Abstract
Perifosine is an alkylphospholipid exhibiting antitumor activity as demonstrated in both preclinical studies and clinical trials. This activity is partly associated with its ability to inhibit Akt activity. It has been shown that the mTOR axis plays a critical role in regulation of cell proliferation and survival, primarily through functioning both downstream and upstream of Akt. The current study reveals a novel mechanism by which perifosine inhibits Akt and the mTOR axis. In addition to inhibition of Akt, perifosine inhibited the assembly of both mTOR/raptor and mTOR/rictor complexes. Strikingly, perifosine reduced the levels of Akt and other major components including mTOR, raptor, rictor, p70S6K, and 4E-BP-1 in the mTOR axis by promoting their degradation through a GSK3/FBXW7-dependent mechanism. These results thus suggest that perifosine inhibits the mTOR axis through a different mechanism from inhibition of mTOR signaling by classical mTOR inhibitors such as rapamycin. Moreover, perifosine substantially increased the levels of type II LC3, a hallmark of autophagy, in addition to increasing PARP cleavage, suggesting that perifosine induces both apoptosis and autophagy. The combination of perifosine with a lysosomal inhibitor enhanced apoptosis and inhibited the growth of xenografts in nude mice, suggesting that perifosine-induced autophagy protects cells from undergoing apoptosis. Collectively, we conclude that perifosine inhibits mTOR signaling and induces autophagy, highlighting a novel mechanism accounting for perifosine’s anticancer activity and a potential strategy to enhance perifosine’s anticancer efficacy by preventing autophagy.
Keywords: Perifosine, Akt, mTOR, degradation, autophagy, lung cancer cells
Introduction
Perifosine is an orally bioavailable alkylphospholipid exhibiting antitumor activity in various preclinical models. Currently, perifosine is being tested in phase II clinical trials (1, 2) and has shown single agent partial responses in certain types of cancer such as renal cell carcinoma (3, 4). In the preclinical settings, perifosine in combination with other antitumor agents such as the PDK1 inhibitor, UCN-01 (5), histone deacetylase inhibitors (6), and the chemotherapeutic agent etoposide (7) show synergistic antitumor effects.
Like other alkylphospholipids, perifosine targets cell membrane and induces growth arrest and apoptosis (1, 8, 9). The mechanisms by which perifosine exerts its antitumor effect have not been fully elucidated, even though it inhibits Akt (8, 10) and MAPK activation (11) while inducing JNK activation (11). Recent studies have shown that inhibition of Akt at least partly accounts for perifosine’s tumor-inhibitory activity (12, 13).
Phosphatidylinositol-3 kinase (PI3K)/Akt signaling is frequently activated in various types of cancers and hence represents a major cell survival pathway. Its activation has long been associated with malignant transformation and apoptotic resistance (14, 15). One well-known downstream signaling of PI3K/Akt is the mammalian target of rapamycin (mTOR) pathway (i.e., mTOR/raptor complex), which is phosphorylated (or activated) in response to stimuli that activate the PI3K/Akt pathway (16, 17). However, the recent discovery of the mTOR/rictor complex as an Akt Ser473 kinase also places mTOR upstream of Akt (18).
mTOR, a PI3K-related serine/theronine kinase, plays a central role in regulating cell growth, proliferation and survival, in part by regulation of translation initiation, through interactions with other proteins such as raptor (forming mTOR complex 1, mTORC1) and rictor (forming mTOR complex 2, mTORC2) (17, 19, 20). The best characterized downstream effectors of mTORC1 are the 70 kD ribosomal S6 kinase (p70S6K) and the eukaryotic translation initiation factor 4E binding protein 1 (4E-BP1) (17). In response to mitogenic stimuli or nutrient availability, mTOR/raptor complex is activated (21), leading to phosphorylation of p70S6K and 4E-BP1, and the subsequent enhanced translation of mRNAs that are critical for cell cycle progression and proliferation (e.g., c-Myc and cyclin D1) (17). Thus, mTOR signaling has emerged as an attractive cancer therapeutic target (17, 22). The potential applications of mTOR inhibitors for treating various types of cancer have been actively studied both pre-clinically and clinically. The most encouraging result from a recent clinical trial is that the mTOR inhibitor CCI-779 improved overall survival among patients with metastatic renal cell carcinoma (23).
Autophagy is a cellular lysosomal degradation pathway that is essential for regulation of cells survival and death to maintain cellular homeostasis (24, 25). One of the key regulators of autophagy is mTOR, which is the major inhibitory signal that shuts off autophagy in the presence of growth factors and abundant nutrients (25). Accordingly, inhibition of mTOR signaling (e.g., by the mTOR inhibitor rapamycin) induces autophagy (26).
We previously showed that perifosine decreases the levels of total Akt in some human lung cancer cell lines (12). The present study followed up this interesting observation in a detailed way and investigated whether perifosine also inhibits the mTOR axis while inhibiting Akt signaling. We found that perifosine inhibited both mTOR/raptor and mTOR/rictor complexes through a different mechanism from the classical mTOR inhibitor rapamycin by facilitating the degradation of the major components in the mTOR axis, thus reveling a unique mechanism by which perifosine inhibits mTOR signaling. Correspondingly, we found that perifosine induced autophagy as well.
Materials and Methods
Reagent
Perifosine was supplied by Keryx Biopharmaceuticals, Inc., dissolved in PBS and stored at −20°C. Rapamycin was purchased from LKT Laboratories, Inc., dissolved in DMSO at a concentration of 10 mM, and stored at −80°C. Other chemicals including MG132, cycloheximide (CHX), LiCl, SB216763, NH4Cl and chloroquine were purchased from Sigma Chemical Co. Rabbit polyclonal antibodies against Akt, mTOR, raptor, p-Akt (S473), p-Akt (T308), p-p70S6K (T389), p70S6K, p-4E-BP1 (Thr37/46), 4E-BP1, and poly(ADP-ribose)polymerase (PARP), respectively, were purchased from Cell Signaling Technology, Inc. Rabbit polyclonal anti-actin antibody was purchased from Sigma Chemical Co. Goat polyclonal mTOR (FRAP; N-19) and mouse monoclonal c-Myc (9E10) antibodies were purchased from Santa Cruz Biotechnology, Inc. Rabbit polyclonal Rictor (BL2178) antibody was purchased from Bethyl Laboratories, Inc. Mouse monoclonal cyclin D1 antibody (clone DCS-6) was purchased from Dako. Rabbit polyclonal microtubule-associated protein light chain 3 (LC3) antibody (NB100-2220) was purchased from Novus Biologicals, Inc.
Cell lines and Cell Culture
The human lung cancer cell lines used in this study were described previously (27). HCT116-FBXW7+/+ and HCT116-FBXW7−/− cell lines were kindly provided by Dr. B. Vogelstein (Johns Hopkins University School of Medicine, Baltimore, MA). These cell lines were grown in monolayer culture in RPMI 1640 medium or McCoy’s medium supplemented with glutamine and 5% fetal bovine serum (FBS) at 37°C in a humidified atmosphere consisting of 5% CO2 and 95% air.
Cell Survival Assay
Cells were cultured in 96-well cell culture plates and treated the next day with the agents indicated. Viable cell number was estimated using the sulforhodamine B assay, as previously described (27).
Detection of Apoptosis
Apoptosis was evaluated by Annexin V staining using Annexin V-PE apoptosis detection kit purchased from BD Biosciences (San Jose, CA) following the manufacturer’s instructions. PARP cleavage was also detected by Western blotting as an additional indicator of apoptosis.
Western Blot Analysis
Preparation of whole cell protein lysates and Western blot analysis were described previously (28, 29).
Immunoprecipitation (IP)
mTOR complexes or rictor were immunoprecipitated with goat polyclonal mTOR (FRAP; N-19) antibody or with rictor antibody (Bethyl Laboratories, Inc.) according to the same procedure described previously (18, 30). At the end, the samples containing an equal amount (20–50 µg) of whole-cell protein lysates and immunoprecipitates from 1–2 mg cell lysates captured with protein A-sepharose were analyzed by Western blotting.
Lung Cancer Xenografts and Treatments
Animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Emory University. Five- to 6- week old (about 20 g of body weight) female athymic (nu/nu) mice were ordered from Taconic and housed under pathogen-free conditions in microisolator cages with laboratory chow and water ad libitum. H460 cells at 5×106 in serum-free medium were injected s.c. into the flank region of nude mice. When tumors reached certain size ranges (~300 mm3), the mice were randomized into four groups (n = 6/group) according to tumor volumes and body weights for the following treatments: vehicle control, perifosine (15–10 mg/kg/day, og), chloroquine (50 mg/kg/day; ip), and the combination of chloroquine and perifosine. Tumor volumes were measured using caliper measurements once every two days and calculated with the formula V = π(length × width2)/6. After a consecutive 14-day treatment, the mice were sacrificed with CO2. The tumors were then removed and weighed.
Statistical Analysis
The statistical significance of differences in tumor sizes or weights between two groups was analyzed with two-sided unpaired Student's t tests when the variances were equal or with Welch's corrected t test when the variances were not equal, by use of Graphpad InStat 3 software. Data were examined as suggested by the same software to verify that the assumptions for use of the t tests held. Results were considered to be statistically significant at P < 0.05.
Results
Perifosine Decreases the Levels of p-Akt and Total Akt and Inhibits mTOR Complex Assembly in Human Lung Cancer Cells
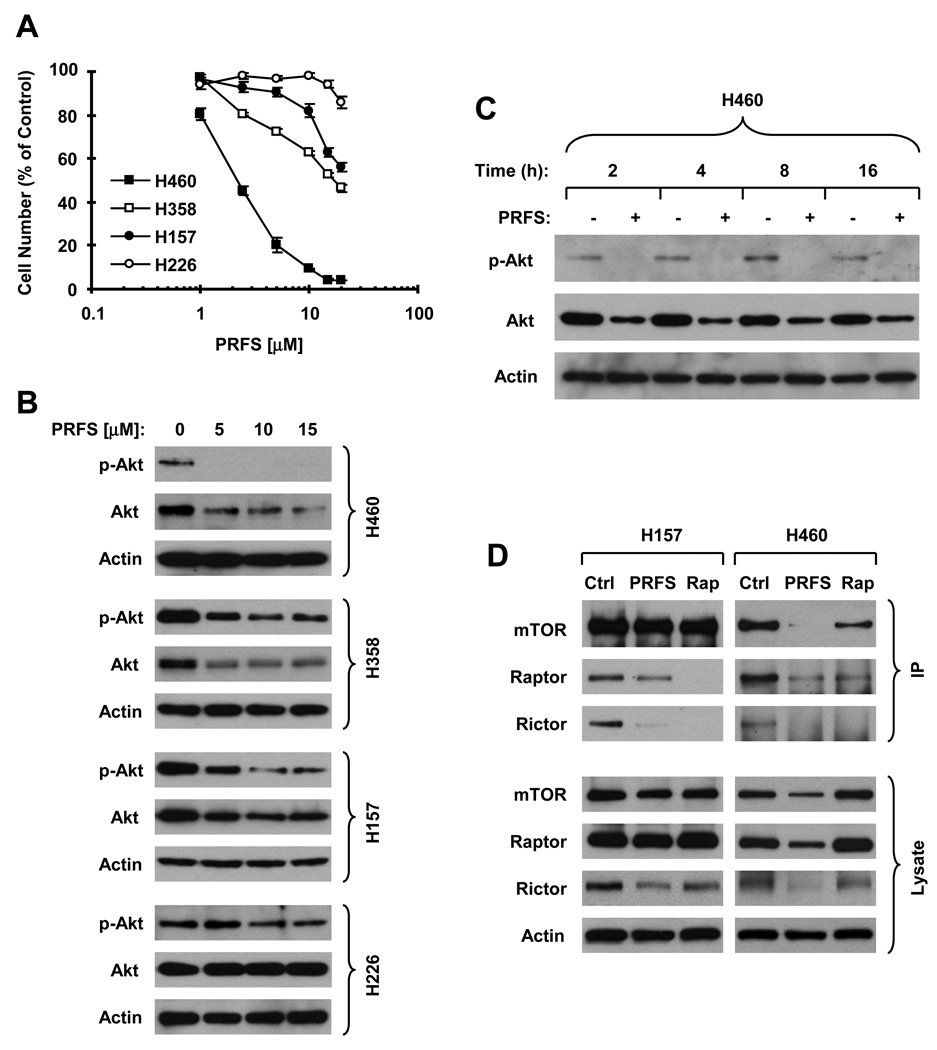
In this study, we used four human lung cancer cell lines with different sensitivities to perifosine. The sensitivities of these cell lines to perifosine are H460>H358>H157>H226 (Fig. 1A). Upon treatment with perifosine, we detected a concentration-dependent decrease of p-Akt (S473) levels in these cell lines. As well, total Akt levels were also decreased in perifosine-sensitive cell lines. The degrees of reduction in p-Akt (S473) and Akt levels in these cell lines were tightly associated with cell sensitivities to perifosine (Fig. 1B). For example, perifosine substantially decreased the levels of both p-Akt (S473) and Akt in H460 cells, which are the most sensitive to perifosine, but only weakly reduced p-Akt (S473) levels in H226 cells, which are the most resistant to perifosine (Fig. 1B). The reduction of Akt levels occurred early within 2 h and was sustained up to 16 h post perifosine treatment (Fig. 1C). We also detected the effects of perifosine on Akt phosphorylation at Thr308 (T308) in these lung cancer cell lines. Perifosine decreased Akt T308 phopshorylation in H460 and H358 cell lines, but not in H157 and H226 cell lines under the tested conditions (see supplementary Fig. S1). It seems that Akt S473 site is more susceptible than Akt T308 site to inhibition by perifoisne. Nonetheless, these results indicate that perifosine not only inhibits Akt phosphorylation, but also decreases the levels of total Akt in human lung cancer cells.
Fig. 1. Perifosine inhibits Akt (B and C) and assembly of mTOR complexes (D) in human lung cancer cell lines with different sensitivities to perifosine (A).
A, The indicated cell lines were treated with different concentrations of perifosine (PRFS) ranging from 1 µM to 20 µM. After 24 h, the cells were subjected to sulforodamine B (SRB) assay for estimating the viable cells. B and C, the indicated cell lines were treated with the increased concentrations of perifosine (0–15 µM) for 8 h (B) or with 5 µM perifosine for the given times. The cells were then subject to preparation of whole cell protein lysates and subsequent Western blot analysis. D, The indicated cell lines were treated with solvent control (Ctrl), 10 µM perifosine or 10 nM rapamycin (Rap). After 6 h, the cells were harvested for preparation of whole cell protein lysates and subsequent immunoprecipitation (IP) with mTOR antibody. The indicated proteins were detected with Western blotting.
Given that mTOR can function both downstream and upstream of Akt and mTOR/rictor acts as an Akt Ser473 kinase (18), we next examined whether perifosine affects the assembly of mTOR complexes. To this end, we immunoprecipitated mTOR complexes with mTOR antibody from cell lysates exposed to perifosine or rapamycin which is known to inhibit mTOR complexes (30, 31) and then detected individual components in the precipitates by Western blot analysis. In H157 cells, we detected less amounts of both raptor and rictor in the immunoprecipitates from cell lysates treated with either perifosine or rapamycin compared with control cell lysates, indicating that perifosine inhibited assembly of both mTOR/raptor and mTOR/rictor complexes. In H460 cells, we observed similar results. Additionally, we detected decreased levels of mTOR in the immunoprecipitate exposed to perifosine as well. Interestingly, we did not find alterations of either mTOR or raptor levels in whole protein lysates from perifosine-treated H157 cells; however, both mTOR and raptor levels were deceased in whole protein lysates from perifosine-treated H460 cells. In whole cell lysates from both H157 and H460 cells treated with perifosine, rictor levels were reduced (Fig. 1D). These results clearly indicate that perifosine inhibits the assembly of both mTOR/raptor and mTOR/rictor complexes, possibly though decreasing the levels of mTOR, raptor, and/or rictor in some cell lines.
Perifosine Decreases the Levels of Major Proteins in the mTOR Axis in Human Lung Cancer Cells
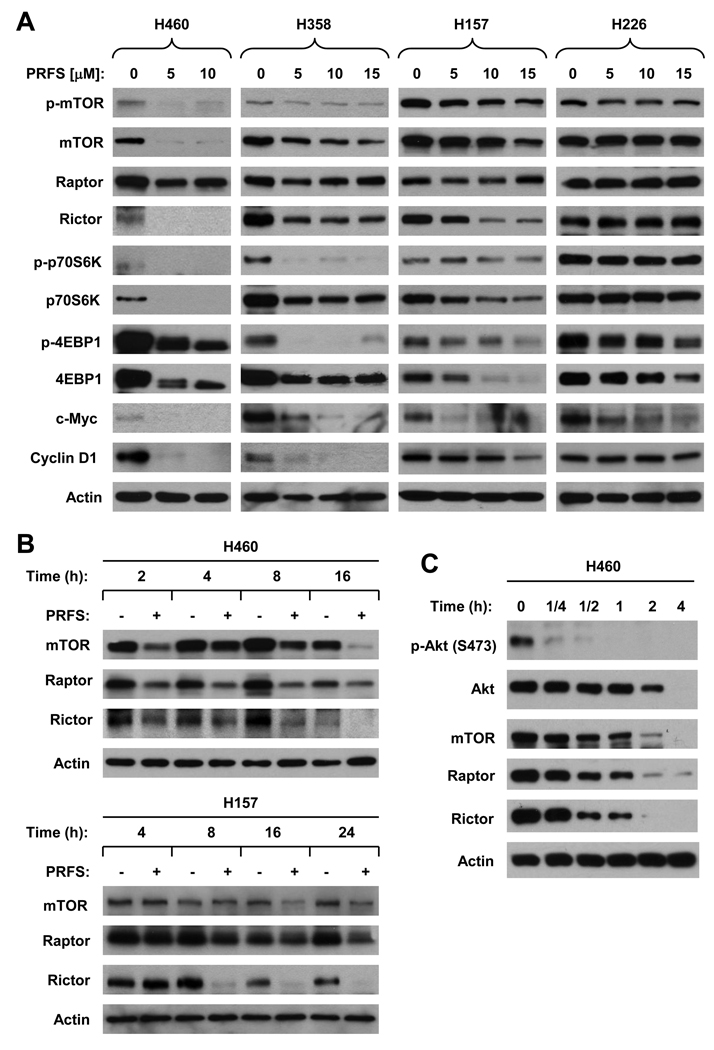
Given that perifosine decreased the levels of mTOR, raptor and rictor in H460 cells during the IP-Western blotting analysis as presented above, we then questioned whether these alterations occurs commonly in human lung cancer cell lines. Thus, we treated the four lung cancer cell lines with different sensitivities to perifosine with different concentrations of perifosine for 8 h and then detected the levels of several key proteins including their phosphorylation in the mTOR axis. As presented in Fig. 2A, the levels of mTOR, p-mTOR, raptor, rictor, p70S6K, p-P70S6K, 4EBP1, and p-4EBP1 were decreased in H460, H358 and H157 cells, but were either not or only minimally reduced in H226 cells. It appeared that the degrees of reduction of these proteins were tightly associated with cell sensitivities to perifosine. Like Akt, the reduction of mTOR, raptor and rictor occurred early at 2 h post perifosine treatment in H460 cells; however, these reductions happened relatively late at either 8 h or 16 h after perifosine treatment in H157 cells (Fig. 2B). Collectively, perifosine decreased the levels of multiple key proteins in the mTOR axis in human lung cancer cells. The degrees of reduction of these proteins in different cell lines are tightly associated with cell sensitivities to perifosine.
Fig. 2. Perifosine induces dose (A)- and time (B and C)-dependent reduction of the levels of multiple components in the mTOR axis in human lung cancer cells.
A, The indicated cell lines were treated with the given concentrations of perifosine (PRFS) for 8 h. B, The given cell lines were treated with 10 µM (H157) or 5 µM (H460) perifosine for the given times. C, H460 cells were exposed to 10 µM perifosine for the indicated times. After the aforementioned treatments, the cells were then harvested for preparation of whole cell protein lysates and subsequent Western blot analysis.
By further conducting a short kinetic analysis of reduction in p-Akt, Akt, mTOR, raptor and rictor, we found that p-Akt reduction occurred at 15 min post perifosine treatment, whereas the reduction of Akt, mTOR, raptor and rictor happened at 30 min or even later after perifosine treatment (Fig. 2C), suggesting that inhibition of p-Akt precedes reduction of Akt, mTOR, raptor and rictor.
Since perifosine inhibits the mTOR axis, we further examined the effects of perifosine on modulation of cyclin D1 and c-Myc, two oncogenic proteins known to be regulated by mTOR signaling (32). As presented in Fig. 2A, cyclin D1 levels were decreased in H460, H358 and H157 cells, but not in H226 cells upon treatment with perifosine. c-Myc levels were decreased in all of these four cell lines when exposed to perifosine. As well, the reduction degrees of both cyclin D1 and c-Myc by perifosine in these cell lines were associated with cell sensitivities to perifosine. For example, perifosine decreased the levels of both proteins to the lowest in H460 cells, but only weakly or minimally in H226 cells.
Perifosine Decreases the Levels of Akt and mTOR Axis Proteins through Promoting Their Degradation
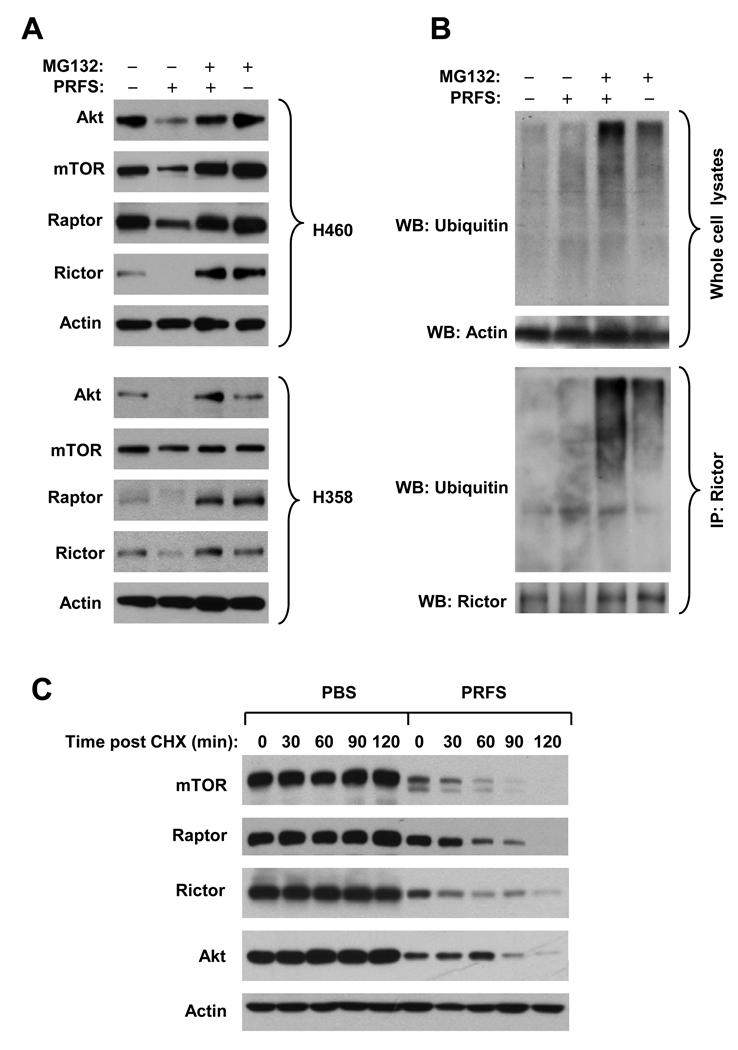
It was recently reported that some proteins including mTOR, raptor, and p70S6K can be regulated through proteasome-mediated protein degradation (33, 34). Therefore, we reasoned whether perifosine induces degradation of these proteins. Thus, we treated both H460 and H358 cells with perifosine in the absence and presence of the proteasome inhibitor MG132 and then detected the levels of some key proteins in the mTOR axis. As shown in Fig. 3A, perifosine decreased the levels of Akt, mTOR, raptor and rictor in both cell lines as presented above. The presence of MG132 could prevent these proteins from reduction by perifosine, suggesting that perifosine decreases the levels of these proteins through facilitating proteasome-mediated protein degradation. Moreover, we determined whether perifosine increased ubiquitination of these proteins using rictor as an example. By Western blotting, we detected the highest levels of ubiquitinated proteins in the whole protein lysates from cells exposed to perifosine and MG132 compared to others treated with solvent, perifosine or MG132 alone (Fig. 3B), suggesting that perifosine increases protein ubiqutination. By IP-Western blotting, we specifically detected rictor ubiqutination in cells exposed to perifosine. Similar to what we observed in whole protein lysates, the highest levels of ubiquitinated rictor were detected in cells exposed to perifsone combined with MG132 in comparison with cells treated with perifosine or MG132 alone (Fig. 3B). These results together suggest that perfosine induces ubiquitin/proteasome-mediated degradation of proteins such as rictor in the mTOR axis.
Fig. 3. Perifosine decreases the levels of Akt and mTOR-associated proteins through ubiquitin/proteasome-mediated protein degradation.
A and B, the given cell lines were pretreated with 20 µM MG132 for 30 minutes prior to the addition of 10 µM perifosine (PRFS). After co-treatment for 4 h, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis (A). In addition, the same lysates were also subjected to immunoprecipitation (IP) with rictor specific antibody followed by Western blotting (B). C, H460 cells were treated with 10 µM perifosine or PBS for 4 h. The cells were then washed with PBS twice and refed with fresh medium containing 20 µg/ml CHX. At the indicated times post CHX, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis.
Additionally, we performed CHX chase assay to determine whether perifosine decreases the stability of mTOR, rapor, rictor and Akt. As presented in Fig. 3C, the levels of these proteins in PBS control cells were not reduced within the tested time ranges, but were substantially decreased as the time increased post addition of CHX in perifosine-treated cells. The half-lives of these proteins in perifosine-treated cells were < 60 min (i.e., mTOR, raptor, and rictor) or < 90 min (i.e., Akt). Thus, it is clear that perifosine decreases the stability of these proteins, further supporting the conclusion that perifosine facilitates the degradation of some key proteins in the mTOR axis.
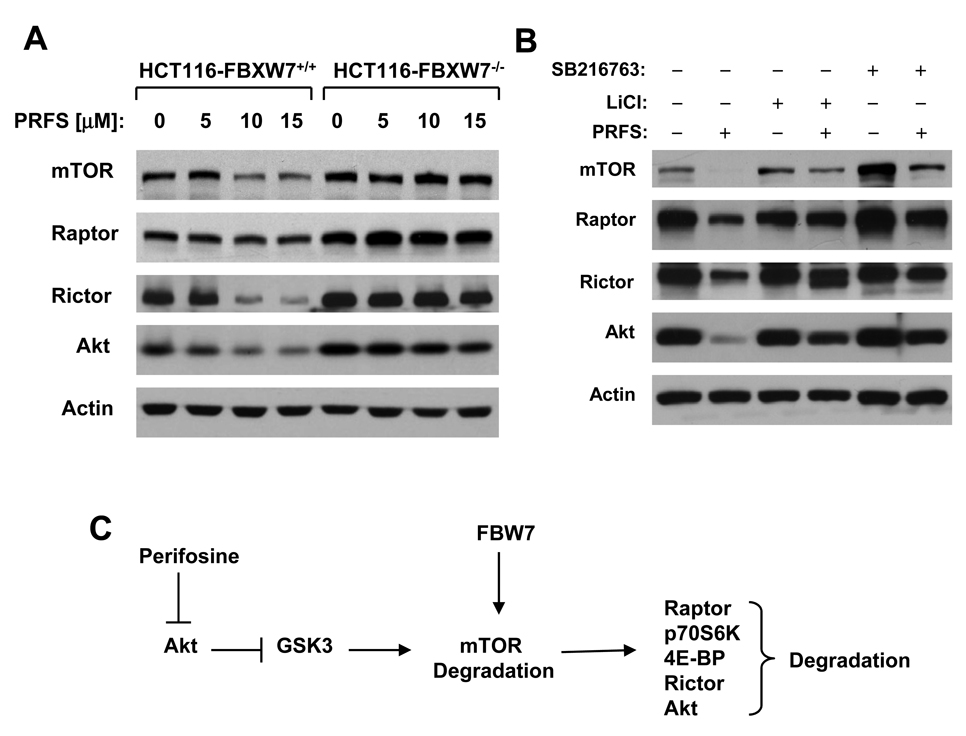
Perifosine Induces the Degradation of Key Proteins in the mTOR Axis through a GSK3/FBXW7-dependent Mechanism
It has been recently shown that mTOR can undergo FBXW7-dependent, proteasome-mediated degradation. We thus asked whether FBWX7 is involved in perifosine-induced degradation of mTOR and its associated proteins. To this end, we took advantage of paired isogenic HCT116 cell lines, in one of which FBXW7 is genetically knocked out (35). We compared the effects of perifosine on the degradation of several key component in the mTOR axis between HCT116-FBXW7+/+ and HCT116-FBXW7−/− cell lines. As shown in Fig. 4A, perifosine decreased the levels of mTOR, raptor, rictor and Akt in a concentration-dependent manner in HCT116-FBXW7+/+ cells, but not in HCT116-FBXW7−/− cells, indicating that perifosine induces a FBXW7-dependent degradation of some key proteins in the mTOR axis. Given that GSK3 is often involved in phosphorylation of the substrates of FBXW7, an essential step for FBXW7-mediated protein ubiquitination and degradation (36), we next questioned whether GSK3 is involved in perifosine-induced FBXW7-dependent degradation of proteins in the mTOR axis. As presented in Fig. 4B, perifosine decreased the levels of mTOR, raptor, rictor and Akt in H460 cells as demonstrated above; however, it failed to do so in the presence of the GSK3 inhibitor, LiCl or SB216763. These data indicate that perifosine-induced degradation of the proteins in the mTOR axis is GSK3-dependent. Collectively, we conclude that perifosine induces degradation of some key proteins in the mTOR axis through a GSK3/FBXW7-dependent mechanism
Fig. 4. Involvement of FBXW7 (A) and GSK3 (B) in perifosine-induced degradation of several proteins in the mTOR axis and a schematic model for the possible mechanism underlying the process (C).
A, The given cell lines were treated with the indicated concentrations of perifosine for 24 h. B, H460 cells were pretreated with 50 mM LiCl or 20 µM SB216763 for 30 min and then co-treated with 10 µM perifosine for an additional 5 h. After the aforementioned treatments, the cells were then harvested for preparation of whole cell protein lysates and subsequent Western blot analysis. C, Hypothetic model for involvement of FBXW7 and GSK3 in perifosine-induced degradation of several proteins in the mTOR axis. Perifosine inhibits Akt, resulting in GSK3 activation, which phosphorylates mTOR and facilitates ubiquitination and degradation of mTOR by FBWX7. As a consequence, mTOR complexes will be destabilized and proteins associated with these complexes will be degraded.
Perifosine Induces Autophagy in Human Lung cancer Cells
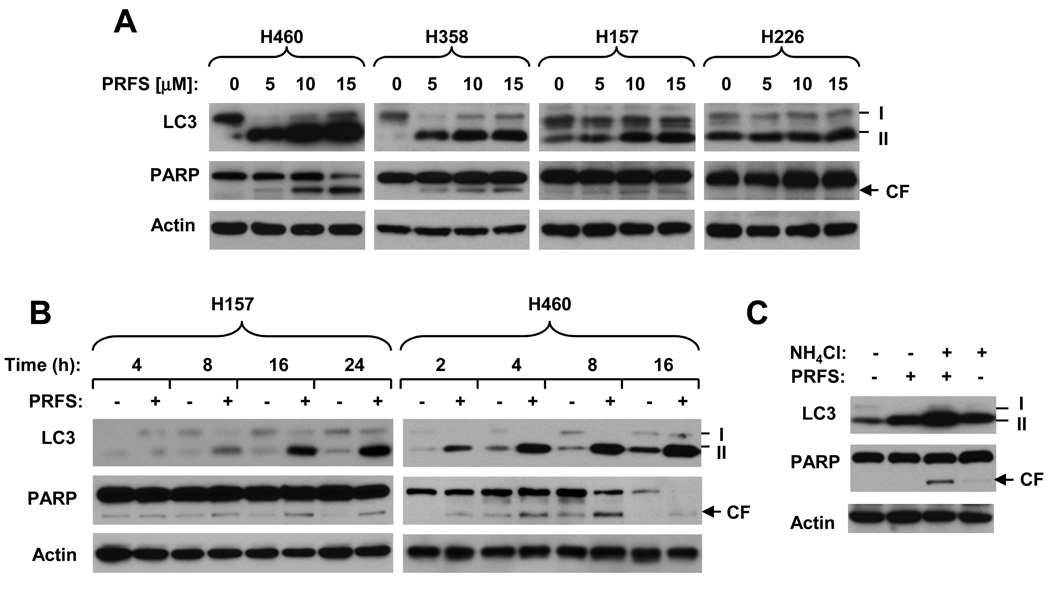
Our above findings clearly show that perifosine inhibits mTOR signaling. Given that mTOR signaling is critical for regulation of autophagy (25), we next determined whether perifosine induces autophagy. In this study, we detected the levels of LC3-II for monitoring autophagy because it is essential for formation of autophagosome and has been widely used for estimating the abundance of the autophagosome or autophagy (37). As presented in Fig. 5A, perifosine potently increased the levels of LC3-II in perifosine-sensitive cell lines (e.g., H460 and H358), but weakly or minimally in the perifosine-resistant cell lines (e.g., H226). The potency of perifosine in inducing LC3-II in these cell lines was H460 > H358 > H157 > H226. We noted that both H157 and H226 have higher basal levels of LC3-II than H460 and H358 cells. Depending on the cell lines, perifosine-induced increase in LC3-II could occur at 2 h (H460 cells) or after 4 h (H157) and could be sustained up to 24 h in a time-dependent manner (Fig. 5B). Since LC3-II itself is degraded by autophagy and its increase is due to the accumulation of the autophagosome by blockage of autophagic degradation (37), we further determined whether perifosine indeed induces autophagic flux. To this end, we compared the modulatory effects of perifosine on LC3-II levels in the absence and presence of the lysosomal protease inhibitor NH4Cl as recommended (37). As shown in Fig. 5C, the presence of NH4Cl, which blocks autophagic degradation, further increased the levels of LC3-II compared to those in cells exposed to perifosine alone, indicating that perifosine indeed induces autophagic flux. Collectively, we conclude that perifosine induces autophagy.
Fig. 5. Perifosine increased the levels of both LC3-II and cleaved PARP (A and B), which are further increased when co-treated with perifosine and NH4Cl (C).
A and B, The indicated cell lines were treated with the given concentrations of perifosine (PRFS) for 8 h (A) or with 10 µM (H157) or 5 µM (H460) perifosine for the given times (B). C, H460 cells were pre-treated with 20 mM NH4Cl for 30 min and then co-treated with 5 µM perifosine for an additional 4 h. After the aforementioned treatment, the cells were then harvested for preparation of whole cell protein lysates and subsequent Western blot analysis.
Moreover, we detected PARP cleavage as an indication of apoptosis in the same cell lines exposed to perifosine. As previously reported (12), perifosine induced PARP cleavage in perifosine-sensitive cells (e.g., H460), but not in perifosine-resistant cell lines (e.g., H226). PARP cleavage was detected and accompanied with an increase in LC3-II (Figs. 5A and 5B). These results indicate that perifosine induces autophagy while triggering apoptosis.
Inhibition of Autophagy Enhances Perifosine’s Apoptosis-inducing Activity and Anticancer Efficacy
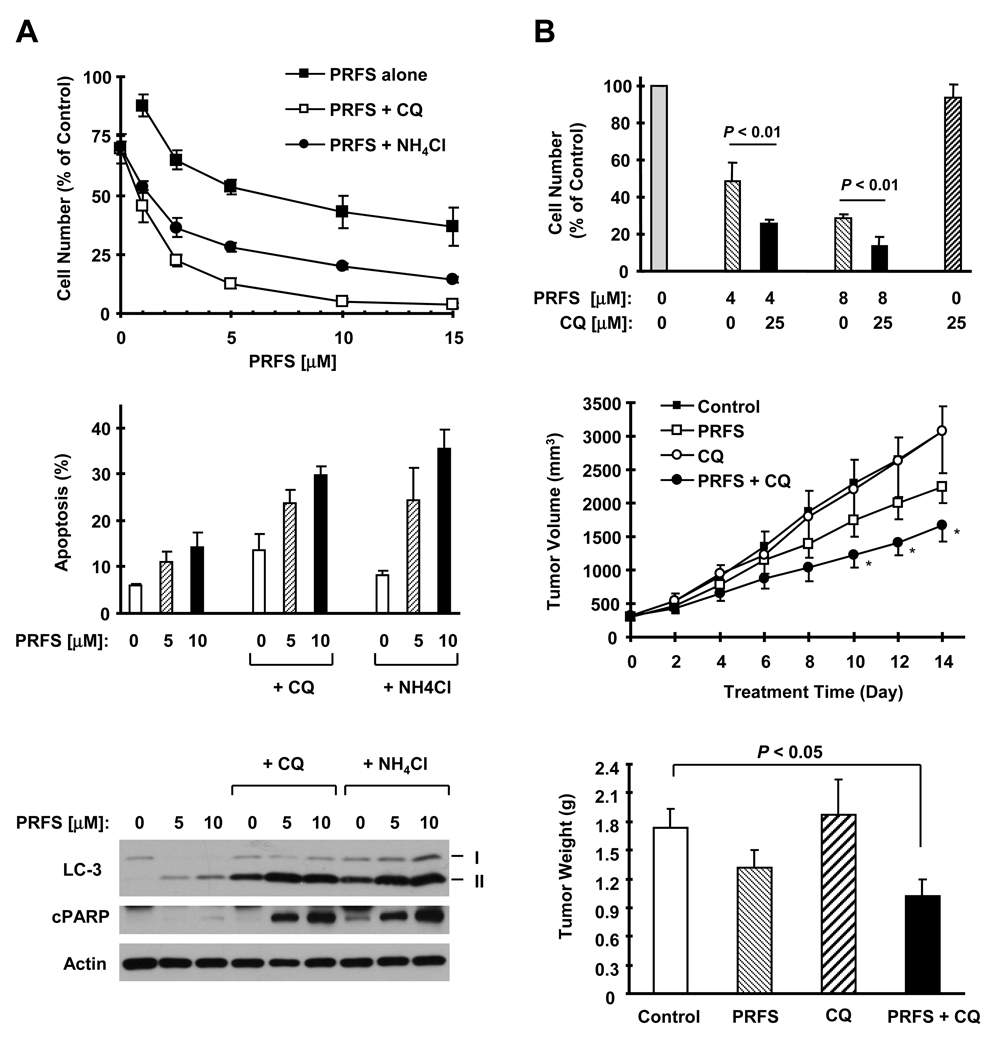
Autophagy plays a critical role in regulation of cell survival or death (38). To determine whether induction of autophagy by perifosine is a survival or death mechanism, we looked at the effects of perifosine on cell survival and apoptosis in the presence of a lysosomal protease inhibitor in several human lung cancer cells. As shown in Fig. 6A (top panel), the combination of perifosine with either NH4Cl or chloroquine was more effective than perifosine alone in decreasing the survival of H157 cells. Similar results were generated in H358 and H226 cell lines as well (data not shown). In agreement, the combination of perifosine with NH4Cl or chloroquine was also more potent than perifosine alone in inducing apoptosis evidenced by increased annexin V positive (e.g., apoptotic ) cells (Fig. 6A, middle panel) and PARP cleavage (Fig. 6A, bottom panel). As we observed in Fig. 5C, the presence of either NH4Cl or chloroquine further increased LC3-II levels (Fig. 6A, bottom panel). These data indicate that perifosine-induced autophagy represents a pro-survival mechanism, inhibition of which drives more cells to die of apoptosis.
Fig. 6. Inhibition of autophagy enhances perifosine’s anticancer activity both in vitro (A and B, tope panel) and in vivo (B, middle and bottom panels).
A, H157 cells on a 96-well cell culture plate were treated with different concentrations of perifosine (PRFS) as indicated, 25 µM chloroquine (CQ) alone, 10 mM NH4Cl alone or their combinations. After 48 h, the cells were fixed and subjected to the SRB assay for estimation of cell numbers (top panel). Points, means of four replicate determinations; bars, ± SDs. Moreover, H157 cells in 10-cm cell culture plates were treated with the indicated concentrations of perifosine alone, 25 µM cholorquine alone, 10 mM NH4Cl alone, or their combinations. After 30 h, the cells were harvested for measurement of apoptosis by Annexing V/flow cytometry (middle panel) and for detection of the given proteins with Western blotting (bottom panel). Columns, means of duplicate assay; bars, ± SDs. B, H460 cells were plated on a 96-well cell culture plate and treated on the second day with different concentrations of perifosine as indicated, 25 µM chloroquine alone, or their combination. After 48 h, the cells were fixed and subjected to the SRB assay for estimation of cell numbers (top panel). Columns, means of four replicate determinations; bars, ± SDs. In addition, four groups of mice with H460 xenografts were treated with the indicated drugs on the same day after grouping. After a consecutive 14-day treatment, the mice were sacrificed and the tumors were removed and weighed (bottom panel). Tumor sizes were measured once every two days (middle panel). Each measurement is a mean ± SD (n=6). * p < 0.05 compared to vehicle control.
We next tested whether inhibition of autophagy enhances perifosine’s anticancer activity in vivo. Given that H157 cells do not form colonies on soft agar (ATCC data sheet) and do not grow well as a xenograft in mice (our unpublished data), we tested the in vivo effect of perifosine combined with autophage inhibition in H460 xenografts, an aggressive tumor model. Before the experiment, we confirmed the effect of perifosine combined with chloroquine on the growth of H460 cells in cell cultures. In agreement with findings in H157 cells, the combination of perifosine and chloroquine was more effective than perifosine alone in decreasing the survival of H460 cells (Fig. 6B, top panel). In the H460 xenograft model, treatment with the perifosine and chloroquine combination significantly (P < 0.05) decreased both tumor size (Fig. 6B, middle panel) and tumor weight (Fig. 6B, bottom panel) compared with vehicle control treatment. Perifosine alone treatment showed a trend to decrease tumor size and weight, but did not achieve statistical significance under the tested schedule (Fig. 6B, middle and bottom panels). These data thus provide in vivo evidence for enhancement of perifosine’s anticancer efficacy by preventing autophagy.
Discussion
Our previous study has demonstrated that perifosine inhibits Akt in human lung cancer cells; this event contributes in part to perifosine-induced apoptosis (12). In the current study, we further show that perifosine not only inhibited Akt phosphorylation, but also decreased the levels of total Akt in perifosine-sensitive cell lines, which appears to be an early event because we could detect Akt reduction even at 15 min post perifosine treatment (Fig. 1 and Fig. 2). Given that perifosine decreased the levels of p-Akt (S473) more profoundly than total Akt levels in some cell lines (e.g., H226 and H157), we believe that perifosine inhibit Akt through both suppressing Akt phosphorylation and reducing total Akt levels, at least in our cell systems. It has recently been demonstrated that mTOR/rictor complex functions as an Akt Ser473 kinase (18). In this study, we found that perifosine clearly inhibited the assembly of not only mTOR/rictor but also mTOR/raptor as rapamycin did (Fig. 1). Thus, it is possible that perifosine inhibits mTOR/rictor, resulting in further inhibition of Akt phosphorylation. In this study, we also observe that perifosine decreased the levels of c-Myc and cyclin D1, likely due to inhibition of mTOR/raptor signaling because these two proteins are well-known to be regulated by mTOR /raptor signaling (32).
The intriguing novel finding in this study is that perifosine decreases the levels of several key components in the mTOR axis including mTOR, rictor, raptor, p70S6K, and 4EBP-1 through promoting ubiquitin/proteasome-mediated protein degradation based on the following lines of evidence: 1) Perifosine decreased the levels of mTOR, rictor, raptor, p70S6K, and 4EBP-1. The activity of perifosine on reducing the levels of these proteins is decreased as the sensitivities of the tested cell lines to perifosine drops (Fig. 2); 2) The presence of the proteasome inhibitor MG132 prevented perifosine-induced reduction of mTOR, raptor and rictor as well as Akt (Fig. 3A); 3) Perifosine increases protein ubiqutination because we detected the highest levels of ubiquitinated proteins in whole cell protein lysates exposed to perifosine in the presence of MG132 and the highest levels of ubiquitinated rictor from the same lysate (Fig. 3B); and 4) Perifosine decreases the stability of mTOR, raptor, rictor and Akt because perifosine shortened the half-lives of these proteins in the CHX chase assay (Fig. 3C). Therefore, it appears that perifosine inhibits mTOR axis through destroying the major components in the mTOR axis; such a mechanism is obviously different from that by which rapamycin inhibits mTOR signaling. Although it has been described in some studies that perifosine inhibits mTOR signaling (e.g., inhibition of p70S6K and 4EBP-1 phosphorylation) (13, 39), our study is the first to demonstrate that perifosine induces degradation of the major components in the mTOR axis, leading to inhibition of the mTOR axis.
The remaining question is how perifosine is able to simultaneously induce degradation of a group of key proteins in the mTOR axis. A recent study has shown that mTOR can be degraded through a FBXW7-dependent mechanism (33). In our study, we have demonstrated that perifosine induced degradation of mTOR, raptor, rictor and Akt is FBXW7-dependent since deficiency in FBXW7 prevents the degradation of these proteins by perifosine (Fig. 4A). Moreover, we have shown that perifosine-induced degradation of mTOR, raptor, rictor and Akt is also GSK3-dependent because the presence of the GSK3 inhibitor (e.g., LiCl or SB216763) abolished perifosine’s ability to reduce the levels of these proteins (Fig. 4B). Collectively, we conclude that perifosine induces the degradation of several key proteins in the mTOR axis through a GSK3-dependent and FBXW7-mediated mechanism. Given that the majority of FBXW7 substrates are phosphorylated by GSK3 (36), our findings also warrant further study on the role of GSK3 in FBXW7-mediated mTOR degradation.
Since these proteins are tightly associated with mTOR, it is possible that the individual proteins in the mTOR complexes will be stabilized and induction of one or two key components in the complexes may result in simultaneous degradation of other components in the complexes. Indeed, mTOR and raptor have been shown to stabilize each other in the complex (40). Our data clearly show that inhibition of Akt phosphorylaton occurs before the reduction of mTOR, raptor, rictor and Akt (Fig. 2C). Given that FBXW7 is the E3 ubiquitin E3 ligase that is responsible for mTOR degradation (33), it is likely that Akt inhibition by perifosine causes GSK3 activation (dephosphorylation) as we demonstrated previously (12), which in turn phosphorylates mTOR and triggers FBXW7-mediated degradation of mTOR. As a consequence, other proteins such as raptor, rictor and Akt that are tightly associated with mTOR are degraded as well (Fig. 4C). Of course, we cannot rule out the possibility that some of these proteins are also directly subjected to FBXW7-mediated degradation since perifosine increased rictor ubiqutination in our study. Nonetheless, our findings warrant further investigation in these directions.
Interestingly, a recent study reported a similar phenomena that activation of forkhead box protein O1 (FOXO1) in mouse myoblasts results in degradation of several mTOR pathway components including mTOR, raptor, p70S6K and 4EBP1 (34). It is well known that FOXO1 is a transcriptional factor known to be phosphorylated or inhibited by Akt (41). We previously showed that perifosine inhibits Akt as well as GSK3 and FOXO1 phosphorylation in these lung cancer cell lines (e.g., H157 and H460) (12). Thus, whether perifosine inhibits Akt, leading to FOXO1 activation (i. e. dephosphorylation), which in turn triggers the degradation of some key proteins in the mTOR axis, needs further investigation as well.
Given the critical role of mTOR signaling in regulation of autophagy, it may not be surprising to find that perifosine induces autophagy evidenced by increased levels of LC3-II in cells, particularly those sensitive to perifosine, when exposed to perifosine (Fig. 5). Our current and previous (12) studies have shown that perifosine induces apoptosis in these lung cancer cells. Thus, it is clear that perifosine induces autophagy while inducing apoptosis. We noted that the abilities of perifosine to induce both apoptosis and autophagy are positively associated with increased cell sensitivities to perifosine. However, the basal levels of IC3-II in perifosine-resistant cells (e.g., H226) were higher than those in perifosine-sensitive cells (e.g., H460) and minimally increased by perifosine. Because autophagy can be either a pro-survival or a death mechanism depending on the circumstances (24, 25), the intriguing question for us is whether induction of autophagy by perifosine in our cell systems is a death or survival mechanism. In our study, the presence of the lysosomal protease inhibitors such as chloroquine and NH4Cl enhanced perifosine’s ability to decrease cell number and increase apoptosis (Fig. 6). Moreover, the combination of perifosine and chloroquine was more potent than perifosine alone in inhibiting the growth of lung cancer xenografts in mice (Fig. 6). Collectively, these results indicate that autophagy is more likely a pro-survival mechanism that protects cells from perifosine-mediated apoptosis. Accordingly, inhibition of autophagy with a lysosomal protease inhibitor such as chloroquine potentiates perifosine’s apoptosis-inducing activity and anticancer activity. Thus, our findings in this study may suggest a strategy in the clinic to enhance perifosine’s anticancer efficacy by combining perifosine with an autophagic inhibitor particularly when single-agent activity of perifosine in many common solid tumors were not encouraging (3).
Supplementary Material
Acknowledgements
We are grateful to Dr. B. Vogelstein for providing FBXW7 wild-type and null HCT116 cell lines and Dr. Heath Elrod in the lab for editing the manuscript.
Grant support: The Georgia Cancer Coalition Distinguished Cancer Scholar award (S-Y. S.), NIH RO1 grant CA118450 (S-Y. S.), NIH SPORE P50 grant CA128613 (S-Y. Sun and F. R. K. for Project 2), NIH lung cancer PO1 grant CA116676 (K.R. Fadlo and S-Y.Sun for Project 1) and DOD BATTLE award W81XWH-06-1-0303 (F.R. K.and S-Y. S. for Project 4).
References
- 1.Hilgard P, Klenner T, Stekar J, Nossner G, Kutscher B, Engel J. D-21266, a new heterocyclic alkylphospholipid with antitumour activity. Eur J Cancer. 1997;33:442–446. doi: 10.1016/s0959-8049(97)89020-x. [DOI] [PubMed] [Google Scholar]
- 2.Vink SR, Schellens JH, van Blitterswijk WJ, Verheij M. Tumor and normal tissue pharmacokinetics of perifosine, an oral anti-cancer alkylphospholipid. Invest New Drugs. 2005;23:279–286. doi: 10.1007/s10637-005-1436-0. [DOI] [PubMed] [Google Scholar]
- 3.Gills JJ, Dennis PA. Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep. 2009;11:102–110. doi: 10.1007/s11912-009-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephenson J, Schreeder M, Waples J, et al. Perifosien (P), active as a single agent for renal cell carcinoma (RCC), now in phase I trials combined with tryosine kinase inhibitors (TKI) Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceddings Part I. 2007;25:15622. [Google Scholar]
- 5.Dasmahapatra GP, Didolkar P, Alley MC, Ghosh S, Sausville EA, Roy KK. In vitro combination treatment with perifosine and UCN-01 demonstrates synergism against prostate (PC-3) and lung (A549) epithelial adenocarcinoma cell lines. Clin Cancer Res. 2004;10:5242–5252. doi: 10.1158/1078-0432.CCR-03-0534. [DOI] [PubMed] [Google Scholar]
- 6.Rahmani M, Reese E, Dai Y, et al. Coadministration of histone deacetylase inhibitors and perifosine synergistically induces apoptosis in human leukemia cells through Akt and ERK1/2 inactivation and the generation of ceramide and reactive oxygen species. Cancer Res. 2005;65:2422–2432. doi: 10.1158/0008-5472.CAN-04-2440. [DOI] [PubMed] [Google Scholar]
- 7.Nyakern M, Cappellini A, Mantovani I, Martelli AM. Synergistic induction of apoptosis in human leukemia T cells by the Akt inhibitor perifosine and etoposide through activation of intrinsic and Fas-mediated extrinsic cell death pathways. Mol Cancer Ther. 2006;5:1559–1570. doi: 10.1158/1535-7163.MCT-06-0076. [DOI] [PubMed] [Google Scholar]
- 8.Ruiter GA, Verheij M, Zerp SF, van Blitterswijk WJ. Alkyl-lysophospholipids as anticancer agents and enhancers of radiation-induced apoptosis. Int J Radiat Oncol Biol Phys. 2001;49:415–419. doi: 10.1016/s0360-3016(00)01476-0. [DOI] [PubMed] [Google Scholar]
- 9.Patel V, Lahusen T, Sy T, Sausville EA, Gutkind JS, Senderowicz AM. Perifosine, a novel alkylphospholipid, induces p21(WAF1) expression in squamous carcinoma cells through a p53-independent pathway, leading to loss in cyclin-dependent kinase activity and cell cycle arrest. Cancer Res. 2002;62:1401–1409. [PubMed] [Google Scholar]
- 10.Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2:1093–1103. [PubMed] [Google Scholar]
- 11.Li X, Luwor R, Lu Y, Liang K, Fan Z. Enhancement of antitumor activity of the anti-EGF receptor monoclonal antibody cetuximab/C225 by perifosine in PTEN-deficient cancer cells. Oncogene. 2006;25:525–535. doi: 10.1038/sj.onc.1209075. [DOI] [PubMed] [Google Scholar]
- 12.Elrod HA, Lin YD, Yue P, et al. The alkylphospholipid perifosine induces apoptosis of human lung cancer cells requiring inhibition of Akt and activation of the extrinsic apoptotic pathway. Mol Cancer Ther. 2007;6:2029–2038. doi: 10.1158/1535-7163.MCT-07-0004. [DOI] [PubMed] [Google Scholar]
- 13.Hennessy BT, Lu Y, Poradosu E, et al. Pharmacodynamic markers of perifosine efficacy. Clin Cancer Res. 2007;13:7421–7431. doi: 10.1158/1078-0432.CCR-07-0760. [DOI] [PubMed] [Google Scholar]
- 14.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 15.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 16.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 18.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 19.Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11:353–361. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 21.Manning BD, Cantley LC. United at last: the tuberous sclerosis complex gene products connect the phosphoinositide 3-kinase/Akt pathway to mammalian target of rapamycin (mTOR) signalling. Biochem Soc Trans. 2003;31:573–578. doi: 10.1042/bst0310573. [DOI] [PubMed] [Google Scholar]
- 22.Sawyers CL. Will mTOR inhibitors make it as cancer drugs? Cancer Cell. 2003;4:343–348. doi: 10.1016/s1535-6108(03)00275-7. [DOI] [PubMed] [Google Scholar]
- 23.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. The New England journal of medicine. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 24.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6:304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- 27.Sun SY, Yue P, Dawson MI, et al. Differential effects of synthetic nuclear retinoid receptor-selective retinoids on the growth of human non-small cell lung carcinoma cells. Cancer Res. 1997;57:4931–4939. [PubMed] [Google Scholar]
- 28.Liu X, Yue P, Zhou Z, Khuri FR, Sun SY. Death receptor regulation and celecoxib-induced apoptosis in human lung cancer cells. J Natl Cancer Inst. 2004;96:1769–1780. doi: 10.1093/jnci/djh322. [DOI] [PubMed] [Google Scholar]
- 29.Sun SY, Yue P, Wu GS, et al. Mechanisms of apoptosis induced by the synthetic retinoid CD437 in human non-small cell lung carcinoma cells. Oncogene. 1999;18:2357–2365. doi: 10.1038/sj.onc.1202543. [DOI] [PubMed] [Google Scholar]
- 30.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 31.Sarbassov dos D, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 32.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 33.Mao JH, Kim IJ, Wu D, et al. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science. 2008;321:1499–1502. doi: 10.1126/science.1162981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu AL, Kim JH, Zhang C, Unterman TG, Chen J. Forkhead box protein O1 negatively regulates skeletal myocyte differentiation through degradation of mammalian target of rapamycin pathway components. Endocrinology. 2008;149:1407–1414. doi: 10.1210/en.2007-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajagopalan H, Jallepalli PV, Rago C, et al. Inactivation of hCDC4 can cause chromosomal instability. Nature. 2004;428:77–81. doi: 10.1038/nature02313. [DOI] [PubMed] [Google Scholar]
- 36.Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- 37.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 38.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Momota H, Nerio E, Holland EC. Perifosine Inhibits Multiple Signaling Pathways in Glial Progenitors and Cooperates With Temozolomide to Arrest Cell Proliferation in Gliomas In vivo. Cancer Res. 2005;65:7429–7435. doi: 10.1158/0008-5472.CAN-05-1042. [DOI] [PubMed] [Google Scholar]
- 40.Kim DH, Sarbassov DD, Ali SM, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 41.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.