late apoptosis or necrosis. Viable cells were negative for both annexin V and PI (lower left quadrant); early apoptotic cells were positive for annexin V and negative for PI (lower right quadrant); late apoptotic or necrotic cells displayed both positive annexin V and PI (upper right quadrant); non-viable cells which underwent necrosis were positive for PI and negative for annexin V (upper left quadrant). After treatment for 48 h, the percentage of early apoptotic cells induced by 50 and 100 µg/ml of SbF1 was 12.4% and 24.2%, respectively, while control was 6.7% (Fig. 5). SbF1 clearly induced apoptosis in MCF-7 cells. However, treatment with 50 and 100 µg/ml of SbF2, SbF3 or SbE did not show apoptotic induction activity. This result suggested that the antiproliferative effect of baicalin-deprived-fraction (SbF1) was mediated by the induction of apoptosis.
Fig. 5.
Apoptosis assay using flow cytometry after annexin V-FITC/propidium iodide (PI) staining. MCF-7 cells were treated with 50 and 100 µg/ml of SbF1 (baicalin-deprived-fraction), SbF2, SbF3, and SbE for 48 h. Viable cells are in the lower left quadrant, early apoptotic cells are in the lower right quadrant, late apoptotic or necrotic cells are in the upper right quadrant and non-viable necrotic cells are in the upper left quadrant.
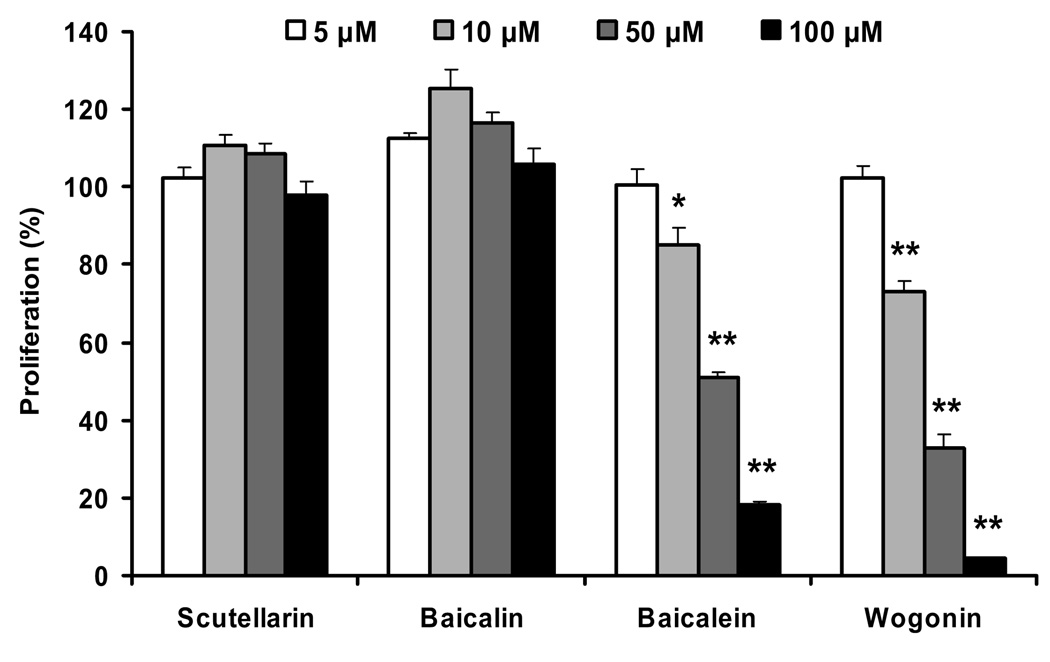
Effect of scutellarin, baicalin, baicalein and wogonin on cell proliferation
To evaluate the contribution of representative single compounds to the proliferation activities of different fractions and SbE, four flavonoids were used to test their antiproliferative effects on MCF-7 cells. Among them, are baicalein and wogonin, the major constituents found in baicalin-deprived-fraction (SbF1), and baicalin, the major constituent found in SbF3. For these three representative compounds, two are aglycons (baicalein and wogonin) and one is a glycoside (baicalin). To balance the compound types, we selected another glycoside, scutellarin, to evaluate the antiproliferative effects on MCF-7 cells, even though the content of this compound in either fractions or SbE was relatively low. As shown in Fig. 6, treatment with 5–100 µM of baicalin and scutellarin for 72 h, yielded no observable cell inhibitory effect, instead, at 10 and 50 µM, baicalin and scutellarin increased cancer cell growth. For the two aglycons, baicalein and wogonin showed significant antiproliferative effect at the treatment concentrations of 10–100 µM. Wogonin showed more potent activity than baicalein (Fig. 6).
Fig. 6.
Effects of scutellarin, baicalin, baicalein and wogonin on the proliferation of MCF-7 human breast cancer cells. Cells were treated with 5, 10, 50, and 100 µM of flavonoids for 72 h, and then assayed by MTS method. Data are shown as means ± standard error of three independent experiments. *p<0.05; and **p<0.01 vs. control.
Discussion
The clinical management of cancer invariably involves diverse conventional modalities, including surgery, radiation, and chemotherapy. The complex characteristics of cancer may also require some alternative management to improve the therapeutic efficacy of conventional treatment (Glynne-Jones et al. 2006; Guo et al. 2008). Numerous effective anticancer drugs have been developed from botanical sources, however there is still a significant untapped resource in herbal medicines, which needs to be investigated (Balunas and Kinghorn 2005; Wang and Yuan 2008). Botanicals could potentially have effective anticancer compounds that may be used alone or as adjuncts to existing chemotherapy to improve efficacy and reduce drug-induced toxicity (Mehendale et al. 2005; Wang et al. 2005).
In our pilot study, we did not find significantly antiproliferative effects of SbE on human breast cancer cells, although SbE showed antiproliferative effects on human prostate cancer and hepatoma cells (Chen et al. 2001; Chang et al. 2002). Based on literatures, baicalin showed insensitivity on breast cancer cells (Ikezoe et al. 2001). Since baicalin is the major constituent in SbE (Ye et al. 2004), we hypothesize that the antiproliferative effect of SbE may be reduced by baicalin. So, we prepared a baicalin-deprived-fraction of SbE.
MCF-7 cell line was used in the breast cancer chemoprevention studies (Kern et al. 1994); it is estrogen receptor (ER)-positive, and has been studied longer than any other breast cancer cell model system. As ER-positive breast cancer constitutes by far the most common type of breast cancer, the challenge of patient selection for adjuvant chemotherapy is critical (Punglia et al. 2005). The usefulness of the MCF-7 cell line as an investigative tool led to its adoption in laboratories worldwide (Simstein et al. 2003; Choi and Kim 2008). In this study, we use the MCF-7 cell line to evaluate the breast cancer inhibitory activities of baicalin-deprived-fraction.
Using MTS assay, the antiproliferative effects of SbE and three fractions were determined. Data showed that the whole SbE did not inhibit MCF-7 cell growth, and even, in some concentrations, SbE increased cell growth. The baicalin-fraction (SbF3) showed significant cell growth exciting activity. In contrast, the baicalin-deprived-fraction (SbF1) showed potent antiproliferative effect. We recognized that the baicalin-deprived-fraction is an active antiproliferative fraction. The whole extract, SbE did not show antiproliferative effect due to the influence of the baicalin-fraction.
Since anti-breast cancer studies of SbE was very limited, the mechanisms of SbE on breast cancer chemoprevention is largely unknown. For the single compounds in SbE, the breast cancer inhibitory effect was tested by using baicalein and wogonin. Baicalein was found to suppress 17β-estradiol-induced transactivation in cells expressing estrogen receptor alpha, and also to inhibit MCF-7 cells growth (Po et al. 2002). Recently, it was found that wogonin possess antiproliferative effects on both ER-positive and -negative breast cancer cells. Wogonin can undergo downregulation of ERa and c-ErbB2 in ER-positive and -negative breast cancer cells, respectively (Chung et al. 2008). From previous literature, wogonin was found to arrest breast cancer cells in G1- and G2-phases. No report found that baicalein affects the cell cycle of breast cancer cells (Li-Weber 2009). In this study, we observed that baicalin-deprived-fraction influenced the cell cycle profile. MCF-7 cells were arrested in S- and G2/M-phases after treatment with 50 µg/ml of SbF1 for 72 h. In addition, we observed significant apoptotic effects of baicalin-deprived-fraction on MCF-7 cells, suggesting that apoptosis plays an important role in the chemoprevention of baicalin-deprived-fraction on MCF-7 cells.
Herbal extract contains many constituents, different compound may have opposing pharmacological activities (Sengupta et al. 2004). To ensure safe use of herbal medicine, these compounds which possess opposing effect should be removed from the extract. For cancer chemoprevention, if a compound can enhance tumor growth, it is dangerous for the cancer patients. In this study, we found that one representative compound in SbE, baicalin, showed cell growth exiting activity on MCF-7 human breast cancer cells. This information is very important for cancer patients, especially breast cancer patients. Fortunately, we obtained a baicalin-deprived-fraction, and observed its positive antiproliferative effects and related mechanisms. From this, we conclude that the baicalin-deprived-fraction, but not the whole extract of S. baicalensis, may be a potent anti-breast cancer herbal medicine.
Acknowledgements
This work was supported in part by the NIH grants AT003255, AT004418, and 5P30DK042086.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;78:431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Bruno M, Piozzi F, Rosselli S. Natural and hemisynthetic neoclerodane diterpenoids from scutellaria and their antifeedant activity. Nat. Prod. Rep. 2002;19:357–378. doi: 10.1039/b111150g. [DOI] [PubMed] [Google Scholar]
- Chang WH, Chen CH, Lu FJ. Different effects of baicalein, baicalin and wogonin on mitochondrial function, glutathione content and cell cycle progression in human hepatoma cell lines. Planta Med. 2002;68:128–132. doi: 10.1055/s-2002-20246. [DOI] [PubMed] [Google Scholar]
- Chen S, Ruan Q, Bedner E, Deptala A, Wang X, Hsieh TC, Traganos F, Darzynkiewicz Z. Effects of the flavonoid baicalin and its metabolite baicalein on androgen receptor expression, cell cycle progression and apoptosis of prostate cancer cell lines. Cell Prolif. 2001;34:293–304. doi: 10.1046/j.0960-7722.2001.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EJ, Kim GH. Daidzein causes cell cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7 and MDA-MB-453 cells. Phytomedicine. 2008;15:683–690. doi: 10.1016/j.phymed.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Chung H, Jung YM, Shin DH, Lee JY, Oh MY, Kim HJ, Jang KS, Jeon SJ, Son KH, Kong G. Anticancer effects of wogonin in both estrogen receptor-positive and - negative human breast cancer cell lines in vitro and in nude mice xenografts. Int. J. Cancer. 2008;122:816–822. doi: 10.1002/ijc.23182. [DOI] [PubMed] [Google Scholar]
- Glynne-Jones R, Mawdsley S, Pearce T, Buyse M. Alternative clinical end points in rectal cancer-are we getting closer? Ann.Oncol. 2006;17:1239–1248. doi: 10.1093/annonc/mdl173. [DOI] [PubMed] [Google Scholar]
- Guo HY, Cai Y, Yang XM, Wang ZH, Wang JL, Zhao XM, Li J, Hu XC. Randomized phase II trial on mitomycin-C/cisplatin KLT in heavily pretreated advanced breast cancer. Am. J. Chin. Med. 2008;36:665–674. doi: 10.1142/S0192415X08006132. [DOI] [PubMed] [Google Scholar]
- Himeji M, Ohtsuki T, Fukazawa H, Tanaka M, Yazaki S, Ui S, Nishio K, Yamamoto H, Tasaka K, Mimura A. Difference of growth-inhibitory effect of Scutellaria baicalensis-producing flavonoid wogonin among human cancer cells and normal diploid cell. Cancer Lett. 2007;245:269–274. doi: 10.1016/j.canlet.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Hong T, Jin GB, Cho S, Cyong JC. Evaluation of the anti-inflammatory effect of baicalein on dextran sulfate sodium-induced colitis in mice. Planta Med. 2002;68:268–271. doi: 10.1055/s-2002-23143. [DOI] [PubMed] [Google Scholar]
- Huang KL, Chen CS, Hsu CW, Li MH, Chang H, Tsai SH, Chu SJ. Therapeutic effects of baicalin on lipopolysaccharide-induced acute lung injury in rats. Am. J. Chin. Med. 2008;36:301–311. doi: 10.1142/S0192415X08005783. [DOI] [PubMed] [Google Scholar]
- Ikezoe T, Chen SS, Heber D, Taguchi H, Koeffler HP. Baicalin is a major component of PC-SPES which inhibits the proliferation of human cancer cells via apoptosis and cell cycle arrest. Prostate. 2001;49:285–292. doi: 10.1002/pros.10024. [DOI] [PubMed] [Google Scholar]
- Kern FG, McLeskey SW, Zhang L, Kurebayashi J, Liu Y, Ding IY, Kharbanda S, Chen D, Miller D, Cullen K, et al. Transfected MCF-7 cells as a model for breast-cancer progression. Breast Cancer Res. Treat. 1994;31:153–165. doi: 10.1007/BF00666149. [DOI] [PubMed] [Google Scholar]
- Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Li HB, Jiang Y, Chen F. Separation methods used for Scutellaria baicalensis active components. J. Chromatogr. B. 2004;812:277–290. doi: 10.1016/j.jchromb.2004.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehendale S, Aung H, Wang A, Yin JJ, Wang CZ, Xie JT, Yuan CS. American ginseng berry extract and ginsenoside Re attenuate cisplatin-induced kaolin intake in rats. Cancer Chemother. Pharmacol. 2005;56:63–69. doi: 10.1007/s00280-004-0956-1. [DOI] [PubMed] [Google Scholar]
- Po LS, Chen ZY, Tsang DS, Leung LK. Baicalein and genistein display differential actions on estrogen receptor (ER) transactivation and apoptosis in MCF-7 cells. Cancer Lett. 2002;187:33–40. doi: 10.1016/s0304-3835(02)00355-5. [DOI] [PubMed] [Google Scholar]
- Punglia RS, Kuntz KM, Winer EP, Weeks JC, Burstein HJ. Optimizing adjuvant endocrine therapy in postmenopausal women with early-stage breast cancer: a decision analysis. J. Clin. Oncol. 2005;23:5178–5187. doi: 10.1200/JCO.2005.02.964. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Toh SA, Sellers LA, Skepper JN, Koolwijk P, Leung HW, Yeung HW, Wong RN, Sasisekharan R, Fan TP. Modulating angiogenesis: the yin and the yang in ginseng. Circulation. 2004;110:1219–1225. doi: 10.1161/01.CIR.0000140676.88412.CF. [DOI] [PubMed] [Google Scholar]
- Simstein R, Burow M, Parker A, Weldon C, Beckman B. Apoptosis, chemoresistance, and breast cancer: insights from the MCF-7 cell model system. Exp. Biol. Med. (Maywood) 2003;228:995–1003. doi: 10.1177/153537020322800903. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Fishbein A, Aung HH, Mehendale SR, Chang WT, Xie JT, Li J, Yuan CS. Polyphenol contents in grape-seed extracts correlate with antipica effects in cisplatin-treated rats. J. Altern. Complement. Med. 2005;11:1059–1065. doi: 10.1089/acm.2005.11.1059. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Mehendale SR, Yuan CS. Commonly used antioxidant botanicals: active constituents and their potential role in cardiovascular illness. Am. J. Chin. Med. 2007;35:543–558. doi: 10.1142/S0192415X07005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Ni M, Sun S, Li XL, He H, Mehendale SR, Yuan CS. Detection of adulteration of notoginseng root extract with other Panax species by quantitative HPLC coupled with PCA. J. Agric. Food. Chem. 2009;57:2363–2367. doi: 10.1021/jf803320d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Yuan CS. Potential role of ginseng in the treatment of colorectal cancer. Am. J. Chin. Med. 2008;36:1019–1028. doi: 10.1142/S0192415X08006545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Wang H, Jiang S, Wu J, Shao J, Cheng X, Tu Y, Zhang DY. Quality evaluation of commercial extracts of Scutellaria baicalensis. Nutr. Cancer. 2004;49:217–222. doi: 10.1207/s15327914nc4902_14. [DOI] [PubMed] [Google Scholar]